Cobalt Encapsulated in Nitrogen-Doped Graphite-like Shells as Efficient Catalyst for Selective Oxidation of Arylalkanes
Abstract
:1. Introduction
2. Results and Discussion
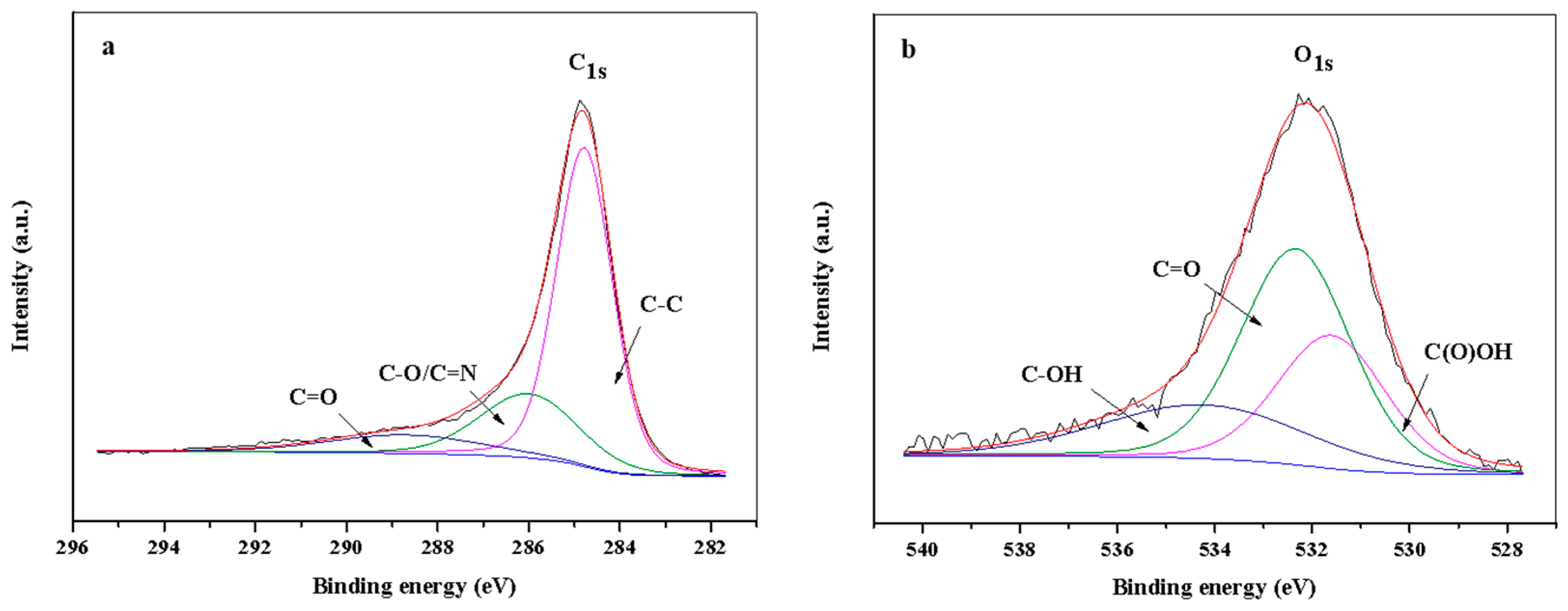
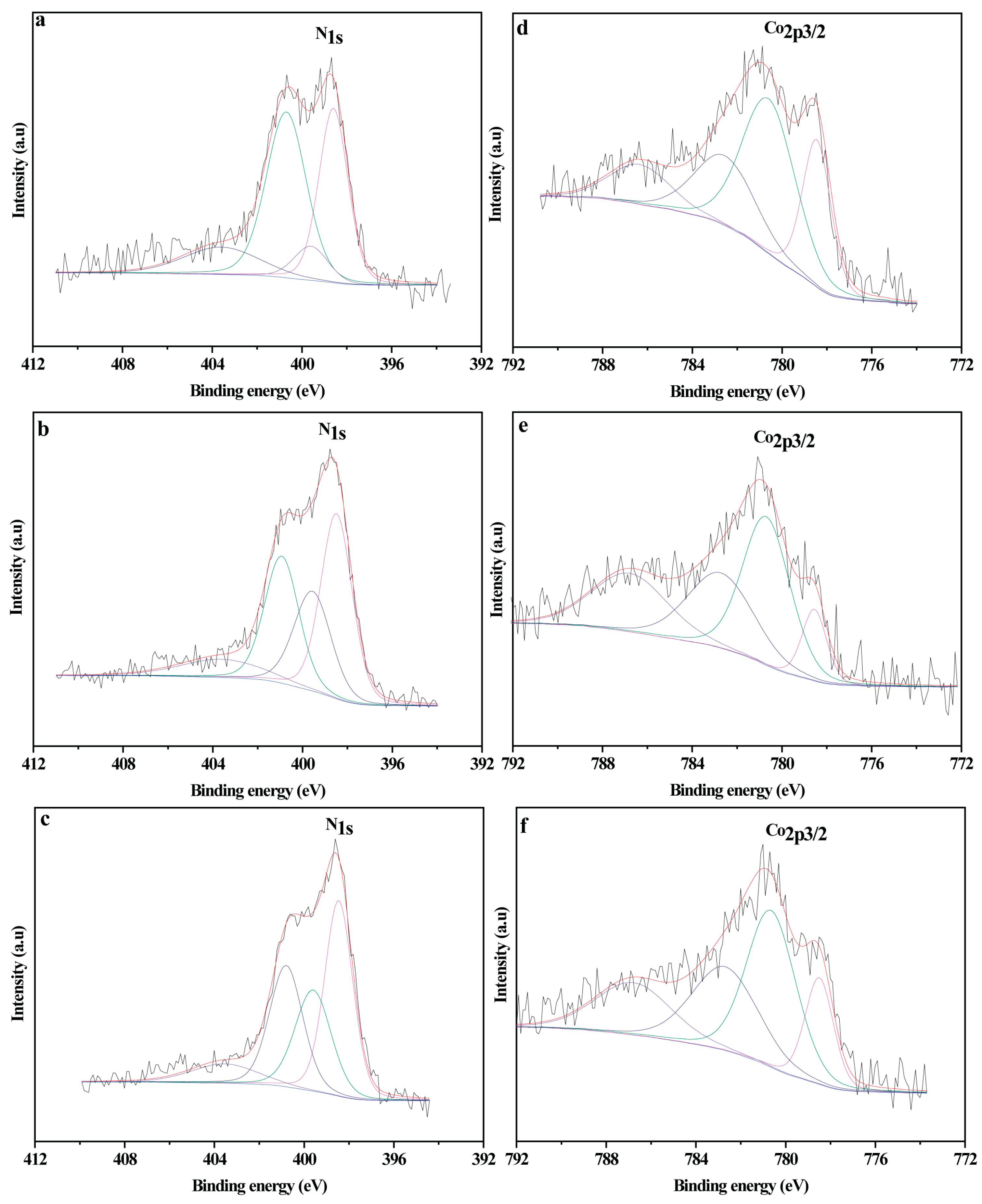
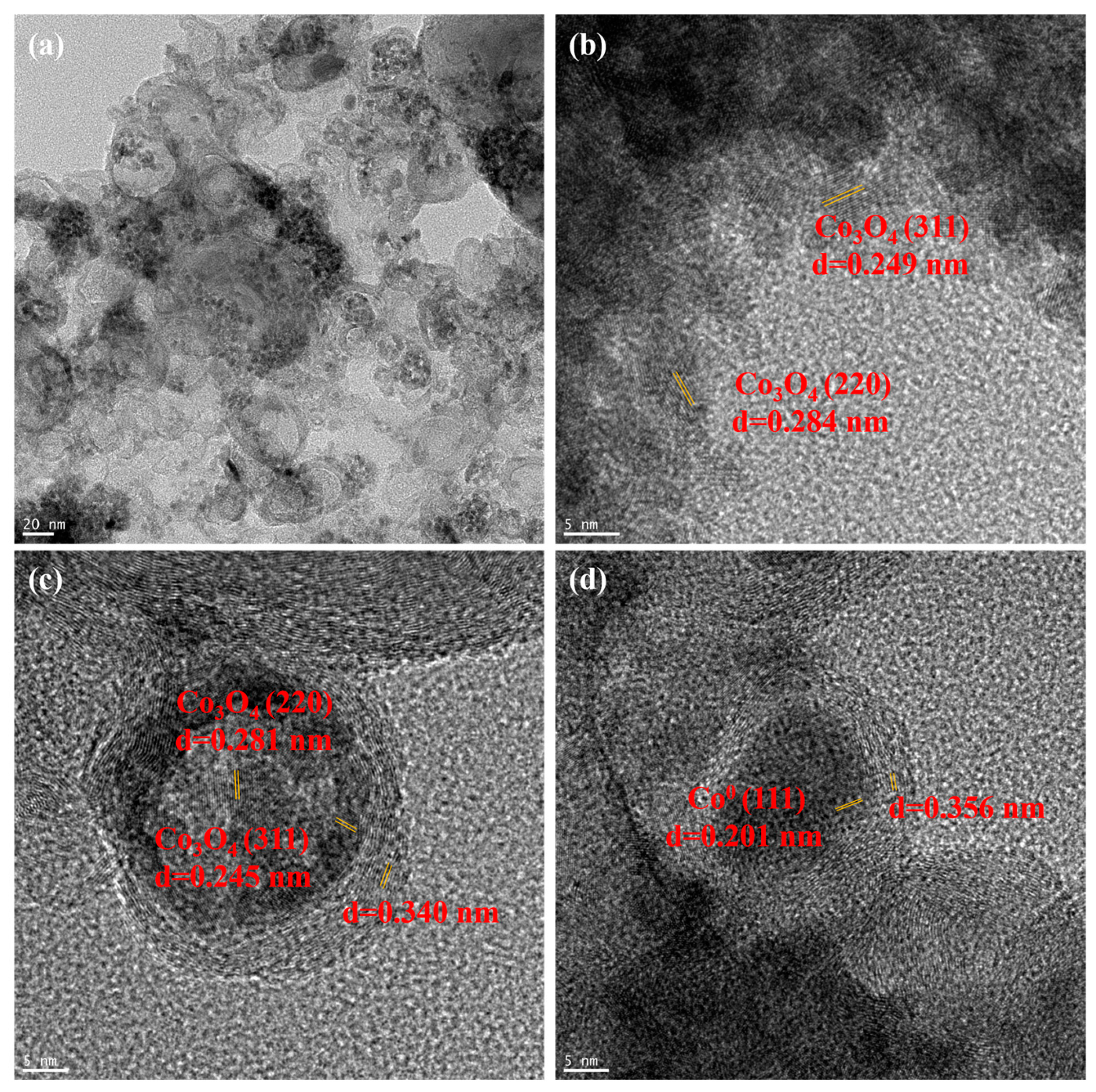
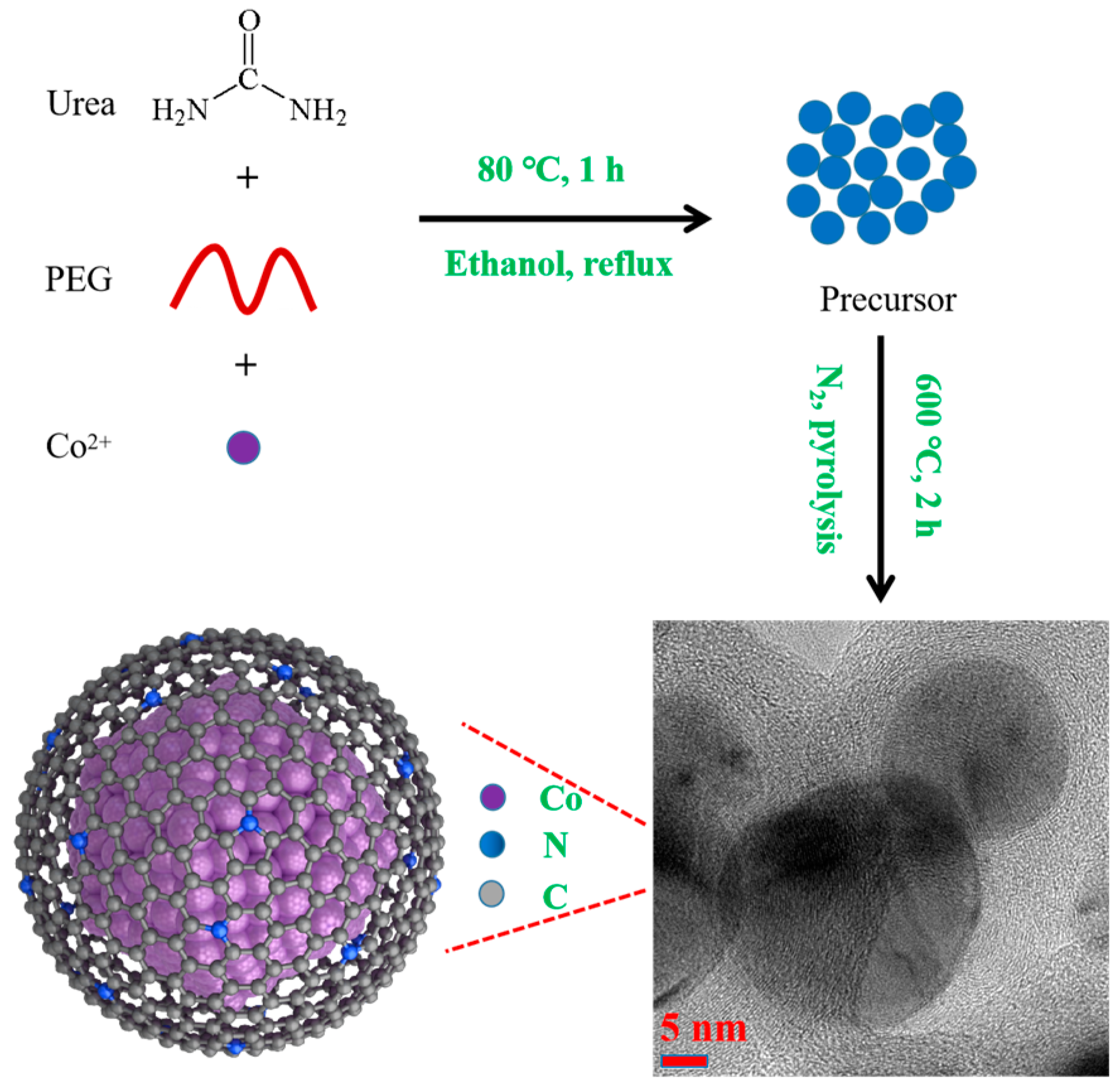
2.1. Catalyst Characterization
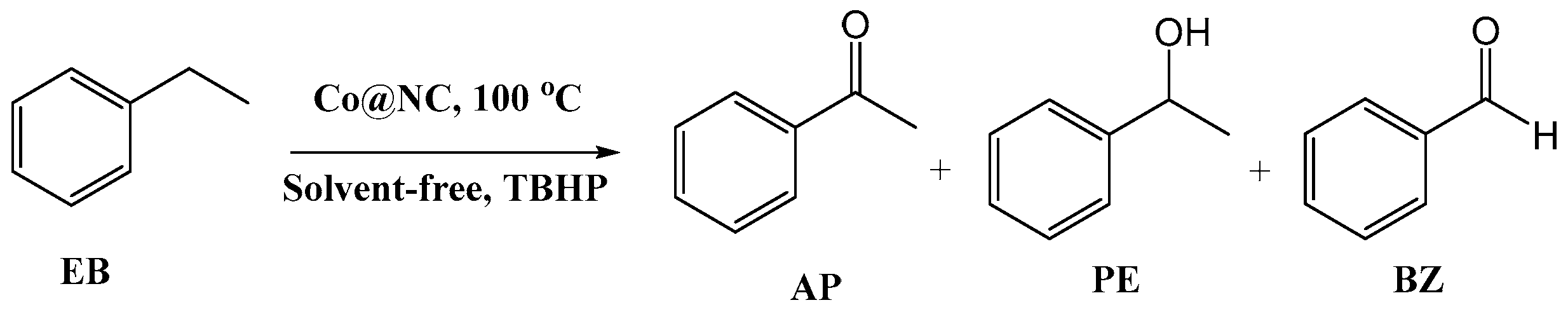
2.2. Selective Oxidation Performance
2.3. Oxidation of Other Arylalkanes
2.4. Kinetic Study
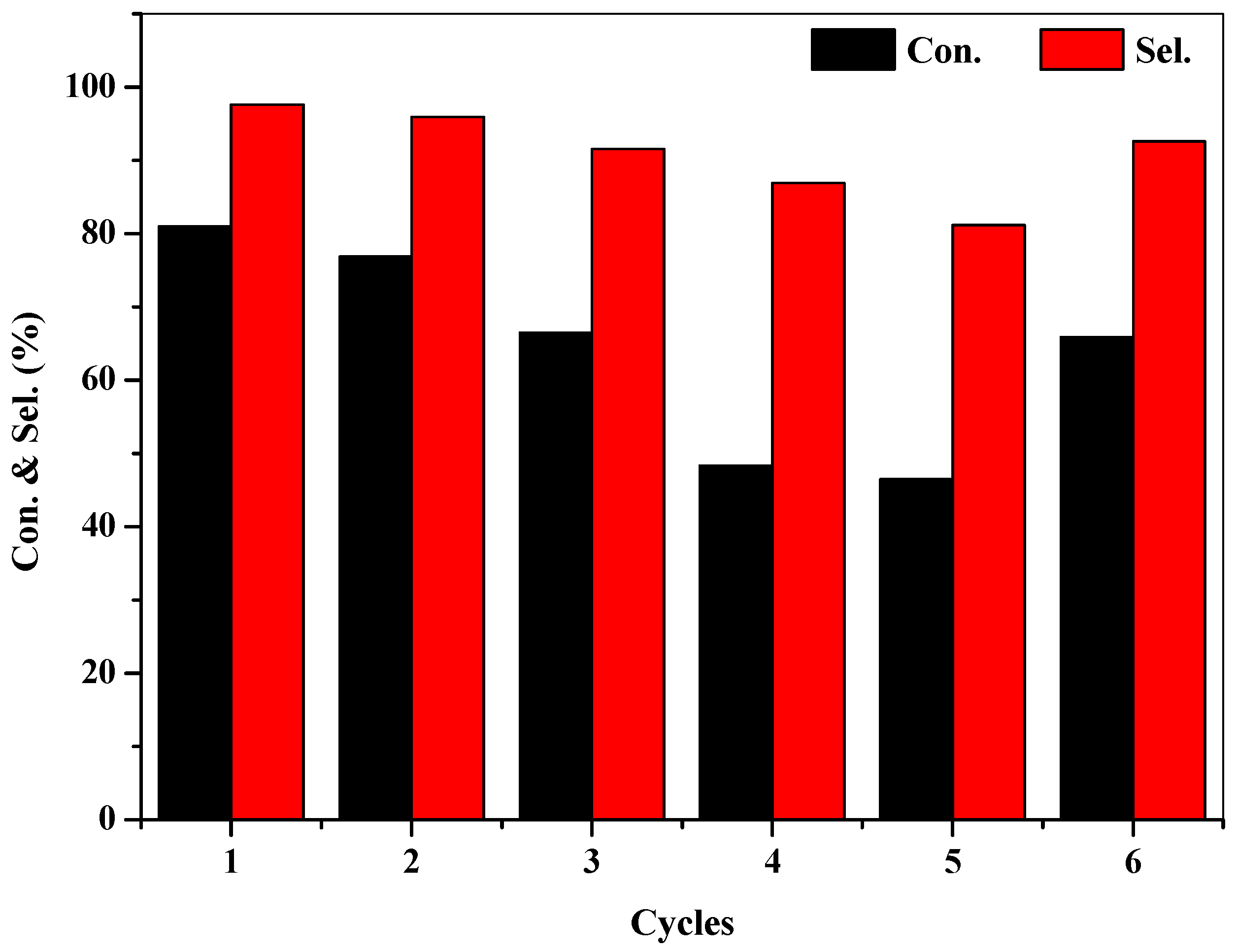
2.5. Gram-Scale Oxidation and Recyclability
2.6. Proposed Mechanism
2.7. Comparison with Other Literature Catalysts
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Materials
3.1.2. Synthesis of UCo@NC and DCo@NC
3.1.3. Synthesis of MCo@NC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.; Peng, L.; Huang, P.; Wang, X.; Sun, Y.; Cao, C.; Song, W. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes. Angew. Chem. Int. Ed. 2016, 128, 4084–4088. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Y.; Feng, D.; Zhang, L.; Zhang, L.; Song, X.; Qiao, Z. Efficient selective oxidation of aromatic alkanes by double cobalt active sites over oxygen vacancy-rich mesoporous Co3O4. Angew. Chem. Int. Ed. 2023, 62, e202306261. [Google Scholar] [CrossRef] [PubMed]
- Xiang, G.; Zhang, L.; Chen, J.; Zhang, B.; Liu, Z. A binary carbon@silica@carbon hydrophobic nanoreactor for highly efficient selective oxidation of aromatic alkanes. Nanoscale 2021, 13, 18140–18147. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, Y.; Huang, H.; Huang, B.; Chai, G.; Xie, Z. Template-free synthesis of graphene-like carbons as efficient carbocatalysts for selective oxidation of alkanes. Green Chem. 2020, 22, 1291–1300. [Google Scholar] [CrossRef]
- Zahedi, S.; Safaei, E. A tetra-cationic tetrapyridinoporphyrazinato iron(Ⅱ) grafted onto sulfonated SBA-15 as a novel heterogeneous catalyst for the aerobic oxidation of C(sp3)-H bonds in alkanes. Appl. Surf. Sci. 2021, 552, 149379. [Google Scholar] [CrossRef]
- Gao, Y.; Hu, G.; Zhong, J.; Shi, Z.; Zhu, Y.; Su, D.; Wang, J.; Bao, X.; Ma, D. Nitrogen-doped sp2-hybridized carbon as a superior catalyst for selective oxidation. Angew. Chem. Int. Ed. 2013, 52, 2109–2113. [Google Scholar] [CrossRef]
