Synthesis of Gold Nanoparticles over CoAl Mixed Oxide for Ethanol Oxidation Reaction
Abstract
1. Introduction
2. Results

3. Materials and Methods
3.1. Synthesis
3.2. Characterization
- -
- XT is the ethanol conversion at T temperature (%);
- -
- [CO2]T is the concentration of CO2 at T temperature (ppm).
- -
- SI,T is the selectivity of I at T temperature (%);
- -
- is the number of C for compound I
- -
- [I]T is the concentration of I at T temperature (ppm).
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochard, G.; Olivet, L.; Tannous, M.; Poupin, C.; Siffert, S.; Cousin, R. Recent advances in the catalytic treatment of volatile organic compounds: A review based on the mixture effect. Catalysts 2021, 11, 1218. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Feng, Q.; Leung, D.Y.C. Low temperature catalytic oxidation of volatile organic compounds: A review. Catal. Sci. Technol. 2015, 5, 2649–2669. [Google Scholar] [CrossRef]
- Min, X.; Guo, M.; Li, K.; Gu, J.N.; Hu, X.; Jia, J.; Sun, T. Boosting the VOCs purification over high-performance α-MnO2 separated from spent lithium-ion battery: Synergistic effect of metal doping and acid treatment. Sep. Purif. Technol. 2022, 295, 121316. [Google Scholar] [CrossRef]
- Brunet, J.; Genty, E.; Barroo, C.; Cazier, F.; Poupin, C.; Siffert, S.; Thomas, D.; de Weireld, G.; de Bocarmé, T.V.; Cousin, R. The CoAlCeO mixed oxide: An alternative to palladium-based catalysts for total oxidation of industrial VOCs. Catalysts 2018, 8, 64. [Google Scholar] [CrossRef]
