An Efficient Synthesis of 1-(1,3-Dioxoisoindolin-2-yl)-3-aryl Urea Analogs as Anticancer and Antioxidant Agents: An Insight into Experimental and In Silico Studies
Abstract
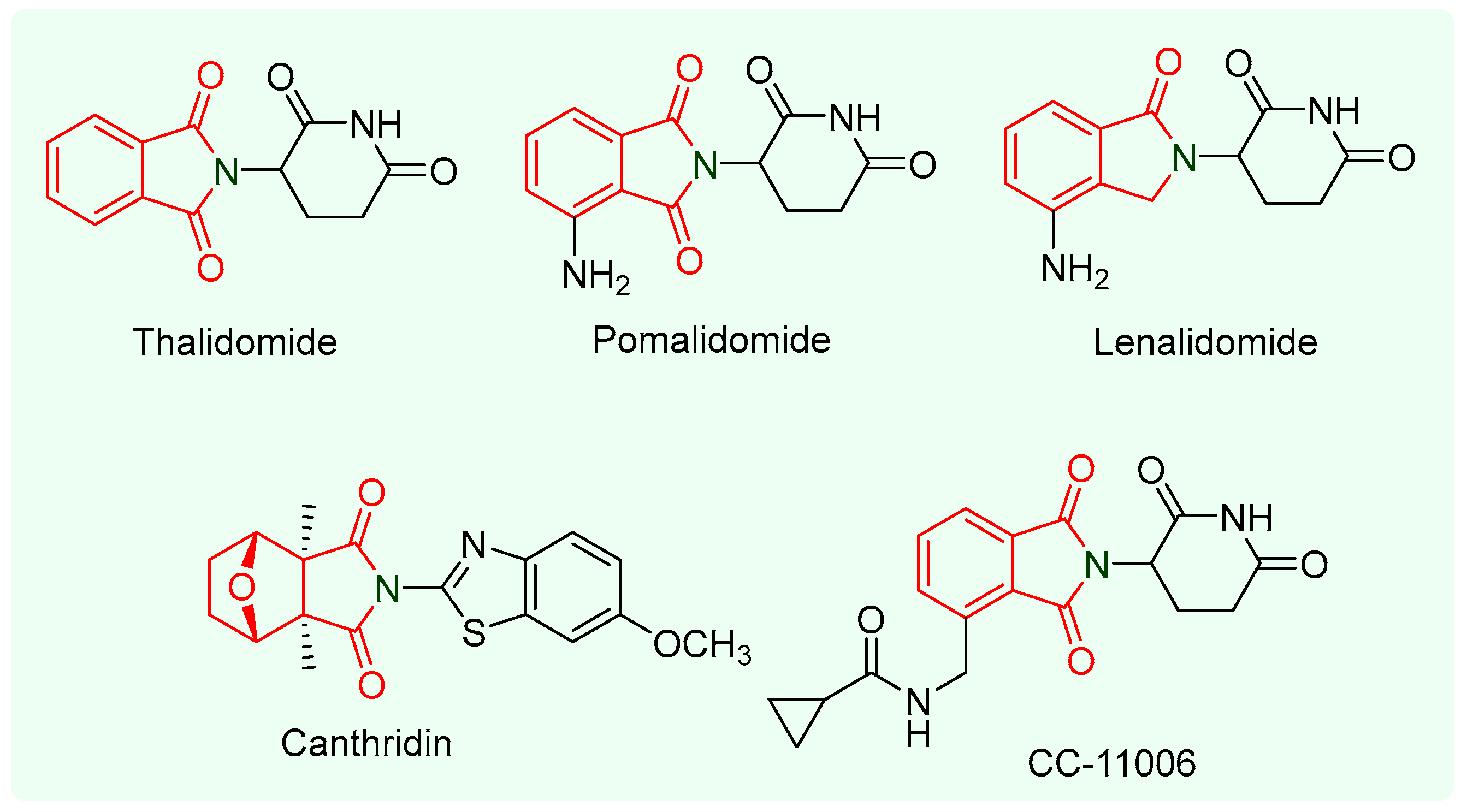
:1. Introduction
2. Results
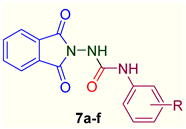
2.1. Chemistry
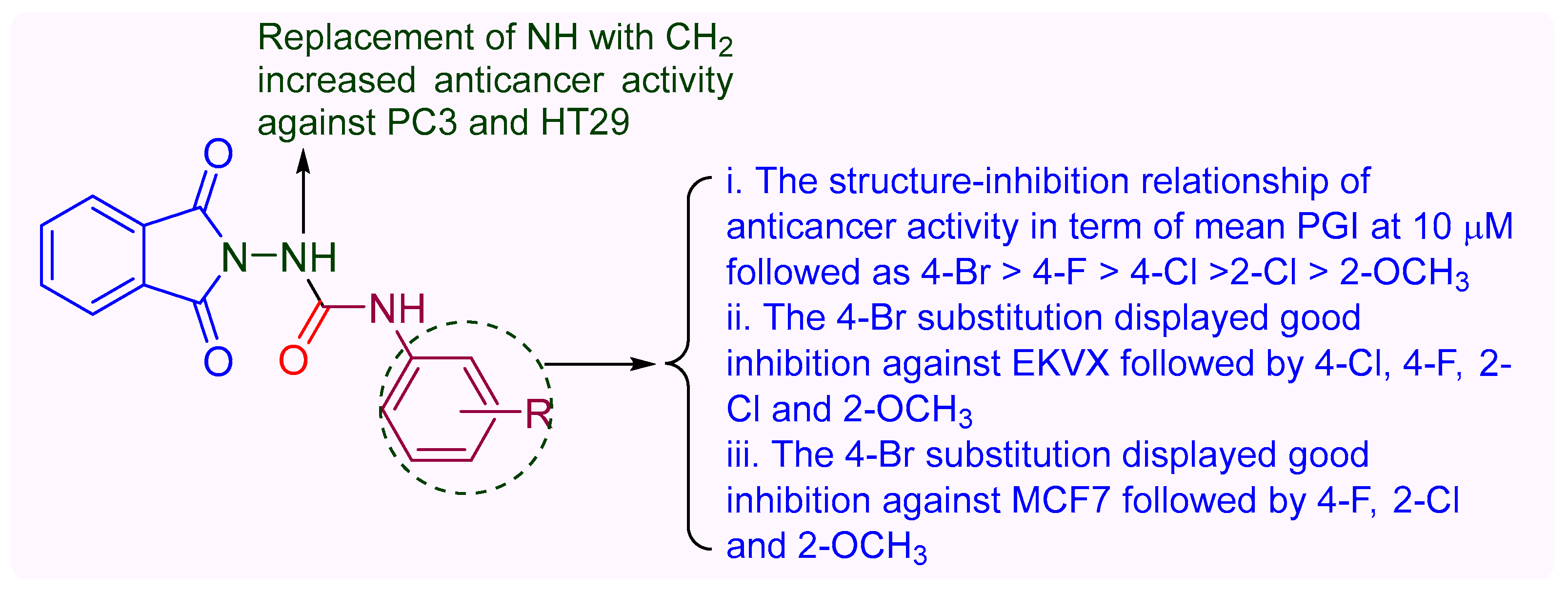
2.2. Anticancer Activity
2.3. In Vitro EGFR Kinase Inhibition Assay
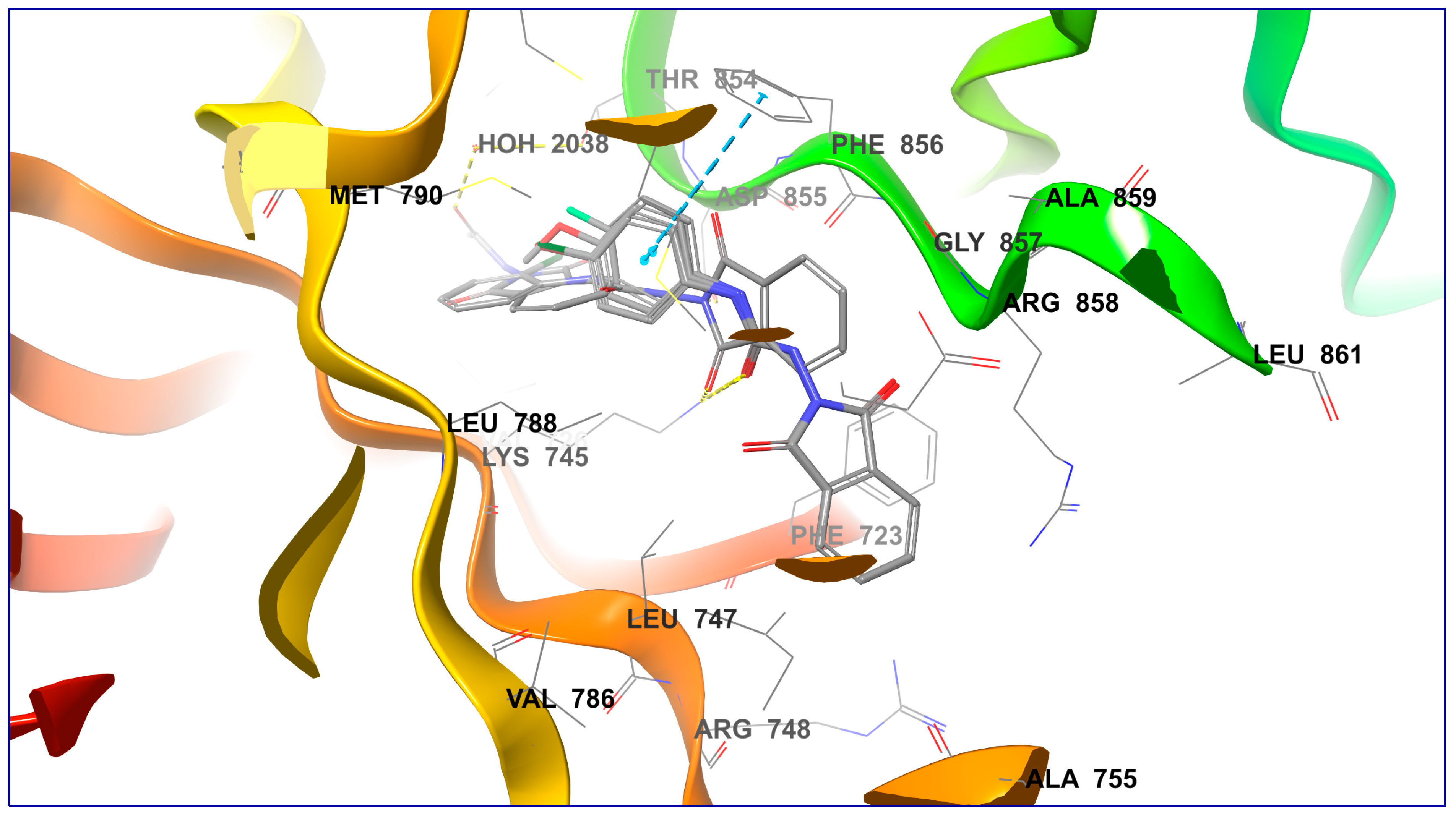
2.4. Molecular Docking Studies
2.5. Antioxidant Activity
2.6. ADMET Prediction
3. Discussion
4. Materials and Methods
4.1. General Procedure for the Synthesis of Phenyl(substituted phenyl)carbamate Analogs (3a–f)
4.2. Procedure for the Synthesis of Phthalimide (5)
4.3. Procedure for the Synthesis of 2-Aminoisoindoline-1,3-dione (6)
4.4. General Procedure for the Synthesis of 1-(1,3-Dioxoisoindolin-2-yl)-3-aryl Urea Analogs (7a–f)
4.5. Anticancer Activity
4.6. In Vitro EGFR Kinase Inhibition Assay
4.7. Molecular Docking Studies
4.8. Antioxidant Activity
4.9. ADMET Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferley, J.; Siegel, R.L.; Laversanne, M.; Soerjomartaram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Xu, L.; Sun, J.; Song, M.; Wang, L.; Yuan, S.; Zhu, Y.; Wan, Z.; Larsson, S.; Tsilidis, K.; et al. Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019. BMJ Oncol. 2023, 2, e000049. [Google Scholar] [CrossRef]
- Ugai, T.; Sasamoto, N.; Lee, H.-Y.; Ando, M.; Song, M.; Tamimi, R.M.; Kawachi, I.; Campbell, P.T.; Giovannucci, E.L.; Weiderpass, E.; et al. Is early-onset cancer an emerging global epidemic? Current evidence and future implications. Nat. Rev. Clin. Oncol. 2022, 19, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Wanga, S.; Zhang, S.; Zeng, H.; Chen, R.; Sun, K.; Li, L.; Bray, F.; Wei, W. Global, regional, and national lifetime probabilities of developing cancer in 2020. Sci. Bull. 2023, 68, 2620–2628. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Malvezzi, M.; Santucci, C.; Boffetta, P.; Collatuzzo, G.; Levi, F.; Vecchia, C.L.; Negri, E. European cancer mortality predictions for the year 2023 with focus on lung cancer. Ann. Oncol. 2023, 34, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; Chaturvedi, M.; Das, P.; Stephen, S.; Mathur, P. Cancer incidence estimates for 2022 & projection for 2025: Result from National Cancer Registry Programme, India. Indian J. Med. Res. 2022, 156, 598–607. [Google Scholar]
