Designed Fabrication of Phloretin-Loaded Propylene Glycol Binary Ethosomes: Stability, Skin Permeability and Antioxidant Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Ethosomes
2.2. Stability Study
2.2.1. Salt Stability
2.2.2. pH Stability
2.2.3. Dilution Multiple Stability
2.2.4. Storage Stability
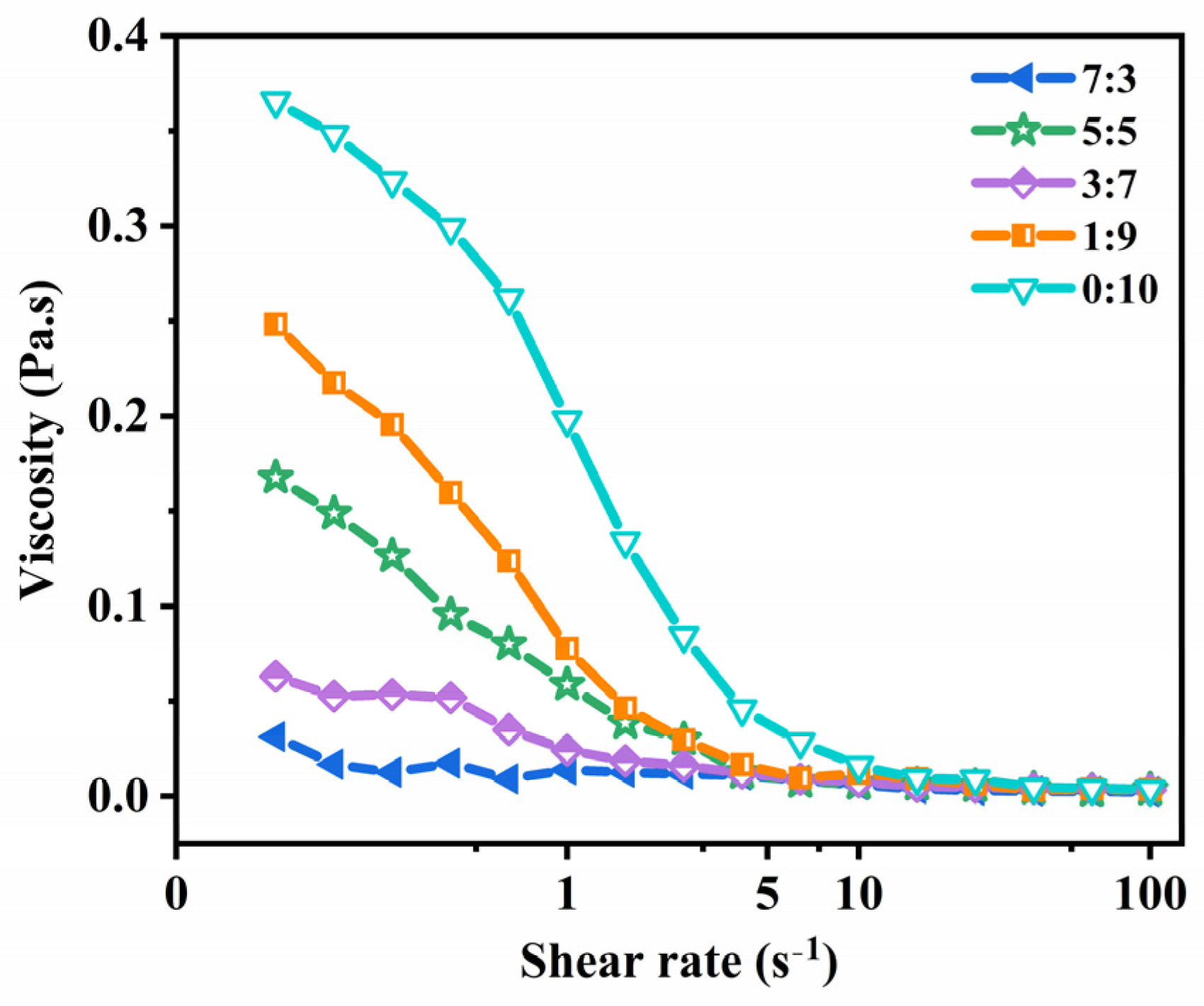
2.3. Rheological Properties Analysis
2.4. Encapsulation Efficiency and Drug Loading Capacity
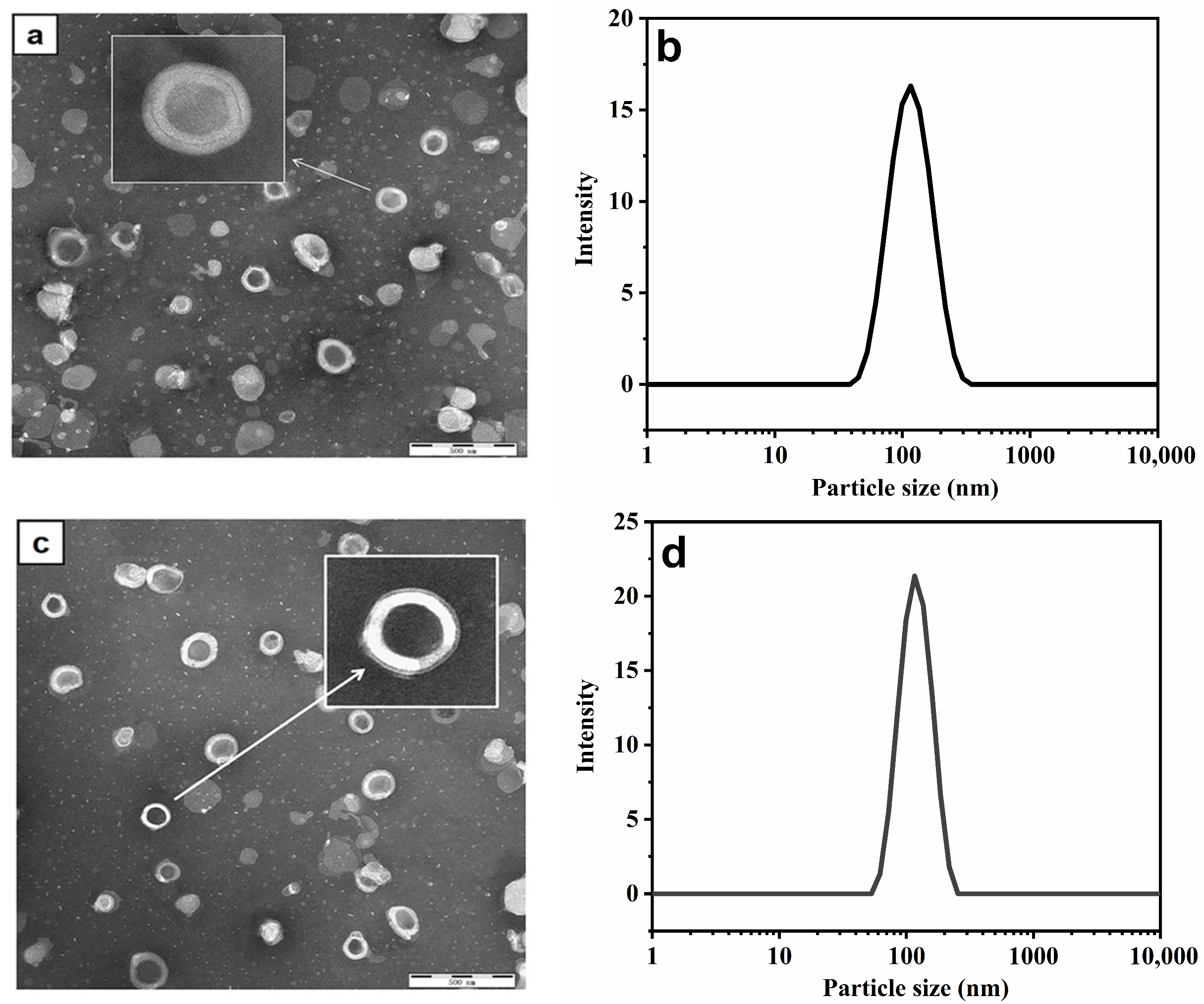
2.5. Transmission Electron Microscopy (TEM) Analysis
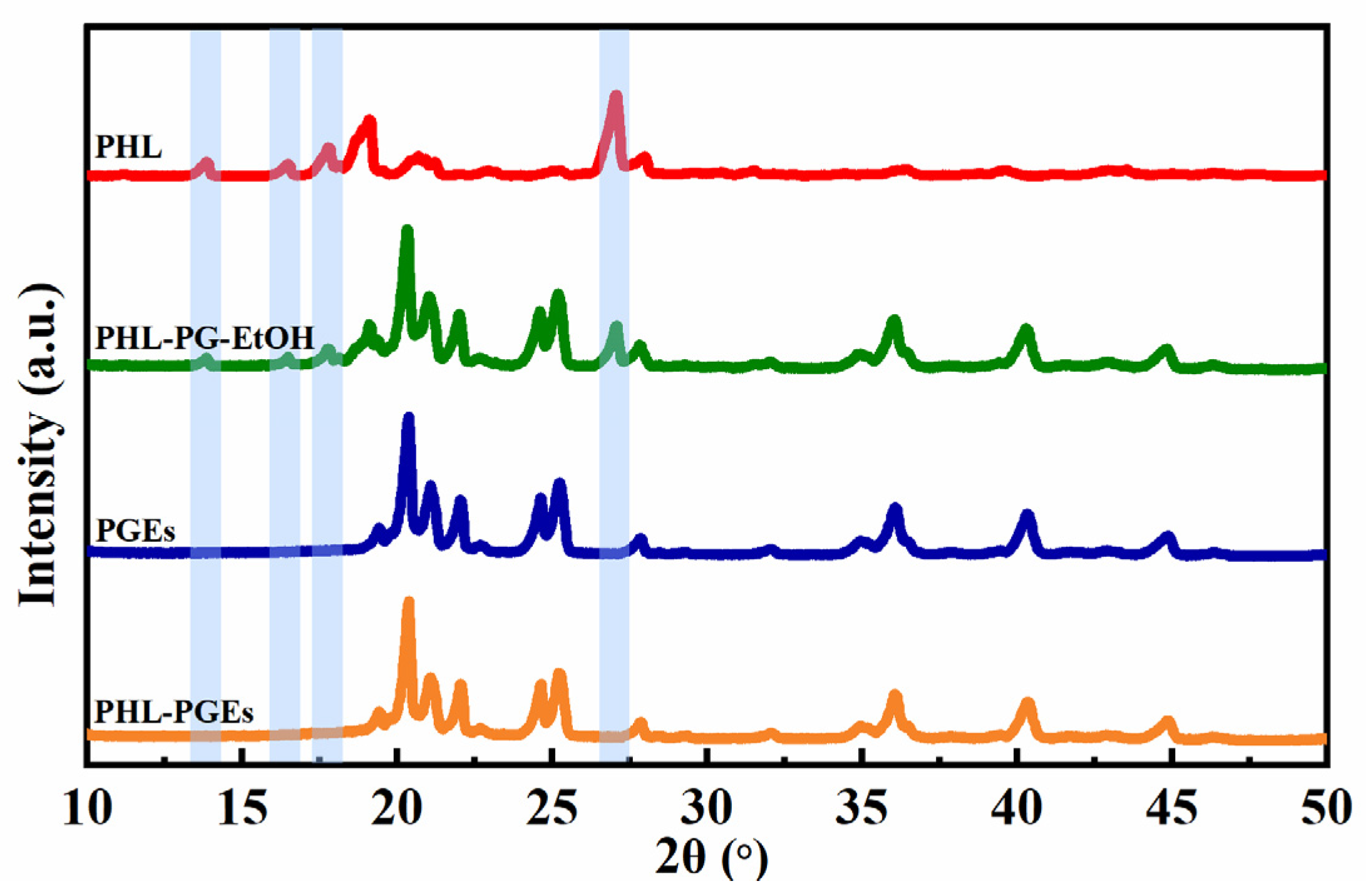
2.6. X-ray Diffractometer (XRD) Analysis
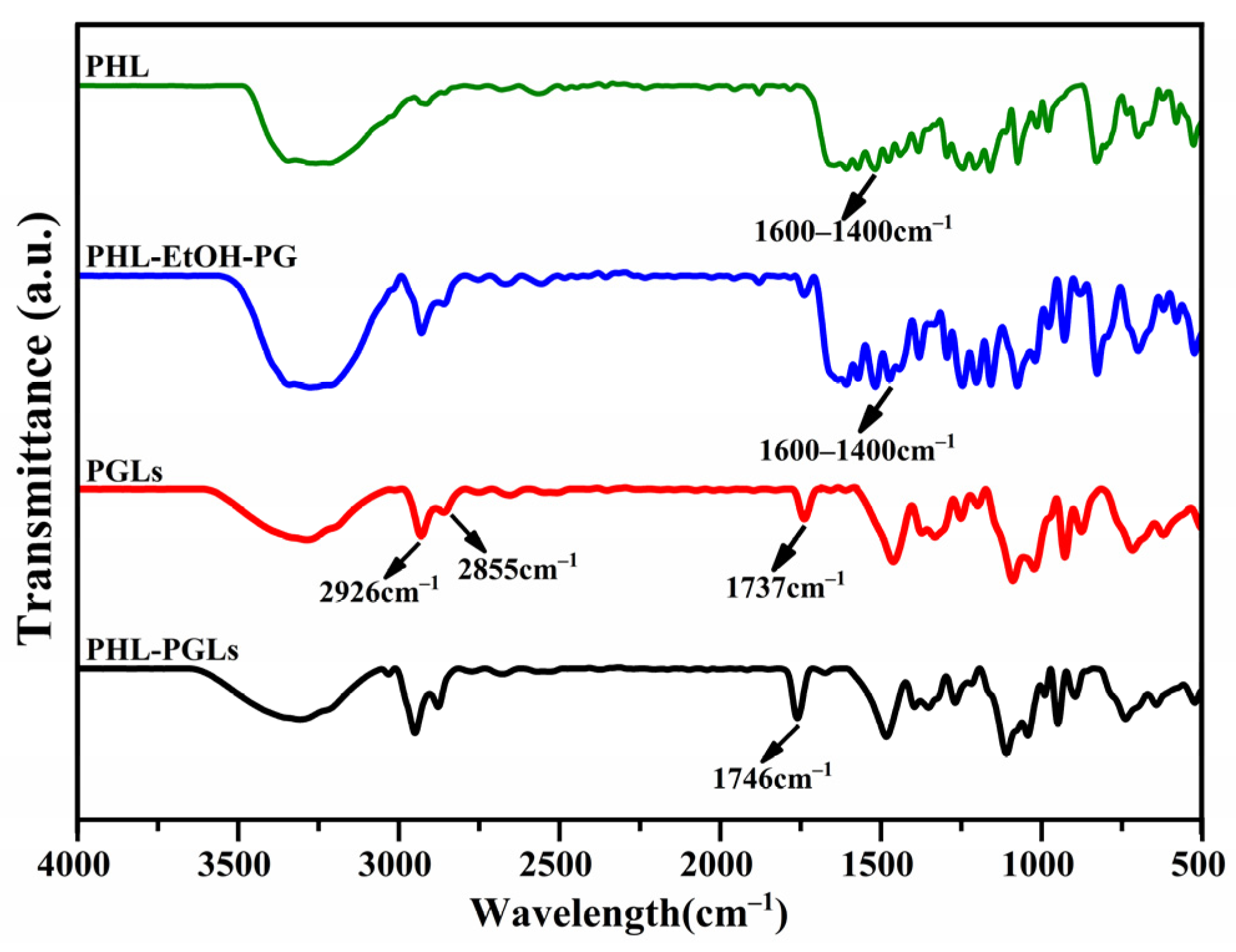
2.7. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
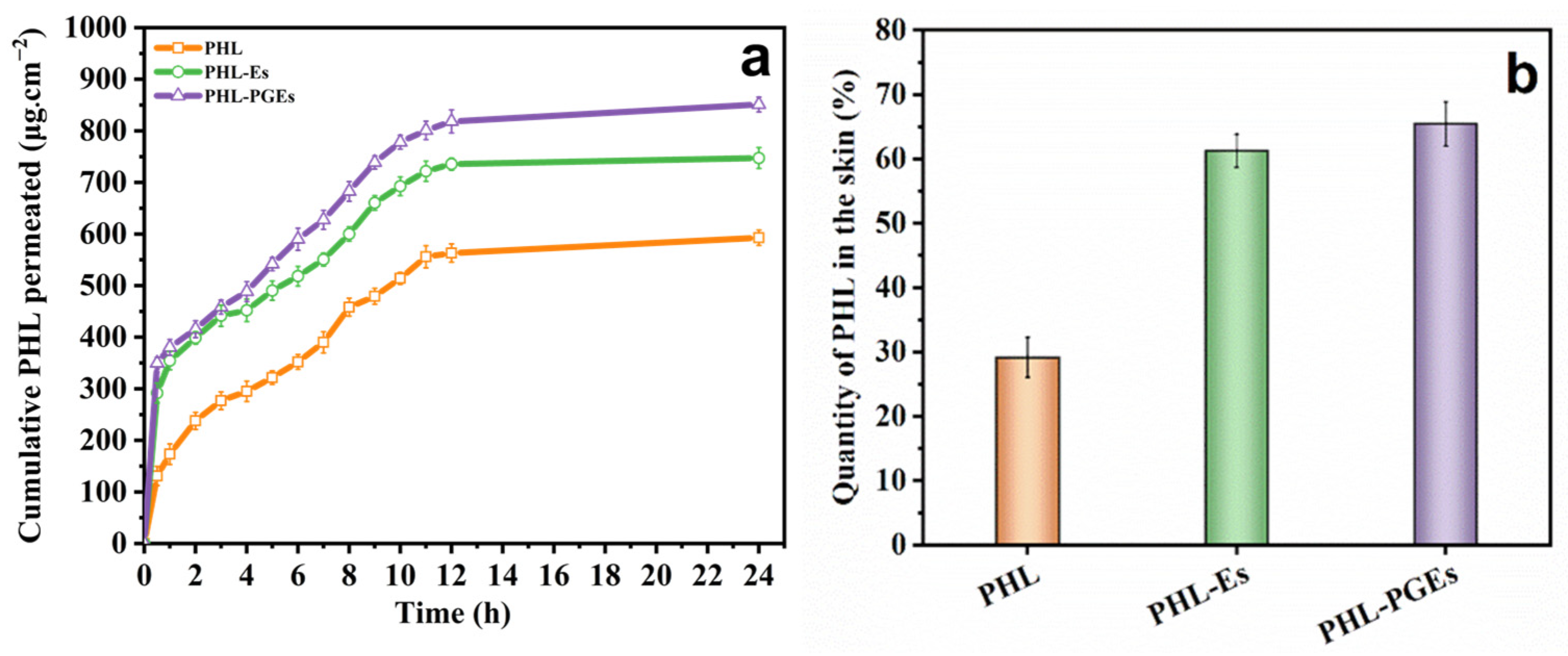
2.8. In Vitro Percutaneous Permeability Test
2.9. In Vitro Antioxidant Activity Study
3. Materials and Methods
3.1. Materials
3.2. Preparation of PGEs
3.3. Characterization of PGEs
3.4. Stability Study
3.4.1. Salt Stability
3.4.2. pH Stability
3.4.3. Dilution Multiples Stability
3.4.4. Storage Stability
3.5. Rheological Properties
3.6. High-Efficiency Liquid Chromatography (HPLC)
3.7. Encapsulation Efficiency and Drug Loading Capacity
3.8. Transmission Electron Microscopy (TEM)
3.9. X-ray Diffractometer (XRD)
3.10. Fourier Transform Infrared (FTIR) Spectroscopy
3.11. In Vitro Percutaneous Permeability Test
3.12. Measurement of Antioxidative Activity
3.12.1. DPPH Radical Scavenging Activity
3.12.2. ABTS Radical Scavenging Activity
3.12.3. Measurement of Ferric Reducing Antioxidant Power
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Apolinário, A.C.; Salata, G.C.; de Souza, M.M.; Chorilli, M.; Lopes, L.B. Rethinking Breast Cancer Chemoprevention: Technological Advantages and Enhanced Performance of a Nanoethosomal-Based Hydrogel for Topical Administration of Fenretinide. AAPS PharmSciTech 2022, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel Vesicular Carriers for Enhanced Delivery: Characterization and Skin Penetration Properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth, S. Rowe Lipid Chain Length and Temperature Dependence of Ethanol-Phosphatidylcholine Interactions. Biochemistry 1983, 22, 3300–3305. [Google Scholar] [CrossRef]
- Adnan, M.; Akhter, M.H.; Afzal, O.; Altamimi, A.S.A.; Ahmad, I.; Alossaimi, M.A.; Jaremko, M.; Emwas, A.-H.; Haider, T.; Haider, M.F. Exploring Nanocarriers as Treatment Modalities for Skin Cancer. Molecules 2023, 28, 5905. [Google Scholar] [CrossRef] [PubMed]