- Khan, H.A.; Abou-Daher, M.; de Freitas, A.L.S.; Subburaj, J.; Tall, O.E.; Farooq, A. Performance studies of Pt, Pd and PtPd supported on SBA-15 for wet CO and hydrocarbon oxidation. Catal. Today 2024, 426, 114370. [Google Scholar] [CrossRef]
- Gong, J.; Wang, X.; W, H.; Dong, X.; Li, J.; Yang, F.; Yuan, A.; Ji, H. MnCo-Layered double hydroxides nanosheets supported Pd nanoparticles for complete catalytic oxidation of formaldehyde at room temperature. Appl. Surf. Sci. 2022, 606, 154702. [Google Scholar] [CrossRef]
- Sangnier, A.; Genty, E.; Iachella, M.; Sautet, P.; Raybaud, P.; Matrat, M.; Dujardin, C.; Chizallet, C. Thermokinetic and Spectroscopic Mapping of Carbon Monoxide Adsorption on Highly Dispersed Pt/γ-Al2O3. ACS Catal. 2021, 11, 13280–13293. [Google Scholar] [CrossRef]
- Haruta, M.; Kobayashi, T.; Sano, H.; Yamada, N. Novel gold catalysts for the oxidation of carbon monoxide at a temperature far below 0 °C. Chem. Lett. 1987, 16, 405–408. [Google Scholar] [CrossRef]
- Taketoshi, A.; Haruta, M. Size- and structure-specificity in catalysis by gold clusters. Chem. Lett. 2014, 43, 380–387. [Google Scholar] [CrossRef]
- Scirè, S.; Liotta, L.F. Supported gold catalysts for the total oxidation of volatile organic compounds. Appl. Catal. B Environ. 2012, 125, 222–246. [Google Scholar] [CrossRef]
- Lamallem, M.; El Ayadi, H.; Gennequin, C.; Cousin, R.; Siffert, S.; Aïssi, F.; Aboukaïs, A. Effect of the preparation method on Au/Ce-Ti-O catalysts activity for VOCs oxidation. Catal. Today 2008, 137, 367–372. [Google Scholar] [CrossRef]
