Design and Development of a Polymeric-Based Curcumin Nanoparticle for Drug Delivery Enhancement and Potential Incorporation into Nerve Conduits
Abstract
1. Introduction
2. Results and Discussion
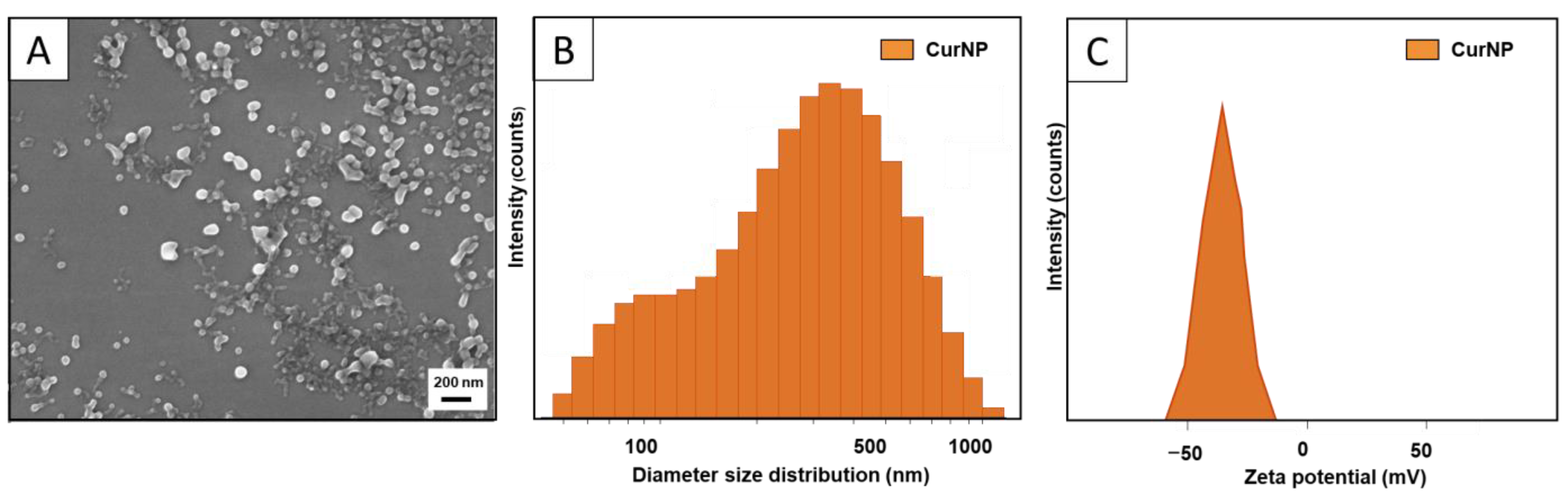
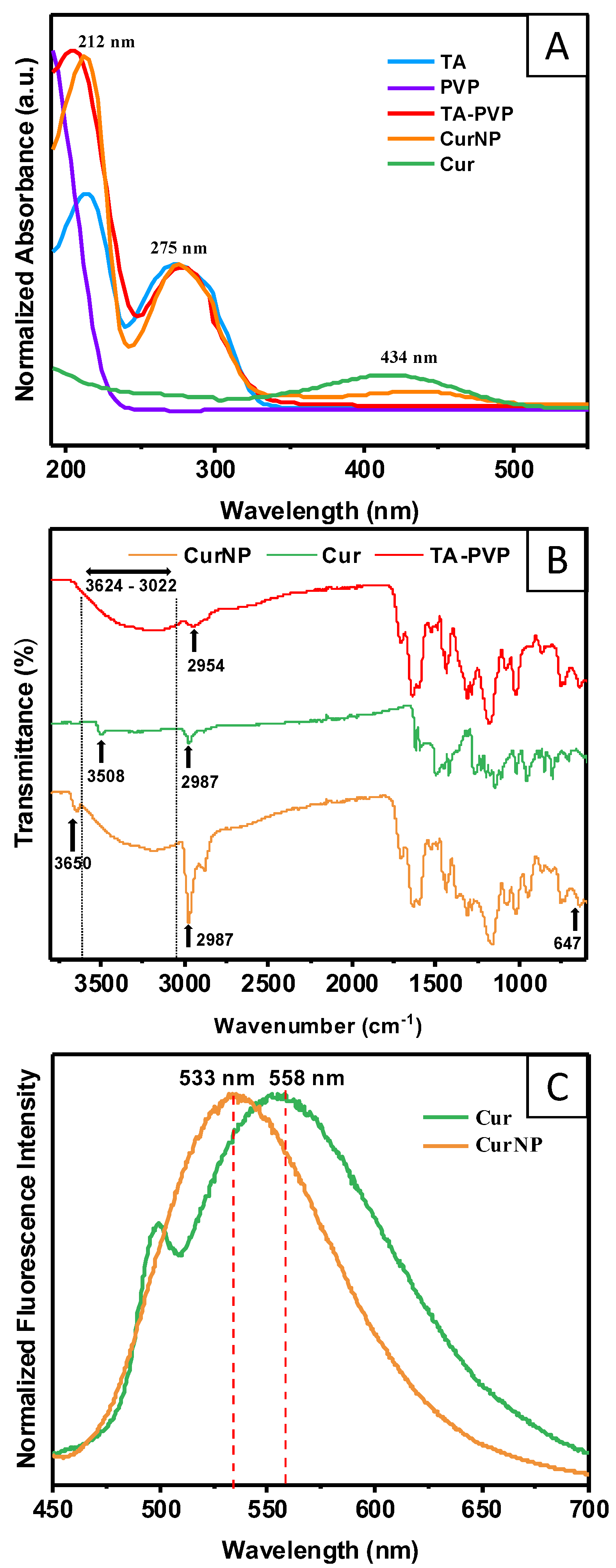
2.1. Material Characterization
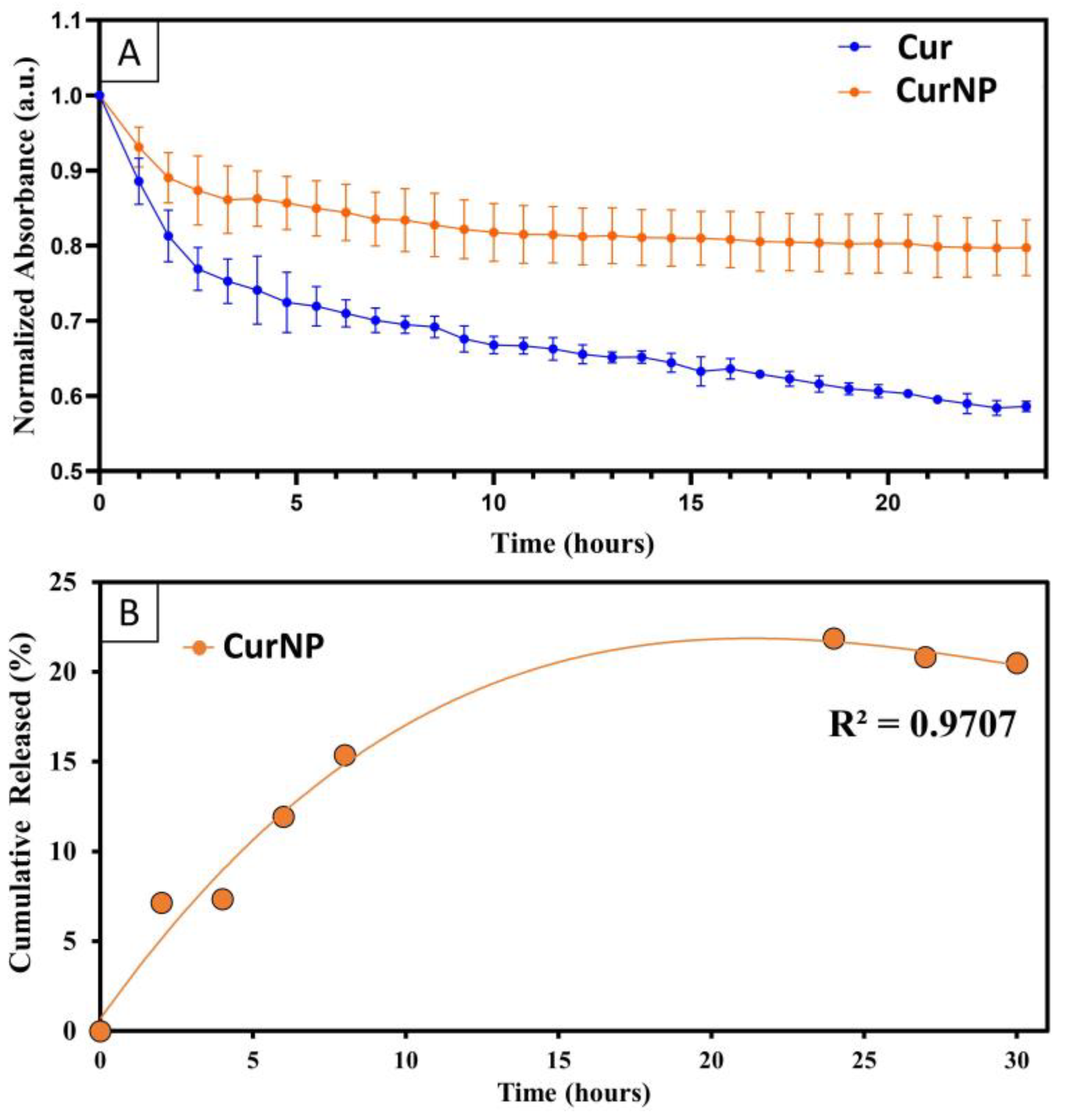
2.2. Degradation Rate, Controlled Release, and Antioxidant Potential of CurNPs
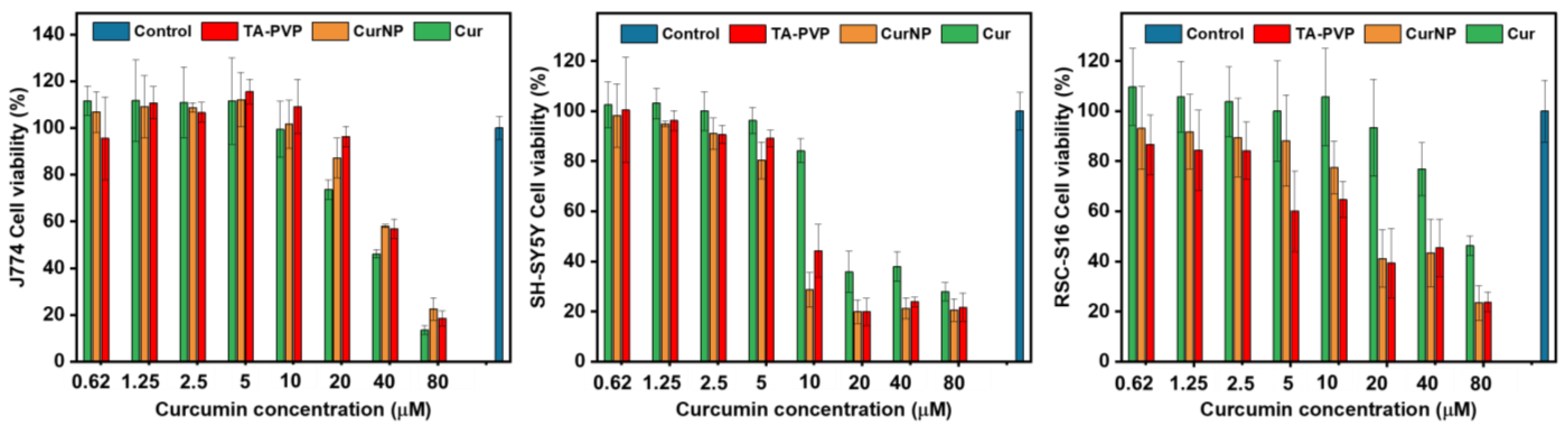
2.3. In Vitro Viability Studies on Cell Models for PNI
2.4. In Vitro Cellular Delivery of Curcumin
2.5. Hydrogen Peroxide (H2O2)-Induced Oxidative Stress and Cell Viability
3. Materials and Methods
3.1. Reagents
3.2. Synthesis of Curcumin Nanoparticles
3.3. Dynamic Light Scattering (DLS) and Zeta Potential (ZP)
3.4. Scanning Electron Microscopy (SEM)
3.5. UV–Visible (UV–Vis) Spectroscopy
3.6. Fourier Transform Infrared Spectroscopy (FTIR)
3.7. Encapsulation Efficiency (EE)
3.8. Fluorescence Spectroscopy
3.9. Curcumin Degradation Rate
3.10. Curcumin Release Rate
3.11. Antioxidant Capacity with 2,2-Diphenyl-1-picryhyldrazyl (DPPH) Radical Scavenging Activity Assay
3.12. Cell Viability Assay
3.13. Confocal Fluorescence Microscopy for In Vitro Cellular Delivery of Curcumin
3.14. Hydrogen Peroxide (H2O2)-Induced Oxidative Stress and Viability Assay
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma Acute Care Surg. 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Gaudin, R.; Knipfer, C.; Henningsen, A.; Smeets, R.; Heiland, M.; Hadlock, T. Approaches to peripheral nerve repair: Generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. BioMed Res. Int. 2016, 2016, 3856262. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Ruijs, A.C.; Jaquet, J.-B.; Kalmijn, S.; Giele, H.; Hovius, S.E. Median and ulnar nerve injuries: A meta-analysis of predictors of motor and sensory recovery after modern microsurgical nerve repair. Plast. Reconstr. Surg. 2005, 116, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; de Aguilar, J.-L.G. Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Int. J. Biol. Sci. 2020, 16, 116. [Google Scholar] [CrossRef]
