Functionalized Ionic Liquids-Modified Metal–Organic Framework Material Boosted the Enzymatic Performance of Lipase
Abstract
1. Introduction
2. Results and Discussion
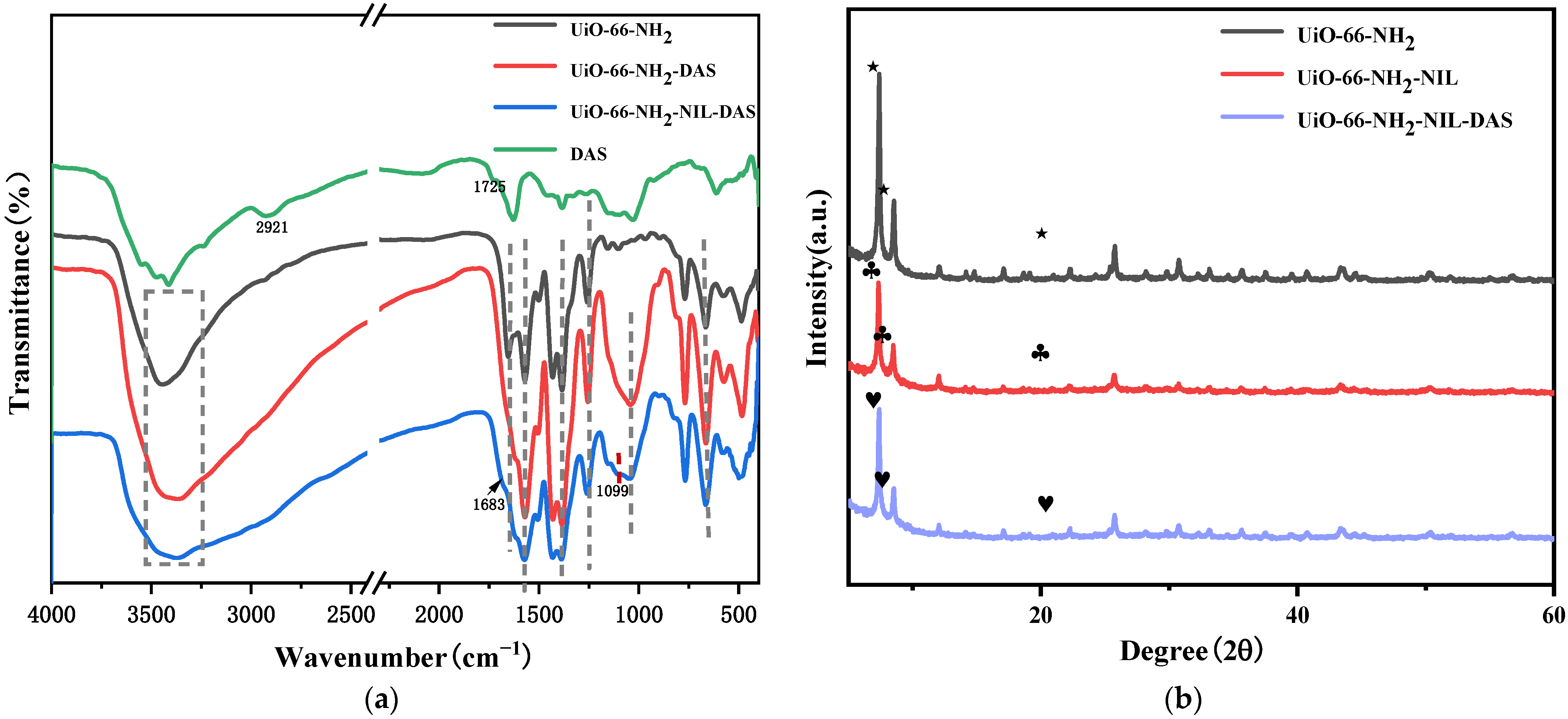
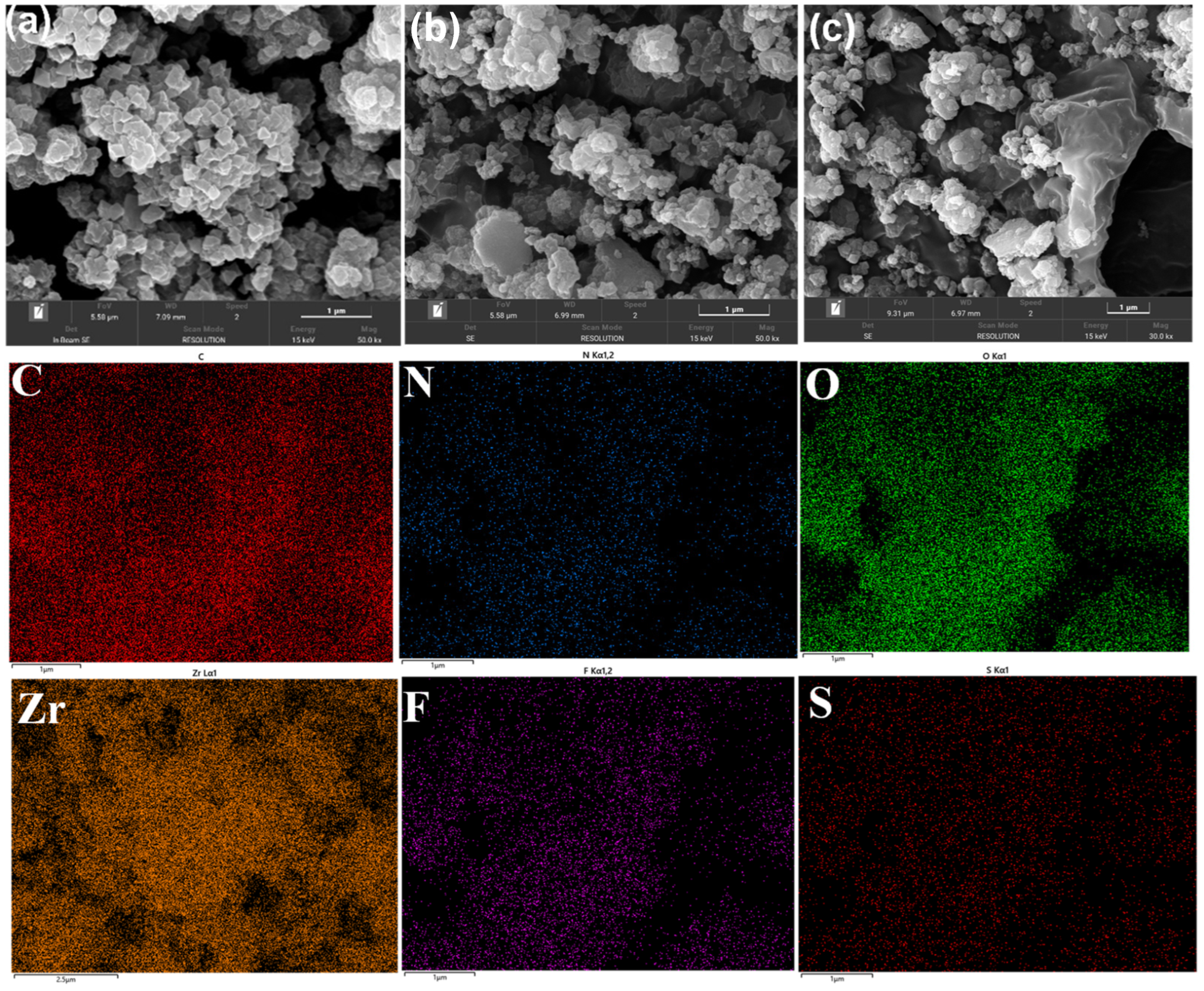
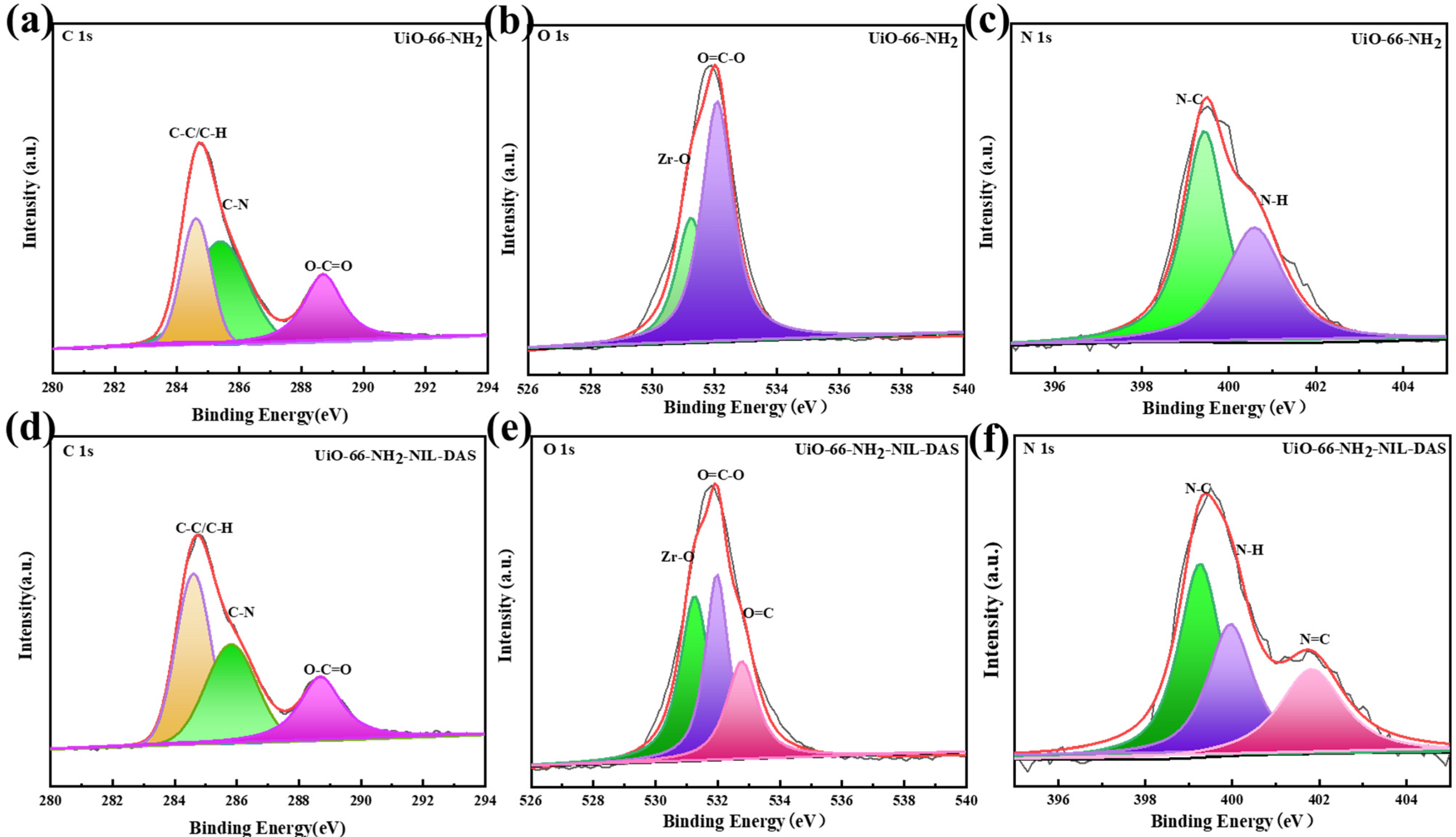
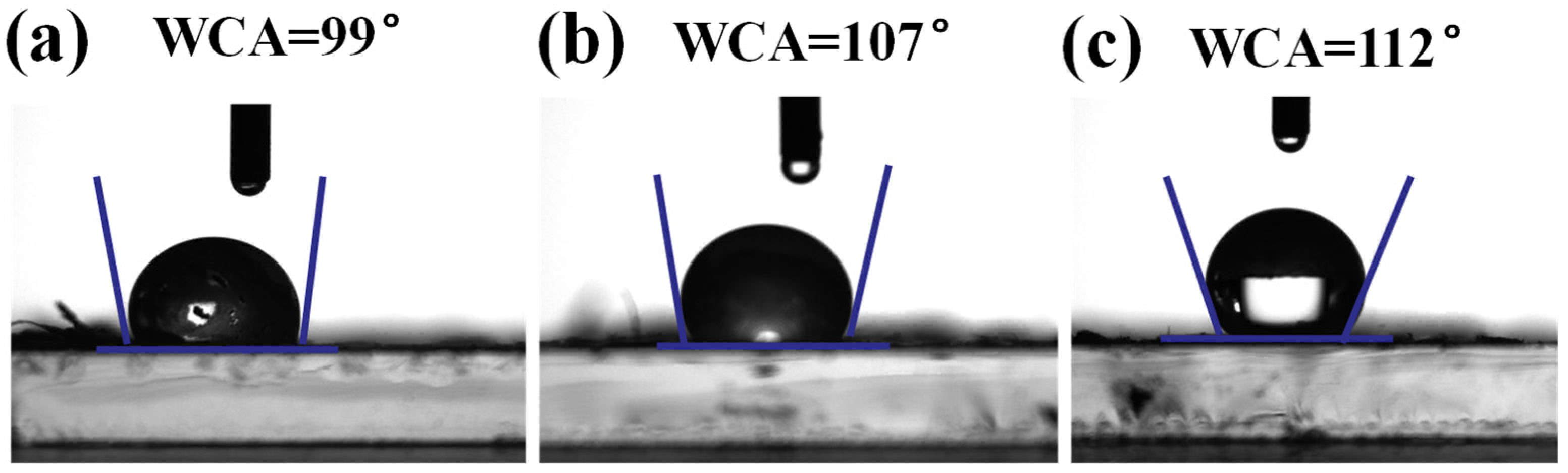
2.1. Characterization Analysis
2.2. The Optimization Conditions of CRL Immobilized on UiO-66-NH2-DAS and UiO-66-NH2-NIL-DAS
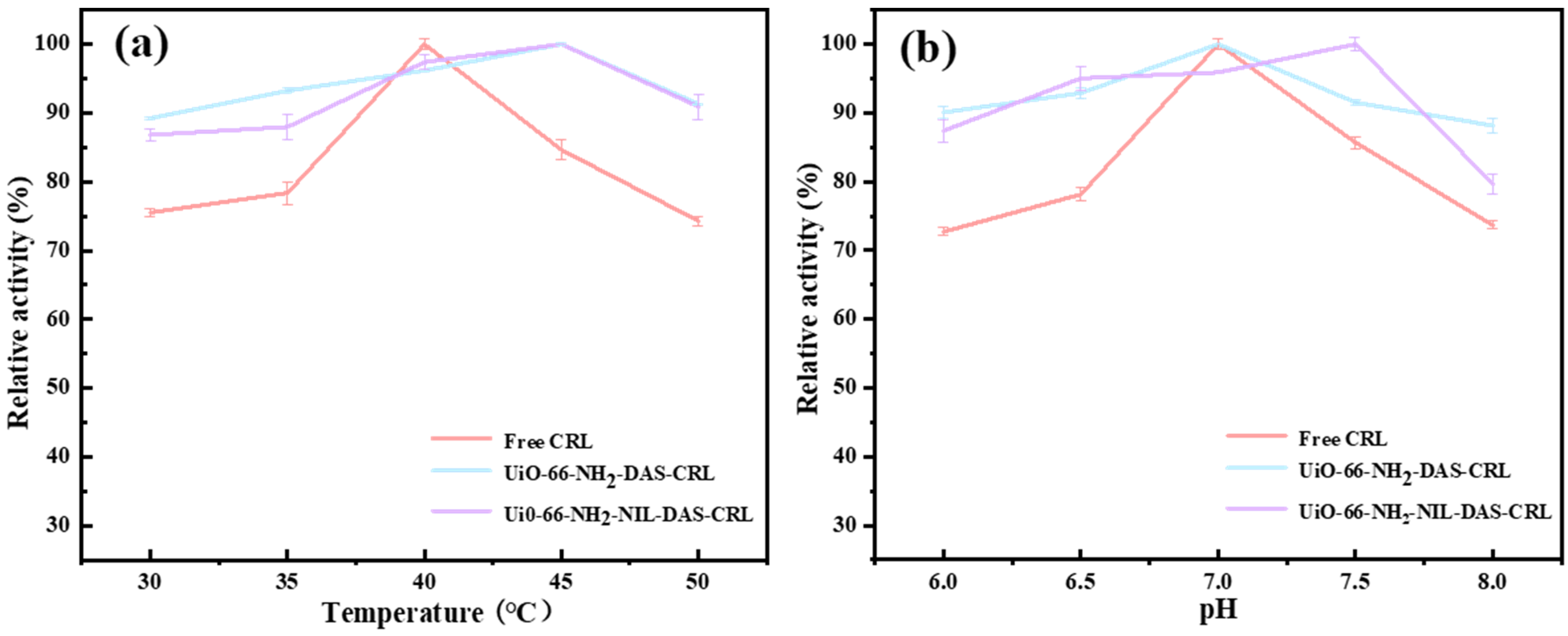
2.3. Effects of ILs-Modified Carrier on the Optimal pH and Temperature of Lipase
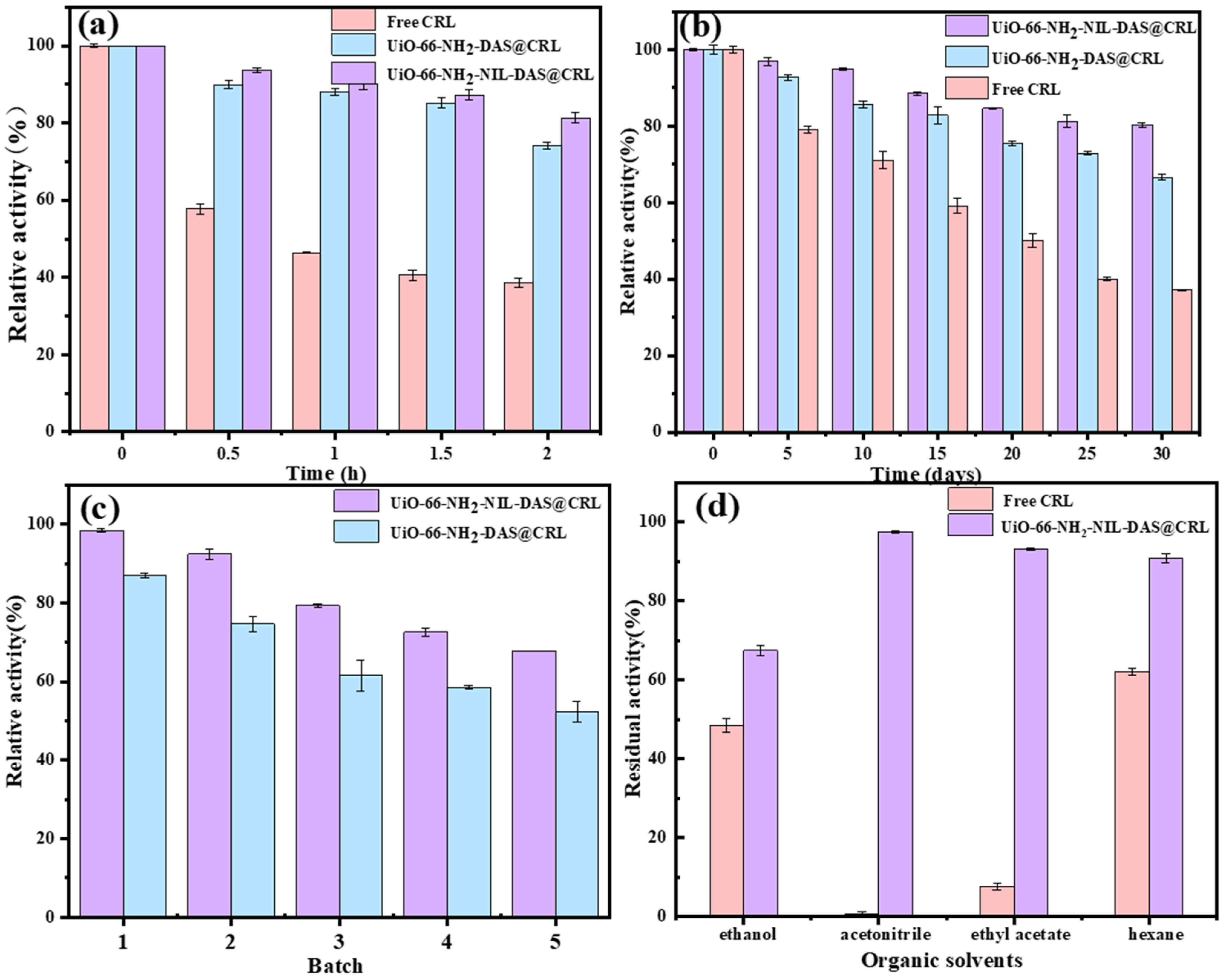
2.4. Stability of Free CRL and Immobilized CRL
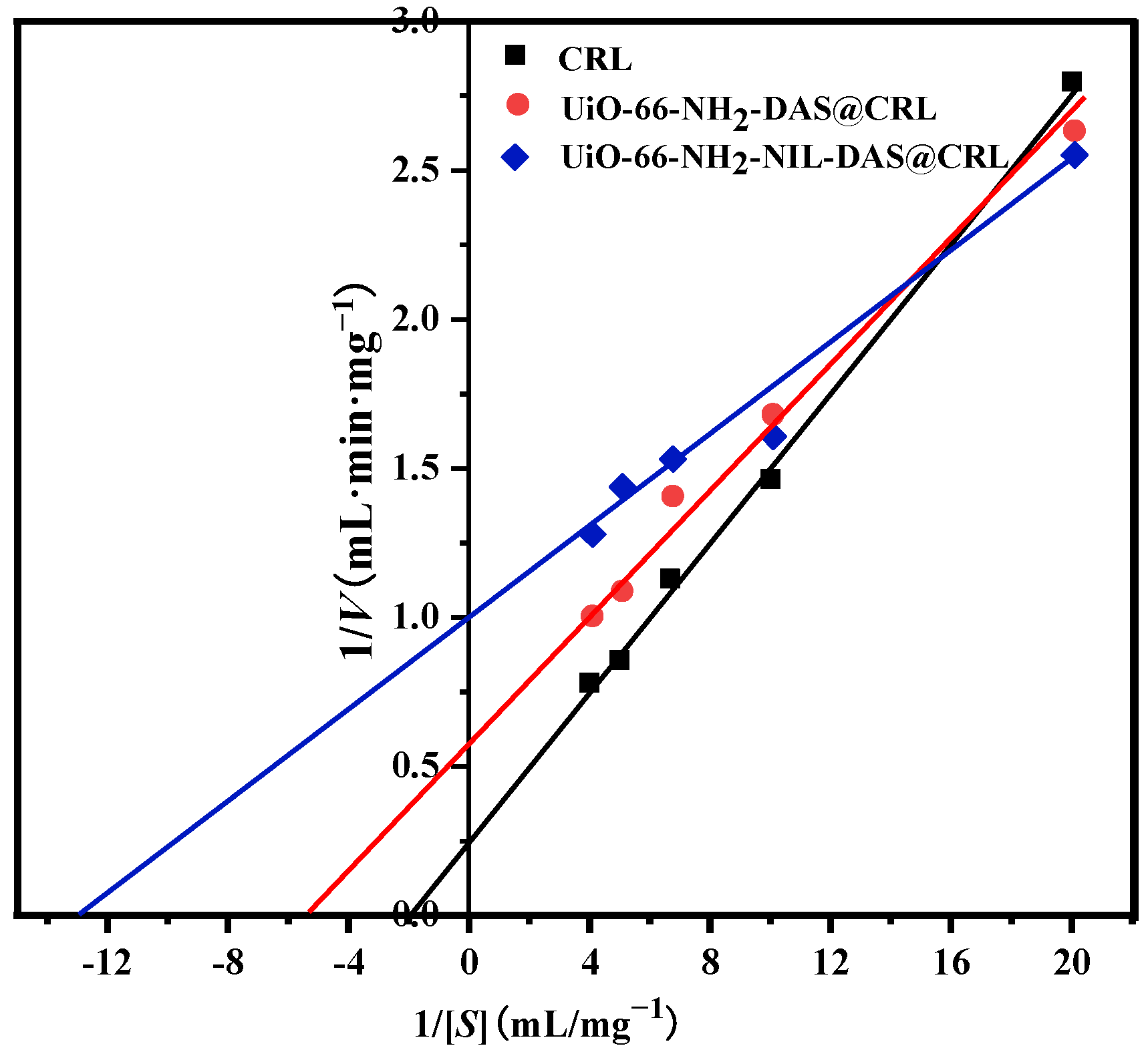
2.5. Michaelis–Menten Kinetic Parameters of Free CRL and Immobilized CRL
3. Material and Method
3.1. Materials
3.2. Characterization
3.3. Synthesis of UiO-66-NH2 and ILs-Modified UiO-66-NH2
3.4. Synthesis of UiO-66-NH2-NIL-DAS
3.5. Lipase Immobilization
3.6. Lipase Activity Assay
3.7. Optimum Temperature and Optimum pH for Free and Immobilized CRL
3.8. Enzyme Kinetics Study of Free and Immobilized CRL
3.9. Study on Stability of Free CRL and Immobilized CRL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, D.-M.; Chen, J.; Shi, Y.-P. Advances on methods and easy separated support materials for enzymes immobilization. Trac-Trend Anal. Chem. 2018, 102, 332–342. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging contaminants of high concern and their enzyme-assisted biodegradation—A review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, M. Immobilization of Candida rugosa lipase onto graphene oxide Fe3O4 nanocomposite: Characterization and application for biodiesel production. Energy. Convers. Manag. 2018, 159, 42–53. [Google Scholar] [CrossRef]
