Soot Erased: Catalysts and Their Mechanistic Chemistry
Abstract
:1. Introduction
1.1. Aim of Review and Distinguishing Features
1.2. Soot Formation and Methodology for Soot Oxidation
2. Effect on Health and the Environment
3. Diversity in Catalysts for Removal of Soot
4. Ceria-Based Mixed Metal Oxides
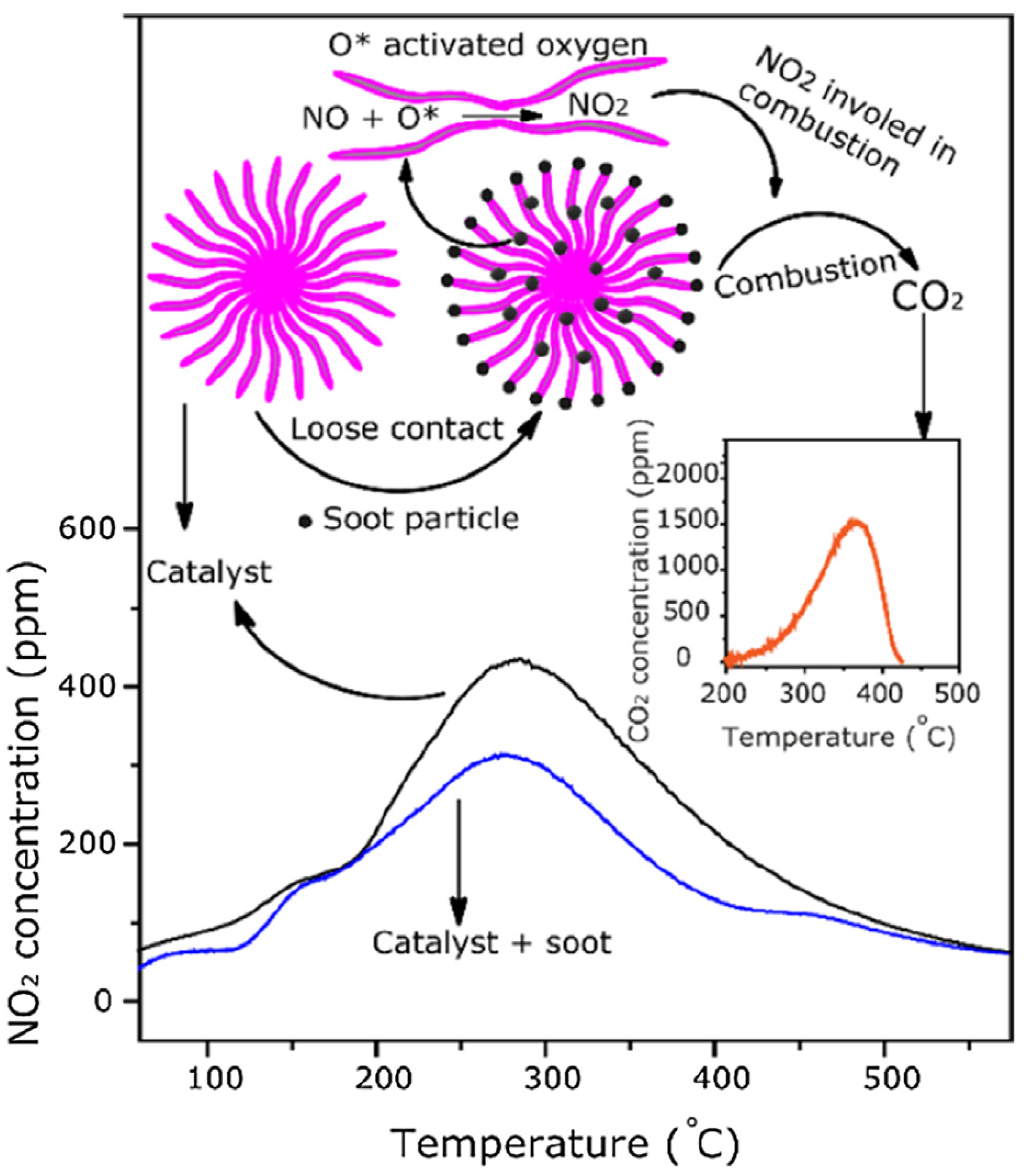
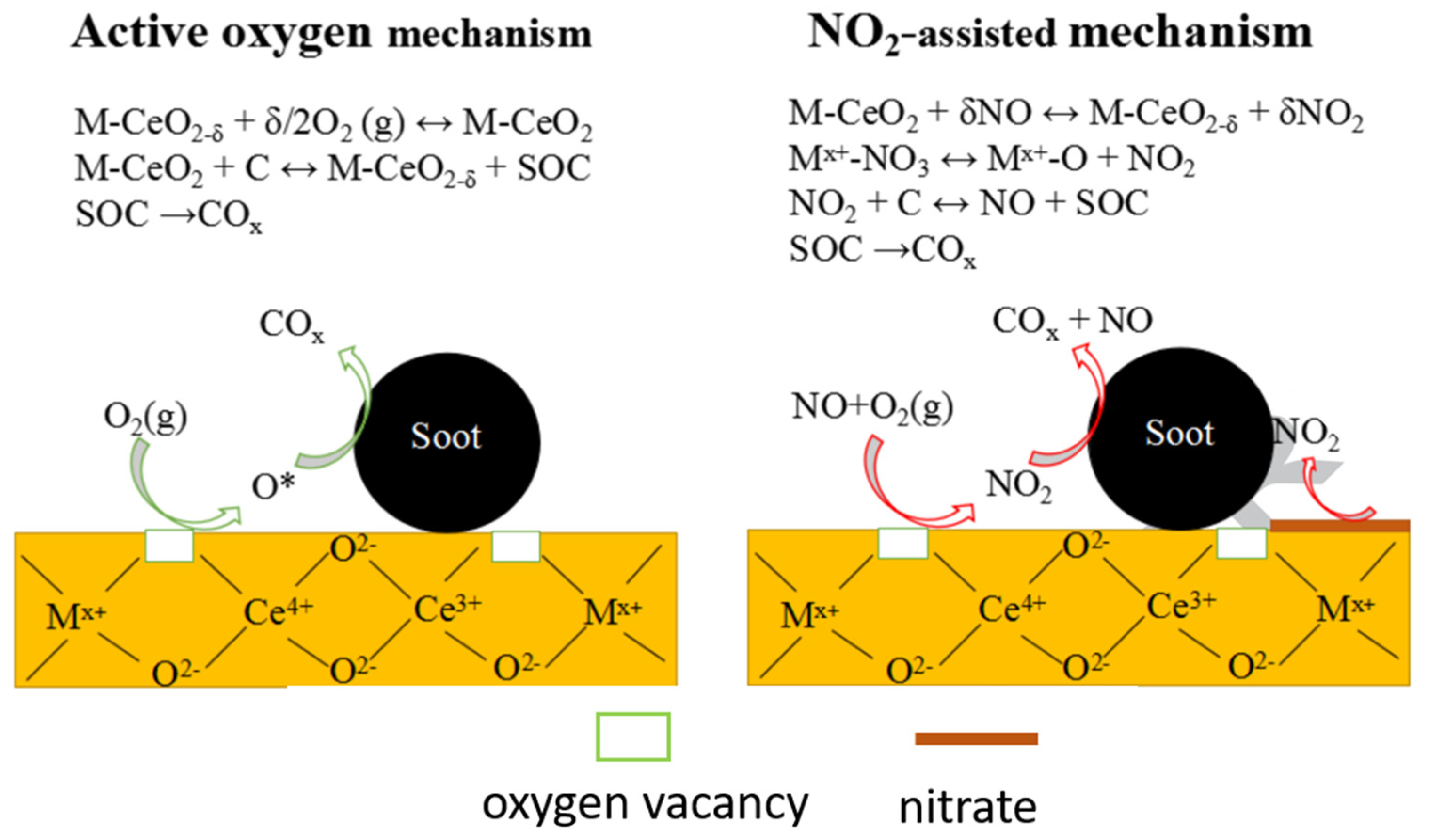
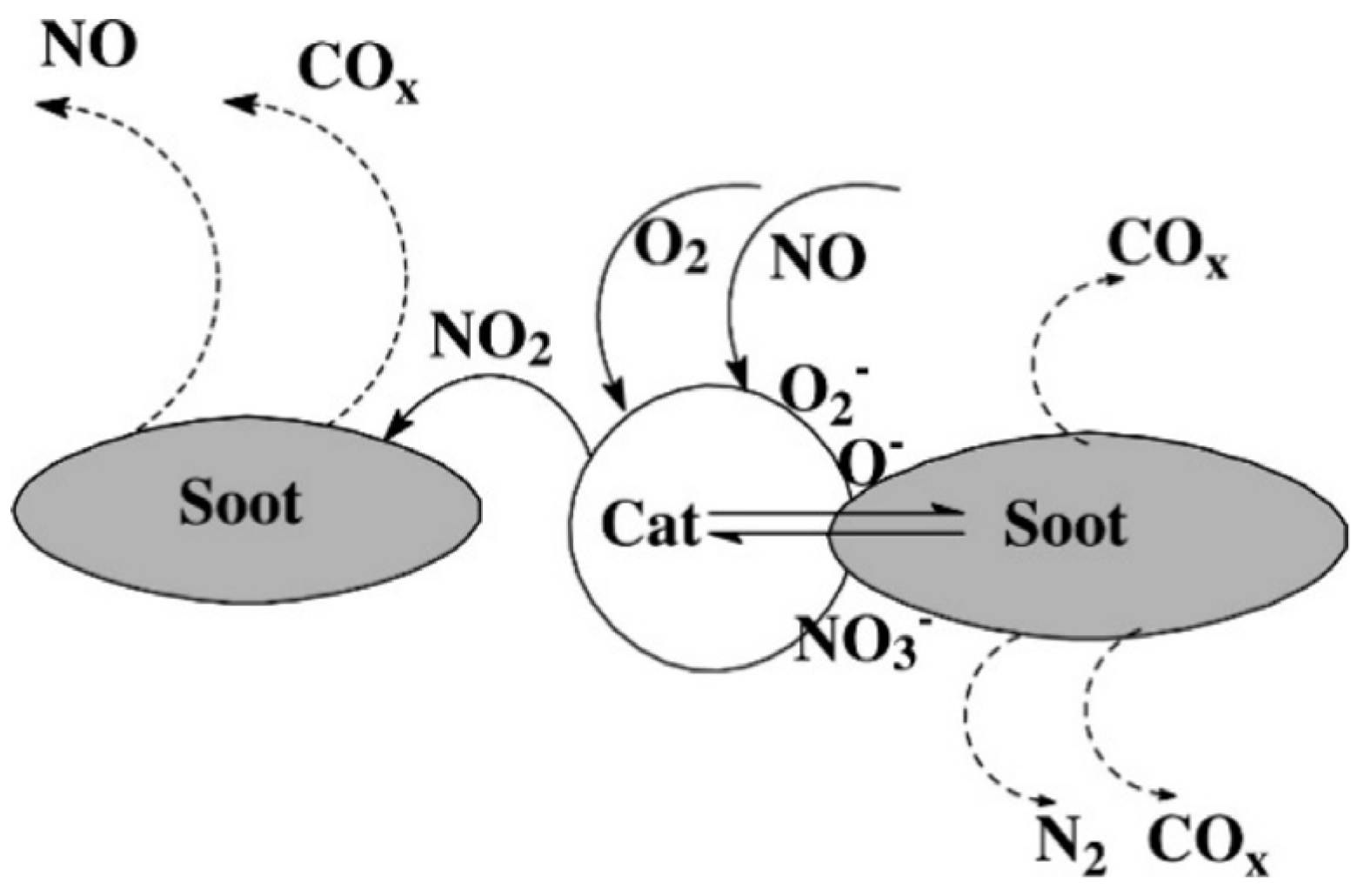
5. Mechanistic Chemistry for Removal of Soot
6. Conclusions, Future Prospects, and Challenges
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pierce, D.; Haynes, A.; Hughes, J.; Graves, R.; Maziasz, P.; Muralidharan, G.; Shyam, A.; Wang, B.; England, R.; Daniel, C. High temperature materials for heavy duty diesel engines: Historical and future trends. Prog. Mater. Sci. 2019, 103, 109–179. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, S.V. A review on catalytic oxidation of soot emitted from diesel fuelled engines. J. Environ. Chem. Eng. 2020, 8, 103945. [Google Scholar]
- Giakoumis, E.G.; Rakopoulos, C.D.; Dimaratos, A.M.; Rakopoulos, D.C. Exhaust emissions of diesel engines operating under transient conditions with biodiesel fuel blends. Prog. Energy Combust. Sci. 2012, 38, 691–715. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Zang, Y.; Liu, C.; Wang, W.; Han, R.; Ji, N.; Zhang, S.; Liu, Q. Promotion of A-site Ag-doped perovskites for the catalytic oxidation of soot: Synergistic catalytic effect of dual active sites. ACS Catal. 2021, 11, 14224–14236. [Google Scholar] [CrossRef]
- Mizushima, N.; Kawano, D.; Ishii, H.; Takada, Y.; Sato, S. Evaluation of Real-World Emissions from Heavy-Duty Diesel Vehicle Fueled with FAME, HVO and BTL Using PEMS; 0148-7191; SAE Technical Paper: Warrendale, PA, USA, 2014. [Google Scholar]
- Sethi, C.; Patnaik, P.; Thatoi, D.; Acharya, S. Performance, Combustion & Emission Analysis on Diesel Engine Utilizing Diethyl Ether as a Fuel Additives. TEST Eng. Manag. 2020, 82, 2391–2408. [Google Scholar]
- Subramanian, K.; Gnanam, A.; Damodharan, D.; Prasanna, N.; Mukilarasan, N. Emission Control and Reduction in Fuel Consumption of Two-Stroke Si Engine Using Nano-Fragment as a Catalyst; AIP Conference Proceedings; AIP Publishing: New York, NY, USA, 2020. [Google Scholar]
- Mahla, S.K.; Ardebili, S.M.S.; Sharma, H.; Dhir, A.; Goga, G.; Solmaz, H. Determination and utilization of optimal diesel/n-butanol/biogas derivation for small utility dual fuel diesel engine. Fuel 2021, 289, 119913. [Google Scholar] [CrossRef]
- Prasad, R.; Bella, V.R. A review on diesel soot emission, its effect and control. Bull. Chem. React. Eng. Catal. 2010, 5, 69–86. [Google Scholar] [CrossRef]
- Mohankumar, S.; Senthilkumar, P. Particulate matter formation and its control methodologies for diesel engine: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 1227–1238. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Khlystov, A.; Norford, L.K.; Tan, Z.-K.; Balasubramanian, R. Characterization of traffic-related ambient fine particulate matter (PM2.5) in an Asian city: Environmental and health implications. Atmos. Environ. 2017, 161, 132–143. [Google Scholar] [CrossRef]
- Dhal, G.C.; Mohan, D.; Prasad, R. Preparation and application of effective different catalysts for simultaneous control of diesel soot and NOX emissions: An overview. Catal. Sci. Technol. 2017, 7, 1803–1825. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, W.; Yu, W. A progress review of practical soot modelling development in diesel engine combustion. J. Traffic Transp. Eng. 2020, 7, 269–281. [Google Scholar] [CrossRef]
- Zygogianni, A.; Syrigou, M.; Konstandopoulos, A.G.; Kostoglou, M. Oxidative reactivity of particulate samples from different diesel combustion systems and its relation to structural and spectral characteristics of soot. Emiss. Control Sci. Technol. 2019, 5, 99–123. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Ye, D.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B Environ. 2016, 197, 105–115. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Lyu, G.; Qiao, Y.; Zhang, W.; Song, C. Effect of the oxidation-induced fragmentation of primary particles on soot oxidation reactivity. Combust. Flame 2022, 240, 112026. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Wang, H.; Zhang, Y.; Peng, Y.; Li, J. Water accelerates and directly participates soot oxidation: An isotopic study. Appl. Catal. B Environ. 2022, 302, 120837. [Google Scholar] [CrossRef]
- Bueno-López, A. Diesel soot combustion ceria catalysts. Appl. Catal. B Environ. 2014, 146, 1–11. [Google Scholar] [CrossRef]
