Use of Commercial Mixed-Mode Stationary Phases and Sorbents in the High-Performance Liquid Chromatography Analysis and Solid-Phase Extraction of Ionized and Hydrophilic Bioactive Compounds
Abstract
1. Introduction
2. Mixed-Mode Stationary Phases for the HPLC Analysis of Low-Molecular-Weight Bioactive Compounds
2.1. Cationic Analytes
2.2. Anionic Analytes
2.3. Hydrophilic Analytes
2.4. Amino Acids
2.5. Chiral Low-Molecular-Weight Drugs and Biological Molecules
3. Mixed-Mode Sorbents for SPE
3.1. Basic Drugs
3.2. Acidic Drugs
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, K.; Liu, X. Mixed-mode chromatography in pharmaceutical and biopharmaceutical applications. J. Pharm. Biomed. Anal. 2016, 128, 73–88. [Google Scholar] [CrossRef]
- Zhang, L.; Dai, Q.; Qiao, X.; Yu, C.; Qin, X.; Yan, H. Mixed-mode chromatographic stationary phases: Recent advancements and its applications for high-performance liquid chromatography. TrAC Trends Anal. Chem. 2016, 82, 143–163. [Google Scholar] [CrossRef]
- Wan, Q.H. Mixed-Mode Chromatography: Principles, Methods and Applications; Springer Nature: Singapore, 2021. [Google Scholar]
- Alechaga, E.; Moyano, E.; Galceran, M.T. Mixed-mode liquid chromatography coupled to tandem mass spectrometry for the analysis of aminoglycosides in meat. Anal. Bioanal. Chem. 2014, 406, 4941–4953. [Google Scholar] [CrossRef]
- Li, Z.-M.; Lakuleswaran, M.; Kannan, K. LC-MS/MS methods for the determination of 30 quaternary ammonium compounds including benzalkonium and paraquat in human serum and urine. J. Chromatogr. B 2023, 1214, 123562. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Hernández, A.; García-Gómez, D.; Pérez-Pavón, J.L.; Rodríguez-Gonzalo, E. Simultaneous determination of favipiravir and surrogates of its metabolites by means of heart-cutting bidimensional liquid chromatography (2D-LC). Anal. Biochem. 2024, 684, 115375. [Google Scholar] [CrossRef]