- Wang, S.; Li, S.; Liu, R.; Zhang, W.; Xu, H.; Hu, Y. Immobilization of interfacial activated Candida rugosa lipase onto magnetic chitosan using Dialdehyde cellulose as cross-linking agent. Front. Bioeng. Biotechnol. 2022, 10, 946117. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.; Guo, L.; Ding, Q.; Gao, C.; Hu, G.; Chen, X.; Li, Y.; Zhang, L.; Chen, W.; Chen, J.; et al. Reprogramming microbial populations using a programmed lysis system to improve chemical production. Nat. Commun. 2021, 12, 6886. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hou, J.; Xu, P.; Guo, L.; Chen, X.; Hu, G.; Ye, C.; Edwards, H.; Chen, J.; Chen, W.; et al. Programmable biomolecular switches for rewiring flux in Escherichia coli. Nat. Commun. 2019, 10, 3751. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rathod, V.K. Magnetic-metal organic framework (magnetic-MOF): A novel platform for enzyme immobilization and nanozyme applications. Int. J. Biol. Macromol. 2018, 120, 2293–2302. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-organic frameworks for energy storage devices: Batteries and supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Liang, W.; Wied, P.; Carraro, F.; Sumby, C.J.; Nidetzky, B.; Tsung, C.-K.; Falcaro, P.; Doonan, C.J. Metal–organic framework-based enzyme biocomposites. Chem. Rev. 2021, 121, 1077–1129. [Google Scholar] [CrossRef]
- Wang, S.; Xia, X.; Chen, F.-E. Engineering of covalent organic framework-based advanced platforms for enzyme immobilization: Strategies, research progress, and prospects. Adv. Mater. Interfaces 2022, 9, 2200874. [Google Scholar] [CrossRef]
- Liu, J.; Xie, Y.; Peng, C.; Yu, G.; Zhou, J. Molecular understanding of laccase adsorption on charged self-assembled monolayers. J. Phys. Chem. B 2017, 121, 10610–10617. [Google Scholar] [CrossRef] [PubMed]
