Determination of Pentacyclic Triterpenoids in Plant Biomass by Porous Graphitic Carbon Liquid Chromatography—Tandem Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Retention of PCTs and Selection of the Chromatographic Conditions
2.2. Quantification and Method Validation
2.3. Plant Biomass Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Plant Materials and Extraction Procedure
3.3. Liquid Chromatography—Mass Spectrometry Analysis
3.4. Method Validation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.; Scheffler, A. Pentacyclic Triterpene Distribution in Various Plants—Rich Sources for a New Group of Multi-Potent Plant Extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Falev, D.I.; Ul’yanovskii, N.V.; Ovchinnikov, D.V.; Faleva, A.V.; Kosyakov, D.S. Screening and semi-quantitative determination of pentacyclic triterpenoids in plants by liquid chromatography–tandem mass spectrometry in precursor ion scan mode. Phytochem. Anal. 2021, 32, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Krasutsky, P.A. Birch bark research and development. Nat. Prod. Rep. 2006, 23, 919–942. [Google Scholar] [CrossRef] [PubMed]
- Kosyakov, D.S.; Ul’yanovskii, N.V.; Falev, D.I. Determination of Triterpenoids from Birch Bark by Liquid Chromatography-Tandem Mass Spectrometry. J. Anal. Chem. 2014, 69, 50–55. [Google Scholar] [CrossRef]
- Ríos, J.; Máñez, S. New Pharmacological Opportunities for Betulinic Acid. Planta Med. 2018, 84, 8–19. [Google Scholar] [CrossRef]
- Sycz, Z.; Tichaczek-Goska, D.; Wojnicz, D. Anti-Planktonic and Anti-Biofilm Properties of Pentacyclic Triterpenes—Asiatic Acid and Ursolic Acid as Promising Antibacterial Future Pharmaceuticals. Biomolecules 2022, 12, 98. [Google Scholar] [CrossRef]
- Laszczyk, M. Pentacyclic Triterpenes of the Lupane, Oleanane and Ursane Group as Tools in Cancer Therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef]
- Hordyjewska, A.; Ostapiuk, A.; Horecka, A.; Kurzepa, J. Betulin and betulinic acid: Triterpenoids derivatives with a powerful biological potential. Phytochem. Rev. 2019, 18, 929–951. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Moreira, V.M.; Gonçalves, B.M.F.; Leal, A.S.; Jing, Y. Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat. Prod. Rep. 2012, 29, 1463. [Google Scholar] [CrossRef]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef]
