Solid State and Solution Structures of Lanthanide Nitrate Complexes of Tris-(1-napthylphosphine oxide)
Abstract
1. Introduction
2. Results and Discussion
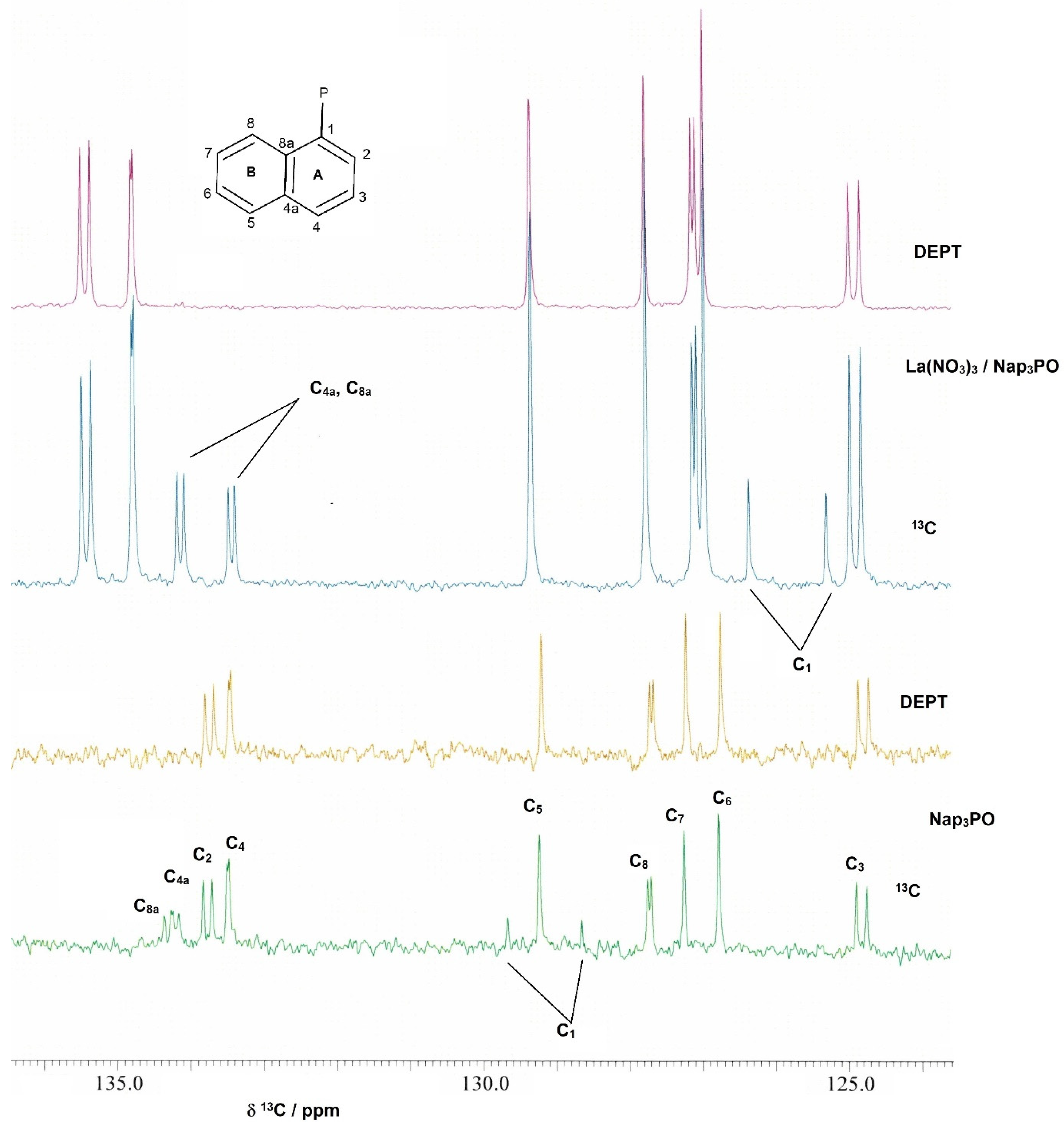
2.1. Solution Studies
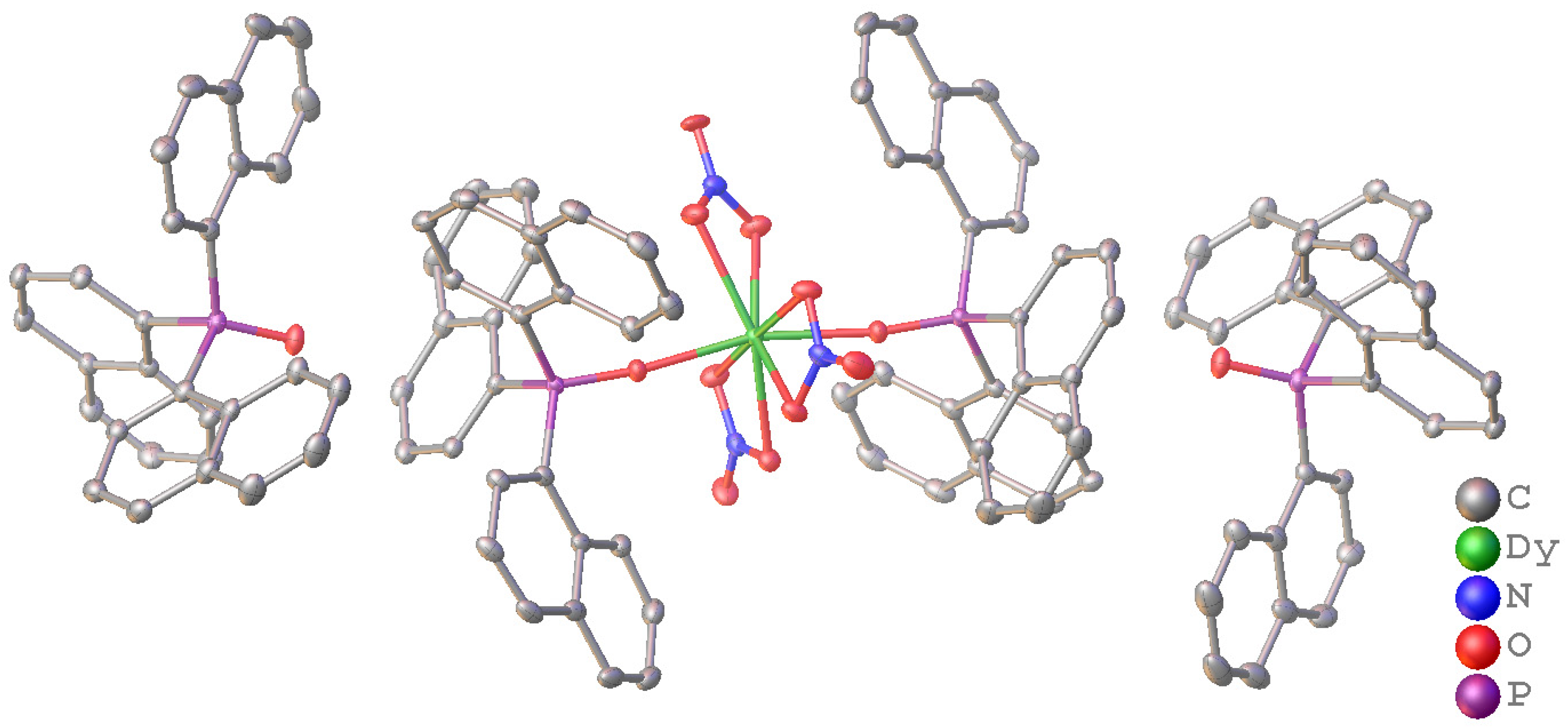
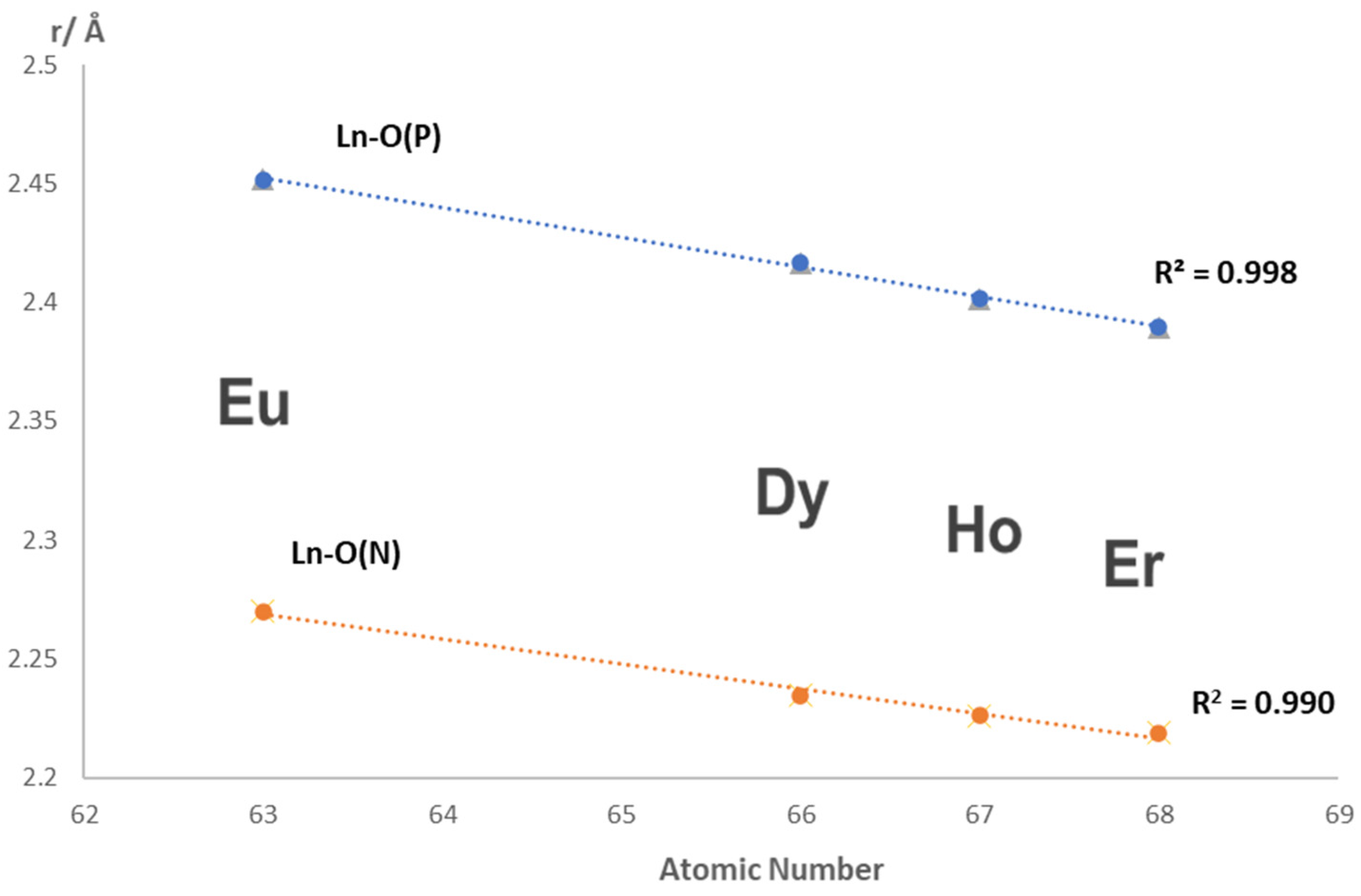
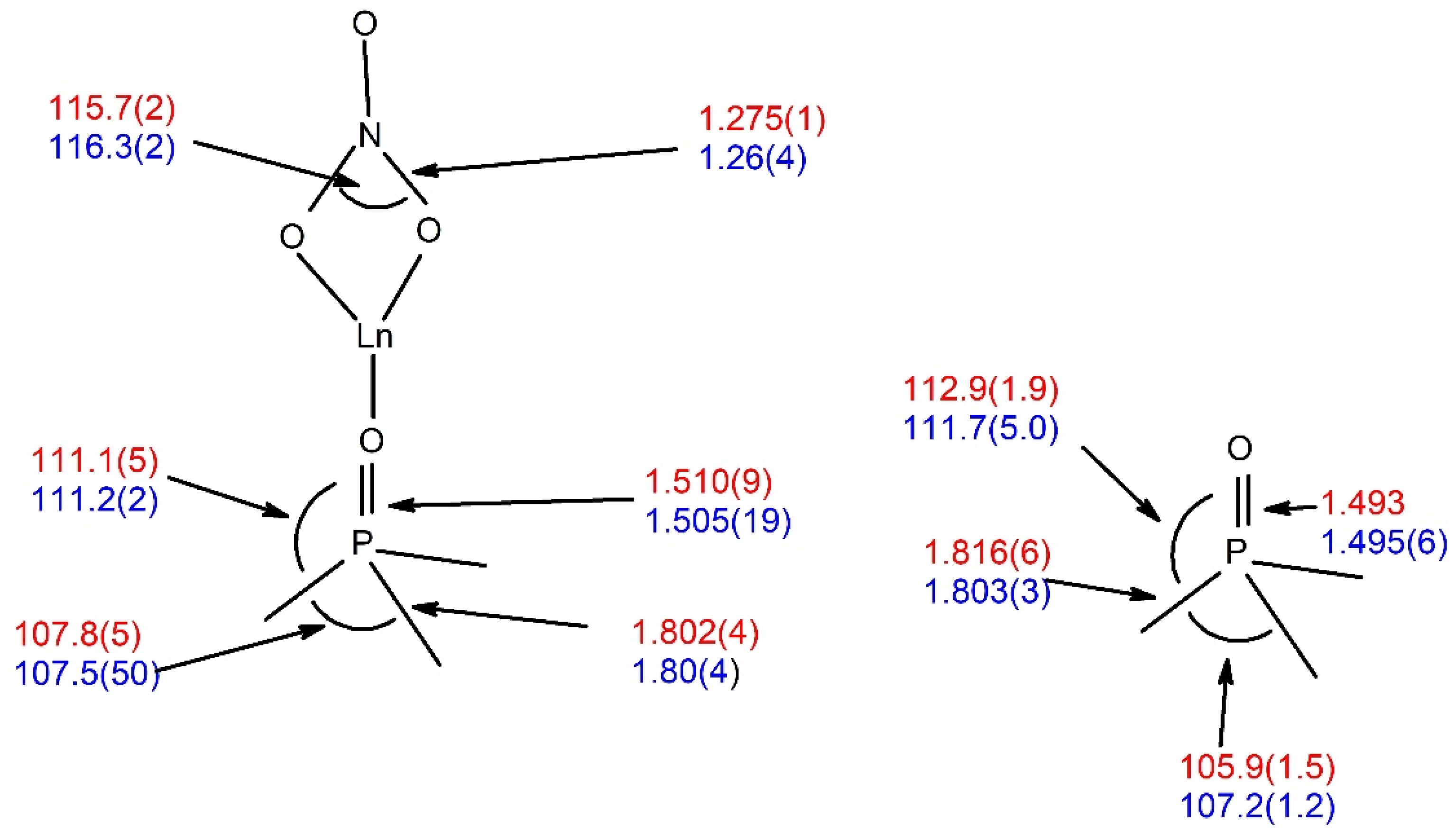
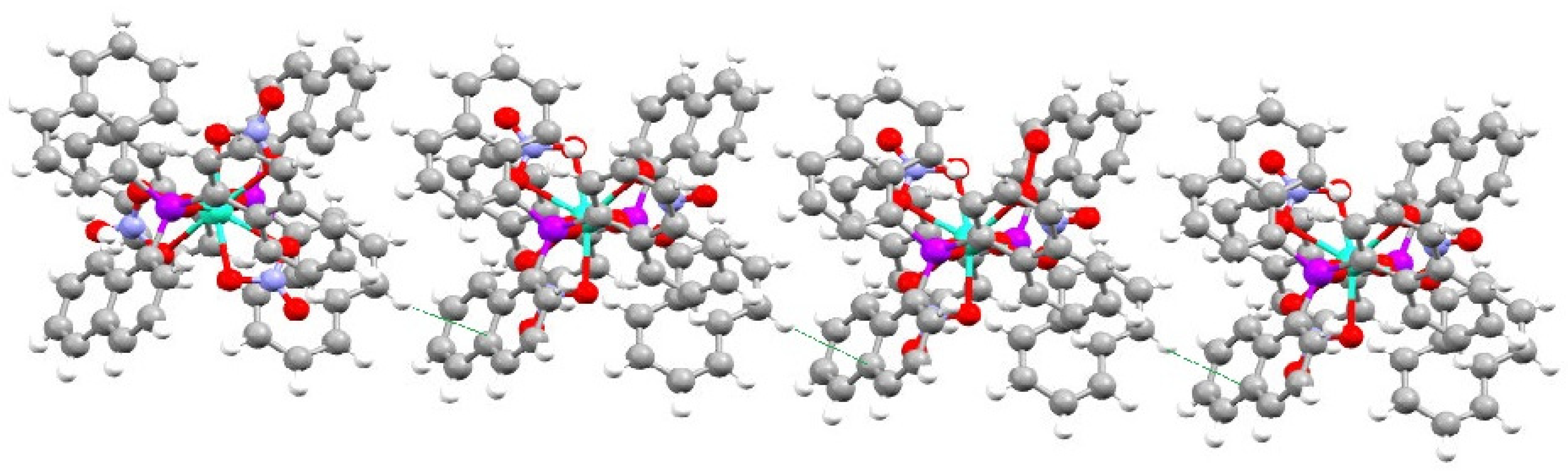
2.2. Crystal Structures of Nap3PO and Its Complexes
3. Materials and Methods
3.1. Crystallography
3.2. NMR Spectroscopy
3.3. Infrared Spectroscopy
3.4. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cousins, D.R.; Hart, F.A. Lanthanide complexes—IV: Complexes of triphenylphosphine oxide with lanthanide and yttrium nitrates. J. Inorg. Nucl. Chem. 1967, 29, 1745–1757. [Google Scholar] [CrossRef]
- Cousins, D.R.; Hart, F.A. Lanthanide complexes—VII: Complexes of yttrium and lanthanide chlorides and thiocyanates with triphenylarsine oxide and triphenylphosphine oxide. J. Inorg. Nucl. Chem. 1968, 30, 3009–3015. [Google Scholar] [CrossRef]
- Levason, W.; Newman, E.H.; Webster, M. (Ethanol-O)tris(nitrato-O,O′)bis(triphenylphosphine oxide-O)cerium(III). Acta Cryst. 2000, C56, 1308–1309. [Google Scholar] [CrossRef] [PubMed]
