Low-Temperature Terpolymerizable Benzoxazine Monomer Bearing Norbornene and Furan Groups: Synthesis, Characterization, Polymerization, and Properties of Its Polymer
Abstract
1. Introduction
2. Results and Discussion
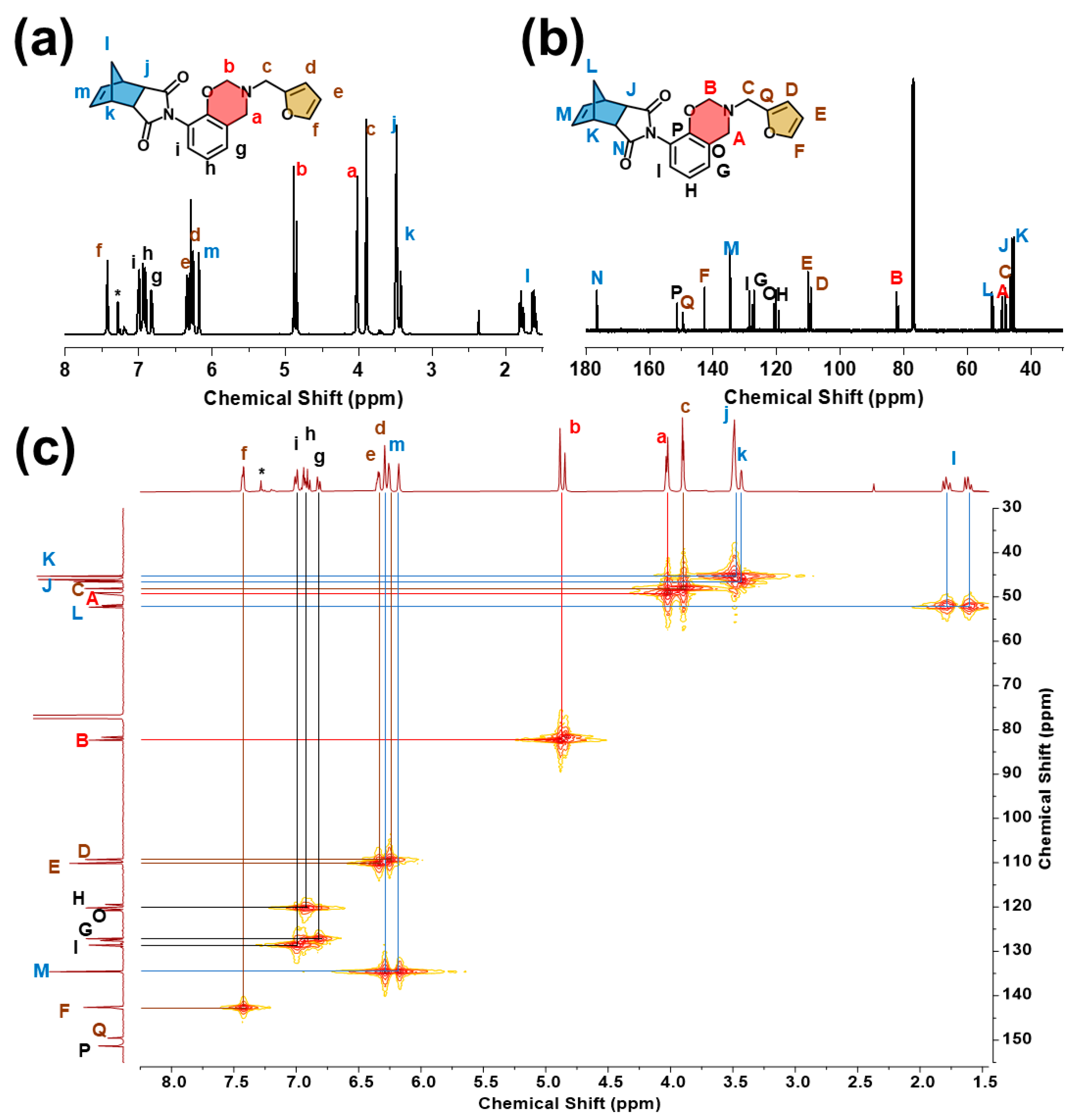
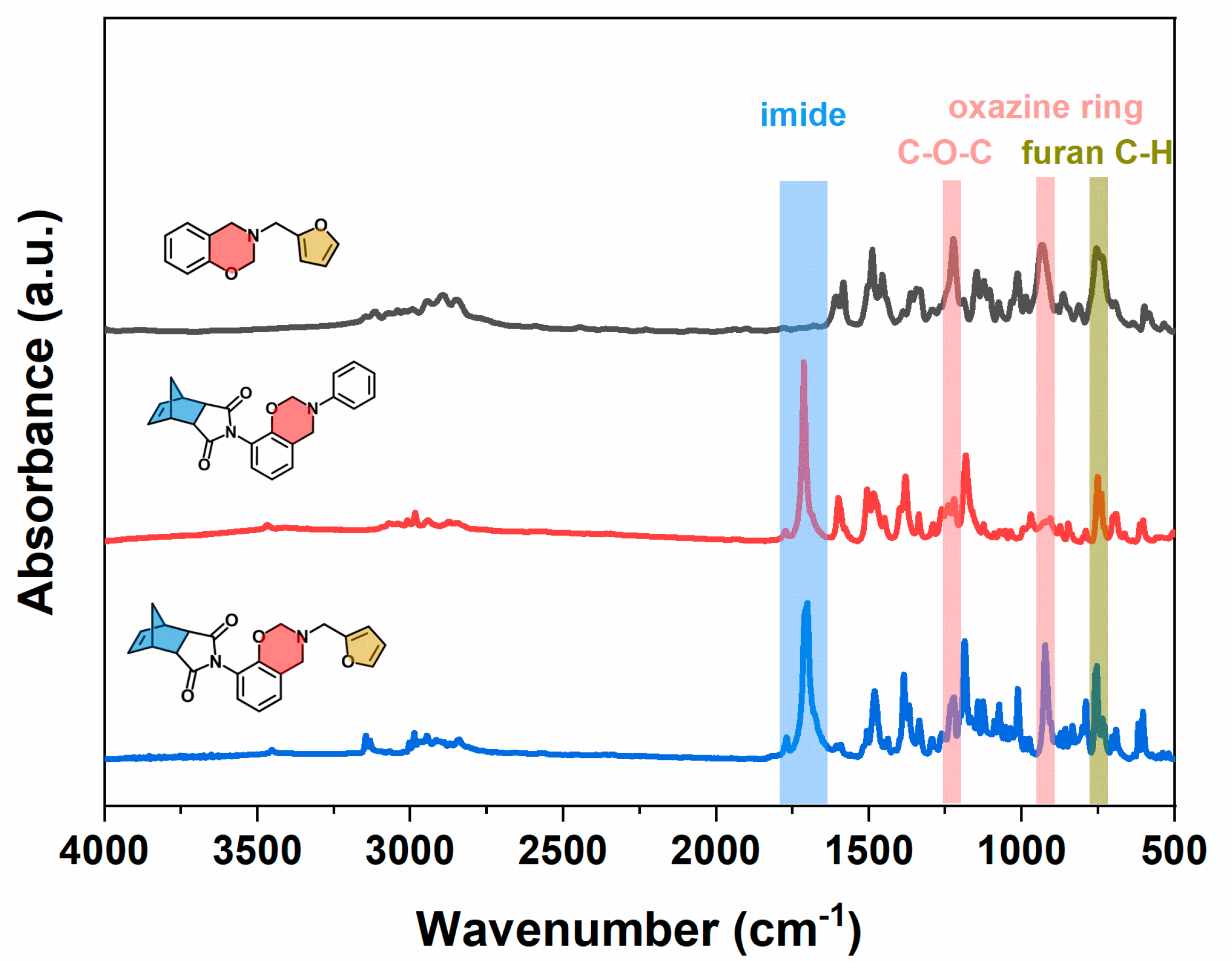
2.1. Design, Synthesis, and Structural Characterization of the Benzoxazine Monomer
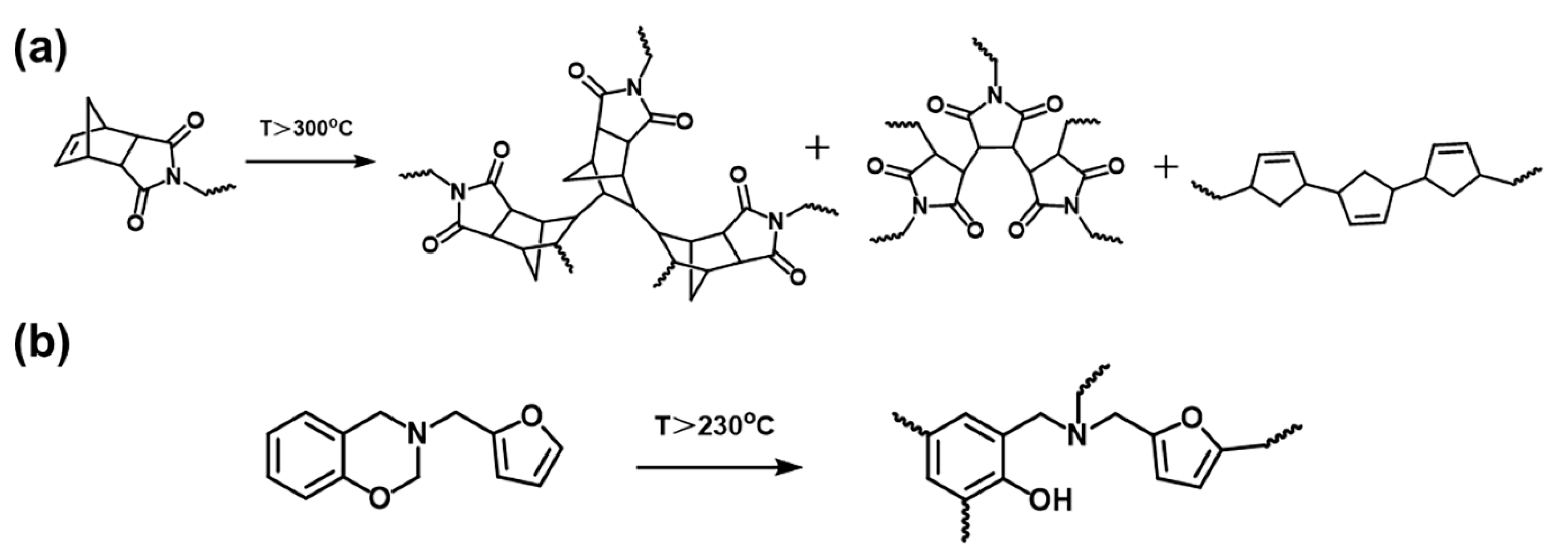
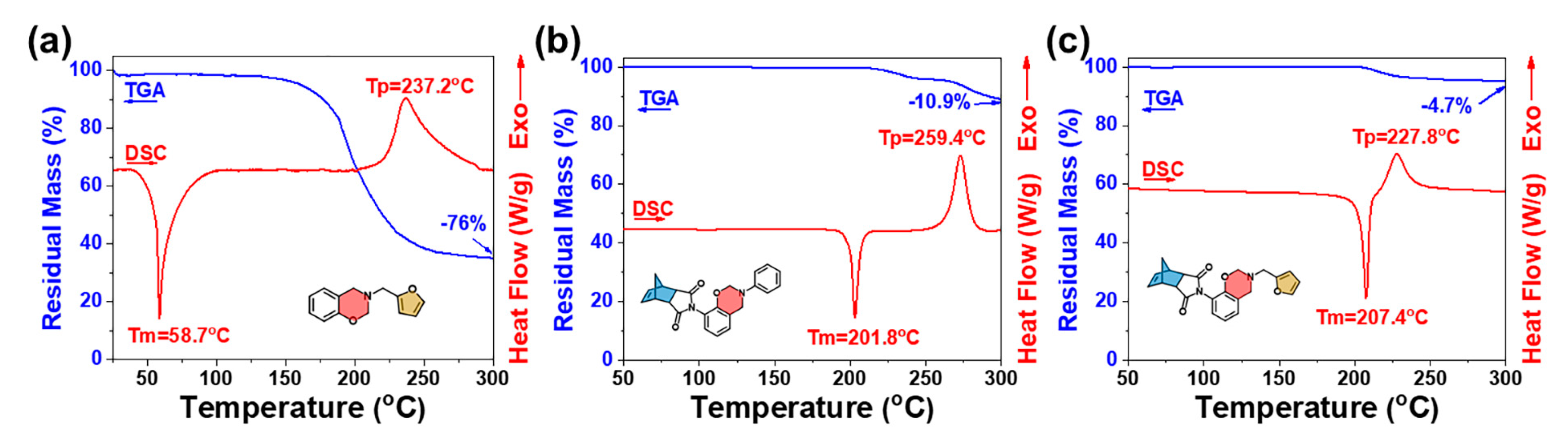
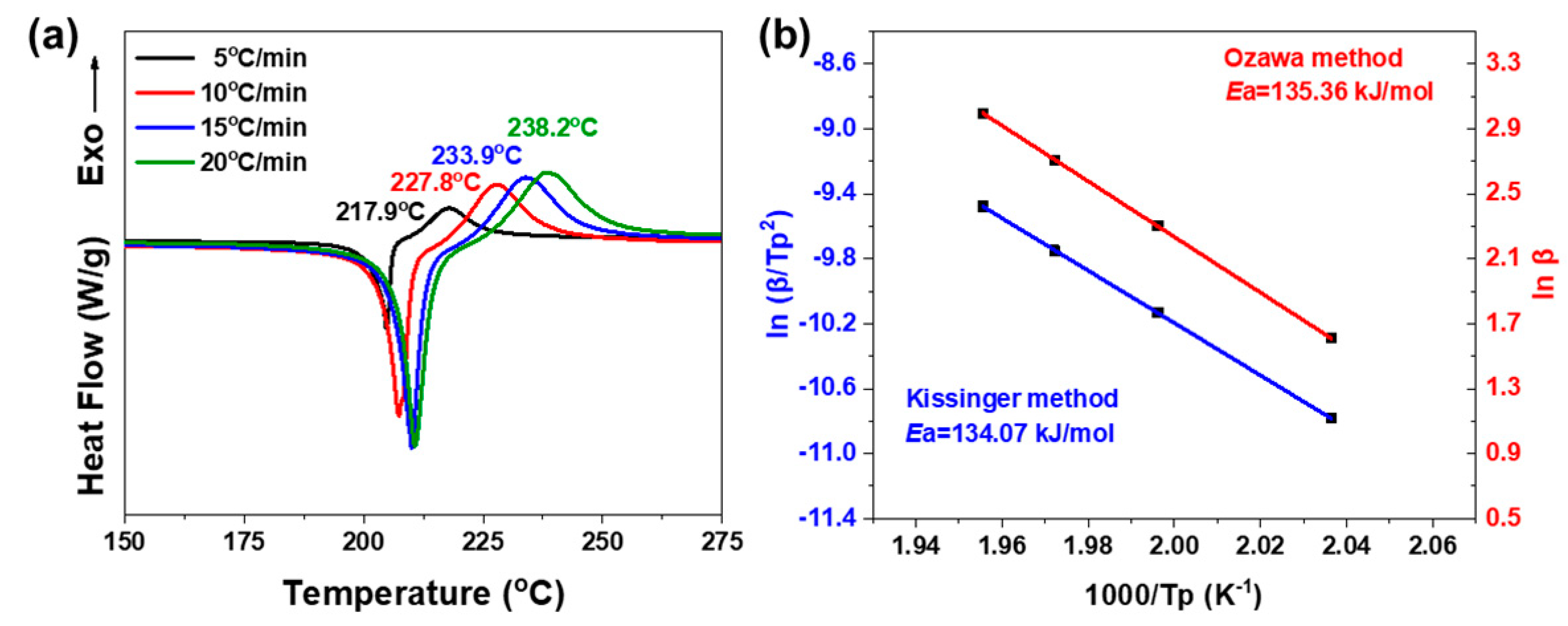
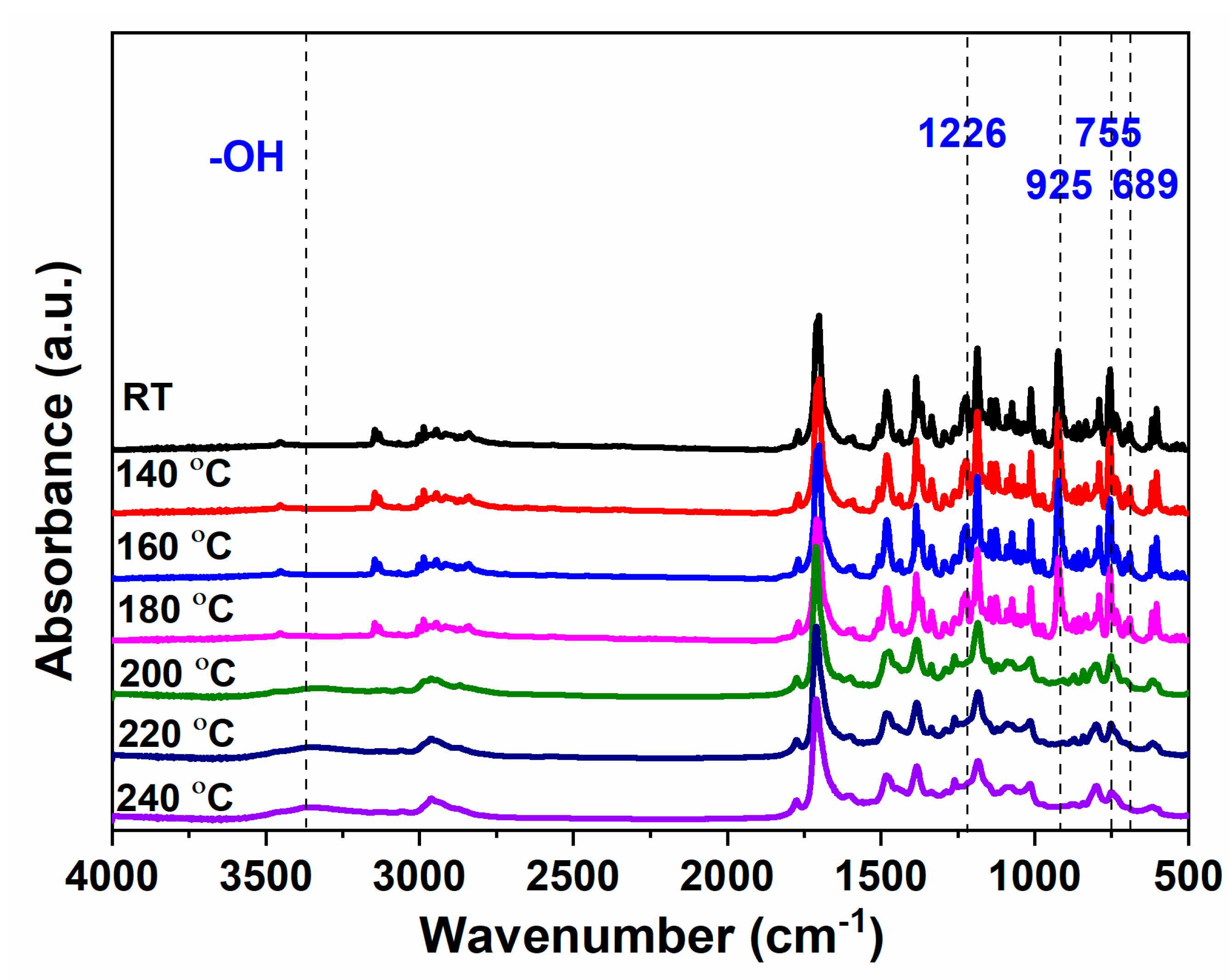
2.2. Polymerization Behaviors of oHPNI-fa
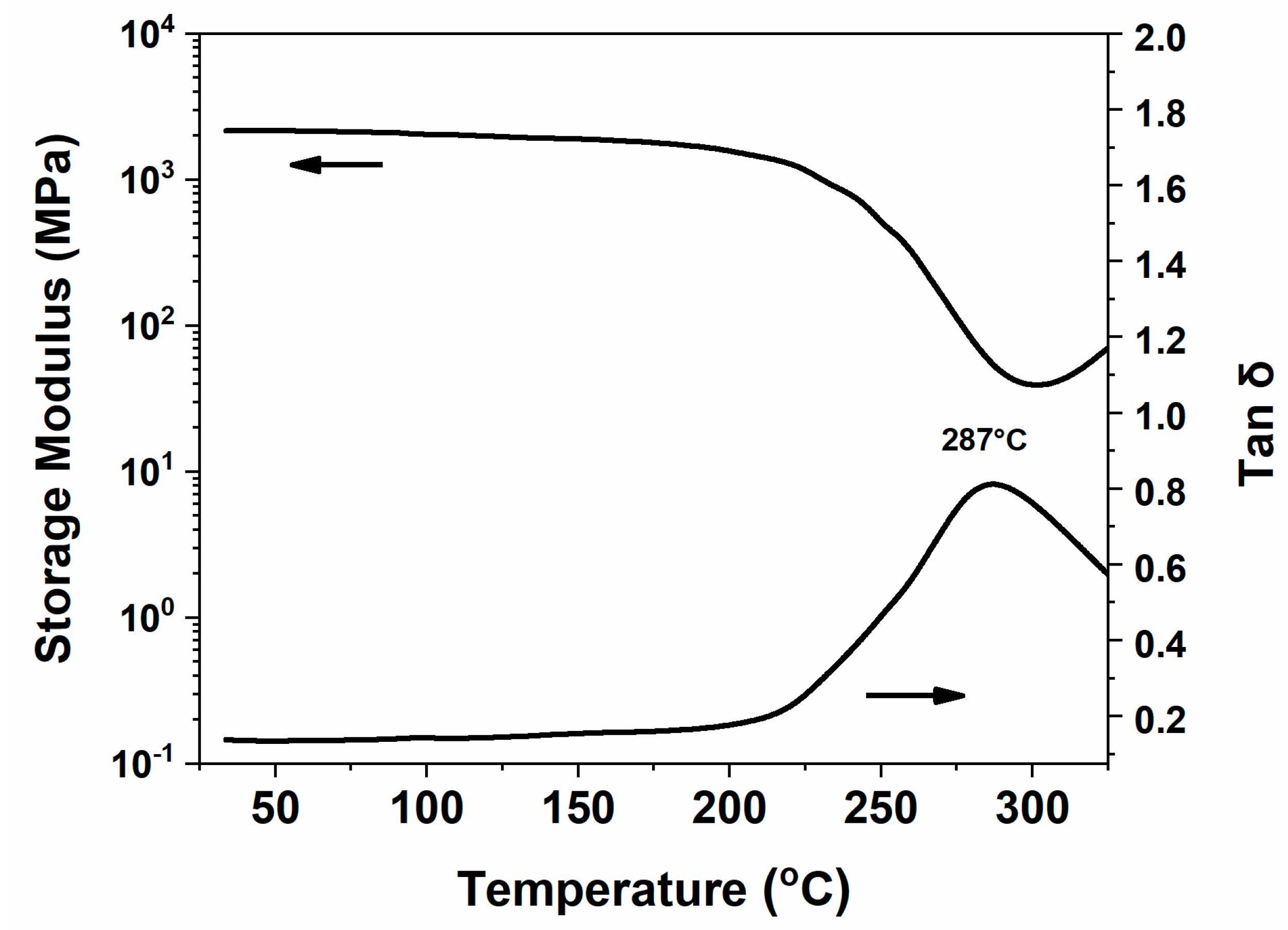
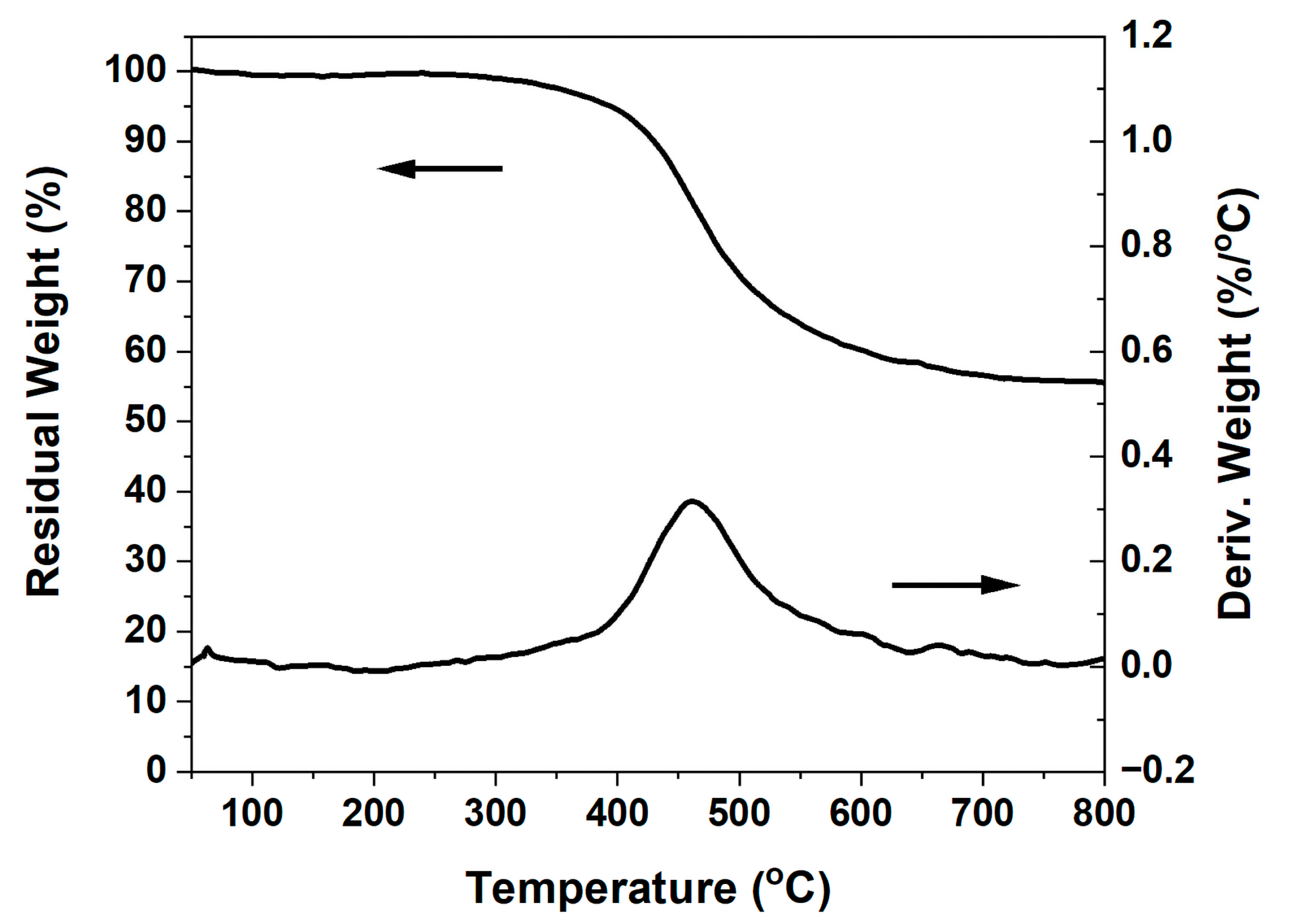
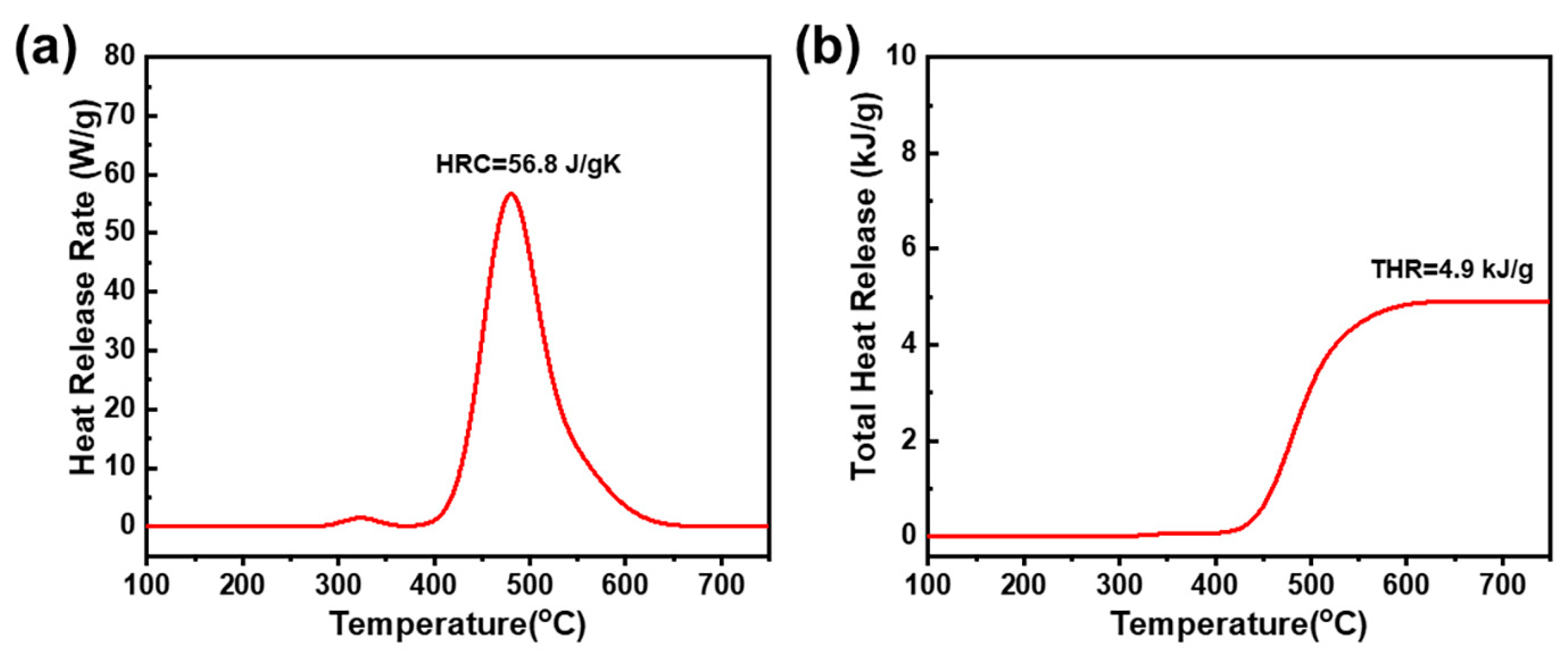
2.3. Thermal Stability and Anti-Flame Properties of Highly Cross-Linked Thermoset
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of (3aR,4S,7R,7aS)-2-(3-(Furan-2-ylmethyl)-3,4-dihydro-2H-benzo[e][1,3]oxazin-8-yl)-3a,4,7,7a-tetrahydro-1H-4,7-methanoisoindole-1,3(2H)-dione (Abbreviated as oHPNI-fa)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Gupta, M. Polymer Composite; New Age International: Chennai, India, 2007. [Google Scholar]
- Lyu, M.-Y.; Choi, T.G. Research trends in polymer materials for use in lightweight vehicles. Int. J. Precis. Eng. Man. 2015, 16, 213–220. [Google Scholar] [CrossRef]
- Kesarwani, S. Polymer composites in aviation sector. Int. J. Eng. Res 2017, 6, 518–525. [Google Scholar] [CrossRef]
- Forintos, N.; Czigany, T. Multifunctional application of carbon fiber reinforced polymer composites: Electrical properties of the reinforcing carbon fibers–A short review. Compos. B Eng. 2019, 162, 331–343. [Google Scholar] [CrossRef]