- Toth, E.; Tolgyesi, A.; Balint, M.; Ma, X.; Sharma, V.K. Separation of fosetyl and phosphonic acid in food matrices with mixed-mode HPLC column coupled with tandem mass spectrometric detection and method application to other highly polar pesticides. J. Chromatogr. B 2022, 1189, 123083. [Google Scholar] [CrossRef]
- Wahl, O.; Holzgrabe, U. Impurity profiling of ibandronate sodium by HPLC-CAD. J. Pharm. Biomed. Anal. 2015, 114, 254–264. [Google Scholar] [CrossRef]
- Pinto, E.C.; Goncalves, M.d.S.; Cabral, L.M.; Armstrong, D.W.; de Sousa, V.P. Development and validation of a stability-indicating HPLC method for topiramate using a mixed-mode column and charged aerosol detector. J. Sep. Sci. 2018, 41, 1716–1725. [Google Scholar] [CrossRef]
- Lee, S.; Chang, N.I.; Yoo, M.; Choi, J.H.; Shin, D. Development and validation of S-allyl-L-cysteine in rat plasma using a mixed-mode reversed-phase and cation-exchange LC-ESI-MS/MS method: Application to pharmacokinetic studies. J. Chromatogr. Sci. 2015, 53, 54–59. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pawellek, R.; Holzgrabe, U. Influence of the mobile phase composition on hyphenated ultraviolet and charged aerosol detection for the impurity profiling of vigabatrin. J. Pharm. Biomed. Anal. 2021, 201, 114110. [Google Scholar] [CrossRef] [PubMed]
- Kuehnreich, R.; Holzgrabe, U. Impurity profiling of L-methionine by HPLC on a mixed mode column. J. Pharm. Biomed. Anal. 2016, 122, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Shiraki, K.; Sakamoto, K.; Smee, D.F.; Barnard, D.L.; Gowen, B.B.; Julander, J.G.; Morrey, J.D. T-705 (favipiravir) and related compounds: Novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009, 82, 95–102. [Google Scholar] [CrossRef]
- Boltz, D.A.; Aldridge, J.R., Jr.; Webster, R.G.; Govorkova, E.A. Drugs in development for influenza. Drugs 2010, 70, 1349–1362. [Google Scholar] [CrossRef]