- D’Adamo, S.; Schiano di Visconte, G.; Lowe, G.; Szaub-Newton, J.; Beacham, T.; Landels, A.; Allen, M.J.; Spicer, A.; Matthijs, M. Engineering the unicellular alga Phaeodactylum tricornutum for high-value plant triterpenoid production. Plant Biotechnol. J. 2018, 17, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Kinghorn, A. Natural Product Triterpenoids and Their Semi-Synthetic Derivatives with Potential Anticancer Activity. Planta Med. 2019, 85, 802–814. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Nguyen, A.H.; Kumar, A.P.; Tan, B.K.H.; Sethi, G. Targeted inhibition of tumor proliferation, survival, and metastasis by pentacyclic triterpenoids: Potential role in prevention and therapy of cancer. Cancer Lett. 2012, 320, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Ding, F.; Jiang, Y.-T.; Peng, Y.-K. Bioavailability and Activity of Natural Food Additive Triterpenoids as Influenced by Protein. J. Agric. Food Chem. 2014, 62, 2271–2283. [Google Scholar] [CrossRef]
- Yogeeswari, P.; Sriram, D. Betulinic Acid and Its Derivatives: A Review on their Biological Properties. Curr. Med. Chem. 2005, 12, 657–666. [Google Scholar] [CrossRef]
- Albuquerque, R.P.; Duarte, G.C.A.; Duarte, N.; Ohana, D.T.; Santos, W.C.; Machado, T.B. Quantitative determination of pentacyclic triterpenic acids in Amazonian species Eugenia punicifolia DC by ATR-FTIR. Nat. Prod. Res. 2019, 35, 15. [Google Scholar] [CrossRef]
- Anikeenko, E.A.; Rakhmatullina, E.N.; Falev, D.I.; Khoroshev, O.Y.; Ul’yanovskii, N.V.; Kosyakov, D.S. Application of Carbon Matrices to Screening Pentacylic Triterpenoids in Plant Feedstock by MALDI Mass Spectrometry. J. Anal. Chem. 2020, 75, 1749–1757. [Google Scholar] [CrossRef]
- Xu, C.; Wang, B.; Pu, Y.; Tao, J.; Zhang, T. Techniques for the analysis of pentacyclic triterpenoids in medicinal plants. J. Sep. Sci. 2017, 41, 6–19. [Google Scholar] [CrossRef]
- Jemmali, Z.; Chartier, A.; Elfakir, C. Development of a gas chromatography–mass spectrometry method to monitor in a single run, mono- to triterpenoid compounds distribution in resinous plant materials. J. Chromatogr. A 2016, 1443, 241–253. [Google Scholar] [CrossRef]
- Tao, Y.; Jiang, Y.; Li, W.; Cai, B. Rapid characterization and determination of isoflavones and triterpenoid saponins in Fu-Zhu-Jiang-Tang tablets using UHPLC-Q-TOF/MS and HPLC-UV. Anal. Methods 2016, 8, 4211–4219. [Google Scholar] [CrossRef]