- Das, S. Life cycle assessment of carbon fiber-reinforced polymer composites. Int. J. Life Cycle. Assess. 2011, 16, 268–282. [Google Scholar] [CrossRef]
- Ogasawara, T.; Hirano, Y.; Yoshimura, A. Coupled thermal–electrical analysis for carbon fiber/epoxy composites exposed to simulated lightning current. Compos. Part A Appl. Sci. Manuf. 2010, 41, 973–981. [Google Scholar] [CrossRef]
- Awasthi, S.; Wood, J.L. Carbon/carbon composite materials for aircraft brakes. In Proceedings of the 12th Annual Conference on Composites and Advanced Ceramic Materials: Ceramic Engineering and Science Proceedings, Cocoa Beach, FL, USA, 17–22 January 1988; pp. 553–559. [Google Scholar]
- Soutis, C. Carbon fiber reinforced plastics in aircraft construction. Mat. Sci. Eng. A 2005, 412, 171–176. [Google Scholar] [CrossRef]
- Soutis, C. Fibre reinforced composites in aircraft construction. Prog. Aerosp. Sci. 2005, 41, 143–151. [Google Scholar] [CrossRef]
- Hu, A.; Hao, J.; He, T.; Yang, S. Synthesis and characterization of high-temperature fluorine-containing PMR polyimides. Macromolecules 1999, 32, 8046–8051. [Google Scholar] [CrossRef]
- Wilson, D. PMR-15 processing, properties and problems—A review. Br. Polym. J. 1988, 20, 405–416. [Google Scholar] [CrossRef]
- Pascal, T.; Audigier, D.; Mercier, R.; Sillion, B. A soluble nadimide end-capped fluorinated resin. Polymer 1991, 32, 1119–1125. [Google Scholar] [CrossRef]
- Turk, M.J.; Ansari, A.S.; Alston, W.B.; Gahn, G.S.; Frimer, A.A.; Scheiman, D.A. Evaluation of the thermal oxidative stability of polyimides via TGA techniques. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 3943–3956. [Google Scholar] [CrossRef]
