Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells
Abstract
1. Introduction
2. Results
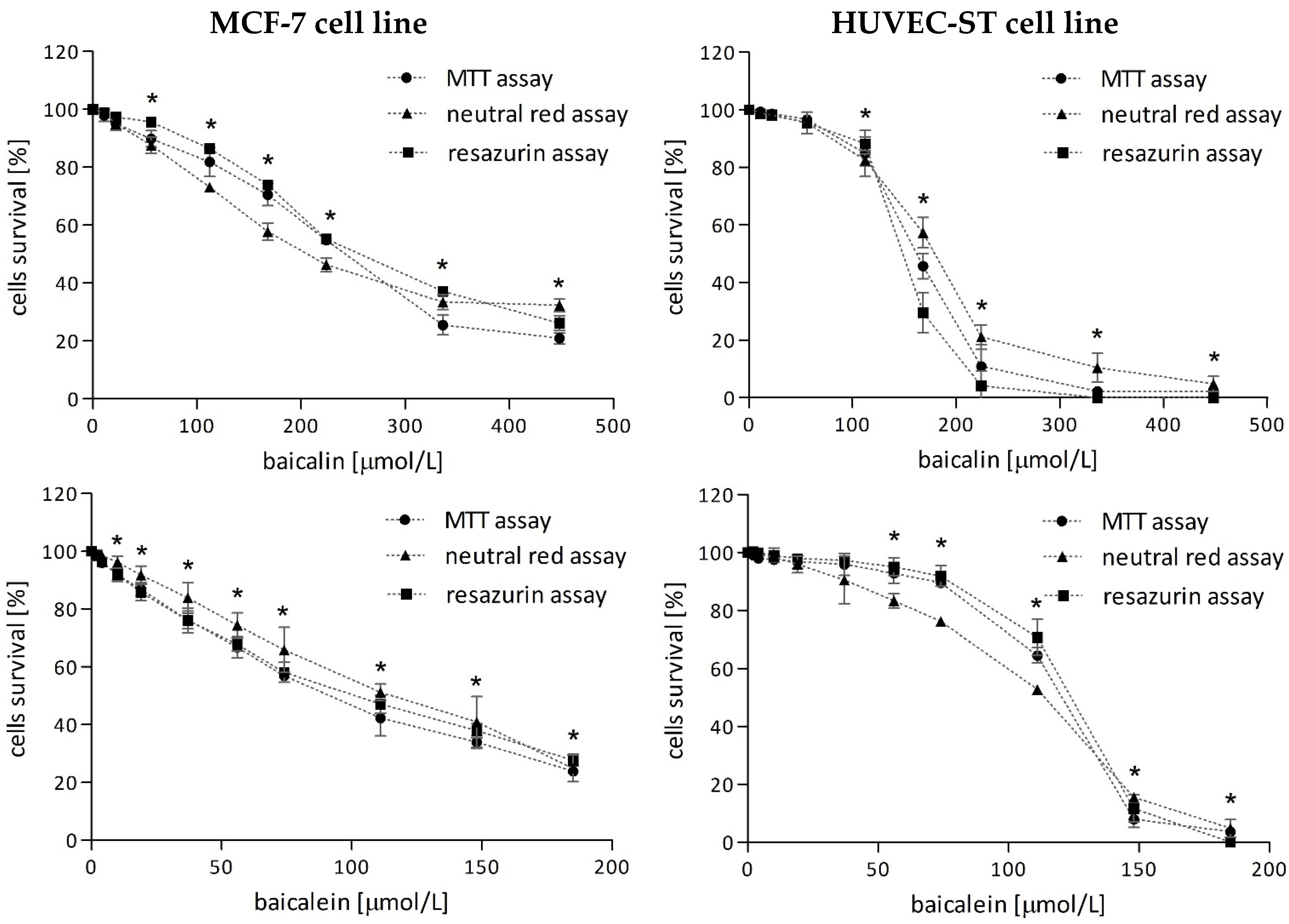
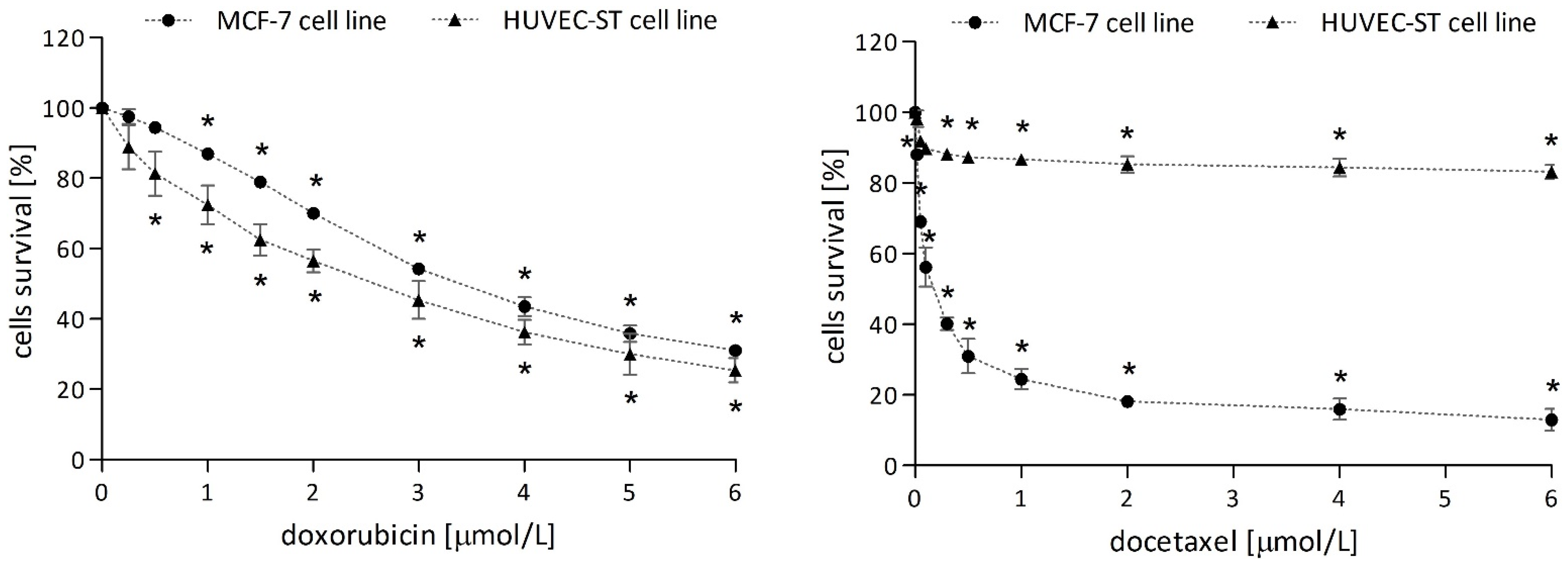
2.1. Cytotoxicity of Investigated Compounds
2.1.1. Baicalin and Baicalein
2.1.2. Anticancer Drugs
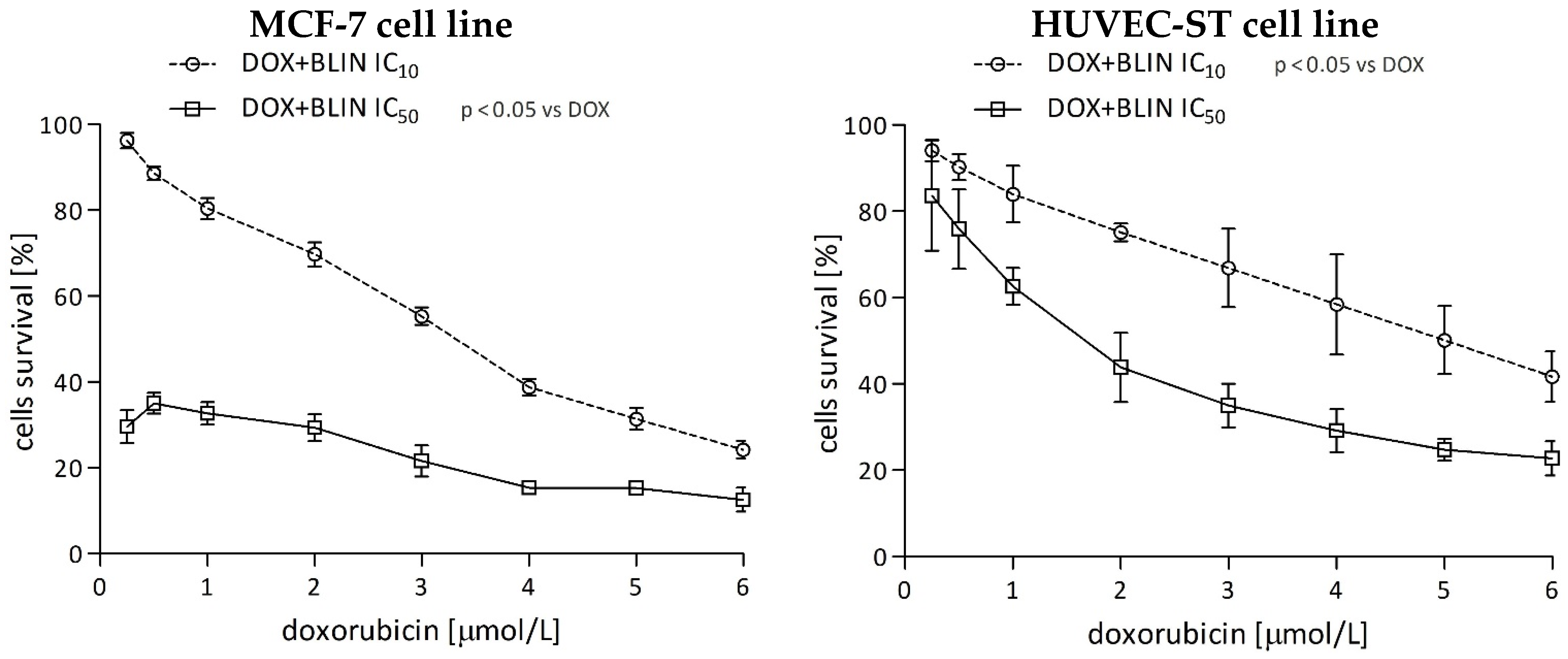
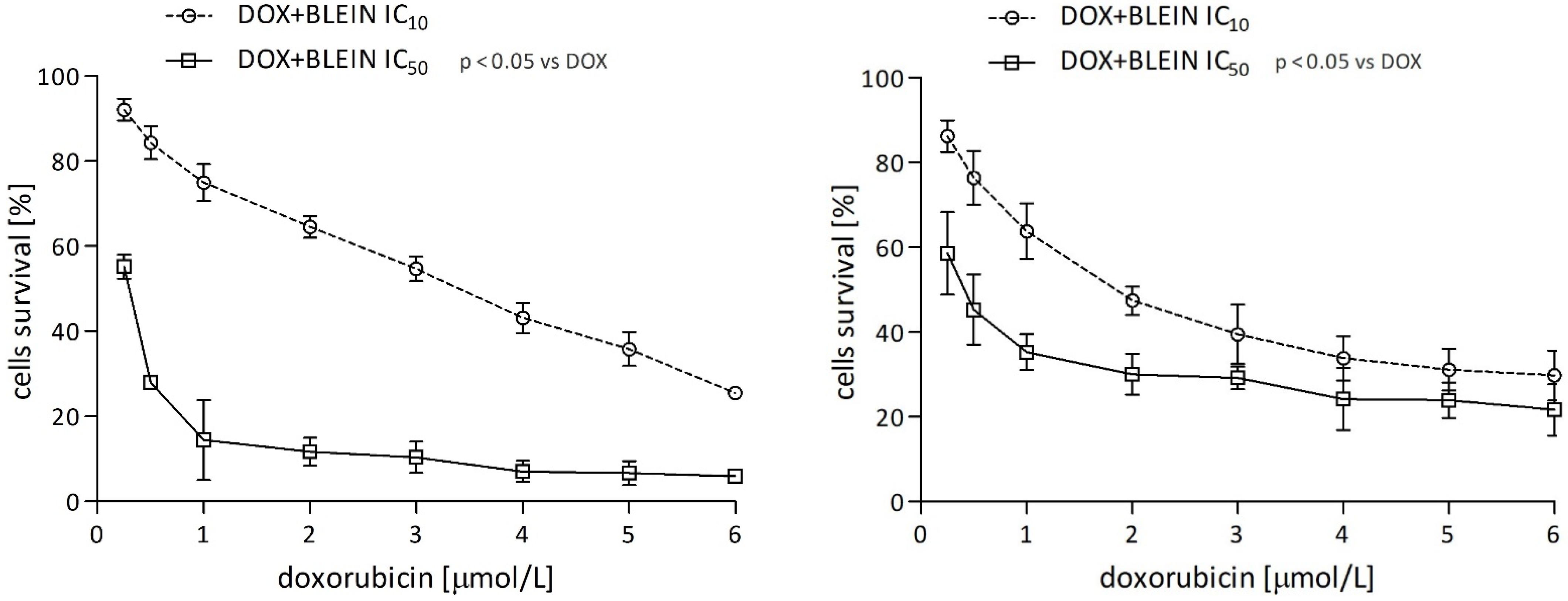
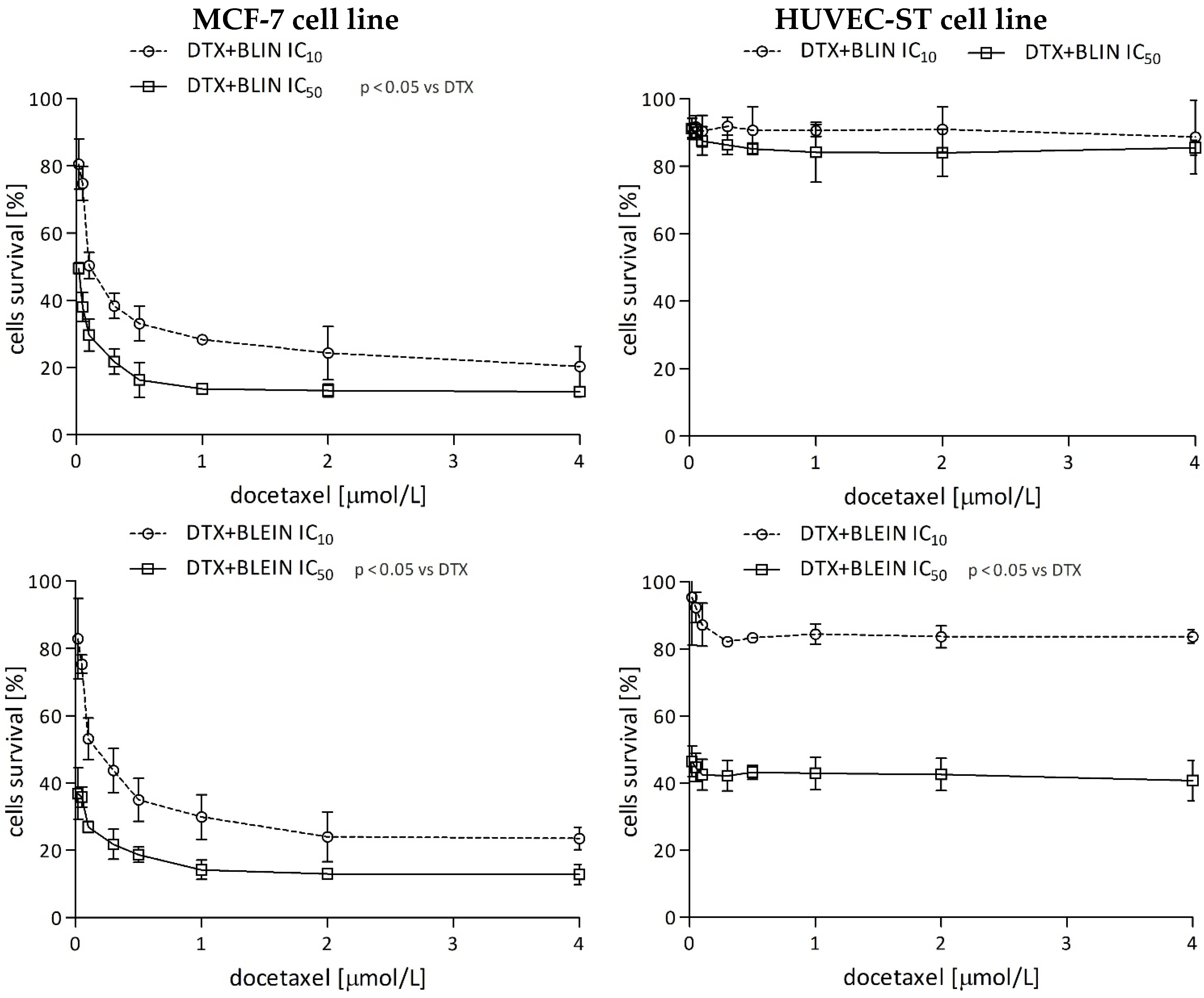
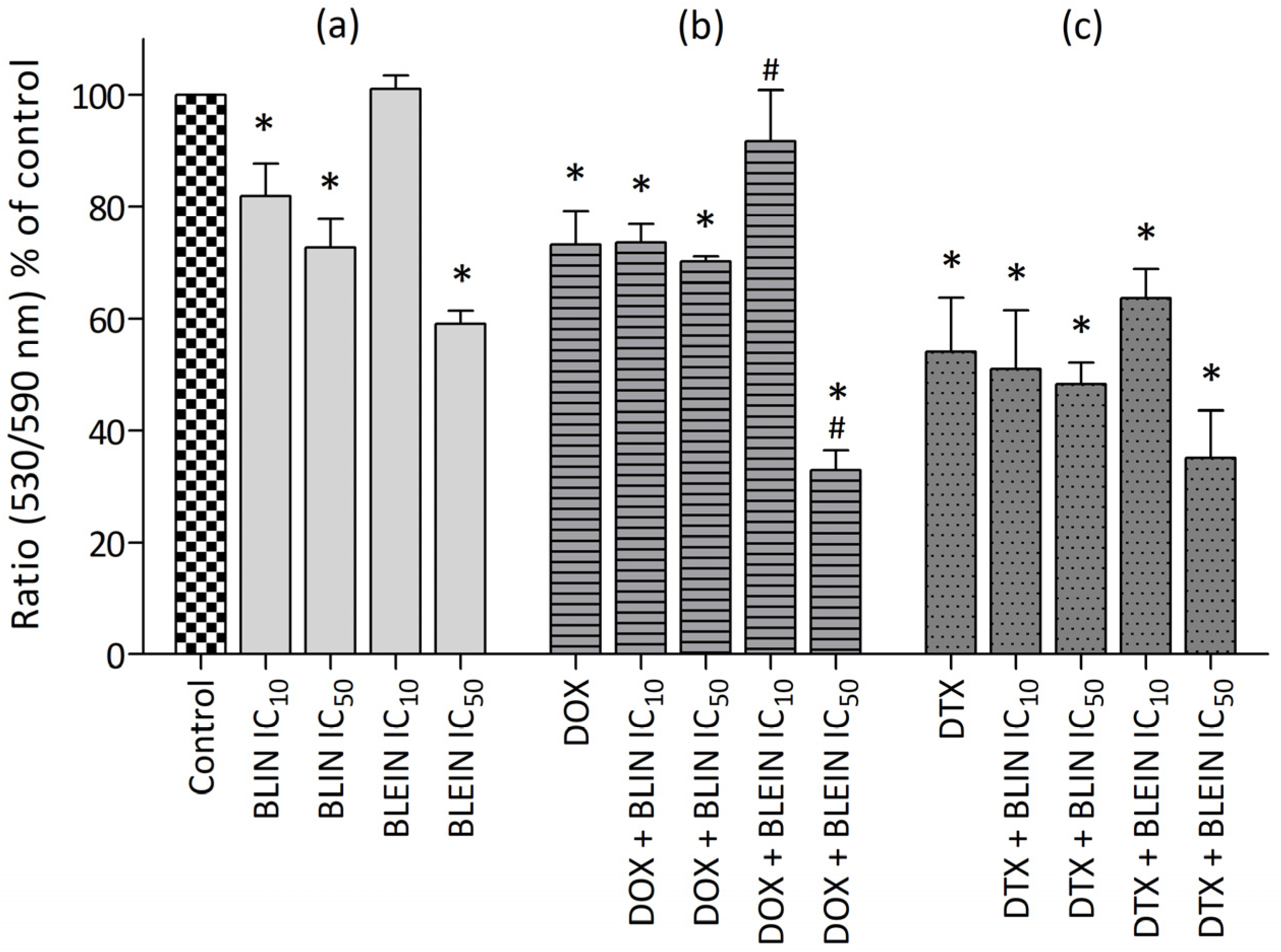
2.1.3. The Effect of Baicalin and Baicalein on the Cytotoxicity of Anticancer Drugs
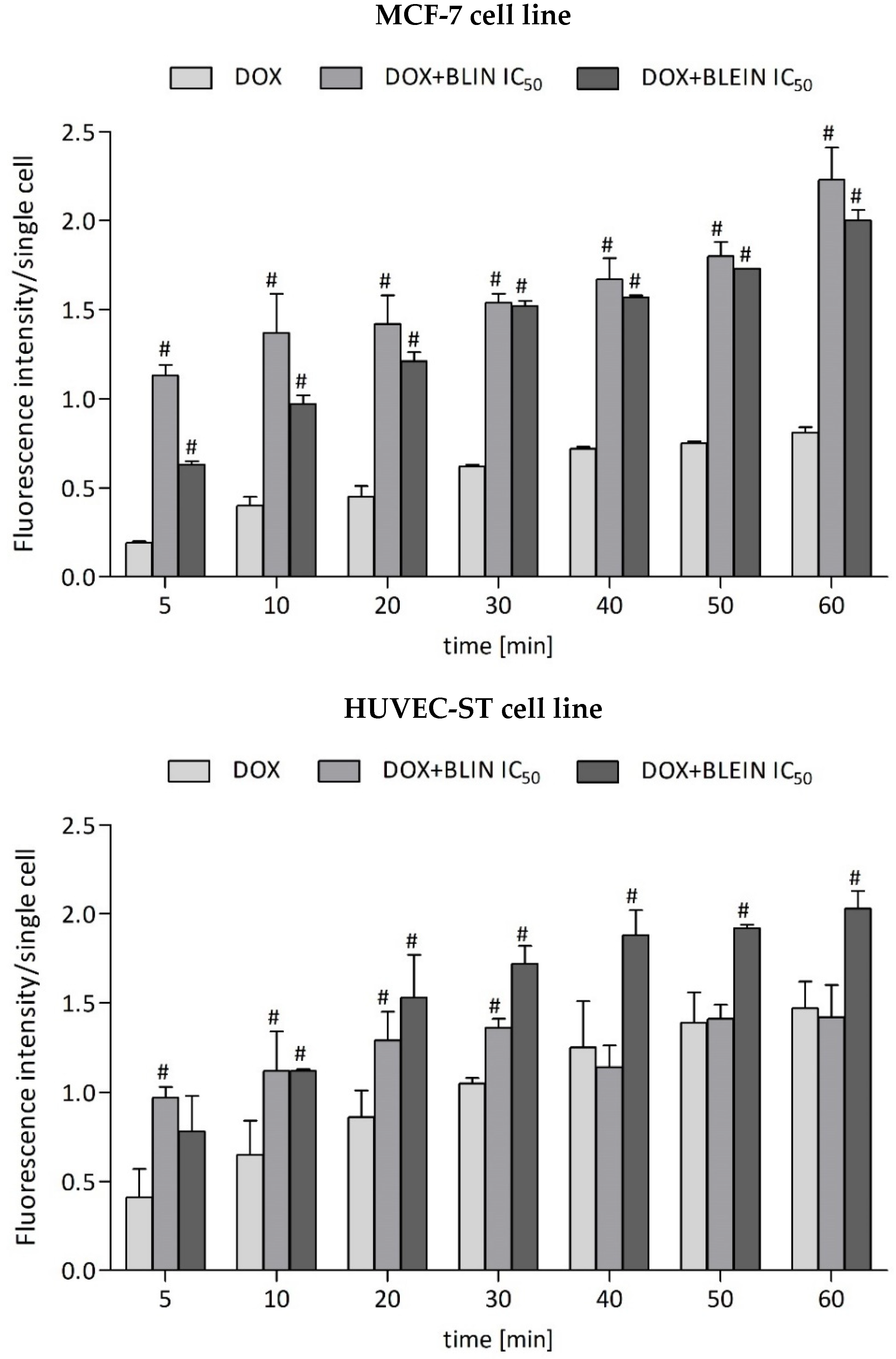
2.2. The Influence of Baicalin and Baicalein on Doxorubicin Accumulation
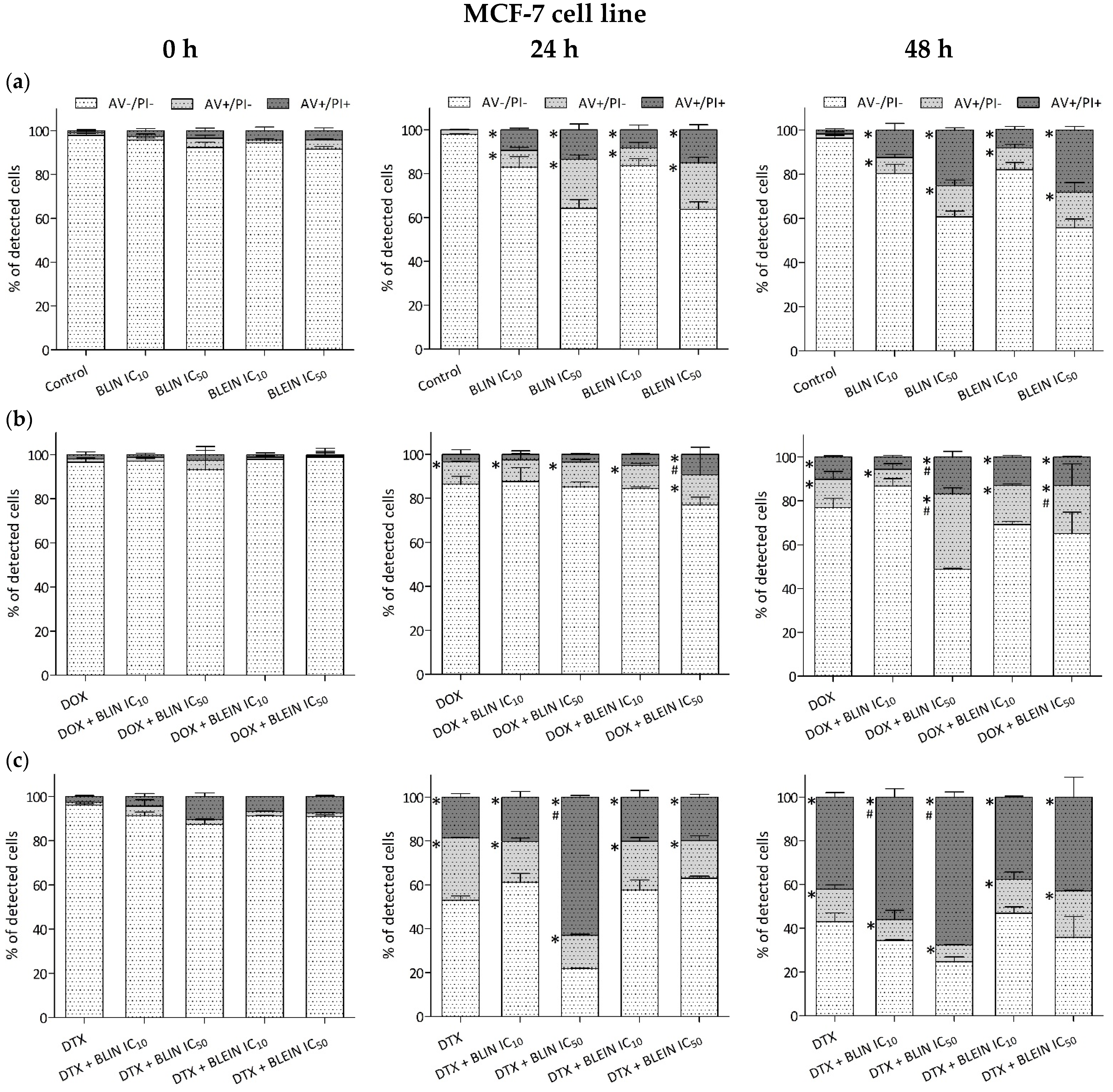
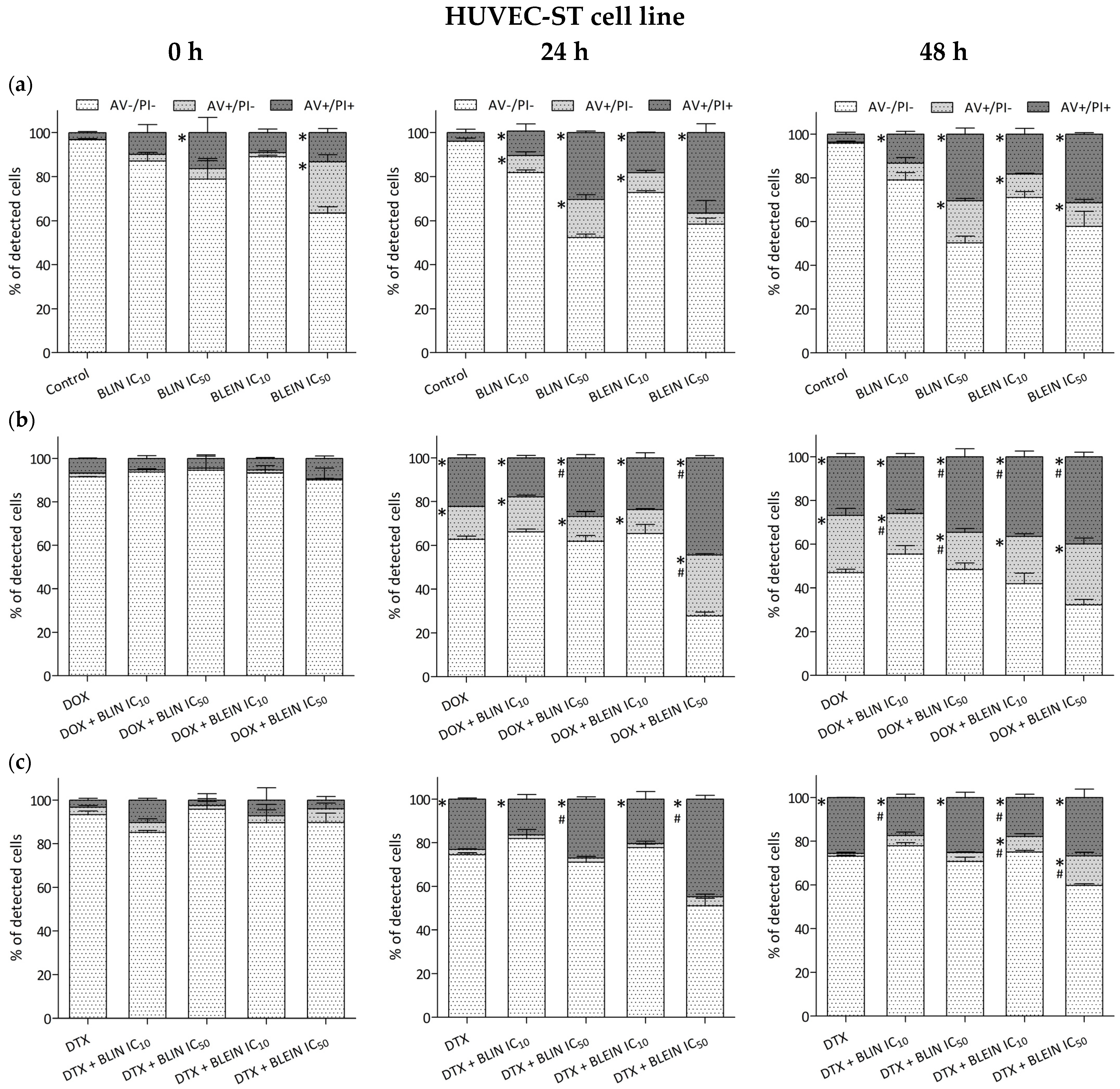
2.3. Induction of Apoptosis and Necrosis by Baicalin and Baicalein Used alone and in Combination with Anticancer Drugs
2.3.1. MCF-7 Cell Line
2.3.2. HUVEC-ST Cell Line
2.4. Changes in Mitochondrial Membrane Potential Caused by Baicalin and Baicalein Used alone and in Combination with Anticancer Drugs
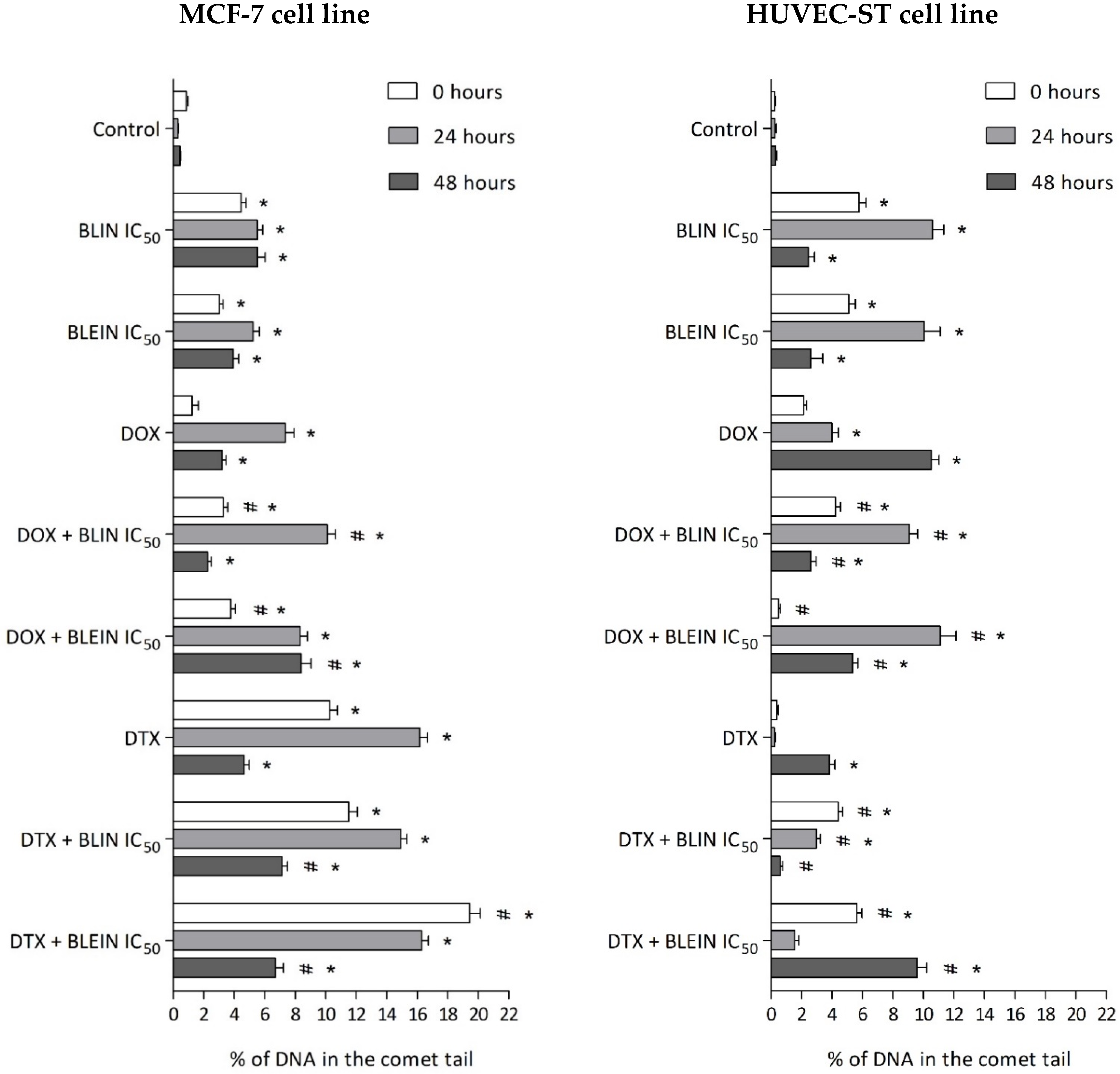
2.5. DNA Damage Induced by Baicalin and Baicalein Used Alone and in Combination with Anticancer Drugs
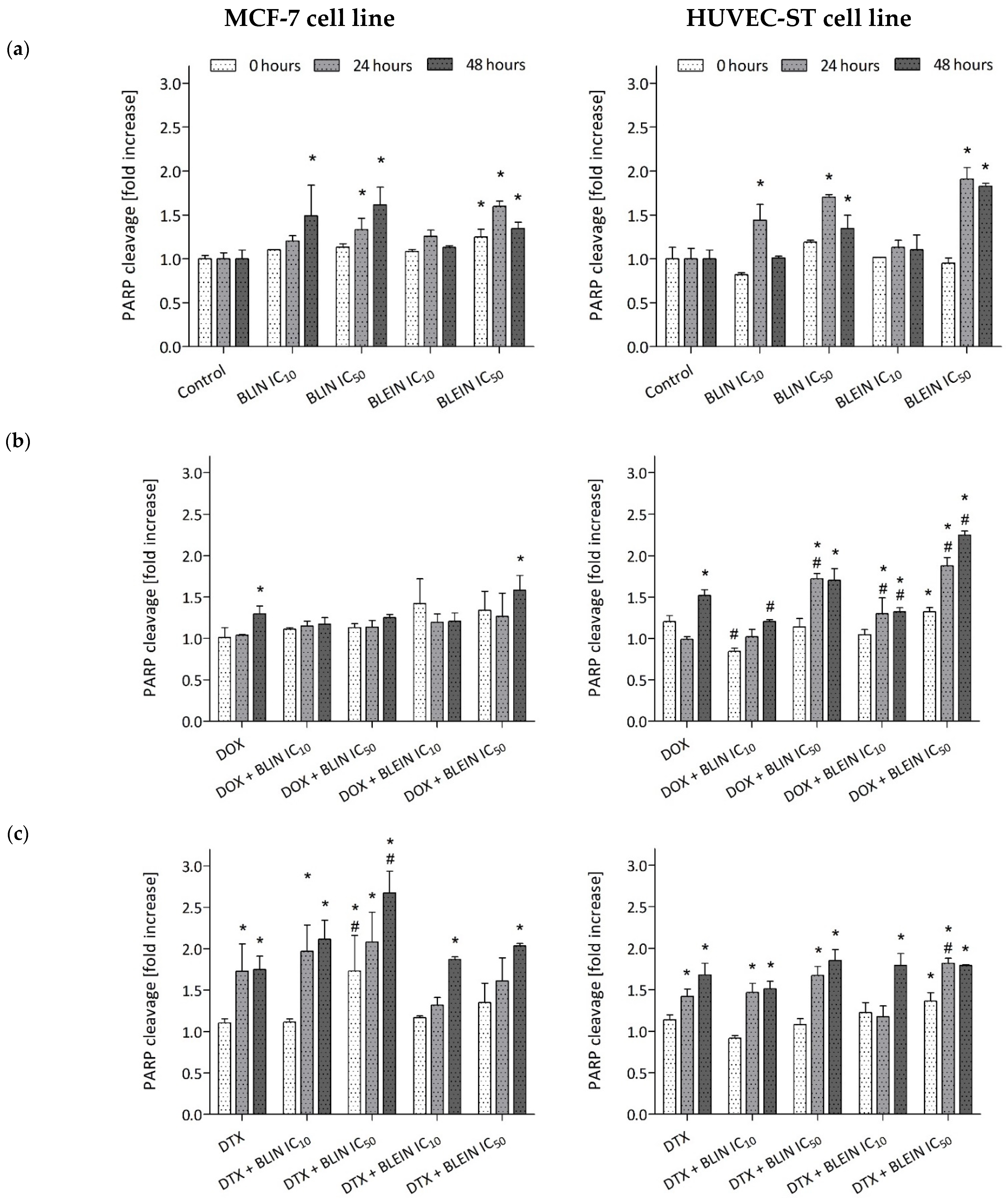
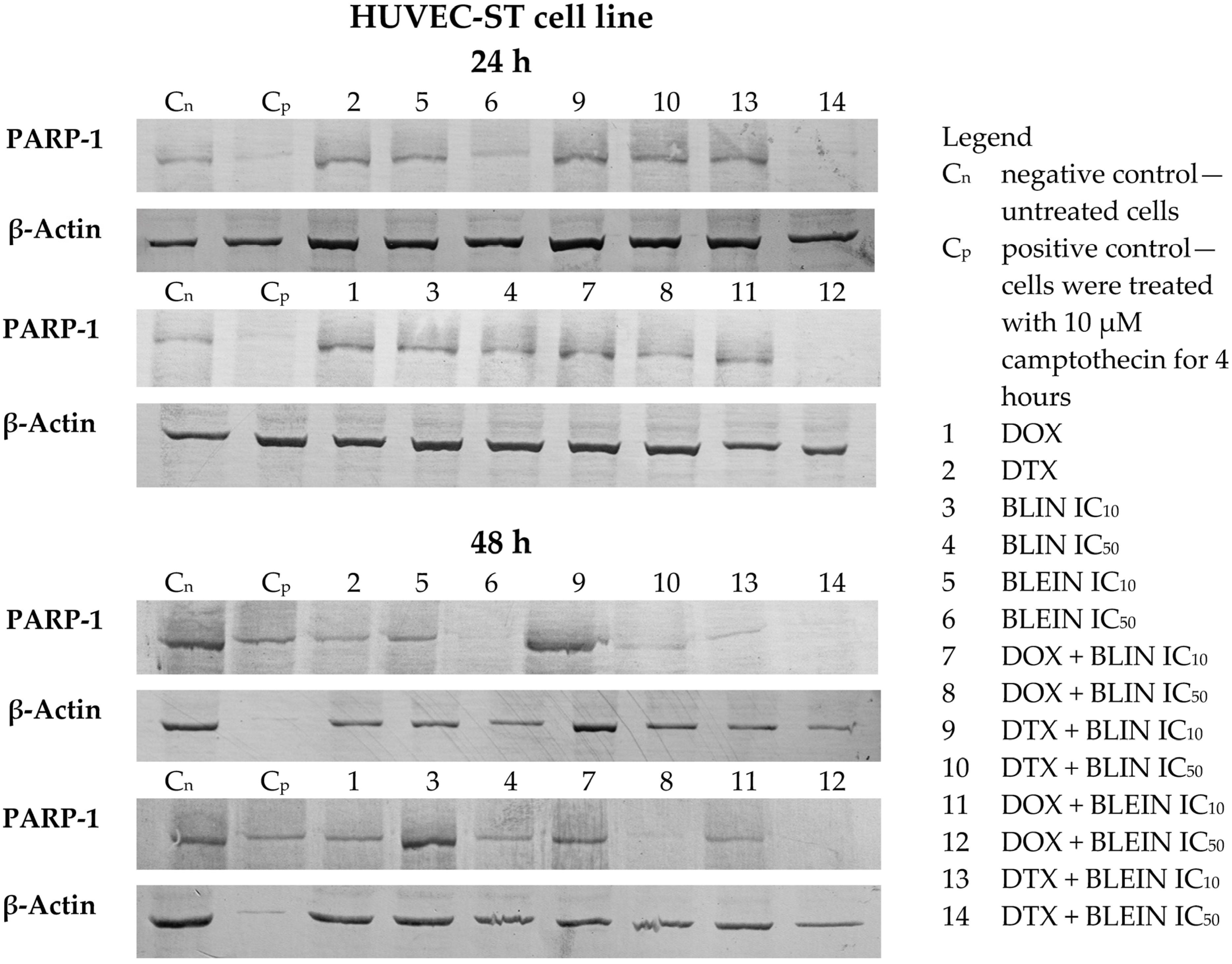
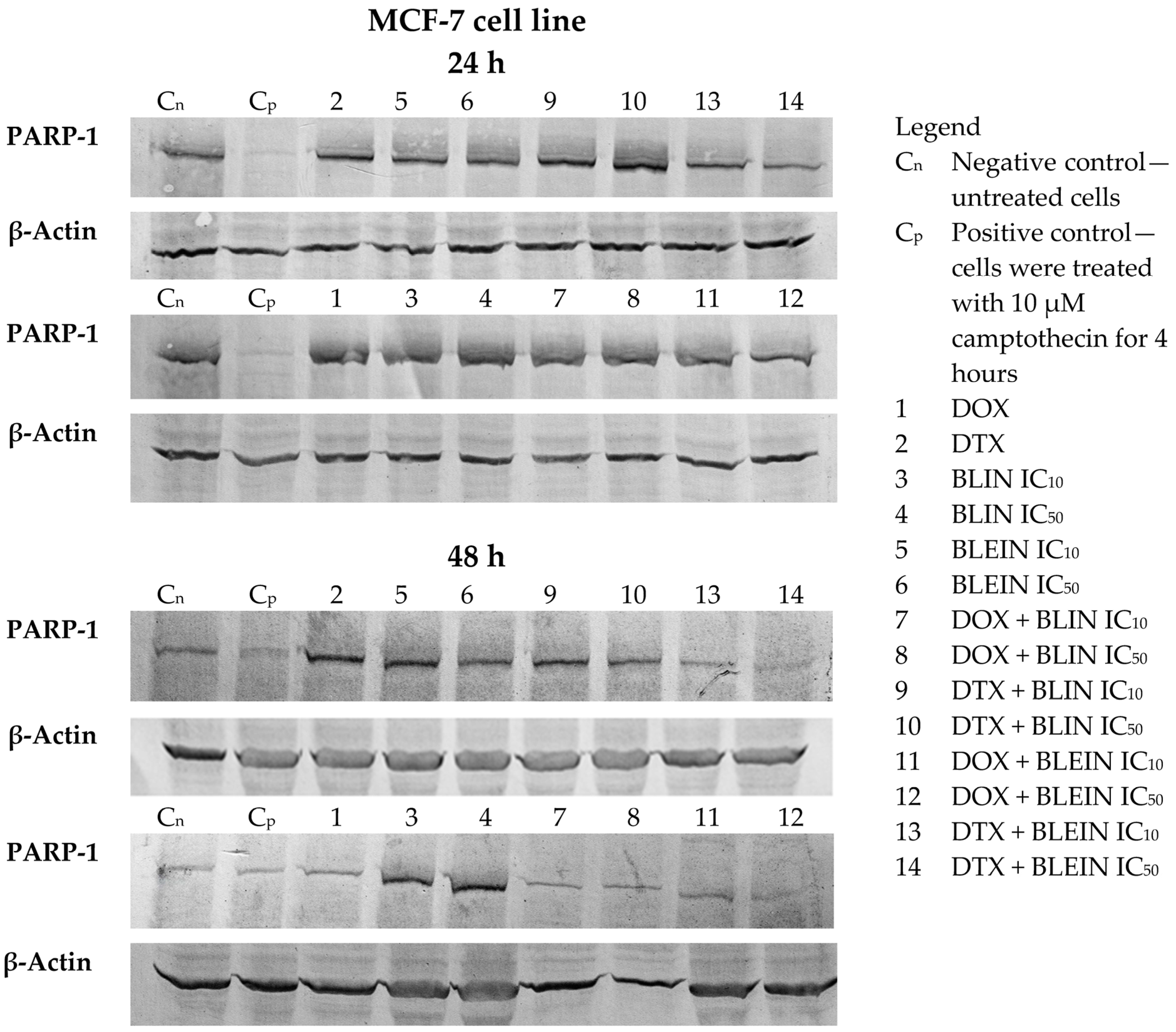
2.6. PARP Cleavage Induced by Baicalin and Baicalein Used Alone and in Combination with Anticancer Drugs
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Chemicals
4.1.2. Cell Culture
4.1.3. Treatment Conditions
4.2. Methods
4.2.1. Cell Proliferation Analysis
- MTT assay
- Neutral red uptake assay
- Resazurin reduction assay
4.2.2. Quantitation of Synergism and Antagonism in Flavonoid–Drug Combinations
4.2.3. Cytometric Measurement of Doxorubicin Accumulation
4.2.4. Annexin V FITC/Propidium Iodide Double Staining Assay
4.2.5. Measurement of Mitochondrial Membrane Potential
4.2.6. The Comet Assay
4.2.7. Cleaved PARP ELISA Assay
4.2.8. Western Blot Detection of PARP Cleavage
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Viale, P.H. The American Cancer Society’s Facts & Figures: 2020 Edition. J. Adv. Pract. Oncol. 2020, 11, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Mutebi, M.; Anderson, B.O.; Duggan, C.; Adebamowo, C.; Agarwal, G.; Ali, Z.; Bird, P.; Bourque, J.-M.; DeBoer, R.; Gebrim, L.H.; et al. Breast cancer treatment: A phased approach to implementation. Cancer 2020, 126 (Suppl. S10), 2365–2378. [Google Scholar] [CrossRef] [PubMed]