- Bhatia, A.; Meena, B.; Shukla, S.K.; Sidhu, O.P.; Upreti, D.K.; Mishra, A.; Roy, R.; Nautiyal, C.S. Determination of Pentacyclic Triterpenes from Betula utilis by High-Performance Liquid Chromatography and High-Resolution Magic Angle Spinning Nuclear Magnetic Resonance Spectroscopy. Anal. Lett. 2017, 50, 233–242. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, J.; Jeong, K.M.; Yoo, D.E.; Han, S.Y.; Choi, S.-Y.; Ko, D.-H.; Kim, H.-J.; Sung, N.H.; Lee, J. A simple and reliable analytical method based on HPLC–UV to determine oleanonic acid content in Chios gum mastic for quality control. Arch. Pharm. Res. 2016, 40, 49–56. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Wu, D.; Zhao, M.; Li, G.; Gong, P.; Wu, Y.; Guo, Y.; Chen, G.; Zhao, X.; Sun, Z.; et al. Development of a facile and sensitive HPLC-FLD method via fluorescence labeling for triterpenic acid bioavailability investigation. Biomed. Chromatogr. 2016, 31, e3894. [Google Scholar] [CrossRef]
- Olmo-García, L.; Bajoub, A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Evaluating the potential of LC coupled to three alternative detection systems (ESI-IT, APCI-TOF and DAD) for the targeted determination of triterpenic acids and dialcohols in olive tissues. Talanta 2016, 150, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Rhourri-Frih, B.; Chaimbault, P.; Claude, B.; Lamy, C.; André, P.; Lafosse, M. Analysis of pentacyclic triterpenes by LC-MS. A comparative study between APCI and APPI. J. Mass Spectrom. 2008, 44, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Falev, D.I.; Kosyakov, D.S.; Ul’yanovskii, N.V.; Ovchinnikov, D.V. Rapid simultaneous determination of pentacyclic triterpenoids by mixed-mode liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2020, 1609, 460458. [Google Scholar] [CrossRef]
- Falev, D.I.; Ovchinnikov, D.V.; Voronov, I.S.; Faleva, A.V.; Ul’yanovskii, N.V.; Kosyakov, D.S. Supercritical Fluid Chromatography—Tandem Mass Spectrometry for Rapid Quantification of Pentacyclic Triterpenoids in Plant Extracts. Pharmaceuticals 2022, 15, 629. [Google Scholar] [CrossRef]
- Pereira, L. Porous Graphitic Carbon as a Stationary Phase in HPLC: Theory and Applications. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 1687–1731. [Google Scholar] [CrossRef]
- West, C.; Elfakir, C.; Lafosse, M. Porous graphitic carbon: A versatile stationary phase for liquid chromatography. J. Chromatogr. A 2010, 1217, 3201–3216. [Google Scholar] [CrossRef]
- Deschamps, F.S.; Gaudin, K.; Baillet, A.; Chaminade, P. Wheat digalactosyldiacylglycerol molecular species profiling using porous graphitic carbon stationary phase. J. Sep. Sci. 2004, 27, 1313–1322. [Google Scholar] [CrossRef]