- Uekusa, S.; Onozato, M.; Sakamoto, T.; Umino, M.; Ichiba, H.; Fukushima, T. Fluorimetric determination of the enantiomers of vigabatrin, an antiepileptic drug, by reversed-phase HPLC with a novel diastereomer derivatization reagent. Biomed. Chromatogr. 2020, 35, e5060. [Google Scholar] [CrossRef] [PubMed]
- Onozato, M.; Uekusa, S.; Sakamoto, T.; Umino, M.; Ichiba, H.; Fukushima, T. Separation of vigabatrin enantiomers using mixed-mode chromatography and its application to determine the vigabatrin enantiomer levels in rat plasma. J. Chromatogr. B 2021, 1179, 122866. [Google Scholar] [CrossRef]
- Onozato, M.; Nakanoue, H.; Sakamoto, T.; Umino, M.; Fukushima, T. Determination of D- and L-amino acids in garlic foodstuffs by liquid chromatography–tandem mass spectrometry. Molecules 2023, 28, 1773. [Google Scholar] [CrossRef]
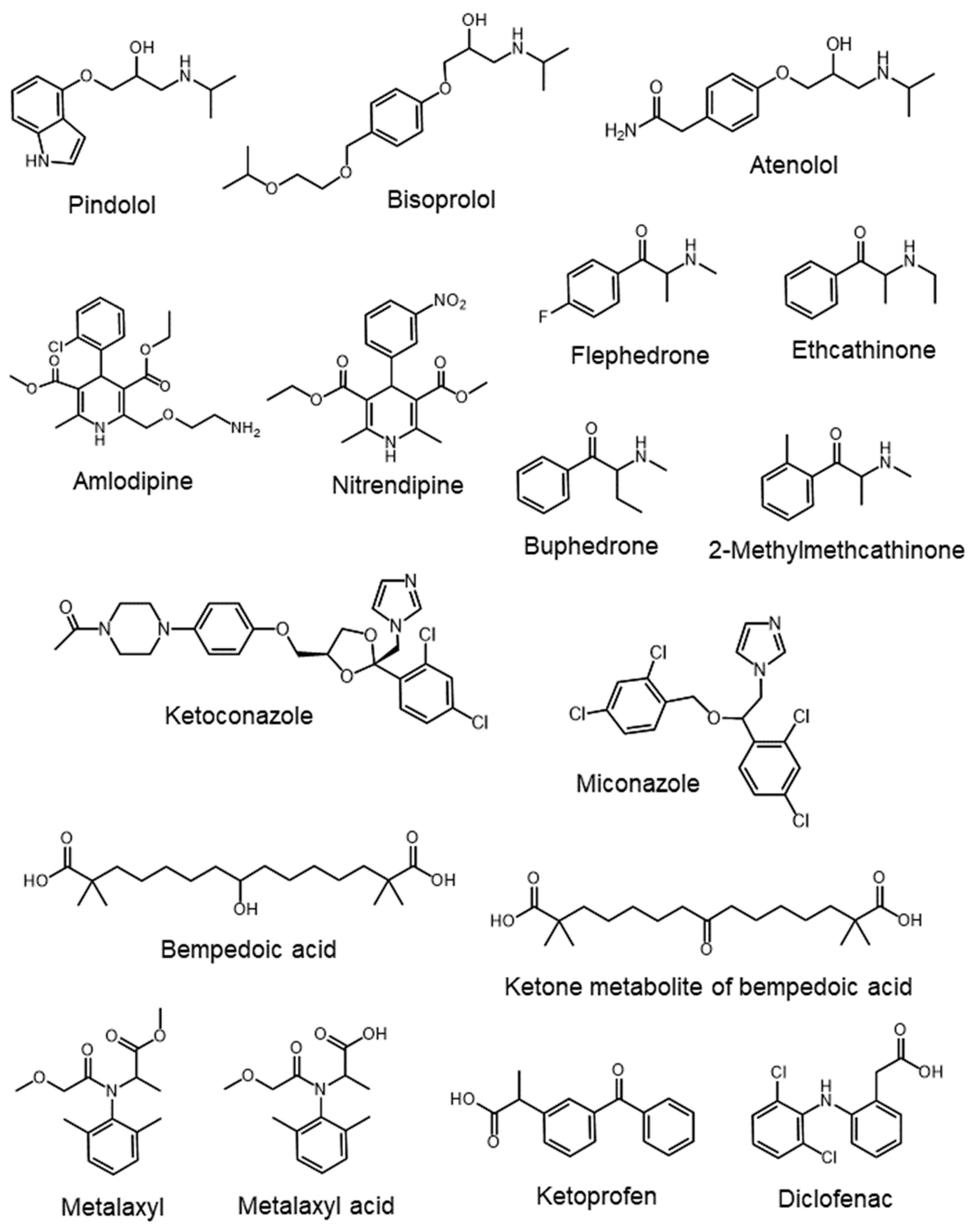
- Bai, H.; Chen, L. Simultaneous separation of atenolol enantiomers and its acid/alkaline degradation impurities on mixed-mode chiral ligand exchange stationary phases. Chirality 2021, 33, 710–721. [Google Scholar] [CrossRef] [PubMed]
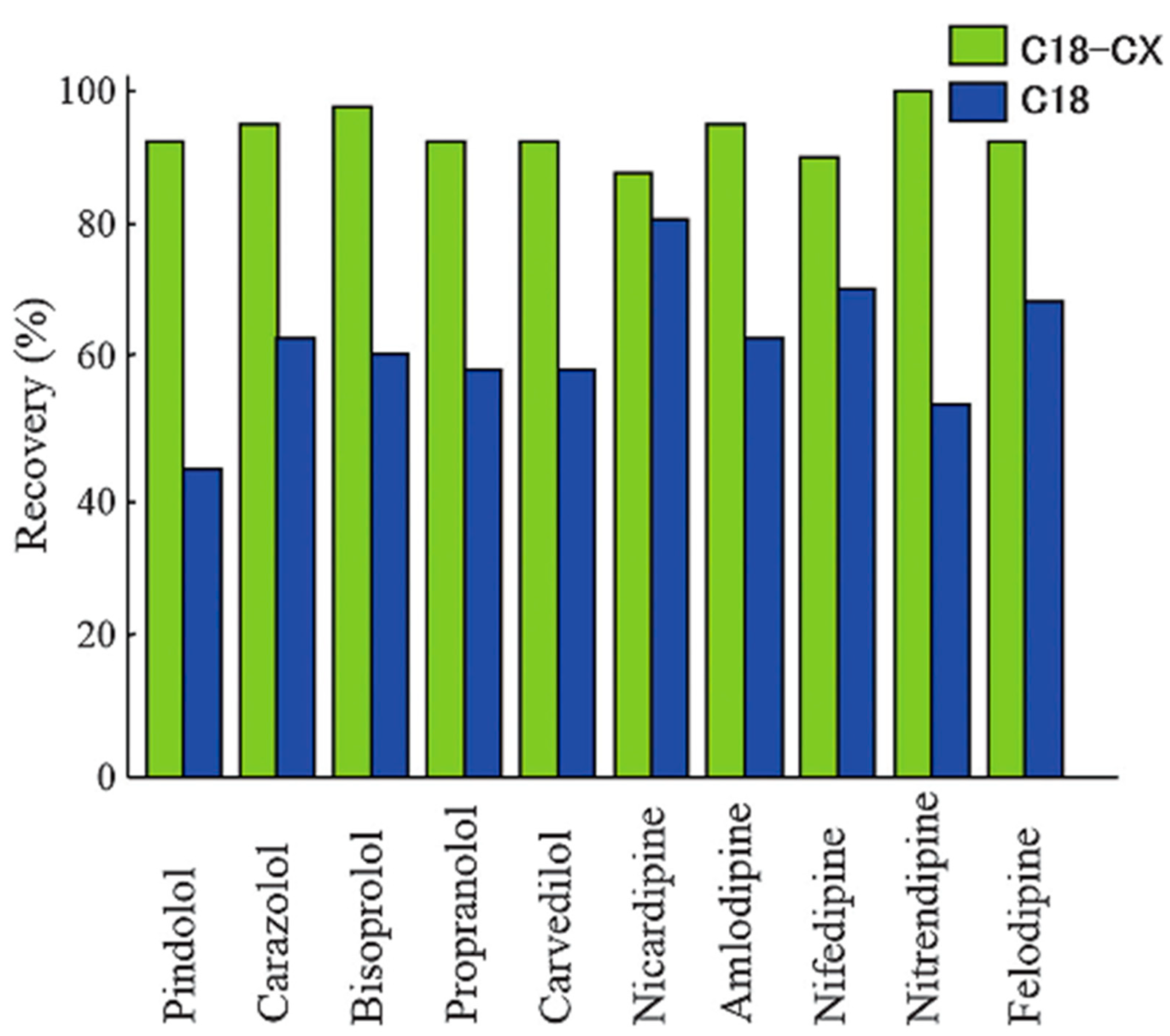
- Namera, A.; Saito, T.; Seki, Y.; Mizutani, T.; Murata, K.; Nagao, M. High-throughput monospin extraction for quantification of cardiovascular drugs in serum coupled to high-performance liquid chromatography–mass spectrometry. Acta Chromatogr. 2019, 31, 294–298. [Google Scholar] [CrossRef]
- Pascual-Caro, S.; Fontanals, N.; Borrull, F.; Aguilar, C.; Calull, M. Solid-phase extraction based on cation-exchange sorbents followed by liquid chromatography high-resolution mass spectrometry to determine synthetic cathinones in urine. Forensic Toxicol. 2020, 38, 185–194. [Google Scholar] [CrossRef]