- Debela, D.T.; Muzazu, S.G.Y.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- GlobalSurg Collaborative; National Institute for Health Research Global Health Research Unit on Global Surgery. Global variation in postoperative mortality and complications after cancer surgery: A multicentre, prospective cohort study in 82 countries. Lancet 2021, 397, 387–397. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Fernandes, J.P.S. The Importance of Medicinal Chemistry Knowledge in the Clinical Pharmacist’s Education. Am. J. Pharm. Educ. 2018, 82, 6083. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.E.A.; Abdel-Salam, H.A.; Shaker, M.A. Synthesis, characterization, molecular modeling, and potential antimicrobial and anticancer activities of novel 2-aminoisoindoline-1,3-dione derivatives. Bioorg. Chem. 2016, 66, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Masoudi, N.A.; Abood, E.; Al-Maliki, Z.T.; Al-Masoudi, W.A.; Pannecouque, C. Amino acid derivatives. Part 6. Synthesis, in vitro antiviral activity and molecular docking study of new N-α-amino acid derivatives conjugated spacer phthalimide backbone. Med. Chem. Res. 2016, 25, 2578–2588. [Google Scholar] [CrossRef]
- Kok, S.H.; Gambari, R.; Chui, C.H.; Yuen, M.C.; Lin, E.; Wong, R.S.; Lau, F.Y.; Cheng, G.Y.; Lam, W.S.; Chan, S.H.; et al. Synthesis and anti-cancer activity of benzothiazole containing phthalimide on human carcinoma cell lines. Bioorg. Med. Chem. 2008, 16, 3626–3631. [Google Scholar] [CrossRef] [PubMed]
- Ihmaid, S.K.; Alraqa, S.Y.; Aouad, M.R.; Aljuhani, A.; Elbadawy, H.M.; Salama, S.A.; Rezki, N.; Ahmed, H.E.A. Design of molecular hybrids of phthalimide-triazole agents with potent selective MCF-7/HepG2 cytotoxicity: Synthesis, EGFR inhibitory effect, and metabolic stability. Bioorg. Med. Chem. 2021, 111, 104835. [Google Scholar] [CrossRef] [PubMed]
- Gunkara, O.T.; Ocal, N. Synthesis of New N-Phthalimide Substituted Tricyclic Imide Containing Isoxazoline and Bispiro Functional Group as Possible Anti-cancer Agents. J. Turk. Chem. Soc. Sec. A Chem. 2018, 5, 73–84. [Google Scholar] [CrossRef]
- Davood, A.; Iman, M.; Pouriaiee, H.; Shafaroodi, H.; Akhbari, S.; Azimidoost, L.; Imani, E.; Rahmatpour, S. Novel derivatives of phthalimide with potent anticonvulsant activity in PTZ and MES seizure models. Iran. J. Basic. Med. Sci. 2017, 20, 430–437. [Google Scholar] [PubMed]
- Bach, D.-H.; Liu, J.-Y.; Kim, W.K.; Hong, J.-Y.; Park, S.H.; Kim, D.; Qin, S.-N.; Luu, T.-T.-T.; Park, H.J.; Xu, Y.-N.; et al. Synthesis and biological activity of new phthalimides as potential anti-inflammatory agents. Bioorg. Med. Chem. 2017, 25, 3396–3405. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Godin, A.M.; Melo, I.S.F.; Coura, G.M.E.; Matsui, T.C.; Dutra, M.M.G.B.; Brito, A.M.S.; Canhestro, W.G.; Alves, R.J.; Araujo, D.P.; et al. The phthalimide analogues N-3-hydroxypropylphthalimide and N-carboxymethyl-3-nitrophthalimide exhibit activity in experimental models of inflammatory and neuropathic pain. Pharmacol. Rep. 2019, 71, 1177–1183. [Google Scholar] [CrossRef]