- Back, N.; Cohen, I.R.; Lajtha, A.; Lambris, J.D.; Paoletti, R.; Yu, D.; Hung, M.-C. Breast Cancer Chemosensitivity; Scholars Portal: New York, NY, USA, 2007; ISBN 978-0-387-74037-9. [Google Scholar]
- Lown, J.W. Anthracycline and anthraquinone anticancer agents: Current status and recent developments. Pharmacol. Ther. 1993, 60, 185–214. [Google Scholar] [CrossRef] [PubMed]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A. A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochem. Pharmacol. 1999, 57, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Grant, D.S.; Williams, T.L.; Zahaczewsky, M.; Dicker, A.P. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere). Int. J. Cancer 2003, 104, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Valero, V.; Hortobagyi, G.N. Are anthracycline-taxane regimens the new standard of care in the treatment of metastatic breast cancer? J. Clin. Oncol. 2003, 21, 959–962. [Google Scholar] [CrossRef]
- Gligorov, J.; Lotz, J.P. Preclinical pharmacology of the taxanes: Implications of the differences. Oncologist 2004, 9 (Suppl. S2), 3–8. [Google Scholar] [CrossRef]
- Rashid, N.; Koh, H.A.; Baca, H.C.; Li, Z.; Malecha, S.; Abidoye, O.; Masaquel, A. Clinical Impact of Chemotherapy-Related Adverse Events in Patients with Metastatic Breast Cancer in an Integrated Health Care System. J. Manag. Care Spec. Pharm. 2015, 21, 863–871. [Google Scholar] [CrossRef]
- Reinisch, M.; von Minckwitz, G.; Harbeck, N.; Janni, W.; Kümmel, S.; Kaufmann, M.; Elling, D.; Nekljudova, V.; Loibl, S. Side effects of standard adjuvant and neoadjuvant chemotherapy regimens according to age groups in primary breast cancer. Breast Care 2013, 8, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, K.A.; Mendonça, C.R.; Noll, M.; Botelho, A.F.; Francischini, C.R.D.; Silva, M.A.M. Antitumor Properties of Curcumin in Breast Cancer Based on Preclinical Studies: A Systematic Review. Cancers 2022, 14, 2165. [Google Scholar] [CrossRef] [PubMed]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar] [PubMed]
- Gu, Y.; Zheng, Q.; Fan, G.; Liu, R. Advances in Anti-Cancer Activities of Flavonoids in Scutellariae radix: Perspectives on Mechanism. Int. J. Mol. Sci. 2022, 23, 11042. [Google Scholar] [CrossRef]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef]