- Levason, W.; Newman, E.H.; Webster, M. Tetrakis(triphenylphosphine oxide) complexes of the lanthanide nitrates; synthesis, characterisation and crystal structures of [La(Ph3PO)4(NO3)3]·Me2CO and [Lu(Ph3PO)4(NO3)2]NO3. Polyhedron 2000, 19, 2697–2705. [Google Scholar] [CrossRef]
- Valle, G.; Casotto, G.; Zanonato, P.L.; Zarbi, B. Crystal and molecular structures of two europium(III) nitrate complexes with triphenylphosphine oxide. Polyhedron 1986, 5, 2093–2096. [Google Scholar] [CrossRef]
- Langley, S.K.; Vignesh, K.R.; Hotton, K.; Benjamin, S.; Hix, G.B.; Phonsri, W.; Manbaraki, B.; Murray, K.S.; Rajaramon, G. Mononuclear Dysprosium(III) Complexes with Triphenylphosphine Oxide Ligands: Controlling the Coordination Environment and Magnetic Anisotropy. Inorganics 2018, 6, 61. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoria-Vazquez, J.; Garcia-Deibe, A.M.; Sanmartin-Matalobos, J.M.; Herrera, J.M.; Colacio, E. Field-Induced Single Molecule Magnets of Phosphine- and Arsine-Oxides. Front. Chem. 2018, 6, 420. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Dey, S.; Gupta, S.K.; Walawakar, M.G.; Rajaraman, G.; Murugard, R. Enhancing the barrier height for Yb(iii) single-ion magnets by modulating axial ligand fields. Chem. Commun. 2020, 56, 11879–11882. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Kitagaura, Y.; Hosegawa, Y.; Imoto, H.; Naka, K. Drastic Enhancement of Photosensitized Energy Transfer Efficiency of a Eu(III) Complex Driven by Arsenic. Inorg. Chem. 2021, 60, 8605–8612. [Google Scholar] [CrossRef]
