Asymmetric Sulfoxidations Catalyzed by Bacterial Flavin-Containing Monooxygenases
Abstract
1. Introduction
2. Results
2.1. Comparision between mFMO and NiFMO in Biocatalysed Sulfoxidations
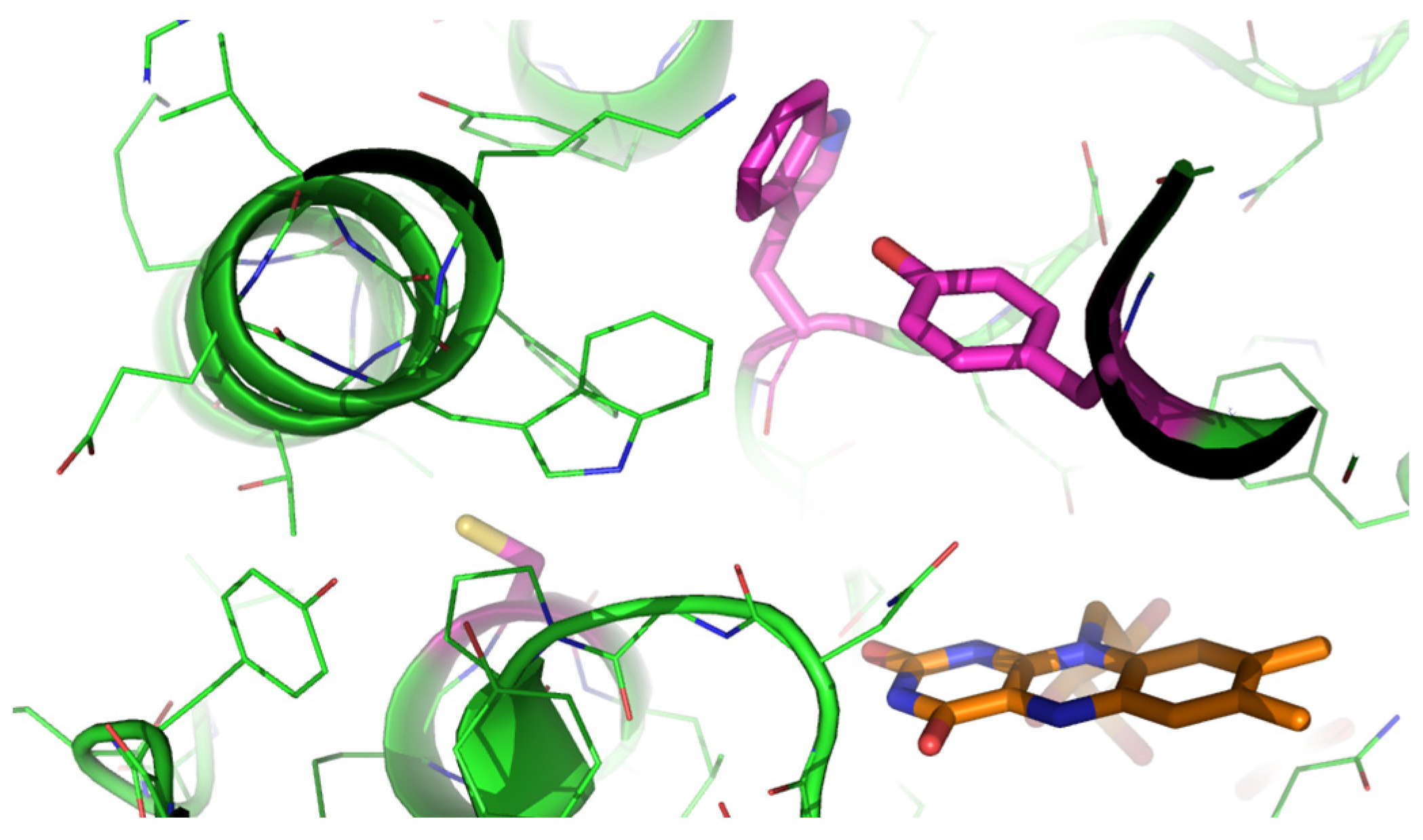
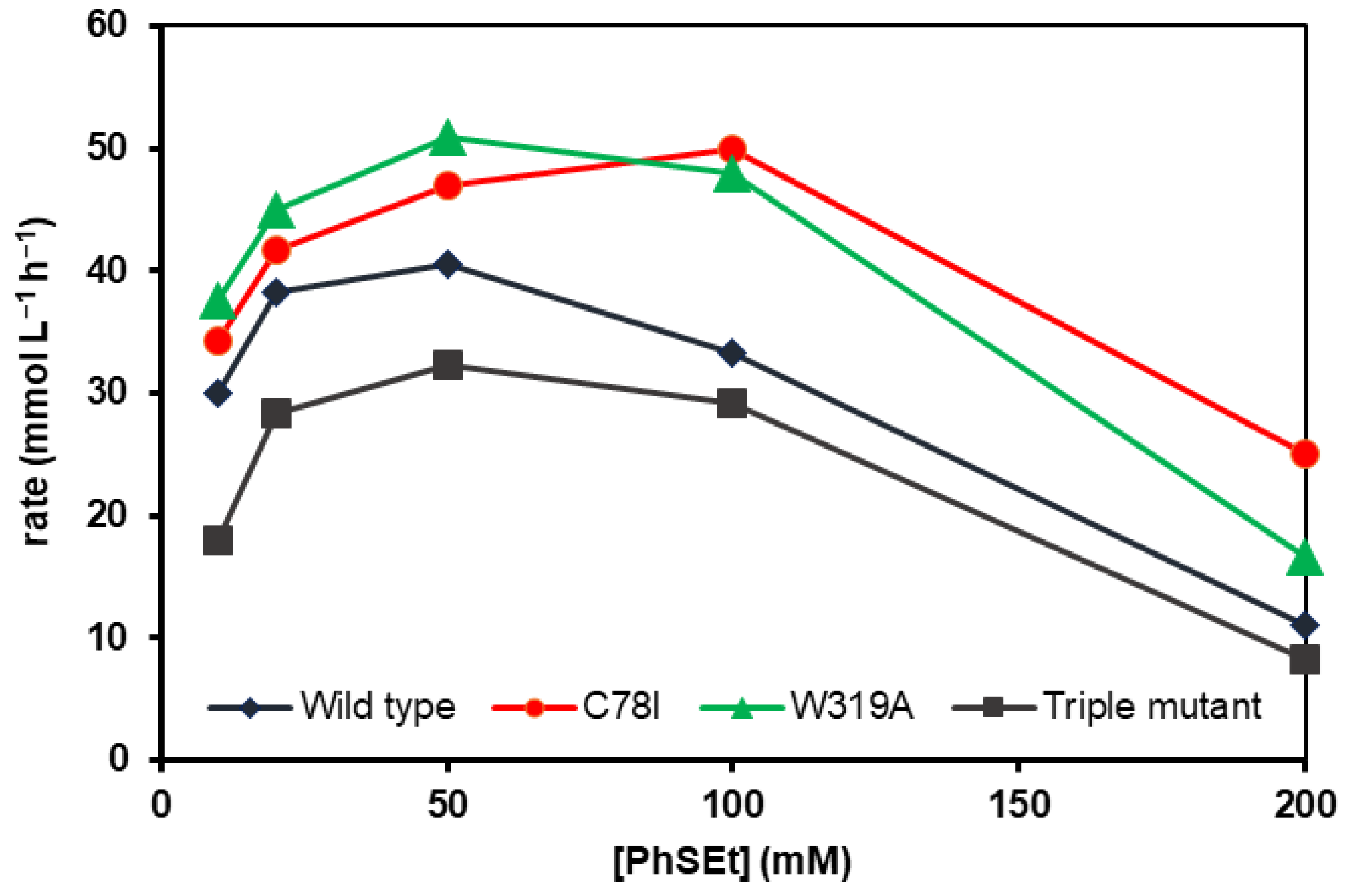
2.2. Performance of mFMO Mutants in Enzymatic Sulfoxidations
3. Materials and Methods
3.1. Materials and Methods
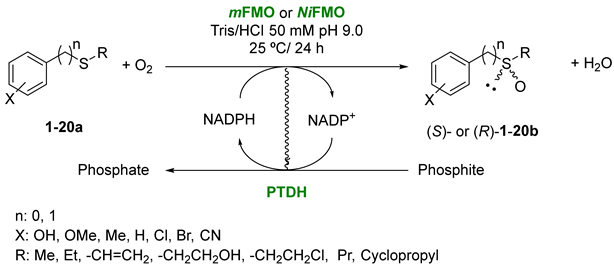
3.2. General Procedure for the FMO-Catalyzed Sulfoxidation of Sulfides 1–20a
3.3. W319A Biocatalyzed Synthesis of (S)-Ethyl Phenyl Sulfoxide (2b) at Multimilligram Scale
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojaczynska, E.; Wojaczynski, J. Sulfoxides in medicine. Curr. Opin. Chem. Biol. 2023, 76, 102340. [Google Scholar] [CrossRef] [PubMed]
- Bentley, R. Role of sulfur chirality in the chemical processes of biology. Chem. Soc. Rev. 2005, 34, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Soloshonok, V.A.; Klika, K.D.; Drabowicz, J.; Wzorek, A. Chiral sulfoxides: Advances in asymmetric synthesis and problems with the accurate determination of the stereochemical outcome. Chem. Soc. Rev. 2018, 47, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- Salom-Reig, X.; Bauder, C. Recent Applications in the Use of Sulfoxides as Chiral Auxiliaries for the Asymmetric Synthesis of Natural and Biologically Active Products. Synthesis 2020, 52, 964–978. [Google Scholar] [CrossRef]
- Trost, B.M.; Rao, M. Development of chiral sulfoxide ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 2015, 54, 5026–5043. [Google Scholar] [CrossRef] [PubMed]
- Wojaczynskak, E.; Wojaczynski, J. Modern stereoselective synthesis of chiral sulfinyl compounds. Chem. Rev. 2020, 120, 4578–4611. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.M.; Wang, S.; Dong, Z.B. Recent advances for chiral sulfoxides in asymmetric catalysis. Synthesis 2022, 54, 5168–5185. [Google Scholar]
