Hydrophobic Flocculation of Fine Cassiterite Using Alkyl Hydroxamic Acids with Different Carbon Chain Lengths as Collectors
Abstract
1. Introduction
2. Results
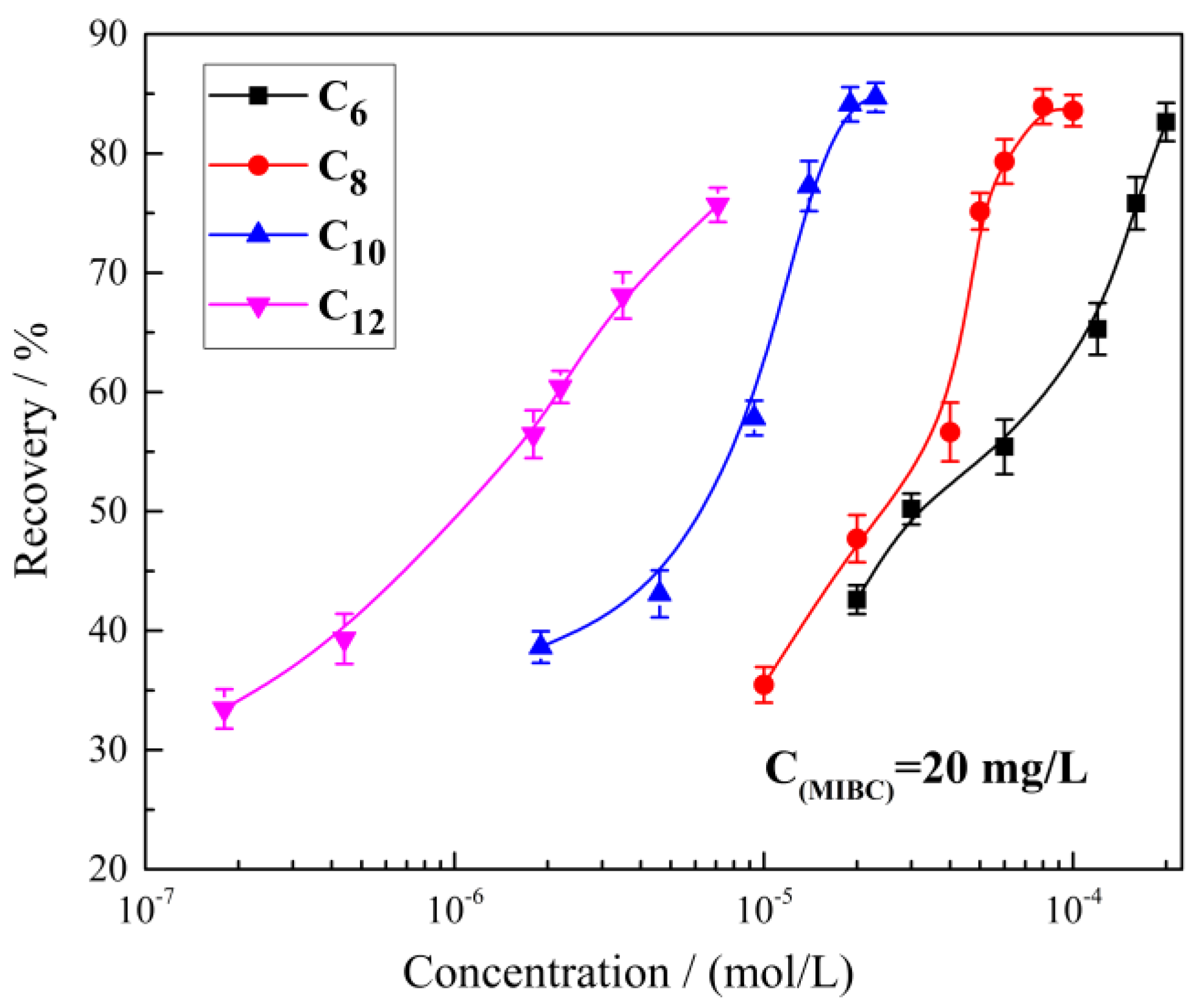
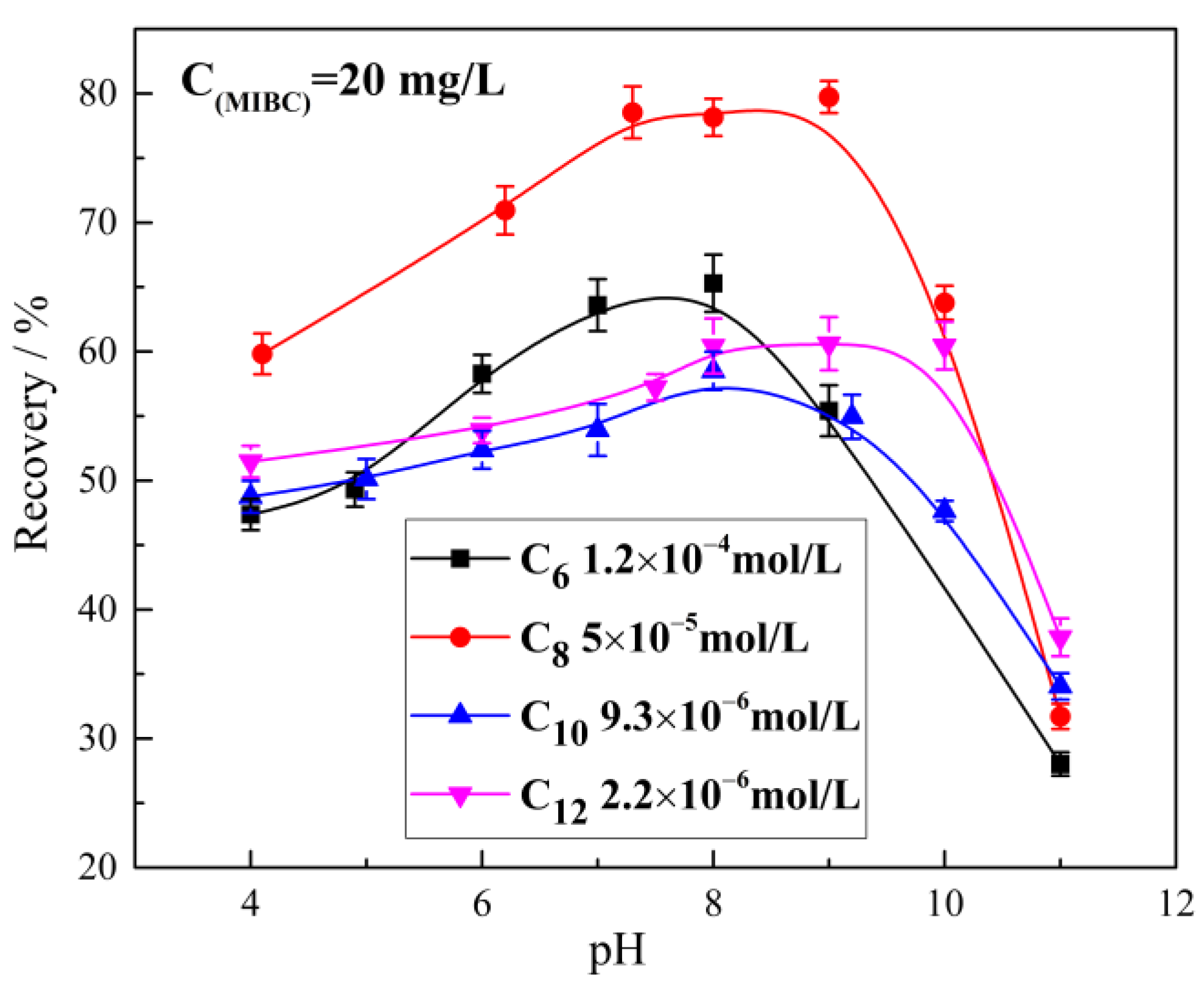
2.1. The Effect of Alkyl Hydroxamic Acids with Various Carbon Chain Lengths on the Microflotation of Cassiterite
2.2. The Effect of Alkyl Hydroxamic Acids with Various Carbon Chain Lengths on the Hydrophobic Flocculation of Cassiterite
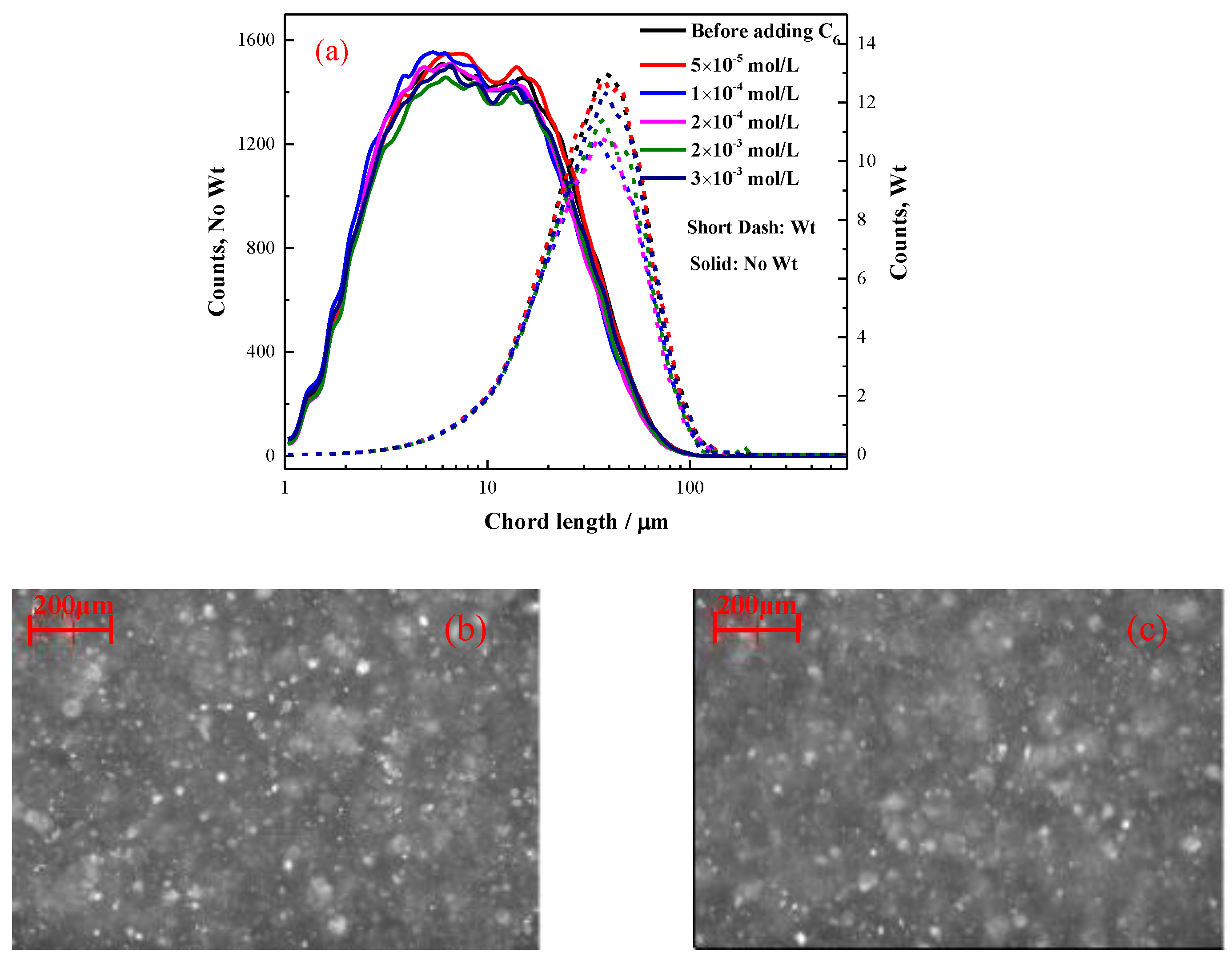
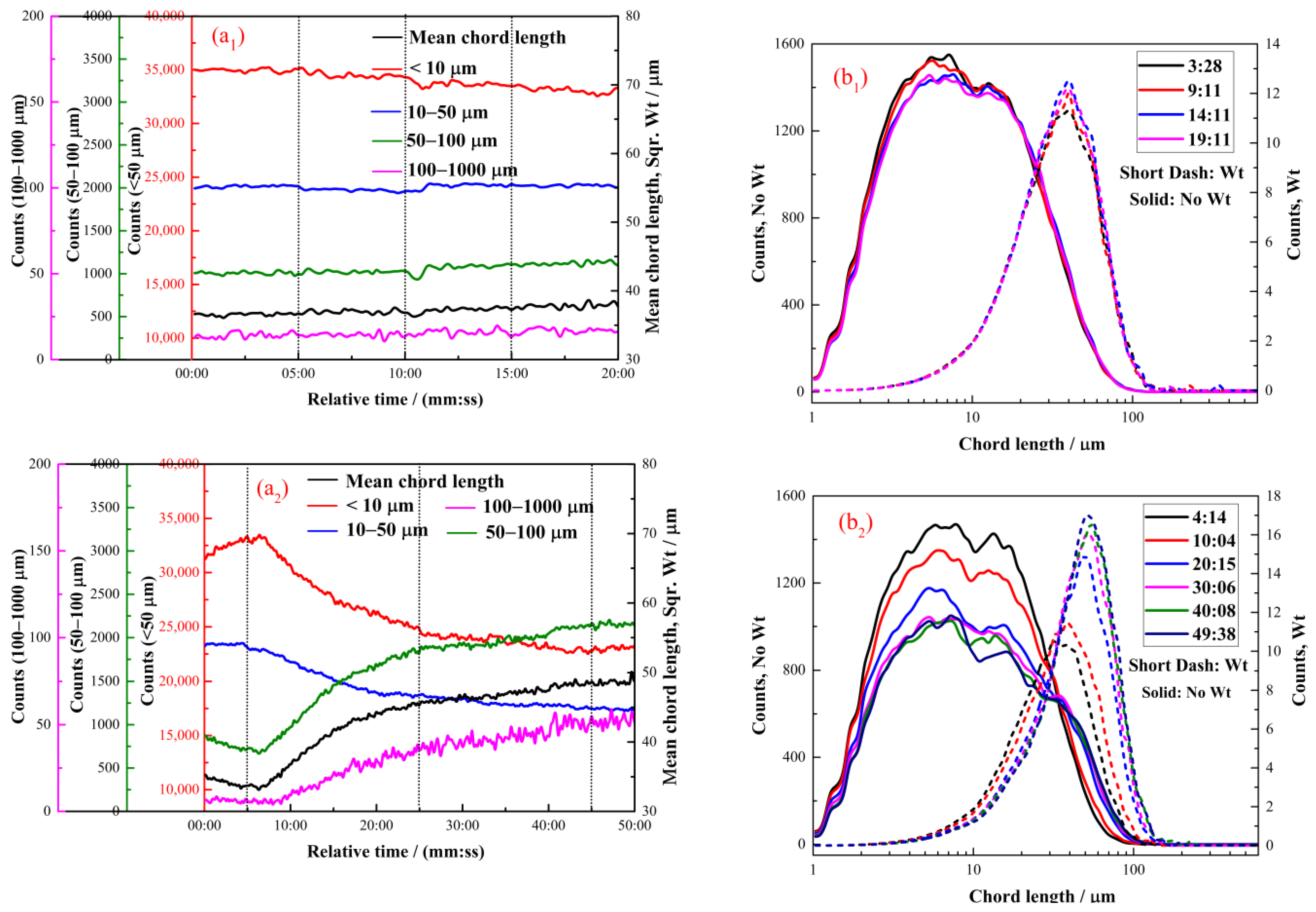
2.2.1. The Effect of Hexyl Hydroxamate on the Hydrophobic Flocculation of Cassiterite
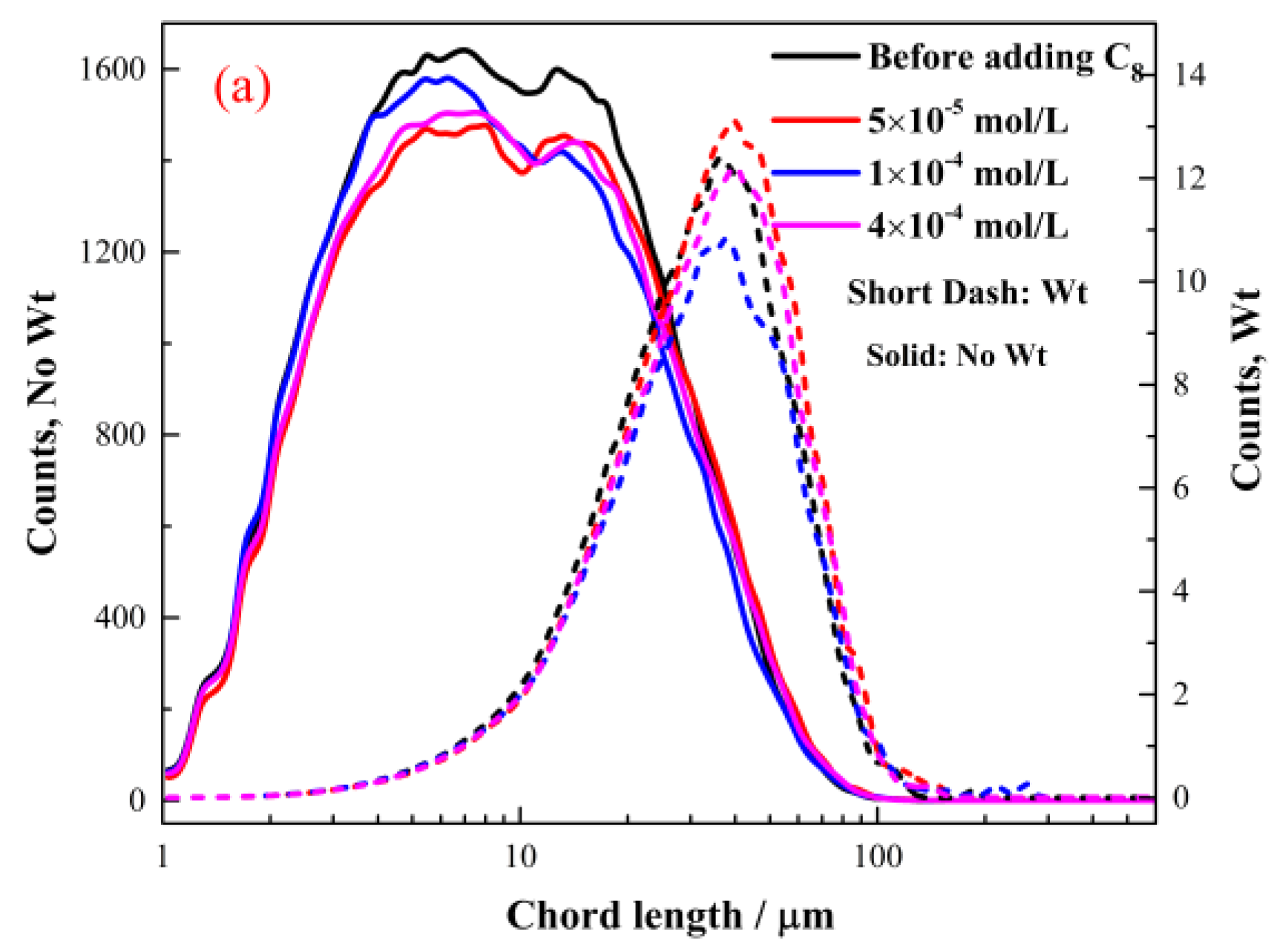
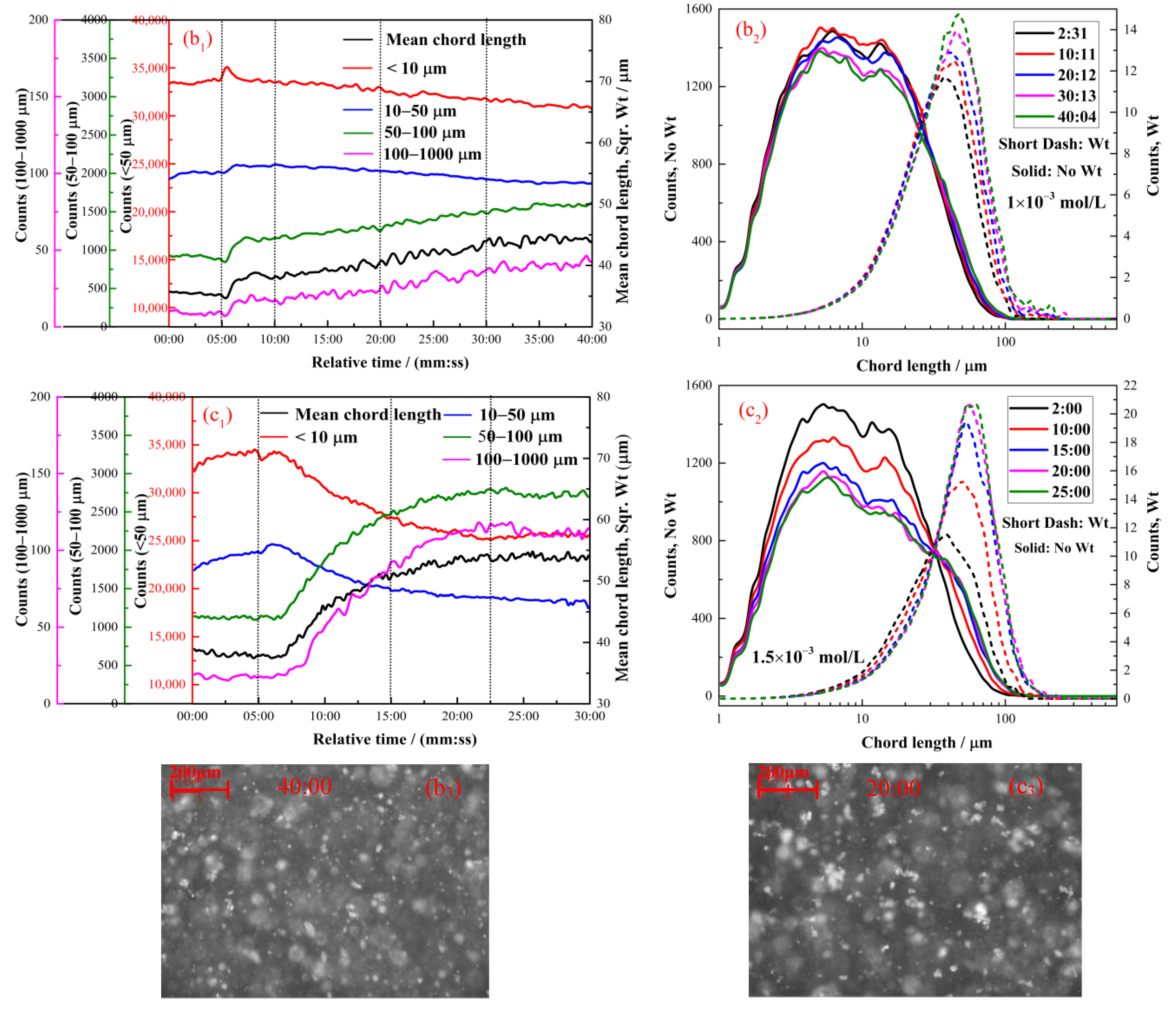
2.2.2. The Effect of Octyl Hydroxamate on the Hydrophobic Flocculation of Cassiterite
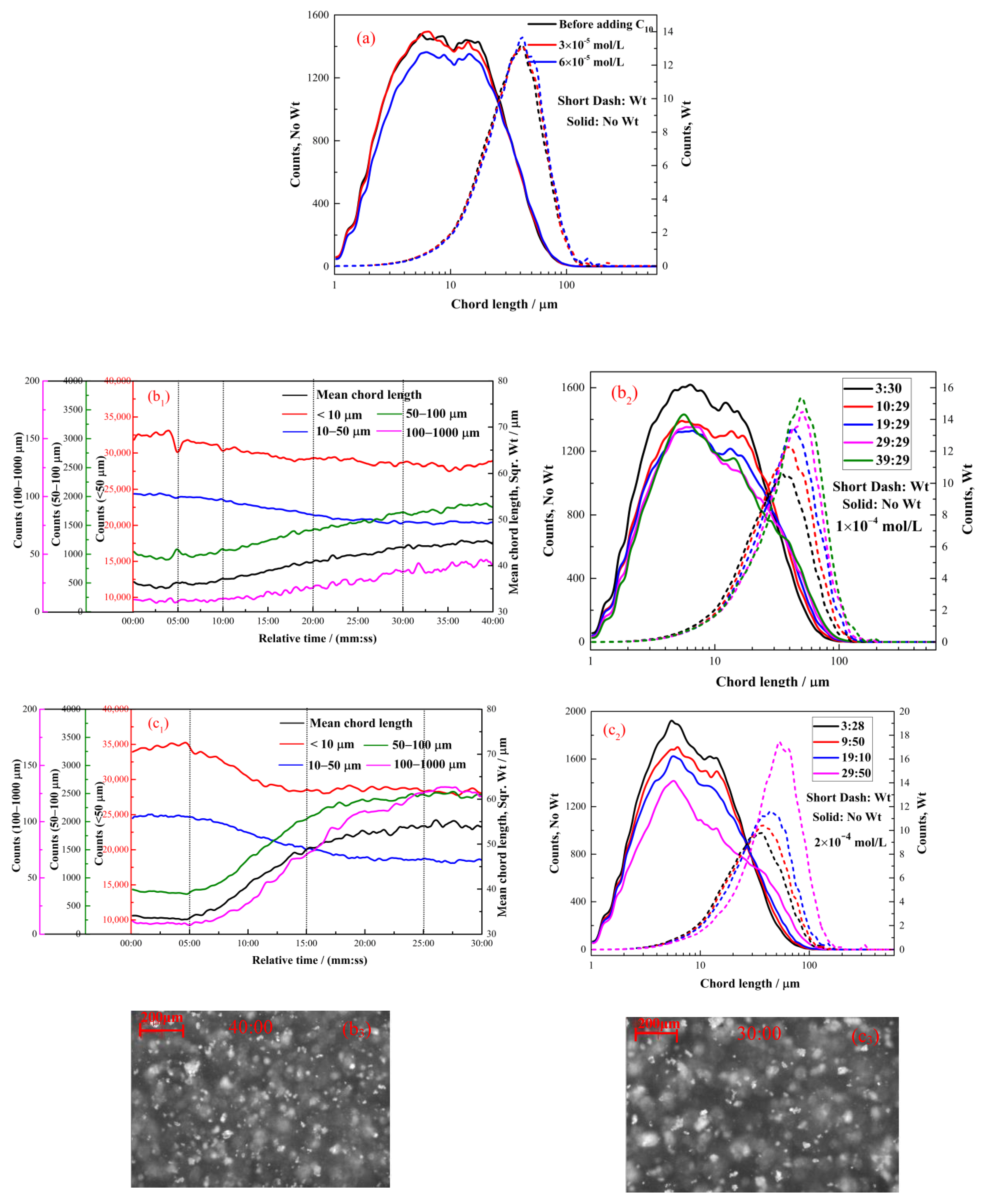
2.2.3. The Effect of Decyl Hydroxamate on the Hydrophobic Flocculation of Cassiterite
2.2.4. The Effect of Dodecyl Hydroxamate on the Hydrophobic Flocculation of Cassiterite
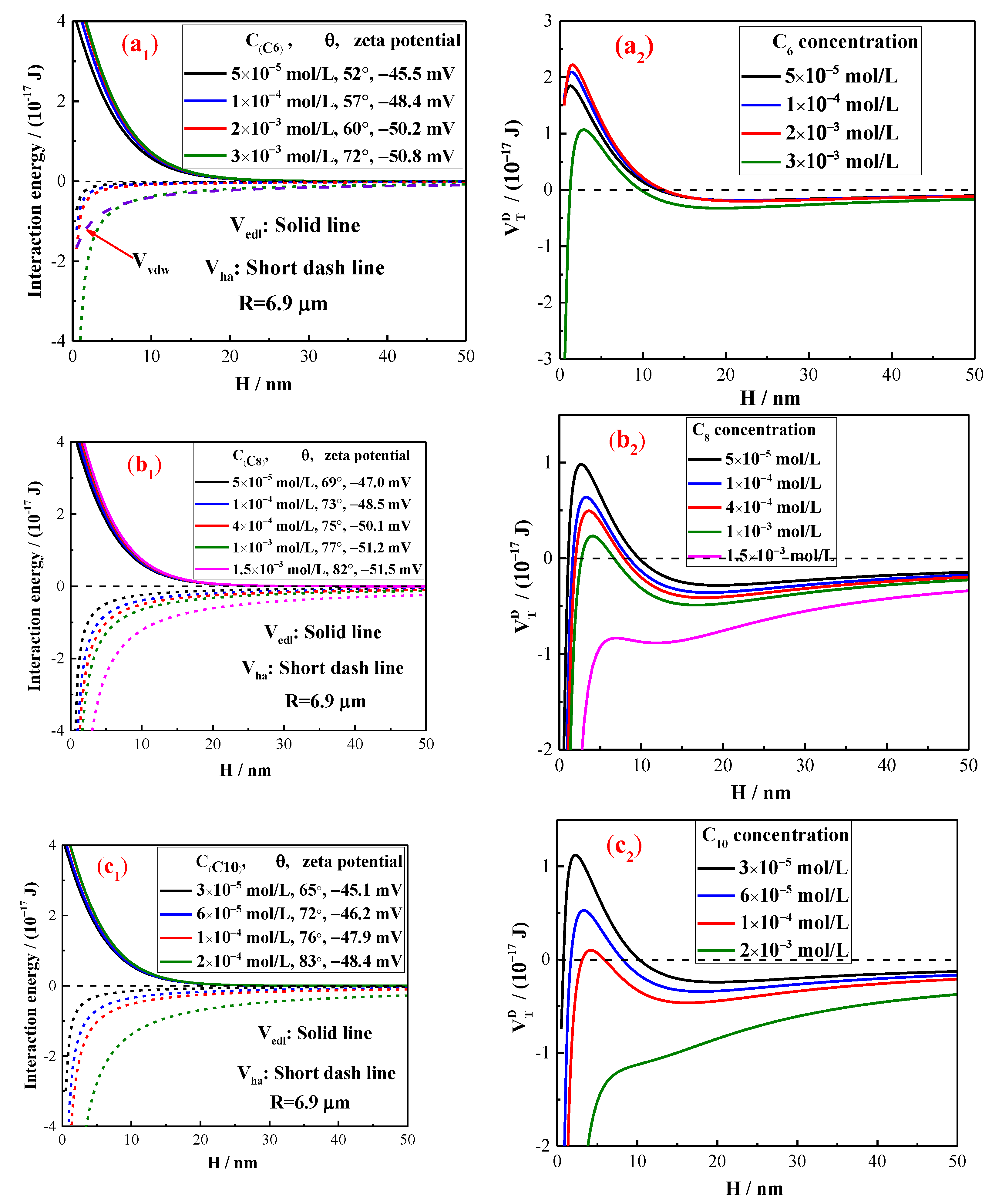
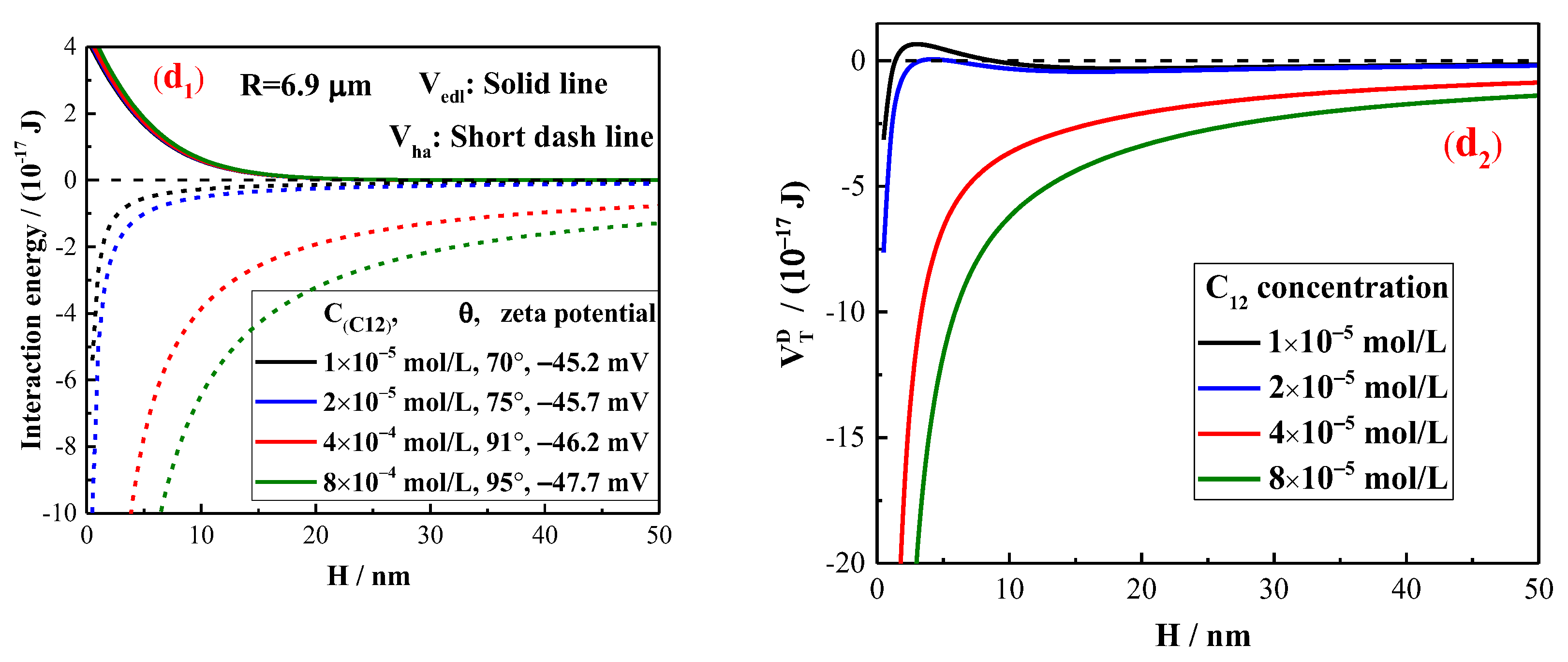
2.3. The Interaction Energy Estimation by Extended DLVO Theory
3. Materials and Methods
3.1. Single Cassiterite Sample and Reagents
