Selective Solid–Liquid Extraction of Lithium Cation Using Tripodal Sulfate-Binding Receptors Driven by Electrostatic Interactions
Abstract
1. Introduction
2. Results and Discussion
3. General Synthetic and Solid–Liquid Extraction Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Farahbakhsh, J.; Arshadi, F.; Mofidi, Z.; Mohseni-Dargah, M.; Kök, C.; Assefi, M.; Soozanipour, A.; Zargar, M.; Asadnia, M.; Boroumand, Y.; et al. Direct lithium extraction: A new paradigm for lithium production and resource utilization. Desalination 2024, 575, 117249. [Google Scholar] [CrossRef]
- Reich, R.; Slunitschek, K.; Danisi, R.M.; Eiche, E.; Kolb, J. Lithium extraction techniques and the application potential of different sorbents for lithium recovery from brines. Min. Proc. Ext. Met. Rev. 2022, 44, 261–280. [Google Scholar] [CrossRef]
- Haddad, A.Z.; Hackl, L.; Akuzum, B.; Pohlman, G.; Magnan, J.-F.; Kostecki, R. How to make lithium extraction cleaner, faster and cheaper—In six steps. Nature 2023, 616, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mo, Y.; Qing, W.; Shao, S.; Tang, C.Y.; Li, J. Membrane-based technologies for lithium recovery from water lithium resources: A review. J. Membr. Sci. 2019, 591, 117317. [Google Scholar] [CrossRef]
- Nie, X.-Y.; Sun, S.-Y.; Sun, Z.; Song, X.; Yu, J.-G. Ion-fractionation of lithium ions from magnesium ions by electrodialysis using monovalent selective ion-exchange membranes. Desalination 2017, 403, 128–135. [Google Scholar] [CrossRef]
