Synthesis and Antitumor Evaluation of Biotin-SN38-Valproic Acid Conjugates
Abstract
1. Introduction
2. Results and Discussion
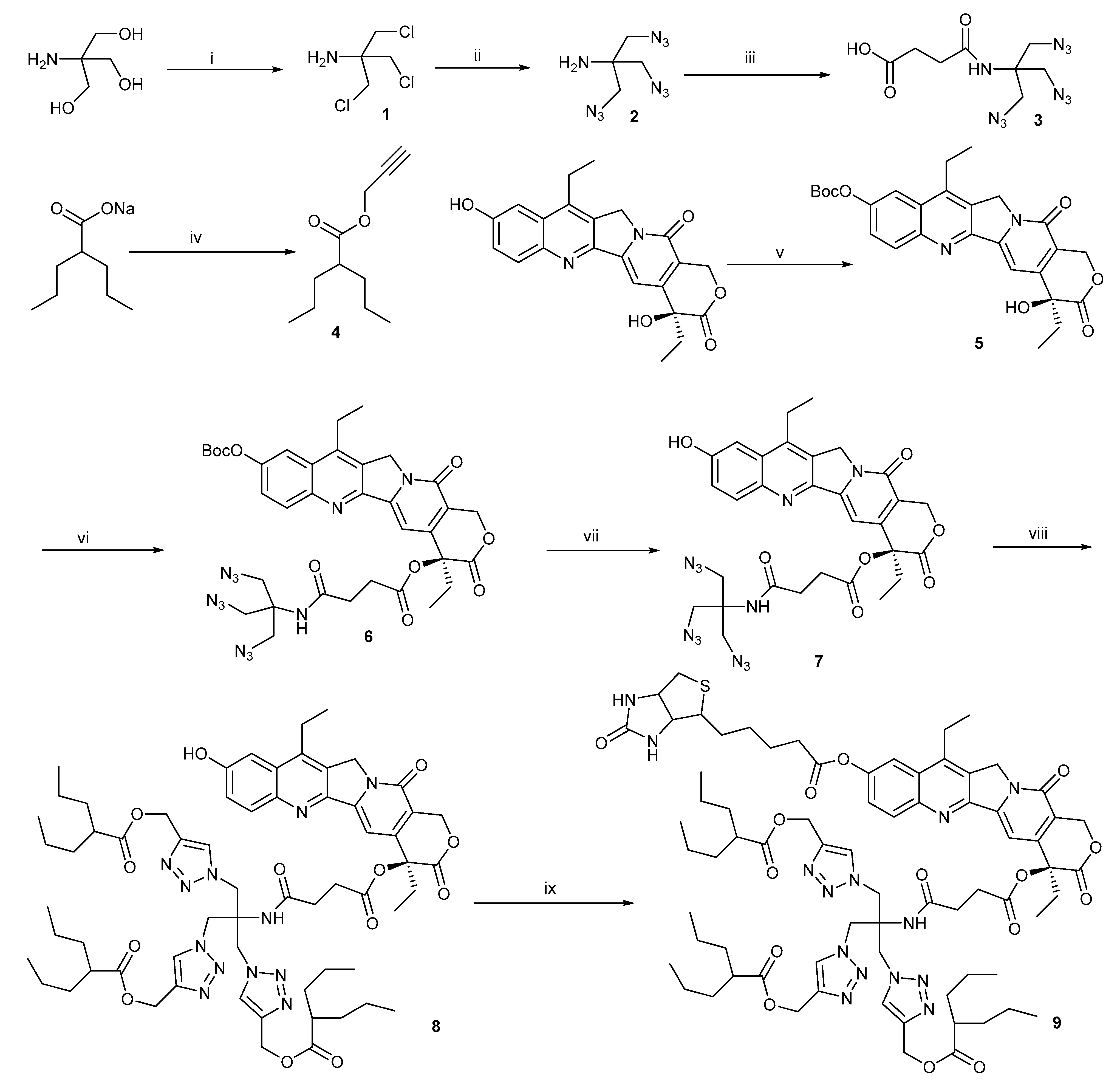
2.1. Chemistry
2.2. In Vitro Biological Evaluation
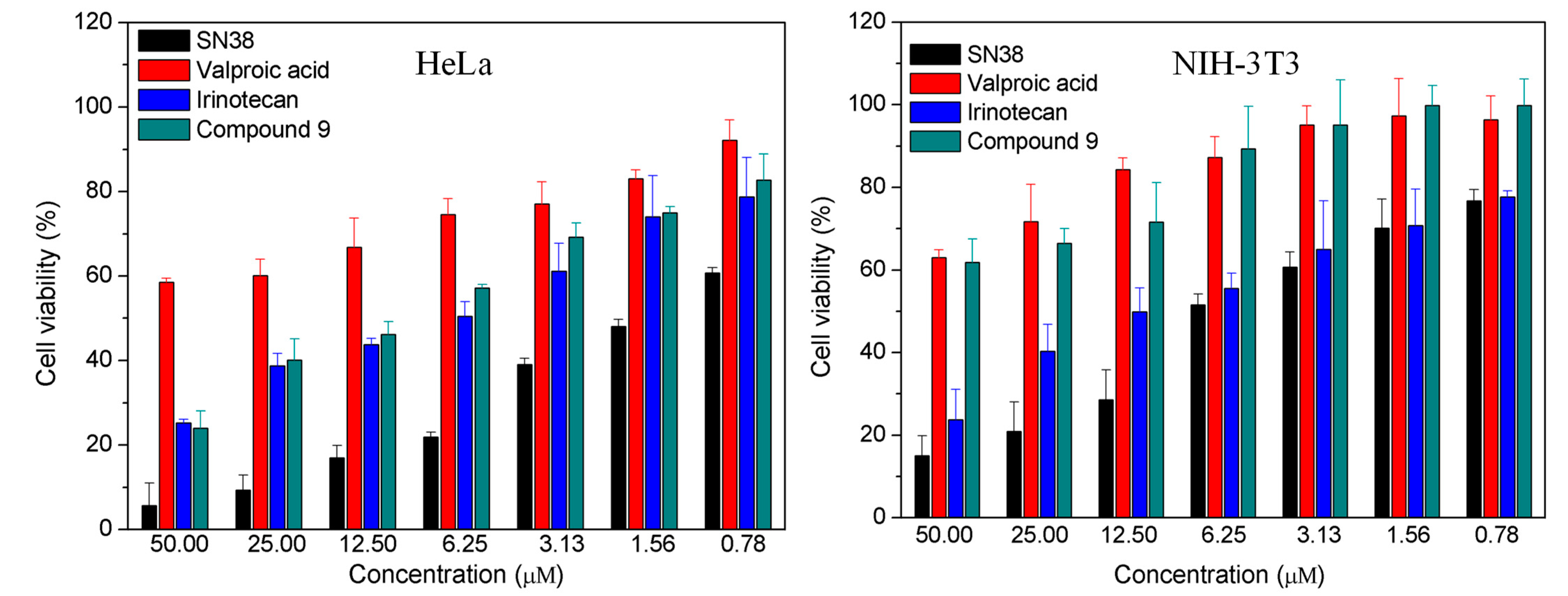
2.2.1. In Vitro Cytotoxicity
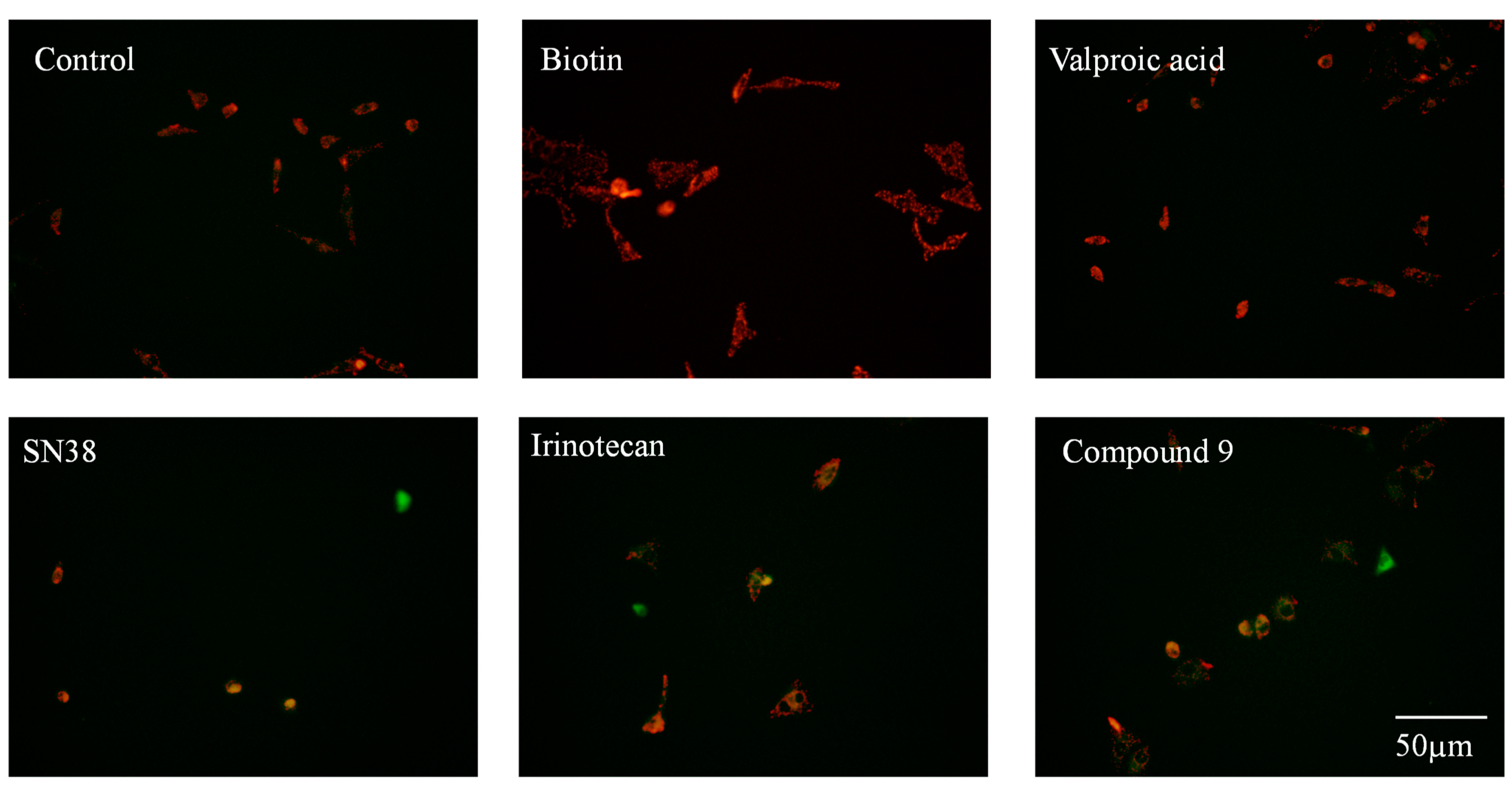
2.2.2. Mitochondrial Membrane Potential Analysis
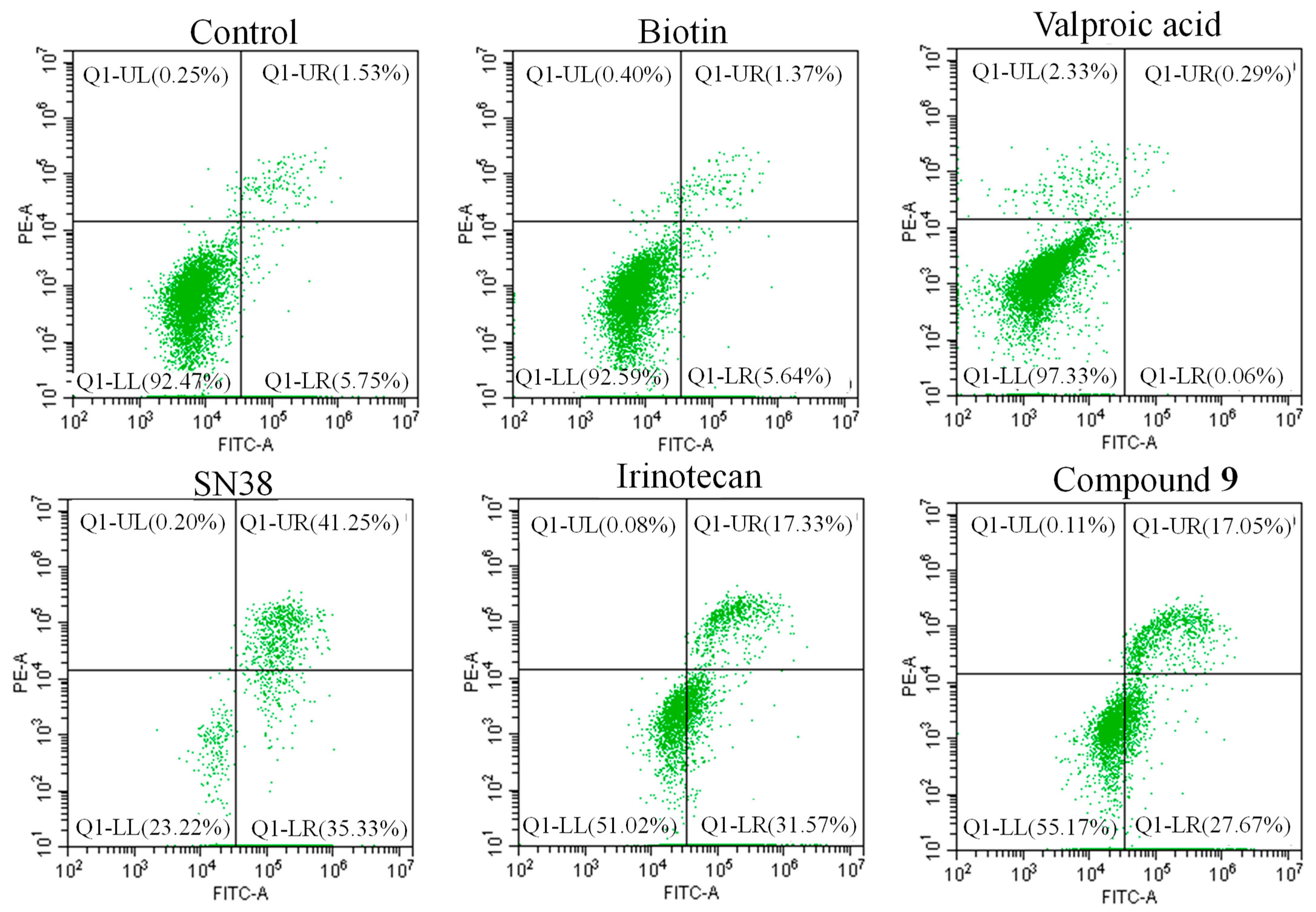
2.2.3. Apoptosis Study
3. Materials and Methods
3.1. Synthesis of Compound 9
3.1.1. The Synthetic Route of Compound 1
3.1.2. The Synthetic Route of Compound 2
3.1.3. The Synthetic Route of Compound 3
3.1.4. The Synthetic Route of Compound 4
3.1.5. The Synthetic Route of Compound 5
3.1.6. The Synthetic Route of Compounds 6 and 7
3.1.7. The Synthetic Route of Compound 8
3.1.8. The Synthetic Route of Compound 9
3.2. In Vitro Biological Evaluation
3.2.1. In Vitro Cytotoxicity Assay
3.2.2. Mitochondrial Membrane Potential Assay
3.2.3. Cell Apoptosis Assay
3.2.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martino, E.; Della Volpe, S.; Terribile, E.; Benetti, E.; Sakaj, M.; Centamore, A.; Sala, A.; Collina, S. The long story of camptothecin: From traditional medicine to drugs. Bioorganic Med. Chem. Lett. 2017, 27, 701–707. [Google Scholar] [CrossRef] [PubMed]
- Bocian, W.; Naumczuk, B.; Urbanowicz, M.; Sitkowski, J.; Bierczynska-Krzysik, A.; Bednarek, E.; Wiktorska, K.; Milczarek, M.; Kozerski, L. The Mode of SN38 Derivatives Interacting with Nicked DNA Mimics Biological Targeting of Topo I Poisons. Int. J. Mol. Sci. 2021, 22, 7471. [Google Scholar] [CrossRef] [PubMed]
- Martin-Encinas, E.; Selas, A.; Palacios, F.; Alonso, C. The design and discovery of topoisomerase I inhibitors as anticancer therapies. Expert. Opin. Drug. Discov. 2022, 17, 581–601. [Google Scholar] [CrossRef] [PubMed]
