Solvent Tolerance Improvement of Lipases Enhanced Their Applications: State of the Art
Abstract
1. Introduction
2. Overview of Lipases
3. Effect of Different Solvents on Stability and Activity of Lipases
3.1. Effect of Organic Solvents on Stability and Activity of Lipases
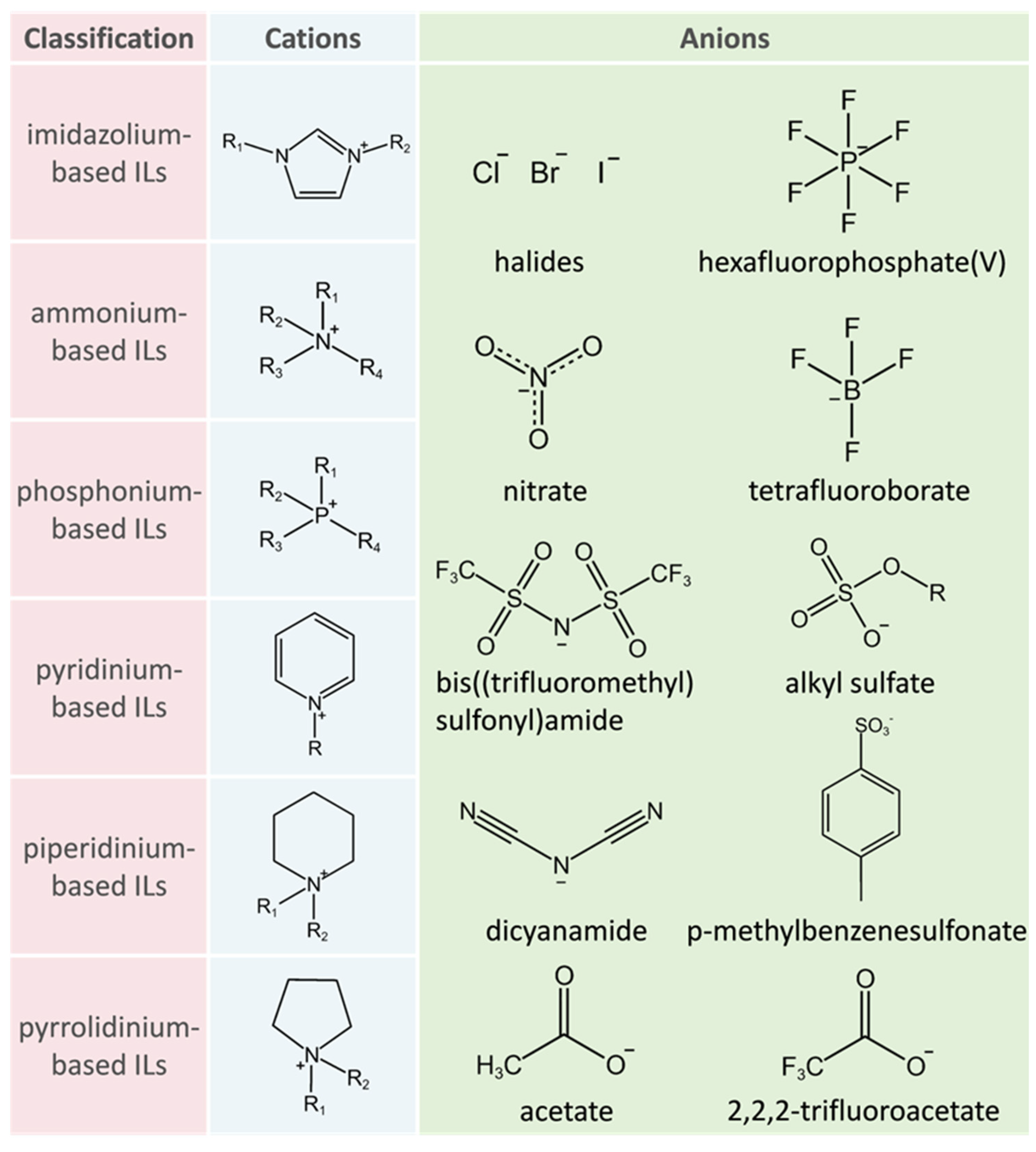
3.2. Effect of Ionic Liquids on Stability and Activity of Lipases
3.3. Effect of Deep Eutectic Solvents on Stability and Activity of Lipases
3.4. Effects of Different Non-Aqueous Solvents on Selectivity of Lipases
3.5. Recovery of Lipases and Separation of Products in Different Non-Aqueous Solvents
4. Protein Engineering for Improving the Solvent Tolerance of Lipases
4.1. Hydration-Based Protein Engineering for Improving the Solvent Tolerance of Lipases
4.2. Hydrogen Bond-Based Protein Engineering for Improving the Solvent Tolerance of Lipases
4.3. Salt Bridge-Based Protein Engineering for Improving the Solvent Tolerance of Lipases
4.4. Disulfide Bond-Based Protein Engineering for Improving the Solvent Tolerance of Lipases
4.5. Hydrophobic Interaction-Based Protein Engineering for Improving the Solvent Tolerance of Lipases
5. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.-J. The Recent Advances in the Utility of Microbial Lipases: A Review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, P.; Tan, Y.; Kinsella, G.K.; Henehan, G.T.; Ryan, B.J. Solvent stable microbial lipases: Current understanding and biotechnological applications. Biotechnol. Lett. 2018, 41, 203–220. [Google Scholar] [CrossRef]
- Carrea, G.; Riva, S. Properties and Synthetic Applications of Enzymes in Organic Solvents. Angew. Chem. Int. Ed. 2000, 39, 2226–2254. [Google Scholar] [CrossRef]
- Pirozzi, D.; Greco, G. Lipase-Catalyzed Transformations for the Synthesis of Butyl Lactate: A Comparison between Esterification and Transesterification. Biotechnol. Prog. 2006, 22, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Ingenbosch, K.N.; Vieyto-Nuñez, J.C.; Ruiz-Blanco, Y.B.; Mayer, C.; Hoffmann-Jacobsen, K.; Sanchez-Garcia, E. Effect of Organic Solvents on the Structure and Activity of a Minimal Lipase. J. Org. Chem. 2021, 87, 1669–1678. [Google Scholar] [CrossRef]
- Cieh, N.L.; Mokhtar, M.N.; Baharuddin, A.S.; Mohammed, M.A.P.; Wakisaka, M. Progress on Lipase Immobilization Technology in Edible Oil and Fat Modifications. Food Rev. Int. 2023, 40, 457–503. [Google Scholar] [CrossRef]
- Sahoo, R.K.; Das, A.; Sahoo, K.; Sahu, A.; Subudhi, E. Characterization of novel metagenomic-derived lipase from Indian hot spring. Int. Microbiol. 2020, 23, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Noro, J.; Cavaco-Paulo, A.; Silva, C. Chemical modification of lipases: A powerful tool for activity improvement. Biotechnol. J. 2022, 17, e2100523. [Google Scholar] [CrossRef]
- Stepankova, V.; Bidmanova, S.; Koudelakova, T.; Prokop, Z.; Chaloupkova, R.; Damborsky, J. Strategies for Stabilization of Enzymes in Organic Solvents. ACS Catal. 2013, 3, 2823–2836. [Google Scholar] [CrossRef]
- Tian, Q.; Hua, Y. Oxidation reactions in model systems simulating the processing of soybeans into soymilk: Role of lipase and lipoxygenase in volatile flavors formation. Int. J. Food Prop. 2021, 24, 192–202. [Google Scholar] [CrossRef]
- Cong, S.; Tian, K.; Zhang, X.; Lu, F.; Singh, S.; Prior, B.; Wang, Z.-X. Synthesis of flavor esters by a novel lipase from Aspergillus niger in a soybean-solvent system. 3 Biotech 2019, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Ghide, M.K.; Yan, Y. 1,3-Dioleoyl-2-palmitoyl glycerol (OPO)-Enzymatic synthesis and use as an important supplement in infant formulas. J. Food Biochem. 2021, 45, e13799. [Google Scholar] [CrossRef]
- Hasibuan, H.A. The synthesis of sn-2 palmitate as human milk fat substitute from palm oil fractions by enzymatic interesterification—A review. J. Oil Palm Res. 2022, 34, 608–621. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.-M.; Sun, C.-F.; Lv, J.-H.; Yang, Y.-J. Comparative Study on Foaming Properties of Egg White with Yolk Fractions and Their Hydrolysates. Foods 2021, 10, 2238. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Chen, W.; Wang, C.-T. Effect of Lipase and Phospholipase A1 on Foaming and Batter Properties of Yolk Contaminated Egg White. Foods 2023, 12, 1289. [Google Scholar] [CrossRef]
- Huang, Z.; Brennan, C.S.; Zheng, H.; Mohan, M.S.; Stipkovits, L.; Liu, W.; Kulasiri, D.; Guan, W.; Zhao, H.; Liu, J. The effects of fungal lipase-treated milk lipids on bread making. LWT 2020, 128, 109455. [Google Scholar] [CrossRef]
- Xiang, M.; Wang, L.; Yan, Q.; Jiang, Z.; Yang, S. Heterologous expression and biochemical characterization of a cold-active lipase from Rhizopus microsporus suitable for oleate synthesis and bread making. Biotechnol. Lett. 2021, 43, 1921–1932. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, Z.; Xie, D.; Cao, Z.; Chen, L.; Wang, H.; Jiang, L.; Shen, Q. Rhizomucor miehei lipase-catalysed synthesis of cocoa butter equivalent from palm mid-fraction and stearic acid: Characteristics and feasibility as cocoa butter alternative. Food Chem. 2021, 343, 128407. [Google Scholar] [CrossRef]
- Hassim, N.A.M.; Ismail, N.H.; Dian, N.L.H.M. Enzymatic interesterification of palm fractions for the production of cocoa butter alternatives. J. Oil Palm Res. 2018, 30, 537–547. [Google Scholar]
- Singh, P.K.; Chopra, R.; Garg, M.; Dhiman, A.; Dhyani, A. Enzymatic interesterification of vegetable oil: A review on physicochemical and functional properties, and its health effects. J. Oleo Sci. 2022, 71, 1697–1709. [Google Scholar] [CrossRef]