- Lan, Y.; Yi, C.; Liu, Z. In situ-synthesized Co and N-doped mesoporous hollow silica spheres for the selective oxidation of ethylbenzene. Phys. Chem. Chem. Phys. 2023, 25, 17207–17213. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, Y.; Miao, H.; Wang, C.; Lv, S.; Song, M.; Li, F.; Feng, M.; Chen, Z. Solvent-free oxidation of benzyl C-H to ketone with Co-Ni layered double hydroxide as the catalyst and O2 as the sole oxidant. Dalton Trans. 2023, 52, 6398–6406. [Google Scholar] [CrossRef]
- Hosseini, S.; Ghiaci, M.; Kulinich, S.; Wunderlich, W.; Ghaziaskar, H.; Koupaei, A. Ethyl benzene oxidation under aerobic conditions using cobalt oxide imbedded in nitrogen-doped carbon fiber felt wrapped by spiral TiO2-SiO2. Appl. Catal. A Gen. 2022, 630, 118456. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, W.; Sun, Z.; Qian, J.; He, M.; Chen, Q.; Sun, S. Fe assisted Co-containing hydrotalcites catalyst for efficient aerobic oxidation of ethylbenzene to acetophenone. Appl. Catal. A Gen. 2021, 624, 118322. [Google Scholar] [CrossRef]
- Azam, S.; Peckh, K.; Orlinska, B. SCILL-SILP hybrid catalytic system by employing carbon nanotubes as a support for the selective oxidation of ethylbenzene to acetophenone. Chem. Eng. J. 2023, 457, 141207. [Google Scholar] [CrossRef]
- Nandanwar, S.; Rathod, S.; Bansal, V.; Bokade, V. A review on selective production of acetophenone from oxidation of ethylbenzene over heterogeneous catalysts in a decade. Catal. Lett. 2021, 151, 2116–2131. [Google Scholar] [CrossRef]
- Mou, X.; Ma, J.; Zheng, S.; Chen, X.; Krumeich, F.; Hauert, R.; Lin, R.; Wu, Z.; Ding, Y. A general synthetic strategy toward highly doped pyridinic nitrogen-rich carbons. Adv. Funct. Mater. 2020, 31, 2006076. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, H.; Yang, Z.; Ji, G.; Yu, B.; Liu, X.; Liu, Z. Mesoporous nitrogen-doped carbon with high nitrogen content and ultrahigh surface areas: Synthesis and applications in catalysis. Green Chem. 2016, 18, 1976–1982. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Huo, H.; Hu, Y.; Xu, X.; Wang, P.; Yang, Y.; Lin, K. Synthesis of three-dimensional nitrogen doped meso/macroporous carbon beads for heterogeneous catalytic solvent-free oxidation of ethylbenzene. Carbon 2020, 158, 226–237. [Google Scholar] [CrossRef]
- Li, S.; Chen, X.; Wang, J.; Yao, N.; Wang, J.; Cen, J.; Li, X. Construction the Ni@Carbon nanostructure with dual-reaction surfaces for the selective hydrogenation reaction. Appl. Surf. Sci. 2019, 489, 786–795. [Google Scholar] [CrossRef]