- Bansal, M.; Upadhyay, C.; Ponam; Kumar, S.; Rathi, B. Phthalimide analogs for antimalarial drug discovery. RSC Med. Chem. 2021, 12, 1854–1867. [Google Scholar] [CrossRef]
- Singh, S.; El-Sakkary, N.; Skinner, D.E.; Sharma, P.P.; Ottilie, S.; Antonova-Koch, Y.; Kumar, P.; Winzeler, E.; Poonam; Caffrey, C.R.; et al. Synthesis and Bioactivity of Phthalimide Analogs as Potential Drugs to Treat Schistosomiasis, a Neglected Disease of Poverty. Pharmaceuticals 2020, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Kumar, P.; Kumar, N.; Singh, B. Recent Advances in the Chemistry of Phthalimide Analogues and their Therapeutic Potential. Mini-Rev. Med. Chem. 2010, 10, 678–704. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.S.; Lopes, J.R.; Santos, J.L.D.; Scarim, C.B. Phthalimide as a versatile pharmacophore scaffold: Unlocking its diverse biological activities. Drug Dev. Res. 2023, 87, 1346–1375. [Google Scholar] [CrossRef] [PubMed]
- Jacques, V.; Czarnik, A.W.; Judge, T.M.; der Ploeg, L.H.T.V.; DeWitt, S.H. Differentiation of anti-inflammatory and antitumorigenic properties of stabilized enantiomers of thalidomide analogs. Proc. Natl. Acad. Sci. USA 2015, 112, E1471–E1479. [Google Scholar] [CrossRef] [PubMed]
- Kotla, V.; Goel, S.; Nischal, S.; Heuck, C.; Vivek, K.; Das, B.; Verma, A. Mechanism of action of lenalidomide in hematological malignancies. J. Hematol. Oncol. 2009, 2, 36. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, M.J. 1,3,4-Oxadiazole containing compounds as therapeutic targets for cancer therapy. Mini-Rev. Med. Chem. 2022, 22, 144–197. [Google Scholar] [CrossRef] [PubMed]
- Staker, B.L.; Feese, M.D.; Cushman, M.; Pommier, Y.; Zembower, D.; Stewart, L.; Burgin, A.B. Structures of three classes of anticancer agents bound to the human topoisomerase I-DNA covalent complex. J. Med. Chem. 2005, 48, 2336–2345. [Google Scholar] [CrossRef]
- AlNeyadi, S.S.; Amer, N.; Thomas, T.G.; Al Ajeil, R.; Breitener, P.; Munawar, N. Synthesis, Characterization, and Antioxidant Activity of Some 2-Methoxyphenols derivatives. Heterocycl. Commun. 2020, 26, 112–122. [Google Scholar] [CrossRef]
- Braughler, J.M.; Duncan, L.A.; Chase, R.L. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratios in initiation. J. Biol. Chem. 1986, 261, 10282–10289. [Google Scholar] [CrossRef]
- Bouyahya, A.; El Menyiy, N.; Oumeslakht, L.; El Allam, A.; Balahbib, A.; Rauf, A.; Muhammad, N.; Kuznetsova, E.; Derkho, M.; Thiruvengadam, M.; et al. Preclinical and Clinical Antioxidant Effects of Natural Compounds against Oxidative Stress-Induced Epigenetic Instability in Tumor Cells. Antioxidants 2021, 10, 1553. [Google Scholar] [CrossRef]