- Meador, M.A. Recent advances in the development of processable high-temperature polymers. Annu. Rev. Mater. Sci. 1998, 28, 599–630. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, X. Catalyst-free and low-temperature terpolymerization in a single-component benzoxazine resin containing both norbornene and acetylene functionalities. Macromolecules 2018, 51, 6524–6533. [Google Scholar] [CrossRef]
- Zhang, K.; Qiu, J.; Li, S.; Shang, Z.; Wang, J. Remarkable improvement of thermal stability of main-chain benzoxazine oligomer by incorporating o-norbornene as terminal functionality. J. Appl. Polym. Sci. 2017, 134, 45408. [Google Scholar] [CrossRef]
- Zhang, K.; Ishida, H. Thermally stable polybenzoxazines via ortho-norbornene functional benzoxazine monomers: Unique advantages in monomer synthesis, processing and polymer properties. Polymer 2015, 66, 240–248. [Google Scholar] [CrossRef]
- Hao, B.; Lu, Y.; Zhang, Y.; Zhang, K. Synthetic approaches to bio-based flame-retardant polymeric materials. In Bio-Based Flame-retardant Technology for Polymeric Materials; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–60. [Google Scholar]
- Zhang, K.; Liu, Y.; Ishida, H. Polymerization of an AB-type benzoxazine monomer toward different polybenzoxazine networks: When diels–alder reaction meets benzoxazine chemistry in a single-component resin. Macromolecules 2019, 52, 7386–7395. [Google Scholar] [CrossRef]
- Machado, I.; Shaer, C.; Hurdle, K.; Calado, V.; Ishida, H. Towards the development of green flame retardancy by polybenzoxazines. Prog. Polym. Sci. 2021, 121, 101435. [Google Scholar] [CrossRef]
- Lyu, Y.; Ishida, H. Natural-sourced benzoxazine resins, homopolymers, blends and composites: A review of their synthesis, manufacturing and applications. Prog. Polym. Sci. 2019, 99, 101168. [Google Scholar] [CrossRef]