- Li, S.; Yao, N.; Fan, L.; Li, Z.; Yang, L.; Cen, J.; Li, X. Supported Ni0@C-N catalyst with dual-reaction surfaces: Structure-performance relation in the selective hydrogenation of p-chloronitrobenzene. Appl. Surf. Sci. 2022, 606, 154786. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, W.; Guo, F.; Dong, X. Fe3C nanoclusters integrated with Fe single-atom planted in nitrogen doped carbon derived from truncated hexahedron zeolitic imidazolate framework for the efficient transfer hydrogenation of halogenated nitrobenzenes. J. Colloid Interface Sci. 2023, 640, 1068–1079. [Google Scholar] [CrossRef]
- Li, J.; Kou, J.; Xiang, Y.; Chen, M.; Zhang, J.; Zhan, X.; Zhang, H.; Wang, F.; Dong, Z. ZIF-8 derived N-doped porous carbon confined ultrafine PdNi bimetallic nanoparticle for semi-hydrogenation of alkynes. Mol. Catal. 2023, 535, 112865. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Feng, L.; Liu, F.; Liang, J.; Wang, X.; Zhang, X. Large-scale and solvent-free synthesis of magnetic bamboo-like nitrogen-doped carbon nanotubes with nickel active sites for photothermally driven CO2 fixation. Green Chem. 2023, 25, 3585–3591. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Zhao, T.; Zhang, B.; Su, H.; Xue, Z.; Li, X.; Chen, J. Schottky barrier induced coupled interface of electron-rich N-doped carbon and electron-deficient Cu: In-built lewis acid-base pairs for highly efficient CO2 fixation. J. Am. Chem. Soc. 2019, 141, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Zou, M.; Long, J.; Li, B.; Zhang, S.; Yang, L.; Wang, D.; Mao, P.; Luo, S.; Luo, X. Nanoporous N-doped carbon/ZnO hybrid derived from zinc aspartate: An acid-base bifunctional catalyst for efficient fixation of carbon dioxide into cyclic carbonates. Appl. Surf. Sci. 2021, 540, 148311. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, T.; Zhou, G.; Liu, P.; Yan, X.; Xu, B.; Guo, J. Cu nanoclusters on N-doped carbon nanotubes as efficient electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2022, 589, 153022. [Google Scholar] [CrossRef]
- Weng, P.; Guo, Y.; Wu, K.; Wang, X.; Huang, G.; Lei, H.; Yuan, Y.; Lu, W.; Li, D. Design of Fe/Ni-doped N/S-rich carbon with advanced bifunctional electrocatalysis for Zn-air batteries. J. Mater. Chem. A 2023, 11, 12194–12201. [Google Scholar] [CrossRef]