- Devine, P.N.; Howard, R.M.; Kumar, R.; Thompson, M.P.; Truppo, M.D.; Turner, N.J. Extending the application of biocatalysis to meet the challenges of drug development. Nat. Chem. Rev. 2018, 2, 409–421. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. 2021, 60, 88–119. [Google Scholar] [CrossRef]
- Simíc, S.; Zukíc, E.; Schmermund, L.; Faber, K.; Winkler, C.K.; Kroutil, W. Shortening synthetic routes to small molecule Active Pharmaceutical Ingredients employing biocatalytic methods. Chem. Rev. 2022, 122, 1052–1126. [Google Scholar] [CrossRef]
- Dong, J.J.; Fernández-Fueyo, E.; Hollmann, F.; Paul, C.E.; Pesic, M.; Schmidt, S.; Wang, Y.; Younes, S.; Zhang, W. Biocatalytic oxidation reactions: A chemist’s perspective. Angew. Chem. Int. Ed. 2018, 57, 9238–9261. [Google Scholar] [CrossRef] [PubMed]
- Maczka, W.; Winska, K.; Grabarczyk, M. Biotechnological Methods of Sulfoxidation: Yesterday, Today, Tomorrow. Catalysts 2018, 8, 624. [Google Scholar] [CrossRef]
- Bassanini, I.; Ferrandi, E.E.; Vanoni, M.; Ottolina, G.; Riva, S.; Crotti, M.; Brenna, E.; Monti, D. Peroxygenase-catalyzed enantioselective sulfoxidations. Eur. J. Org. Chem. 2017, 7186–7189. [Google Scholar] [CrossRef]
- Linde, D.; Cañella, M.; Coscolín, C.; Davó-Siguero, I.; Romero, A.; Lucas, F.; Ruiz-Dueñas, F.J.; Guallar, V.; Martínez, A.T. Asymmetric sulfoxidation by engineering the heme pocket of a dye-decolorizing peroxidase. Catal. Sci. Technol. 2016, 6, 6277–6285. [Google Scholar] [CrossRef]
- Sanfilippo, C.; Cernuto, F.; Patti, A. Expanding the Use of Peroxygenase from Oat Flour in Organic Synthesis: Enantioselective Oxidation of Sulfides. Int. J. Mol. Sci. 2023, 24, 7464. [Google Scholar] [CrossRef] [PubMed]
- Boyd, D.R.; Sharma, N.D.; Stevenson, P.J.; Hoering, P.; Allen, C.C.R.; Dansette, P.M. Monooxygenase- and Dioxygenase-Catalyzed Oxidative Dearomatization of Thiophenes by Sulfoxidation, cis-Dihydroxylation and Epoxidation. Int. J. Mol. Sci. 2022, 23, 909. [Google Scholar] [CrossRef] [PubMed]