- Casado, J.; Rodríguez, I.; Ramil, M.; Cela, R. Selective determination of antimycotic drugs in environmental water samples by mixed-mode solid-phase extraction and liquid chromatography quadrupole time-of-flight mass spectrometry. J. Chromatogr. A 2014, 1339, 42–49. [Google Scholar] [CrossRef]
- Zhou, H.; Shan, Q.; He, L.M.; Zhang, M.Y.; Zhao, C.; Zheng, G.M.; Li, L.C.; Xu, F.; Ma, L.S.; Yin, Y. Simultaneous determination of enantiomer residues of metalaxyl and its metabolite metalaxyl acid in animal muscle tissues by chiral liquid chromatography-tandem mass spectrometry. Food Chem. 2024, 435, 137599. [Google Scholar] [CrossRef] [PubMed]
- Engel, B.J.; Preusch, K.; Brown, C.; Cramer, C.T.; Shoup, R. Measurement of bempedoic acid and its keto metabolite in human plasma and urine using solid phase extraction and electrospray LC-MS/MS. J. Chromatogr. B 2020, 1154, 122291. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, Y.; Yang, J.; Peng, J.; Tan, J.; Fan, Y.; Wang, L.; Chen, J. Hyperbranched mixed-mode anion-exchange polymeric sorbent for highly selective extraction of nine acidic non-steroidal anti-inflammatory drugs from human urine. Talanta 2018, 190, 15–22. [Google Scholar] [CrossRef]
- Kazarian, A.A.; Barnhart, W.; Long, J.; Sham, K.; Wu, B.; Murray, J.K. Purification of N-acetylgalactosamine-modified-oligonucleotides using orthogonal anion-exchange and mixed-mode chromatography approaches. J. Chromatogr. A 2022, 1661, 462679. [Google Scholar] [CrossRef]
- Donegan, M.; Nguyen, J.M.; Gilar, M. Effect of ion-pairing reagent hydrophobicity on liquid chromatography and mass spectrometry analysis of oligonucleotides. J. Chromatogr. A 2022, 1666, 462860. [Google Scholar] [CrossRef]


| Analyte | Mixed-Mode Column | Mobile Phase | Detection | LOD/LOQ | Sample | Ref. |
|---|---|---|---|---|---|---|
| Cationic drugs and herbicides | ||||||
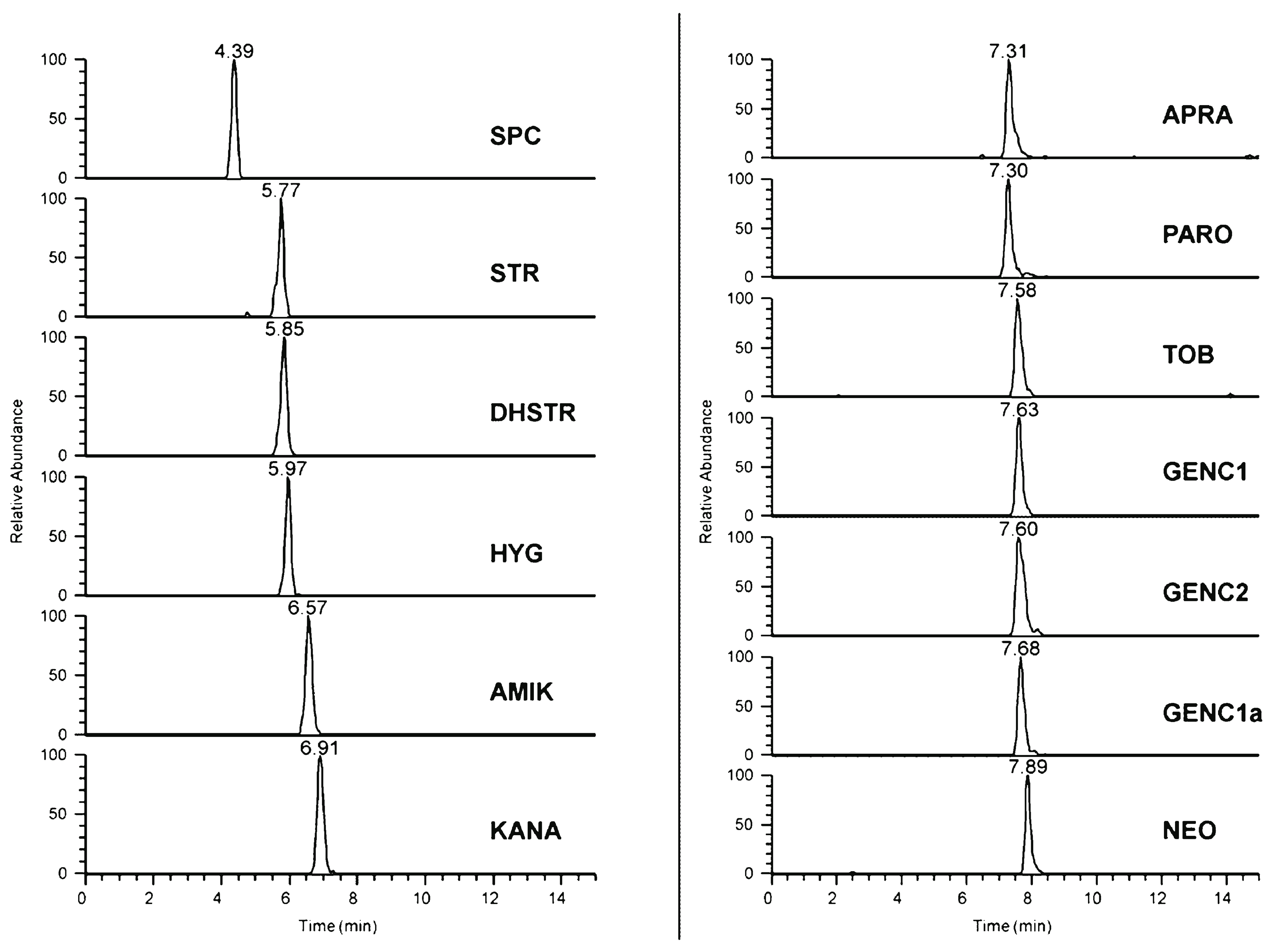
| Aminoglycoside antibiotics | Obelisc® R column (SIELC) (150 × 2.1 mm, 5 μm) | Gradient elution with A: acetonitrile, B: water, C: 1% formic acid in water | MS | Spectinomycin: 3 pg (LOD) Gentamicin C2: 30 pg (LOD) | Minced meat (veal, pork, and chicken) | [4] |