3.2. Microflotation Tests
3.3. Focused Beam Reflectance Measurement and Particle Video Microscope Observation
3.4. Zeta Potential and Contact Angle Measurements
4. Theoretical Background
5. Discussion
6. Conclusions
- (1)
- The hydroxamic acid concentration required to float cassiterite decreased with the increase in carbon chain length. The cassiterite flotation recoveries were between 30% and 85%.
- (2)
- When C6 was used as a collector, the cassiterite particles could not form hydrophobic flocs. The lowest concentrations of C8, C10 and C12 required to induce the hydrophobic aggregation of cassiterite particles decreased with the increase in the carbon chain length. The lowest concentrations of C8, C10 and C12 were approximately 1 × 10−3, 1 × 10−4 and 2 × 10−5 mol/L, respectively.
- (3)
- The aggregation growth rate and apparent floc size increased with the increase in the hydroxamic acid concentration. Compared with other collectors, the hydroxamic acid with a longer carbon chain could induce cassiterite particles to form larger flocs at a lower concentration within a shorter time.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Baldauf, H.; Schoenherr, J.; Schubert, H. Alkane dicarboxylic acids and aminonaphthol-sulfonic acids—A new reagent regime for cassiterite flotation. Int. J. Miner. Process. 1985, 15, 117–133. [Google Scholar] [CrossRef]