- Genty, E.; Cousin, R.; Capelle, S.; Gennequin, C.; Siffert, S. Catalytic Oxidation of Toluene and CO over Nanocatalysts Derived from Hydrotalcite-Like Compounds (X62+Al23+): Effect of the Bivalent Cation. Eur. J. Inorg. Chem. 2012, 2012, 2802–2811. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Casale, S.; Capelle, S.; Massiani, P.; Siffert, S.; Cousin, R. Co-Al mixed oxides prepared via LDH route using microwaves or ultrasound: Application for catalytic toluene total oxidation. Catalysts 2015, 5, 851–867. [Google Scholar] [CrossRef]
- Genty, E.; Brunet, J.; Poupin, C.; Ojala, S.; Siffert, S.; Cousin, R. Influence of CO addition on the toluene total oxidation over Co based mixed oxide catalysts. Appl. Catal. B Environ. 2019, 247, 163–172. [Google Scholar] [CrossRef]
- La Parola, V.; Pantaleo, G.; Liotta, L.F.; Venezia, A.M.; Gabrovska, M.; Nikolova, D.; Tabakova, T. Gold and Ceria Modified NiAl Hydrotalcite Materials as Catalyst Precursors for Dry Reforming of Methane. Catalysts 2023, 13, 606. [Google Scholar] [CrossRef]
- Li, S.; Ezugwu, C.I.; Zhang, S.; Xiong, Y.; Liu, S. Co-doped MgAl-LDHs nanosheets supported Au nanoparticles for complete catalytic oxidation of HCHO at room temperature. Appl. Surf. Sci. 2019, 487, 260–271. [Google Scholar] [CrossRef]
- Dobrosz, I.; Jiratova, K.; Pitchon, V.; Rynkowski, J.M. Effect of the preparation of supported gold particles on the catalytic activity in CO oxidation reaction. J. Mol. Catal. A Chem. 2005, 234, 187–197. [Google Scholar] [CrossRef]
- Ivanova, S.; Petit, C.; Pitchon, V. A new preparation method for the formation of gold nanoparticles on an oxide support. Appl. Catal. A Gen. 2004, 267, 191–201. [Google Scholar] [CrossRef]
- Moreau, F.; Bond, G.; Taylor, A. Gold on titania catalysts for the oxidation of carbon monoxide: Control of pH during preparation with various gold contents. J. Catal. 2005, 231, 105–114. [Google Scholar] [CrossRef]