- Liu, H.; Deng, C.; Chen, Q.; Shen, X. Europium-containing ionic liquids and crystal complexes based on phosphine oxides. Polyhedron 2016, 117, 309–317. [Google Scholar] [CrossRef]
- Hou, S.; Liu, M.; Han, H.-L.; Jin, Q.-H.; Hou, J.; Su, W.; Chen, Y.-Y.; Yao, J.-Y. Synthesis, structures and luminescence properties of nine lanthanide complexes with triphenylphospine oxide and phenanthroline. Polyhedron 2015, 85, 69–75. [Google Scholar]
- Mu, Y.; Xu, S.; Wang, X.; Liu, M.; Li, Y.-X.; Xin, X.-L.; Jin, Q.-H. Triphenylphosphane Oxide Complexes of Lanthanide Nitrates: Polymorphs and Photophysics. Z. Allorg. Allg. Chem. 2017, 643, 780–788. [Google Scholar]
- Barnes, F.H.; Kelly, A.W.; Melzer, H.; Patterson, H.H.; Pike, R.D. Synthesis, Structures, and Luminescent Properties of Lanthanide Complexes with Triphenylphospine Oxide. Z. Anorg. Allg. Chem. 2018, 644, 525–533. [Google Scholar] [CrossRef]
- Arumugama, R.; Shankar, B.; Sathiyendiranc, T.A.M. The first use of tri(1-naphthyl)phosphine oxide as ligand for rhenium(I)-complexes from phosphine via a one-pot approach. J. Organomet. Chem. 2021, 933, 121657. [Google Scholar] [CrossRef]