- Mineo, S.; Takahashi, N.; Yamada-Hara, M.; Tsuzuno, T.; Aoki-Nonaka, Y.; Tabeta, K. Rice bran-derived protein fractions enhance sulforaphane-induced anti-oxidative activity in gingival epithelial cells. Arch. Oral. Biol. 2021, 129, 105215. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.-N.; Cheng, F.; Yang, Z.; Shang, X.-F.; Liang, J.-P.; Shang, R.-F.; Hao, B.-C.; Wang, X.-H.; Zhang, H.-J.; Wali, A.; et al. Antioxidant Activity and the Potential Mechanism of the Fruit From Ailanthus altissima Swingle. Front. Vet. Sci. 2021, 8, 784898. [Google Scholar] [CrossRef] [PubMed]
- Bădiceanu, C.D.; Mares, C.; Nuță, D.C.; Avram, S.; Drăghici, C.; Udrea, A.-M.; Zarafu, I.; Chiriță, C.; Hovaneț, M.V.; Limban, C. N-Substituted (Hexahydro)-1H-isoindole-1,3(2H)-dione Derivatives: New Insights into Synthesis and Characterization. Processes 2023, 11, 1616. [Google Scholar] [CrossRef]
- Perveen, S.; Orfali, R. L-Proline-Catalyzed Synthesis of Phthalimide Derivatives and Evaluation of Their Antioxidant, Anti-Inflammatory, and Lipoxygenase Inhibition Activities. J. Chem. 2018, 2018, 5198325. [Google Scholar] [CrossRef]
- Karthik, C.S.; Mallesha, L.; Mallu, P. Investigation of antioxidant properties of phthalimide derivatives. Can. Chem. Trans. 2015, 3, 199–206. [Google Scholar]
- Yogeeswari, P.; Sriram, D.; Saraswat, V.; Ragavendran, J.V.; Kumar, M.M.; Murugesan, S.; Thirumurugan, R.; Stables, J.P. Synthesis and anticonvulsant and neurotoxicity evaluation of N4-phthalimido phenyl (thio) semicarbazides. Eur. J. Pharm. Sci. 2003, 20, 341–346. [Google Scholar] [CrossRef]
- Verma, M.; Singh, K.N.; Clercq, E.D. Synthesis of some heterocycle containing urea derivatives and their antiviral activity. Heterocycles 2006, 68, 11–22. [Google Scholar]
- Moradi, M.; Golmohammadi, R.; Najafi, A.; Moghaddam, M.M.; Fasihi-Ramandi, M.; Mirnejad, R. A contemporary review on the important role of in silico approaches for managing different aspects of COVID-19 crisis. Inform. Med. Unlocked. 2022, 28, 100862. [Google Scholar] [CrossRef]
- Puttaswamy, H.; Gowtham, H.G.; Ojha, M.D.; Yadav, A.; Choudhir, G.; Raguraman, V.; Kongkham, B.; Selvaraju, K.; Shareef, S.; Gehlot, P.; et al. In silico studies evidenced the role of structurally diverse plant secondary metabolites in reducing SARS-CoV-2 pathogenesis. Sci. Rep. 2020, 10, 20584. [Google Scholar] [CrossRef]
- Huanca, P.I.J.; Veras, B.A.F.; de Sousa Oliveira, I.; Ferreira, S.B. In Silico Analysis Applied to the Study of Cytotoxicity in Natural Products. Chem. Proc. 2022, 12, 69. [Google Scholar] [CrossRef]
- A Phase 1, Evaluate the Safety, Tolerability, and Pharmacokinetics of INS018_055 in Healthy Subjects. Available online: https://clinicaltrials.gov/study/NCT05154240 (accessed on 2 December 2023).