- Limongi, T.; Susa, F.; Marini, M.; Allione, M.; Torre, B.; Pisano, R.; Di Fabrizio, E. Lipid-Based Nanovesicular Drug Delivery Systems. Nanomaterials 2021, 11, 3391. [Google Scholar] [CrossRef]
- Lu, J.; Guo, T.; Fan, Y.; Li, Z.; He, Z.; Yin, S.; Feng, N. Recent Developments in the Principles, Modification and Application Prospects of Functionalized Ethosomes for Topical Delivery. Curr. Drug Deliv. 2021, 18, 570–582. [Google Scholar] [CrossRef]
- Zhigaltsev, I.V.; Maurer, N.; Akhong, Q.F.; Leone, R.; Leng, E.; Wang, J.; Semple, S.C.; Cullis, P.R. Liposome-Encapsulated Vincristine, Vinblastine and Vinorelbine: A Comparative Study of Drug Loading and Retention. J. Control. Release 2005, 104, 103–111. [Google Scholar] [CrossRef]
- Ruan, S.; Zhang, Y.; Feng, N. Microneedle-Mediated Transdermal Nanodelivery Systems: A Review. Biomater. Sci. 2021, 9, 8065–8089. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Silva, A.L.; Guerra, C.; Peixoto, D.; Pereira-Silva, M.; Zeinali, M.; Mascarenhas-Melo, F.; Castro, R.; Veiga, F. Ethosomes as Nanocarriers for the Development of Skin Delivery Formulations. Pharm. Res. 2021, 38, 947–970. [Google Scholar] [CrossRef]
- Sguizzato, M.; Ferrara, F.; Hallan, S.S.; Baldisserotto, A.; Drechsler, M.; Malatesta, M.; Costanzo, M.; Cortesi, R.; Puglia, C.; Valacchi, G.; et al. Ethosomes and Transethosomes for Mangiferin Transdermal Delivery. Antioxidants 2021, 10, 768. [Google Scholar] [CrossRef]
- Ma, H.; Guo, D.; Fan, Y.; Wang, J.; Cheng, J.; Zhang, X. Paeonol-Loaded Ethosomes as Transdermal Delivery Carriers: Design, Preparation and Evaluation. Molecules 2018, 23, 1756. [Google Scholar] [CrossRef] [PubMed]
- Soleymani, J.; Jouyban-Gharamaleki, V.; Suleymanov, T.A.; Jouyban-Gharamaleki, K.; Jouyban, A. Solubilization of Lamotrigine Using Tween 80 and Ethylene Glycol or Propylene Glycol. J. Mol. Liq. 2017, 236, 249–253. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid Vesicles for Skin Delivery of Drugs: Reviewing Three Decades of Research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Mura, S.; Sinico, C.; Fadda, A.M.; Vila, A.O.; Molina, F. Development and Characterization of Liposomes Containing Glycols as Carriers for Diclofenac. Colloids Surfaces A Physicochem. Eng. Asp. 2009, 342, 53–58. [Google Scholar] [CrossRef]
- Aljohani, A.A.; Alanazi, M.A.; Munahhi, L.A.; Hamroon, J.D.; Mortagi, Y.; Qushawy, M.; Soliman, G.M. Binary Ethosomes for the Enhanced Topical Delivery and Antifungal Efficacy of Ketoconazole. OpenNano 2023, 11, 100145. [Google Scholar] [CrossRef]