- Xing, C.; Mei, P.; Mu, Z.; Li, B.; Feng, X.; Zhang, Y.; Wang, B. Enhancing enzyme activity by the modulation of covalent interactions in the confined channels of covalent organic frameworks. Angew. Chem. Int. Ed. Engl. 2022, 61, e202201378. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.R.; Chen, M.; Zhang, W.; Nian, B.B.; Hu, Y. Comprehensive applications of ionic liquids in enzyme immobilization: Current status and prospects. Mol. Catal. 2024, 552, 113675. [Google Scholar] [CrossRef]
- Wan, X.; Tang, S.; Xiang, X.; Huang, H.; Hu, Y. Immobilization of Candida antarctic lipase B on functionalized ionic liquid modified MWNTs. Appl. Biochem. Biotechnol. 2017, 183, 807–819. [Google Scholar] [CrossRef]
- Suo, H.; Gao, Z.; Xu, L.; Xu, C.; Yu, D.; Xiang, X.; Huang, H.; Hu, Y. Synthesis of functional ionic liquid modified magnetic chitosan nanoparticles for porcine pancreatic lipase immobilization. Mater. Sci. Eng. C 2019, 96, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lu, Z.; Liu, R.; Ji, L.; Nian, B.; Hu, Y. Ionic liquid modulation of metal-organic framework immobilized laccase and boosted its catalytic performance for organic pollutants removal. J. Environ. Chem. Eng. 2023, 11, 110880. [Google Scholar] [CrossRef]
- Suo, H.B.; Geng, X.Y.; Sun, Y.H.; Zhang, L.; Yang, J.; Yang, F.; Yan, H.; Hu, Y.; Xu, L. Surface modification of magnetic ZIF-90 nanoparticles improves the microenvironment of immobilized lipase and its application in esterification. Langmuir 2022, 38, 15384–15393. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.S.; Rathod, V.K. Immobilization of pectinase onto chitosan magnetic nanoparticles by macromolecular cross-linker. Carbohydr. Polym. 2017, 157, 677–685. [Google Scholar] [CrossRef]