- First Drug Discovered and Designed with Generative AI Enters Phase II Trials, with First Patients Dosed. Available online: https://www.eurekalert.org/news-releases/993844 (accessed on 2 December 2023).
- Yogeeswari, P.; Sriram, D.; Thirumurugan, R.; Saxena, A.; Stables, J.; Vaigunda Raghuvendran, J.; Suddan, K.; Pavana, R.K. Discovery of N-(2,6-Dimethyphenyl)-substituted semicarbazones as Anticonvulsants: Hybrid Pharmacophore-Based Design. J. Med. Chem. 2005, 48, 6202–6211. [Google Scholar] [CrossRef] [PubMed]
- DTP Developmental Therapeutic Programs. Available online: http://dtp.nci.nih.gov (accessed on 10 October 2023).
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a highflux anticancer drug screening using a diverse panel of cultured human tumor cell lines. J. Nat. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Aliabadi, A.; Mohammadi-Farani, A.; Seydi-Kangarshahi, S.; Ahmadi, F. Discovery of 2-(1,3-dioxoisoindolin-2-yl)-n-phenylacetamide derivatives as probable 15-lipoxygenase-1 Inhibitors with potential anticancer effects. Farmacia 2017, 65, 268–274. [Google Scholar]
- Aliabadi, A.; Mohammadi-Farani, A.; Hosseinzadeh, Z.; Nadri, H.; Moradi, A.; Ahmadi, F. Phthalimide analogs as probable 15-lipoxygenase-1 inhibitors: Synthesis, biological evaluation and docking studies. DARU J. Pharm. Sci. 2015, 23, 36. [Google Scholar] [CrossRef] [PubMed]
- Mourad, A.A.; Farouk, N.A.; El-Sayed, E.S.H.; Mahdy, A.R. EGFR/VEGFR-2 dual inhibitor and apoptotic inducer: Design, synthesis, anticancer activity and docking study of new 2-thioxoimidazolidin-4one derivatives. Life Sci. 2021, 277, 119531. [Google Scholar] [CrossRef]
- Takahashi, D.T.; Gadelle, D.; Agama, K.; Kiselev, E.; Zhang, H.; Yab, E.; Petrella, S.; Forterre, P.; Pommier, Y.; Mayer, C. Topoisomerase I (TOP1) dynamics: Conformational transition from open to closed states. Nat. Comm. 2022, 13, 59. [Google Scholar] [CrossRef]
- Capranico, G.; Marinello, J.; Chillemi, G. Type I DNA Topoisomerases. J. Med. Chem. 2017, 60, 2169–2192. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. 2006, 6, 789–802. [Google Scholar] [CrossRef]
- Saulnier, M.G.; Balasubramanian, B.N.; Long, B.H.; Frennesson, D.B.; Ruediger, E.; Zimmermann, K.; Eummer, J.T.; St. Laurent, D.R.; Stoffan, K.M.; Naidu, B.N.; et al. Discovery of a Fluoroindolo[2,3-a]carbazole Clinical Candidate with Broad Spectrum Antitumor Activity in Preclinical Tumor Models Superior to the Marketed Oncology Drug, CPT-11. J. Med. Chem. 2005, 48, 2258–2261. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. DNA Topoisomerase I Inhibitors: Chemistry, Biology, and Interfacial Inhibition. Chem. Rev. 2009, 109, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Shuai, W.; Wang, G.; Zhang, Y.; Bu, F.; Zhang, S.; Miller, D.D.; Li, W.; Ouyang, L.; Wang, Y. Recent Progress on Tubulin Inhibitors with Dual Targeting Capabilities for Cancer Therapy. J. Med. Chem. 2021, 64, 7963–7990. [Google Scholar] [CrossRef] [PubMed]
- Hawash, M. Recent Advances of Tubulin Inhibitors Targeting the Colchicine Binding Site for Cancer Therapy. Biomolecules 2022, 12, 1843. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lu, X.; Feng, B. A review of research progress of antitumor drugs based on tubulin targets. Transl. Cancer Res. 2020, 9, 4020–4027. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, T.; Noguchi, T.; Miyachi, H.; Kobayashi, H.; Hashimoto, Y. Tubulin polymerization inhibitors with a fluorinated phthalimide skeleton derived from thalidomide. Bioorg. Med. Chem. Lett. 2006, 16, 4748–4751. [Google Scholar] [CrossRef] [PubMed]
- X-ray Crystal Structure of DNA Topoisomerase I. Available online: https://www.rcsb.org/structure/1sc7 (accessed on 10 October 2023).
- X-ray Crystal Structure of Tubulin. Available online: https://www.rcsb.org/structure/1sa0 (accessed on 10 October 2023).
- Zubair, T.; Bandyopadhyay, D. Small Molecule EGFR Inhibitors as Anti-Cancer Agents: Discovery, Mechanisms of Action, and Opportunities. Int. J. Mol. Sci. 2023, 24, 2651. [Google Scholar] [CrossRef] [PubMed]
- X-ray Crystal Structure of EGFR. Available online: https://www.rcsb.org/structure/3W2R (accessed on 10 October 2023).