- Leppard, J.B.; Champoux, J.J. Human DNA topoisomerase I: Relaxation, roles, and damage control. Chromosoma 2005, 114, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Khaiwa, N.; Maarouf, N.R.; Darwish, M.H.; Alhamad, D.W.M.; Sebastian, A.; Hamad, M.; Omar, H.A.; Orive, G.; Al-Tel, T.H. Camptothecin’s journey from discovery to WHO Essential Medicine: Fifty years of promise. Eur. J. Med. Chem. 2021, 223, 113639. [Google Scholar] [CrossRef] [PubMed]
- Cronin, A.; Barnes, J.; Pedersen, J.; Heathcote, D.; Collins, T. Mitigating exacerbation of Irinotecan-induced gastrointestinal toxicity in combination with an ATM inhibitor in the rat. Toxicol. Lett. 2016, 258, S243. [Google Scholar] [CrossRef]
- Venditto, V.J.; Simanek, E.E. Cancer Therapies Utilizing the Camptothecins: A Review of the in Vivo Literature. Mol. Pharm. 2010, 7, 307–349. [Google Scholar] [CrossRef]
- Bala, V.; Rao, S.; Li, P.; Wang, S.; Prestidge, C.A. Lipophilic Prodrugs of SN38: Synthesis and in Vitro Characterization toward Oral Chemotherapy. Mol. Pharm. 2016, 13, 287–294. [Google Scholar] [CrossRef]
- Shi, L.; Wu, X.; Li, T.; Wu, Y.; Song, L.; Zhang, W.; Yin, L.; Wu, Y.; Han, W.; Yang, Y. An esterase-activatable prodrug formulated liposome strategy: Potentiating the anticancer therapeutic efficacy and drug safety. Nanoscale Adv. 2022, 4, 952–966. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Wang, H.; Liu, X.; Zhang, Q.; Li, K.; Chen, Y.; Zhu, Q.; Shen, Y.; Sui, M. A nanotherapeutic strategy to overcome chemoresistance to irinotecan/7-ethyl-10-hydroxy-camptothecin in colorectal cancer. Acta Biomater. 2022, 137, 262–275. [Google Scholar] [CrossRef]
