O-Phthalaldehyde Derivatization for the Paper-Based Fluorometric Determination of Glutathione in Nutritional Supplements
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization Procedures
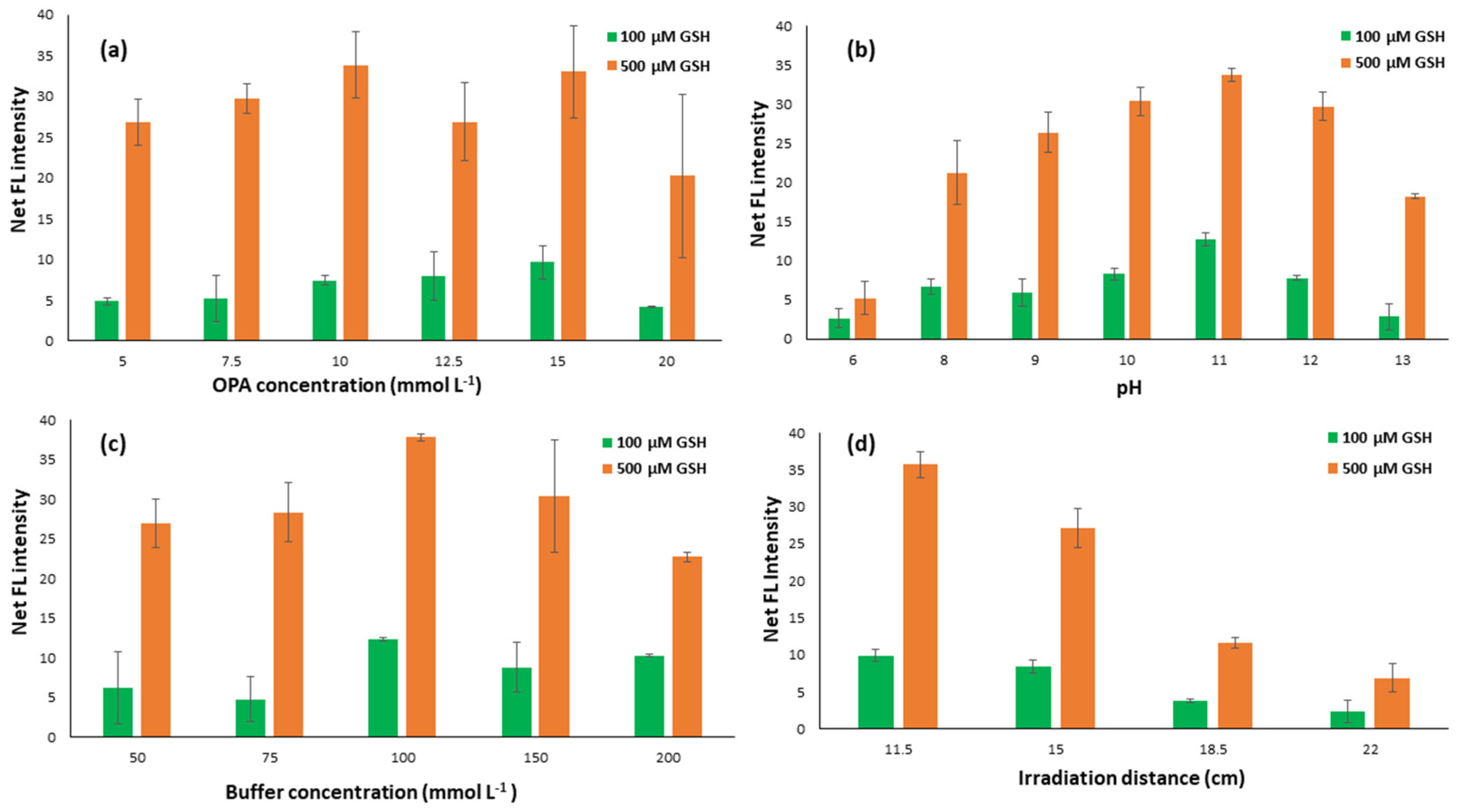
2.1.1. Effect of OPA Concentration
2.1.2. Effect of pH and Buffer Concentration
2.1.3. Effect of UV Irradiation Distance
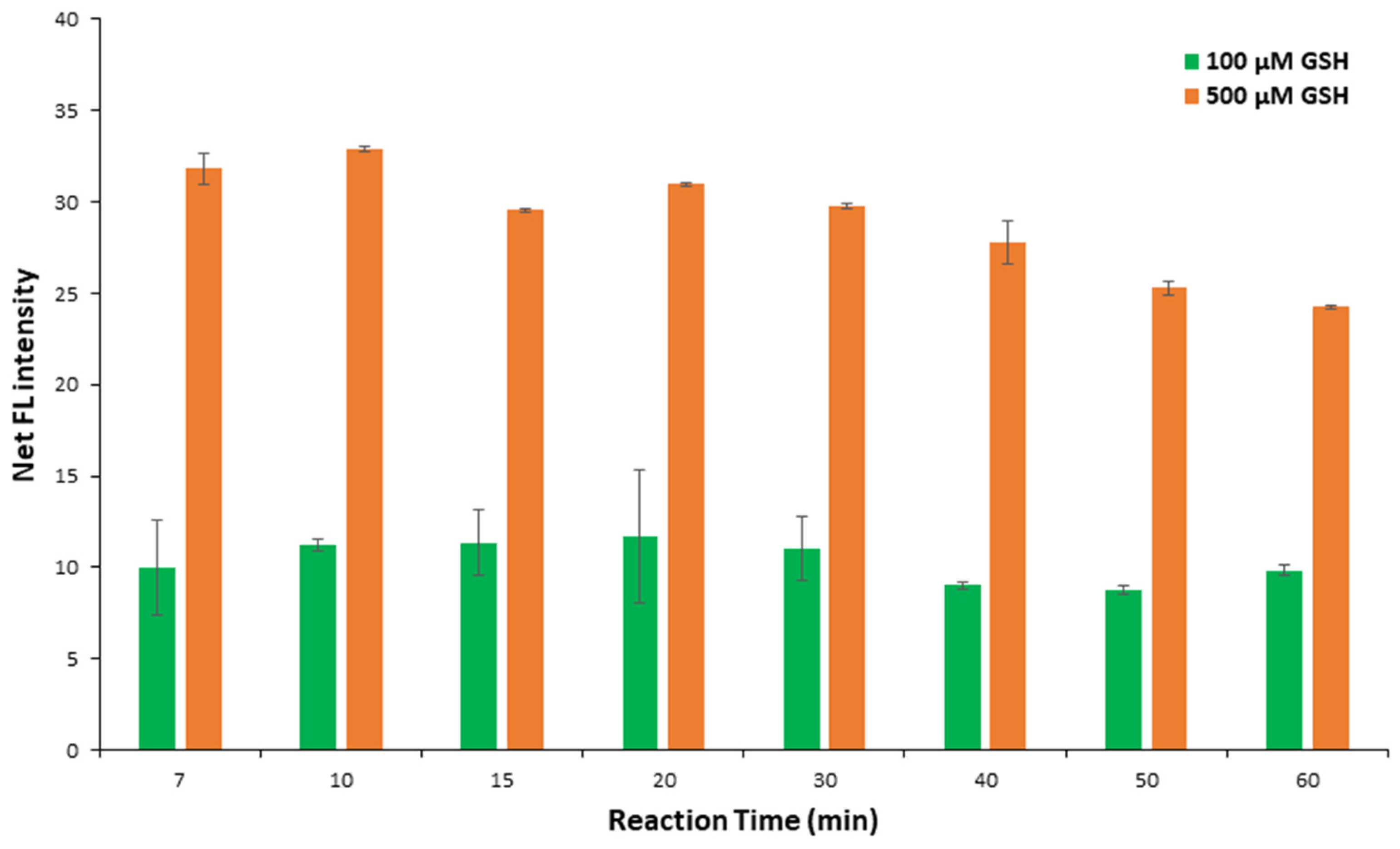
2.1.4. Effect of Reaction Time
2.1.5. Effect of Reagent Sequence
2.2. Analytical Parameters
2.2.1. Figures of Merit
2.2.2. Selectivity
2.2.3. Stability of the Devices
2.3. Application in Nutritional Supplement Samples
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Apparatus
3.3. Fabrication of the Paper Devices
3.4. Experimental Procedure
3.5. Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Richie, J.P., Jr.; Skowronski, L.; Abraham, P.; Leutzinger, Y. Blood glutathione concentrations in a large-scale human study. Clin. Chem. 1996, 42, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M.; Brown, B.I. A review of dietary (phyto)nutrients for glutathione support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Kanďár, R.; Vrbová, M.; Čandová, J. An assay of total glutathione and glutathione disulfide in human whole blood and plasma using a high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. Relat Technol. 2013, 36, 2013–2028. [Google Scholar] [CrossRef]
- Tsiasioti, A.; Tzanavaras, P.D. Determination of glutathione and glutathione disulfide using liquid chromatography: A review on recent applications. Microchem. J. 2023, 193, 109157. [Google Scholar] [CrossRef]
- Marchand, S.; de Revel, G. A HPLC fluorescence-based method for glutathione derivatives quantification in must and wine. Anal. Chim. Acta 2010, 660, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Tsiasioti, A.; Iakovidou, I.; Zacharis, C.K.; Tzanavaras, P.D. Automated fluorimetric sensor for glutathione based on zone fluidics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 229, 117963. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-W.; Tseng, W.-L. Gold nanoparticle extraction followed by capillary electrophoresis to determine the total, free, and protein-bound aminothiols in plasma. Anal. Chem. 2010, 82, 2696–2702. [Google Scholar] [CrossRef]