- Li, W.-Z.; Hao, X.-L.; Zhao, N.; Han, W.-X.; Zhai, X.-F.; Zhao, Q.; Wang, Y.-E.; Zhou, Y.-Q.; Cheng, Y.-C.; Yue, Y.-H.; et al. Propylene Glycol-Embodying Deformable Liposomes as a Novel Drug Delivery Carrier for Vaginal Fibrauretine Delivery Applications. J. Control. Release 2016, 226, 107–114. [Google Scholar] [CrossRef]
- Manconi, M.; Petretto, G.; D’hallewin, G.; Escribano, E.; Milia, E.; Pinna, R.; Palmieri, A.; Firoznezhad, M.; Peris, J.E.; Usach, I.; et al. Thymus Essential Oil Extraction, Characterization and Incorporation in Phospholipid Vesicles for the Antioxidant/Antibacterial Treatment of Oral Cavity Diseases. Colloids Surfaces B Biointerfaces 2018, 171, 115–122. [Google Scholar] [CrossRef]
- Dickey, A.N.; Faller, R. How Alcohol Chain-Length and Concentration Modulate Hydrogen Bond Formation in a Lipid Bilayer. Biophys. J. 2007, 92, 2366–2376. [Google Scholar] [CrossRef]
- Wang, H.; Shao, Q.; Zhang, Y.; Ding, J.; Yang, M.; Yang, L.; Wang, W.; Cui, P.; Dai, Z.; Ma, L. Preparation and Evaluation of Liposomes Containing Ethanol and Propylene Glycol as Carriers for Nicotine. Curr. Drug Deliv. 2023, 21, 249–260. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N. Development of Stable Tocopherol Succinate-loaded Ethosomes to Enhance Transdermal Permeation: In Vitro and in Vivo Characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef]
- Ferrara, F.; Benedusi, M.; Sguizzato, M.; Cortesi, R.; Baldisserotto, A.; Buzzi, R.; Valacchi, G.; Esposito, E. Ethosomes and Transethosomes as Cutaneous Delivery Systems for Quercetin: A Preliminary Study on Melanoma Cells. Pharmaceutics 2022, 14, 1038. [Google Scholar] [CrossRef] [PubMed]
- Kusumawati, I.; Kurniawan, K.O.; Rohmania, R.; Pratama, B.A.; Pratama, Y.A.; Rullyansyah, S.; Warsito, M.F.; Widyowati, R.; Hestianah, E.P.; Matsunami, K. Comparative Study of Liposomal and Ethosomal Formulations of Curcuma Heyneana Rhizome Extract in a Transdermal Delivery System. Pharm. Nanotechnol. 2023, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Anunciato Casarini, T.P.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S. Dermatological Applications of the Flavonoid Phloretin. Eur. J. Pharmacol. 2020, 889, 173593. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska-Pawlega, B.; Ignacy Gruszecki, W.; Misiak, L.; Paduch, R.; Piersiak, T.; Zarzyka, B.; Pawelec, J.; Gawron, A. Modification of Membranes by Quercetin, a Naturally Occurring Flavonoid, via Its Incorporation in the Polar Head Group. Biochim. Biophys. Acta Biomembr. 2007, 1768, 2195–2204. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.D.; Shah, D.S.; Gurram, S.; Jha, D.K.; Batabyal, P.; Amin, P.D.; Sathaye, S. Formulation, Characterization, Pharmacokinetics and Antioxidant Activity of Phloretin Oral Granules. Int. J. Pharm. 