- Yang, X.Y.; Zhao, H.Y.; Lei, H.; Yuan, B.; Mao, S.; Xin, M.; Zhang, S.Q. Synthesis and Biological Evaluation of 10-Substituted Camptothecin Derivatives with Improved Water Solubility and Activity. ChemMedChem 2021, 16, 1000–1010. [Google Scholar] [CrossRef]
- Liu, L.; Xie, F.; Xiao, D.; Xu, X.; Su, Z.; Wang, Y.; Fan, S.; Zhou, X.; Li, S. Synthesis and evaluation of highly releasable and structurally stable antibody-SN-38-conjugates. Drug. Deliv. 2021, 28, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Shen, Q.; Gao, Y.; Wang, L.; Fang, Y.; Chen, Y.; Lu, W. Design, Synthesis, and Biological Evaluation of HSP90 Inhibitor-SN38 Conjugates for Targeted Drug Accumulation. J. Med. Chem. 2020, 63, 5421–5441. [Google Scholar] [CrossRef] [PubMed]
- Botta, L.; Filippi, S.; Zippilli, C.; Cesarini, S.; Bizzarri, B.M.; Cirigliano, A.; Rinaldi, T.; Paiardini, A.; Fiorucci, D.; Saladino, R.; et al. Artemisinin Derivatives with Antimelanoma Activity Show Inhibitory Effect against Human DNA Topoisomerase 1. ACS Med. Chem. Lett. 2020, 11, 1035–1040. [Google Scholar] [CrossRef]
- Verza, F.A.; Das, U.; Fachin, A.L.; Dimmock, J.R.; Marins, M. Roles of Histone Deacetylases and Inhibitors in Anticancer Therapy. Cancers 2020, 12, 1664. [Google Scholar] [CrossRef]
- Autin, P.; Blanquart, C.; Fradin, D. Epigenetic Drugs for Cancer and microRNAs: A Focus on Histone Deacetylase Inhibitors. Cancers 2019, 11, 1530. [Google Scholar] [CrossRef] [PubMed]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef]
- Molife, L.R.; de Bono, J.S. Belinostat: Clinical applications in solid tumors and lymphoma. Expert. Opin. Investig. Drugs 2011, 20, 1723–1732. [Google Scholar] [CrossRef] [PubMed]
- Garnock-Jones, K.P. Panobinostat: First Global Approval. Drugs 2015, 75, 695–704. [Google Scholar] [CrossRef]