- Haav, K.; Saame, J.; Kütt, A.; Leito, I. Basicity of Phosphanes and Diphosphanes in Acetonitrile. Eur. J. Org. Chem. 2012, 2012, 2167–2172. [Google Scholar] [CrossRef]
- Chahma, M.; Myles, D.J.T.; Hicks, R.G. Synthesis, characterisation and coordination chemistry of phosphines with ethylenedioxythiophene substituents. Can. J. Chem. 2005, 83, 150–155. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 5th ed.; John Wiley and Sons: New York, NY, USA, 1997. [Google Scholar]
- Emsley, J.W.; Salman, S.R.; Storey, R.A. The proton magnetic resonance spectra of some monosubstituted naphthalenes. J. Chem. Soc. B 1970, 1513–1516. [Google Scholar] [CrossRef]
- Barfield, M.; Chakrabarti, B. Valence-bond studies of contact nuclear spin-spin coupling. III. pi.-Electron coupling in aromatic and cyclic unsaturated hydrocarbons. J. Am. Chem. Soc. 1969, 91, 4346–4352. [Google Scholar] [CrossRef]
- Quali, N.; Rivera, J.-P.; Chapon, D.; Delange, P.; Piguet, C. The Solution Structure of Rhombic Lanthanide Complexes Analyzed with a Model-Free and Crystal-Field Independent Paramagnetic NMR Method: Application to Nonaxial Trimetallic Complexes [LnxLu3-x(TACI-3H)2(H2O)6]3+ (x = 1−3). Inorg. Chem. 2004, 43, 1517–1529. [Google Scholar]