- White, P.C.; Lawrence, N.S.; Davis, J.; Compton, R.G. Electrochemical determination of thiols: A perspective. Electroanalysis 2002, 14, 89–98. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Chen, J.; Wang, S.; Huang, X. Sensitive and selective detection of glutathione based on resonance light scattering using sensitive gold nanoparticles as colorimetric probes. Analyst 2012, 137, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Seiwert, B.; Karst, U. Simultaneous LC/MS/MS determination of thiols and disulfides in urine samples based on differential labeling with ferrocene-based maleimides. Anal. Chem. 2007, 79, 7131–7138. [Google Scholar] [CrossRef] [PubMed]
- Thiel, A.; Weishaupt, A.-K.; Nicolai, M.M.; Lossow, K.; Kipp, A.P.; Schwerdtle, T.; Bornhorst, J. Simultaneous quantitation of oxidized and reduced glutathione via LC-MS/MS to study the redox state and drug-mediated modulation in cells, worms and animal tissue. J. Chromatogr. B 2023, 1225, 123742. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhang, Y.; Guo, Z.; Liu, D.; Zuo, G. A Sensitive chemiluminescence sensor for glutathione detection based on Au supported carbon nitride catalyst. Microchem. J. 2020, 159, 105435. [Google Scholar] [CrossRef]
- Liu, X.; Fan, Q.; Zhang, X.; Li, M.; Huan, Y.; Ma, P.; Song, D.; Fei, Q. A FeS2NPs-Luminol-MnO2NSs system based on chemiluminescence resonance energy transfer platform for sensing glutathione. Talanta 2022, 240, 123171. [Google Scholar] [CrossRef] [PubMed]
- Tsogas, G.Z.; Kappi, F.A.; Vlessidis, A.G.; Giokas, D.L. Recent advances in nanomaterial probes for optical biothiol sensing: A review. Anal. Lett. 2018, 51, 443–468. [Google Scholar] [CrossRef]
- Li, L.; Wang, Q.; Chen, Z. Colorimetric detection of glutathione based on its inhibitory effect on the peroxidase-mimicking properties of WS2 nanosheets. Microchim. Acta 2019, 186, 257. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Zhang, Y.; Zhang, L.; Su, Y.; Lv, Y. Colorimetric detection of glutathione in human blood serum based on the reduction of oxidized TMB. N. J. Chem. 2013, 37, 2174–2178. [Google Scholar] [CrossRef]
- Xu, H.-H.; Deng, H.-H.; Lin, X.-Q.; Wu, Y.-Y.; Lin, X.-L.; Peng, H.-P.; Liu, A.-L.; Xia, X.-H.; Chen, W. Colorimetric glutathione assay based on the peroxidase-like activity of a nanocomposite consisting of platinum nanoparticles and graphene oxide. Microchim. Acta 2017, 184, 3945–3951. [Google Scholar] [CrossRef]
- Di, W.; Zhang, X.; Qin, W. Single-layer MnO2 nanosheets for sensitive and selective detection of glutathione by a colorimetric method. Appl. Surf. Sci. 2017, 400, 200–205. [Google Scholar] [CrossRef]