- Moberg, M.; Holmström, S.J.M.; Lundström, U.S.; Markides, K.E. Novel approach to the determination of structurally similar hydroxamate siderophores by column-switching capillary liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2003, 1020, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, A.J.; Lafontaine, S.; Dailey, J.; Varnum, S.; Lerno, L.A.; Zweigenbaum, J.; Heymann, H.; Ebeler, S.E. Characterization of Humulus lupulus glycosides with porous graphitic carbon and sequential high performance liquid chromatography quadrupole time-of-flight mass spectrometry and high performance liquid chromatography fractionation. J. Chromatogr. A 2022, 1674, 463130. [Google Scholar] [CrossRef] [PubMed]
- Rhourri-Frih, B.; Chaimbault, P.; Dequeral, D.; André, P.; Lafosse, M. Investigation of porous graphitic carbon for triterpenoids and natural resinous materials analysis by high performance liquid chromatography hyphenated to mass spectrometry. J. Chromatogr. A 2012, 1240, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Fish, B.J. Use of a porous graphitic carbon column to separate cis and trans isomers in a novel anti-asthma compound. J. Pharm. Biomed. Anal. 1993, 11, 517–521. [Google Scholar] [CrossRef]
- Viron, C.; André, P.; Dreux, M.; Lafosse, M. Evaluation of porous graphitic carbon as stationary phase for the analysis of fatty acid methyl esters by liquid chromatography. Chromatographia 1999, 49, 137–141. [Google Scholar] [CrossRef]
- Azenha, I.S.; Simões, M.M.Q.; Mendes, A.; Silva, C.M. Adsorbents, mobile phases, and strategies for the chromatographic separation of betulinic, oleanolic, and ursolic acids. Biomass Convers. Biorefin. 2023. [Google Scholar] [CrossRef]
- Mus, A.A.; Goh, L.P.W.; Marbawi, H.; Gansau, J.A. The Biosynthesis and Medicinal Properties of Taraxerol. Biomedicines 2022, 10, 807. [Google Scholar] [CrossRef]
- Faleva, A.V.; Ul’yanovskii, N.V.; Falev, D.I.; Onuchina, A.A.; Budaev, N.A.; Kosyakov, D.S. New Oligomeric Dihydrochalcones in the Moss Polytrichum commune: Identification, Isolation, and Antioxidant Activity. Metabolites 2022, 12, 974. [Google Scholar] [CrossRef]
- Heck, M.A.; Lüth, V.M.; van Gessel, N.; Krebs, M.; Kohl, M.; Prager, A.; Joosten, H.; Decker, E.L.; Reski, R. Axenic in-vitro cultivation of nineteen peat-moss (Sphagnum L.) species as a resource for basic biology, biotechnology and paludiculture. New Phytol. 2020, 229, 861–876. [Google Scholar] [CrossRef]