- Bagi, S.; Sharma, V.; Patel, M.; Aswath, P.B. Effects of diesel soot composition and accumulated vehicle mileage on soot oxidation characteristics. Energy Fuels 2016, 30, 8479–8490. [Google Scholar] [CrossRef]
- Wei, J.; Fan, C.; Zhuang, Y.; Fu, Z.; Guan, Z.; Li, H.; Li, D.; Qian, Y. Diesel soot combustion over ceria catalyst: Evolution of functional groups on soot surfaces. Fuel 2023, 338, 127391. [Google Scholar] [CrossRef]
- Xi, J.; Zhong, B.J. Soot in diesel combustion systems. Chem. Eng. Technol. Ind. Chem.-Plant Equip.-Process Eng.-Biotechnol. 2006, 29, 665–673. [Google Scholar] [CrossRef]
- Altenhoff, M.; Aßmann, S.; Teige, C.; Huber, F.J.; Will, S. An optimized evaluation strategy for a comprehensive morphological soot nanoparticle aggregate characterization by electron microscopy. J. Aerosol Sci. 2020, 139, 105470. [Google Scholar] [CrossRef]
- Kennedy, I.M. Models of soot formation and oxidation. Prog. Energy Combust. Sci. 1997, 23, 95–132. [Google Scholar] [CrossRef]
- De Falco, G.; Picca, F.; Commodo, M.; Minutolo, P. Probing soot structure and electronic properties by optical spectroscopy. Fuel 2020, 259, 116244. [Google Scholar] [CrossRef]
- Mathis, U.; Mohr, M.; Kaegi, R.; Bertola, A.; Boulouchos, K. Influence of diesel engine combustion parameters on primary soot particle diameter. Env. Sci Technol 2005, 39, 1887–1892. [Google Scholar] [CrossRef]
- Wu, X.; Liu, S.; Weng, D.; Lin, F.; Ran, R. MnOx–CeO2–Al2O3 mixed oxides for soot oxidation: Activity and thermal stability. J. Hazard. Mater. 2011, 187, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Howard, J.B. Formation of polycyclic aromatic hydrocarbons and their growth to soot—A review of chemical reaction pathways. Prog. Energy Combust. Sci. 2000, 26, 565–608. [Google Scholar] [CrossRef]
- Siegmann, K.; Siegmann, H.C. Molecular Precursor of Soot and Quantification of the Associated Health Risk. In Current Problems in Condensed Matter; Morán-López, J.L., Ed.; Springer: Boston, MA, USA, 1998; pp. 143–160. [Google Scholar]
- Richter, H.; Granata, S.; Green, W.H.; Howard, J.B. Detailed modeling of PAH and soot formation in a laminar premixed benzene/oxygen/argon low-pressure flame. Proc. Combust. Inst. 2005, 30, 1397–1405. [Google Scholar] [CrossRef]
- Tree, D.R.; Svensson, K.I. Soot processes in compression ignition engines. Prog. Energy Combust. Sci. 2007, 33, 272–309. [Google Scholar] [CrossRef]
- Jaramillo, I.C.; Gaddam, C.K.; Vander Wal, R.L.; Huang, C.-H.; Levinthal, J.D.; Lighty, J.S. Soot oxidation kinetics under pressurized conditions. Combust. Flame 2014, 161, 2951–2965. [Google Scholar] [CrossRef]
- Yang, Y.; Zheng, S.; He, Y.; Liu, H.; Sui, R.; Lu, Q. Effects of simultaneous CO2 addition to the fuel and oxidizer streams on soot formation in co-flow diffusion ethylene flame. Fuel 2023, 353, 129181. [Google Scholar] [CrossRef]
- Wu, Q.; Jing, M.; Wei, Y.; Zhao, Z.; Zhang, X.; Xiong, J.; Liu, J.; Song, W.; Li, J. High-efficient catalysts of core-shell structured Pt@ transition metal oxides (TMOs) supported on 3DOM-Al2O3 for soot oxidation: The effect of strong Pt-TMO interaction. Appl. Catal. B Environ. 2019, 244, 628–640. [Google Scholar] [CrossRef]
- Orihuela, M.P.; Miceli, P.; Ramirez-Rico, J.; Fino, D.; Chacartegui, R. Ceria-based catalytic coatings on biomorphic silicon carbide: A system for soot oxidation with enhanced properties. Chem. Eng. J. 2021, 415, 128959. [Google Scholar] [CrossRef]
- Aneggi, E.; Boaro, M.; Colussi, S.; de Leitenburg, C.; Trovarelli, A. Ceria-based materials in catalysis: Historical perspective and future trends. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 209–242. [Google Scholar]
- Liu, S.; Wu, X.; Tang, J.; Cui, P.; Jiang, X.; Chang, C.; Liu, W.; Gao, Y.; Li, M.; Weng, D. An exploration of soot oxidation over CeO2-ZrO2 nanocubes: Do more surface oxygen vacancies benefit the reaction? Catal. Today 2017, 281, 454–459. [Google Scholar] [CrossRef]
- Vita, A. Catalytic applications of CeO2-based materials. Catalysts 2020, 10, 576. [Google Scholar] [CrossRef]
- Vander Wal, R.L.; Tomasek, A.J. Soot oxidation: Dependence upon initial nanostructure. Combust. Flame 2003, 134, 1–9. [Google Scholar] [CrossRef]
- Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. Nanostructured ceria-based catalysts for soot combustion: Investigations on the surface sensitivity. Appl. Catal. B Environ. 2015, 165, 742–751. [Google Scholar] [CrossRef]