- Ma, Z.; Wu, T.; Li, P.; Liu, M.; Huang, S.; Li, H.; Zhang, Y.; Yao, S. A dual (colorimetric and fluorometric) detection scheme for glutathione and silver (I) based on the oxidase mimicking activity of MnO2 nanosheets. Microchim. Acta 2019, 186, 498. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, Y.; Wei, W.; Huang, J.; Lu, D.; Liu, S.; Shi, X. A ratiometric fluorescence-based colorimetric sensor for the portable analysis of antioxidants via smartphone. Microchem. J. 2023, 190, 108625. [Google Scholar] [CrossRef]
- He, M.; Shang, N.; Zheng, B.; Yue, G. An ultrasensitive colorimetric and fluorescence dual-readout assay for glutathione with a carbon dot–MnO2 nanosheet platform based on the inner filter effect. RSC Adv. 2021, 11, 21137–21144. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Lin, X.; Zhang, J.; Hu, Z.; Wu, F. Facile preparation of carbon dots with multicolor emission for fluorescence detection of ascorbic acid, glutathione and moisture content. J. Lumin. 2023, 264, 120169. [Google Scholar] [CrossRef]
- Zacharis, C.K.; Tzanavaras, P.D.; Zotou, A. Ethyl Propiolate as a post-column derivatization reagent for thiols: Development of a green liquid chromatographic method for the determination of glutathione in vegetables. Anal. Chim. Acta 2011, 690, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, A.; Tsiasioti, A.; Tzanavaras, P.D. Extraction and determination of free glutathione and its disulfide in various flour samples using liquid chromatography and on-line post column derivatization. Microchem. J. 2023, 193, 109145. [Google Scholar] [CrossRef]
- Tsiasioti, A.; Tzanavaras, P.D. Pulsed-post column derivatization coupled to green liquid chromatography for the determination of glutathione and cysteine based on thioacrylates formation. Talanta 2023, 259, 124488. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, Z.; Malekian Jebeli, F.; Ghasemzadeh, S. Peroxidase-mimetic activity of FeOCl nanosheets for the colorimetric determination of glutathione and cysteine. Microchim. Acta 2021, 188, 239. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Cai, R.; Chen, X.; Wu, Q.; Zhang, L.; Jiang, Y.; Cui, C.; Wan, S.; Tan, W. Facile approach to prepare HSA-templated MnO2 nanosheets as oxidase mimic for colorimetric detection of gutathione. Talanta 2019, 195, 40–45. [Google Scholar] [CrossRef]
- Luo, N.; Yang, Z.; Tang, F.; Wang, D.; Feng, M.; Liao, X.; Yang, X. Fe3O4/Carbon nanodot hybrid nanoparticles for the indirect colorimetric detection of glutathione. ACS Appl. Nano Mater. 2019, 2, 3951–3959. [Google Scholar] [CrossRef]