- Shang, X.; He, X.; He, X.; Li, M.; Zhang, R.; Fan, P.; Zhang, Q.; Jia, Z. The genus Scutellaria an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2010, 128, 279–313. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-B.; Jiang, Y.; Chen, F. Separation methods used for Scutellaria baicalensis active components. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004, 812, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Gong, W.-Y.; Zhao, Z.-X.; Liu, B.-J.; Lu, L.-W.; Dong, J.-C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef]
- Hu, Z.; Guan, Y.; Hu, W.; Xu, Z.; Ishfaq, M. An overview of pharmacological activities of baicalin and its aglycone baicalein: New insights into molecular mechanisms and signaling pathways. Iran. J. Basic Med. Sci. 2022, 25, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Martín, M.D.; Tejedor-Bueno, M.S.; Correa-Casado, M. Effectiveness of Complementary Therapies in Cancer Patients: A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 1017. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-S.; Chen, J.; Tan, H.-Y.; Wang, N.; Chen, Z.; Feng, Y. Scutellaria baicalensis and Cancer Treatment: Recent Progress and Perspectives in Biomedical and Clinical Studies. Am. J. Chin. Med. 2018, 46, 25–54. [Google Scholar] [CrossRef]
- Kiartivich, S.; Wei, Y.; Liu, J.; Soiampornkul, R.; Li, M.; Zhang, H.; Dong, J. Regulation of cytotoxicity and apoptosis-associated pathways contributes to the enhancement of efficacy of cisplatin by baicalein adjuvant in human A549 lung cancer cells. Oncol. Lett. 2017, 13, 2799–2804. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, J.; Shi, B.; Tie, J. Baicalein enhanced cisplatin sensitivity of gastric cancer cells by inducing cell apoptosis and autophagy via Akt/mTOR and Nrf2/Keap 1 pathway. Biochem. Biophys. Res. Commun. 2020, 531, 320–327. [Google Scholar] [CrossRef] [PubMed]
- He, G.-P.; Liu, X.-Z.; Xue, N.; Yu, T.; Li, Y.-X.; Zhang, C.-Y.; Wang, H.-Q. Baicalin reduces the stemness potential of hepatoblastoma rather than hepatocellular carcinoma and improves its chemo-sensitivity. Gastroenterol. Hepatol. Res. 2021, 3, 1–11. [Google Scholar] [CrossRef]
- Yu, M.; Qi, B.; Xiaoxiang, W.; Xu, J.; Liu, X. Baicalein increases cisplatin sensitivity of A549 lung adenocarcinoma cells via PI3K/Akt/NF-κB pathway. Biomed. Pharmacother. 2017, 90, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, L.; Wang, P.; Yang, L.; Zhu, X.; Li, C.G. Drug-herb interactions between Scutellaria baicalensis and pharmaceutical drugs: Insights from experimental studies, mechanistic actions to clinical applications. Biomed. Pharmacother. 2021, 138, 111445. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-H.; Luo, N.; Zhong, M.-Z.; Xiao, Z.-Q.; Wang, J.-X.; Yao, X.-Y.; Peng, Y.; Cao, J. Inhibition of microRNA-196a might reverse cisplatin resistance of A549/DDP non-small-cell lung cancer cell line. Tumour Biol. 2016, 37, 2387–2394. [Google Scholar] [CrossRef] [PubMed]