- Swain, B. Separation and purification of lithium by solvent extraction and supported liquid membrane, analysis of their mechanism: A review. J. Chem. Technol. Biotechnol. 2016, 91, 2549–2562. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Zeng, X.; Deng, T.; Wang, J. Membranes for separation of alkali/alkaline earth metal ions: A review. Sep. Purif. Technol. 2021, 278, 119640. [Google Scholar] [CrossRef]
- Razmjou, A.; Asadnia, M.; Hosseini, E.; Habibnejad Korayem, A.; Chen, V. Design principles of ion selective nanostructured membranes for the extraction of lithium ions. Nat. Commun. 2019, 10, 5793. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Zhao, W.; Wu, B. Separation of sulfate anion from aqueous solution governed by recognition chemistry: A minireview. Front. Chem. 2022, 10, 905563. [Google Scholar] [CrossRef] [PubMed]
- Lein, G.M.; Cram, D.J. Spherand complexation and decomplexation rates with sodium and lithium picrates, and activation parameters for decomplexation. J. Chem. Soc. Chem. Commun. 1982, 5, 301–304. [Google Scholar] [CrossRef]
- Cram, D.J.; Lein, G.M. Host-guest complexation. 36. spherand and lithium and sodium ion complexation rates and equilibria. J. Am. Chem. Soc. 1985, 107, 3657–3668. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Xue, Z.; Mao, L.; Sun, J.; Shao, F.; Qi, M.; Jing, Y.; Jia, Y. Extraction separation of lithium isotopes with Bromobenzene-15-crown-5/ionic liquids system: Experimental and theoretical study. J. Mol. Liq. 2022, 364, 120020. [Google Scholar] [CrossRef]
- Bezdomnikov, A.A.; Sharov, V.E.; Demina, L.I.; Skrebtsov, M.I.; Ilyukhin, A.B.; Tsivadze, A.Y. Specific features of lithium solvent extraction from perchlorate media with benzo-15-crown-5. Polyhedron 2023, 244, 116612. [Google Scholar] [CrossRef]
- Xu, C.; Tran, Q.; Wojtas, L.; Liu, W. Harnessing ion–dipole interactions: A simple and effective approach to high-performance lithium receptors. J. Mater. Chem. A 2023, 11, 12214–12222. [Google Scholar] [CrossRef]
- Talanova, G.G.; Elkarim, N.S.A.; Talanov, V.S.; Hanes, R.E.; Hwang, H.-S.; Bartsch, R.A.; Rogers, R.D. The “picrate effect” on extraction selectivities of aromatic group-containing crown ethers for alkali metal cations. J. Am. Chem. Soc. 1999, 121, 11281–11290. [Google Scholar] [CrossRef]
- Lehn, J.M. Cryptate inclusion complexes, effects on solute-solute and solute-solvent interactions and on ionic reactivity. Pure Appl. Chem. 1980, 52, 2303–2319. [Google Scholar] [CrossRef]
- Mahoney, J.M.; Beatty, A.M.; Smith, B.D. Selective solid-liquid extraction of lithium halide salts using a ditopic macrobicyclic receptor. Inorg. Chem. 2004, 43, 7617–7621. [Google Scholar] [CrossRef]