| Diquat, paraquat | AcclaimTM Trinity Q1 (Thermo Fisher Scientific) (100 × 2.1 mm, 3 µm) | Isocratic elution (A:B = 25:75) with A: 100 mM ammonium formate (pH 5.0), B: acetonitrile | MS | Diquat: 0.04 ng/mL (LOD) Paraquat: 0.05 ng/mL (LOD) | Human serum and urine | [5] |
| Anionic drugs and impurities | ||||||
| Favipiravir-RMP, RDP, RTP | Primesep SB column (SIELC) (150 × 4.6 mm, 5 μm) | Isocratic elution (A:B = 99:1) with A: 180 mM ammonium acetate (pH 4.6), B: acetonitrile | DAD | Favipiravir: 0.4 mg/L (LLOQ; serum, plasma, PBMCs) Favipiravir-ribofuranose: 10 mg/L (LLOQ, PBMCs) Favipiravir-ribofuranosyl-monophosphate: 16 mg/L (LLOQ, PBMCs) Favipiravir-ribofuranosyl-diphosphate: 20 mg/L (LLOQ, PBMCs) Favipiravir-ribofuranosyl-triphosphate: 4 mg/L (LLOQ, PBMCs) 5-hydroxyfavipiravir: 5.0 mg/L (LLOQ, urine) | Human serum, plasma, urine, and PBMCs | [6] |
| Fosetyl and phosphonic acid | Luna Omega PS C18 (Phenomenex) (100 mm × 3 mm, 3 μm) | Gradient elution with A: 10% methanol in water B: 20 mM ammonium formate and 0.1% formic acid in water (pH 3.5) | MS | Fosetyl: 0.02 mg/kg (LOQ, spinach), 0.02 mg/kg (LOQ, cherry), 0.20 mg/kg (LOQ, wheat flour) Phosphonic acid: 0.02 mg/kg in spinach, 0.02 mg/kg in cherry, 0.20 mg/kg in wheat flour (LOQ) | Food (spinach, cherry, arugula, lettuce, wheat, and oat flour) | [7] |
| Ibandronate sodium and its impurities | Coresep® SB (SIELC) (150 × 4.6 mm, 2.7 μm) | Gradient elution with A: ultrapure water, B: 15% acetonitrile containing 15 mM trifluoroacetic acid | CAD MS | N-Pentyl-N-methyl-β-alanine: 0.03% (LOQ) Phosphate: 0.02% (LOQ) Despentylibandronate: 0.02% (LOQ) Olpadronate: 0.03% (LOQ) Phosphite: 0.02% (LOQ) Desmethylibandronate: 0.02% (LOQ) | Batch test | [8] |
| Hydrophilic drugs and impurities | ||||||