- Sarker, M.; Naghieh, S.; McInnes, A.D.; Schreyer, D.J.; Chen, X. Regeneration of peripheral nerves by nerve guidance conduits: Influence of design, biopolymers, cells, growth factors, and physical stimuli. Prog. Neurobiol. 2018, 171, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Arslantunali, D.; Dursun, T.; Yucel, D.; Hasirci, N.; Hasirci, V. Peripheral nerve conduits: Technology update. Med. Devices Evid. Res. 2014, 7, 405–424. [Google Scholar]
- Carvalho, C.R.; Oliveira, J.M.; Reis, R.L. Modern trends for peripheral nerve repair and regeneration: Beyond the hollow nerve guidance conduit. Front. Bioeng. Biotechnol. 2019, 7, 337. [Google Scholar] [CrossRef]
- Evans, G.R.; Brandt, K.; Katz, S.; Chauvin, P.; Otto, L.; Bogle, M.; Wang, B.; Meszlenyi, R.K.; Lu, L.; Mikos, A.G. Bioactive poly (L-lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 2002, 23, 841–848. [Google Scholar] [CrossRef]
- Ramburrun, P.; Kumar, P.; Choonara, Y.E.; Bijukumar, D.; du Toit, L.C.; Pillay, V. A review of bioactive release from nerve conduits as a neurotherapeutic strategy for neuronal growth in peripheral nerve injury. BioMed Res. Int. 2014, 2014, 132350. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Z.; Wang, Y.; Xia, L.; Yu, S.; Li, H.; Zhang, W.; Liu, W.; Shao, K.; Han, B. Nerve Regeneration Effect of a Composite Bioactive Carboxymethyl Chitosan-Based Nerve Conduit with a Radial Texture. Molecules 2022, 27, 9039. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Gouda, M.; Cai, E.; Wang, R.; Xu, W.; Wu, Y.; Munekata, P.E.; Lorenzo, J.M. The antioxidant phytochemical schisandrin a promotes neural cell proliferation and differentiation after ischemic brain injury. Molecules 2021, 26, 7466. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Yu, H.; Liu, J.; Chen, Y.; Wang, Q.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery after sciatic nerve crush injury in diabetic rats. Neurosci. Lett. 2016, 610, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, R.; Li, H.; Lao, J. Green tea polyphenols promote functional recovery from peripheral nerve injury in rats. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2020, 26, e923806-1–e923806-9. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Fan, Z.; Wang, X.; Li, Z.; Wen, J.; Deng, J.; Tan, D.; Pan, M.; Hu, X. Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front. Cell. Neurosci. 2019, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Aung Myo, Y.P.; McKiver, B.D.; Osinska Warncke, U.; Thompson, D.; Mann, J.; Del Fabbro, E.; Desmoulière, A.; Billet, F.; Damaj, M.I. Key developments in the potential of curcumin for the treatment of peripheral neuropathies. Antioxidants 2020, 9, 950. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Yu, H.; Wang, Q.; Chen, Y.; Xiang, L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci. Lett. 2013, 547, 26–31. [Google Scholar] [CrossRef]
- Moattari, M.; Moattari, F.; Kouchesfahani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M.; Mansouri, K. Curcumin and biodegradable membrane promote nerve regeneration and functional recovery after sciatic nerve transection in adult rats. Ann. Plast. Surg. 2018, 81, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guan, Z.; Wang, X.; Sun, D.; Wang, D.; Li, Y.; Pei, B.; Ye, M.; Xu, J.; Yue, X. Curcumin alleviates oxaliplatin-induced peripheral neuropathic pain through inhibiting oxidative stress-mediated activation of NF-κB and mitigating inflammation. Biol. Pharm. Bull. 2020, 43, 348–355. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–125. [Google Scholar]
- Trigo-Gutierrez, J.K.; Vega-Chacón, Y.; Soares, A.B.; Mima, E.G.d.O. Antimicrobial activity of curcumin in nanoformulations: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer. Res. 2003, 23, 363–398. [Google Scholar]
- Mahakunakorn, P.; Tohda, M.; Murakami, Y.; Matsumoto, K.; Watanabe, H.; Vajaragupta, O. Cytoprotective and cytotoxic effects of curcumin: Dual action on H2O2-induced oxidative cell damage in NG108-15 cells. Biol. Pharm. Bull. 2003, 26, 725–728. [Google Scholar] [CrossRef]
- Gagliardi, S.; Morasso, C.; Stivaktakis, P.; Pandini, C.; Tinelli, V.; Tsatsakis, A.; Prosperi, D.; Hickey, M.; Corsi, F.; Cereda, C. Curcumin formulations and trials: What’s new in neurological diseases. Molecules 2020, 25, 5389. [Google Scholar] [CrossRef] [PubMed]