- Zhang, Y.; Wong, C.H.; Loong, H.H.F. Synergistic activities of the histone deacetylase inhibitors with conventional cytotoxic chemotherapies in angiosarcomas. Investig. New. Drugs 2022, 40, 868–869. [Google Scholar] [CrossRef] [PubMed]
- Cincinelli, R.; Musso, L.; Artali, R.; Guglielmi, M.B.; La Porta, I.; Melito, C.; Colelli, F.; Cardile, F.; Signorino, G.; Fucci, A.; et al. Hybrid topoisomerase I and HDAC inhibitors as dual action anticancer agents. PLoS ONE 2018, 13, e0205018. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Miyanishi, K.; Kubo, T.; Hamaguchi, K.; Osuga, T.; Tanaka, S.; Ohnuma, H.; Murase, K.; Takada, K.; Nagayama, M.; et al. Synergistic antitumor effect of histone deacetylase class IIa inhibitor with lenvatinib in hepatocellular carcinoma. Hepatol. Int. 2023, 17, 1–10. [Google Scholar] [CrossRef]
- Ovejero-Sanchez, M.; Asensio-Juarez, G.; Gonzalez, M.; Puebla, P.; Vicente-Manzanares, M.; Pelaez, R.; Gonzalez-Sarmiento, R.; Herrero, A.B. Panobinostat Synergistically Enhances the Cytotoxicity of Microtubule Destabilizing Drugs in Ovarian Cancer Cells. Int. J. Mol. Sci. 2022, 23, 13019. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, X.; Shen, Q.; Zhang, Q.; Su, M.; Zhou, Y.; Li, J.; Chen, Y.; Lu, W. A series of camptothecin prodrugs exhibit HDAC inhibition activity. Bioorganic Med. Chem. 2018, 26, 4706–4715. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, G.Y.; Wang, L.B.; Kang, J.H.; Wang, J.M. Curcumin p38-dependently enhances the anticancer activity of valproic acid in human leukemia cells. Eur. J. Pharm. Sci. 2010, 41, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Lai, H.C.; Lee, H.Y.; Lin, W.H.; Chang, C.C.; Chu, T.Y.; Lin, Y.W.; Lee, K.D.; Yu, M.H. Valproic acid resensitizes cisplatin-resistant ovarian cancer cells. Cancer Sci. 2008, 99, 1218–1226. [Google Scholar] [CrossRef]