- Wei, J.; Lu, W.; Pan, M.; Liu, Y.; Cheng, X.; Wang, C. Physical properties of exhaust soot from dimethyl carbonate-diesel blends: Characterizations and impact on soot oxidation behavior. Fuel 2020, 279, 118441. [Google Scholar] [CrossRef]
- Michelsen, H.A.; Colket, M.B.; Bengtsson, P.-E.; D’anna, A.; Desgroux, P.; Haynes, B.S.; Miller, J.H.; Nathan, G.J.; Pitsch, H.; Wang, H. A review of terminology used to describe soot formation and evolution under combustion and pyrolytic conditions. ACS Nano 2020, 14, 12470–12490. [Google Scholar] [CrossRef]
- Jiaqiang, E.; Xu, W.; Ma, Y.; Tan, D.; Peng, Q.; Tan, Y.; Chen, L. Soot formation mechanism of modern automobile engines and methods of reducing soot emissions: A review. Fuel Process. Technol. 2022, 235, 107373. [Google Scholar]
- Corbin, J.C.; Modini, R.L.; Gysel-Beer, M. Mechanisms of soot-aggregate restructuring and compaction. Aerosol Sci. Technol. 2023, 57, 89–111. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, S.; Tan, D.; Zhang, Z.; Wang, Z.; Wang, B. A Review of the Development and Application of Soot Modelling for Modern Diesel Engines and the Soot Modelling for Different Fuels. Process Saf. Environ. Prot. 2023, 178, 836–859. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Chu, B.; Ma, Q.; He, H. Atmospheric heterogeneous reactions on soot: A review. Fundam. Res. 2023, 3, 579–591. [Google Scholar] [CrossRef]
- Li, Q.; Xin, Y.; Zhang, Z.; Cao, X. Electron donation mechanism of superior Cs-supported oxides for catalytic soot combustion. Chem. Eng. J. 2018, 337, 654–660. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, S.; Wang, J.; Gong, M.; Chen, Y. Effects of contact model and NOx on soot oxidation activity over Pt/MnOx-CeO2 and the reaction mechanisms. Chem. Eng. J. 2017, 327, 1066–1076. [Google Scholar] [CrossRef]
- Wang, C.; Yuan, H.; Lu, G.; Wang, H. Oxygen vacancies and alkaline metal boost CeO2 catalyst for enhanced soot combustion activity: A first-principles evidence. Appl. Catal. B Environ. 2021, 281, 119468. [Google Scholar] [CrossRef]
- Wagloehner, S.; Nitzer-Noski, M.; Kureti, S. Oxidation of soot on manganese oxide catalysts. Chem. Eng. J. 2015, 259, 492–504. [Google Scholar] [CrossRef]
- Zou, G.; Xu, Y.; Wang, S.; Chen, M.; Shangguan, W. The synergistic effect in Co–Ce oxides for catalytic oxidation of diesel soot. Catal. Sci. Technol. 2015, 5, 1084–1092. [Google Scholar] [CrossRef]
- Singer, C.; Kureti, S. Soot oxidation in diesel exhaust on manganese oxide catalyst prepared by flame spray pyrolysis. Appl. Catal. B Environ. 2020, 272, 118961. [Google Scholar] [CrossRef]
- Gao, Y.; Jin, B.; Xi, Z.; Li, Z.; Wu, X.; Ran, R.; Si, Z.; Weng, D. Nitration Poisoning of Silver on Al2O3 for the Catalytic Oxidation of Soot in NO x-Containing Atmospheres. Ind. Eng. Chem. Res. 2022, 61, 15893–15899. [Google Scholar] [CrossRef]
- Yang, F. Under the Dome: “Chinese” smog as a viral media event. Crit. Stud. Media Commun. 2016, 33, 232–244. [Google Scholar] [CrossRef]
- Kerr, R.A. Soot is Warming the World Even More than Thought; American Association for the Advancement of Science: Washington, DC, USA, 2013. [Google Scholar]
- Tollefson, J. Soot a major contributor to climate change. Nature 2013, 15, 15. [Google Scholar] [CrossRef]
- Lohmann, U.; Friebel, F.; Kanji, Z.A.; Mahrt, F.; Mensah, A.A.; Neubauer, D. Future warming exacerbated by aged-soot effect on cloud formation. Nat. Geosci. 2020, 13, 674–680. [Google Scholar] [CrossRef]
- Manosh, P. Soot: Sources, Formation and Health Effects; Nova Science Publishers: New York, NY, USA, 2012. [Google Scholar]
- Harrison, P.G.; Ball, I.K.; Daniell, W.; Lukinskas, P.; Céspedes, M.A.; Miró, E.E.; Ulla, M.A. Cobalt catalysts for the oxidation of diesel soot particulate. Chem. Eng. J. 2003, 95, 47–55. [Google Scholar] [CrossRef]
- Van Setten, B.A.A.L.; Makkee, M.; Moulijn, J.A. Science and technology of catalytic diesel particulate filters. Catal. Rev.—Sci. Eng. 2001, 43, 489–564. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Weng, D.; Li, M.; Lee, H.-R. Combined promoting effects of platinum and MnOx-CeO2 supported on alumina on NOx-assisted soot oxidation: Thermal stability and sulfur resistance. Chem. Eng. J. 2012, 203, 25–35. [Google Scholar] [CrossRef]
- Lisi, L.; Landi, G.; Di Sarli, V. The issue of soot-catalyst contact in regeneration of catalytic diesel particulate filters: A critical review. Catalysts 2020, 10, 1307. [Google Scholar] [CrossRef]
- Ritchie, H.; Roser, M. Outdoor Air Pollution. Our World Data. 2019. Available online: https://ourworldindata.org/outdoor-air-pollution (accessed on 1 November 2019).