- Wiggers, H.J.; Zaioncz, S.; Cheleski, J.; Mainardes, R.M.; Khalil, N.M. Chapter 7—Curcumin, a Multitarget Phytochemical: Challenges and Perspectives. In Studies in Natural Products Chemistry; Atta ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 53, pp. 243–276. [Google Scholar]
- Khan, N.S.; Ahmad, A.; Hadi, S.M. Anti-oxidant, pro-oxidant properties of tannic acid and its binding to DNA. Chem. Biol. Interact. 2000, 125, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef]
- Le, Z.; Chen, Y.; Han, H.; Tian, H.; Zhao, P.; Yang, C.; He, Z.; Liu, L.; Leong, K.W.; Mao, H.-Q. Hydrogen-bonded tannic acid-based anticancer nanoparticle for enhancement of oral chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 42186–42197. [Google Scholar] [CrossRef]
- Lee, H.; Bang, J.-B.; Na, Y.-G.; Lee, J.-Y.; Cho, C.-W.; Baek, J.-S.; Lee, H.-K. Development and Evaluation of Tannic Acid-Coated Nanosuspension for Enhancing Oral Bioavailability of Curcumin. Pharmaceutics 2021, 13, 1460. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhang, X.; Cheng, X.; Zhang, Y.; Zan, X.; Zhang, L. Medical Applications Based on Supramolecular Self-Assembled Materials From Tannic Acid. Front. Chem. 2020, 8, 583484. [Google Scholar] [CrossRef]
- Jahromi, H.K.; Farzin, A.; Hasanzadeh, E.; Barough, S.E.; Mahmoodi, N.; Najafabadi, M.R.H.; Farahani, M.S.; Mansoori, K.; Shirian, S.; Ai, J. Enhanced sciatic nerve regeneration by poly-L-lactic acid/multi-wall carbon nanotube neural guidance conduit containing Schwann cells and curcumin encapsulated chitosan nanoparticles in rat. Mater. Sci. Eng. C 2020, 109, 110564. [Google Scholar] [CrossRef]
- Wang, L.; Hao, L.; Hou, H.; Wang, L.; Liu, S.; Tang, R.; Mao, J.; Zhou, Q.; Deng, Q.; Yan, L. Carboxymethylpachymaran-Coated Zein Nanoparticles for Oral Delivery of Curcumin: Formation, Characterization, Physicochemical Stability, and Controlled Release Properties. ACS Food Sci. Technol. 2023, 3, 170–181. [Google Scholar] [CrossRef]