- Ghosh, N.; Kiskan, B.; Yagci, Y. Polybenzoxazines—New high performance thermosetting resins: Synthesis and properties. Prog. Polym. Sci. 2007, 32, 1344–1391. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Li, C.-J.; Khan, M.A.R.; Liaw, C.-C.; Zhang, K.; Kuo, S.-W. Formaldehyde-Free Synthesis of Fully Bio-Based Multifunctional Bisbenzoxazine Resins from Natural Renewable Starting Materials. Macromolecules 2022, 55, 3106–3115. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Kotzebue, L.R.V.; Freitas, D.B.; Mattos, A.L.A.; da Costa Júnior, A.E.; Mazzetto, S.E.; Lomonaco, D. Towards novel high-performance bio-composites: Polybenzoxazine-based matrix reinforced with Manicaria saccifera fabrics. Compos. B Eng. 2020, 194, 108060. [Google Scholar] [CrossRef]
- Machado, I.; Rachita, E.; Fuller, E.; Calado, V.N.M.; Ishida, H. Very High-Char-Yielding Elastomers Based on the Copolymers of a Catechol/Furfurylamine Benzoxazine and Polydimethylsiloxane Oligomers. ACS Sustain. Chem. Eng. 2021, 9, 16637–16650. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, X.; Han, L.; Zhang, K. Recent Progress of High Performance Thermosets Based on Norbornene Functional Benzoxazine Resins. Polymers 2021, 13, 1417. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, X.; Wang, Y.; Liu, Y. Thermally Activated Structural Changes of a Norbornene–Benzoxazine–Phthalonitrile Thermosetting System: Simple Synthesis, Self-Catalyzed Polymerization, and Outstanding Flame Retardancy. ACS Appl. Polym. Mater. 2019, 1, 2713–2722. [Google Scholar] [CrossRef]