2023, 645, 123386. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kum, H.; Ryu, D.; Kim, M.; Jung, E.; Park, D. Protective Effects of a New Phloretin Derivative against UVB-Induced Damage in Skin Cell Model and Human Volunteers. Int. J. Mol. Sci. 2014, 15, 18919–18940. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.-C.; Zhu, J.-J.; You, X.-M.; Yang, X.-Q.; Yin, S.-W. Biocompatible Gliadin-Sericin Complex Colloidal Particles Used for Topical Delivery of the Antioxidant Phloretin. Colloids Surfaces B Biointerfaces 2023, 225, 113244. [Google Scholar] [CrossRef]
- Li, J.; Chang, C.; Chen, W.; Su, Y.; Gu, L.; Yang, Y.; Zhai, J. Hybrid Liposomes Composed of Hydrophilic Emulsifiers and Lecithin: Physicochemical, Interaction and Curcumin Loading Properties. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 655, 130210. [Google Scholar] [CrossRef]
- Elmoslemany, R.M.; Abdallah, O.Y.; El-Khordagui, L.K.; Khalafallah, N.M. Propylene Glycol Liposomes as a Topical Delivery System for Miconazole Nitrate: Comparison with Conventional Liposomes. AAPS PharmSciTech 2012, 13, 723–731. [Google Scholar] [CrossRef]
- Jafari, A.; Daneshamouz, S.; Ghasemiyeh, P.; Mohammadi-Samani, S. Ethosomes as Dermal/Transdermal Drug Delivery Systems: Applications, Preparation and Characterization. J. Liposome Res. 2023, 33, 34–52. [Google Scholar] [CrossRef]
- Hajare, A.A.; Dol, H.S. Screening of Effective Formulation Techniques for Designing and Fabrication of Terbinafine Hydrochloride Ethosomes. Res. J. Pharm. Technol. 2021, 14, 1353–1359. [Google Scholar] [CrossRef]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming Skin Barriers through Advanced Transdermal Drug Delivery Approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, O.G. Lipids, Curvature, and Nano-medicine. Eur. J. Lipid Sci. Technol. 2011, 113, 1174–1187. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Xie, J.; Feng, X.; Zhang, X.; Ren, Z.; Zheng, Y.; Yang, J. Preparation and Evaluation of a Rubropunctatin-Loaded Liposome Anticancer Drug Carrier. RSC Adv. 2020, 10, 10352–10360. [Google Scholar] [CrossRef] [PubMed]
- Hassane Hamadou, A.; Zhang, J.; Chao, C.; Xu, B. Stability of Rutin Using Pectin-Chitosan Dual Coating Nanoliposomes. LWT 2022, 170, 114084. [Google Scholar] [CrossRef]