- Willrodt, C.; Gröning, J.A.D.; Nerke, P.; Koch, R.; Scholtissek, A.; Heine, T.; Schmid, A.; Bühler, B.; Tischler, D. Highly Efficient Access to (S)-Sulfoxides Utilizing a Promiscuous Flavoprotein Monooxygenase in a Whole-Cell Biocatalyst Format. ChemCatChem 2020, 17, 4664–4671. [Google Scholar] [CrossRef]
- Liu, F.; Shou, C.; Geng, Q.; Zhao, C.; Xu, J.; Yu, H. A Baeyer-Villiger monooxygenase from Cupriavidus basilensis catalyzes asymmetric synthesis of (R)-lansoprazole and other pharmaco-sulfoxides. Appl. Microbiol. Biotechnol. 2020, 105, 3169–3180. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-B.; Huang, Q.; Peng, W.; Wu, P.; Yu, D.; Chen, B.; Wang, B.; Reetz, M.T. P450-BM3-Catalyzed Sulfoxidation versus Hydroxylation: A Common or Two Different Catalytically Active Species? J. Am. Chem. Soc. 2020, 142, 2068–2073. [Google Scholar] [CrossRef]
- Boredwick, S.; Beier, A.; Balke, K.; Bornscheuer, U.T. Baeyer-Villiger monooxygenases from Yarrowia lipolytica catalyze preferentially sulfoxidations. Enzyme Microb. Technol. 2018, 109, 31–42. [Google Scholar] [CrossRef]
- Romero, E.; Gómez Castellanos, J.R.; Gadda, G.; Fraaije, M.W.; Mattevi, A. Same Substrate, Many Reactions: Oxygen Activation in Flavoenzymes. Chem. Rev. 2018, 118, 1742–1769. [Google Scholar] [CrossRef] [PubMed]
- Catucci, C.; Gao, C.; Sadeghi, S.J.; Gilardi, G. Chemical applications of Class B flavoprotein monooxygenases. Rend. Fis. Acc. Lincei 2017, 28, 195–206. [Google Scholar] [CrossRef]
- Hamman, M.A.; Haehner-Daniels, B.D.; Wrighton, S.A.; Rettie, A.E.; Hall, S.D. Stereoselective sulfoxidation of sulindac sulfide by flavin-containing monooxygenases. Comparison of human liver and kidney microsomes and mammalian enzymes. Biochem. Pharmacol. 2000, 60, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Phillips, I.R.; Sheppard, E.A. Endogenous Roles of Mammalian Flavin-Containing Monooxygenases. Catalysts 2019, 9, 1001. [Google Scholar] [CrossRef]