- Li, M.; Dai, X.; Li, A.; Qi, Q.; Wang, W.; Cao, J.; Jiang, Z.; Liu, R.; Suo, H.; Xu, L. Preparation and characterization of magnetic metal–organic frameworks functionalized by ionic liquid as supports for immobilization of Pancreatic lipase. Molecules 2022, 27, 6800. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, R.; Yang, X.; Nian, B.; Hu, Y. Immobilization of laccase on organic-inorganic nanocomposites and its application in the removal of phenolic pollutants. Front. Chem. Sci. Eng. 2023, 17, 867–879. [Google Scholar] [CrossRef]
- Walia, S.; Kaur, M.; Kansal, S.K. Adsorptive removal of 2,4-dinitrophenol from aqueous phase using amine functionalized metal organic framework (NH2-MIL-101(Cr)). Mater. Chem. Phys. 2022, 289, 126493. [Google Scholar] [CrossRef]
- Aldawsari, A.M.; Alsohaimi, I.H.; Hassan, H.M.A.; Berber, M.R.; Abdalla, Z.E.A.; Hassan, I.; Saleh, E.A.M.; Hameed, B.H. Activated carbon/MOFs composite: AC/NH2-MIL-101(Cr), synthesis and application in high performance adsorption of p-nitrophenol. J. Saudi Chem. Soc. 2020, 24, 693–703. [Google Scholar] [CrossRef]
- Huang, X.; Lu, J.; Wang, W.; Wei, X.; Ding, J. Experimental and computational investigation of CO2 capture on amine grafted metal-organic framework NH2-MIL-101. Appl. Surf. Sci. 2016, 371, 307–313. [Google Scholar] [CrossRef]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. A novel and universal metal-organic frameworks sensing platform for selective detection and efficient removal of heavy metal ions. Chem. Eng. J. 2019, 375, 122111. [Google Scholar] [CrossRef]
- Jamshidifard, S.; Koushkbaghi, S.; Hosseini, S.; Rezaei, S.; Karamipour, A.; Jafari rad, A.; Irani, M. Incorporation of UiO-66-NH2 MOF into the PAN/chitosan nanofibers for adsorption and membrane filtration of Pb(II), Cd(II) and Cr(VI) ions from aqueous solutions. J. Hazard. Mater. 2019, 368, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Wang, L.; Shi, Y.; Li, L.; Xu, Z.; Sun, H.; Guo, F.; Shi, W. Construction of nanodiamonds/UiO-66-NH2 heterojunction for boosted visible-light photocatalytic degradation of antibiotics. Sep. Purif. Technol. 2022, 284, 120270. [Google Scholar] [CrossRef]