- Pei, F.; Chen, M.; Kong, F.; Huang, Y.; Cui, X. In-situ coupling FeN nanocrystals with Fe/Fe3C nanoparticles to N-doped carbon nanosheets for efficient oxygen electrocatalysis. Appl. Surf. Sci. 2022, 587, 152922. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, P.; Mou, X.; Zhu, H.; Jiang, Z.; Lin, R. Trace single-atom iron-decorated nitrogen-doped carbons enable highly efficient selective oxidation of ethylbenzene. ChemCatChem 2021, 13, 5084–5088. [Google Scholar] [CrossRef]
- Xia, M.; Huang, H.; Zhang, X.; Wei, Q.; Xie, Z. Single-atom cobalt-fused biomolecule-derived nitrogen-doped carbon nanosheets for selective oxidation reactions. Phys. Chem. Chem. Phys. 2021, 23, 14276–14283. [Google Scholar] [CrossRef]
- Xiong, Y.; Sun, W.; Han, Y.; Xin, P.; Zheng, X.; Yan, W.; Dong, J.; Zhang, J.; Wang, D.; Li, Y. Cobalt single atom site catalysts with ultrahigh metal loading for enhanced aerobic oxidation of ethylbenzene. Nano Res. 2021, 14, 2418–2423. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, S.; Cheng, N.; Liu, Z. Solvent-free melting-assisted pyrolysis strategy applied on the Co/N codoped porous carbon catalyst. ACS Sustain. Chem. Eng. 2019, 7, 19474–19482. [Google Scholar] [CrossRef]
- Pendem, S.; Singuru, R.; Sarkar, C.; Joseph, B.; Lee, J.; Shinde, D.; Lai, Z.; Mondal, J. Zeolitic imidazolate framework-mediated synthesis of Co3O4 nanoparticles encapsulated in N-doped graphitic carbon as an efficient catalyst for selective oxidation of hydrocarbons. ACS Appl. Nano Mater. 2018, 1, 4836–4851. [Google Scholar] [CrossRef]
- Jie, S.; Lin, X.; Chen, Q.; Zhu, R.; Zhang, L.; Zhang, B.; Liu, Z. Montmorillonite-assisted synthesis of cobalt-nitrogen-doped carbon nanosheets for high-performance selective oxidation of alkyl aromatics. Appl. Surf. Sci. 2018, 456, 951–958. [Google Scholar] [CrossRef]
- Chen, Y.; Jie, S.; Yang, C.; Liu, Z. Active and efficient Co-N/C catalysts derived from cobalt porphyrin for selective oxidation of alkylaromatics. Appl. Surf. Sci. 2017, 419, 98–106. [Google Scholar] [CrossRef]
- Lin, X.; Nie, Z.; Zhang, L.; Mei, S.; Chen, Y.; Zhang, B.; Zhu, R.; Liu, Z. Nitrogen-doped carbon nanotubes encapsulate cobalt nanoparticles as efficient catalysts for aerobic and solvent-free selective oxidation of hydrocarbons. Green Chem. 2017, 19, 2164–2173. [Google Scholar] [CrossRef]
- Wang, R.; Lu, K.; Zhang, J.; Li, X.; Zheng, Z. Regulation of the Co-Nx active sites of MOF-templated Co@NC catalysts via Au doping for boosting oxidative esterification of alcohols. ACS Catal. 2022, 12, 14290–14303. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Bai, C.; Liao, J.; Li, Y. Base-free oxidation of alcohols to esters at room temperature and atmospheric conditions using nanoscale Co-based catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