- Nicol, C.R.; Mascotti, M.L. Investigating the biochemical signatures and physiological roles of the FMO family using molecular phylogeny. BBA Adv. 2023, 4, 100108. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Kim, J.K.; Cho, E.H.; Kim, Y.C.; Kim, J.I.; Kim, S.W. A novel flavin-containing monooxygenase from Methylophaga sp. strain SK1 and its indigo synthesis in Escherichia coli. Biochem. Biophys. Res. Commun. 2003, 306, 930–936. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, A.; Malito, E.; Orru, R.; Fraaije, M.W.; Mattevi, A. Revealing the moonlighting role of NADP in the structure of a flavin-containing monooxygenase. Proc. Natl. Acad. Sci. USA 2008, 105, 6572–6577. [Google Scholar] [CrossRef]
- Rioz-Martínez, A.; Kopacz, M.; de Gonzalo, G.; Torres Pazmiño, D.E.; Gotor, V.; Fraaije, M.W. Exploring the biocatalytic scope of a bacterial flavin-containing monooxygenase. Org. Biomol. Chem. 2010, 9, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Lončar, N.; van Beek, H.L.; Fraaije, M.W. Structure-based redesign of a self-sufficient flavin-containing monooxygenase towards indigo production. Int. J. Mol. Sci. 2019, 20, 6148. [Google Scholar] [CrossRef]
- Lončar, N.; Fiorentini, F.; Bailleul, G.; Savino, S.; Romero, E.; Mattevi, A.; Fraaije, M.W. Characterization of a thermostable flavin-containing monooxygenase from Nitrincola lacisaponensis (NiFMO). Appl. Microbiol. Biotechnol. 2019, 103, 1755–1764. [Google Scholar] [CrossRef]
- Torres Pazmiño, D.E.; Snajdrova, R.; Baas, B.-J.; Ghobrial, M.; Mihovilovic, M.D.; Fraaije, M.W. Self-sufficient Baeyer-Villiger monooxygenases: Effective Coenzyme Regeneration for Biooxygenation by Fusion Engineering. Angew. Chem. Int. Ed. 2008, 47, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Zambianchi, F.; Fraaije, M.W.; Carrea, G.; de Gonzalo, G.; Rodríguez, C.; Gotor, V.; Ottolina, G. Titration and assignment of residues that regulate the enantioselectivity of phenylacetone monooxygenase. Adv. Synth. Catal. 2007, 349, 1327–1331. [Google Scholar] [CrossRef]
- de Gonzalo, G.; Torres Pazmiño, D.E.; Ottolina, G.; Fraaije, M.W.; Carrea, G. Oxidations catalyzed by phenylacetone monooxygenase from Thermobifida fusca. Tetrahedron Asymmetry 2005, 16, 3077–3083. [Google Scholar] [CrossRef]