- Hugon, A.; Kolli, N.; Louis, C. Advances in the preparation of supported gold catalysts: Mechanism of deposition, simplification of the procedures and relevance of the elimination of chlorine. J. Catal. 2010, 274, 239–250. [Google Scholar] [CrossRef]
- Bond, G.C.; Louis, C.; Thompson, D.T. Catalysis by Gold; Hutchings, G.H., Ed.; Imperial College Press: London, UK, 2006. [Google Scholar]
- Wu, H.; Liotta, L.F. Metal-support interaction effects on gold catalysts over reducible oxides. In Heterogeneous Gold Catalysts and Catalysis; RSC Catalysis Series; The Royal Society of Chemistry: London, UK, 2014; pp. 462–488. [Google Scholar]
- Pérez-Ramírez, J.; Mul, G.; Kapteijn, F.; Moulijn, J.A. In situ investigation of the thermal decomposition of Co–Al hydrotalcite in different atmospheres. J. Mater. Chem. 2001, 11, 821–830. [Google Scholar] [CrossRef]
- Guan, D.; Shi, C.; Xu, H.; Gu, Y.; Zhong, J.; Sha, Y.; Hu, Z.; Ni, M.; Shao, Z. Simultaneously mastering operando strain and reconstruction effects via phase-segregation strategy for enhanced oxygen-evolving electrocatalysis. J. Energy Chem. 2023, 82, 572–580. [Google Scholar] [CrossRef]
- Genty, E.; Cousin, R.; Capelle, S.; Siffert, S. Influence of gold on hydrotalcite-like compound catalysts for toluene and CO total oxidation. Catalysts 2013, 3, 966–977. [Google Scholar] [CrossRef]
- El Khawaja, R.; Rochard, G.; Genty, E.; Poupin, C.; Siffert, S.; Cousin, R. Optimization of Mn-Mg-Al Mixed Oxides Composition on their Activity towards the Total Oxidation of Aromatic and Oxygenated VOCs. Eur. J. Inorg. Chem. 2023, 26, e202300213. [Google Scholar] [CrossRef]