- Fu, L.; Lu, Y.; Liu, Z.; Zhu, R. Influence of the metal sites of M-N-C (M = Co, Fe, Mn) catalysts derived from metalloporphyrins in ethylbenzene oxidation. Chin. J. Catal. 2016, 37, 398–404. [Google Scholar] [CrossRef]
- Zhang, L.; Jie, S.; Liu, Z. Bicontinuous mesoporous Co, N co-doped carbon catalysts with high catalytic performance for ethylbenzene oxidation. New J. Chem. 2019, 43, 7275–7281. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, L.; Liu, H.; Fu, Y.; Yin, S.; Yang, W. Microporous cobaltporphyrin covalent polymer mediated Co3O4@PNC nanocomposites for efficient catalytic C-H bond activation. Appl. Catal. A Gen. 2021, 614, 118035. [Google Scholar] [CrossRef]
- Liu, T.; Cheng, H.; Sun, L.; Liang, F.; Zhang, C.; Ying, Z.; Lin, W.; Zhao, F. Synthesis of acetophenone from aerobic catalytic oxidation of ethylbenzene over Ti-Zr-Co alloy catalyst: Influence of annealing conditions. Appl. Catal. A Gen. 2016, 512, 9–14. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, Y.; Wang, J.; Su, D.; Tang, M.; Mao, S.; Wang, Y. Cobalt encapsulated in N-doped graphene layers: An efficient and stable catalyst for hydrogenation of quinoline compounds. ACS Catal. 2016, 6, 5816–5822. [Google Scholar] [CrossRef]
- Sheng, Y.; Peng, J.; Ma, L.; Zhang, Y.; Jiang, T.; Li, X. Nickel nanoparticles embedded in porous carbon-coated honeycomb ceramics: A potential monolithic catalyst for continuous hydrogenation reaction. Carbon 2022, 197, 171–182. [Google Scholar] [CrossRef]
- Long, J.; Shen, K.; Li, Y. Bifunctional N-doped Co@C catalysts for base-free transfer hydrogenations of nitriles: Controllable selectivity to primary amines vs imines. ACS Catal. 2017, 7, 275–284. [Google Scholar] [CrossRef]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 14969. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, P.; Zhang, Z.; Zhou, Y.; Zhang, J.; Zhang, H.; Shi, Z.; Han, R.; Huang, F.; Ma, D. Nickel catalyst stabilization via graphene encapsulation for enhanced methanation reaction. J. Catal. 2016, 334, 42–51. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Li, G.; Zhang, L.; Yin, C.; Yang, Y.; Wang, H.; Feng, F.; Wei, L.; Zhang, Q.; et al. A highly sulfur resistant and stable heterogeneous catalyst for liquid-phase hydrogenation. Appl. Catal. B Environ. 2022, 315, 121566. [Google Scholar] [CrossRef]