- Li, L.; Pei, Y.; Cheng, K.; Deng, Y.; Dong, X.; Fang, R.; Chu, B.; Wei, P.; Chen, Q.; Xiao, G.; et al. Production and evaluation of enzyme-modified cheese adding protease or lipase to improve quality properties. J. Biosci. Bioeng. 2023, 135, 389–394. [Google Scholar] [CrossRef]
- Guan, T.; Liu, B.; Wang, R.; Huang, Y.; Luo, J.; Li, Y. The enhanced fatty acids flavor release for low-fat cheeses by carrier immobilized lipases on O/W Pickering emulsions. Food Hydrocoll. 2021, 116, 106651. [Google Scholar] [CrossRef]
- Shoaib, M.; Bhatti, S.A.; Nawaz, H.; Saif-Ur-Rehman, M. Effect of lipase and bile acids on growth performance, nutrient digestibility, and meat quality in broilers on energy-diluted diets. Turk. J. Vet. Anim. Sci. 2021, 45, 148–157. [Google Scholar] [CrossRef]
- Zou, Z.; Dai, L.; Liu, D.; Du, W. Research Progress in Enzymatic Synthesis of Vitamin E Ester Derivatives. Catalysts 2021, 11, 739. [Google Scholar] [CrossRef]
- Panpan, W.; Hongwei, Y. Application of enzyme catalysis in the preparation of vitamins and their derivatives. Synth. Biol. J. 2022, 3, 500. [Google Scholar]
- El-Fiky, A.F.; Khalil, E.M.; Mowafi, S.; Zaki, R.A.; El-Sayed, H. A Novel Approach towards Removal of Lipid Barrier from Wool Fibers’ Surface Using Thermophilic Lipase. J. Nat. Fibers 2022, 19, 9471–9485. [Google Scholar] [CrossRef]
- Kumar, J.A.; Kumar, M.S. A study on improving dyeability of polyester fabric using lipase enzyme. Autex Res. J. 2020, 20, 243–249. [Google Scholar] [CrossRef]
- Sundaramahalingam, M.A.; Vijayachandran, P.; Rajeshbanu, J.; Sivashanmugam, P. Production of lipase from Priestia endophytica SSP strain and its potential application in deinking of printed paper. Biomass-Convers. Biorefinery 2022, 1–15. [Google Scholar] [CrossRef]
- Nathan, V.K.; Rani, M.E. A cleaner process of deinking waste paper pulp using Pseudomonas mendocina ED9 lipase supplemented enzyme cocktail. Environ. Sci. Pollut. Res. 2020, 27, 36498–36509. [Google Scholar] [CrossRef] [PubMed]
- Ben Rejeb, I.; Khemir, H.; Messaoudi, Y.; Miled, N.; Gargouri, M. Optimization of Enzymatic Degreasing of Sheep Leather for an Efficient Approach and Leather Quality Improvement Using Fractional Experimental Design. Appl. Biochem. Biotechnol. 2022, 194, 2251–2268. [Google Scholar] [CrossRef] [PubMed]
- Moujehed, E.; Zarai, Z.; Khemir, H.; Miled, N.; Bchir, M.S.; Gablin, C.; Bessueille, F.; Bonhommé, A.; Leonard, D.; Carrière, F.; et al. Cleaner degreasing of sheepskins by the Yarrowia lipolytica LIP2 lipase as a chemical-free alternative in the leather industry. Colloids Surf. B Biointerfaces 2022, 211, 112292. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, M.; Li, X.; Zhang, Y.; Wang, Z.; Zheng, J. A novel lipase from Aspergillus oryzae WZ007 catalyzed synthesis of brivaracetam intermediate and its enzymatic characterization. Chirality 2021, 33, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Fang, B. Synthesizing Chiral Drug Intermediates by Biocatalysis. Appl. Biochem. Biotechnol. 2020, 192, 146–179. [Google Scholar] [CrossRef] [PubMed]
- Yoneten, K.K.; Kasap, M.; Arga, K.Y.; Akpinar, G.; Utkan, N.Z. Decreased serum levels of glycerol-3-phosphate dehydrogenase 1 and monoacylglycerol lipase act as diagnostic biomarkers for breast cancer. Cancer Biomark. 2022, 34, 67–76. [Google Scholar] [CrossRef]
- Jawed, A.; Singh, G.; Kohli, S.; Sumera, A.; Haque, S.; Prasad, R.; Paul, D. Therapeutic role of lipases and lipase inhibitors derived from natural resources for remedies against metabolic disorders and lifestyle diseases. S. Afr. J. Bot. 2019, 120, 25–32. [Google Scholar] [CrossRef]
- Alves, A.A.; De Queiroz, A.A.A.E.; Jorge Soares, C.R.; de Queiroz, A.A. Polymer Edition. Microfluidic caging lipase in hyperbranched polyglycerol microcapsules for extracorporeal treatment of enzyme pancreatic insufficiency. J. Biomater. Sci. Polym. Ed. 2021, 32, 2349–2368. [Google Scholar] [CrossRef] [PubMed]
- Ungcharoenwiwat, P.; Aran, H. Enzymatic synthesis of coconut oil based wax esters by immobilized lipase EQ3 and commercial lipozyme RMIM. Electron. J. Biotechnol. 2020, 47, 10–16. [Google Scholar] [CrossRef]
- Abdelgawad, A.; Eid, M.; Abou-Elmagd, W.; Abou-Elregal, M. Lipase catalysed transesterification of palm stearin with ferulic acid in solvent-free media. Biocatal. Biotransform. 2022, 40, 378–385. [Google Scholar] [CrossRef]
- Baek, Y.; Lee, J.; Son, J.; Lee, T.; Sobhan, A.; Lee, J.; Koo, S.-M.; Shin, W.H.; Oh, J.-M.; Park, C. Enzymatic Synthesis of Formate Ester through Immobilized Lipase and Its Reuse. Polymers 2020, 12, 1802. [Google Scholar] [CrossRef]
- Mehta, A.; Gupta, A.; Bhardwaj, K.K.; Gupta, R. A Purified Alkaline and Detergent-Tolerant Lipase from Aspergillus fumigatus with Potential Application in Removal of Mustard Oil Stains from Cotton Fabric. Tenside Surfactants Deterg. 2021, 58, 442–451. [Google Scholar] [CrossRef]
- Bredai, R.; Ben Romdhane, I.B.; Bouchaala, I.; Belghith, K.S.; Belghith, H. Purification of Bacillus licheniformis Lipase and its Application as an Additive in Detergent for Destaining. J. Surfactants Deterg. 2021, 24, 835–853. [Google Scholar] [CrossRef]
- Abdulmalek, S.A.; Yan, Y. Recent developments of lipase immobilization technology and application of immobilized lipase mixtures for biodiesel production. Biofuels Bioprod. Biorefining 2022, 16, 1062–1094. [Google Scholar] [CrossRef]