- Behravesh, E.; Kumar, N.; Balme, Q.; Roine, J.; Salonen, J.; Schukarev, A.; Mikkola, J.P.; Peurla, M.; Aho, A.; Eränen, K.; et al. Synthesis and characterization of Au nano particles supported catalysts for partial oxidation of ethanol: Influence of solution pH, Au nanoparticle size, support structure and acidity. J. Catal. 2017, 353, 223–238. [Google Scholar] [CrossRef]
- Cherepanova, S.V.; Koemets, E.G.; Gerasimov, E.Y.; Simentsova, I.I.; Bulavchenko, O.A. Reducibility of Al3+-Modified Co3O4: Influence of Aluminum Distribution. Materials 2023, 16, 6216. [Google Scholar] [CrossRef]
- Arnoldy, P.; Moulijn, J.A. Temperature-Programmed Reduction of CoO/Al2O3 Catalysts. J. Catal. 1985, 93, 38–54. [Google Scholar] [CrossRef]
- Sexton, B.A.; Hughes, A.E.; Turney, T.W. An XPS and TPR Study of the Reduction of Promoted Cobalt-Kieselguhr Fisher-Tropsch Catalysts. J. Catal. 1986, 406, 390–406. [Google Scholar] [CrossRef]
- Solsona, B.; Aylón, E.; Murillo, R.; Mastral, A.M.; Monzonís, A.; Agouram, S.; Davies, T.E.; Taylor, S.H.; Garcia, T. Deep oxidation of pollutants using gold deposited on a high surface area cobalt oxide prepared by a nanocasting route. J. Hazard. Mater. 2011, 187, 544–552. [Google Scholar] [CrossRef]
- Liu, Y.; Dai, H.; Deng, J.; Xie, S.; Yang, H.; Tan, W.; Han, W.; Jiang, Y.; Guo, G. Mesoporous Co3O4-supported gold nanocatalysts: Highly active for the oxidation of carbon monoxide, benzene, toluene, and o-xylene. J. Catal. 2014, 309, 408–418. [Google Scholar] [CrossRef]