| Topiramate and its main degradation products | AcclaimTM Trinity P1 (Dionex) (150 × 3.0 mm, 2.7 μm) | Isocratic elution (A:B = 80:20) with A: 20 mM ammonium acetate buffer (pH 4.0), B: methanol | CAD | Topiramate: 2.97 μg/mL (LOD), 11.15 μg/mL (LOQ) Fructose: 12.08 μg/mL (LOD), 40.28 μg/mL (LOQ) Sulfate: 4.02 μg/mL (LOD), 13.41 μg/mL (LOQ) Sulfamate: 13.91 μg/mL (LOD), 46.38 μg/mL (LOQ) Compound A: 3.94 μg/mL (LOD), 13.13 μg/mL (LOQ) | Standard | [9] |
| Amino acids and their impurities | ||||||
| S-Allyl-L-cysteine | CAPCELL PAK CR 1:4 (Shiseido) (100 × 2.0 mm, 3 μm) | Isocratic elution with A: 2 mM ammonium acetate buffer (pH 3.5), B: 0.1% formic acid in acetonitrile | MS | S-Allyl-L-cysteine: 5 ng/mL (LOQ) | Rat plasma | [10] |
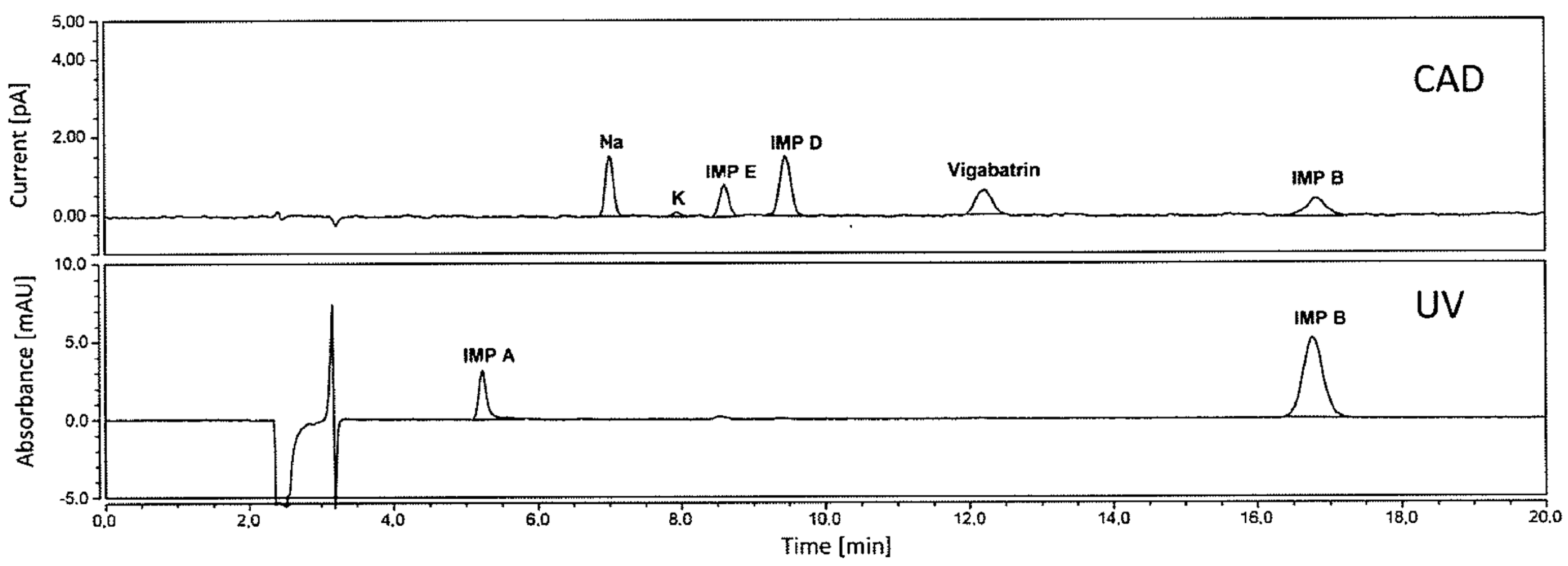
| Vigabatrin impurities | Primesep® 100 (SIELC) (250 × 4.6 mm, 5 μm) | Isocratic elution with water/acetonitrile (85:15) containing 0.1 vol% trifluoroacetic acid | CAD | Impurity A: 9 ng (0.006%) Impurity B: 6 ng (0.004%) Impurity D: 12 ng (0.008%) Impurity E: 18 ng (0.012%) | Standard | [11] |
| L-Methionine impurities | Primesep® 100 (SIELC) (250 × 4.6 mm, 5 μm) | Isocratic elution (A:B = 80:20) with A: 12.5 mM aqueous phosphoric acid, B: acetonitrile | Ultraviolet (210 nm) | AcMet: 0.06 μg/mL (LOD), 0.30 μg/mL (LOQ) AcMetMet 1: 0.06 μg/mL (LOD), 0.30 μg/mL (LOQ) AcMetMet 2: 0.06 μg/mL (LOD), 0.30 μg/mL (LOQ) MetOx: 0.30 μg/mL (LOD), 0.75 μg/mL (LOQ) | Standard | [12] |
| Analyte | SPE Process | Chromatography | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SPE Sorbent | Preconditioning | Washing | Elution | Column | Mobile Phase | Detection | Sample Matrix | Recovery | Ref. | |
| Basic drugs | ||||||||||
| Cardiovascular drugs | MonoSpin C18-CX® | 0.5 mL of methanol, 0.5 mL of citrate buffer (pH 3) | 0.4 mL of citrate buffer (pH 3) | 0.1 mL of 2% NH3 in methanol | InertSustain® C8 (150 × 2.1 mm, 3 μm) | Elution with A: 10 mM ammonium formate containing 0.1% formic acid (pH 3.3) B: acetonitrile or methanol | MS | Human serum | 76–108% | [19] |