- Sogabe, S.; Kawakita, Y.; Igaki, S.; Iwata, H.; Miki, H.; Cary, D.R.; Takagi, T.; Takagi, S.; Ohta, Y.; Ishikawa, T. Structure-Based Approach for the Discovery of Pyrrolo[3,2-d]pyrimidine-Based EGFR T790M/L858R Mutant Inhibitors. ACS Med. Chem. Lett. 2013, 4, 201–205. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defence. World Allergy Organ J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef] [PubMed]
- Vaibhav, D.A.; Arunkumar, W.; Abhijit, M.P.; Arvind, S. Antioxidants as an immunomodulator. Int. J. Curr. Pharm. Res. 2011, 1, 8–10. [Google Scholar]
- Koleva, I.I.; Van-Beek, T.A.; Linssen, J.P.; de-Groot, A.; Evstatieva, L.N. Screening of plant extracts for antioxidant activity: A comparative study on three testing methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Tumosienė, I.; Kantminienė, K.; Klevinskas, A.; Petrikaitė, V.; Jonuškienė, I.; Mickevičius, V. Antioxidant and Anticancer Activity of Novel Derivatives of 3-[(4-Methoxyphenyl)amino]propanehydrazide. Molecules 2020, 25, 2980. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, druglikeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg To Predict Gastrointestinal Absorption and Brain Penetration of Small Molecules. ChemMedChem 2016, 11, 1117–1121. [Google Scholar] [CrossRef]
- ADME Prediction. Available online: http://www.swissadme.ch/ (accessed on 12 October 2023).
- Toxicity Prediction. Available online: https://tox-new.charite.de/protox_II/ (accessed on 12 October 2023).
- Lipinski, C.A.; Lombardo, L.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Delivery Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Tahir, A.; Bakht, M.A.; Salahuddin; Ahsan, M.J. Molecular Engineering of Curcumin, an Active Constituent of Curcuma longa L. (Turmeric) of the Family Zingiberaceae with Improved Antiproliferative Activity. Plants 2021, 10, 1559. [Google Scholar] [CrossRef]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]




 | ||||||||
|---|---|---|---|---|---|---|---|---|
| S. No. | Compound | R | NSC Code | Rf * | % Yield | Mp (°C) | ||
| Found | Reported | Found | Reported | |||||
| 1 | 7a | 4-Fluoro | 838925 | 0.69 | 89 | 67.7 a | 248–250 | >245 a |
| 2 | 7b | 4-Chloro | 838926 | 0.71 | 90 | 86.2 a | 252–254 | >250 a |
| 3 | 7c | 4-Bromo | 838927 | 0.72 | 92 | 73.1 a | 250–252 | 250 a |
| 4 | 7d | 4-Methoxy | - | 0.67 | 83 | 35.9 a | >260 | >260 a |
| 5 | 7e | 2-Chloro | 838928 | 0.68 | 81 | 46 b | 140–142 | 139 b |
| 6 | 7f | 2-Methoxy | 838934 | 0.73 | 85 | - | 156–158 | - |
| Panel | Cell Line | 7a | 7b | 7c | 7e | 7f |
|---|---|---|---|---|---|---|
| Leukemia | CCRF-CEM | 103.9 | 104.8 | 101.2 | 103.37 | 95.13 |
| HL-60(TB) | 86.23 | 83.07 | 92.99 | 101.97 | 101.22 | |
| K-562 | 100.3 | 91.98 | 92.8 | 110.7 | 92.54 | |
| MOLT-4 | 98.29 | 101.1 | 99.67 | 97.49 | 106.2 | |
| RPMI-8226 | 105.9 | 106.5 | 108.3 | 109.46 | 103.93 | |
| SR | 94.86 | 96.2 | 94.2 | 92.66 | 93.35 | |
| Non-small cell lung cancer | A549/ATCC | 99.08 | 102.7 | 101.2 | 103.02 | 103.59 |
| EKVX | 89.44 | 80.73 | 75.46 | 90.83 | 91.2 | |
| HOP-62 | 107.5 | 107.1 | 108.4 | 99.95 | 115.38 | |
| HOP-92 | 120.9 | 109.3 | 107.7 | 110.12 | 115.4 | |
| NCI-H226 | 99.82 | 94.57 | 91.94 | 96.96 | 100.71 | |
| NCI-H23 | 102.4 | 93.59 | 93.98 | 100.32 | 97.03 | |
| NCI-H322M | 94 | 96.1 | 98.49 | 101.21 | 95.89 | |
| NCI-H460 | 116.7 | 121.1 | 107.8 | 106.44 | 118.73 | |
| NCI-H522 | 96.78 | 94.82 | 96.41 | 97.3 | 96.7 | |
| Colon cancer | COLO 205 | 111.6 | 111.5 | 108.9 | 100.1 | 113.79 |
| HCC-2998 | 105.2 | 107.6 | 104.5 | 101.52 | 104.81 | |
| HCT-116 | 97.82 | 100.6 | 97.06 | 104.92 | 110.15 | |
| HCT-15 | 99.39 | 94.99 | 94.12 | 101.91 | 100.49 | |