- Huang, K.; Huang, X.; Jia, Y.; Wang, S.; Cao, Z.; Zhong, H. A novel surfactant styryl phosphonate mono-iso-octyl ester with improved adsorption capacity and hydrophobicity for cassiterite flotation. Miner. Eng. 2019, 142, 105895. [Google Scholar] [CrossRef]
- Yin, W.Z.; Tang, Y. Interactive effect of minerals on complex ore flotation: A brief review. Int. J. Miner. Met. Mater. 2020, 27, 571–582. [Google Scholar] [CrossRef]
- Cai, J.; Deng, J.; Wang, L.; Hu, M.; Xu, H.; Hou, X.; Wu, B.; Li, S. Reagent types and action mechanisms in ilmenite flotation: A review. Int. J. Miner. Met. Mater. 2022, 29, 1656–1668. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, L.; Zhang, W.; Wang, H.; Gao, Z. Mechanism of calcium lignosulfonate in apatite and dolomite flotation system. Int. J. Miner. Met. Mater. 2022, 29, 1697–1740. [Google Scholar] [CrossRef]
- Rubio, J.; Capponi, F.; Rodrigues, R.T.; Matiolo, E. Enhanced flotation of sulfide fines using the emulsified oil extender technique. Int. J. Miner. Process. 2007, 84, 41–50. [Google Scholar] [CrossRef]