- Chen, C.; Sun, W.; Lv, H.; Li, H.; Wang, Y.; Wang, P. Spacer arm-facilitated tethering of laccase on magnetic polydopamine nanoparticles for efficient biocatalytic water treatment. Chem. Eng. J. 2018, 350, 949–959. [Google Scholar] [CrossRef]
- Lou, X.; Zhi, F.; Sun, X.; Wang, F.; Hou, X.; Lv, C.; Hu, Q. Construction of co-immobilized laccase and mediator based on MOFs membrane for enhancing organic pollutants removal. Chem. Eng. J. 2023, 451, 138080. [Google Scholar] [CrossRef]
- Ka, D.; Jang, S.; Kim, M.-K.; Jung, H.; Lee, J.; Jung, H.; Jin, Y. UiO-66-NH2/graphene oxide nanocomposites as reactive adsorbents for soman upon long-term exposure to high-humidity environment. Mater. Lett. 2021, 285, 129105. [Google Scholar] [CrossRef]
- Chen, L.; Yu, F.; Shen, X.; Duan, C. N-CND modified NH2-UiO-66 for photocatalytic CO2 conversion under visible light by a photo-induced electron transfer process. Chem. Commun. 2019, 55, 4845–4848. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.-W.; Liu, J.-M.; Li, C.-Y.; Zhao, N.; Wang, Z.-H.; Wang, S. In situ growth of benzothiadiazole functionalized UiO-66-NH2 on carboxyl modified g-C3N4 for enhanced photocatalytic degradation of sulfamethoxazole under visible light. Catal. Sci. Technol. 2020, 10, 4703–4711. [Google Scholar] [CrossRef]
- Luo, R.; Zhang, K.; Qin, Y.; Xie, L.; Chai, X.; Zhang, L.; Du, G.; Ge, S.; Rezakazemi, M.; Aminabhavi, T.M.; et al. Amine-functionalized UiO-66 incorporated electrospun cellulose/chitosan porous nanofibrous membranes for removing copper ions. Chem. Eng. J. 2024, 480, 148077. [Google Scholar] [CrossRef]