| HT29 | 103.4 | 109.3 | 107.1 | 104.06 | 113.18 | |
| KM12 | 100.2 | 99.16 | 98.57 | 99.5 | 100.71 | |
| SW-620 | 103.7 | 108.3 | 99.5 | 96.91 | 101.88 | |
| CNS cancer | SF-268 | 97.06 | 97.16 | 96.17 | 94.28 | 104.11 |
| SF-295 | 94.85 | 98.09 | 99.52 | 96.86 | 101.54 | |
| SF-539 | 95.37 | 92.59 | 92.35 | 94.08 | 95.62 | |
| SNB-19 | 97.85 | 96.23 | 96.31 | 98.24 | 97.73 | |
| U251 | 103.1 | 107.2 | 105.5 | 104.47 | 104.72 | |
| Melanoma | LOX IMVI | 96.84 | 87.19 | 84.52 | 92.19 | 96.42 |
| MALME-3M | 93.65 | 94.22 | 96.35 | 94.53 | 94.69 | |
| M14 | 101.2 | 101.9 | 101.8 | 101.76 | 104.17 | |
| MDA-MB-435 | 97.22 | 96.66 | 96.81 | 100.53 | 99.08 | |
| SK-MEL-28 | 99.56 | 102.6 | 104.4 | 104.47 | 101.02 | |
| SK-MEL-5 | 92.01 | 86.59 | 90.69 | 92.19 | 93.38 | |
| UACC-257 | 102 | 105.8 | 104.4 | 94.53 | 103.72 | |
| UACC-62 | 90.53 | 85.6 | 80.81 | 101.76 | 93.41 | |
| Ovarian cancer | IGROV1 | 105 | 106.3 | 104.3 | 100.53 | 110.36 |
| OVCAR-3 | 109.2 | 113.9 | 111.8 | 104.47 | 106.37 | |
| OVCAR-4 | 97.78 | 96.67 | 93.93 | 94.18 | 99.61 | |
| OVCAR-5 | 104.8 | 102.9 | 101.1 | 105.8 | 102.63 | |
| OVCAR-8 | 99.87 | 103.1 | 102.2 | 92.98 | 97.77 | |
| NCI/ADR-RES | 102.4 | 102.1 | 103.1 | 108.87 | 102.13 | |
| SK-OV-3 | 102.6 | 105.6 | 99.28 | 107.11 | 104.8 | |
| Renal cancer | 786-0 | 98.32 | 100.2 | 100.1 | 100.59 | 104.72 |
| A498 | 103.9 | 117 | 114.4 | 108.88 | 115.67 | |
| ACHN | 98.77 | 92.38 | 89.61 | 99.89 | 99.4 | |
| CAKI-1 | 92.91 | 82.3 | 78.52 | 99.75 | 89.58 | |
| RXF 393 | 96.91 | 102 | 105.8 | 114.5 | 105.46 | |
| SN 12C | 93.94 | 93.88 | 94.56 | 93.07 | 99.81 | |
| TK-10 | 96.11 | 109.1 | 113.5 | 102.14 | 111.49 | |
| Prostate cancer | PC-3 | 99.99 | 102.2 | 99.48 | 96.67 | 104.34 |
| DU-145 | 104.9 | 104.6 | 103.3 | 105.71 | 104.85 | |
| Breast cancer | MCF7 | 84.87 | 90.11 | 83.48 | 87.43 | 91.63 |
| HS 578T | 98.14 | 96.97 | 92.76 | 93.19 | 100.05 | |
| BT-549 | 103.5 | 117.7 | 112.2 | 117.25 | 115.87 | |
| T-47D | 103.8 | 102.3 | 98.97 | 103.41 | 105.35 | |
| MDA-MB-468 | 99.41 | 97.99 | 100 | 98.35 | 97.8 | |
| Mean GP | 99.92 | 100.11 | 98.79 | 100.64 | 102.35 | |
| Compound | Anticancer Activity in One Dose (10 µM) | ||
|---|---|---|---|
| The Most Sensitive Cell Lines | GP | PGI | |
| 7a | MCF7 (Breast cancer) | 84.87 | 15.13 |
| HL-60(TB) (Leukemia) | 86.23 | 13.77 | |
| EKVX (Non-small cell lung cancer) | 89.44 | 10.56 | |
| SK-MEL-5 (Melanoma) | 92.01 | 7.99 | |
| CAKI-1 (Renal cancer) | 92.91 | 7.09 | |
| 7b | EKVX (Non-small cell lung cancer) | 80.73 | 19.27 |
| CAKI-1 (Renal cancer) | 82.3 | 17.7 | |
| HL-60(TB) (Leukemia) | 83.07 | 16.93 | |
| UACC-62 (Melanoma) | 85.6 | 14.4 | |
| LOX IMVI (Melanoma) | 87.19 | 12.81 | |
| 7c | EKVX (Non-small cell lung cancer) | 75.46 | 24.54 |
| CAKI-1 (Renal cancer) | 78.52 | 21.48 | |
| UACC-62 (Melanoma) | 80.81 | 19.19 | |
| MCF7 (Breast cancer) | 83.48 | 16.52 | |
| LOX IMVI (Melanoma) | 84.52 | 15.48 | |
| 7e | MCF7 (Breast cancer) | 87.43 | 12.57 |
| EKVX (Non-small cell lung cancer) | 90.83 | 9.17 | |
| LOX IMVI (Melanoma) | 92.19 | 7.81 | |
| SK-MEL-5 (Melanoma) | 92.19 | 7.81 | |
| SR (Leukemia) | 92.66 | 7.34 | |
| 7f | CAKI-1 (Renal cancer) | 89.58 | 10.42 |
| EKVX (Non-small cell lung cancer) | 91.2 | 8.8 | |
| MCF7 (Breast cancer) | 91.63 | 8.37 | |
| K-562 (Leukemia) | 92.54 | 7.46 | |
| SR (Leukemia) | 93.35 | 6.47 | |
| Panels | 7a | 7b | 7c | 7e | 7f | Imatinib * | Gefitinib * | Thalidomide * |
|---|---|---|---|---|---|---|---|---|
| Leukemia | 1.76 | 2.72 | 1.82 | −2.6 | 1.27 | 9 | 79.68 | 15.28 |
| Non-small cell lung cancer | −2.94 | 0.01 | 2.07 | −0.7 | −3.85 | 15.68 | 63.97 | 0.06 |
| Colon cancer | −3.05 | −4.5 | −1.39 | −1.3 | −6.43 | 5.34 | 52.19 | −0.27 |