- Geraldes, C.F.G.C.; Zhang, S.; Sherry, A.D. Comparison of crystal field dependent and independent methods to analyse lanthanide induced NMR shifts in axially symmetric complexes. Part I. Systems with a C3 symmetry axis. Inorg. Chim. Acta 2004, 357, 381–395. [Google Scholar] [CrossRef]
- Rubini, P.; Ben Nasr, C.; Rodehuser, L.; Delpuech, J.-J. Complexation of lanthanide(III) ions with a β-diphosphorylated ligand: Nonamethylimidodiphosphoramide (NIPA). A 1H and 31P NMR study. Magn. Reson. Chem. 1987, 25, 609–618. [Google Scholar] [CrossRef]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE—Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Version 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Mantina, M.; Chamberlin, A.C.; Valero, R.; Cramer, C.J.; Truhlar, D.G. Consistent van der Waals radii for the whole main group. J. Phys. Chem. A 2009, 113, 5806–5812. [Google Scholar] [CrossRef] [PubMed]
- Bowden, A.; Coles, S.J.; Pitak, M.B.; Platt, A.W.G. Complexes of Lanthanide Nitrates with tri-tert Butlyphosphine Oxide. Inorg. Chem. 2012, 51, 4379–4390. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gomez-Coca, S.; Dolinar, B.S.; Yang, L.; Yu, F.; Kong, M.; Yang, Y.-Q.; Song, Y.; Dunbar, K.R. Hexagonal Bipyramidal Complexes as Structural Archetypes for Single Molecule Magnets. Inorg. Chem. 2019, 58, 2610–2617. [Google Scholar] [CrossRef]