- Rochard, G.; Giraudon, J.M.; Liotta, L.F.; La Parola, V.; Lamonier, J.F. Au/Co promoted CeO2 catalysts for formaldehyde total oxidation at ambient temperature: Role of oxygen vacancies. Catal. Sci. Technol. 2019, 9, 3203–3213. [Google Scholar] [CrossRef]
- Wan, C.; Wei, X.; Cai, G.; Li, D.; Zhan, Y.; Xiao, Y.; Jiang, L. Hydrotalcite-Derived Aluminum-Doped Cobalt Oxides for Catalytic Benzene Combustion: Effect of Calcination Atmosphere. Mol. Catal. 2022, 520, 112160. [Google Scholar] [CrossRef]
- Zhao, Q.; Ge, Y.; Fu, K.; Ji, N.; Song, C.; Liu, Q. Oxidation of Acetone over Co-Based Catalysts Derived from Hierarchical Layer Hydrotalcite: Influence of Co/Al Molar Ratios and Calcination Temperatures. Chemosphere 2018, 204, 257–266. [Google Scholar] [CrossRef]
- Zou, D.; Yi, Y.; Song, Y.; Guan, D.; Xu, M.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. The BaCe0.16Y0.04Fe0.8O3−δ nanocomposite: A new high-performance cobalt-free triple-conducting cathode for protonic ceramic fuel cells operating at reduced temperatures. J. Mater. Chem. A 2022, 10, 5381–5390. [Google Scholar] [CrossRef]
- Wei, W.; Chen, W.; Ivey, D.G. Rock Salt-Spinel Structural Transformation in Anodically Electrodeposited Mn-Co-O Nanocrystals. Chem. Mater. 2008, 20, 1941–1947. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.; Sapi, A.; Tatsumi, H.; Zherebetskyy, D.; Han, H.-L.; Carl, L.; Somorjai, G. Molecular Orientations Change Reaction Kinetics and Mechanism: A Review on Catalytic Alcohol Oxidation in Gas Phase and Liquid Phase on Size-Controlled Pt Nanoparticles. Catalysts 2018, 8, 226. [Google Scholar] [CrossRef]