- Luna-Palencia, G.R.; Correa-Basurto, J.; Vasquez-Moctezuma, I. Valproic acid as a sensitizing agent for cancer treatment. Gac. Med. Mex. 2019, 155, 417–422. [Google Scholar] [CrossRef]
- Sanaei, M.; Kavoosi, F. Profound Inhibitory and Apoptotic Effects of Histone Deacetylase Inhibitor Valproic Acid on Different Cancers. Crescent J. Med. Biol. Sci. 2019, 6, 441–448. [Google Scholar]
- Wawruszak, A.; Halasa, M.; Okon, E.; Kukula-Koch, W.; Stepulak, A. Valproic Acid and Breast Cancer: State of the Art in 2021. Cancers 2021, 13, 3409. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Cheng, Z.; Yang, B.; Yu, J.; Chen, Y.; Lu, W. SN38-based albumin-binding prodrug for efficient targeted cancer chemotherapy. J. Control. Release 2021, 339, 297–306. [Google Scholar] [CrossRef]
- Zhang, Q.; Che, R.; Lu, W. Enhanced cellular uptake efficiency of DCM probes or SN38 conjugating with phenylboronic acids. Bioorganic Med. Chem. 2020, 28, 115377. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, J.; Jin, X.; Liu, L.; Tian, X. Folate Receptor Targeting and Cathepsin B-Sensitive Drug Delivery System for Selective Cancer Cell Death and Imaging. ACS Med. Chem. Lett. 2020, 11, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, D.; Choi, M.; Lee, S.; Kang, S.; Lee, Y.; Kim, S.; Chung, J.; Jon, S. Antibody-Assisted Delivery of a Peptide-Drug Conjugate for Targeted Cancer Therapy. Mol. Pharm. 2019, 16, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Andrikopoulou, A.; Zografos, E.; Liontos, M.; Koutsoukos, K.; Dimopoulos, M.A.; Zagouri, F. Trastuzumab Deruxtecan (DS-8201a): The Latest Research and Advances in Breast Cancer. Clin. Breast Cancer 2021, 21, e212–e219. [Google Scholar] [CrossRef]
- Koo, S.; Bobba, K.N.; Cho, M.Y.; Park, H.S.; Won, M.; Velusamy, N.; Hong, K.S.; Bhuniya, S.; Kim, J.S. Molecular Theranostic Agent with Programmed Activation for Hypoxic Tumors. ACS Appl. Bio Mater. 2019, 2, 4648–4655. [Google Scholar] [CrossRef]