- Toldrá-Reig, F.; Mora, L.; Toldrá, F. Developments in the Use of Lipase Transesterification for Biodiesel Production from Animal Fat Waste. Appl. Sci. 2020, 10, 5085. [Google Scholar] [CrossRef]
- Patel, G.B.; Rakholiya, P.; Shindhal, T.; Varjani, S.; Tabhani, N.; Shah, K.R. Lipolytic Nocardiopsis for reduction of pollution load in textile industry effluent and SWISS model for structural study of lipase. Bioresour. Technol. 2021, 341, 125673. [Google Scholar] [CrossRef] [PubMed]
- Marchut-Mikolajczyk, O.; Drożdżyński, P.; Struszczyk-Świta, K. Biodegradation of slop oil by endophytic Bacillus cereus EN18 coupled with lipase from Rhizomucor miehei (Palatase®). Chemosphere 2020, 250, 126203. [Google Scholar] [CrossRef]
- Malunavicius, V.; Padaiga, A.; Stankeviciute, J.; Pakalniskis, A.; Gudiukaite, R. Engineered Geobacillus lipolytic enzymes—Attractive polyesterases that degrade polycaprolactones and simultaneously produce esters. Int. J. Biol. Macromol. 2023, 253, 127656. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Oh, Y.-R.; Kim, D.-M.; Eom, G.T.; Song, J.K. Screening and efficient production of engineered lipase B from Candida Antarctica for eco-friendly recycling of waste polycaprolactone. Polym. Degrad. Stab. 2022, 195, 109807. [Google Scholar] [CrossRef]
- Zhong, W.; Zhang, M.; Li, X.; Zhang, Y.; Wang, Z.; Zheng, J. Enantioselective Resolution of (R, S)-2-Phenoxy-Propionic Acid Methyl Ester by Covalent Immobilized Lipase from Aspergillus oryzae. Appl. Biochem. Biotechnol. 2020, 190, 1049–1059. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef]
- Zhao, J.; Ma, M.; Zeng, Z.; Wan, D.; Yan, X.; Xia, J.; Yu, P.; Gong, D. Effects of Mutating the Alanine Residue in the Consensus Pentapeptide on Biochemical and Structural Characteristics of Bacillus licheniformis Lipase. Food Biotechnol. 2023, 37, 280–300. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; de Aguiar Falcão, I.R.; da Silva Souza, J.E.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Casas-Godoy, L.; Gasteazoro, F.; Duquesne, S.; Bordes, F.; Marty, A.; Sandoval, G.J.L. Lipases: An overview. Lipases Phospholipases Methods Protoc. 2018, 1835, 3–38. [Google Scholar] [CrossRef]
- Brandao, L.M.d.S.; Barbosa, M.S.; Souza, R.L.; Pereira, M.M.; Lima, A.S.; Soares, C.M. Lipase activation by molecular bioimprinting: The role of interactions between fatty acids and enzyme active site. Biotechnol. Prog. 2021, 37, e3064. [Google Scholar] [CrossRef] [PubMed]
- Contesini, F.J.; Davanço, M.G.; Borin, G.P.; Vanegas, K.G.; Cirino, J.P.G.; de Melo, R.R.; Mortensen, U.H.; Hildén, K.; Campos, D.R.; Carvalho, P.d.O. Advances in Recombinant Lipases: Production, Engineering, Immobilization and Application in the Pharmaceutical Industry. Catalysts 2020, 10, 1032. [Google Scholar] [CrossRef]
- Albayati, S.H.; Masomian, M.; Ishak, S.N.H.; Mohamad Ali, M.S.b.; Thean, A.L.; Mohd Shariff, F.b.; Muhd Noor, N.D.b.; Raja Abd Rahman, R.N.Z. Main Structural Targets for Engineering Lipase Substrate Specificity. Catalysts 2020, 10, 747. [Google Scholar] [CrossRef]
- Fernandez-Lopez, L.; Roda, S.; Robles-Martín, A.; Muñoz-Tafalla, R.; Almendral, D.; Ferrer, M.; Guallar, V. Enhancing the Hydrolytic Activity of a Lipase towards Larger Triglycerides through Lid Domain Engineering. Int. J. Mol. Sci. 2023, 24, 13768. [Google Scholar] [CrossRef] [PubMed]
- Ma’ruf, I.F.; Widhiastuty, M.P.; Moeis, M.R. Effect of mutation at oxyanion hole residu (H110F) on activity of Lk4 lipase. Biotechnol. Rep. 2021, 29, e00590. [Google Scholar] [CrossRef]
- Infanzón, B.; Sotelo, P.H.; Martínez, J.; Diaz, P. Rational evolution of the unusual Y-type oxyanion hole of Rhodococcus sp. CR53 lipase LipR. Enzyme Microb. Technol. 2018, 108, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Secundo, F.; Carrea, G. Optimization of Hydrolase Efficiency in Organic Solvents. Chem. Eur. J. 2003, 9, 3194–3199. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Meng, X.; Zhou, H.; Liu, Y.; Secundo, F.; Liu, Y. Enzyme Stability and Activity in Non-Aqueous Reaction Systems: A Mini Review. Catalysts 2016, 6, 32. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Brady, D. The limits to biocatalysis: Pushing the envelope. Chem. Commun. 2018, 54, 6088–6104. [Google Scholar] [CrossRef]
- Cui, H.; Vedder, M.; Zhang, L.; Jaeger, K.; Schwaneberg, U.; Davari, M.D. Polar Substitutions on the Surface of a Lipase Substantially Improve Tolerance in Organic Solvents. ChemSusChem 2022, 15, e202102551. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Zhu, H.; Shi, B.; Huang, A.; Gou, L. Catalytic synthesis of sucrose-6-acetate by lipase in DMF composite solvent. J. Chem. Technol. Biotechnol. 2022, 98, 381–386. [Google Scholar] [CrossRef]
- Shehata, M.; Timucin, E.; Venturini, A.; Sezerman, O.U. Understanding thermal and organic solvent stability of thermoalkalophilic lipases: Insights from computational predictions and experiments. J. Mol. Model. 2020, 26, 122. [Google Scholar] [CrossRef] [PubMed]
- Sadaf, A.; Grewal, J.; Jain, I.; Kumari, A.; Khare, S.K. Stability and structure of Penicillium chrysogenum lipase in the presence of organic solvents. Prep. Biochem. Biotechnol. 2018, 48, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Mohtashami, M.; Fooladi, J.; Haddad-Mashadrizeh, A.; Housaindokht, M.R.; Monhemi, H. Molecular mechanism of enzyme tolerance against organic solvents: Insights from molecular dynamics simulation. Int. J. Biol. Macromol. 2019, 122, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Yenenler, A.; Venturini, A.; Burduroglu, H.C.; Sezerman, O.U. Investigating the structural properties of the active conformation BTL2 of a lipase from Geobacillus thermocatenulatus in toluene using molecular dynamic simulations and engineering BTL2 via in-silico mutation. J. Mol. Model. 2018, 24, 229. [Google Scholar] [CrossRef] [PubMed]