- Uhlenbruck, N.; Dietrich, B.; Hofberger, C.; Stoppel, L.; Wetzel, T. Methane Pyrolysis in a Liquid Metal Bubble Column Reactor: A Model Approach Combining Bubble Dynamics with Byproduct and Soot Formation. Energy Technology 2022, 10, 2200654. [Google Scholar] [CrossRef]
- Buseck, P.; Adachi, K.; Gelencsér, A.; Tompa, É.; Pósfai, M. Are black carbon and soot the same? Atmos. Chem. Phys. Discuss. 2012, 12, 24821–24846. [Google Scholar]
- Lambert, C.K. Current state of the art and future needs for automotive exhaust catalysis. Nat. Catal. 2019, 2, 554–557. [Google Scholar] [CrossRef]
- Baldelli, A.; Esmeryan, K.D.; Popovicheva, O. Turning a negative into a positive: Trends, guidelines and challenges of developing multifunctional non-wettable coatings based on industrial soot wastes. Fuel 2021, 301, 121068. [Google Scholar] [CrossRef]
- Chi, H.; Zhang, P.; Xiong, J.; Wei, Y.; Li, Y.; Zhao, Z.; Liu, J.; Jiao, J. Single-crystalline α-MnO2 catalysts with tailored exposed crystal facets for boosting catalytic soot oxidation: The crystal facet-dependent activity. Appl. Surf. Sci. 2023, 608, 155116. [Google Scholar] [CrossRef]
- Hu, C.; Dai, P.; Chen, Z.; Zhang, H. Property and Reactivity Relationships of Co3O4 with Diverse Nanostructures for Soot Oxidation. ACS Omega 2022, 7, 44116–44123. [Google Scholar] [CrossRef] [PubMed]
- Jampaiah, D.; Velisoju, V.K.; Venkataswamy, P.; Coyle, V.E.; Nafady, A.; Reddy, B.M.; Bhargava, S.K. Nanowire morphology of mono-and bidoped α-MnO2 catalysts for remarkable enhancement in soot oxidation. ACS Appl. Mater. Interfaces 2017, 9, 32652–32666. [Google Scholar] [CrossRef] [PubMed]
- Miceli, P.; Bensaid, S.; Russo, N.; Fino, D. Effect of the morphological and surface properties of CeO2-based catalysts on the soot oxidation activity. Chem. Eng. J. 2015, 278, 190–198. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. The role of metal-support interaction in Ag/CeO2 catalysts for CO and soot oxidation. Appl. Catal. B Environ. 2020, 260, 118148. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Ai, L.; Liu, W.; Li, Q.; Wang, X.; Wang, L. High performance of K-supported Pr2Sn2O7 pyrochlore catalysts for soot oxidation. Fuel 2022, 317, 123467. [Google Scholar] [CrossRef]
- Uppara, H.P.; Singh, S.K.; Labhsetwar, N.K.; Murari, M.S.; Dasari, H. The decisive factor of hollow spherical network morphology of Nd1-xCexCo1-yCuyO3±δ perovskites towards soot oxidation. Chem. Pap. 2022, 76, 3771–3787. [Google Scholar] [CrossRef]
- Li, Y.; Guo, H.; Xiong, J.; Ma, Y.; Li, X.; Zhang, P.; Zhang, S.; Wei, Y. The Catalyst of Ruthenium Nanoparticles Decorated Silicalite-1 Zeolite for Boosting Catalytic Soot Oxidation. Catalysts 2023, 13, 1167. [Google Scholar] [CrossRef]
- Voskanyan, A.A.; Chan, K.Y. Scalable synthesis of three-dimensional meso/macroporous NiO with uniform ultralarge randomly packed mesopores and high catalytic activity for soot oxidation. ACS Appl. Nano Mater. 2018, 1, 556–563. [Google Scholar] [CrossRef]
- Shuang, L.; Xiaodong, W.; Duan, W.; Rui, R. Ceria-based catalysts for soot oxidation: A review. J. Rare Earths 2015, 33, 567–590. [Google Scholar]
- Lou, D.; Xiang, B.; Zhang, Y.; Fang, L.; Tan, P.; Hu, Z. Study on the Catalytic Characteristics of Precious Metal Catalysts with Different Pt/Pd Ratios for Soot Combustion. ACS Omega 2023, 8, 20834–20844. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.U.; Ullah, S.; Yuan, Q.; Ali, S.; Ahmad, A.; Khan, Z.U.H.; Rahman, A.U. In situ fabrication of Au–CoFe2O4: An efficient catalyst for soot oxidation. Appl. Nanosci. 2020, 10, 3901–3910. [Google Scholar] [CrossRef]
- Oh, D.-K.; Lee, Y.-J.; Lee, K.-Y.; Park, J.-S. Nitrogen Monoxide and Soot Oxidation in Diesel Emissions with Platinum–Tungsten/Titanium Dioxide Catalysts: Tungsten Loading Effect. Catalysts 2020, 10, 1283. [Google Scholar] [CrossRef]
- Mukherjee, D.; Reddy, B.M. Noble metal-free CeO2-based mixed oxides for CO and soot oxidation. Catal. Today 2018, 309, 227–235. [Google Scholar] [CrossRef]
- Gálvez, M.E.; Ascaso, S.; Moliner, R.; Lázaro, M.J. Me (Cu, Co, V)-K/Al2O3 supported catalysts for the simultaneous removal of soot and nitrogen oxides from diesel exhausts. Chem. Eng. Sci. 2013, 87, 75–90. [Google Scholar] [CrossRef]
- Ferkel, H.; Bachmann, M.; Volpp, H.-R.; Stöwe, K.; Hensgen, L. Precious-metal-free Nano Catalysts for diesel Particulate Filters. ATZautotechnology 2010, 10, 58–63. [Google Scholar] [CrossRef]
- Matti Maricq, M. Chemical characterization of particulate emissions from diesel engines: A review. J. Aerosol Sci. 2007, 38, 1079–1118. [Google Scholar] [CrossRef]
- Martinovic, F.; Galletti, C.; Bensaid, S.; Pirone, R.; Deorsola, F.A. Soot oxidation in low-O2 and O2-free environments by lanthanum-based perovskites: Structural changes and the effect of Ag doping. Catal. Sci. Technol. 2022, 12, 5453–5464. [Google Scholar] [CrossRef]
- Mishra, A.; Prasad, R. Preparation and application of perovskite catalysts for diesel soot emissions control: An overview. Catal. Rev. 2014, 56, 57–81. [Google Scholar] [CrossRef]
- Zhang, R.; Alamdari, H.; Kaliaguine, S. Fe-based perovskites substituted by copper and palladium for NO+ CO reaction. J. Catal. 2006, 242, 241–253. [Google Scholar] [CrossRef]
- Hernández, W.; Tsampas, M.; Zhao, C.; Boreave, A.; Bosselet, F.; Vernoux, P. La/Sr-based perovskites as soot oxidation catalysts for Gasoline Particulate Filters. Catal. Today 2015, 258, 525–534. [Google Scholar] [CrossRef]
- Shao, W.; Wang, Z.; Zhang, X.; Wang, L.; Ma, Z.; Li, Q.; Zhang, Z. Promotion effects of cesium on perovskite oxides for catalytic soot combustion. Catal. Lett. 2016, 146, 1397–1407. [Google Scholar] [CrossRef]
- Jiménez, R.; Zamora, R.; Pecchi, G.; García, X.; Gordon, A. Effect of Ca-substitution in La1—xCaxFeO3 perovskites on the catalytic activity for soot combustion. Fuel Process. Technol. 2010, 91, 546–549. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sanchez-Adsuar, M.-S.; Illan-Gomez, M.-J. Analyzing the role of copper in the soot oxidation performance of BaMnO3-perovskite-based catalyst obtained by modified sol-gel synthesis. Fuel 2022, 328, 125258. [Google Scholar] [CrossRef]
- Labhasetwar, N.; Saravanan, G.; Megarajan, S.K.; Manwar, N.; Khobragade, R.; Doggali, P.; Grasset, F. Perovskite-type catalytic materials for environmental applications. Sci. Technol. Adv. Mater. 2015, 16, 036002. [Google Scholar] [CrossRef]
- Pecchi, G.; Dinamarca, R.; Campos, C.M.; Garcia, X.; Jimenez, R.; Fierro, J.L. Soot oxidation on silver-substituted LaMn0.9Co0.1O3 perovskites. Ind. Eng. Chem. Res. 2014, 53, 10090–10096. [Google Scholar] [CrossRef]
- Wang, M.; Han, Z.; Liu, Y.; Gao, C.; Pan, X.; Zhou, S. The influence of partial substitution of Ce with K in CeMO3 (M= Mn, Fe, Co, Ni, Cu) perovskite catalysts on soot combustion performance. J. Environ. Chem. Eng. 2023, 11, 110850. [Google Scholar] [CrossRef]
- Mishra, A.; Prasad, R. Synthesis and performance of transition metal based perovskite catalysts for diesel soot oxidation. Bull. Chem. React. Eng. Catal. 2017, 12, 469–477. [Google Scholar] [CrossRef]
- Fan, Q.; Zhang, S.; Sun, L.; Dong, X.; Zhang, L.; Shan, W.; Zhu, Z. Catalytic oxidation of diesel soot particulates over Ag/LaCoO3 perovskite oxides in air and NOx. Chin. J. Catal. 2016, 37, 428–435. [Google Scholar] [CrossRef]
- Feng, N.; Wu, Y.; Meng, J.; Chen, C.; Wang, L.; Wan, H.; Guan, G. Catalytic combustion of soot over Ce and Co substituted three-dimensionally ordered macroporous La 1–x CexFe1–yCoyO3 perovskite catalysts. RSC Adv. 2015, 5, 91609–91618. [Google Scholar] [CrossRef]
- Luo, J.; Zhu, X.; Wu, H.; Zhou, Z.; Chen, G.; Yang, G. Soot oxidation over V/ZSM-5 catalysts in a dielectric barrier discharge (DBD) reactor: Performance enhancement by transition metal (Mn, Co and Fe) doping. Catal. Today 2023, 419, 114139. [Google Scholar] [CrossRef]
- Tao, F.; Golovitchev, V.I.; Chomiak, J. A phenomenological model for the prediction of soot formation in diesel spray combustion. Combust. Flame 2004, 136, 270–282. [Google Scholar] [CrossRef]
- Chang, H.; Bjørgum, E.; Mihai, O.; Yang, J.; Lein, H.L.; Grande, T.; Raaen, S.; Zhu, Y.-A.; Holmen, A.; Chen, D. Effects of oxygen mobility in La–Fe-based perovskites on the catalytic activity and selectivity of methane oxidation. ACS Catal. 2020, 10, 3707–3719. [Google Scholar] [CrossRef]
- Gill, S.S.; Chatha, G.S.; Tsolakis, A. Analysis of reformed EGR on the performance of a diesel particulate filter. Int. J. Hydrog. Energy 2011, 36, 10089–10099. [Google Scholar] [CrossRef]
- Yamada, I. Novel catalytic properties of quadruple perovskites. Sci. Technol. Adv. MaTerialS 2017, 18, 541–548. [Google Scholar] [CrossRef]
- Legutko, P.; Jakubek, T.; Kaspera, W.; Stelmachowski, P.; Sojka, Z.; Kotarba, A. Soot oxidation over K-doped manganese and iron spinels—How potassium precursor nature and doping level change the catalyst activity. Catal. Commun. 2014, 43, 34–37. [Google Scholar] [CrossRef]
- Liu, H.; Dai, X.; Wang, K.; Yan, Z.; Qian, L. Highly efficient catalysts of Mn1−xAgxCo2O4 spinel oxide for soot combustion. Catal. Commun. 2017, 101, 134–137. [Google Scholar] [CrossRef]
- Islam, R. Performance of Nanostructured Ternary Metal Spinel Oxides as Efficient Electrocatalysts. 2019. Available online: https://hdl.handle.net/10315/37441 (accessed on 11 May 2019).