| Synthetic cathinones (β-keto phenethylamines) | Oasis® MCX (150 mg/6 mL) | 5 mL of methanol, 5 mL of phosphate buffer solution (pH 6) | 2 mL of methanol | 2 mL of 5% NH4OH in methanol | Luna Omega 5 μm Polar C18 (150 × 4.6 mm, 5 μm) | Gradient elution with A: 0.1% formic acid in ultrapure water B: 0.1% formic acid in acetonitrile | High-resolution MS | Human urine | 84–101% | [20] |
| Antimycotic drugs | Oasis® MCX (150 mg) | 5 mL of methanol, 5 mL of ultrapure water, adjusted to the same pH as water samples | 5 mL of methanol:water (10:90) 2.5 mL of methanol (0.1% formic acid) | 2 mL of methanol containing 2% NH3 (v/v) | Zorbax Eclipse XDB C18 (100 × 2 mm, 3.5 μm) | Gradient elution with A: 5 mM ammonium acetate in ultrapure water B: 5 mM ammonium acetate in methanol | MS | Environmental water (sewage treatment plants, river water) | 84–104% (river water) 71–109% (treated water) 72–92% (raw wastewater) | [21] |
| Acidic drugs or metabolites | ||||||||||
| Metalaxyl, metalaxyl acid | Oasis® MAX (60 mg, 3 mL) | 3 mL of methanol, 3 mL of water, 3 mL of 0.2% aqueous ammonia | 3 mL of 20% aqueous methanol, 3 mL of 0.2% aqueous ammonia | 3 mL of 0.5% formic acid in methanol | EnantioPak® Y1R (150 × 4.6 mm, 5 μm) | Acetonitrile:H2O:formic acid (60:40:0.1) | MS/MS | Muscle tissues (veal, pork, chicken, fish) | 89.5–110.3% | [22] |
| Bempedoic acid, ketone metabolite | Oasis® MAX (30 mg/well, 30 μm) | 1.0 mL of water:methanol (5:95), 1.0 mL of 100 mM ammonium formate buffer (pH 3.8) | 1.0 mL of 100 mM ammonium formate buffer (pH 3.8), 1.0 mL of water | 800 μL of formic acid:ethanol (2:98) | BEH C18 (50 × 2.1 mm, 1.7 μm) | Gradient elution with A: water:methanol:formic acid (90:10:0.1) B: water:methanol:formic acid (10:90:0.1) | MS/MS | Human urine | 88.2–96.9% (bempedoic acid), 89.9–94.1% (ketone metabolite) | [23] |