- Sibalic, D.; Salic, A.; Zelic, B.; Tran, N.N.; Hessel, V.; Nigam, K.D.P.; Tisma, M. Synergism of ionic liquids and lipases for lignocellulosic biomass valorization. Chem. Eng. J. 2023, 461, 142011. [Google Scholar] [CrossRef]
- Kumar, A.; Bhakuni, K.; Venkatesu, P. Strategic planning of proteins in ionic liquids: Future solvents for the enhanced stability of proteins against multiple stresses. Phys. Chem. Chem. Phys. 2019, 21, 23269–23282. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zou, X.; Jin, Q.; Wang, X. Advance in influence of ionic liquids system on properties and structure of lipase. China Oils Fats 2017, 42, 26–30. [Google Scholar]
- Han, P.; An, Z.; Ouyang, P. Research progress in lipase catalyzed synthesis of sugar ester. Mod. Chem. Ind. 2005, 24, 4. [Google Scholar]
- Ventura, S.P.M.; Santos, L.D.F.; Saraiva, J.A.; Coutinho, J.A.P. Concentration effect of hydrophilic ionic liquids on the enzymatic activity of Candida antarctica lipase B. World J. Microbiol. Biotechnol. 2012, 28, 2303–2310. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.-S.; Wen, Q.; Huang, Z.-L.; Cai, Y.-Z.; Halling, P.J.; Yang, Z. Impacts of ionic liquids on enzymatic synthesis of glucose laurate and optimization with superior productivity by response surface methodology. Process Biochem. 2015, 50, 1852–1858. [Google Scholar] [CrossRef]
- Kim, H.S.; Eom, D.; Koo, Y.-M.; Yingling, Y.G. The effect of imidazolium cations on the structure and activity of the Candida antarctica Lipase B enzyme in ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 22062–22069. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, Z.; Moniruzzaman, M. Ionic liquids as a potential solvent for lipase-catalysed reactions: A review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Frauenkron-Machedjou, V.J.; Fulton, A.; Zhu, L.; Anker, C.; Bocola, M.; Jaeger, K.; Schwaneberg, U. Towards Understanding Directed Evolution: More than Half of All Amino Acid Positions Contribute to Ionic Liquid Resistance of Bacillus subtilis Lipase A. ChemBioChem 2015, 16, 937–945. [Google Scholar] [CrossRef]
- Klähn, M.; Lim, G.S.; Wu, P. How ion properties determine the stability of a lipase enzyme in ionic liquids: A molecular dynamics study. Phys. Chem. Chem. Phys. 2011, 13, 18647–18660. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2020, 121, 1232–1285. [Google Scholar] [CrossRef]
- Nian, B.; Li, X. Can deep eutectic solvents be the best alternatives to ionic liquids and organic solvents: A perspective in enzyme catalytic reactions. Int. J. Biol. Macromol. 2022, 217, 255–269. [Google Scholar] [CrossRef]
- Bellou, M.G.; Gkantzou, E.; Skonta, A.; Moschovas, D.; Spyrou, K.; Avgeropoulos, A.; Gournis, D.; Stamatis, H. Development of 3D Printed Enzymatic Microreactors for Lipase-Catalyzed Reactions in Deep Eutectic Solvent-Based Media. Micromachines 2022, 13, 1954. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Wang, J.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. A novel strategy for biodiesel production by combination of liquid lipase, deep eutectic solvent and ultrasonic-assistance in scaled-up reactor: Optimization and kinetics. J. Clean. Prod. 2022, 372, 133740. [Google Scholar] [CrossRef]
- Nian, B.; Cao, C.; Liu, Y. How Candida antarctica lipase B can be activated in natural deep eutectic solvents: Experimental and molecular dynamics studies. J. Chem. Technol. Biotechnol. 2020, 95, 86–93. [Google Scholar] [CrossRef]
- Alvarez, M.S.; Longo, M.A.; Deive, F.J.; Rodriguez, A. Synthesis and characterization of a lipase-friendly DES based on cholinium dihydrogen phosphate. J. Mol. Liq. 2021, 340, 117230. [Google Scholar] [CrossRef]
- Hollenbach, R.; Bindereif, B.; van der Schaaf, U.S.; Ochsenreither, K.; Syldatk, C. Optimization of Glycolipid Synthesis in Hydrophilic Deep Eutectic Solvents. Front. Bioeng. Biotechnol. 2020, 8, 382. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, Z.; Lu, Z.; Qi, Y.; Nian, B.; Hu, Y. Hydrophobic deep eutectic solvents-lipase synergistically catalyze the synthesis of Vitamin E succinate via hydrogen bonds. J. Mol. Liq. 2023, 394, 123711. [Google Scholar] [CrossRef]
- Soares, G.A.; Alnoch, R.C.; dos Santos, L.A.; Mafra, M.R.; Mitchell, D.A.; Krieger, N. High hydrolytic activity of the metagenomic lipase LipC12 in deep eutectic solvents. J. Mol. Liq. 2023, 391, 123383. [Google Scholar] [CrossRef]
- Abed, K.M.; Hayyan, A.; Elgharbawy, A.A.M.; Hizaddin, H.F.; Hashim, M.A.; Abu Hasan, H.; Hamid, M.D.; Zuki, F.M.; Saleh, J.; Aldaihani, A.G. Palm Raceme as a Promising Biomass Precursor for Activated Carbon to Promote Lipase Activity with the Aid of Eutectic Solvents. Molecules 2022, 27, 8734. [Google Scholar] [CrossRef]
- Guajardo, N.; Schrebler, R.A.; Domínguez de María, P. From batch to fed-batch and to continuous packed-bed reactors: Lipase-catalyzed esterifications in low viscous deep-eutectic-solvents with buffer as cosolvent. Bioresour. Technol. 2019, 273, 320–325. [Google Scholar] [CrossRef]
- Hümmer, M.; Kara, S.; Liese, A.; Huth, I.; Schrader, J.; Holtmann, D. Synthesis of (−)-menthol fatty acid esters in and from (−)-menthol and fatty acids—Novel concept for lipase catalyzed esterification based on eutectic solvents. Mol. Catal. 2018, 458, 67–72. [Google Scholar] [CrossRef]