- Nazal, M.K.; Olakunle, O.S.; Al-Ahmed, A.; Merzougui, B.; Abualkibash, A.; Sultan, A.; Yousaf, A.B.; Zaidi, S.J. Precious metal free Ni/Cu/Mo trimetallic nanocomposite supported on multi-walled carbon nanotubes as highly efficient and durable anode-catalyst for alkaline direct methanol fuel cells. J. Electroanal. Chem. 2018, 823, 98–105. [Google Scholar] [CrossRef]
- Yousef, R.; Nassif, A.; Al-Zoubi, A.; Al-Din, N.S. Synthesis and Characterisation of Structural and Electrical Properties of CuMn2O4 Spinel Compound. Journal of King Faisal University: Basic and Applied Sciences 2021, 22, 47–50. [Google Scholar] [CrossRef]
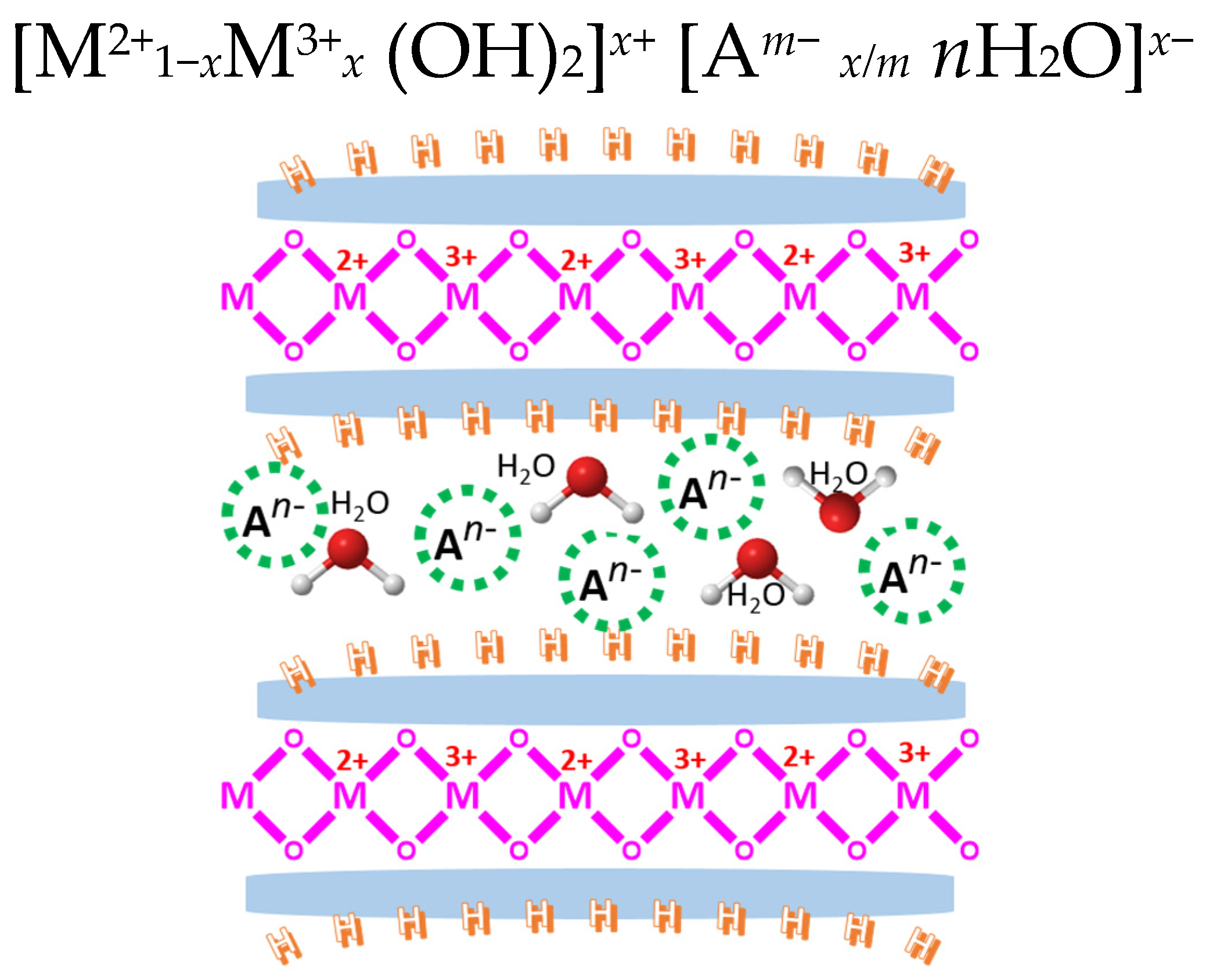
- Karim, A.V.; Hassani, A.; Eghbali, P.; Nidheesh, P. Nanostructured modified layered double hydroxides (LDHs)-based catalysts: A review on synthesis, characterization, and applications in water remediation by advanced oxidation processes. Curr. Opin. Solid State Mater. Sci. 2022, 26, 100965. [Google Scholar] [CrossRef]
- Bukhtiyarova, M. A review on effect of synthesis conditions on the formation of layered double hydroxides. J. Solid State Chem. 2019, 269, 494–506. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Tzou, Y.M.; Wang, M.K.; Liu, C.H.; Chiang, P.N. Removal of 2-Chlorophenol from Aqueous Solution by Mg/Al Layered Double Hydroxide (LDH) and Modified LDH. Ind. Eng. Chem. Res. 2008, 47, 3813–3819. [Google Scholar] [CrossRef]
- Yang, Z.-Z.; Wei, J.-J.; Zeng, G.-M.; Zhang, H.-Q.; Tan, X.-F.; Ma, C.; Li, X.-C.; Li, Z.-H.; Zhang, C. A review on strategies to LDH-based materials to improve adsorption capacity and photoreduction efficiency for CO2. Coord. Chem. Rev. 2019, 386, 154–182. [Google Scholar] [CrossRef]
- Ren, W.; Ding, T.; Yang, Y.; Xing, L.; Cheng, Q.; Zhao, D.; Zhang, Z.; Li, Q.; Zhang, J.; Zheng, L. Identifying oxygen activation/oxidation sites for efficient soot combustion over silver catalysts interacted with nanoflower-like hydrotalcite-derived CoAlO metal oxides. ACS Catal. 2019, 9, 8772–8784. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Xiong, J.; Wei, Y.; Jiang, N.; Li, Y.; Chi, H.; Zhao, Z.; Liu, J.; Jiao, J. Synergistic effect of binary Co and Ni cations in hydrotalcite-derived Co2-xNixAlO catalysts for promoting soot combustion. Fuel 2022, 320, 123888. [Google Scholar] [CrossRef]
- Long, X.; Wang, Z.; Xiao, S.; An, Y.; Yang, S. Transition metal based layered double hydroxides tailored for energy conversion and storage. Mater. Today 2016, 19, 213–226. [Google Scholar] [CrossRef]
- Ali, S.; Wu, X.; Zuhra, Z.; Ma, Y.; Abbas, Y.; Jin, B.; Ran, R.; Weng, D. Cu-Mn-Ce mixed oxides catalysts for soot oxidation and their mechanistic chemistry. Appl. Surf. Sci. 2020, 512, 145602. [Google Scholar] [CrossRef]
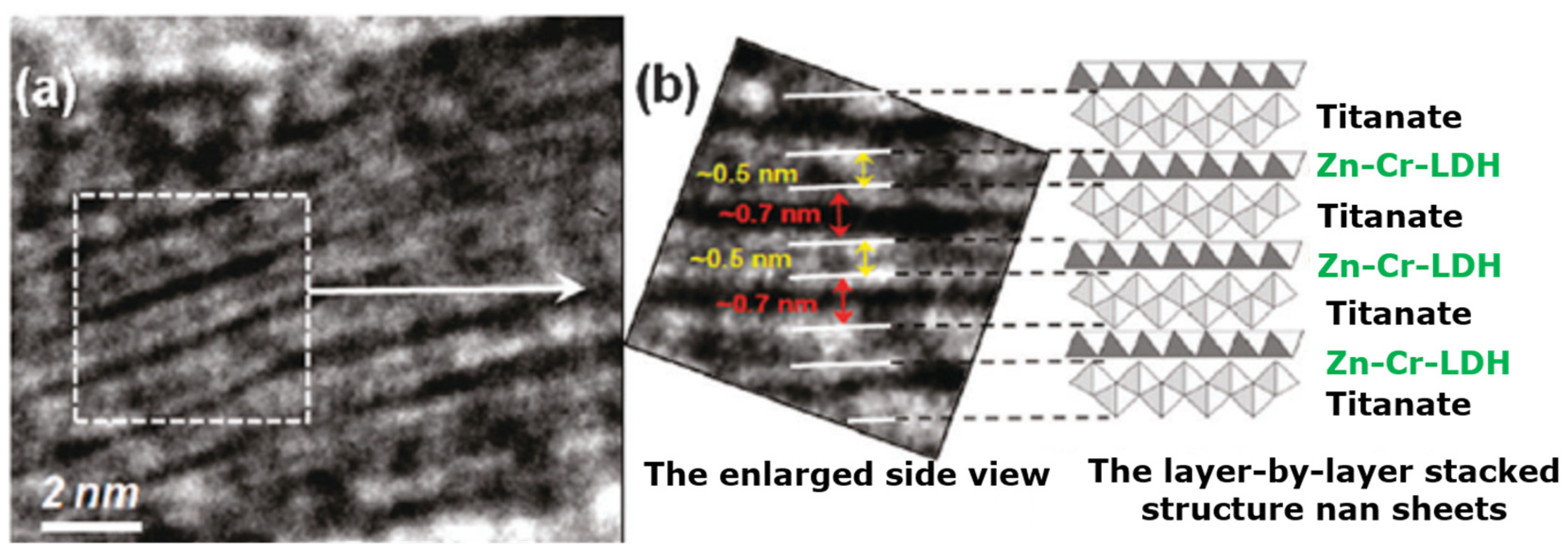
- Liu, J.; Li, J.; Bing, X.; Ng, D.H.L.; Cui, X.; Ji, F.; Kionga, D.D. ZnCr-LDH/N-doped graphitic carbon-incorporated g-C3N4 2D/2D nanosheet heterojunction with enhanced charge transfer for photocatalysis. Mater. Res. Bull. 2018, 102, 379–390. [Google Scholar] [CrossRef]
- Luo, B.; Song, R.; Jing, D. ZnCr LDH nanosheets modified graphitic carbon nitride for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2017, 42, 23427–23436. [Google Scholar] [CrossRef]