- Xian, Z.; Zhang, L.; Yu, Y.; Lin, B.; Wang, Y.; Guo, M.; Cao, Y. Nanozyme based on CoFe2O4 modified with MoS2 for colorimetric determination of cysteine and glutathione. Microchim. Acta 2021, 188, 65. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Liu, P.; Jin, C.; Shi, Z.; Luo, X.; Liu, Q. Perylene diimide-functionalized CeO2 nanocomposite as a peroxidase mimic for colorimetric determination of hydrogen peroxide and glutathione. Microchim. Acta 2019, 186, 332. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yang, T.; Lan, J.; Huang, C. Use of the peroxidase mimetic activity of erythrocyte-like Cu1.8S nanoparticles in the colorimetric determination of glutathione. Anal. Methods 2017, 9, 841–846. [Google Scholar] [CrossRef]
- Guan, H.; Han, B.; Peng, B.; Ye, H.; Zhang, Q.; Wang, D. Magnetic supported gold-copper bimetallic organic framework nanocomposite as a novel nanozyme for ultra-fast point-of-care colorimetric assay of glutathione. Microchem. J. 2023, 194, 109311. [Google Scholar] [CrossRef]
- Chen, X.; Zhuang, Y.; Chen, J.; Lin, J.; Chen, J.; Han, Z. Novel ratiometric fluorescence and colorimetric dual-mode biosensors for Cu2+ and biothiols detection based on norepinephrine modified carbon dots. Microchem. J. 2022, 181, 107828. [Google Scholar] [CrossRef]
- Liang, B.; Wang, B.; Ma, Q.; Xie, C.; Li, X.; Wang, S. A Lysosome-targetable turn-on fluorescent probe for the detection of thiols in living cells based on a 1,8-Naphthalimide derivative. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2018, 192, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kand’ár, R.; Žáková, P.; Lotková, H.; Kučera, O.; Červinková, Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J. Pharm. Biomed. Anal. 2007, 43, 1382–1387. [Google Scholar] [CrossRef]
- Tarara, M.; Tsiasioti, A.; Tzanavaras, P.D.; Tsogas, G.Z. Development of an equipment free paper based fluorimetric method for the selective determination of histidine in human urine samples. Talanta 2022, 249, 123685. [Google Scholar] [CrossRef]
- Kappi, F.A.; Tsogas, G.Z.; Routsi, A.-M.; Christodouleas, D.C.; Giokas, D.L. Paper-based devices for biothiols sensing using the photochemical reduction of silver halides. Anal. Chim. Acta 2018, 1036, 89–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, M.; Shao, Z.; Xu, H.; Chen, Y.; Yang, Y.; Xu, W.; Liao, X. A paper-based fluorescent test for determination and visualization of cysteine and glutathione by using gold-silver nanoclusters. Microchem. J. 2020, 158, 105327. [Google Scholar] [CrossRef]