- Liu, X.; Meng, X.-Y.; Xu, Y.; Dong, T.; Zhang, D.-Y.; Guan, H.-X.; Zhuang, Y.; Wang, J. Enzymatic synthesis of 1-caffeoylglycerol with deep eutectic solvent under continuous microflow conditions. Biochem. Eng. J. 2019, 142, 41–49. [Google Scholar] [CrossRef]
- Hollenbach, R.; Ochsenreither, K.; Syldatk, C. Enzymatic Synthesis of Glucose Monodecanoate in a Hydrophobic Deep Eutectic Solvent. In Int. J. Mol. Sci. 2020, 21, 4342. [Google Scholar] [CrossRef]
- Zhou, P.; Liu, W.; Zhang, Y.; Liu, G.; Zhao, Z.; Zhang, M.; Wei, Z.; Zhang, Y.; Tang, X.; Li, P.; et al. Tuning multi-purpose glycerol-based deep eutectic solvents for biocatalytic deacidification of high-acid rice bran oil: Kinetics, mechanism, and inhibition of side glycerolysis. LWT 2023, 189, 115535. [Google Scholar] [CrossRef]
- Mazur, M.; Janeczko, T.; Gładkowski, W. Lipase-mediated Baeyer–Villiger oxidation of benzylcyclopentanones in ester solvents and deep eutectic solvents. Sci. Rep. 2022, 12, 14795. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko Bubalo, M.; Jurinjak Tušek, A.; VinkoviĿ, M.; RadoševiĿ, K.; Gaurina SrĿek, V.; RadojĿiĿ RedovnikoviĿ, I. Cholinium-based deep eutectic solvents and ionic liquids for lipase-catalyzed synthesis of butyl acetate. J. Mol. Catal. B Enzym. 2015, 122, 188–198. [Google Scholar] [CrossRef]
- ZitianWang; Dai, L.; Liu, D.; Liu, H.; Du, W. Kinetics and Mechanism of Solvent Influence on the Lipase-Catalyzed 1,3-Diolein Synthesis. ACS Omega 2020, 5, 24708–24716. [Google Scholar] [CrossRef] [PubMed]
- Ehlert, J.; Kronemann, J.; Zumbrägel, N.; Preller, M. Lipase-Catalyzed Chemoselective Ester Hydrolysis of Biomimetically Coupled Aryls for the Synthesis of Unsymmetric Biphenyl Esters. Molecules 2019, 24, 4272. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.S.; Potdar, M.K.; Harjani, J.R.; Nara, S.J.; Salunkhe, M.M. Ionic liquids: Efficient additives for Candida rugosa lipase-catalysed enantioselective hydrolysis of butyl 2-(4-chlorophenoxy)propionate. J. Mol. Catal. B Enzym. 2004, 30, 185–188. [Google Scholar] [CrossRef]
- Kołodziejska, R.; Studzińska, R.; Pawluk, H. Lipase-catalyzed enantioselective transesterification of prochiral 1-((1,3-dihydroxypropan-2-yloxy)methyl)-5,6,7,8-tetrahydroquinazoline-2,4(1H,3H)-dione in ionic liquids. Chirality 2018, 30, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Jaimes, I.; García-Torres, Y.; Santillán-Uribe, H.C.; Montiel, C. Immobilization and enantioselectivity of Bacillus pumilus lipase in ionic liquids. J. Mol. Catal. B Enzym. 2013, 89, 137–141. [Google Scholar] [CrossRef]
- Fredes, Y.; Chamorro, L.; Cabrera, Z. Increased Selectivity of Novozym 435 in the Asymmetric Hydrolysis of a Substrate with High Hydrophobicity through the Use of Deep Eutectic Solvents and High Substrate Concentrations. Molecules 2019, 24, 792. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Qi, S.-J.; Xin, R.-P.; Yang, B.; Wang, Y.-H. Enzymatic selective synthesis of 1,3-DAG based on deep eutectic solvent acting as substrate and solvent. Bioprocess Biosyst. Eng. 2015, 38, 2053–2061. [Google Scholar] [CrossRef]
- Sankaran, R.; Show, P.L.; Yap, Y.J.; Lam, H.L.; Ling, T.C.; Pan, G.-T.; Yang, T.C.K. Sustainable approach in recycling of phase components of large scale aqueous two-phase flotation for lipase recovery. J. Clean. Prod. 2018, 184, 938–948. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, K.-P.; Huang, Y.; Wang, Z. Both hydrolytic and transesterification activities of Penicillium expansum lipase are significantly enhanced in ionic liquid [BMIm][PF6]. J. Mol. Catal. B Enzym. 2010, 63, 23–30. [Google Scholar] [CrossRef]
- Abdi, Y.; Shomal, R.; Taher, H.; Al-Zuhair, S. Improving the reusability of an immobilized lipase-ionic liquid system for biodiesel production. Biofuels 2019, 10, 635–641. [Google Scholar] [CrossRef]
- Pätzold, M.; Burek, B.O.; Liese, A.; Bloh, J.Z.; Holtmann, D. Product recovery of an enzymatically synthesized (−)-menthol ester in a deep eutectic solvent. Bioprocess Biosyst. Eng. 2019, 42, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Lozano, P.; Alvarez, E.; Nieto, S.; Villa, R.; Bernal, J.M.; Donaire, A. Biocatalytic synthesis of panthenyl monoacyl esters in ionic liquids and deep eutectic solvents. Green Chem. 2019, 21, 3353–3361. [Google Scholar] [CrossRef]
- Panić, M.; Radović, M.; Maros, I.; Jurinjak Tušek, A.; Cvjetko Bubalo, M.; Radojčić Redovniković, I. Development of environmentally friendly lipase-catalysed kinetic resolution of (R,S)-1-phenylethyl acetate using aqueous natural deep eutectic solvents. Process Biochem. 2021, 102, 1–9. [Google Scholar] [CrossRef]
- He, Y.; Li, K.; Bo, G.; Wang, J.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Enhancing biodiesel production via liquid Yarrowia lipolytica lipase 2 in deep eutectic solvents. Fuel 2022, 316, 123342. [Google Scholar] [CrossRef]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef]
- Cantone, S.; Hanefeld, U.; Basso, A. Biocatalysis in non-conventional media—Ionic liquids, supercritical fluids and the gas phase. Green Chem. 2007, 9, 954–971. [Google Scholar] [CrossRef]
- Yao, J.; Li, C.; Xiao, L.; Wu, Y.; Wu, Q.; Cui, Z.; Wang, B. Influence of natural deep eutectic solvents on stability and structure of cellulase. J. Mol. Liq. 2022, 346, 118238. [Google Scholar] [CrossRef]