- Li, D.; Wei, Z.; Sun, J.; Xue, C. Tremella polysaccharides-coated zein nanoparticles for enhancing stability and bioaccessibility of curcumin. Curr. Res. Food Sci. 2022, 5, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.G.; Jiao, W.-Q.; Yang, H.; Liu, J.; Liu, D. Phenylboronic acid-conjugated chitosan nanoparticles for high loading and efficient delivery of curcumin. Carbohydr. Polym. 2021, 256, 117497. [Google Scholar] [CrossRef] [PubMed]
- Anbari, H.; Maghsoudi, A.; Hosseinpour, M.; Yazdian, F. Acceleration of antibacterial activity of curcumin loaded biopolymers against methicillin-resistant Staphylococcus aureus: Synthesis, optimization, and evaluation. Eng. Life Sci. 2022, 22, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhan, Y.; Luo, G.; Zeng, Y.; McClements, D.J.; Hu, K. Curcumin encapsulated zein/caseinate-alginate nanoparticles: Release and antioxidant activity under in vitro simulated gastrointestinal digestion. Curr. Res. Food Sci. 2023, 6, 100463. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Rananaware, P.; Brahmkhatri, V.; Mishra, M. Polyvinylpyrrolidone-curcumin nanoconjugate as a biocompatible, non-toxic material for biological applications. J. Clust. Sci. 2023, 34, 395–414. [Google Scholar] [CrossRef]
- Wang, H.; Song, B.; Zhou, J.; Gao, G.; Ding, Y.; Meng, X.; Ke, L.; Ding, W.; Zhang, S.; Chen, T. Fabrication and characterization9 of curcumin-loaded nanoparticles using licorice protein isolate from Radix Glycyrrhizae. Int. J. Biol. Macromol. 2024, 255, 128235. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Rutan, S. Principles and applications of solvatochromism. Appl. Spectrosc. 2001, 55, 362A–370A. [Google Scholar] [CrossRef]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of curcumin: From mechanism to biological implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef] [PubMed]
- Griesser, M.; Pistis, V.; Suzuki, T.; Tejera, N.; Pratt, D.A.; Schneider, C. Autoxidative and cyclooxygenase-2 catalyzed transformation of the dietary chemopreventive agent curcumin. J. Biol. Chem. 2011, 286, 1114–1124. [Google Scholar] [CrossRef]