- Hunter, A.P.; Lees, A.M.J.; Platt, A.W.G. Synthesis, structures and mass spectrometry of lanthanide nitrate Complexes with tricyclohexylphosphine oxide. Polyhedron 2007, 26, 4865–4876. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with ShelXL. Acta Cryst. 2015, C27, 3–8. [Google Scholar]
- Sheldrick, G.M. ShelXT-Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlisPro Software System; Rigaku Oxford Diffraction: Cedar Park, TX, USA, 2019.
- Coles, S.J.; Gale, P.A. Changing and challenging times for service crystallography. Chem. Sci. 2012, 3, 683–689. [Google Scholar] [CrossRef]


| CD3CN a | CDCl3 b | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 °C | 80 °C | 30 °C | 60 °C | |||||||||
| δ c | w1/2 d | δ c | w1/2 d | δfree c | w1/2 d | δcoord c | w1/2 d | δfree c | w1/2 d | δcoord c | w1/2 d | |
| La | 42.3 | 19 | 42.9 | 13 | ||||||||
| Ce | 79.3 | 65 | 73.9 | 44 | ||||||||
| Pr | 119.7 | 200 | 113.2 | 120 | ||||||||
| Nd | 111.1 | 300 | 108.9 | 100 | ||||||||
| Eu | −115.2 | 450 | −99.2 | 400 | 41.8 | 80 | Not Obs | 40.3 | 45 | −67.6 | 300 | |
| Tb | −206 | 5500 | −185 | 1100 | ||||||||
| Dy | −86.6 | 2500 | −94 | 600 | 41.0 | 40 | −11.2 | 4500 | 40.4 | 110 | −52.1 | 1300 |
| Ho | −113.3 | 3000 | −111.1 | 750 | 41.0 | 175 | −204.0 | 600 | ||||
| Er | 41.0 | 60 | −235.7 | 200 | 40.3 | 40.3 | −198.5 | |||||