- Jangili, P.; Won, M.; Kim, S.J.; Chun, J.; Shim, I.; Kang, C.; Ren, W.X.; Kim, J.S. Binary Drug Reinforced First Small-Molecule-Based Prodrug for Synergistic Anticancer Effects. ACS Appl. Bio Mater. 2019, 2, 3532–3539. [Google Scholar] [CrossRef]
- Xie, H.; Xu, X.; Chen, J.; Li, L.; Wang, J.; Fang, T.; Zhou, L.; Wang, H.; Zheng, S. Rational design of multifunctional small-molecule prodrugs for simultaneous suppression of cancer cell growth and metastasis in vitro and in vivo. Chem. Commun. 2016, 52, 5601–5604. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Xie, K.; Fang, T.; Chen, C.; Xie, H.; Zhou, L.; Zheng, S. Precise Engineering of Prodrug Cocktails into Single Polymeric Nanoparticles for Combination Cancer Therapy: Extended and Sequentially Controllable Drug Release. ACS Appl. Mater. Interfaces 2017, 9, 10567–10576. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, W.; He, R.; Ismail, M.; Ling, L.; Yao, C.; Fu, Z.; Li, X. Dual 7-ethyl-10-hydroxycamptothecin conjugated phospholipid prodrug assembled liposomes with in vitro anticancer effects. Bioorganic Med. Chem. 2017, 25, 3247–3258. [Google Scholar] [CrossRef]
- de Groot, F.; Busscher, G.; Aben, R.; Scheeren, H. Novel 20-carbonate linked prodrugs of camptothecin and 9-aminocamptothecin designed for activation by tumour-associated plasmin. Bioorganic Med. Chem. Lett. 2002, 12, 2371–2376. [Google Scholar] [CrossRef]
- Fam, K.T.; Collot, M.; Klymchenko, A.S. Probing biotin receptors in cancer cells with rationally designed fluorogenic squaraine dimers. Chem. Sci. 2020, 11, 8240–8248. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.Y.; Huo, F.J.; Yue, Y.K.; Chen, T.G.; Yin, C.X. Cancer cell recognition by a Cys-reactive turn-on significant enhanced fluorescent emission targeting biotin receptors. Sens. Actuators B-Chem. 2020, 304, 127431. [Google Scholar] [CrossRef]