- Akrivi, E.; Kappi, F.; Gouma, V.; Vlessidis, A.G.; Giokas, D.L.; Kourkoumelis, N. Biothiol modulated growth and aggregation of gold nanoparticles and their determination in biological fluids using digital photometry. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2021, 249, 119337. [Google Scholar] [CrossRef]
- Lai, Y.; Li, M.; Liao, X.; Zou, L. Smartphone-assisted colorimetric detection of glutathione and glutathione reductase activity in human serum and mouse liver using hemin/G-Quadruplex DNAzyme. Molecules 2021, 26, 5016. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Zhang, X.; Ding, M.; Zhang, A.; Wang, C. A Smartphone-assisted colorimetric and photothermal probe for glutathione detection based on enhanced oxidase-mimic CoFeCe three-atom nanozyme in food. Food Chem. 2023, 423, 136296. [Google Scholar] [CrossRef]
- Zuman, P. Reactions of orthophthalaldehyde with nucleophiles. Chem. Rev. 2004, 104, 3217–3238. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Kamataki, T.; Miura, T. Difference between histidine and histamine in the mechanistic pathway of the fluorescence reaction with ortho-phthalaldehyde. Anal Biochem. 1990, 188, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Czauderna, M.; Kowalczyk, J.; Niedźwiedzka, K.M.; Wąsowska, I. A highly efficient method for determination of some amino acids and glutathione by liquid chromatography. J. Anim. Feed Sci. 2003, 12, 199–215. [Google Scholar] [CrossRef]
- Nedeljko, P.; Turel, M.; Lobnik, A. Turn-On fluorescence detection of glutathione based on o-phthaldialdehyde-assisted SiO2 particles. J. Sens. 2018, 2018, e1692702. [Google Scholar] [CrossRef]
- Molnár-Perl, I.; Vasanits, A. Stability and characteristics of the o-phthaldialdehyde/3-mercaptopropionic acid and o-phthaldialdehyde/N-Acetyl-l-cysteine reagents and their amino acid derivatives measured by high-performance liquid chromatography. J. Chromatogr. A 1999, 835, 73–91. [Google Scholar] [CrossRef]
- Fekkes, D.; van Dalen, A.; Edelman, M.; Voskuilen, A. Validation of the determination of amino acids in plasma by high-performance liquid chromatography using automated pre-column derivatization with o-phthaldialdehyde. J. Chromatogr. B Biomed. Sci. Appl. 1995, 669, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Duan, Q.; Li, Y.; Zhang, Y.; Che, M.; Zhang, W.; Sang, S. A “Turn-on” fluorescent probe for glutathione detection based on the polyethylenimine-carbon dots-Cu2+ system. J. Photochem. Photobiol. B Biol. 2019, 197, 111532. [Google Scholar] [CrossRef]