- Qu, W.R.; Zhu, Z.; Liu, J.; Song, D.B.; Tian, H.; Chen, B.P.; Li, R.; Deng, L.X. Interaction between Schwann cells and other cells during repair of peripheral nerve injury. Neural Regen. Res. 2021, 16, 93–98. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Popovich, P.G.; Ramer, M.S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J. Neuroinflamm. 2011, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Peng, J.; Han, G.H.; Ding, X.; Wei, S.; Gao, G.; Huang, K.; Chang, F.; Wang, Y. Role of macrophages in peripheral nerve injury and repair. Neural Regen. Res. 2019, 14, 1335–1342. [Google Scholar] [PubMed]
- Al-Ali, H.; Beckerman, S.R.; Bixby, J.L.; Lemmon, V.P. In vitro models of axon regeneration. Exp. Neurol. 2017, 287, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Gordon, T.; Chan, K.M.; Sulaiman, O.A.; Udina, E.; Amirjani, N.; Brushart, T.M. Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery 2009, 65, A132–A144. [Google Scholar] [CrossRef] [PubMed]
- Standardization, I. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- Li, A.; Pereira, C.; Hill, E.E.; Vukcevich, O.; Wang, A. In Vitro, In Vivo and Ex Vivo Models for Peripheral Nerve Injury and Regeneration. Curr. Neuropharmacol. 2022, 20, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.; Giannelli, G.G.; Davidson, E.; Santra, S.; Leon, S. Intermolecularly-Fabricated Composition for Encapsulating Plant Materials, Agrochemicals and Pharmaceuticals. U.S. Patent Application 18/121,401, 21 September 2023. [Google Scholar]
- Pundarikakshudu, K.; Dave, H.N. Simultaneous determination of curcumin and berberine in their pure form and from the combined extracts of Curcuma longa and Berberis aristata. Int. J. Appl. Sci. Eng. 2010, 8, 19–26. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Davidson, E.; Pereira, J.; Gan Giannelli, G.; Murphy, Z.; Anagnostopoulos, V.; Santra, S. Multi-Functional Chitosan Nanovesicles Loaded with Bioactive Manganese for Potential Wound Healing Applications. Molecules 2023, 28, 6098. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Pantoja-Castro, M.A.; González-Rodríguez, H. Study by infrared spectroscopy and thermogravimetric analysis of tannins and tannic acid. Rev. Latinoam. Química 2011, 39, 107–112. [Google Scholar]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. β-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B Biointerfaces 2010, 79, 113–125. [Google Scholar] [CrossRef]