- Li, J.; Tang, X.; Yi, H.; Yu, Q.; Gao, F.; Zhang, R.; Li, C.; Chu, C. Effects of copper-precursors on the catalytic activity of Cu/graphene catalysts for the selective catalytic oxidation of ammonia. Appl. Surf. Sci. 2017, 412, 37–44. [Google Scholar] [CrossRef]
- Ito, Y.; Cong, W.; Fujita, T.; Tang, Z.; Chen, M. High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew. Chem. Int. Ed. 2015, 54, 2131–2136. [Google Scholar] [CrossRef]
- Xiong, C.; Xue, C.; Yu, X.; He, Y.; Liang, Y.; Zhou, X.; Ji, H. Tuning the olefin-VOCs epoxidation performance of ceria by mechanochemical loading of coinage metal. J. Hazard. Mater. 2023, 441, 129888. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Cheng, F.; Tao, Z.; Chen, J. Cobalt nanoparticles embedded in porous N-doped carbon as long-life catalysts for hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 3443–3448. [Google Scholar] [CrossRef]
- Tang, P.; Gao, Y.; Yang, J.; Li, W.; Zhao, H.; Ma, D. Growth mechanism of N-doped graphene materials and their catalytic behavior in the selective oxidation of ethylbenzene. Chin. J. Catal. 2014, 35, 922–928. [Google Scholar] [CrossRef]
- Chaudhary, V.; Sharma, S. Study of ethylbenzene oxidation over polymer-silica hybrid supported Co(Ⅱ) and Cu(Ⅱ) complexes. Catal. Today 2021, 375, 601–613. [Google Scholar] [CrossRef]
- Dai, X.; Li, X.; Tang, S.; Peng, X.; Zheng, X.; Jiang, O. Efficient aerobic oxidation of ethylbenzene accelerated by Cu species in hydrotalcite. Catal. Commun. 2021, 149, 106184. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Jian, P.; Wang, L.; Yan, X. Promoted selective oxidation of ethylbenzene in liquid phase achieved by hollow CeVO4 microspheres. J. Colloid Interface Sci. 2022, 614, 102–109. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, S.; Liu, Z. Influence of the synergistic effect between Co-N-C and ceria on the catalytic performance for selective oxidation of ethylbenzene. Phys. Chem. Chem. Phys. 2015, 17, 14012–14020. [Google Scholar] [CrossRef]
- Thangasamy, P.; Selvakumar, K.; Sathish, M.; Kumar, S.; Thangamuthu, R. Anchoring of ultrafine Co3O4 nanoparticles on MWCNTs using supercritical fluid processing and its performance evaluation towards electrocatalytic oxygen reduction reaction. Catal. Sci. Technol. 2017, 7, 1227–1234. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, L.; Yu, J.; Yang, L.; Zhang, J.; Zhang, X.; Han, F.; Zhao, L.; Li, X.; Liu, H.; et al. Charge redistribution of Ru nanoclusters on Co3O4 porous nanowire via the oxygen regulation for enhanced hydrogen evolution reaction. Nano Energy 2021, 85, 105940. [Google Scholar] [CrossRef]