- Mo, Z.; Zhang, H.; Shahab, A.; Alam khan, F.; Chen, J.; Huang, C. Functionalized metal-organic framework UIO-66 nanocomposites with ultra-high stability for efficient adsorption of heavy metals: Kinetics, thermodynamics, and isothermal adsorption. J. Taiwan Inst. Chem. Eng. 2023, 146, 104778. [Google Scholar] [CrossRef]
- Sarker, M.; Song, J.Y.; Jhung, S.H. Carboxylic-acid-functionalized UiO-66-NH2: A promising adsorbent for both aqueous- and non-aqueous-phase adsorptions. Chem. Eng. J. 2018, 331, 124–131. [Google Scholar] [CrossRef]
- Xiao, F.; Cao, M.; Chen, Y. MOFs-mediated nanoscale Turing structure in polyamide membrane for enhanced nanofiltration. Desalination 2022, 544, 116146. [Google Scholar] [CrossRef]
- Askari, S.; Jafarzadeh, M.; Christensen, D.B.; Kegnæs, S. A synergic activity of urea/butyl imidazolium ionic liquid supported on UiO-66-NH2 metal–organic framework for synthesis of oximes. Catal. Lett. 2020, 150, 3159–3173. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Alcantara, A.R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports: Immobilization mechanism, advantages, problems, and solutions. Biotechnol. Adv. 2019, 37, 746–770. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Polyethylenimine: A very useful ionic polymer in the design of immobilized enzyme biocatalysts. J. Mater. Chem. B 2017, 5, 7461–7490. [Google Scholar] [CrossRef]
- Shahin, R.; Yousefi, M.; Ziyadi, H.; Bikhof, M.; Hekmati, M. pH-Responsive and magnetic Fe3O4@UiO-66-NH2@PEI nanocomposite as drug nanocarrier: Loading and release study of Imatinib. Inorg. Chem. Commun. 2023, 147, 110186. [Google Scholar] [CrossRef]
- Rahimpoor, R.; Bahrami, A.; Nematollahi, D.; Ghorbani-Shahna, F.; Farhadian, M. Sensitive determination of urinary muconic acid using magnetic dispersive-solid-phase extraction by magnetic amino-functionalised UiO-66. Int. J. Environ. Anal. Chem. 2020, 102, 1–14. [Google Scholar] [CrossRef]