- Sawant, T.; Prabhavalkar, K. Amelioration of cisplatin-induced toxicity in experimental animals by baicalin. World J. Pharm. Res. 2020, 9, 1864–1879. [Google Scholar]
- Li, J.; Duan, B.; Guo, Y.; Zhou, R.; Sun, J.; Bie, B.; Yang, S.; Huang, C.; Yang, J.; Li, Z. Baicalein sensitizes hepatocellular carcinoma cells to 5-FU and Epirubicin by activating apoptosis and ameliorating P-glycoprotein activity. Biomed. Pharmacother. 2018, 98, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Zhang, M.; Song, Y.; Zhang, Y.; Fan, S.; Ren, S.; Fu, L.; Zhang, N.; Hui, H.; et al. Baicalein resensitizes tamoxifen-resistant breast cancer cells by reducing aerobic glycolysis and reversing mitochondrial dysfunction via inhibition of hypoxia-inducible factor-1α. Clin. Transl. Med. 2021, 11, e577. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Xiao, Y.-Y.; Qu, X.-H.; Li, S.-S.; Zhang, K.; Zhou, C.; He, J.-L.; Zhu, Y.; Wan, Y.-Y.; Jiang, L.-P.; et al. Baicalein sensitizes triple negative breast cancer MDA-MB-231 cells to doxorubicin via autophagy-mediated down-regulation of CDK1. Mol. Cell. Biochem. 2023, 478, 1519–1531. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Liang, X.; Zhu, S.; Liu, C.; Luo, X.; Zhang, Q.; Song, L. Baicalin, a Potent Inhibitor of NF-κB Signaling Pathway, Enhances Chemosensitivity of Breast Cancer Cells to Docetaxel and Inhibits Tumor Growth and Metastasis Both In Vitro and In Vivo. Front. Pharmacol. 2020, 11, 879. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-Y.; Cheng, W.-T.; Cheng, H.-C.; Chou, W.-C.; Chen, H.-I.; Ou, H.-C.; Tsai, K.-L. Baicalin Enhances Chemosensitivity to Doxorubicin in Breast Cancer Cells via Upregulation of Oxidative Stress-Mediated Mitochondria-Dependent Apoptosis. Antioxidants 2021, 10, 1506. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Li, J.; Haung, H.-H.; Liu, H.; Han, M.; Ramachandran, S.; Li, C.-Q.; Sharp, W.W.; Hamann, K.J.; Yuan, C.-S.; et al. Baicalein protects against doxorubicin-induced cardiotoxicity by attenuation of mitochondrial oxidant injury and JNK activation. J. Cell. Biochem. 2011, 112, 2873–2881. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Yang, Y.; Liu, N.; Wang, S. Baicalin regulates TLR4/IκBα/NFκB signaling pathway to alleviate inflammation in Doxorubicin related cardiotoxicity. Biochem. Biophys. Res. Commun. 2022, 637, 1–8. [Google Scholar] [CrossRef]