- Wang, X.; Swing, C.J.; Feng, T.; Xia, S.; Yu, J.; Zhang, X. Effects of Environmental PH and Ionic Strength on the Physical Stability of Cinnamaldehyde-Loaded Liposomes. J. Dispers. Sci. Technol. 2020, 41, 1568–1575. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, S.; Liu, Y.; Luo, X.; Di, D.; Song, Y.; Liu, X.; Deng, Y. Polysialic Acid and Pluronic F127 Mixed Polymeric Micelles of Docetaxel as New Approach for Enhanced Antitumor Efficacy. Drug Dev. Ind. Pharm. 2017, 43, 1827–1835. [Google Scholar] [CrossRef]
- Huang, S.; Wang, X.; Liu, M.; Lin, Z.; Gu, W.; Zhao, H.; Zhang, Y.; Ding, B.; Liu, J.; Wu, X.; et al. Modification of Sodium Aescinate into a Safer, More Stable and Effective Water-Soluble Drug by Liposome-Encapsulation: An in Vitro and in Vivo Study. Drug Deliv. 2022, 29, 1132–1141. [Google Scholar] [CrossRef]
- Sakdiset, P.; Amnuaikit, T.; Pichayakorn, W.; Pinsuwan, S. Formulation Development of Ethosomes Containing Indomethacin for Transdermal Delivery. J. Drug Deliv. Sci. Technol. 2019, 52, 760–768. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Singhsa, P.; Suksiriworapong, J.; Chantasart, D. Physicochemical Properties and Skin Permeation of Span 60/Tween 60 Niosomes of Ellagic Acid. Int. J. Pharm. 2012, 423, 303–311. [Google Scholar] [CrossRef]
- Ghiasi, F.; Eskandari, M.H.; Golmakani, M.T.; Rubio, R.G.; Ortega, F. Build-Up of a 3D Organogel Network within the Bilayer Shell of Nanoliposomes. A Novel Delivery System for Vitamin D3: Preparation, Characterization, and Physicochemical Stability. J. Agric. Food Chem. 2021, 69, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Pilch, E.; Musiał, W. Liposomes with an Ethanol Fraction as an Application for Drug Delivery. Int. J. Mol. Sci. 2018, 19, 3806. [Google Scholar] [CrossRef] [PubMed]
- Vecher, O.V.; Diskaeva, E.I.; Bazikov, I.A.; Elbekyan, K.S.; Diskaeva, E.N. Study of Some Rheological Properties of Niosomal Dispersions of Various Concentrations Based on PEG-12 Dimethicone. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045007. [Google Scholar] [CrossRef]
- Hasan, M.; Ben Messaoud, G.; Michaux, F.; Tamayol, A.; Kahn, C.J.F.; Belhaj, N.; Linder, M.; Arab-Tehrany, E. Chitosan-Coated Liposomes Encapsulating Curcumin: Study of Lipid-Polysaccharide Interactions and Nanovesicle Behavior. RSC Adv. 2016, 6, 45290–45304. [Google Scholar] [CrossRef]
- Ali, S.; Davinelli, S.; Mencucci, R.; Fusi, F.; Scuderi, G.; Costagliola, C.; Scapagnini, G. Crosslinked Hyaluronic Acid with Liposomes and Crocin Confers Cytoprotection in an Experimental Model of Dry Eye. Molecules 2021, 26, 849. [Google Scholar] [CrossRef]
- Sun, Y.; Tang, W.; Pu, C.; Li, R.; Sun, Q.; Wang, H. Improved Stability of Liposome-Stabilized Emulsions as a Co-Encapsulation Delivery System for Vitamin B2, Vitamin E and β-Carotene. Food Funct. 2022, 13, 2966–2984. [Google Scholar] [CrossRef] [PubMed]