- Yu, X.; Shang, Z.; Zhang, K. Thermally stable polybenzoxazines via tetrahydrophthalimide-functional monobenzoxazines: Synthesis, characterization and thermally activated polymerization kinetics. Thermochim. Acta 2019, 675, 29–37. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, J.; Ohashi, S.; Liu, X.; Han, Z.; Ishida, H. Synthesis of high thermal stability polybenzoxazoles via ortho-imide-functional benzoxazine monomers. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1330–1338. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, K. Studies on the isomeric effect of nitrile functionality on the polymerization and thermal properties of ortho-norbornene-based benzoxazine resins. J. Polym. Res. 2020, 27, 130. [Google Scholar] [CrossRef]
- Lu, Y.; Yu, X.; Evans, C.J.; Yang, S.; Zhang, K. Elucidating the role of acetylene in ortho-phthalimide functional benzoxazines: Design, synthesis, and structure–property investigations. Polym. Chem. 2021, 12, 5059–5068. [Google Scholar] [CrossRef]
- Zhao, W.; Hao, B.; Lu, Y.; Zhang, K. Thermal latent and Low-Temperature polymerization of a Bio-Benzoxazine resin from natural renewable chrysin and furfurylamine. Eur. Polym. J. 2022, 166, 111041. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, Y.; Zhang, K. A highly thermally stable benzoxazine resin derived from norbornene and natural renewable tyramine and furfurylamine. Eur. Polym. J. 2023, 187, 111895. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Zhao, W.; Zhang, K. Bio-benzoxazine structural design strategy toward highly thermally stable and intrinsically flame-retardant thermosets. Chem. Eng. J. 2023, 457, 141232. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Zhang, K. Renewable Biomass Resources to Access Halogen-and Phosphorus-Free Flame Retardant Thermosets with Ultra-Low Heat Release Capacity. Chem. Eng. J. 2022, 22, 137670. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Han, M.; Froimowicz, P. Smart and sustainable design of latent catalyst-containing benzoxazine-bio-resins and application studies. Green Chem. 2020, 22, 1209–1219. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, K. Synthesis and properties of biobased mono-benzoxazine resins from natural renewable pterostilbene. Eur. Polym. J. 2021, 156, 110607. [Google Scholar] [CrossRef]
- Mukherjee, S.; Amarnath, N.; Lochab, B. Oxazine Ring-Substituted 4th Generation Benzoxazine Monomers & Polymers: Stereoelectronic Effect of Phenyl Substituents on Thermal Properties. Macromolecules 2021, 54, 10001–10016. [Google Scholar]
- Zhang, K.; Han, M.; Liu, Y.; Froimowicz, P. Design and synthesis of bio-based high-performance trioxazine benzoxazine resin via natural renewable resources. ACS Sustain. Chem. Eng. 2019, 7, 9399–9407. [Google Scholar] [CrossRef]
- Kotzebue, L.R.; de Oliveira, J.S.R.; da Silva, J.B.; Mazzetto, S.E.; Ishida, H.; Lomonaco, D. Development of fully biobased high-performance bis-benzoxazine under environmentally friendly conditions. ACS Sustain. Chem. Eng. 2018, 6, 5485–5494. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chou, C.I. High performance benzoxazine monomers and polymers containing furan groups. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 5267–5282. [Google Scholar] [CrossRef]
- Hao, B.; Han, L.; Liu, Y.; Zhang, K. An apigenin-based bio-benzoxazine with three polymerizable functionalities: Sustainable synthesis, thermal latent polymerization, and excellent thermal properties of its thermosets. Polym. Chem. 2020, 11, 5800–5809. [Google Scholar] [CrossRef]