- Han, H.; Liu, A.; Wang, C.; Yang, R.; Li, S.; Wang, H. Flotation kinetics performance of different coal size fractions with nanobubbles. Int. J. Miner. Met. Mater. 2022, 29, 1502–1510. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, P.; Tao, D. Surface nanobubble characterization and its enhancement mechanisms for fine-particle flotation: A review. Int. J. Miner. Met. Mater. 2022, 29, 727–737. [Google Scholar] [CrossRef]
- Qin, W.Q.; Ren, L.Y.; Wang, P.P.; Yang, C.R.; Zhang, Y.S. Electro-flotation and collision-attachment mechanism of fine cassiterite. Trans. Nonferrous Met. Soc. China 2012, 22, 917–924. [Google Scholar] [CrossRef]
- Ren, L.; Zeng, W.; Nguyen, A.V.; Ma, X. Effects of bubble size, velocity, and particle agglomeration on the electro-flotation kinetics of fine cassiterite. Asia-Pac. J. Chem. Eng. 2019, 14, e2333. [Google Scholar] [CrossRef]
- Zhou, W.; Chen, H.; Ou, L.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Lara-Valenzuela, C. Floc flotation of galena and sphalerite fines. Miner. Process. 2001, 14, 87–98. [Google Scholar] [CrossRef]
- Yan, X.; Wei, L.; Meng, Q.; Wang, J.; Yang, Q.; Zhai, S.; Lu, J. A study on the mechanism of calcium ion in promoting the sedimentation of illite particles. J. Water Process. Eng. 2021, 42, 102153. [Google Scholar] [CrossRef]