| Tm | −110.2 | 2000 | −85.8 | 300 | ||||||||
| Yb | −1.6 | 1600 | 13.1 | 1000 | 40.8 | 75 | 31.8 | 440 | 40.1 | 150 | 33.7 | 1000 |
| Lu | 42.8 | 20 | 43.8 | 13 | 40.8 | 30 | 49.2 | 190 | 40.3 | 180 | 47.9 | 200 |
| Nap3PO b | 39.2 | 7 | 41.1 | 12 | 40.1 | 7 | ||||||
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| δ (ppm)/JC–P (Hz) | ||||||||||
| C1 | C2 | C3 | C4 | C4a | C5 | C6 | C7 | C8 | C8a | |
| Nap3PO | 129.0/101.6 | 133.7/11.5 | 124.7/14.4 | 133.4/2.8 | 134.1/7.6 | 129.1 | 126.7 | 127.2 | 127.6/5.8 | 134.2/9.5 |
| La | 125.8/106.4 | 135.3/12.5 | 124.8/15.3 | 134.7/2.9 | 133.4/7.7 | 129.2 | 126.9 | 127.7 | 127.0/5.7 | 134.1/9.5 |
| Ce | 127.6/104.4 | 136.5/12.5 | 125.3/14.4 | 135.0/2.9 | 134.3/8.6 | 129.2 | 126.7 | 127.3 | 127.1/2.4 | 134.4/10.4 |
| Pr | 130.7/111.2 | 139.1/12.5 | 126.3/15.4 | 135.3 | 135.1 | 129.5 | 126.7 | 127.1 | 128.3/4.8 | 136.5 |
| Nd | 123.9/126.5 | 135.0/14.4 | 125.1/11.5 | 136.1 | 133.6 | 129.3 | 127.4 | 126.8 | 126.2 | 134.0 |
| Eu | 128.6/110.3 | 133.9/12.5 | 124.5/15.3 | 134.4 | 132.1/7.6 | 129.2 | 127.1 | 127.9 | 127.8 | 133.6/8.6 |
| Lu | 125.8/107.3 | 135.3/12.5 | 124.9/14.8 | 136.7/2.9 | 133.2/8.6 | 129.3 | 126.9 | 127.6 | 127.0/5.8 | 133.9/9.5 |
| Eu | Dy | Ho | Er | Average | ||
|---|---|---|---|---|---|---|