- Xie, R.; Fan, G.; Yang, L.; Li, F. Hierarchical flower-like Co-Cu mixed metal oxide microspheres as highly efficient catalysts for selective oxidation of ethylbenzene. Chem. Eng. J. 2016, 288, 169–178. [Google Scholar] [CrossRef]
- Zhao, H.; Fang, J.; Xu, D.; Li, J.; Li, B.; Zhao, H.; Dong, Z. Multistep protection strategy for preparation of atomically dispersed Fe-N catalysts for selective oxidation of ethylbenzene to acetophenone. Catal. Sci. Technol. 2022, 12, 641–651. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Liu, X.; Liu, X.; Yang, X.; Miao, S.; Wang, W.; Wang, A.; Zhang, T. Discriminating catalytically active FeNx species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C-H bond. J. Am. Chem. Soc. 2017, 139, 10790–10798. [Google Scholar] [CrossRef]
- Xiang, G.; Zhang, L.; Yi, C.; Liu, Z. One-pot pyrolysis method to fabricate Co/N co-doped hollow mesoporous spheres with carbon/silica binary shells for selective oxidation of arylalkanes. Appl. Surf. Sci. 2022, 577, 151829. [Google Scholar] [CrossRef]
- Li, J.; Zhao, S.; Yang, S.; Wang, S.; Sun, H.; Jiang, S.; Johannessen, B.; Liu, S. Atomically dispersed cobalt on graphitic carbon nitride as a robust catalyst for selective oxidation of ethylbenzene by peroxymonosulfate. J. Mater. Chem. A 2021, 9, 3029–3035. [Google Scholar] [CrossRef]
- Gao, L.; Zhuge, W.; Feng, X.; Sun, W.; Sun, X.; Zheng, G. Co/rGO synthesized via the alcohol-thermal method as a heterogeneous catalyst for the highly efficient oxidation of ethylbenzene with oxygen. New J. Chem. 2019, 43, 8189–8194. [Google Scholar] [CrossRef]
- Nakatsuka, K.; Yoshii, T.; Kuwahara, Y.; Mori, K.; Yamashita, H. Controlled synthesis of carbon-supported Co catalysts from single-sites to nanoparticles: Characterization of the structural transformation and investigation of their oxidation catalysis. Phys. Chem. Chem. Phys. 2017, 19, 4967–4974. [Google Scholar] [CrossRef]
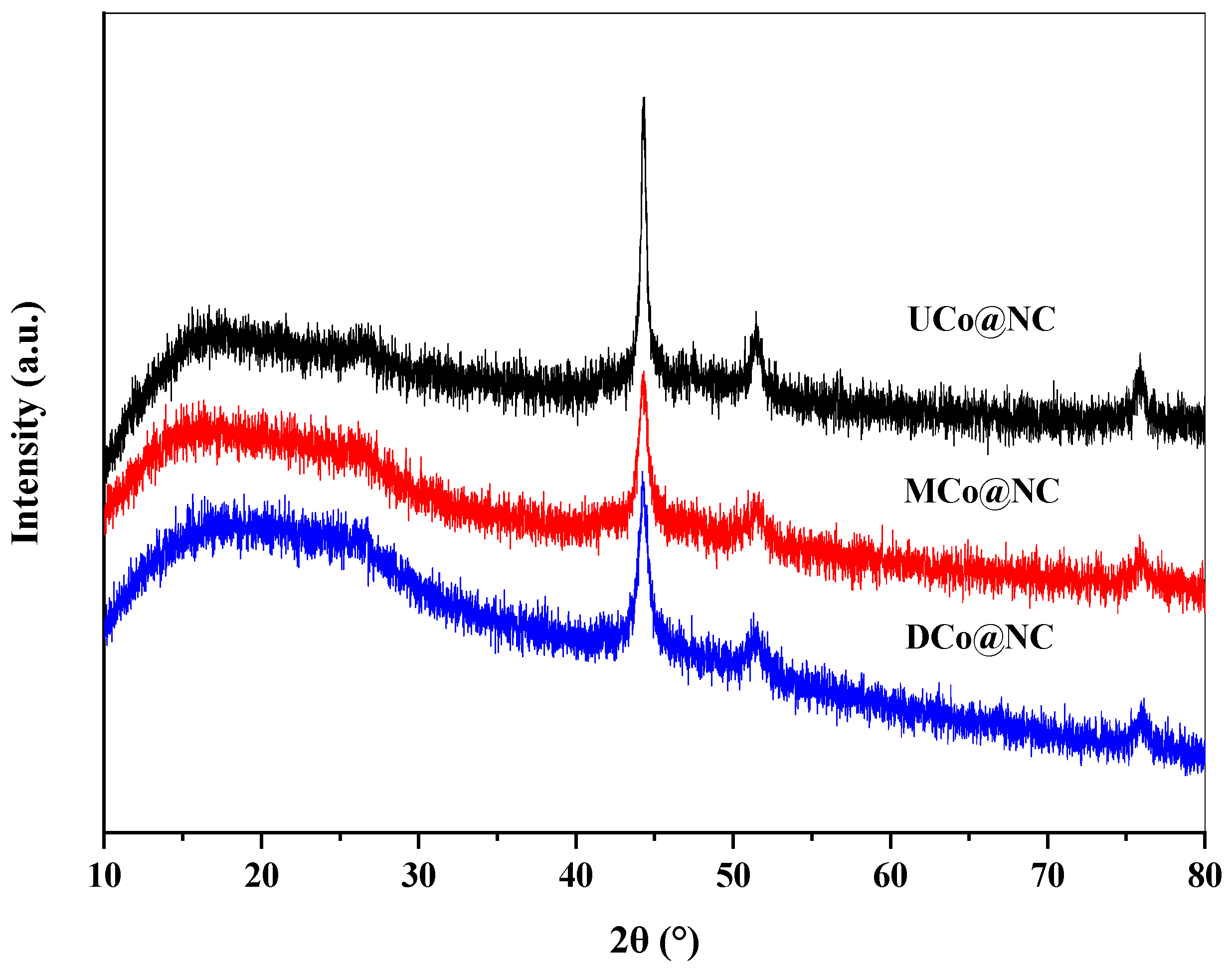

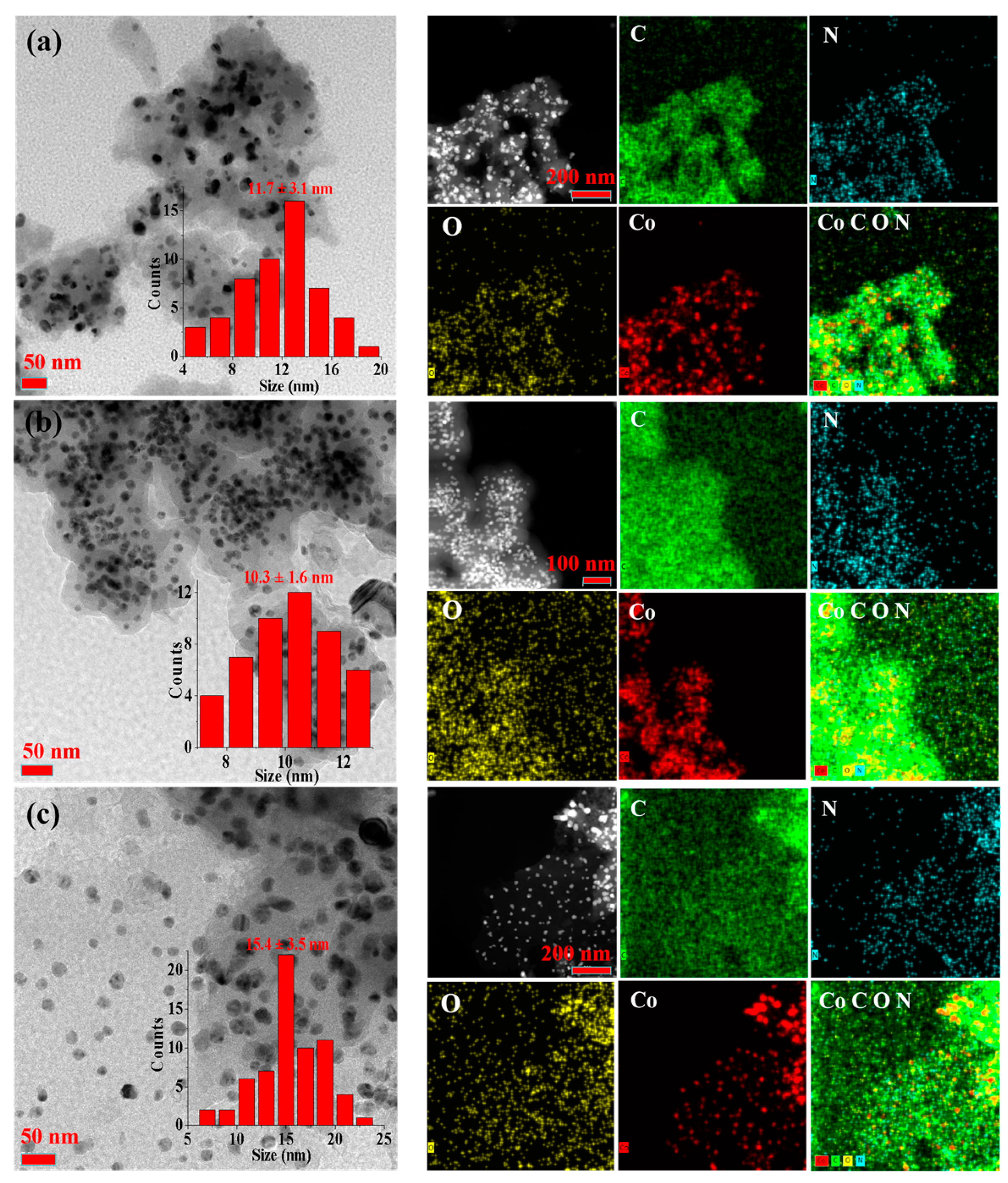
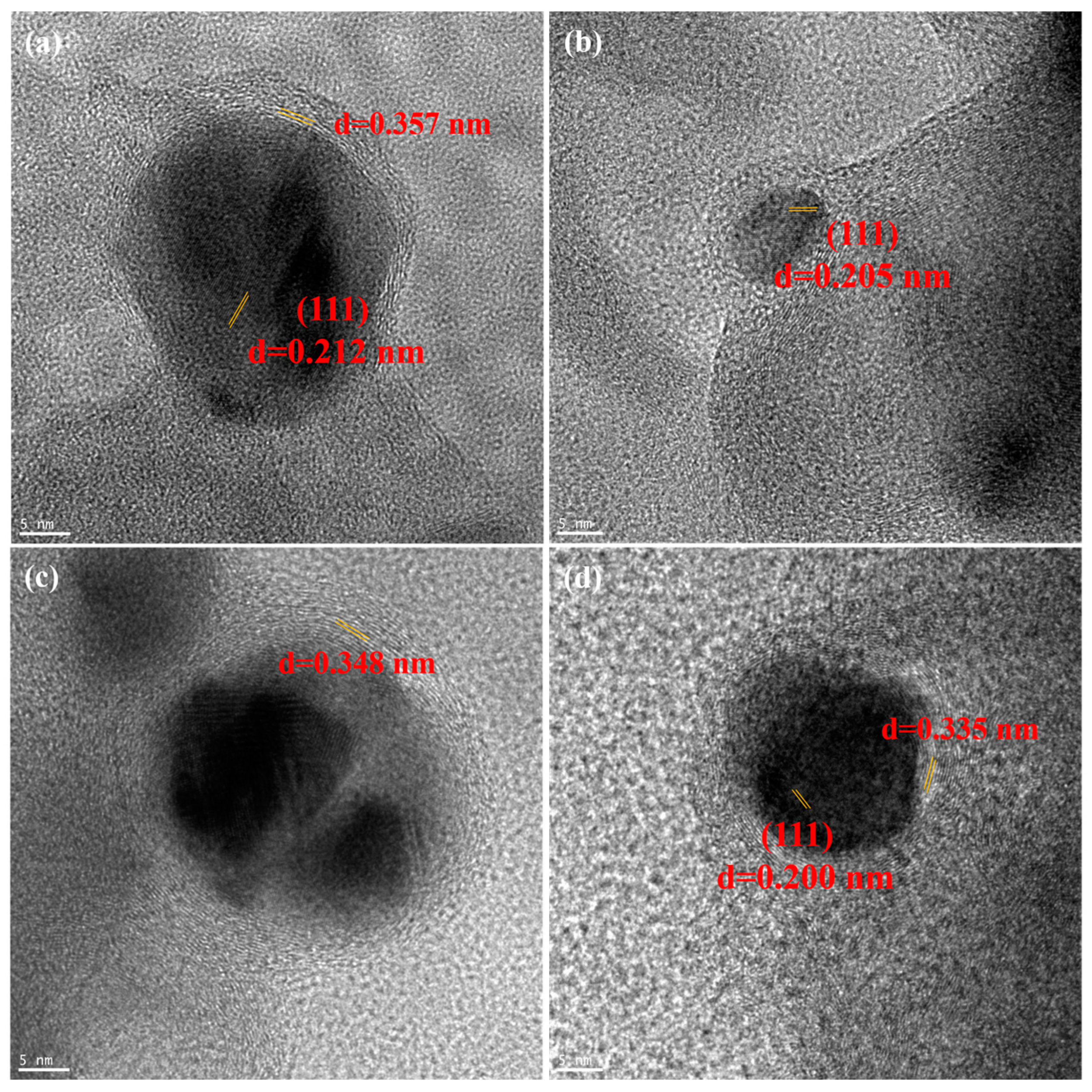
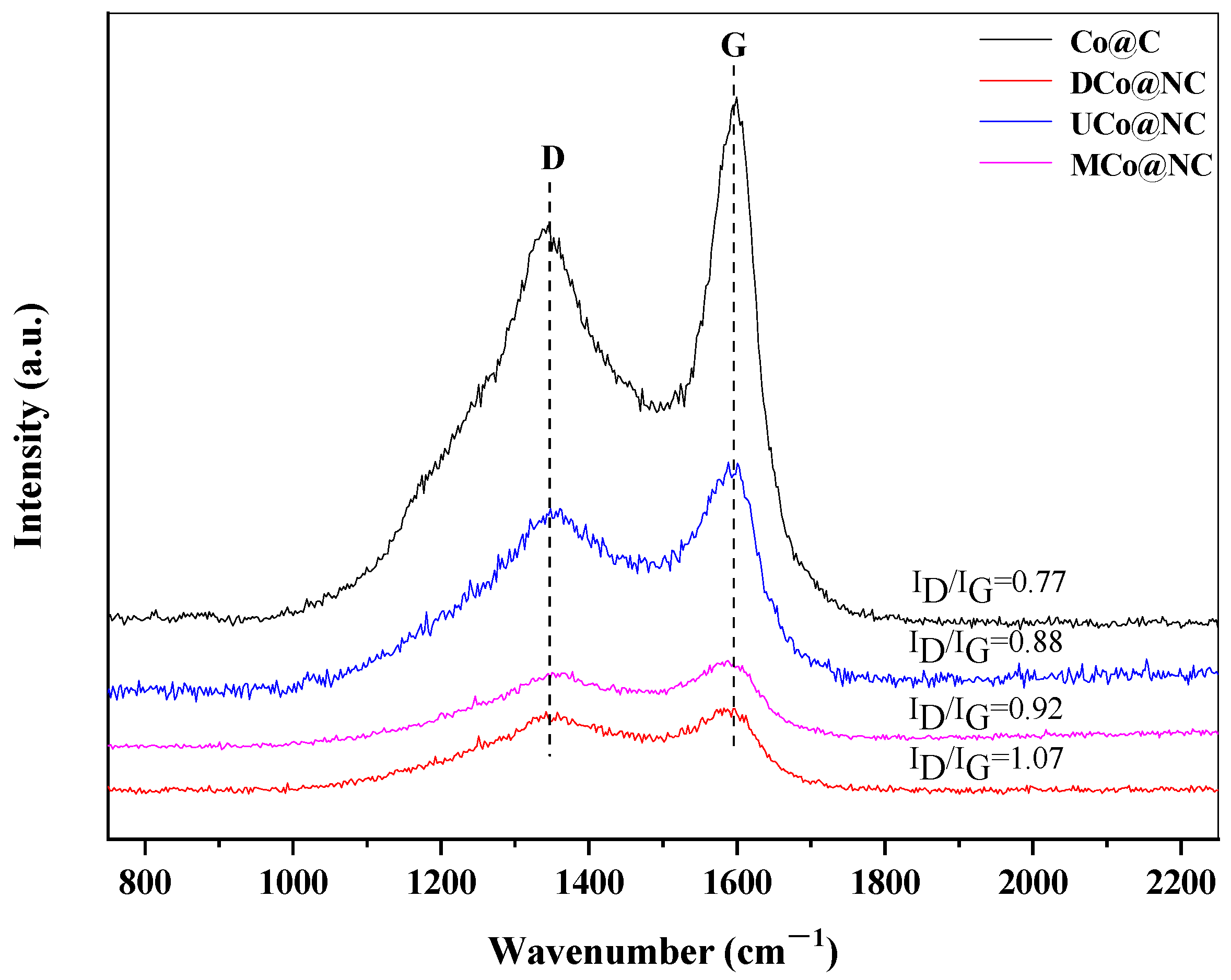




| Catalyst | SBET (m2/g) | V (cm3/g) | D (nm) a | DCo (nm) b | d111 (nm) c |
|---|---|---|---|---|---|
| Co@C | 221 | 0.15 | 2.67 | 10.9 | 0.204 |