- Elgharbawy, A.A.; Riyadi, F.A.; Alam, M.Z.; Moniruzzaman, M. Ionic liquids as a potential solvent for lipase-catalysed reactions: A review. J. Mol. Liq. 2018, 251, 150–166. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Z.; Zhang, P.; Qiao, M.; Hu, Y.; Shen, B.; Li, B.; Zhang, X. Enzyme-functionalized magnetic framework composite fabricated by one-pot encapsulation of lipase and Fe3O4 nanoparticle into metal–organic framework. Biochem. Eng. J. 2021, 169, 107962. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Sun, Y. Zwitterionic polymer-coated porous poly(vinyl acetate–divinyl benzene) microsphere: A new support for enhanced performance of immobilized lipase. Chin. J. Chem. Eng. 2020, 28, 242–248. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wu, D. Fabrication of microencapsulated phase change materials with TiO2/Fe3O4 hybrid shell as thermoregulatory enzyme carriers: A novel design of applied energy microsystem for bioapplications. Appl. Energ. 2017, 201, 20–33. [Google Scholar] [CrossRef]
- Jafarian, F.; Bordbar, A.-K.; Razmjou, A.; Zare, A. The fabrication of a high performance enzymatic hybrid membrane reactor (EHMR) containing immobilized Candida rugosa lipase (CRL) onto graphene oxide nanosheets-blended polyethersulfone membrane. J. Membr. Sci. 2020, 613, 118435. [Google Scholar] [CrossRef]
- Cea, M.; González, M.E.; Abarzúa, M.; Navia, R. Enzymatic esterification of oleic acid by Candida rugosa lipase immobilized onto biochar. J. Environ. Manage. 2019, 242, 171–177. [Google Scholar] [CrossRef]