- Xu, Z.P.; Zhang, J.; Adebajo, M.O.; Zhang, H.; Zhou, C. Catalytic applications of layered double hydroxides and derivatives. Appl. Clay Sci. 2011, 53, 139–150. [Google Scholar] [CrossRef]
- Chaillot, D.; Bennici, S.; Brendlé, J. Layered double hydroxides and LDH-derived materials in chosen environmental applications: A review. Environ. Sci. Pollut. Res. 2021, 28, 24375–24405. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qin, T.; Ma, Y.; Xiong, J.; Zhang, P.; Lai, K.; Liu, X.; Zhao, Z.; Liu, J.; Chen, L. Revealing active edge sites induced by oriented lattice bending of Co-CeO2 nanosheets for boosting auto-exhaust soot oxidation. J. Catal. 2023, 421, 351–364. [Google Scholar] [CrossRef]
- Tang, S.; Yao, Y.; Chen, T.; Kong, D.; Shen, W.; Lee, H.K. Recent advances in the application of layered double hydroxides in analytical chemistry: A review. Anal. Chim. Acta 2020, 1103, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, A.; Wagle, P.G.; Jagtiani, E.; More, A.P. Layered double hydroxides (LDHs) for coating applications. J. Coat. Technol. Res. 2022, 19, 1009–1032. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Yu, Y.; Shan, W.; He, H. Insight into the better performance of Co than Pt on Ce-Sn catalyst for soot oxidation. Fuel 2023, 346, 128379. [Google Scholar] [CrossRef]
- Gawande, M.B.; Pandey, R.K.; Jayaram, R.V. Role of Mixed Metal Oxides in Catalysis Science—Versatile Applications in Organic Synthesis. Catal. Sci. Technol. 2012, 2, 1113–1125. [Google Scholar] [CrossRef]
- Shukla, M.; Balyan, Y.; Kumar, A.; Bhaskar, T.; Dhar, A. Catalytic oxidation of soot by CeO2–ZrO2 catalysts: Role of Zr. Mater. Chem. Phys. 2022, 286, 126161. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic Properties of Ceria and CeO2-Containing Materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, N.; Yin, X.; Wang, W.; Zhao, Y.; Zheng, Z.; Li, S.; Wang, J.; Chen, Y. Highlights on the key roles of interfaces between CeO2-based oxide and perovskite (LaMnO3/LaFeO3) in creating active oxygen species for soot oxidation. Fuel 2024, 356, 129444. [Google Scholar] [CrossRef]
- Zhang, D.; Du, X.; Shi, L.; Gao, R. Shape-controlled synthesis and catalytic application of ceria nanomaterials. Dalton Trans. 2012, 41, 14455–14475. [Google Scholar] [CrossRef]
- Tang, W.-X.; Gao, P.-X. Nanostructured cerium oxide: Preparation, characterization, and application in energy and environmental catalysis. Mrs Commun. 2016, 6, 311–329. [Google Scholar] [CrossRef]
- Wu, K.; Sun, L.-D.; Yan, C.-H. Recent Progress in Well-Controlled Synthesis of Ceria-Based Nanocatalysts towards Enhanced Catalytic Performance. Adv. Energy Mater. 2016, 6, 1600501. [Google Scholar] [CrossRef]
- Trovarelli, A.; Llorca, J. Ceria Catalysts at Nanoscale: How Do Crystal Shapes Shape Catalysis? Acs Catal. 2017, 7, 4716–4735. [Google Scholar] [CrossRef]
- Huang, W.; Gao, Y. Morphology-dependent surface chemistry and catalysis of CeO2 nanocrystals. Catal. Sci. Technol. 2014, 4, 3772–3784. [Google Scholar] [CrossRef]