- Baas, M.; Pancost, R.; van Geel, B.; Sinninghe Damsté, J.S. A comparative study of lipids in Sphagnum species. Org. Geochem. 2000, 31, 535–541. [Google Scholar] [CrossRef]
- Guo, Z.; Bi, G.; Zhang, Y.; Li, J.; Meng, D. Rare benzonaphthoxanthenones from Chinese folk herbal medicine Polytrichum commune and their anti-neuroinflammatory activities in vitro. Bioorg. Chem. 2020, 102, 104087. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Materska, M.; Łupina, K. Corn starch and methylcellulose edible films incorporated with fireweed (Chamaenerion angustifolium L.) extract: Comparison of physicochemical and antioxidant properties. Int. J. Biol. Macromol. 2021, 190, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Kukina, T.P.; Frolova, T.S.; Salnikova, O.I. Neutral Constituents of Chamaenerion angustifolium Leaves. Chem. Nat. Comp. 2014, 50, 233–236. [Google Scholar] [CrossRef]
- Falev, D.I.; Kosyakov, D.S.; Ul’yanovskii, N.V.; Ovchinnikov, D.V.; Shestakov, S.L. Subcritical extraction of birch bark penta-cyclic triterpenes. Russ. Chem. Bull. 2017, 66, 875–881. [Google Scholar] [CrossRef]

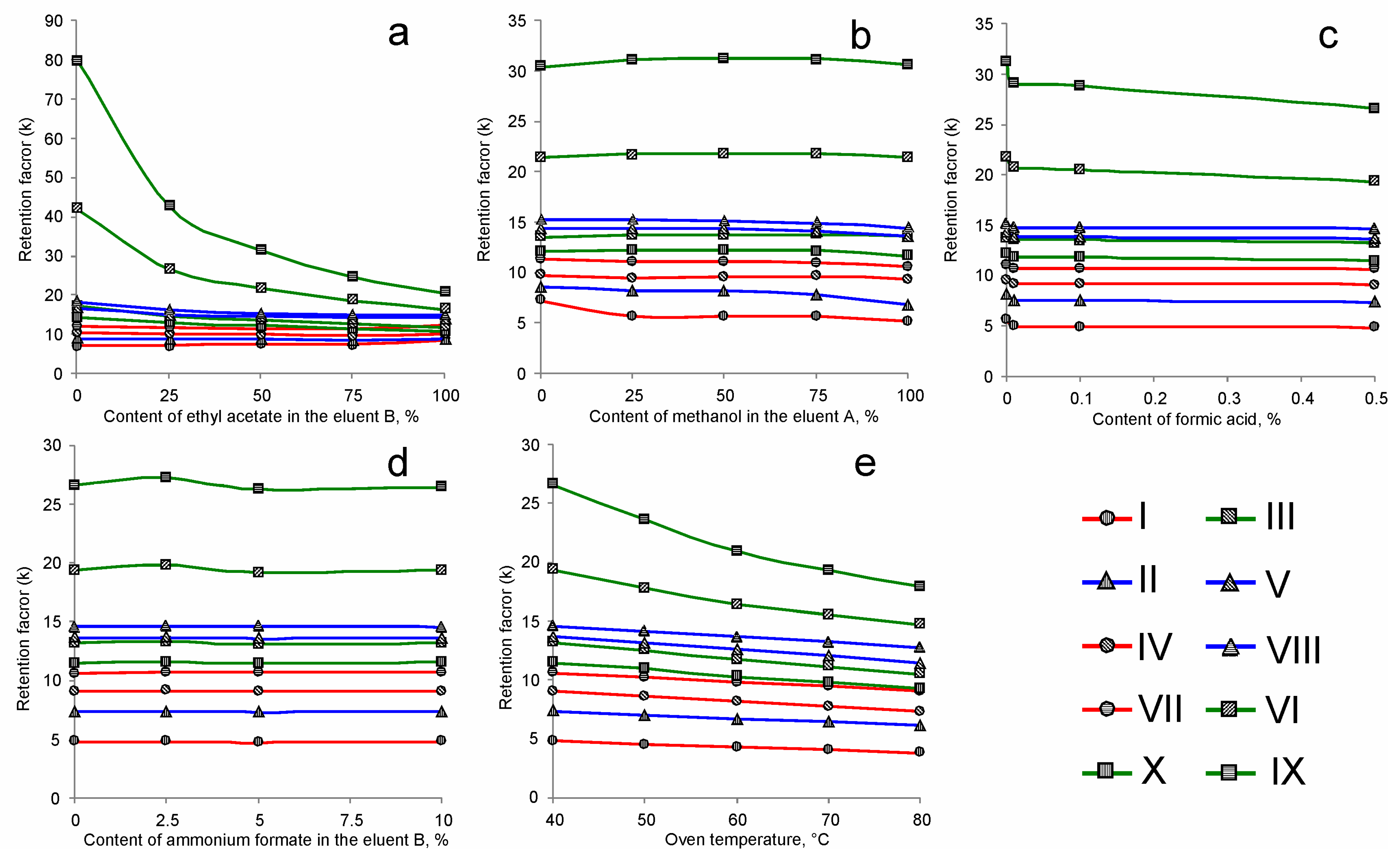

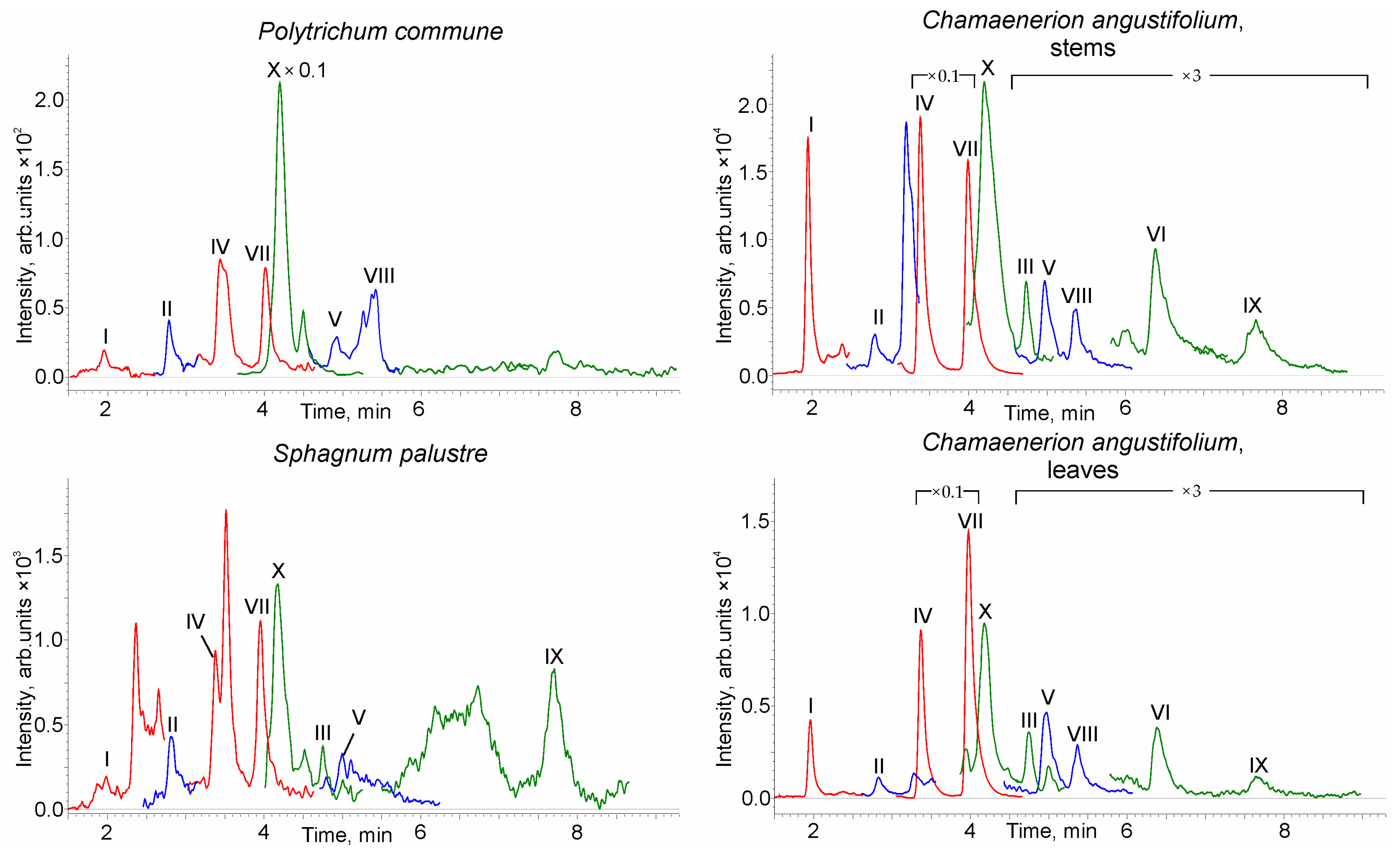
| Analyte | tR | k | α | N | Rs | As10% | As50% |
|---|---|---|---|---|---|---|---|
| I | 1.93 | 4.2 | - | 3750 | - | 2.12 | 1.15 |
| II | 2.81 | 6.6 | 1.56 | 4410 | 5.98 | 2.81 | 1.30 |
| III | 4.74 | 11.8 | 1.15 | 11,900 | 2.91 | 2.68 | 1.49 |
| IV | 3.38 | 8.1 | 1.23 | 8030 | 3.57 | 2.09 | 1.24 |
| V | 4.96 | 12.4 | 1.05 | 7330 | 1.09 | 2.92 | 1.69 |
| VI | 6.38 | 16.2 | 1.20 | 5130 | 3.59 | 3.05 | 1.46 |
| VII | 3.97 | 9.7 | 1.20 | 11,400 | 3.95 | 2.84 | 1.37 |
| VIII | 5.36 | 13.5 | 1.09 | 10,100 | 1.80 | 2.76 | 1.23 |
| IX | 7.69 | 19.8 | 1.22 | 1730 | 2.40 | 3.86 | 2.62 |
| X | 4.18 | 10.3 | 1.06 | 6210 | 1.17 | 2.89 | 1.53 |
| Analyte | tR, min | Linear Range, μg L−1 | a | R2 | LOD, μg L−1 | LOQ, μg L−1 |
|---|---|---|---|---|---|---|
| I | 1.93 | 16–5000 | 74.9 | 0.999 | 4.69 | 15.6 |
| II | 2.81 | 39–10,000 | 52.2 | 0.999 | 11.7 | 38.9 |
| III | 4.74 | 23–5000 | 78.4 | 0.994 | 6.85 | 22.8 |
| IV | 3.38 | 14–5000 | 100 | 0.999 | 4.26 | 14.2 |
| V | 4.96 | 66–15,000 | 35.9 | 0.999 | 19.9 | 66.3 |
| VI | 6.38 | 200–40,000 | 28.8 | 0.999 | 60.6 | 202 |
| VII | 3.97 | 34–10,000 | 50.5 | 0.999 | 10.2 | 34.1 |
| VIII | 5.36 | 110–25,000 | 24.2 | 0.999 | 32.8 | 109 |
| IX | 7.69 | 350–70,000 | 29.0 | 0.999 | 104 | 347 |
| X | 4.18 | 34–10,000 | 64.8 | 0.999 | 10.0 | 33.5 |