- Nuhu, F.; Gordon, A.; Sturmey, R.; Seymour, A.-M.; Bhandari, S. Measurement of glutathione as a tool for oxidative stress studies by high performance liquid chromatography. Molecules 2020, 25, 4196. [Google Scholar] [CrossRef] [PubMed]
- Hanczkó, R.; Molnár-Perl, I. Derivatization, stability and chromatographic behavior ofo-phthaldialdehyde amino acid and amine derivatives:o-Phthaldialdehyde/2- mercaptoethanol reagent. Chromatographia 2003, 57, S103–S113. [Google Scholar] [CrossRef]
- Choleva, T.G.; Kappi, F.A.; Giokas, D.L.; Vlessidis, A.G. Paper-based assay of antioxidant activity using analyte-mediated on-paper nucleation of gold nanoparticles as colorimetric probes. Anal. Chim. Acta 2015, 860, 61–69. [Google Scholar] [CrossRef] [PubMed]



| Time (Days) | |||
|---|---|---|---|
| 2 | 4 | 6 | |
| Temperature (°C) | Recovery % | ||
| 25 | 76.5 ± 6.7 | 73.8 ± 5.9 | 71.2 ± 5.5 |
| 4 | 94.7 ± 7.8 | 88.8 ± 7.3 | 85.2 ± 8.4 |
| −18 | 73.3 ± 6.5 | 69.6 ± 6.0 | 63.9 ± 10.6 |
| Samples | Paper-Based Method GSH (μmol L−1) (S.D.) | GSH Concentration of the Nutritional Supplements (μmol L−1) | Relative Error (RE%) |
|---|---|---|---|
| Sample 1 | 3143 (±161) | 3253 | −3.4 |
| Sample 2 | 4605 (±235) | 4880 | −5.6 |
| Sample 3 | 1620 (±66) | 1627 | −0.4 |
| Samples | Paper-Based Method GSH (μmol L−1) (S.D.) | GSH Concentration Determined with HPLC-PCD Method (μmol L−1) | Relative Error (RE%) |
|---|---|---|---|
| Sample 1 | 3143 (±161) | 3218 (±31) | −2.4 |
| Sample 2 | 4605 (±235) | 4780 (±86) | −3.8 |
| Sample 3 | 1620 (±66) | 1649 (±18) | −1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarara, M.; Tzanavaras, P.D.; Tsogas, G.Z. O-Phthalaldehyde Derivatization for the Paper-Based Fluorometric Determination of Glutathione in Nutritional Supplements. Molecules 2024, 29, 2550. https://doi.org/10.3390/molecules29112550
Tarara M, Tzanavaras PD, Tsogas GZ. O-Phthalaldehyde Derivatization for the Paper-Based Fluorometric Determination of Glutathione in Nutritional Supplements. Molecules. 2024; 29(11):2550. https://doi.org/10.3390/molecules29112550
Chicago/Turabian StyleTarara, Maria, Paraskevas D. Tzanavaras, and George Z. Tsogas. 2024. "O-Phthalaldehyde Derivatization for the Paper-Based Fluorometric Determination of Glutathione in Nutritional Supplements" Molecules 29, no. 11: 2550. https://doi.org/10.3390/molecules29112550
APA StyleTarara, M., Tzanavaras, P. D., & Tsogas, G. Z. (2024). O-Phthalaldehyde Derivatization for the Paper-Based Fluorometric Determination of Glutathione in Nutritional Supplements. Molecules, 29(11), 2550. https://doi.org/10.3390/molecules29112550