- Kemppainen, K.; Suopajärvi, T.; Laitinen, O.; Ämmälä, A.; Liimatainen, H.; Illikainen, M. Flocculation of fine hematite and quartz suspensions with anionic cellulose nanofibers. Chem. Eng. Sci. 2016, 148, 256–266. [Google Scholar] [CrossRef]
- Ozkan, A.; Dudnik, V.; Esmeli, K. Hydrophobic flocculation of talc with kerosene and effects of anionic surfactants. Part. Sci. Technol. 2015, 34, 235–240. [Google Scholar] [CrossRef]
- Li, Y.; Wu, F.; Xia, W.; Mao, Y.; Peng, Y.; Xie, G. The bridging action of microbubbles in particle-bubble adhesion. Powder Technol. 2020, 375, 271–274. [Google Scholar] [CrossRef]
- Warren, L.J. Flocculation of stirred suspensions of cassiterite and tourmaline. Colloids Surf. 1982, 5, 301–319. [Google Scholar] [CrossRef]
- Wang, D.; Qiu, G.; Hu, Y. Resource Processing Science, 3rd ed.; China Science Publishing & Medium Ltd.: Beijing, China, 2005; pp. 191–196. [Google Scholar]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Bermejo-Perez, H.I.; Trass, O. Hydrophobic flocculation of galena fines in aqueous suspensions. J. Colloid Interf. Sci. 2000, 227, 272–281. [Google Scholar] [CrossRef]
- Warren, L.J. Shear-flocculation of ultrafine scheelite in sodium oleate solutions. J. Colloid Interf. Sci. 1975, 50, 307–318. [Google Scholar] [CrossRef]