 | |||||
|---|---|---|---|---|---|
| mFMO | NiFMO | ||||
| Entry | Sulfide | Conv. (%) 2 | ee (%) 3 | Conv. (%) 2 | ee (%) 3 |
| Entry 1 4 | PhSMe (1a) | 90 | 35 (S) | 17 | 9 (R) |
| Entry 2 4 | PhSEt (2a) | 18 | 79 (S) | 25 | 57 (S) |
| Entry 3 | PhSCH=CH2 (3a) | ≤3 | n.d. | ≤3 | n.d. |
| Entry 4 | PhSPr (4a) | 12 | 64 (S) | 10 | 55 (S) |
| Entry 5 | PhSCyclopropyl (5a) | 39 | 19 (S) | ≤3 | n.d. |
| Entry 6 | PhSCH2CH2OH (6a) | 78 | 28 (S) | 43 | 41 (S) |
| Entry 7 | PhSCH2CH2Cl (7a) | 70 | 33 (S) | 40 | 49 (S) |
| Entry 8 | p-HO-PhSMe (8a) | 56 | 72 (S) | 8 | 17 (S) |
| Entry 9 4 | p-MeO-PhSMe (9a) | 78 | 70 (S) | 12 | 29 (S) |
| Entry 10 4 | p-Me-PhSMe (10a) | 66 | 92 (S) | 97 | 76 (S) |
| Entry 11 4 | p-Cl-PhSMe (11a) | 80 | 95 (S) | 32 | 83 (S) |
| Entry 12 4 | m-Cl-PhSMe (12a) | 69 | 15 (S) | 31 | 12 (S) |
| Entry 13 4 | o-Cl-PhSMe (13a) | 20 | 64 (S) | 23 | 15 (S) |
| Entry 14 | p-Br-PhSMe (14a) | 75 | 85 (S) | 22 | 75 (S) |
| Entry 15 4 | p-NC-PhSMe (15a) | 50 | 22 (S) | 19 | 32 (S) |
| Entry 16 5 | NaphSMe (16a) | 8 | 39 (S) | ≤3 | n.d. |
| Entry 17 5 | PhSBn (17a) | ≤3 | n.d. | ≤3 | n.d. |
| Entry 18 5 | Pyrmetazole (18a) | ≤3 | n.d. | ≤3 | n.d. |
| Entry 19 4 | BnSMe (19a) | 85 | 17 (S) | 38 | 51 (R) |
| Entry 20 4 | BnSEt (20a) | 51 | 15 (S) | 12 | 41 (R) |
| Entry | Sulfide | T (°C) | Time (h) | Conv. (%) 1 | ee (%) 2 |
|---|---|---|---|---|---|
| Entry 1 | PhSEt (2a) | 45 | 24 | 40 | 55 (S) |
| Entry 2 | p-Me-PhSMe (10a) | 45 | 14 | 97 | 75 (S) |
| Entry 3 | p-Cl-PhSMe (11a) | 45 | 24 | 61 | 84 (S) |
| Entry 4 | p-Cl-PhSMe (11a) | 60 | 24 | 15 | 27 (S) |
| Entry 5 | p-Br-PhSMe (14a) | 45 | 24 | 35 | 72 (S) |
| Entry 6 | BnSMe (19a) | 45 | 24 | 54 | 50 (S) |
| Sulfide | Wild Type | C78I | W319A | Triple Mutant |
|---|---|---|---|---|
| PhSMe (1a) | 90% 35% (S) | 40% 10% (S) | 13% 64% (S) | 57% 63% (S) |
| PhSEt (2a) | 72% 75% (S) | 82% 75% (S) | 90% 94% (S) | 43% 83% (S) |
| PhSPr (4a) | 32% 64% (S) | 25% 60% (S) | 37% 77% (S) | 15% 65% (S) |