- Park, H.J.; Joo, J.C.; Park, K.; Yoo, Y.J. Stabilization of Candida antarctica lipase B in hydrophilic organic solvent by rational design of hydrogen bond. Biotechnol. Bioprocess Eng. 2012, 17, 722–728. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, L.; Eltoukhy, L.; Jiang, Q.; Korkunc, S.K.; Jaeger, K.-E.; Schwaneberg, U.; Davari, M.D. Enzyme Hydration Determines Resistance in Organic Cosolvents. ACS Catal. 2020, 10, 14847–14856. [Google Scholar] [CrossRef]
- Pramanik, S.; Cui, H.; Dhoke, G.V.; Yildiz, C.B.; Vedder, M.; Jaeger, K.-E.; Schwaneberg, U.; Davari, M.D. How Does Surface Charge Engineering of Bacillus subtilis Lipase A Improve Ionic Liquid Resistance? Lessons Learned from Molecular Dynamics Simulations. ACS Sustain. Chem. Eng. 2022, 10, 2689–2698. [Google Scholar] [CrossRef]
- Wang, X.; Sheng, Y.; Cui, H.; Qiao, J.; Song, Y.; Li, X.; Huang, H. Corner Engineering: Tailoring Enzymes for Enhanced Resistance and Thermostability in Deep Eutectic Solvents. Angew. Chem. 2024, 136, e202315125. [Google Scholar] [CrossRef]
- Markel, U.; Zhu, L.; Frauenkron-Machedjou, V.J.; Zhao, J.; Bocola, M.; Davari, M.D.; Jaeger, K.-E.; Schwaneberg, U. Are Directed Evolution Approaches Efficient in Exploring Nature’s Potential to Stabilize a Lipase in Organic Cosolvents? Catalysts 2017, 7, 142. [Google Scholar] [CrossRef]
- Min, K.; Kim, H.T.; Park, S.J.; Lee, S.; Jung, Y.J.; Lee, J.-S.; Yoo, Y.J.; Joo, J.C. Improving the organic solvent resistance of lipase a from Bacillus subtilis in water–ethanol solvent through rational surface engineering. Bioresour. Technol. 2021, 337, 125394. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.; Shemesh, E.; Dayan, N.; Fishman, A. protein engineering by random mutagenesis and structure-guided consensus of geobacillus stearothermophilus lipase T6 for enhanced stability in methanol. Appl. Environ. Microbiol. 2014, 80, 1515–1527. [Google Scholar] [CrossRef]
- Sheng, Y.; Cui, H.; Wang, X.; Wang, M.; Song, P.; Huang, H.; Li, X. Harnessing solvation-guided engineering to enhance deep eutectic solvent resistance and thermostability in enzymes. Green Chem. 2024. [Google Scholar] [CrossRef]
- Siemers, M.; Bondar, A.-N. interactive interface for graph-based analyses of dynamic H-bond networks: Application to Spike protein S. J. Chem. Inf. Model. 2021, 61, 2998–3014. [Google Scholar] [CrossRef]
- Simón, L.; Goodman, J.M. Enzyme Catalysis by Hydrogen Bonds: The Balance between Transition State Binding and Substrate Binding in Oxyanion Holes. J. Org. Chem. 2009, 75, 1831–1840. [Google Scholar] [CrossRef]
- Reetz, M.T.; Soni, P.; Fernández, L.; Gumulya, Y.; Carballeira, J.D. Increasing the stability of an enzyme toward hostile organic solvents by directed evolution based on iterative saturation mutagenesis using the B-FIT method. Chem. Commun. 2010, 46, 8657–8658. [Google Scholar] [CrossRef]
- Kawata, T.; Ogino, H. Amino acid residues involved in organic solvent-stability of the LST-03 lipase. Biochem. Biophys. Res. Commun. 2010, 400, 384–388. [Google Scholar] [CrossRef]
- Zhao, J.; Frauenkron-Machedjou, V.J.; Fulton, A.; Zhu, L.; Davari, M.D.; Jaeger, K.-E.; Schwaneberg, U.; Bocola, M. Unraveling the effects of amino acid substitutions enhancing lipase resistance to an ionic liquid: A molecular dynamics study. Phys. Chem. Chem. Phys. 2018, 20, 9600–9609. [Google Scholar] [CrossRef]
- Frauenkron-Machedjou, V.J.; Fulton, A.; Zhao, J.; Weber, L.; Jaeger, K.-E.; Schwaneberg, U.; Zhu, L. Exploring the full natural diversity of single amino acid exchange reveals that 40–60% of BSLA positions improve organic solvents resistance. Bioresour. Bioprocess. 2018, 5, 2. [Google Scholar] [CrossRef]
- Dror, A.; Kanteev, M.; Kagan, I.; Gihaz, S.; Shahar, A.; Fishman, A. Structural insights into methanol-stable variants of lipase T6 from Geobacillus stearothermophilus. Appl. Microbiol. Biotechnol. 2015, 99, 9449–9461. [Google Scholar] [CrossRef] [PubMed]
- Ban, X.; Lahiri, P.; Dhoble, A.S.; Li, D.; Gu, Z.; Li, C.; Cheng, L.; Hong, Y.; Li, Z.; Kaustubh, B. Evolutionary Stability of Salt Bridges Hints Its Contribution to Stability of Proteins. Comput. Struct. Biotechnol. J. 2019, 17, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Kordes, S.; Romero-Romero, S.; Lutz, L.; Höcker, B. A newly introduced salt bridge cluster improves structural and biophysical properties of de novoTIM barrels. Protein Sci. 2022, 31, 513–527. [Google Scholar] [CrossRef] [PubMed]
- Korman, T.P.; Sahachartsiri, B.; Charbonneau, D.M.; Huang, G.L.; Beauregard, M.; Bowie, J.U. Dieselzymes: Development of a stable and methanol tolerant lipase for biodiesel production by directed evolution. Biotechnol. Biofuels 2013, 6, 70. [Google Scholar] [CrossRef]
- Dachuri, V.; Jang, S.-H.; Lee, C. Different Effects of Salt Bridges near the Active Site of Cold-Adapted Proteus mirabilis Lipase on Thermal and Organic Solvent Stabilities. Catalysts 2022, 12, 761. [Google Scholar] [CrossRef]
- Cui, H.; Eltoukhy, L.; Zhang, L.; Markel, U.; Jaeger, K.; Davari, M.D.; Schwaneberg, U. Less Unfavorable Salt Bridges on the Enzyme Surface Result in More Organic Cosolvent Resistance. Angew. Chem. Int. Ed. 2021, 60, 11448–11456. [Google Scholar] [CrossRef]
- Landeta, C.; Boyd, D.; Beckwith, J. Disulfide bond formation in prokaryotes. Nat. Microbiol. 2018, 3, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Ittisoponpisan, S.; Jeerapan, I. In Silico Analysis of Glucose Oxidase from Aspergillus niger: Potential Cysteine Mutation Sites for Enhancing Protein Stability. Bioengineering 2021, 8, 188. [Google Scholar] [CrossRef] [PubMed]
- Jahangirizadeh, Z.; Taghdir, M. Tuning the active site dynamic properties and substrate affinity in Pseudomonas aeruginosa elastase by Cys270–Cys297 disulfide bond. Chem. Phys. 2020, 531, 110615. [Google Scholar] [CrossRef]