| NSAIDs | G4-QHMs MAX sorbent | 5 mL of methanol, 5 mL of 10 mM phosphate buffer (pH 2.0) | 3 mL of 15% acetic acid, 5 mL of 5% methanol in 50 mM sodium acetate (pH 7) 5 mL of methanol | 4 mL of 1% formic acid in methanol | Agilent Zorbax SB-C18 (250 × 4.6 mm, 5 μm) | Gradient elution with A: 20 mM KH2PO4 (pH 2.7); B: methanol Isocratic elution for ibuprofen; 20 mM KH2PO4 (pH 2.7):methanol (20:80) | Ultraviolet | Human urine | 81.9–104.0% | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukushima, T.; Koishi, M.; Sakamoto, T.; Onozato, M. Use of Commercial Mixed-Mode Stationary Phases and Sorbents in the High-Performance Liquid Chromatography Analysis and Solid-Phase Extraction of Ionized and Hydrophilic Bioactive Compounds. Molecules 2024, 29, 2341. https://doi.org/10.3390/molecules29102341
Fukushima T, Koishi M, Sakamoto T, Onozato M. Use of Commercial Mixed-Mode Stationary Phases and Sorbents in the High-Performance Liquid Chromatography Analysis and Solid-Phase Extraction of Ionized and Hydrophilic Bioactive Compounds. Molecules. 2024; 29(10):2341. https://doi.org/10.3390/molecules29102341
Chicago/Turabian StyleFukushima, Takeshi, Mikoto Koishi, Tatsuya Sakamoto, and Mayu Onozato. 2024. "Use of Commercial Mixed-Mode Stationary Phases and Sorbents in the High-Performance Liquid Chromatography Analysis and Solid-Phase Extraction of Ionized and Hydrophilic Bioactive Compounds" Molecules 29, no. 10: 2341. https://doi.org/10.3390/molecules29102341
APA StyleFukushima, T., Koishi, M., Sakamoto, T., & Onozato, M. (2024). Use of Commercial Mixed-Mode Stationary Phases and Sorbents in the High-Performance Liquid Chromatography Analysis and Solid-Phase Extraction of Ionized and Hydrophilic Bioactive Compounds. Molecules, 29(10), 2341. https://doi.org/10.3390/molecules29102341