| PO-Ln-r a | Ph3PO | 1.199 | 1.199 | 1.185 | 1.194 | 1.194(6) |
| PO-Ln-r b | Nap3PO | 1.204 | 1.208 | 1.213 | 1.212 | 1.209(4) |
| NO-Ln-r b | Ph3PO | 1.378 | 1.389 | 1.377 | 1.389 | 1.383(5) |
| NO-Ln-r b | Nap3PO | 1.386 | 1.390 | 1.387 | 1.386 | 1.387(2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coles, S.J.; McCormick McPherson, L.J.; Platt, A.W.G.; Singh, K. Solid State and Solution Structures of Lanthanide Nitrate Complexes of Tris-(1-napthylphosphine oxide). Molecules 2024, 29, 2580. https://doi.org/10.3390/molecules29112580
Coles SJ, McCormick McPherson LJ, Platt AWG, Singh K. Solid State and Solution Structures of Lanthanide Nitrate Complexes of Tris-(1-napthylphosphine oxide). Molecules. 2024; 29(11):2580. https://doi.org/10.3390/molecules29112580
Chicago/Turabian StyleColes, Simon J., Laura J. McCormick McPherson, Andrew W. G. Platt, and Kuldip Singh. 2024. "Solid State and Solution Structures of Lanthanide Nitrate Complexes of Tris-(1-napthylphosphine oxide)" Molecules 29, no. 11: 2580. https://doi.org/10.3390/molecules29112580
APA StyleColes, S. J., McCormick McPherson, L. J., Platt, A. W. G., & Singh, K. (2024). Solid State and Solution Structures of Lanthanide Nitrate Complexes of Tris-(1-napthylphosphine oxide). Molecules, 29(11), 2580. https://doi.org/10.3390/molecules29112580