- Qiao, Z.-A.; Wu, Z.; Dai, S. Shape-Controlled Ceria-based Nanostructures for Catalysis Applications. Chemsuschem 2013, 6, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Tana; Zhang, M.; Li, J.; Li, H.; Li, Y.; Shen, W. Morphology-dependent redox and catalytic properties of CeO2 nanostructures: Nanowires, nanorods and nanoparticles. Catal. Today 2009, 148, 179–183. [Google Scholar] [CrossRef]
- He, H.; Yang, P.; Li, J.; Shi, R.; Chen, L.; Zhang, A.; Zhu, Y. Controllable synthesis, characterization, and CO oxidation activity of CeO2 nanostructures with various morphologies. Ceram. Int. 2016, 42, 7810–7818. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, J.; Yichuan, Y.; Wang, Z.; Gao, Y.; Liu, W.; Yin, H. Catalytic oxidation of soot on mesoporous ceria-based mixed oxides with cetyltrimethyl ammonium bromide (CTAB)-assisted synthesis. J. Colloid Interface Sci. 2017, 508, 1–13. [Google Scholar] [CrossRef]
- Shan, W.; Guo, H.; Liu, C.; Wang, X. Controllable preparation of CeO2 nanostructure materials and their catalytic activity. J. Rare Earths 2012, 30, 665–669. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Z.; Liu, C.; Wang, F.; Zhang, Y.; Wang, M. Biotemplated fabrication of hierarchical mesoporous CeO2 derived from diatom and its application for catalytic oxidation of CO. Chin. Sci. Bull. 2014, 59, 3260–3265. [Google Scholar] [CrossRef]
- Alammar, T.; Noei, H.; Wang, Y.; Gruener, W.; Mudring, A.-V. Ionic Liquid-Assisted Sonochemical Preparation of CeO2 Nanoparticles for CO Oxidation. Acs Sustain. Chem. Eng. 2015, 3, 42–54. [Google Scholar] [CrossRef]
- Tang, W.; Li, W.; Shan, X.; Wu, X.; Chen, Y. Template-free synthesis of hierarchical layer-stacking CeO2 nanostructure with enhanced catalytic oxidation activity. Mater. Lett. 2015, 140, 95–98. [Google Scholar] [CrossRef]
- Kittelson, D.B. Engines and nanoparticles: A review. J. Aerosol Sci. 1998, 29, 575–588. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Dai, F.; Zha, Y.; Xie, Y.; Hu, T.; Zhang, J. Multifunctional hydrotalcite-derived K/MnMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot–NOx removal. Chem. Eng. J. 2012, 184, 106–112. [Google Scholar] [CrossRef]
- Li, Q.; Meng, M.; Tsubaki, N.; Li, X.; Li, Z.; Xie, Y.; Hu, T.; Zhang, J. Performance of K-promoted hydrotalcite-derived CoMgAlO catalysts used for soot combustion, NOx storage and simultaneous soot–NOx removal. Appl. Catal. B Environ. 2009, 91, 406–415. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Di Benedetto, A.; Lisi, L. Synergy between ceria and metals (Ag or Cu) in catalytic diesel particulate filters: Effect of the metal content and of the preparation method on the regeneration performance. Top. Catal. 2021, 64, 256–269. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Di Benedetto, A. Ceria-coated diesel particulate filters for continuous regeneration. AIChE J. 2017, 63, 3442–3449. [Google Scholar] [CrossRef]
- Di Sarli, V.; Landi, G.; Lisi, L.; Saliva, A.; Di Benedetto, A. Catalytic diesel particulate filters with highly dispersed ceria: Effect of the soot-catalyst contact on the regeneration performance. Appl. Catal. B Environ. 2016, 197, 116–124. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.-M.; Duan, A.-J. Simultaneous removal of NOx and diesel soot over nanometer Ln-Na-Cu-O perovskite-like complex oxide catalysts. Appl. Catal. B Environ. 2008, 78, 61–72. [Google Scholar] [CrossRef]