| CNS cancer | 2.36 | 1.74 | 2.02 | 2.41 | −0.74 | 5.8 | 46.13 | −4.95 |
| Melanoma | 3.38 | 4.93 | 5.03 | 2.26 | 1.76 | −0.87 | 44.99 | −0.39 |
| Ovarian cancer | −3.08 | −4.38 | −2.23 | −1.99 | −3.38 | −7.16 | 60.93 | 1.90 |
| Renal cancer | 2.74 | 0.45 | 0.50 | −2.69 | −3.733 | 3.25 | 77.89 | −2.37 |
| Prostate cancer | −2.46 | −3.40 | −1.41 | −1.19 | −4.59 | 12.5 | 59.60 | −8.60 |
| Breast cancer | 2.06 | −0.99 | 2.25 | 0.07 | −2.35 | 12.15 | 52.88 | −2.04 |
| S. No. | Compound | PDB: 1SA0 | PDB ID: 1SC7 | PDB ID: 3W2R | ||||
|---|---|---|---|---|---|---|---|---|
| Docking Score | Emodel Score | Docking Score | Emodel Score | Docking Score | Emodel Score | Interaction | ||
| 1 | 7a | −6.469 | −54.331 | −7.169 | −59.500 | −7.934 | −66.771 | H-bond (Lys745; 2.05 Å), π-π-stacking (Phe856; 5.21 Å) |
| 2 | 7b | −6.426 | −56.002 | −7.473 | −63.130 | −7.816 | −64.552 | H-bond (Lys745; 1.97 Å) |
| 3 | 7c | −6.480 | −57.825 | −7.318 | −64.802 | −7.558 | −64.096 | H-bond (Lys745; 2.04 Å) |
| 4 | 7d | −7.069 | −57.606 | −7.565 | −66.006 | −7.702 | −65.761 | H-bond (Lys745; 2.04 Å), π-π-stacking (Phe856; 5.48 Å) |
| 5 | 7e | −7.292 | −62.624 | −6.363 | −65.436 | −7.119 | −65.715 | H-bond (Lys745; 1.64 Å), H-bond (Asp855; 2.13 Å) |
| 6 | 7f | −5.830 | −54.813 | −7.306 | −63.183 | −8.644 | −65.599 | H-bond (Thr854 via H2O; 1.76 and 2.39 Å) |
| S. No. | Compound | Free Radical Scavenging Activity IC50 (μM) |
|---|---|---|
| 1 | 7a | 36.18 ± 0.31 |
| 2 | 7b | 39.22 ± 0.12 |
| 3 | 7c | 24.02 ± 0.29 |
| 4 | 7d | 16.05 ± 0.15 |
| 5 | 7e | 54.27 ± 0.49 |
| 6 | 7f | 15.99 ± 0.10 |
| 7 | Ascorbic acid | 14.02 ± 0.15 |
| S. No. | Compound | % ABS | TPSA | HBA (<10) | HBD (<5) | NROTB | Log p (<5) | MW (500) | GI Absorption | Lipinski’s Violation (≤1) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7a | 81.91 | 78.51 | 4 | 2 | 4 | 1.74 | 299.26 | High | 0 |
| 2 | 7b | 81.51 | 78.51 | 3 | 2 | 4 | 1.92 | 315.71 | High | 0 |
| 3 | 7c | 81.51 | 78.51 | 3 | 2 | 4 | 2.03 | 360.16 | High | 0 |
| 4 | 7d | 78.73 | 87.74 | 4 | 2 | 5 | 1.93 | 311.29 | High | 0 |
| 5 | 7e | 81.51 | 78.51 | 3 | 2 | 4 | 1.94 | 315.71 | High | 0 |
| 6 | 7f | 78.73 | 87.74 | 4 | 2 | 5 | 2.05 | 311.29 | High | 0 |
| S. No. | Compound | HEP | IMMUNO | MUTAGEN | CYTOTOX | LD50 (mg/Kg) | Toxicity Class |
|---|---|---|---|---|---|---|---|
| 1 | 7a | + | − | − | − | 1000 | IV |
| 2 | 7b | + | − | − | − | 5000 | V |
| 3 | 7c | + | − | − | − | 1000 | IV |
| 4 | 7d | + | − | − | − | 5000 | V |
| 5 | 7e | + | − | − | − | 1500 | IV |
| 6 | 7f | + | − | − | − | 5000 | V |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afzal, O.; Ahsan, M.J. An Efficient Synthesis of 1-(1,3-Dioxoisoindolin-2-yl)-3-aryl Urea Analogs as Anticancer and Antioxidant Agents: An Insight into Experimental and In Silico Studies. Molecules 2024, 29, 67. https://doi.org/10.3390/molecules29010067
Afzal O, Ahsan MJ. An Efficient Synthesis of 1-(1,3-Dioxoisoindolin-2-yl)-3-aryl Urea Analogs as Anticancer and Antioxidant Agents: An Insight into Experimental and In Silico Studies. Molecules. 2024; 29(1):67. https://doi.org/10.3390/molecules29010067
Chicago/Turabian StyleAfzal, Obaid, and Mohamed Jawed Ahsan. 2024. "An Efficient Synthesis of 1-(1,3-Dioxoisoindolin-2-yl)-3-aryl Urea Analogs as Anticancer and Antioxidant Agents: An Insight into Experimental and In Silico Studies" Molecules 29, no. 1: 67. https://doi.org/10.3390/molecules29010067
APA StyleAfzal, O., & Ahsan, M. J. (2024). An Efficient Synthesis of 1-(1,3-Dioxoisoindolin-2-yl)-3-aryl Urea Analogs as Anticancer and Antioxidant Agents: An Insight into Experimental and In Silico Studies. Molecules, 29(1), 67. https://doi.org/10.3390/molecules29010067