- Park, I.W.; Yoo, J.; Adhikari, S.; Park, J.S.; Sessler, J.L.; Lee, C.H. Calix[4]pyrrole-based heteroditopic ion-pair receptor that displays anion-modulated, cation-binding behavior. Chem. Eur. J. 2012, 18, 15073–15078. [Google Scholar] [CrossRef]
- He, Q.; Zhang, Z.; Brewster, J.T.; Lynch, V.M.; Kim, S.K.; Sessler, J.L. Hemispherand-strapped calix[4]pyrrole: An ion-pair receptor for the recognition and extraction of lithium nitrite. J. Am. Chem. Soc. 2016, 138, 9779–9782. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Williams, N.J.; Oh, J.H.; Lynch, V.M.; Kim, S.K.; Moyer, B.A.; Sessler, J.L. Selective solid-liquid and liquid-liquid extraction of lithium chloride using strapped calix[4]pyrroles. Angew. Chem. Int. Ed. 2018, 57, 11924–11928. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-I.; Kim, H.; Kim, Y.; Choi, M.-G.; Jang, W.-D. Strapped calix[4]pyrrole as a lithium salts selective receptor through separated ion-pair binding. Chem. Commun. 2020, 56, 10541–10544. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jones, L.O.; Hwang, I.; Allen, M.J.; Tao, D.; Lynch, V.M.; Freeman, B.D.; Khashab, N.M.; Schatz, G.C.; Page, Z.A.; et al. Selective separation of lithium chloride by organogels containing strapped calix[4]pyrroles. J. Am. Chem. Soc. 2021, 143, 20403–20410. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Yeon, Y.; Lee, A.; Lynch, V.M.; He, Q.; Sessler, J.L.; Kim, S.K. Tetraamidoindolyl calix[4]arene as a selective ion pair receptor for LiCl. Org. Chem. Front. 2022, 9, 6888–6893. [Google Scholar] [CrossRef]