- Chen, X.; Zou, L.-Q.; Niu, J.; Liu, W.; Peng, S.-F.; Liu, C.-M. The Stability, Sustained Release and Cellular Antioxidant Activity of Curcumin Nanoliposomes. Molecules 2015, 20, 14293–14311. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.-F. Magnetic and optical studies on polyvinylpyrrolidone thin films doped with rare earth metal salts. Polym. J. 2010, 42, 728–734. [Google Scholar] [CrossRef]

| Molar Ratio (Cur:TA:PVP) | Water (mL) | Ethanol, 95% (mL) | Hydrodynamic Size Average (nm) | Encapsulation Efficiency (%) |
|---|---|---|---|---|
| 1:2:0.021 | 14 | 1 | 207 | 66 |
| 1:2:0.021 | 9 | 1 | 210 | 77 |
| 1:3:0.032 | 14 | 1 | 229 | 66 |
| 1:3:0.032 | 9 | 1 | 214 | 82 |
| 1:3.3:0.035 | 9 | 1 | 220 | 88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannelli, G.G.; Davidson, E.; Pereira, J.; Santra, S. Design and Development of a Polymeric-Based Curcumin Nanoparticle for Drug Delivery Enhancement and Potential Incorporation into Nerve Conduits. Molecules 2024, 29, 2281. https://doi.org/10.3390/molecules29102281
Giannelli GG, Davidson E, Pereira J, Santra S. Design and Development of a Polymeric-Based Curcumin Nanoparticle for Drug Delivery Enhancement and Potential Incorporation into Nerve Conduits. Molecules. 2024; 29(10):2281. https://doi.org/10.3390/molecules29102281
Chicago/Turabian StyleGiannelli, Giuliana Gan, Edwin Davidson, Jorge Pereira, and Swadeshmukul Santra. 2024. "Design and Development of a Polymeric-Based Curcumin Nanoparticle for Drug Delivery Enhancement and Potential Incorporation into Nerve Conduits" Molecules 29, no. 10: 2281. https://doi.org/10.3390/molecules29102281
APA StyleGiannelli, G. G., Davidson, E., Pereira, J., & Santra, S. (2024). Design and Development of a Polymeric-Based Curcumin Nanoparticle for Drug Delivery Enhancement and Potential Incorporation into Nerve Conduits. Molecules, 29(10), 2281. https://doi.org/10.3390/molecules29102281