- Chong, S.Y.; Wang, T.T.; Cheng, L.C.; Lv, H.Y.; Ji, M. Metal–organic framework MIL-101-NH2-supported acetate-based butylimidazolium ionic liquid as a highly efficient heterogeneous catalyst for the synthesis of 3-Aryl-2-oxazolidinones. Langmuir 2019, 35, 495–503. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Pencreac’h, G.; Baratti, J.C. Activity of Pseudomonas cepacia lipase in organic media is greatly enhanced after immobilization on a polypropylene support. Appl. Microbiol. Biotechnol. 1997, 47, 630–635. [Google Scholar] [CrossRef]


| Support | Lipase Loading (mg/g) | Specific Activity (U/g) | Activity Recovery (%) |
|---|---|---|---|
| UiO-66-NH2-DAS | 62.4 | 1918 | 52.9 |
| UiO-66-NH2-NIL-DAS | 65.0 | 2872 | 79.3 |
| Support | Km (mg/mL) | Vmax (mg/(mL·min)) | Vmax/Km |
|---|---|---|---|
| CRL | 0.51 | 4.02 | 7.88 |
| UiO-66-NH2-DAS@CRL | 0.181 | 1.702 | 9.4 |
| UiO-66-NH2-NIL-DAS@CRL | 0.077 | 1.01 | 13.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, L.; Zhang, W.; Zhang, Y.; Nian, B.; Hu, Y. Functionalized Ionic Liquids-Modified Metal–Organic Framework Material Boosted the Enzymatic Performance of Lipase. Molecules 2024, 29, 2381. https://doi.org/10.3390/molecules29102381
Ji L, Zhang W, Zhang Y, Nian B, Hu Y. Functionalized Ionic Liquids-Modified Metal–Organic Framework Material Boosted the Enzymatic Performance of Lipase. Molecules. 2024; 29(10):2381. https://doi.org/10.3390/molecules29102381
Chicago/Turabian StyleJi, Liran, Wei Zhang, Yifei Zhang, Binbin Nian, and Yi Hu. 2024. "Functionalized Ionic Liquids-Modified Metal–Organic Framework Material Boosted the Enzymatic Performance of Lipase" Molecules 29, no. 10: 2381. https://doi.org/10.3390/molecules29102381
APA StyleJi, L., Zhang, W., Zhang, Y., Nian, B., & Hu, Y. (2024). Functionalized Ionic Liquids-Modified Metal–Organic Framework Material Boosted the Enzymatic Performance of Lipase. Molecules, 29(10), 2381. https://doi.org/10.3390/molecules29102381