| Analyte | Common Haircap | Prairie Sphagnum | Fireweed Stems | Fireweed Leaves |
|---|---|---|---|---|
| I | 0.68 ± 0.13 | 0.65 ± 0.03 | 49 ± 4 | 87 ± 5 |
| II | 1.8 ± 0.1 | 2.7 ± 0.3 | 15 ± 1 | 32 ± 1 |
| III | <LOQ | 1.6 ± 0.2 | 4.7 ± 0.3 | 21 ± 1 |
| IV | 2.5 ± 0.2 | 3.0 ± 0.1 | 550 ± 30 | 1500 ± 100 |
| V | 3.6 ± 1.5 | 3.6 ± 0.3 | 21 ± 1 | 110 ± 10 |
| VI | <LOQ | <LOQ | 48 ± 2 | 140 ± 10 |
| VII | 4.3 ± 0.3 | 8.4 ± 0.1 | 840 ± 10 | 4500 ± 100 |
| VIII | 12 ± 1 | <LOQ | 22 ± 1 | 90 ± 4 |
| IX | <LOQ | 15 ± 2 | 32 ± 7 | 79 ± 6 |
| X | 180 ± 1 | 8.0 ± 0.3 | 210 ± 10 | 800 ± 10 |
| Analyte | Nominal Mass, Da | Precursor Ion, [M − H2O + H]+ m/z | Product Ion, m/z | Q1 Bias, V | Collision Energy, eV | Q2 Bias, V |
|---|---|---|---|---|---|---|
| I | 456 | 439 | 95 | −46.8 | 40 | −40.3 |
| II | 442 | 425 | 95 | −43.5 | 32 | −37.1 |
| III | 426 | 409 | 95 | −43.5 | 33 | −14.5 |
| IV | 456 | 439 | 203 | −50.0 | 27 | −43.5 |
| V | 442 | 425 | 191 | −46.8 | 14 | −46.8 |
| VI | 426 | 409 | 95 | −43.5 | 36 | −43.5 |
| VII | 456 | 439 | 203 | −46.8 | 26 | −40.3 |
| VIII | 442 | 425 | 191 | −46.8 | 17 | −37.1 |
| IX | 426 | 409 | 95 | −40.3 | 40 | −37.1 |
| X | 426 | 409 | 95 | −10.3 | 38 | −36.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voronov, I.S.; Falev, D.I.; Faleva, A.V.; Ul’yanovskii, N.V.; Kosyakov, D.S. Determination of Pentacyclic Triterpenoids in Plant Biomass by Porous Graphitic Carbon Liquid Chromatography—Tandem Mass Spectrometry. Molecules 2023, 28, 3945. https://doi.org/10.3390/molecules28093945
Voronov IS, Falev DI, Faleva AV, Ul’yanovskii NV, Kosyakov DS. Determination of Pentacyclic Triterpenoids in Plant Biomass by Porous Graphitic Carbon Liquid Chromatography—Tandem Mass Spectrometry. Molecules. 2023; 28(9):3945. https://doi.org/10.3390/molecules28093945
Chicago/Turabian StyleVoronov, Ilya S., Danil I. Falev, Anna V. Faleva, Nikolay V. Ul’yanovskii, and Dmitry S. Kosyakov. 2023. "Determination of Pentacyclic Triterpenoids in Plant Biomass by Porous Graphitic Carbon Liquid Chromatography—Tandem Mass Spectrometry" Molecules 28, no. 9: 3945. https://doi.org/10.3390/molecules28093945
APA StyleVoronov, I. S., Falev, D. I., Faleva, A. V., Ul’yanovskii, N. V., & Kosyakov, D. S. (2023). Determination of Pentacyclic Triterpenoids in Plant Biomass by Porous Graphitic Carbon Liquid Chromatography—Tandem Mass Spectrometry. Molecules, 28(9), 3945. https://doi.org/10.3390/molecules28093945