- Manconi, M.; Caddeo, C.; Nacher, A.; Diez-Sales, O.; Peris, J.E.; Ferrer, E.E.; Fadda, A.M.; Manca, M.L. Eco-Scalable Baicalin Loaded Vesicles Developed by Combining Phospholipid with Ethanol, Glycerol, and Propylene Glycol to Enhance Skin Permeation and Protection. Colloids Surfaces B Biointerfaces 2019, 184, 110504. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, T.; Zhang, L.; Cheng, X.; Wang, C. The Transdermal Performance, Pharmacokinetics, and Anti-Inflammatory Pharmacodynamics Evaluation of Harmine-Loaded Ethosomes. Drug Dev. Ind. Pharm. 2020, 46, 101–108. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured Lipid Carrier-Embedded Polyacrylic Acid Transdermal Patches for Improved Transdermal Delivery of Capsaicin. Eur. J. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef]
- Song, F.; Yang, G.; Wang, Y.; Tian, S. Effect of Phospholipids on Membrane Characteristics and Storage Stability of Liposomes. Innov. Food Sci. Emerg. Technol. 2022, 81, 103155. [Google Scholar] [CrossRef]
- Li, X.M.; Zhu, J.; Pan, Y.; Meng, R.; Zhang, B.; Chen, H.Q. Fabrication and Characterization of Pickering Emulsions Stabilized by Octenyl Succinic Anhydride -Modified Gliadin Nanoparticle. Food Hydrocoll. 2019, 90, 19–27. [Google Scholar] [CrossRef]
- Ding, L.; Yang, J.; Yin, K.; Cheng, H.; Li, J.; Xue, C. The Spatial Arrangement of Astaxanthin in Bilayers Greatly Influenced the Structural Stability of DPPC Liposomes. Colloids Surfaces B Biointerfaces 2022, 212, 112383. [Google Scholar] [CrossRef] [PubMed]
- Forutan, M.; Hasani, M.; Hasani, S.; Salehi, N.; Sabbagh, F. Liposome System for Encapsulation of Spirulina Platensis Protein Hydrolysates: Controlled-Release in Simulated Gastrointestinal Conditions, Structural and Functional Properties. Materials 2022, 15, 8581. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.J.; Park, H.J.; Kang, M.J. Neutral Oil-Incorporated Liposomal Nanocarrier for Increased Skin Delivery of Ascorbic Acid. Materials 2023, 16, 2294. [Google Scholar] [CrossRef] [PubMed]
- Nainwal, N.; Jawla, S.; Singh, R.; Saharan, V.A. Transdermal Applications of Ethosomes—A Detailed Review. J. Liposome Res. 2019, 29, 103–113. [Google Scholar] [CrossRef]
- Cassano, R.; Curcio, F.; Sole, R.; Trombino, S. Transdermal Delivery of Phloretin by Gallic Acid Microparticles. Gels 2023, 9, 226. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, Y.; Zhai, B.; Cheng, J.; Sun, J.; Zhang, X.; Guo, D. Phloretin Transfersomes for Transdermal Delivery: Design, Optimization, and In Vivo Evaluation. Molecules 2023, 28, 6790. [Google Scholar] [CrossRef]