- Maiti, S.; Paira, P. Biotin conjugated organic molecules and proteins for cancer therapy: A review. Eur. J. Med. Chem. 2018, 145, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Khoury, A.; Sakoff, J.A.; Gilbert, J.; Karan, S.; Gordon, C.P.; Aldrich-Wright, J.R. Potent Platinum(IV) Prodrugs That Incorporate a Biotin Moiety to Selectively Target Cancer Cells. Pharmaceutics 2022, 14, 2780. [Google Scholar] [CrossRef] [PubMed]
- Perumal, D.; Golla, M.; Pillai, K.S.; Raj, G.; Krishna, P.K.A.; Varghese, R. Biotin-decorated NIR-absorbing nanosheets for targeted photodynamic cancer therapy. Org. Biomol. Chem. 2021, 19, 2804–2810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.M.; Luo, Y.; Du, C.H.; Wu, L.; Wang, Y.K.; Chen, Y.D.; Li, S.Q.; Jiang, X.; Xie, Y.M. Synthesis and biological evaluation of novel SN38-glucose conjugate for colorectal cancer treatment. Bioorganic Med. Chem. Lett. 2023, 81, 129128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fu, W.; Zhu, L.-Z.; Liu, X.-F.; Li, L.; Peng, L.-Z.; Kai, G.-Y.; Liu, Y.-Q.; Zhang, Z.-J.; Xu, C.-R. Anti-tumor effects and mechanism of a novel camptothecin derivative YCJ100. Life Sci. 2022, 311, 121105. [Google Scholar] [CrossRef]
- Zhang, G.R.; Yin, R.J.; Dai, X.F.; Wu, G.Z.; Qi, X.; Li, J.; Jiang, T. Design, synthesis, and biological evaluation of novel 7-substituted 10,11-methylenedioxy-camptothecin derivatives against drug-resistant small-cell lung cancer in vitro and in vivo. Eur. J. Med. Chem. 2022, 241, 114610. [Google Scholar] [CrossRef]