- Torbina, V.V.; Vodyankin, A.A.; Ten, S.; Mamontov, G.V.; Salaev, M.A.; Sobolev, V.I.; Vodyankina, O.V. Ag-Based Catalysts in Heterogeneous Selective Oxidation of Alcohols: A Review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef]
- Liotta, L.F.; Ousmane, M.; Di Carlo, G.; Pantaleo, G.; Deganello, G.; Marcì, G.; Retailleau, L.; Giroir-Fendler, A. Total oxidation of propene at low temperature over Co3O4–CeO2 mixed oxides: Role of surface oxygen vacancies and bulk oxygen mobility in the catalytic activity. Appl. Catal. A Gen. 2008, 347, 81–88. [Google Scholar] [CrossRef]

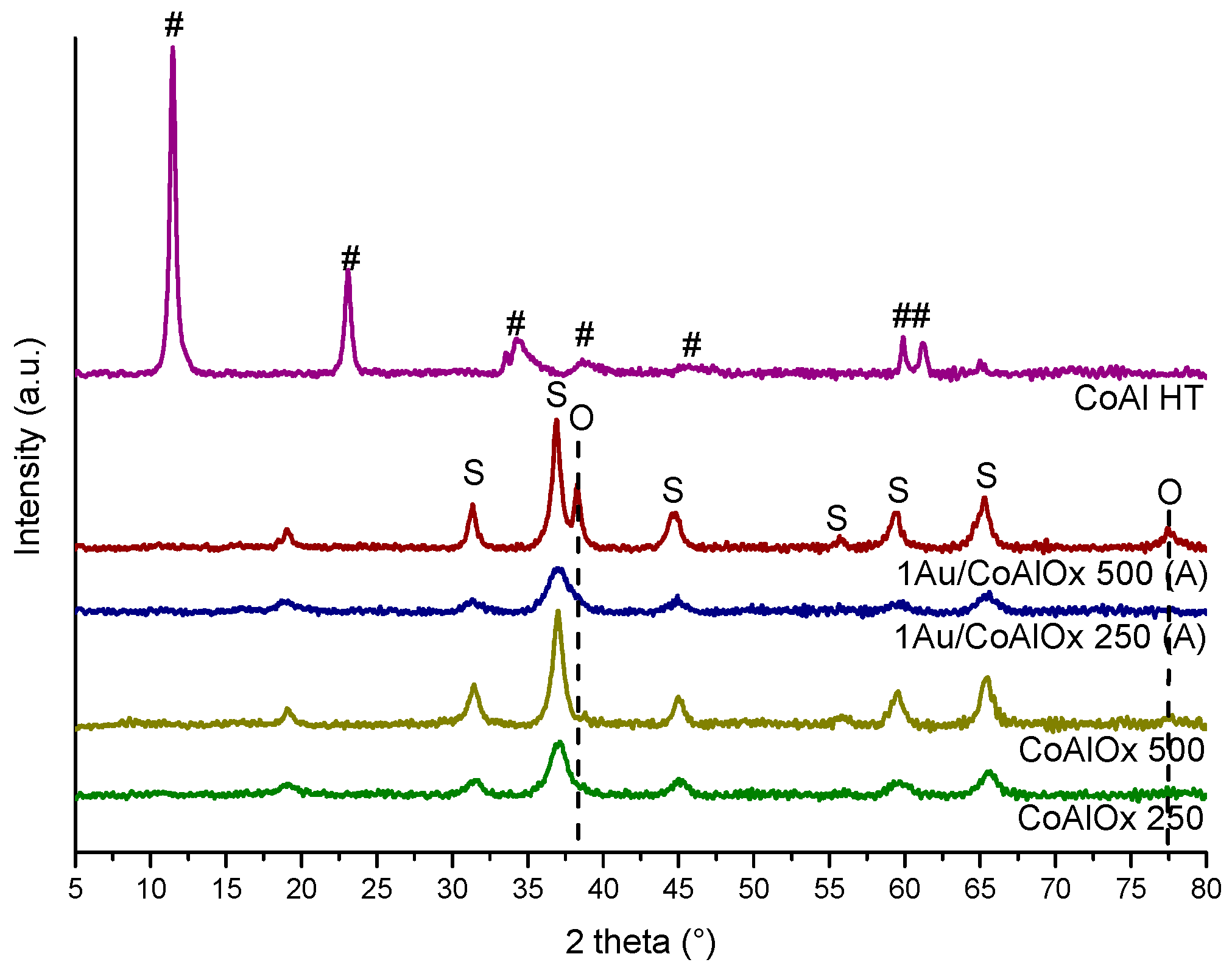

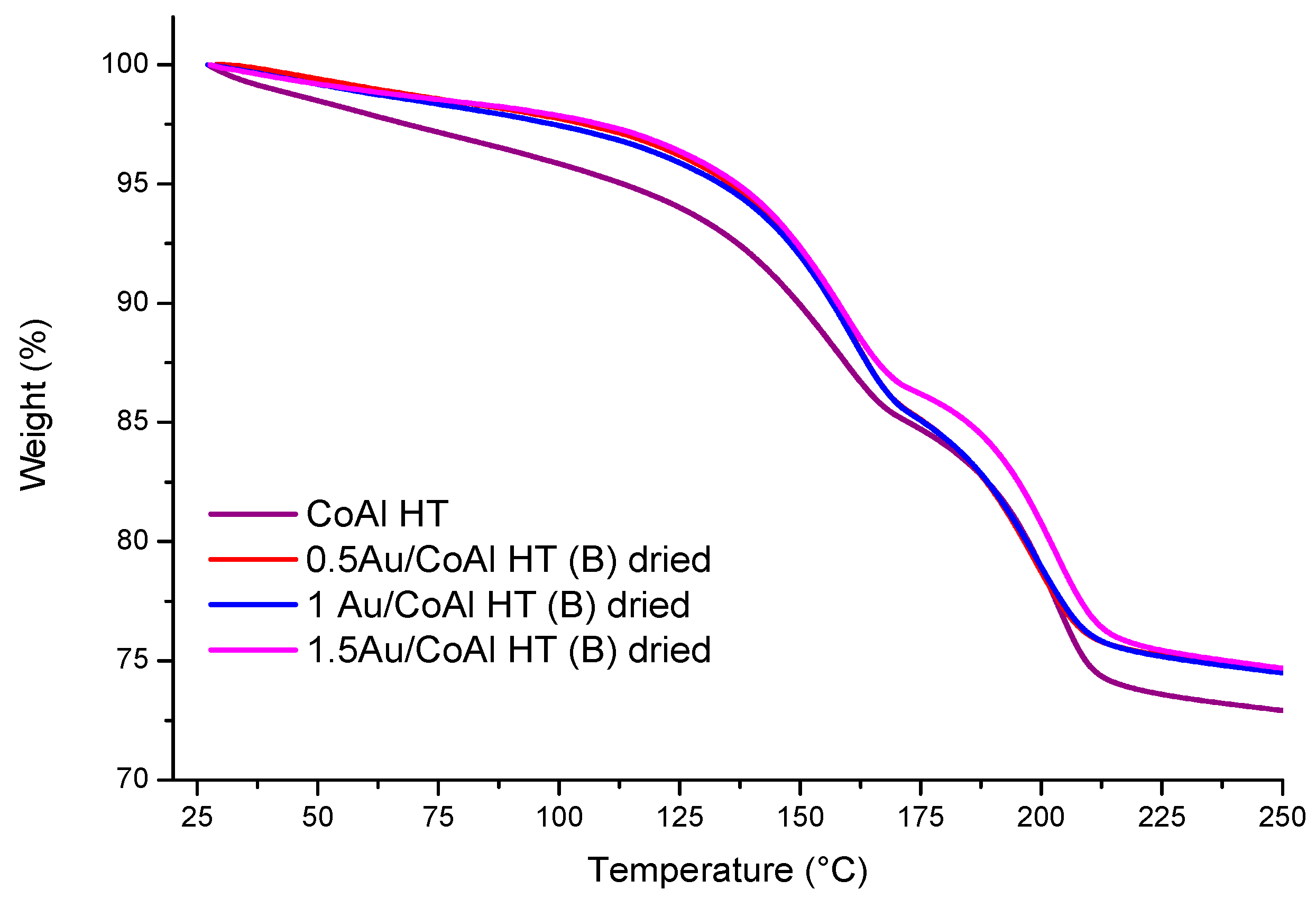


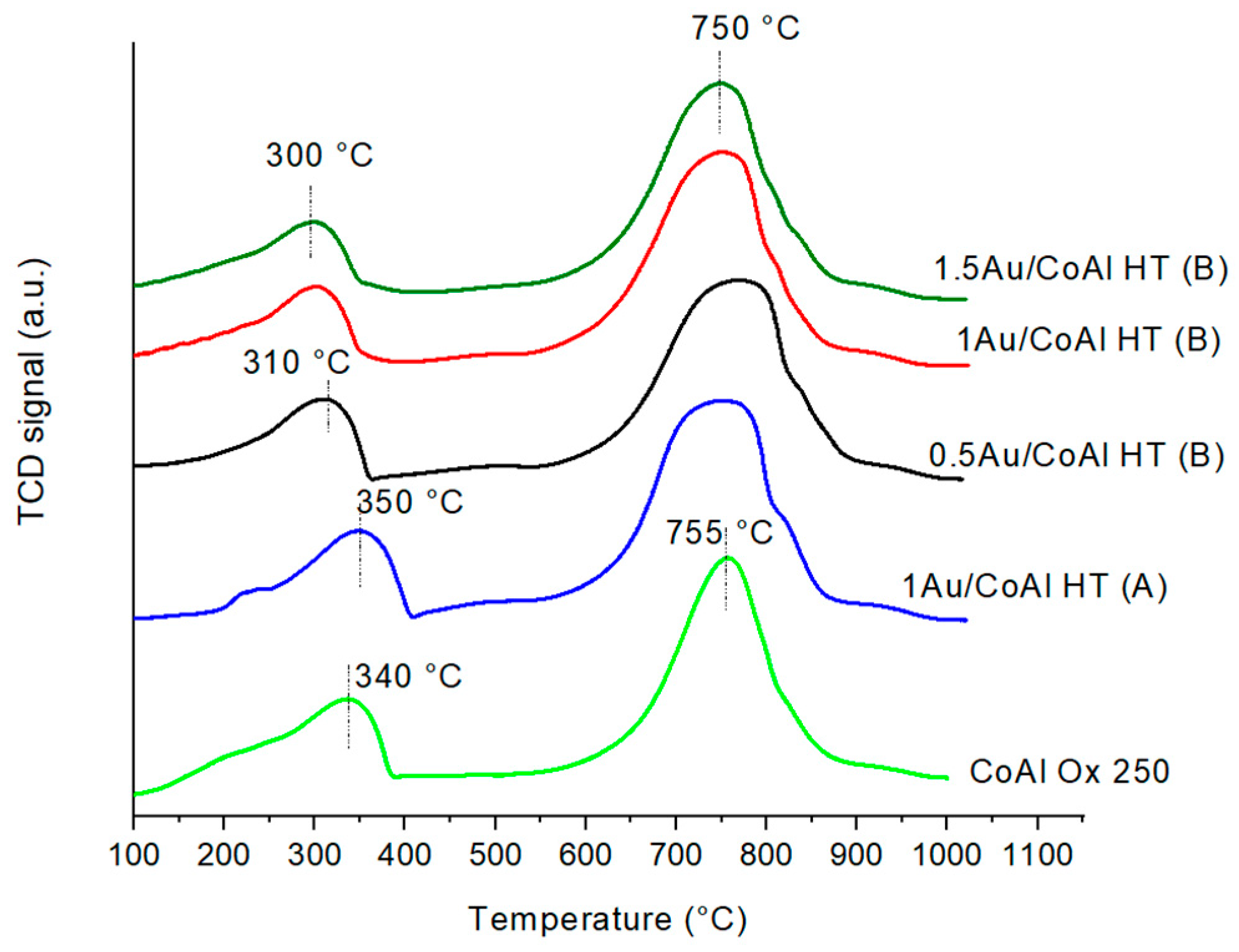
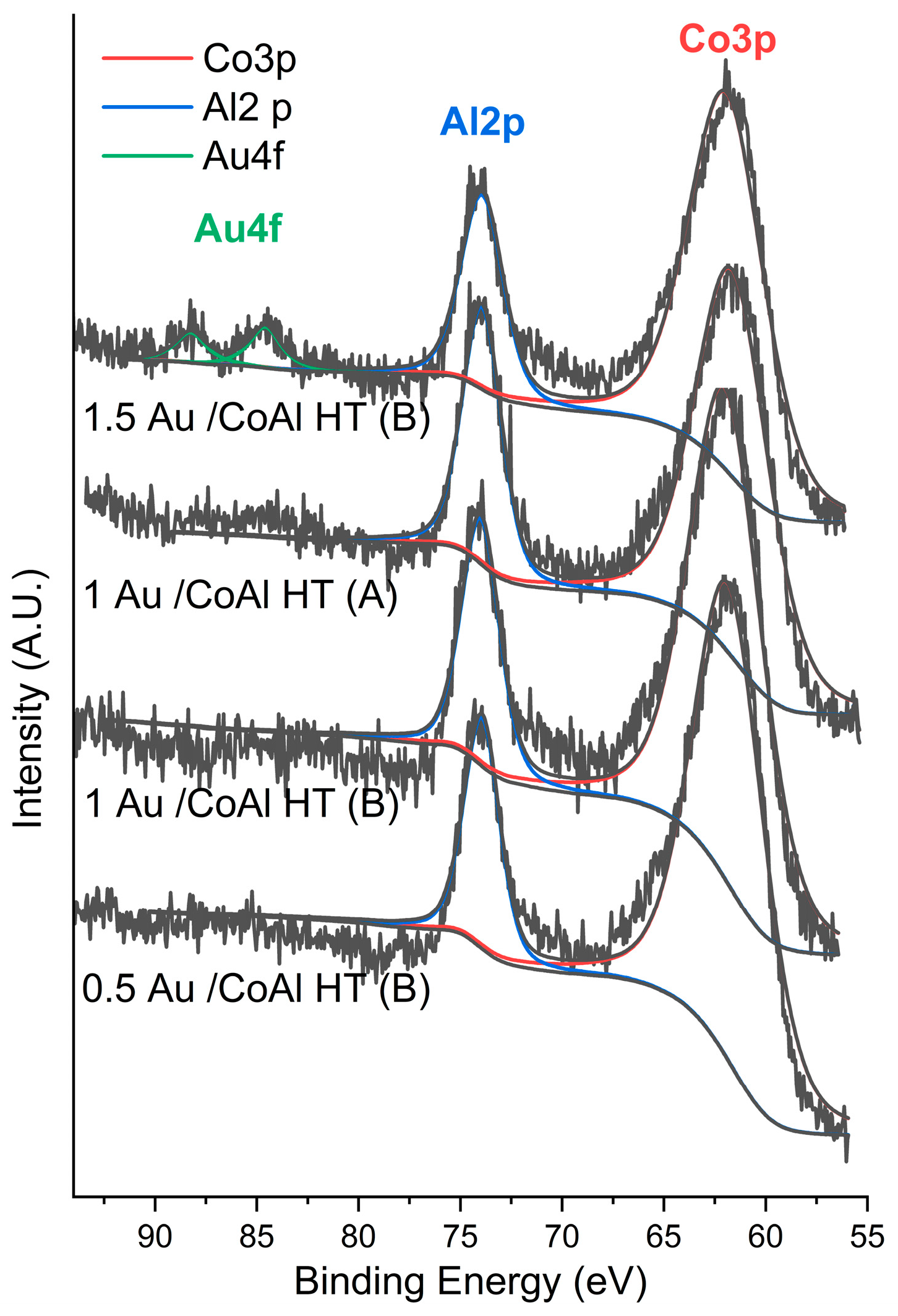

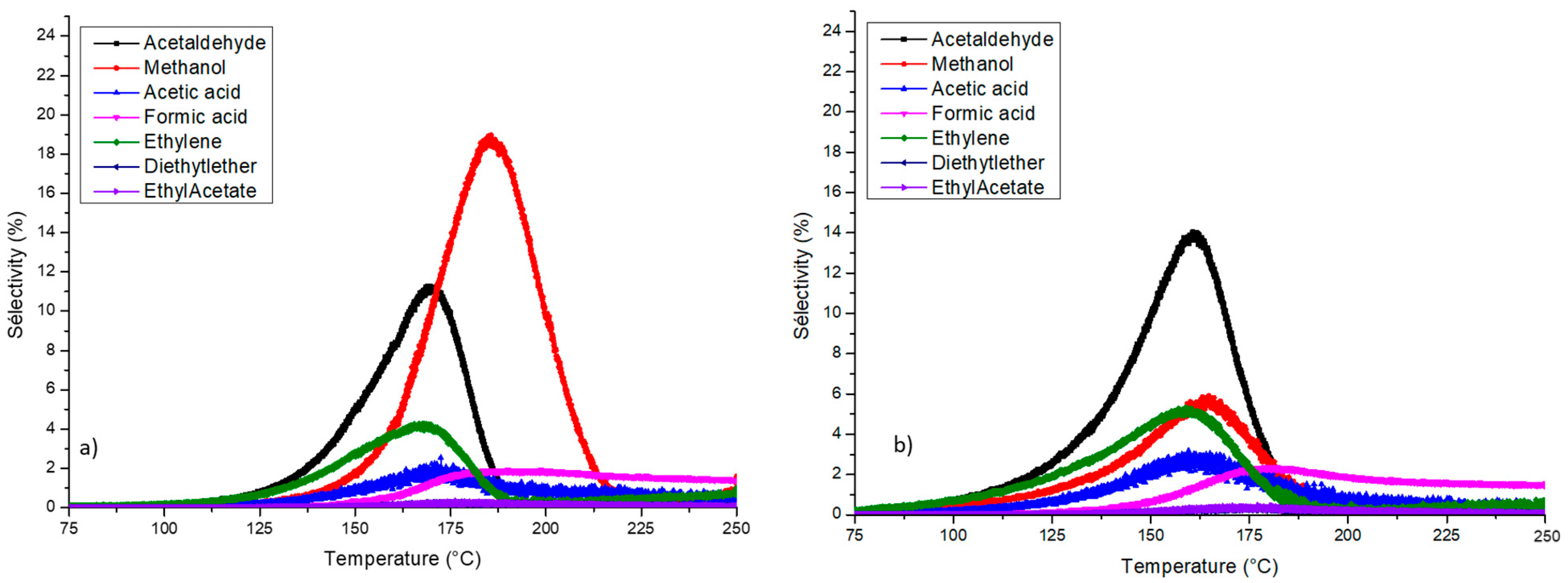
| Samples | Specific Surface Area (m2·g−1) | Mean Pore Size (nm) | Pore Volume (cm3·g−1) | Ethanol Oxidation | ||||
|---|---|---|---|---|---|---|---|---|
| T10 (°C) | T50 (°C) | Selectivity Max (%) | Carbon Balance (%) | |||||
| Methanol | Acetaldehyde | |||||||
| CoAl Ox 250 | 275 | 16.7 | 0.95 | 155 | 176 | 11 | 18 | 96.7 |
| CoAl Ox 500 | 113 | 20.0 | 0.67 | 174 | 196 | 10 | 17 | 97.2 |
| 1Au/CoAl Ox 250 (A) | 155 | 11.5 | 0.53 | 165 | 188 | 7 | 13 | 97.5 |
| 1Au/CoAl Ox 500 (A) | 108 | 15.6 | 0.55 | 165 | 190 | 6 | 14 | 96.8 |
| 1Au/CoAl HT (A) | 180 | 12.0 | 0.52 | 159 | 184 | 7 | 13 | 96.5 |
| 0.5Au/CoAl HT (B) | 224 | 10.9 | 0.60 | 148 | 171 | 3 | 9 | 98.0 |
| 1Au/CoAl HT (B) | 206 | 11.2 | 0.57 | 143 | 166 | 7 | 14 | 98.9 |
| 1.5Au/CoAl HT (B) | 197 | 11.1 | 0.56 | 146 | 168 | 5 | 16 | 98.4 |
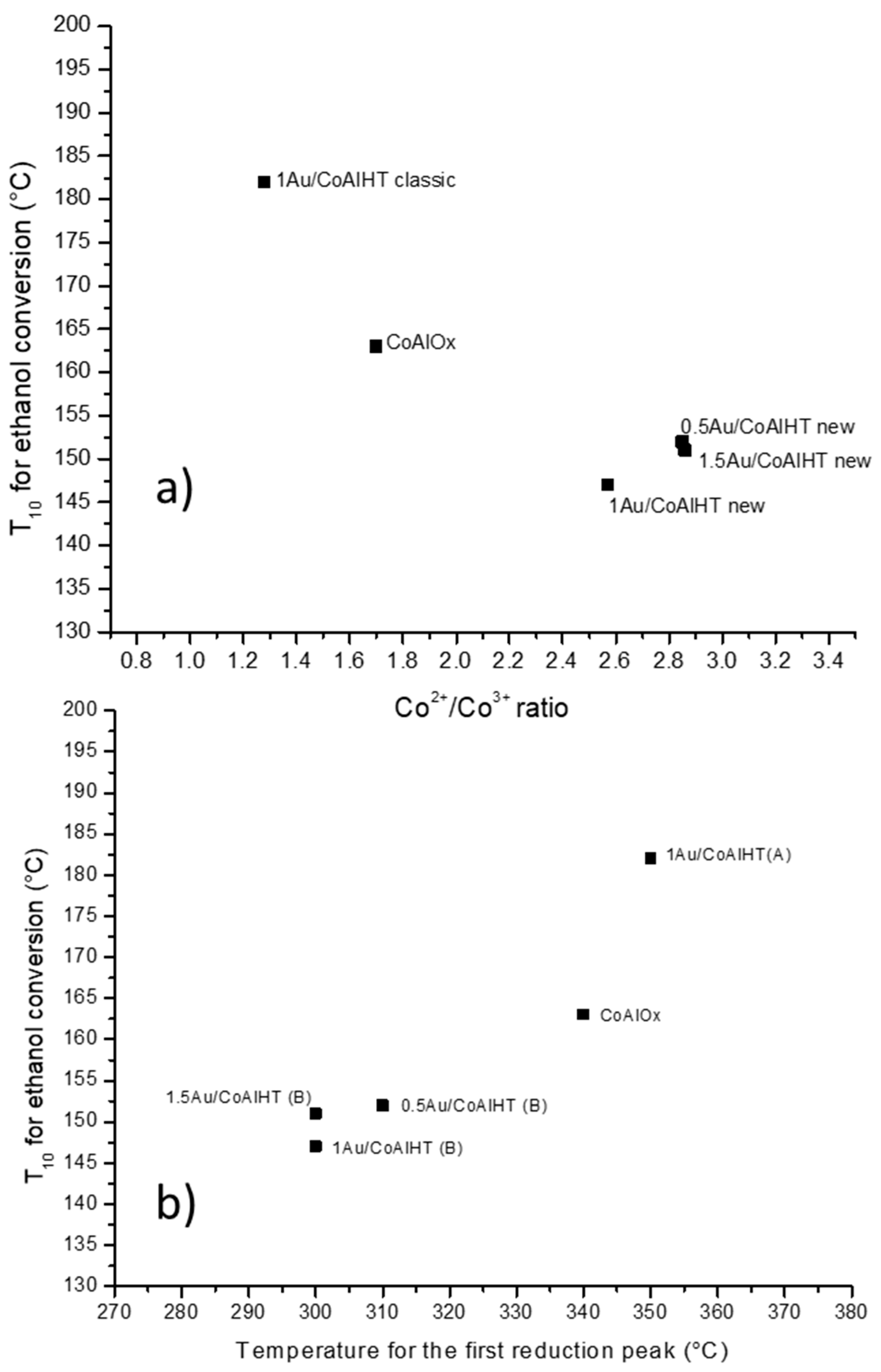
| Samples | H2 Consumption (mL/g) | XPS Atomic Ratio | ||||
|---|---|---|---|---|---|---|