| PhSCyclopropyl (5a) | 39% 19% (S) | 43% 15% (S) | 41% 17% (S) | 30% 35% (S) |
| PhSCH2CH2OH (6a) | 78% 28% (S) | 81% 5% (S) | 17% 9% (S) | ≤3% n.d. |
| PhSCH2CH2Cl (7a) | 70% 33% (S) | 61% 12% (S) | ≤3% n.d. | ≤3% n.d. |
| p-HO-PhSMe (8a) | 56% 72% (S) | 33% 69% (S) | ≤3% n.d. | 28% 45% (S) |
| p-MeO-PhSMe (9a) | 78% 70% (S) | 43% 85% (S) | 18% 30% (S) | 56% 65% (S) |
| p-Me-PhSMe (10a) | 66% 92% (S) | 70% 90% (S) | 33% 9% (S) | 58% 63% (S) |
| p-Cl-PhSMe (11a) | 80% 95% (S) | 77% 95% (S) | 66% 5% (S) | 43% 61% (S) |
| m-Cl-PhSMe (12a) | 69% 15% (S) | 73% 70% (S) | 47% 65% (S) | 49% 27% (S) |
| o-Cl-PhSMe (13a) | 20% 64% (S) | 24% 62% (S) | 27% 15% (S) | 8% 22% (S) |
| p-Br-PhSMe (14a) | 75% 85% (S) | 67% 83% (S) | 80% 7% (S) | 37% 59% (S) |
| p-NC-PhSMe (15a) | 50% 22% (S) | 37% 41% (S) | 63% 7% (S) | 15% 37% (S) |
| NaphSMe (16a) | 8% 39% (S) | 37% 35% (S) | ≤3% n.d. | 8% 27% (S) |
| BnSMe (19a) | 58% 14% (S) | 31% 17% (R) | 35% 43% (S) | 20% 20% (R) |
| BnSEt (20a) | 51% 15% (S) | 69% 12% (R) | 76% 35% (S) | 69% 10% (R) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Gonzalo, G.; Coto-Cid, J.M.; Lončar, N.; Fraaije, M.W. Asymmetric Sulfoxidations Catalyzed by Bacterial Flavin-Containing Monooxygenases. Molecules 2024, 29, 3474. https://doi.org/10.3390/molecules29153474
de Gonzalo G, Coto-Cid JM, Lončar N, Fraaije MW. Asymmetric Sulfoxidations Catalyzed by Bacterial Flavin-Containing Monooxygenases. Molecules. 2024; 29(15):3474. https://doi.org/10.3390/molecules29153474
Chicago/Turabian Stylede Gonzalo, Gonzalo, Juan M. Coto-Cid, Nikola Lončar, and Marco W. Fraaije. 2024. "Asymmetric Sulfoxidations Catalyzed by Bacterial Flavin-Containing Monooxygenases" Molecules 29, no. 15: 3474. https://doi.org/10.3390/molecules29153474
APA Stylede Gonzalo, G., Coto-Cid, J. M., Lončar, N., & Fraaije, M. W. (2024). Asymmetric Sulfoxidations Catalyzed by Bacterial Flavin-Containing Monooxygenases. Molecules, 29(15), 3474. https://doi.org/10.3390/molecules29153474