- Sahu, B.D.; Kumar, J.M.; Kuncha, M.; Borkar, R.M.; Srinivas, R.; Sistla, R. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sci. 2016, 144, 8–18. [Google Scholar] [CrossRef]
- El-Ela, S.R.A.; Zaghloul, R.A.; Eissa, L.A. Promising cardioprotective effect of baicalin in doxorubicin-induced cardiotoxicity through targeting toll-like receptor 4/nuclear factor-κB and Wnt/β-catenin pathways. Nutrition 2022, 102, 111732. [Google Scholar] [CrossRef]
- Torki, Z.; Ghavi, D.; Hashemi, S.; Rahmati, Y.; Rahmanpour, D.; Pornour, M.; Alivand, M.R. The related miRNAs involved in doxorubicin resistance or sensitivity of various cancers: An update. Cancer Chemother. Pharmacol. 2021, 88, 771–793. [Google Scholar] [CrossRef]
- Tacar, O.; Dass, C.R. Doxorubicin-induced death in tumour cells and cardiomyocytes: Is autophagy the key to improving future clinical outcomes? J. Pharm. Pharmacol. 2013, 65, 1577–1589. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Saleem, S.; Chaudhuri, A.; Ali, J.; Baboota, S. Docetaxel: An update on its molecular mechanisms, therapeutic trajectory and nanotechnology in the treatment of breast, lung and prostate cancer. J. Drug Deliv. Sci. Technol. 2020, 60, 101959. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, Z.; Peng, J.; You, F.; Ren, Y.; Li, X.; Xiao, C. Multiple roles of baicalin and baicalein in the regulation of colorectal cancer. Front. Pharmacol. 2024, 15, 1264418. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Wu, T.; Peng, Z.; Tan, Q.; Peng, X.; Zhan, Z.; Song, L.; Wei, B. Baicalin induces apoptosis and autophagy in human osteosarcoma cells by increasing ROS to inhibit PI3K/Akt/mTOR, ERK1/2 and β-catenin signaling pathways. J. Bone Oncol. 2022, 33, 100415. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Meena, A.; Luqman, S. Baicalin mediated regulation of key signaling pathways in cancer. Pharmacol. Res. 2021, 164, 105387. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-H.; Yang, C.-X.; Zhang, L.; Yang, C.-Y.; Xu, X.-Q. Baicalein, as a Prooxidant, Triggers Mitochondrial Apoptosis in MCF-7 Human Breast Cancer Cells through Mobilization of Intracellular Copper and Reactive Oxygen Species Generation. OncoTargets Ther. 2019, 12, 10749–10761. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Li, X.