- Li, L.; Zhang, S.; Wu, W.; Guan, W.; Deng, Z.; Qiao, H. Enhancing thermostability of Yarrowia lipolytica lipase 2 through engineering multiple disulfide bonds and mitigating reduced lipase production associated with disulfide bonds. Enzym. Microb. Technol. 2019, 126, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, S.; Xu, Y.; Yu, X. Engineering of a thermo-alkali-stable lipase from Rhizopus chinensis by rational design of a buried disulfide bond and combinatorial mutagenesis. J. Ind. Microbiol. Biotechnol. 2020, 47, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, W.; Deng, Z.; Zhang, S.; Guan, W. Improved thermostability of lipase Lip2 from Yarrowia lipolytica through disulfide bond design for preparation of medium-long-medium structured lipids. LWT 2022, 166, 113786. [Google Scholar] [CrossRef]
- Huang, J.; Dai, S.; Chen, X.; Xu, L.; Yan, J.; Yang, M.; Yan, Y. Alteration of Chain-Length Selectivity and Thermostability of Rhizopus oryzae Lipase via Virtual Saturation Mutagenesis Coupled with Disulfide Bond Design. Appl. Environ. Microbiol. 2023, 89, e01878-22. [Google Scholar] [CrossRef]
- Ogino, H.; Uchiho, T.; Yokoo, J.; Kobayashi, R.; Ichise, R.; Ishikawa, H. Role of Intermolecular Disulfide Bonds of the Organic Solvent-Stable PST-01 Protease in Its Organic Solvent Stability. Appl. Environ. Microbiol. 2001, 67, 942–947. [Google Scholar] [CrossRef] [PubMed]
- Pulido, I.Y.; Prieto, E.; Pieffet, G.P.; Méndez, L.; Jiménez-Junca, C.A. Functional Heterologous Expression of Mature Lipase LipA from Pseudomonas aeruginosa PSA01 in Escherichia coli SHuffle and BL21 (DE3): Effect of the Expression Host on Thermal Stability and Solvent Tolerance of the Enzyme Produced. Int. J. Mol. Sci. 2020, 21, 3925. [Google Scholar] [CrossRef]
- Gihaz, S.; Bash, Y.; Rush, I.; Shahar, A.; Pazy, Y.; Fishman, A. Bridges to Stability: Engineering Disulfide Bonds towards Enhanced Lipase Biodiesel Synthesis. ChemCatChem 2020, 12, 181–192. [Google Scholar] [CrossRef]
- Hamdan, S.H.; Maiangwa, J.; Nezhad, N.G.; Ali, M.S.M.; Normi, Y.M.; Shariff, F.M.; Rahman, R.N.Z.R.A.; Leow, T.C. Knotting terminal ends of mutant T1 lipase with disulfide bond improved structure rigidity and stability. Appl. Microbiol. Biotechnol. 2023, 107, 1673–1686. [Google Scholar] [CrossRef]
- Malleshappa Gowder, S.; Chatterjee, J.; Chaudhuri, T.; Paul, K. Prediction and Analysis of Surface Hydrophobic Residues in Tertiary Structure of Proteins. Sci. World J. 2014, 2014, 971258. [Google Scholar] [CrossRef]
- Katsura, S.; Furuishi, T.; Ueda, H.; Yonemochi, E. Synthesis and Characterization of Cholesteryl Conjugated Lysozyme (CHLysozyme). Molecules 2020, 25, 3704. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Park, S.; Jiménez, R.H.F.; Rinehart, D.; Tamm, L.K. Role of Aromatic Side Chains in the Folding and Thermodynamic Stability of Integral Membrane Proteins. J. Am. Chem. Soc. 2007, 129, 8320–8327. [Google Scholar] [CrossRef]
- Gihaz, S.; Kanteev, M.; Pazy, Y.; Fishman, A. Filling the Void: Introducing Aromatic Interactions into Solvent Tunnels to Enhance Lipase Stability in Methanol. Appl. Environ. Microbiol. 2018, 84, e02143-18. [Google Scholar] [CrossRef]
- Shokri, M.M.; Ahmadian, S.; Akbari, N.; Khajeh, K. Hydrophobic Substitution of Surface Residues Affects Lipase Stability in Organic Solvents. Mol. Biotechnol. 2014, 56, 360–368. [Google Scholar] [CrossRef]
- Nordwald, E.M.; Armstrong, G.S.; Kaar, J.L. NMR-Guided Rational Engineering of an Ionic-Liquid-Tolerant Lipase. ACS Catal. 2014, 4, 4057–4064. [Google Scholar] [CrossRef]
- Kawata, T.; Ogino, H. Enhancement of the organic solvent-stability of the LST-03 lipase by directed evolution. Biotechnol. Prog. 2009, 25, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Yagonia, C.F.J.; Park, H.J.; Hong, S.Y.; Yoo, Y.J. Simultaneous improvements in the activity and stability of Candida antarctica lipase B through multiple-site mutagenesis. Biotechnol. Bioprocess Eng. 2015, 20, 218–224. [Google Scholar] [CrossRef]
- Zhu, C.-L.; Lü, X.; Xia, X.-L. Effect of site-directed mutagenesis of amino acids in lid region on the enzymatic properties of T1 lipase. Biotechnol. Bull. 2020, 36, 94. [Google Scholar]



| Application Fields | Specific Aspects | References |
|---|---|---|
| Food | Flavorings | [10,11] |
| Production of human milk fat substitutes | [12,13] | |
| Egg processing | [14,15] | |
| Bread processing | [16,17] | |
| Cocoa butter substitute processing | [18,19] | |
| Edible oil processing | [6,20] | |
| Cheese processing | [21,22] | |
| Meat and fish processing | [23] | |
| Vitamin production | [24,25] | |
| Textile | Textile processing | [26,27] |
| Papermaking | De-inking of wastepaper | [28,29] |
| Leather | Leather degreasing | [30,31] |
| Pharmaceuticals | Chiral drug preparation | [32,33] |
| Diagnostic tools | [34] | |
| Anti-obesity treatment | [35] | |
| Medical device composition | [36] | |
| Cosmetics | Personal care products production | [37,38] |
| Perfume production | [39] | |
| Detergents | Detergent production | [40,41] |
| Bioenergy | Biodiesel production | [42,43] |
| Environment | Wastewater treatment | [44,45] |
| Plastic degradation | [46,47] | |
| Agriculture | Herbicide synthesis | [48] |
| DES System | Lipase | Substrate | Temperature | Time | Relative Enzyme Activity | References |
|---|---|---|---|---|---|---|
| Bet:Gly (1:3) | CALB | pNPB | 40 °C | 5 min | 95.00% | [80] |