- Moolakkadath, T.; Aqil, M.; Ahad, A.; Imam, S.S.; Praveen, A.; Sultana, Y.; Mujeeb, M.; Iqbal, Z. Fisetin Loaded Binary Ethosomes for Management of Skin Cancer by Dermal Application on UV Exposed Mice. Int. J. Pharm. 2019, 560, 78–91. [Google Scholar] [CrossRef]
- Abouhussein, D.M.N. Enhanced Transdermal Permeation of BCS Class IV Aprepitant Using Binary Ethosome: Optimization, Characterization and Ex Vivo Permeation. J. Drug Deliv. Sci. Technol. 2021, 61, 102185. [Google Scholar] [CrossRef]
- Kubiliene, A.; Munius, E.; Songailaite, G.; Kokyte, I.; Baranauskaite, J.; Liekis, A.; Sadauskiene, I. A Comparative Evaluation of Antioxidant Activity of Extract and Essential Oil of Origanum Onites L. In Vivo. Molecules 2023, 28, 5302. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A.; Panyachariwat, N.; Chaiwut, P. In Vitro and In Vivo Anti-Aging Effect of Coffee Berry Nanoliposomes. Molecules 2023, 28, 6830. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, H.; Wu, Z.; Al-Kassas, R.; Alany, R.G. Niosomes and Discomes for Ocular Delivery of Naltrexone Hydrochloride: Morphological, Rheological, Spreading Properties and Photo-Protective Effects. Int. J. Pharm. 2012, 433, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wang, M.; Chen, F.; Gong, T.; Jian, Y.; Zhang, Z.; Huang, Y. Lung-Targeting Delivery of Dexamethasone Acetate Loaded Solid Lipid Nanoparticles. Arch. Pharm. Res. 2007, 30, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fang, W.; Liu, W.; Liu, J.; Gong, P. Microcapsules and Nanoliposomes Based Strategies to Improve the Stability of Blueberry Anthocyanins. Molecules 2023, 28, 7344. [Google Scholar] [CrossRef] [PubMed]
- Palchoudhury, S.; Das, P.; Ghasemi, A.; Tareq, S.M.; Sengupta, S.; Han, J.; Maglosky, S.; Almanea, F.; Jones, M.; Cox, C.; et al. A Novel Experimental Approach to Understand the Transport of Nanodrugs. Materials 2023, 16, 5485. [Google Scholar] [CrossRef] [PubMed]
- Tiţa, B.; Fuliaş, A.; Bandur, G.; Marian, E.; Tiţa, D. Compatibility Study between Ketoprofen and Pharmaceutical Excipients Used in Solid Dosage Forms. J. Pharm. Biomed. Anal. 2011, 56, 221–227. [Google Scholar] [CrossRef]
- Kumar, B.; Sahoo, P.K. Augmented Transdermal Delivery of Curcumin for the Effective Management of Plaque Psoriasis—Design, Formulation, Characterisation, and In Vivo Studies. AAPS PharmSciTech 2023, 24, 1–14. [Google Scholar] [CrossRef]
- Jo, Y.J.; Cho, H.S.; Chun, J.Y. Antioxidant Activity of β-Cyclodextrin Inclusion Complexes Containing Trans-Cinnamaldehyde by DPPH, ABTS and FRAP. Food Sci. Biotechnol. 2021, 30, 807–814. [Google Scholar] [CrossRef]