- Vilar, R. Anion Recognition and Templation in Coordination Chemistry. Eur. J. Inorg. Chem. 2008, 2008, 357–367. [Google Scholar] [CrossRef]
- Jin, C.; Zhang, M.; Wu, L.; Guan, Y.; Pan, Y.; Jiang, J.; Lin, C.; Wang, L. Squaramide-based tripodal receptors for selective recognition of sulfate anion. Chem. Commun. 2013, 49, 2025–2027. [Google Scholar] [CrossRef]
- Valls, A.; Altava, B.; Burguete, M.I.; Escorihuela, J.; Martí-Centelles, V.; Luis, S.V. Supramolecularly assisted synthesis of chiral tripodal imidazolium compounds. Org. Chem. Front. 2019, 6, 1214–1225. [Google Scholar] [CrossRef]
- Chen, L.; Berry, S.N.; Wu, X.; Howe, E.N.W.; Gale, P.A. Advances in Anion Receptor Chemistry. Chem 2020, 6, 61–141. [Google Scholar] [CrossRef]
- Jagleniec, D.; Wilczek, M.; Romański, J. Tripodal, Squaramide-Based Ion Pair Receptor for Effective Extraction of Sulfate Salt. Molecules 2021, 26, 2751. [Google Scholar] [CrossRef]
- Jia, C.; Wu, B.; Li, S.; Huang, X.; Zhao, Q.; Li, Q.S.; Yang, X.J. Highly efficient extraction of sulfate ions with a tripodal hexaurea receptor. Angew. Chem. Int. Ed. 2011, 50, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Zuo, W.; Jia, C.; Zhang, H.; Zhao, Y.; Yang, X.