- Fino, D.; Russo, N.; Saracco, G.; Specchia, V. Catalytic removal of NOx and diesel soot over nanostructured spinel-type oxides. J. Catal. 2006, 242, 38–47. [Google Scholar] [CrossRef]
- Fino, D.; Fino, P.; Saracco, G.; Specchia, V. Studies on kinetics and reactions mechanism of La2−xKxCu1−yVyO4 layered perovskites for the combined removal of diesel particulate and NOx. Appl. Catal. B Environ. 2003, 43, 243–259. [Google Scholar] [CrossRef]
- Milt, V.G.; Ulla, M.A.; Miró, E.E. NOx trapping and soot combustion on BaCoO3−y perovskite: LRS and FTIR characterization. Appl. Catal. B Environ. 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Suzuki, J.; Matsumoto, S.I. Development of Catalysts for Diesel Particulate NOxReduction. Top. Catal. 2004, 28, 171–176. [Google Scholar] [CrossRef]
- Tichit, D.; Coq, B. Catalysis by Hydrotalcites and Related Materials. Cattech 2003, 7, 206–217. [Google Scholar] [CrossRef]
- Silletti, B.A.; Adams, R.T.; Sigmon, S.M.; Nikolopoulos, A.; Spivey, J.J.; Lamb, H.H. A novel Pd/MgAlOx catalyst for NOx storage-reduction. Catal. Today 2006, 114, 64–71. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Su, Q.; Wang, Z.; Li, Q.; Gao, X. Determination of Intermediates and Mechanism for Soot Combustion with NOx/O2 on Potassium-Supported Mg−Al Hydrotalcite Mixed Oxides by In Situ FTIR. Environ. Sci. Technol. 2010, 44, 8254–8258. [Google Scholar] [CrossRef]
- Yang, R.; Gao, Y.; Wang, J.; Wang, Q. Layered double hydroxide (LDH) derived catalysts for simultaneous catalytic removal of soot and NOx. Dalton Trans. 2014, 43, 10317–10327. [Google Scholar] [CrossRef]
- Castoldi, L. An overview on the catalytic materials proposed for the simultaneous removal of NOx and soot. Materials 2020, 13, 3551. [Google Scholar] [CrossRef]
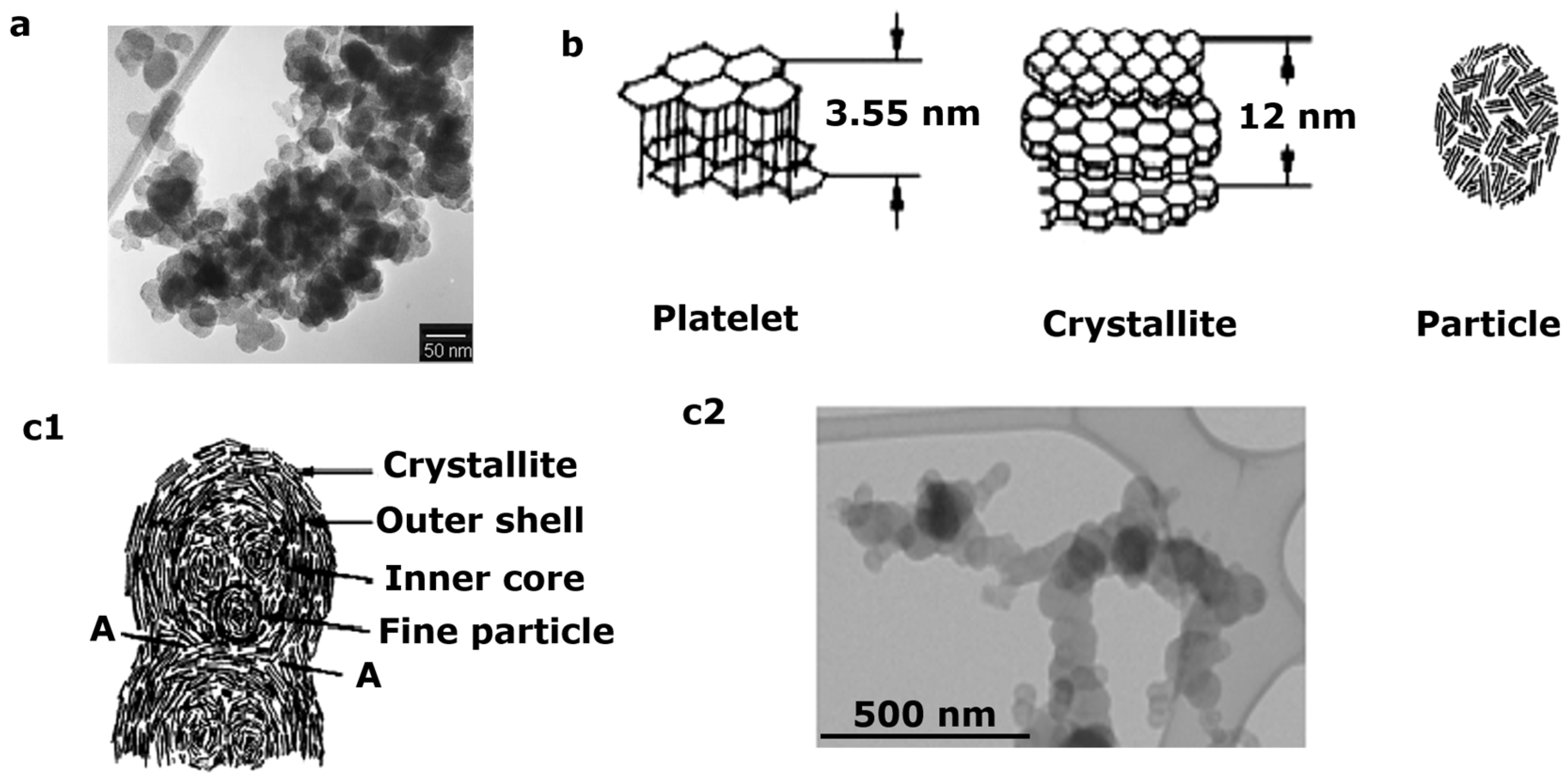
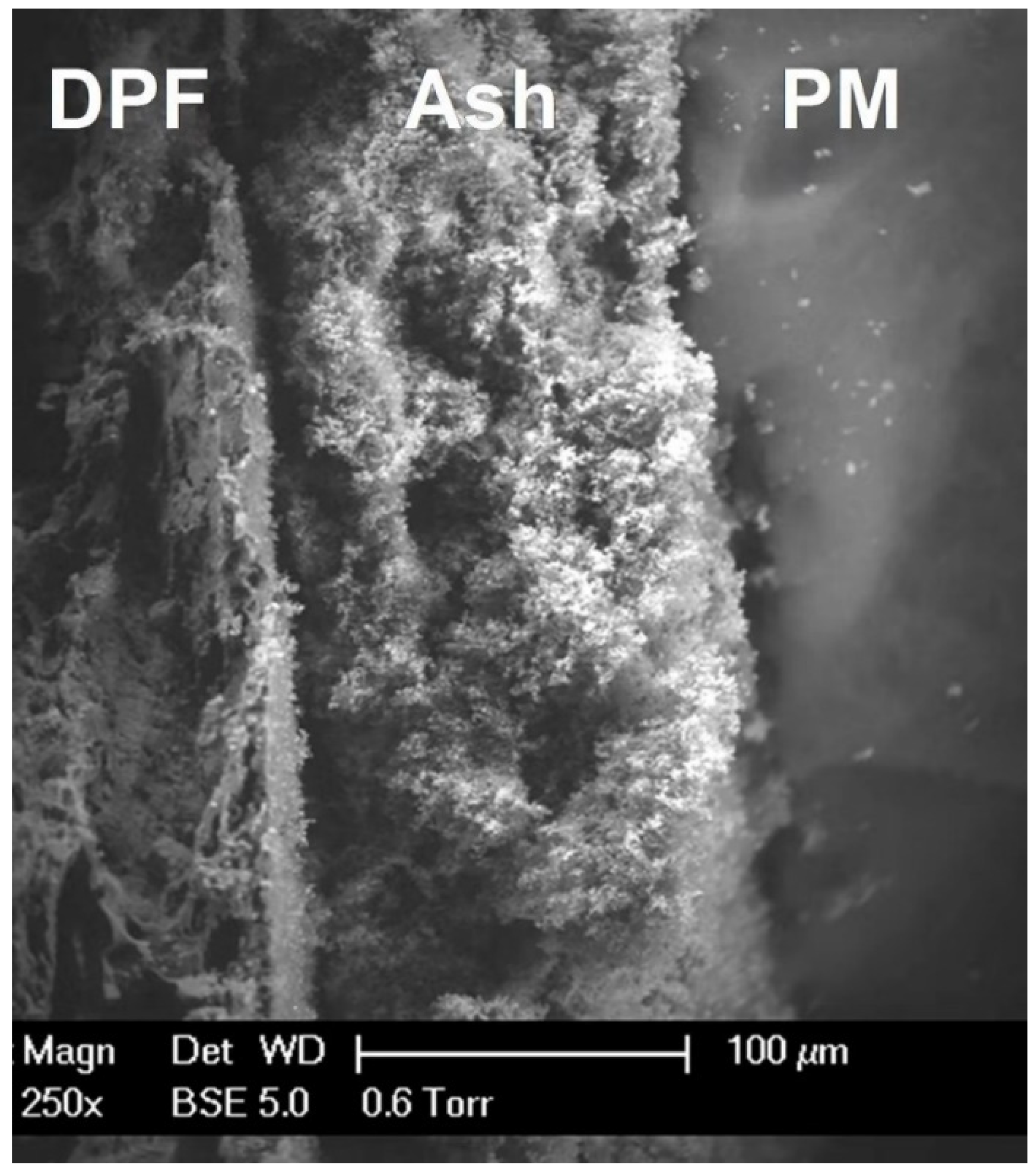

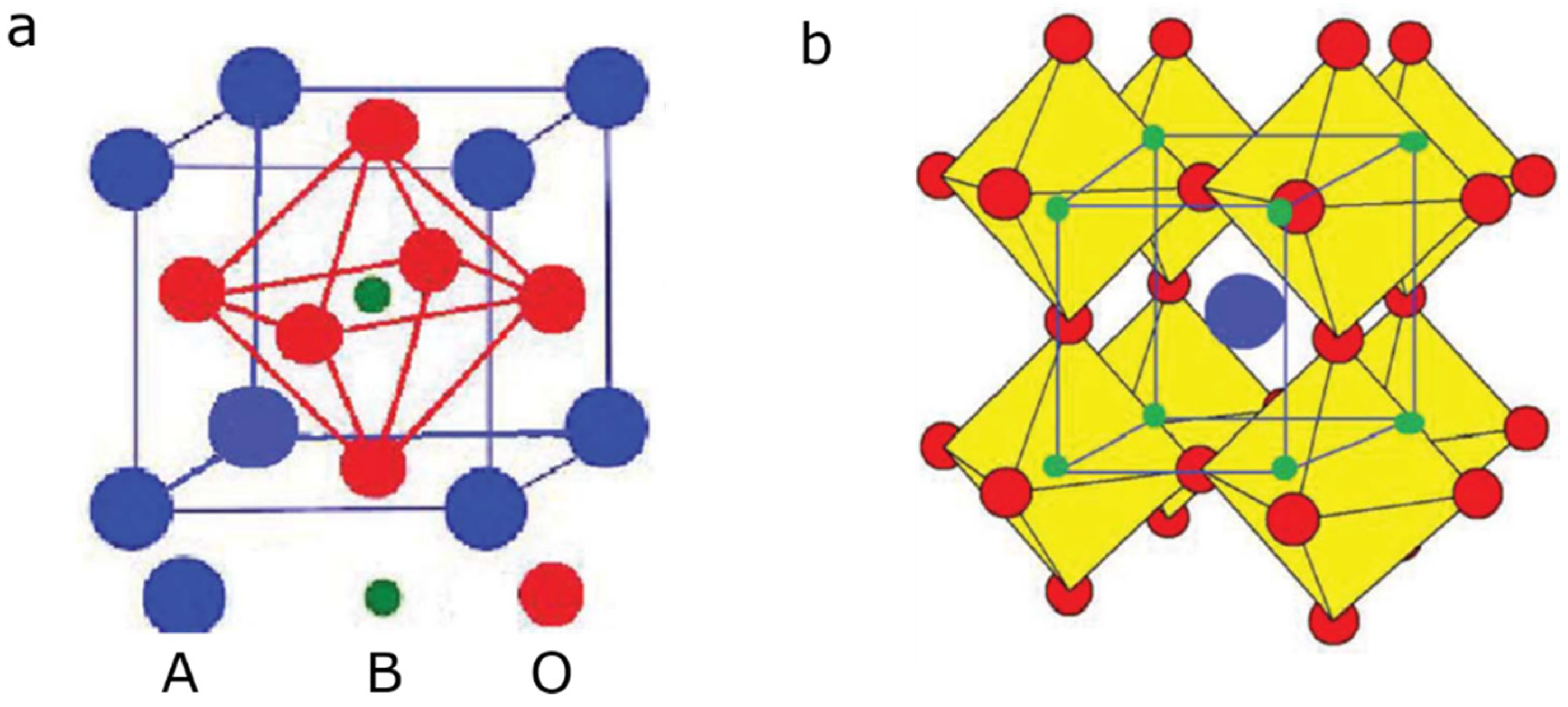
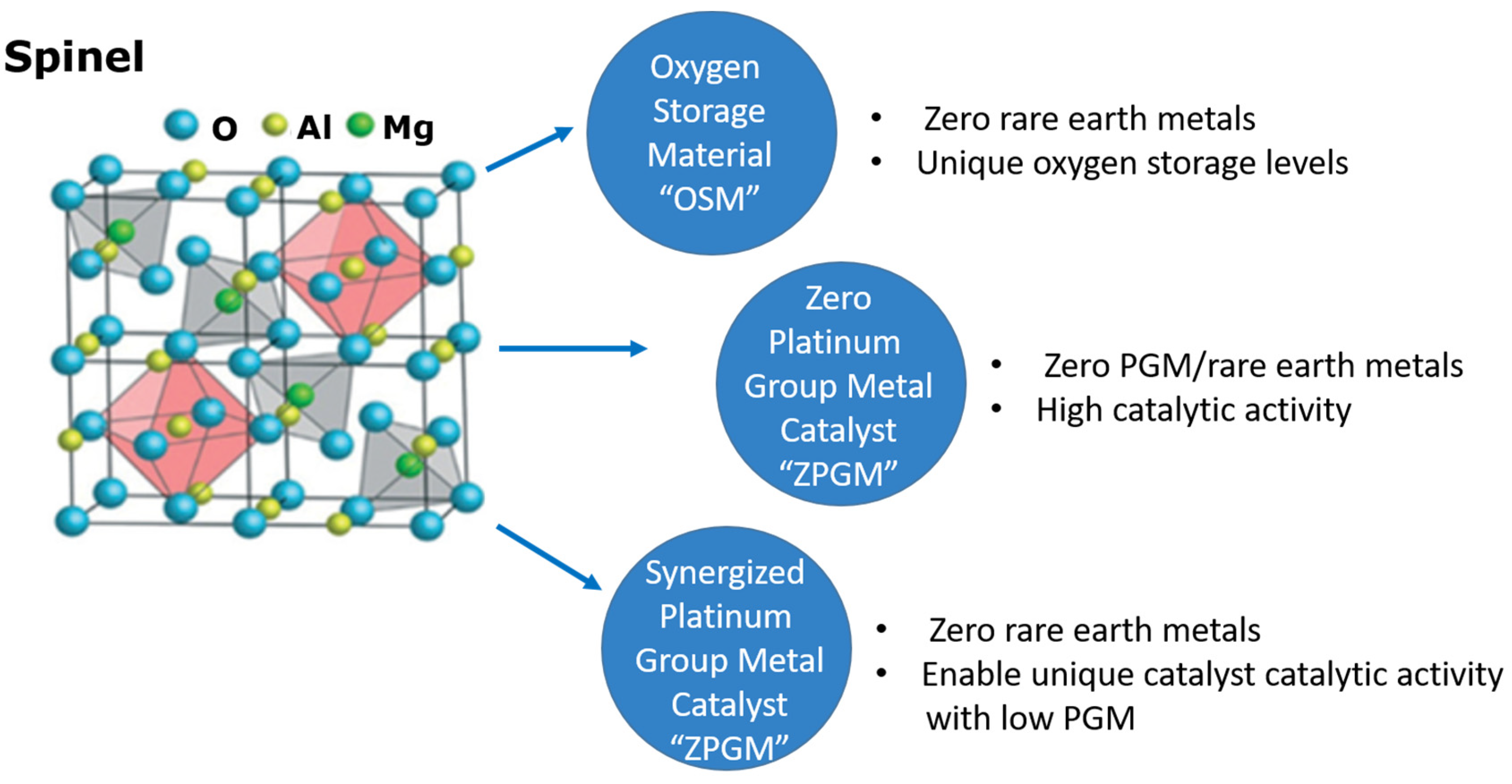
| Pollutants | Concentration | Threats |
|---|---|---|
| Soot | 20–200 mg/m3 | Eyes problems, cancer, asthma, skin infections, lung damage, heart issues |
| NOx | 30–1000 ppm | Chest pain, respiratory and lungs problems, cough |
| SOx | Proportional to fuel S content | Acid rain, skin problems |
| CO2 | 2–12 vol% | Green house effect, acid rain, lung disease |
| CO | 100–1000 ppm | Hpertension, head pressure, lung disease |
| HC | 50–500 ppm | Eyes irritation, lungs issues, respiratory problems |
| PAH | 0.3 mg/mil | Kindney and liver damage |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuhra, Z.; Li, S.; Xie, G.; Wang, X. Soot Erased: Catalysts and Their Mechanistic Chemistry. Molecules 2023, 28, 6884. https://doi.org/10.3390/molecules28196884
Zuhra Z, Li S, Xie G, Wang X. Soot Erased: Catalysts and Their Mechanistic Chemistry. Molecules. 2023; 28(19):6884. https://doi.org/10.3390/molecules28196884
Chicago/Turabian StyleZuhra, Zareen, Shuo Li, Guanqun Xie, and Xiaoxia Wang. 2023. "Soot Erased: Catalysts and Their Mechanistic Chemistry" Molecules 28, no. 19: 6884. https://doi.org/10.3390/molecules28196884
APA StyleZuhra, Z., Li, S., Xie, G., & Wang, X. (2023). Soot Erased: Catalysts and Their Mechanistic Chemistry. Molecules, 28(19), 6884. https://doi.org/10.3390/molecules28196884