- Müller, L.; Fröhlich, K.; Böhm, V. Comparative Antioxidant Activities of Carotenoids Measured by Ferric Reducing Antioxidant Power (FRAP), ABTS Bleaching Assay (ATEAC), DPPH Assay and Peroxyl Radical Scavenging Assay. Food Chem. 2011, 129, 139–148. [Google Scholar] [CrossRef]
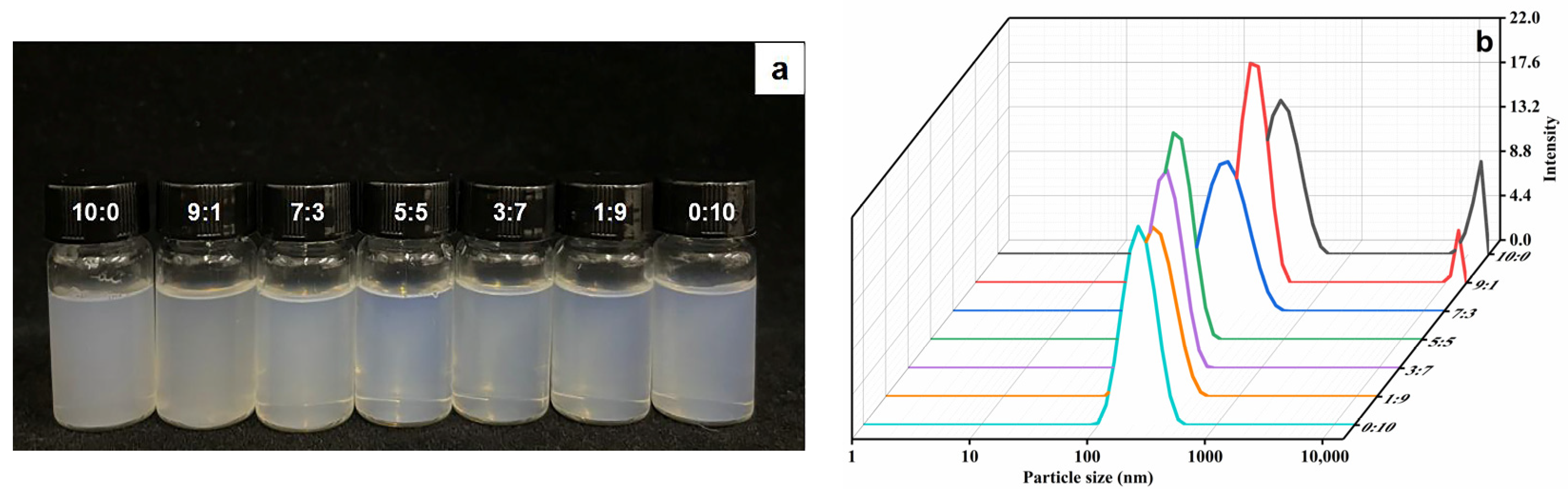
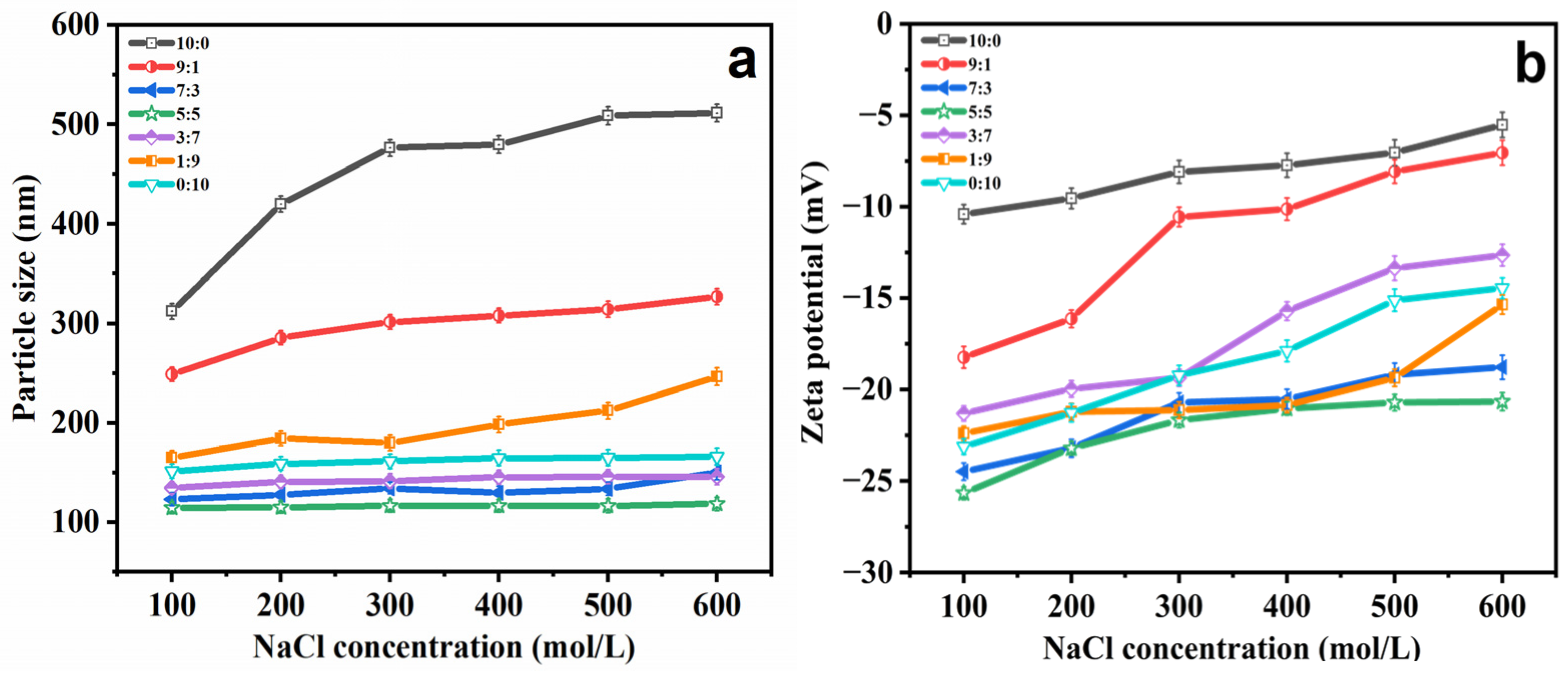
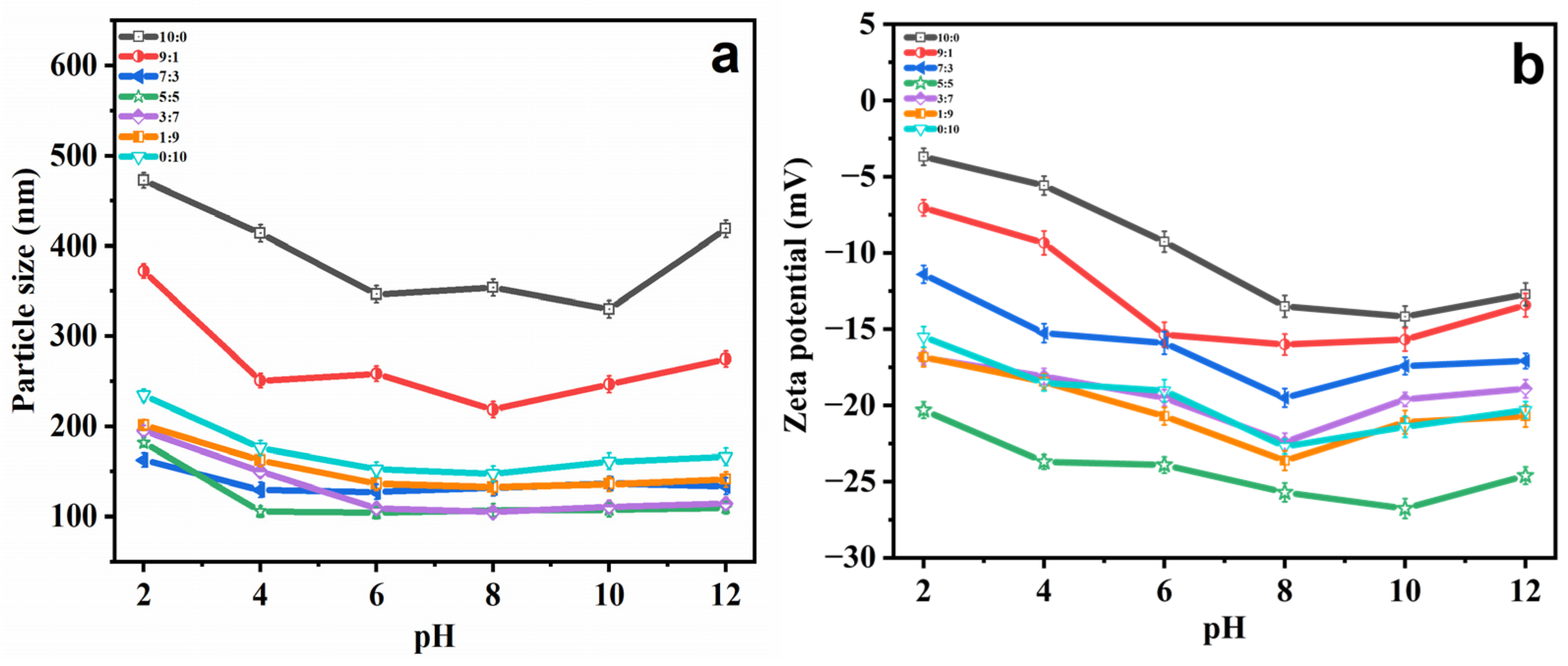
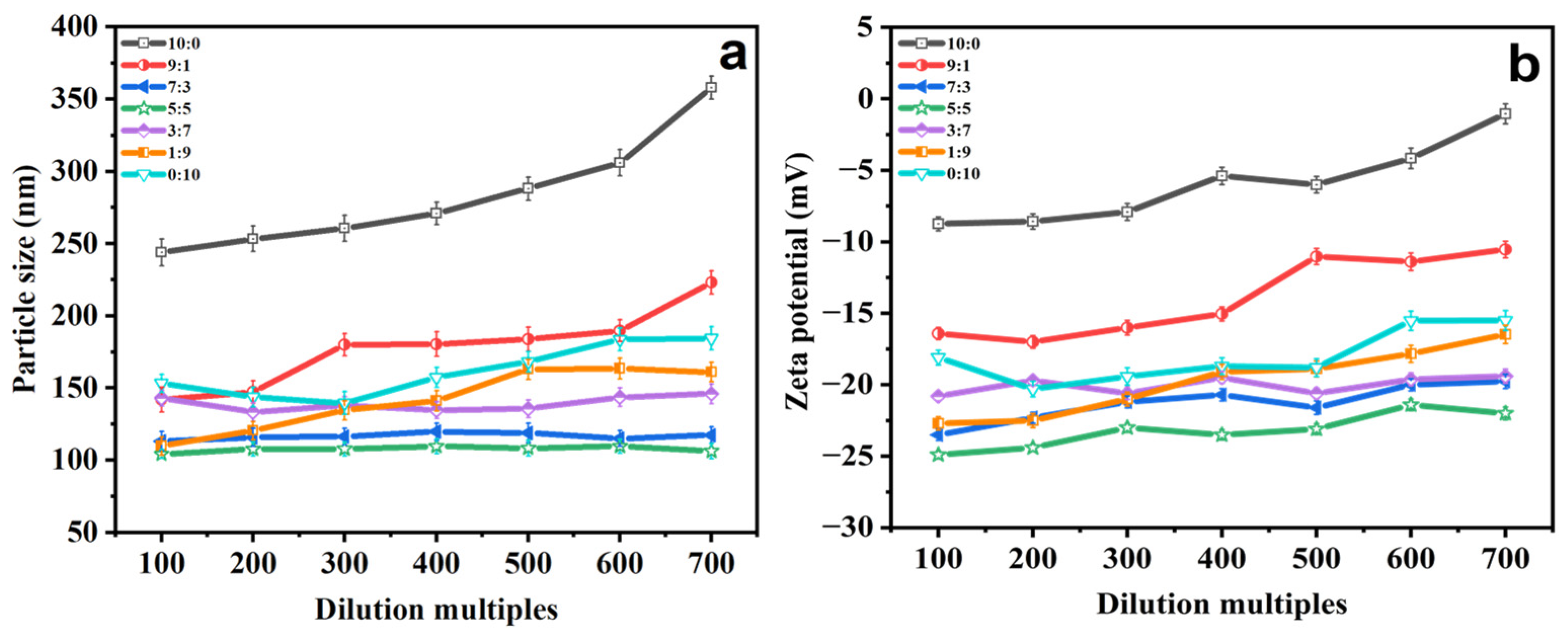



| Ethosomes Formulations | Particle Size (nm) | PDI | Zeta Potential (mV) |
|---|---|---|---|
| 10:0 | 345.25 ± 8.25 | 0.443 ± 0.008 | −9.83 ± 0.67 |
| 9:1 | 273.32 ± 6.43 | 0.318 ± 0.009 | −15.8 ± 0.58 |
| 7:3 | 193.78 ± 4.74 | 0.201 ± 0.004 | −23.4 ± 0.32 |
| 5:5 | 119.27 ± 4.50 | 0.161 ± 0.002 | −25.7 ± 0.41 |
| 3:7 | 153.26 ± 5.67 | 0.064 ± 0.003 | −22.3 ± 0.52 |
| 1:9 | 184.98 ± 6.31 | 0.072 ± 0.005 | −18.4 ± 0.49 |
| 0:10 | 212.54 ± 6.19 | 0.075 ± 0.004 | −17.4 ± 0.68 |
| PHL Concentration (mg/mL) | Particle Size (nm) | PDI | EE% | DL% |
|---|---|---|---|---|
| 0.50 | 114.87 ± 4.65 | 0.125 ± 0.003 | 79.03 ± 2.13 | 3.16 ± 0.03 |
| 0.75 | 117.00 ± 3.75 | 0.129 ± 0.002 | 82.37 ± 1.94 | 3.75 ± 0.02 |
| 1.00 | 122.94 ± 4.34 | 0.154 ± 0.004 | 89.42 ± 2.42 | 4.21 ± 0.04 |
| 1.25 | 129.73 ± 3.98 | 0.158 ± 0.007 | 89.12 ± 2.17 | 4.16 ± 0.05 |
| 1.5 | 127.87 ± 4.12 | 0.156 ± 0.006 | 89.23 ± 2.25 | 4.19 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.; Zhuang, X.; Li, S.; Wang, Y.; Zhang, X.; Li, J.; Wu, D. Designed Fabrication of Phloretin-Loaded Propylene Glycol Binary Ethosomes: Stability, Skin Permeability and Antioxidant Activity. Molecules 2024, 29, 66. https://doi.org/10.3390/molecules29010066
Zhang M, Zhuang X, Li S, Wang Y, Zhang X, Li J, Wu D. Designed Fabrication of Phloretin-Loaded Propylene Glycol Binary Ethosomes: Stability, Skin Permeability and Antioxidant Activity. Molecules. 2024; 29(1):66. https://doi.org/10.3390/molecules29010066
Chicago/Turabian StyleZhang, Meng, Xue Zhuang, Siqi Li, Yansong Wang, Xiangyu Zhang, Jinlian Li, and Dongmei Wu. 2024. "Designed Fabrication of Phloretin-Loaded Propylene Glycol Binary Ethosomes: Stability, Skin Permeability and Antioxidant Activity" Molecules 29, no. 1: 66. https://doi.org/10.3390/molecules29010066
APA StyleZhang, M., Zhuang, X., Li, S., Wang, Y., Zhang, X., Li, J., & Wu, D. (2024). Designed Fabrication of Phloretin-Loaded Propylene Glycol Binary Ethosomes: Stability, Skin Permeability and Antioxidant Activity. Molecules, 29(1), 66. https://doi.org/10.3390/molecules29010066