- Huang, D.; Liu, Q.; Zhang, M.; Guo, Y.; Cui, Z.; Li, T.; Luo, D.; Xu, B.; Huang, C.; Guo, J.; et al. A Mitochondria-Targeted Phenylbutyric Acid Prodrug Confers Drastically Improved Anticancer Activities. J. Med. Chem. 2022, 65, 9955–9973. [Google Scholar] [CrossRef]
- Joo, Y.H.; Shreeve, J.M. 1,3-Diazido-2-(azidomethyl)-2-propylammonium salts. Inorg. Chem. 2009, 48, 8431–8438. [Google Scholar] [CrossRef]
- Das, K.K.; Ghosh, A.K.; Hajra, A. Late-stage ortho-C-H alkenylation of 2-arylindazoles in aqueous medium by Manganese(i)-catalysis. RSC Adv. 2022, 12, 19412–19416. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Z.; Zhang, Q.; Zhu, Q.; Chen, Y.; Lu, W. Co-Prodrugs of 7-Ethyl-10-hydroxycamptothecin and Vorinostat with in Vitro Hydrolysis and Anticancer Effects. ACS Omega 2019, 5, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, Y.; Kang, S.; Choi, M.; Lee, S.; Kim, S.; Gujrati, V.; Kim, J.; Jon, S. Self-assembled nanoparticles comprising aptide-SN38 conjugates for use in targeted cancer therapy. Nanotechnology 2016, 27, 48LT01. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Y.; Zhang, Y.; Ye, T.; Chen, Y. Synthesis and Antitumor Evaluation of Biotin-SN38-Valproic Acid Conjugates. Molecules 2023, 28, 3936. https://doi.org/10.3390/molecules28093936
Dai Y, Zhang Y, Ye T, Chen Y. Synthesis and Antitumor Evaluation of Biotin-SN38-Valproic Acid Conjugates. Molecules. 2023; 28(9):3936. https://doi.org/10.3390/molecules28093936
Chicago/Turabian StyleDai, Yi, Yang Zhang, Tianxiang Ye, and Yue Chen. 2023. "Synthesis and Antitumor Evaluation of Biotin-SN38-Valproic Acid Conjugates" Molecules 28, no. 9: 3936. https://doi.org/10.3390/molecules28093936
APA StyleDai, Y., Zhang, Y., Ye, T., & Chen, Y. (2023). Synthesis and Antitumor Evaluation of Biotin-SN38-Valproic Acid Conjugates. Molecules, 28(9), 3936. https://doi.org/10.3390/molecules28093936