| ChCl:Fru:H2O (5:2:5) | CALB | pNPB | 40 °C | 5 min | 92.00% | [80] |
| ChCl:U (1:2) | CALB | pNPB | 40 °C | 5 min | 87.00% | [80] |
| Bet:Gly (1:2) | CALB | pNPP | 59.85 °C | 5 min | 115.48% | [82] |
| Bet:Xyl (1:1) | CALB | pNPP | 59.85 °C | 5 min | 91.69% | [82] |
| DecA:Lid (2:1) | CRL | pNPP | 40 °C | 10 min | 81.02% | [85] |
| ChCl:EG (1:2) H2O/DES (% v/v) = 70/25 | LipC12 | pNPP | 25 °C | 2 min | 1050.00% | [86] |
| ChCl:Gly (1:2) H2O/DES (% v/v) = 70/25 | LipC12 | pNPP | 25 °C | 2 min | 1233.00% | [86] |
| ChCl:Sor (2:1) H2O/DES (% v/v) = 70/25 | LipC12 | pNPP | 25 °C | 2 min | 1795.00% | [86] |
| Ala:NaOH (1:1) | PPL | pNPP | 40 °C | 15 min | 162.50% | [87] |
| DES | Lipase | Substrates | Temperature | Time | Conversion Rate | Reference |
|---|---|---|---|---|---|---|
| ChCl:glycerol (1:2) H2O/DES (% v/v) = 10/90 | Novozym 435 | benzoic acid, glycerol | 60 °C | 24 h | 90% | [88] |
| (−)-menthol:decanoic acid (65:35) H2O/DES (% w/w) = 10/90 | CRL | decanoic acid, (−)-menthol | 35 °C | 7 d | 83% | [89] |
| ChCl:urea (7:6) (w:w) | Novozym 435 | methyl caffeate, glycerin | 65 °C | 2.5 h | 96.46% (yield rate) | [90] |
| Decanoic acid:(−)-menthol (1:1) | immobilized lipase B from Candida antarctica | vinyl decanoate, glucose | 50 °C | 24 h | 10.95% (yield rate) | [91] |
| ChCl:glycerol (1:2) | Lipozyme RM IM | high-acid rice bran oil, glycerol | 50 °C | 24 h | 94.5% | [92] |
| ChCl:urea (1:2) | immobilized lipase B from Candida antarctica | α-benzylcyclopentanones | 55 °C | 3 d | exceed 90% | [93] |
| ChCl:ethylene glycol (1:2) H2O/DES (% w/w) = 5/95 | immobilized lipase B from Candida antarctica | acetic anhydride, 1-butanol | 25 °C | 2 h | 80% (yield rate) | [94] |
| Lipases | Solvent | Beneficial Mutants | Stabilizing Factors | References |
|---|---|---|---|---|
| Proteus mirabilis lipase | methanol | a variant with 13 mutation sites (Dieselzyme 4) | hydrogen bond, salt bridge | [129] |
| Bacillus subtilis lipase A | 12% (v/v) 2,2,2-trifluoroethanol | I12R/M137H/N166E | hydration | [113] |
| Bacillus subtilis lipase A | water-miscible organic cosolvents (1,4-dioxane, 2,2,2-trifluoroethanol, dimethyl sulfoxide) | introduction of charged and polar amino acids | hydration | [116] |
| Bacillus subtilis lipase A | 1,4-dioxane, 2,2,2-trifluoroethanol, dimethyl sulfoxide | introduction of charged and polar amino acids | hydrogen bond | [125] |
| lipase from Bacillus subtilis | acetonitrile, dimethyl sulfoxide, N, N-dimethylformamide | M134D/I157M/Y139C/K112D/R33G | hydrogen bond, salt bridge | [122] |
| Geobacillus stearothermophilus lipase T6 | methanol | H86Y, Q185L, and A269T | hydrogen bond, hydration | [118] |
| lipase LST-03 from Pseudomonas aeruginosa | dimethyl sulfoxide, straight-chain alkanes | S155L, S164K, S194R, and D209N (dimethyl sulfoxide), G157R, S194R, and D209N (straight-chain alkanes) | hydrogen bond, salt bridge, and hydration | [123,149] |
| Geobacillus stearothermophilus lipase T6 | water-methanol cosolvent | H86Y/A269T/R374W | hydrogen bond | [126] |
| Bacillus subtilis lipase A | water-ethanol co-solvent | S16G, A38G, A38T, and L108N | hydration | [117] |
| a novel lipase from Pseudomonas sp. (GQ243724) | methanol, ethanol, n-propanol, N, N-dimethylformamide | N219L, N219I | hydrophobic interaction | [147] |
| Candida antarctica lipase B | 40% (v/v) ethanol | V139E/A92E | flexibility | [150] |
| Geobacillus stearothermophilus lipase T6 | methanol | E251C/G332C | disulfide bond | [141] |
| Geobacillus zalihae T1 lipase | methanol | S2C/A384C | disulfide bond | [142] |
| Geobacillus zalihae T1 lipase | dimethyl sulfoxide, n-hexadecane | L188M, A190L, A190Y, L188M/A190L, and L188M/A190Y | hydrophobic interaction | [151] |
| Candida antarctica lipase B | water-methanol cosolvent | N97Q, N264Q, and D265E | hydrogen bond, hydration | [113] |
| Bacillus subtilis lipase A | water-miscible organic cosolvents (1,4-dioxane, 2,2,2-trifluoroethanol, dimethyl sulfoxide) | D34K, D34R, K112D, K112E, K112D, and D144K | hydration | [131] |
| Bacillus subtilis lipase A | [Bmim][Cl], [Bmim][Br], [Bmim][I], [Bmim][TfO] | introduction of charged and polar amino acids | hydrogen bond, hydration | [76] |
| Bacillus subtilis lipase A | [Bmim][Cl] | G158E/K44E/R57E/Y49E | ionic interaction | [148] |
| Bacillus subtilis lipase A | ChCl: acetamide, ChCl: ethylene glycol, tetrabutylphosphonium bromide: ethylene glycol | T66H/G67D, K88E/N89K, M137D/N138D, M137D/N138H, Y161D/S162E/S163E | hydration | [115] |
| Bacillus subtilis lipase A | ChCl: acetamide, ChCl: ethylene glycol, tetrabutylphosphonium bromide: ethylene glycol | D64H/R107L/E171Y, D64H/R142L/E171Y | hydration | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Jin, T.; Nian, B.; Cheng, W. Solvent Tolerance Improvement of Lipases Enhanced Their Applications: State of the Art. Molecules 2024, 29, 2444. https://doi.org/10.3390/molecules29112444
Chen M, Jin T, Nian B, Cheng W. Solvent Tolerance Improvement of Lipases Enhanced Their Applications: State of the Art. Molecules. 2024; 29(11):2444. https://doi.org/10.3390/molecules29112444
Chicago/Turabian StyleChen, Mei, Tongtong Jin, Binbin Nian, and Wenjun Cheng. 2024. "Solvent Tolerance Improvement of Lipases Enhanced Their Applications: State of the Art" Molecules 29, no. 11: 2444. https://doi.org/10.3390/molecules29112444
APA StyleChen, M., Jin, T., Nian, B., & Cheng, W. (2024). Solvent Tolerance Improvement of Lipases Enhanced Their Applications: State of the Art. Molecules, 29(11), 2444. https://doi.org/10.3390/molecules29112444