- Han, L.; Iguchi, D.; Gil, P.; Heyl, T.R.; Sedwick, V.M.; Arza, C.R.; Ohashi, S.; Lacks, D.J.; Ishida, H. Oxazine ring-related vibrational modes of benzoxazine monomers using fully aromatically substituted, deuterated, 15N isotope exchanged, and oxazine-ring-substituted compounds and theoretical calculations. J. Phys. Chem. A 2017, 121, 6269–6282. [Google Scholar] [CrossRef]
- Han, L.; Salum, M.L.; Zhang, K.; Froimowicz, P.; Ishida, H. Intrinsic self-initiating thermal ring-opening polymerization of 1, 3-benzoxazines without the influence of impurities using very high purity crystals. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 3434–3445. [Google Scholar] [CrossRef]
- Zhang, K.; Han, L.; Nie, Y.; Szigeti, M.L.; Ishida, H. Examining the effect of hydroxyl groups on the thermal properties of polybenzoxazines: Using molecular design and Monte Carlo simulation. RSC Adv. 2018, 8, 18038–18050. [Google Scholar] [CrossRef] [PubMed]
- Sini, N.; Endo, T. Toward elucidating the role of number of oxazine rings and intermediates in the benzoxazine backbone on their thermal characteristics. Macromolecules 2016, 49, 8466–8478. [Google Scholar] [CrossRef]
- Ozawa, T. Kinetics of non-isothermal crystallization. Polymer 1971, 12, 150–158. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Van Krevelen, D. Some basic aspects of flame resistance of polymeric materials. Polymer 1975, 16, 615–620. [Google Scholar] [CrossRef]
- Shan, F.; Ohashi, S.; Erlichman, A.; Ishida, H. Non-flammable thiazole-functional monobenzoxazines: Synthesis, polymerization, thermal and thermomechanical properties, and flammability studies. Polymer 2018, 157, 38–49. [Google Scholar] [CrossRef]