| DCo@C | 366 | 0.31 | 3.44 | 12.7 | 0.205 |
| UCo@C | 80 | 0.12 | 6.06 | 20.7 | 0.204 |
| MCo@C | 362 | 0.25 | 2.75 | 11.0 | 0.205 |
| Catalyst | C (at.%) | O (at.%) | N (at.%) | N1s | Co (at.%) | Co2p3/2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N1 | N2 | N3 | N4 | Co0 | Co3+ | Co2+ | |||||
| Co@C | 86.6 | 10.4 | - | - | - | - | - | 3.0 | 29.4 | 34.1 | 36.5 |
| DCo@NC | 80.2 | 9.8 | 8.9 | 37.0 | 23.6 | 29.6 | 9.7 | 1.1 | 12.8 | 51.1 | 36.1 |
| UCo@NC | 83.6 | 8.0 | 7.3 | 34.0 | 7.8 | 44.3 | 13.9 | 1.2 | 25.2 | 49.4 | 25.4 |
| MCo@NC | 79.6 | 10.2 | 9.1 | 38.0 | 25.2 | 27.9 | 8.9 | 1.1 | 18.6 | 47.3 | 34.1 |
| UCo@NC a | 73.6 | 20.2 | 3.0 | 15.6 | 21.9 | 54.5 | 8.1 | 3.3 | 4.6 | 51.3 | 44.1 |
| Entry | Catalyst | t (h) | Con. (%) | Sel. (%) |
|---|---|---|---|---|
| 1 | blank | 3 | trace | - |
| 2 | NC | 3 | 22.9 | 68.3 |
| 3 | Co@C | 3 | 55.0 | 77.0 |
| 4 | DCo@NC | 3 | 61.6 | 84.5 |
| 5 | UCo@NC | 3 | 81.6 | 93.2 |
| 6 | MCo@NC | 3 | 65.3 | 83.6 |
| 7 | DCo@NC | 12 | 90.2 | 95.4 |
| 8 | UCo@NC | 12 | 95.2 | 96.0 |
| 9 | MCo@NC | 12 | 90.3 | 95.3 |
| 10 a | UCo@NC | 12 | 94.0 | 96.3 |
| 11 b | UCo@NC | 24 | 90.6 | 95.8 |
| 12 c | UCo@NC | 3 | trace | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Ali, S.; Zuhra, Z.; Shen, H.; Qiu, J.; Zeng, Y.; Zheng, K.; Wang, X.; Xie, G.; Ding, S. Cobalt Encapsulated in Nitrogen-Doped Graphite-like Shells as Efficient Catalyst for Selective Oxidation of Arylalkanes. Molecules 2024, 29, 65. https://doi.org/10.3390/molecules29010065
Li S, Ali S, Zuhra Z, Shen H, Qiu J, Zeng Y, Zheng K, Wang X, Xie G, Ding S. Cobalt Encapsulated in Nitrogen-Doped Graphite-like Shells as Efficient Catalyst for Selective Oxidation of Arylalkanes. Molecules. 2024; 29(1):65. https://doi.org/10.3390/molecules29010065
Chicago/Turabian StyleLi, Shuo, Shafqat Ali, Zareen Zuhra, Huahuai Shen, Jiaxiang Qiu, Yanbin Zeng, Ke Zheng, Xiaoxia Wang, Guanqun Xie, and Shujiang Ding. 2024. "Cobalt Encapsulated in Nitrogen-Doped Graphite-like Shells as Efficient Catalyst for Selective Oxidation of Arylalkanes" Molecules 29, no. 1: 65. https://doi.org/10.3390/molecules29010065
APA StyleLi, S., Ali, S., Zuhra, Z., Shen, H., Qiu, J., Zeng, Y., Zheng, K., Wang, X., Xie, G., & Ding, S. (2024). Cobalt Encapsulated in Nitrogen-Doped Graphite-like Shells as Efficient Catalyst for Selective Oxidation of Arylalkanes. Molecules, 29(1), 65. https://doi.org/10.3390/molecules29010065