-L.; Wang, Q.-F.; Mehendale, S.R.; Yuan, C.-S. Selective fraction of Scutellaria baicalensis and its chemopreventive effects on MCF-7 human breast cancer cells. Phytomedicine 2010, 17, 63–68. [Google Scholar] [CrossRef]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Moussa, M.; Carroll, K.K. Inhibition of human breast cancer cell proliferation and delay of mammary tumorigenesis by flavonoids and citrus juices. Nutr. Cancer 1996, 26, 167–181. [Google Scholar] [CrossRef]
- So, F.V.; Guthrie, N.; Chambers, A.F.; Carroll, K.K. Inhibition of proliferation of estrogen receptor-positive MCF-7 human breast cancer cells by flavonoids in the presence and absence of excess estrogen. Cancer Lett. 1997, 112, 127–133. [Google Scholar] [CrossRef]
- Shang, D.; Li, Z.; Zhu, Z.; Chen, H.; Zhao, L.; Wang, X.; Chen, Y. Baicalein suppresses 17-β-estradiol-induced migration, adhesion and invasion of breast cancer cells via the G protein-coupled receptor 30 signaling pathway. Oncol. Rep. 2015, 33, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-J.; Huang, T.-S.; Cheng, W.-F.; Lu, F.-J. Baicalein and baicalin are potent inhibitors of angiogenesis: Inhibition of endothelial cell proliferation, migration and differentiation. Int. J. Cancer 2003, 106, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.-X.; Xu, M.; Huang, S.-H.; Wu, Q.-Q.; Yuan, Y.; Deng, W.; Tang, Q.-Z. Baicalein protects against endothelial cell injury by inhibiting the TLR4/NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 3085–3091. [Google Scholar] [CrossRef]
- Zhang, A.; Hou, Y.; Sun, C.; Pu, Y.; Sun, Y.; Zhang, Y.; Shen, Y.; Zhou, Q. Baicalin Protects against Thrombin-Induced Cell Injury in Human Umbilical Vein Endothelial Cells. BioMed Res. Int. 2019, 2019, 2187306. [Google Scholar] [CrossRef]
- Zhang, J. Biomarkers of endothelial activation and dysfunction in cardiovascular diseases. Rev. Cardiovasc. Med. 2022, 23, 73. [Google Scholar] [CrossRef] [PubMed]
- Miocinovic, R.; McCabe, N.P.; Keck, R.W.; Jankun, J.; Hampton, J.A.; Selman, S.H. In Vivo and In Vitro effect of baicalein on human prostate cancer cells. Int. J. Oncol. 2005, 26, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Lu, J.; Mori, T.; Smith-Powell, L.; Synold, T.W.; Chen, S.; Wen, W. Baicalin increases VEGF expression and angiogenesis by activating the ERR{alpha}/PGC-1{alpha} pathway. Cardiovasc. Res. 2011, 89, 426–435. [Google Scholar] [CrossRef]