- Lyon, R.; Walters, R.; Stoliarov, S. Thermal analysis of flammability. J. Therm. Anal. Calorim. 2007, 89, 441–448. [Google Scholar] [CrossRef]


| Monomer | Ea (KJ/mol) | |
|---|---|---|
| Kissinger | Ozawa | |
| PH-fa | 77.13 | 81.41 |
| oHPNI-a | 117.92 | 120.51 |
| oHPNI-fa | 134.07 | 135.36 |
| Sample | Tg (DMA) (°C) | Td5 (°C) | Td10 (°C) | Yc (wt.%) | HRC (Jg−1K−1) | THR (kJg−1) |
|---|---|---|---|---|---|---|
| poly(oHPNI-fa) | 287 | 395 | 425 | 56 | 56.8 | 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Peng, Y.; Yang, Y.; Liu, J.; Zhang, K. Low-Temperature Terpolymerizable Benzoxazine Monomer Bearing Norbornene and Furan Groups: Synthesis, Characterization, Polymerization, and Properties of Its Polymer. Molecules 2023, 28, 3944. https://doi.org/10.3390/molecules28093944
Lu Y, Peng Y, Yang Y, Liu J, Zhang K. Low-Temperature Terpolymerizable Benzoxazine Monomer Bearing Norbornene and Furan Groups: Synthesis, Characterization, Polymerization, and Properties of Its Polymer. Molecules. 2023; 28(9):3944. https://doi.org/10.3390/molecules28093944
Chicago/Turabian StyleLu, Yin, Yaliang Peng, Yi Yang, Jiahao Liu, and Kan Zhang. 2023. "Low-Temperature Terpolymerizable Benzoxazine Monomer Bearing Norbornene and Furan Groups: Synthesis, Characterization, Polymerization, and Properties of Its Polymer" Molecules 28, no. 9: 3944. https://doi.org/10.3390/molecules28093944
APA StyleLu, Y., Peng, Y., Yang, Y., Liu, J., & Zhang, K. (2023). Low-Temperature Terpolymerizable Benzoxazine Monomer Bearing Norbornene and Furan Groups: Synthesis, Characterization, Polymerization, and Properties of Its Polymer. Molecules, 28(9), 3944. https://doi.org/10.3390/molecules28093944