-J.; Wu, B. Selective recognition of choline phosphate by tripodal hexa-urea receptors with dual binding sites: Crystal and solution evidence. Chem. Sci. 2019, 10, 2483–2488. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Q.; Yu, S.-N.; Zhao, W.; Liang, L.; Gong, Y.; Yuan, L.; Tang, J.; Yang, X.-J.; Wu, B. Recognition-guided sulfate extraction and transport using tripodal hexaurea receptors. Inorg. Chem. Front. 2022, 9, 6091–6101. [Google Scholar] [CrossRef]
- Sun, Z.-Y.; Chen, S.-Q.; Liang, L.; Zhao, W.; Yang, X.-J.; Wu, B. pH-Dependent phosphate separation using a tripodal hexaurea receptor. Chem. Commun. 2023, 59, 12923–12926. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y. A conductance study of alkali metal ion–18-crown-6 complexes in N,N-dimethylformamide. Bull. Chem. Soc. Jpn. 1981, 54, 3133–3136. [Google Scholar] [CrossRef]
- Pasgreta, E.; Puchta, R.; Galle, M.; van Eikema Hommes, N.; Zahl, A.; van Eldik, R. Ligand-Exchange Processes on Solvated Lithium Cations: DMSO and Water/DMSO Mixtures. ChemPhysChem 2007, 8, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Liu, W.; Das Partha, J.; Colquhoun Howard, M.; Stoddart, J.F. Whither second-sphere coordination? CCS Chem. 2021, 4, 755–784. [Google Scholar] [CrossRef]

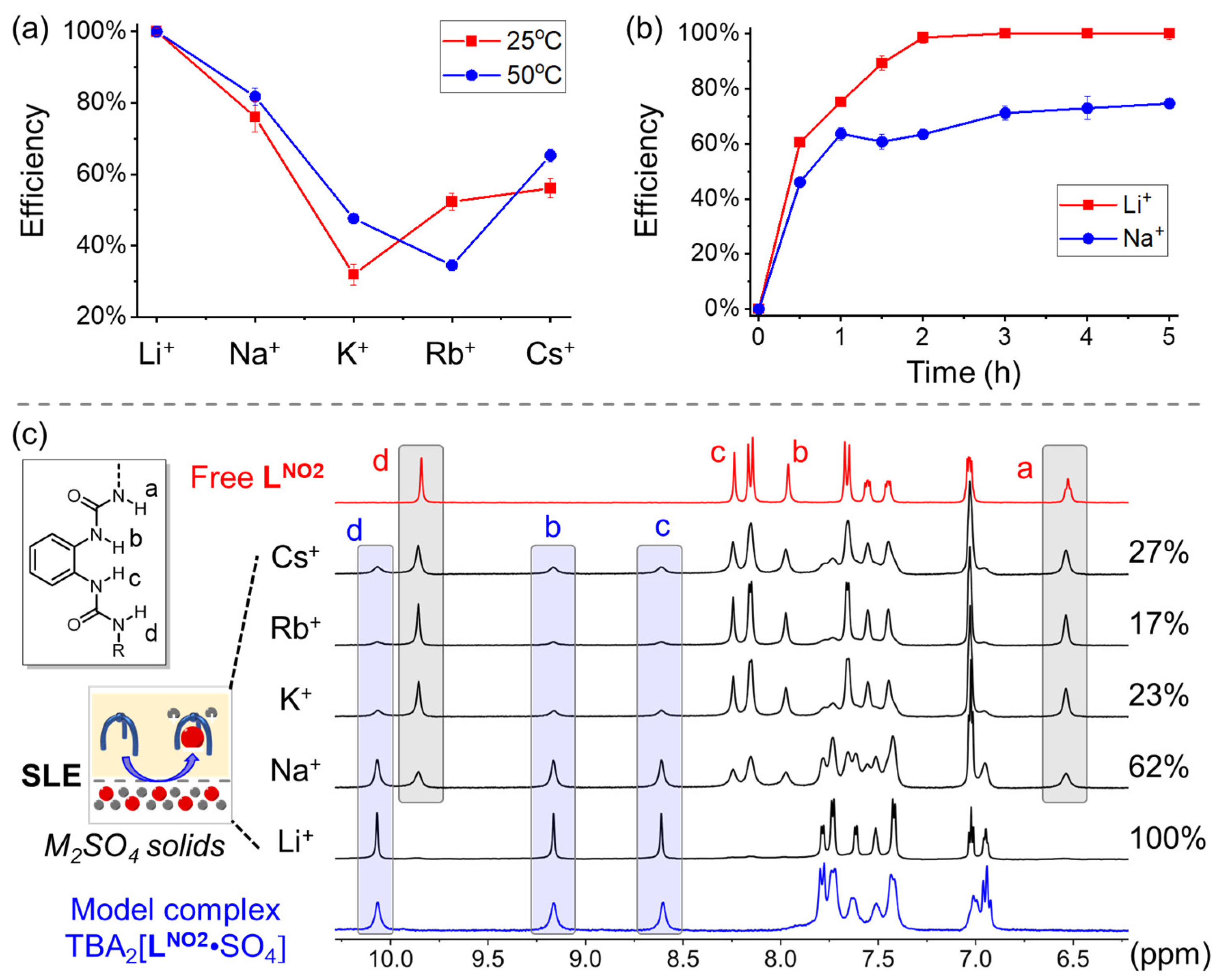

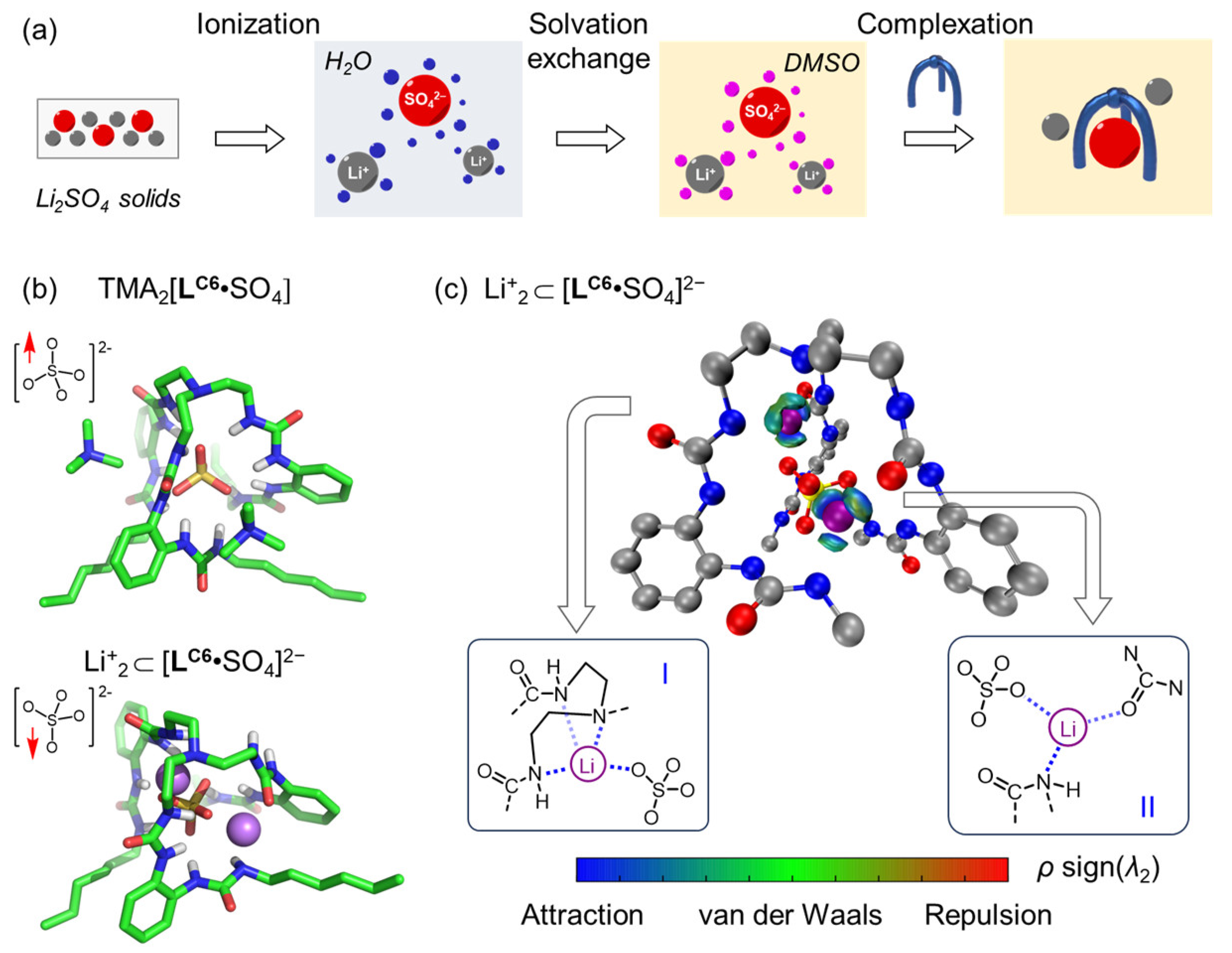
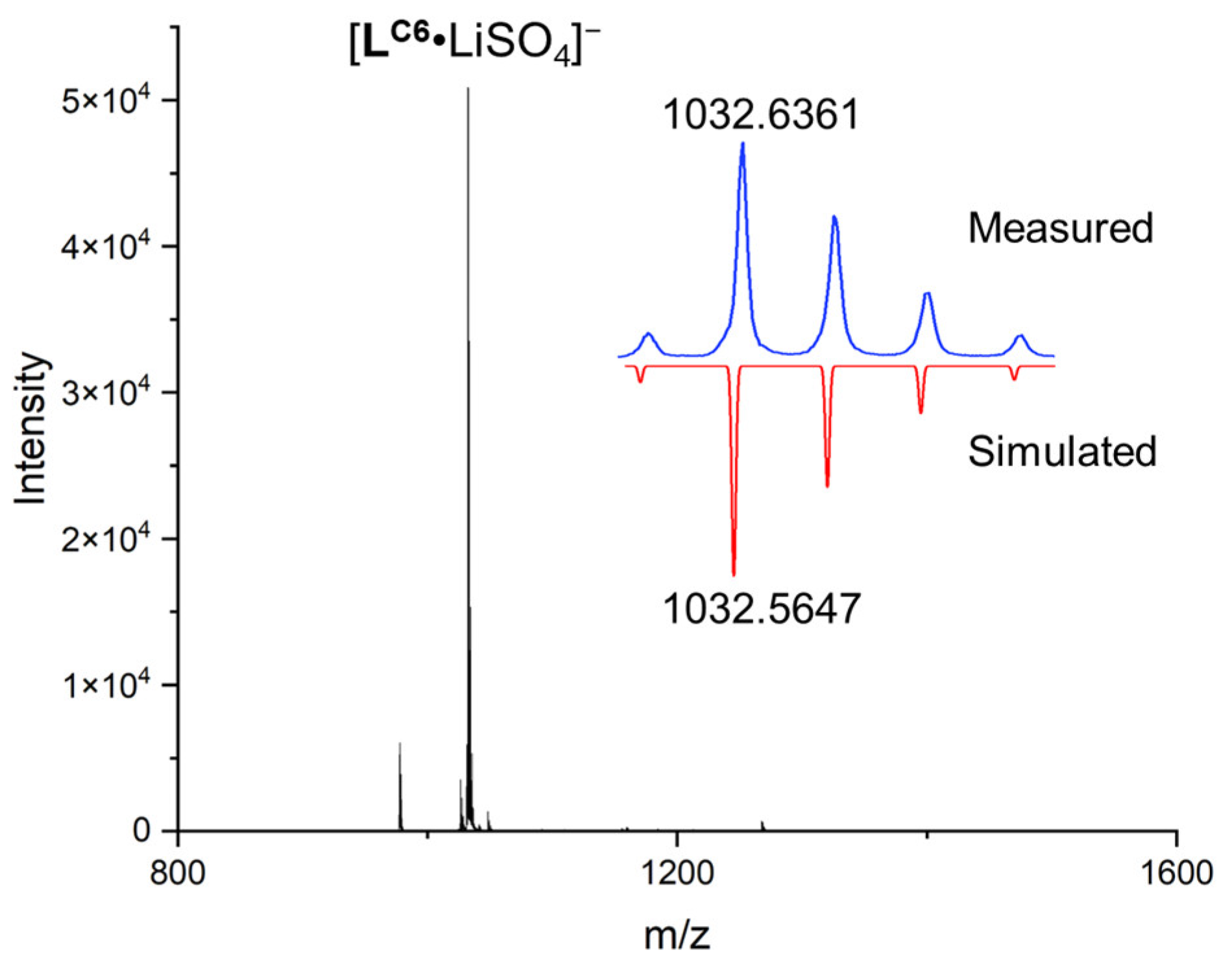