- Huang, Y.; Miao, Z.; Hu, Y.; Yuan, Y.; Zhou, Y.; Wei, L.; Zhao, K.; Guo, Q.; Lu, N. Baicalein reduces angiogenesis in the inflammatory microenvironment via inhibiting the expression of AP-1. Oncotarget 2017, 8, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, T.; Su, Z.; Pi, C.; Wei, Y.; Zhao, L. Latest research progress on anticancer effect of baicalin and its aglycone baicalein. Arch. Pharm. Res. 2022, 45, 535–557. [Google Scholar] [CrossRef]
- Touil, Y.S.; Fellous, A.; Scherman, D.; Chabot, G.G. Flavonoid-induced morphological modifications of endothelial cells through microtubule stabilization. Nutr. Cancer 2009, 61, 310–321. [Google Scholar] [CrossRef]
- Kalapos-Kovács, B.; Magda, B.; Jani, M.; Fekete, Z.; Szabó, P.T.; Antal, I.; Krajcsi, P.; Klebovich, I. Multiple ABC Transporters Efflux Baicalin. Phytother. Res. 2015, 29, 1987–1990. [Google Scholar] [CrossRef] [PubMed]
- Boumendjel, A.; Di Pietro, A.; Dumontet, C.; Barron, D. Recent advances in the discovery of flavonoids and analogs with high-affinity binding to P-glycoprotein responsible for cancer cell multidrug resistance. Med. Res. Rev. 2002, 22, 512–529. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.E.; Zhang, S. Flavonoid-drug interactions: Effects of flavonoids on ABC transporters. Life Sci. 2006, 78, 2116–2130. [Google Scholar] [CrossRef] [PubMed]
- van Zanden, J.J.; Wortelboer, H.M.; Bijlsma, S.; Punt, A.; Usta, M.; van Bladeren, P.J.; Rietjens, I.M.C.M.; Cnubben, N.H.P. Quantitative structure activity relationship studies on the flavonoid mediated inhibition of multidrug resistance proteins 1 and 2. Biochem. Pharmacol. 2005, 69, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Mattioli, R.; Ilari, A.; Colotti, B.; Mosca, L.; Fazi, F.; Colotti, G. Doxorubicin and other anthracyclines in cancers: Activity, chemoresistance and its overcoming. Mol. Aspects Med. 2023, 93, 101205. [Google Scholar] [CrossRef] [PubMed]
- Duriez, P.J.; Shah, G.M. Cleavage of poly(ADP-ribose) polymerase: A sensitive parameter to study cell death. Biochem. Cell Biol. 1997, 75, 337–349. [Google Scholar] [CrossRef]
- Nuydens, R.; Novalbos, J.; Dispersyn, G.; Weber, C.; Borgers, M.; Geerts, H. A rapid method for the evaluation of compounds with mitochondria-protective properties. J. Neurosci. Methods 1999, 92, 153–159. [Google Scholar] [CrossRef]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Towbin, H.; Staehelin, T.; Gordon, J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc. Natl. Acad. Sci. USA 1979, 76, 4350–4354. [Google Scholar] [CrossRef] [PubMed]