| Low Temperature (<400 °C) | High Temperature (>400 °C) | Total | Co2+/Co3+ | Co/Al | Au/Al | |
| CoAl Ox 250 | 69 | 215 | 284 | 1.70 | 1.05 | - |
| 1Au/CoAl HT (A) | 72 | 221 | 293 | 1.28 | 0.97 | n.d. * |
| 0.5Au/CoAl HT (B) | 63 | 200 | 263 | 2.85 | 1.19 | n.d. |
| 1Au/CoAl HT (B) | 63 | 201 | 264 | 2.57 | 1.07 | n.d. |
| 1.5Au/CoAl HT (B) | 66 | 195 | 261 | 2.86 | 1.08 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rochard, G.; Genty, E.; Giraudon, J.-M.; Poupin, C.; Lamonier, J.-F.; Siffert, S.; La Parola, V.; Liotta, L.F.; Cousin, R. Synthesis of Gold Nanoparticles over CoAl Mixed Oxide for Ethanol Oxidation Reaction. Molecules 2024, 29, 2285. https://doi.org/10.3390/molecules29102285
Rochard G, Genty E, Giraudon J-M, Poupin C, Lamonier J-F, Siffert S, La Parola V, Liotta LF, Cousin R. Synthesis of Gold Nanoparticles over CoAl Mixed Oxide for Ethanol Oxidation Reaction. Molecules. 2024; 29(10):2285. https://doi.org/10.3390/molecules29102285
Chicago/Turabian StyleRochard, Guillaume, Eric Genty, Jean-Marc Giraudon, Christophe Poupin, Jean-François Lamonier, Stéphane Siffert, Valeria La Parola, Leonarda Francesca Liotta, and Renaud Cousin. 2024. "Synthesis of Gold Nanoparticles over CoAl Mixed Oxide for Ethanol Oxidation Reaction" Molecules 29, no. 10: 2285. https://doi.org/10.3390/molecules29102285
APA StyleRochard, G., Genty, E., Giraudon, J.-M., Poupin, C., Lamonier, J.-F., Siffert, S., La Parola, V., Liotta, L. F., & Cousin, R. (2024). Synthesis of Gold Nanoparticles over CoAl Mixed Oxide for Ethanol Oxidation Reaction. Molecules, 29(10), 2285. https://doi.org/10.3390/molecules29102285