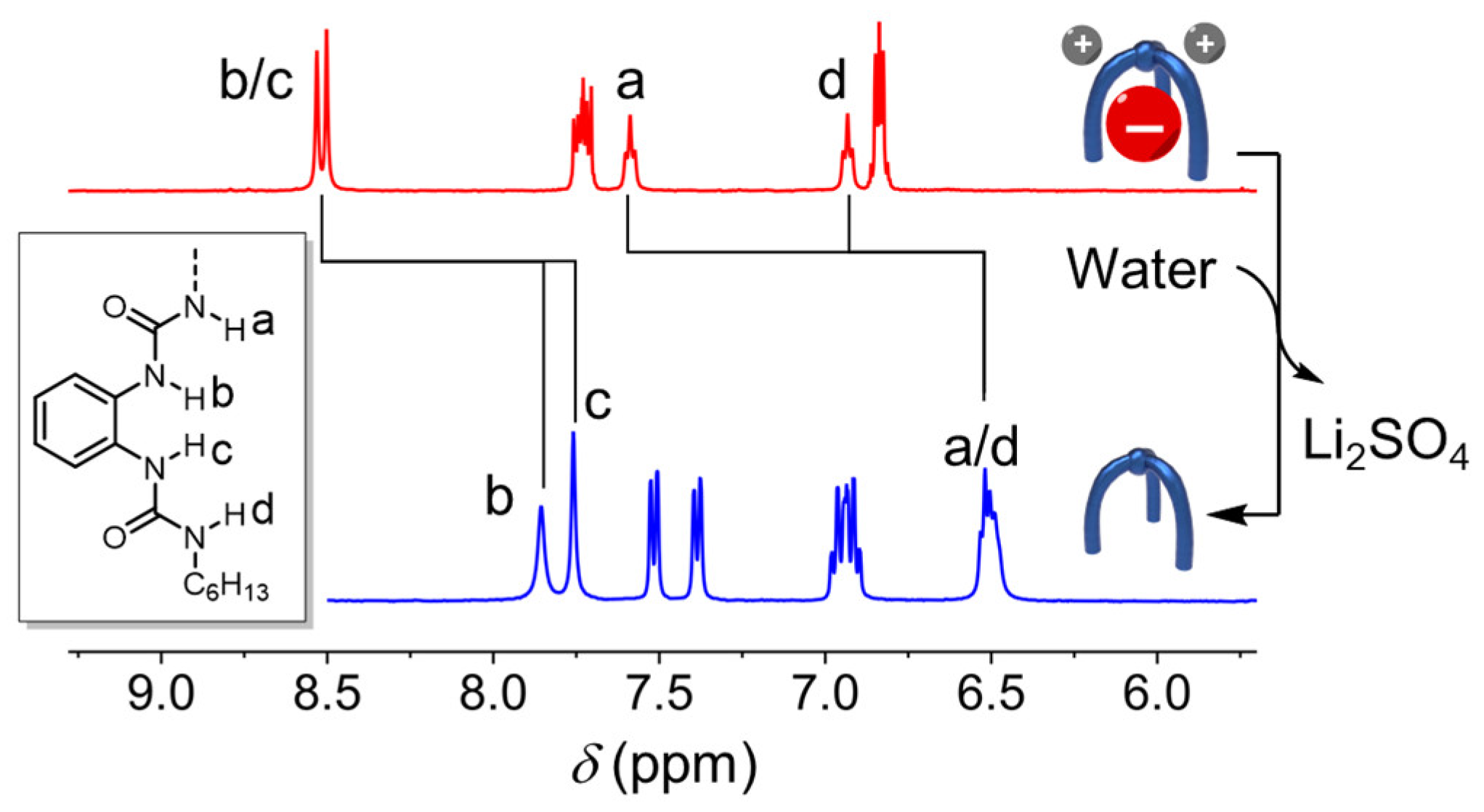
| Li+ | Na+ | K+ | Rb+ | Cs+ | |
|---|---|---|---|---|---|
| b Control | 2% | 2% | 3% | 1% | 3% |
| LNO2 | 100% | 62% | 23% | 17% | 27% |
| LMe | 100% | 50% | 18% | 30% | 64% |
| LC6 | 100% | 48% | 8% | 15% | 38% |
| c TLC6 | 80% | 33% | 17% | 15% | 6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Z.; He, Y.-C.; Yan, L.; Zhao, W.; Wu, B. Selective Solid–Liquid Extraction of Lithium Cation Using Tripodal Sulfate-Binding Receptors Driven by Electrostatic Interactions. Molecules 2024, 29, 2445. https://doi.org/10.3390/molecules29112445
Chen Y-Z, He Y-C, Yan L, Zhao W, Wu B. Selective Solid–Liquid Extraction of Lithium Cation Using Tripodal Sulfate-Binding Receptors Driven by Electrostatic Interactions. Molecules. 2024; 29(11):2445. https://doi.org/10.3390/molecules29112445
Chicago/Turabian StyleChen, Ya-Zhi, Ying-Chun He, Li Yan, Wei Zhao, and Biao Wu. 2024. "Selective Solid–Liquid Extraction of Lithium Cation Using Tripodal Sulfate-Binding Receptors Driven by Electrostatic Interactions" Molecules 29, no. 11: 2445. https://doi.org/10.3390/molecules29112445
APA StyleChen, Y.-Z., He, Y.-C., Yan, L., Zhao, W., & Wu, B. (2024). Selective Solid–Liquid Extraction of Lithium Cation Using Tripodal Sulfate-Binding Receptors Driven by Electrostatic Interactions. Molecules, 29(11), 2445. https://doi.org/10.3390/molecules29112445