- Leary, J.J.; Brigati, D.J.; Ward, D.C. Rapid and sensitive colorimetric method for visualizing biotin-labeled DNA probes hybridized to DNA or RNA immobilized on nitrocellulose: Bio-blots. Proc. Natl. Acad. Sci. USA 1983, 80, 4045–4049. [Google Scholar] [CrossRef] [PubMed]


| Inhibitory Concentration (µmol/L) | ||
|---|---|---|
| MCF-7 cell line | HUVEC-ST cell line | |
| baicalin IC10 | 36 ± 3.4 | 101 ± 10.7 |
| baicalin IC50 | 250 ± 10.5 | 167 ± 6.7 |
| baicalein IC10 | 9 ± 0.7 | 75 ± 1.8 |
| baicalein IC50 | 95 ± 4.8 | 115 ± 2.6 |
| Combination Index (CI) | ||||
|---|---|---|---|---|
| MCF-7 cell line | HUVEC-ST cell line | |||
| drug + flavonoid | DOX IC50 | DTX IC50 | DOX IC50 | DTX IC50 |
| baicalin IC10 | 1.15812 | 0.96524 | 2.91523 | 0.99482 |
| baicalin IC50 | 0.64347 | 0.54206 | 1.79361 | 3.13654 |
| baicalein IC10 | 1.07317 | 1.07966 | 0.87213 | 0.96110 |
| baicalein IC50 | 0.34117 | 0.62760 | 0.68010 | 0.44278 |
| (a) DOX (24 h) MCF-7 cell line | (b) DOX (48 h) HUVEC-ST cell line | (c) DTX (24 h) MCF-7 cell line |
 | 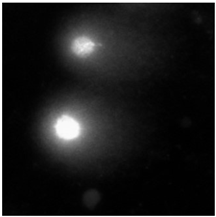 | 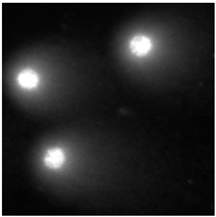 |
| (d) DTX (24 h) HUVEC-ST cell line | (e) BLIN IC50 (24 h) MCF-7 cell line | (f) BLIN IC50 (24 h) HUVEC-ST cell line |
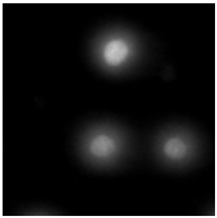 |  |  |
| (g) DTX+ BLEIN IC50 (0 h) MCF-7 cell line | (h) DOX+ BLIN IC50 (48 h) HUVEC-ST cell line | (i) Control cells MCF-7 cell line |
 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernasinska-Slomczewska, J.; Hikisz, P.; Pieniazek, A.; Koceva-Chyla, A. Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells. Molecules 2024, 29, 2503. https://doi.org/10.3390/molecules29112503
Bernasinska-Slomczewska J, Hikisz P, Pieniazek A, Koceva-Chyla A. Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells. Molecules. 2024; 29(11):2503. https://doi.org/10.3390/molecules29112503
Chicago/Turabian StyleBernasinska-Slomczewska, Joanna, Pawel Hikisz, Anna Pieniazek, and Aneta Koceva-Chyla. 2024. "Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells" Molecules 29, no. 11: 2503. https://doi.org/10.3390/molecules29112503
APA StyleBernasinska-Slomczewska, J., Hikisz, P., Pieniazek, A., & Koceva-Chyla, A. (2024). Baicalin and Baicalein Enhance Cytotoxicity, Proapoptotic Activity, and Genotoxicity of Doxorubicin and Docetaxel in MCF-7 Breast Cancer Cells. Molecules, 29(11), 2503. https://doi.org/10.3390/molecules29112503