- Warren, L.J. Slime coating and shear-flocculation in the scheelite-sodium oleate system. Trans. Inst. Min. Met. 1975, 84, C99–C104. [Google Scholar]
- Yoon, R.H.; Luttrell, G.H. Development of the Selective Coagulation Process; Virginia Center for Coal and Minerals Processing: Blacksburg, VA, USA, 1992; pp. 64–66. [Google Scholar]
- Pascoe, R.D.; Doherty, E. Shear flocculation and flotation of hematite using sodium oleate. Int. J. Miner. Process. 1997, 51, 269–282. [Google Scholar] [CrossRef]
- Akdemir, Ü. Shear flocculation of fine hematite particles and correlation between flocculation, flotation and contact angle. Powder Technol. 1997, 94, 1–4. [Google Scholar] [CrossRef]
- Jin, S.; Ou, L. Comparison of the effects of sodium oleate and benzohydroxamic acid on fine scheelite and cassiterite hydrophobic flocculation. Minerals 2022, 12, 687. [Google Scholar] [CrossRef]
- Song, S.; Lu, S. Hydrophobic flocculation of fine hematite, siderite, and rhodochrosite particles in aqueous solution. J. Colloids Interf. Sci. 1994, 166, 35–42. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Qin, W.; Xu, Y.; Liu, H.; Ren, L.; Yang, C. Flotation and surface behavior of cassiterite with salicylhydroxamic acid. Ind. Eng. Chem. Res. 2011, 50, 10778–10783. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Sun, W.; Han, H.; Sun, L.; Hu, Y. Activation role of lead ions in benzohydroxamic acid flotation of oxide minerals: New perspective and new practice. J. Colloids Interf. Sci. 2018, 529, 150–160. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; He, J.; Hu, Y.; Sun, W.; Yuan, D. Novel insights into adsorption mechanism of benzohydroxamic acid on lead (II)-activated cassiterite surface: An integrated experimental and computational study. Miner. Eng. 2018, 122, 327–338. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Wang, P.; Qin, W.; Ren, L.; Wei, Q.; Liu, R.; Yang, C.; Zhong, S. Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite. Trans. Nonferrous Met. Soc. China 2013, 23, 1789–1796. [Google Scholar] [CrossRef]
- Jin, S.; Z’hang, P.; Ou, L.; Zhang, Y.; Chen, J. Flotation of cassiterite using alkyl hydroxamates with different carbon chain lengths: A theoretical and experimental study. Miner. Eng. 2021, 170, 107025. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; Wang, L.; Li, Y.; Ji, B.; Hu, Y.; et al. Effects of the preassembly of benzohydroxamic acid with Fe (III) ions on its adsorption on cassiterite surface. Miner. Eng. 2018, 127, 32–41. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, L.; Gao, Z.; Sun, W.; Cao, X. Activation mechanism of zinc ions in cassiterite flotation with benzohydroxamic acid as a collector. Miner. Eng. 2020, 156, 106523. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, X.; Tong, X.; Feng, D.; Lv, J.; Chen, Y.; Song, Q. The activation mechanism of Fe(II) ion-modified cassiterite surface to promote salicylhydroxamic acid adsorption. Miner. Eng. 2021, 160, 106707. [Google Scholar] [CrossRef]
- Shuai, S.; Huang, Z.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Selective separation of wolframite from calcite by froth flotation using a novel amidoxime surfactant: Adsorption mechanism and DFT calculation. Miner. Eng. 2022, 185, 107716. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Xia, W.; Li, Y.; Wu, F.; Niu, C. Enhanced flotation selectivity of fine coal from kaolinite by anionic polyacrylamide pre-conditioning. J. Mol. Liq. 2021, 334, 116083. [Google Scholar] [CrossRef]
- Li, H.; Liu, M.; Liu, Q. The effect of non-polar oil on fine hematite flocculation and flotation using sodium oleate or hydroxamic acids as a collector. Miner. Eng. 2018, 119, 105–115. [Google Scholar] [CrossRef]
- Zhang, S.; Huang, Z.; Wang, H.; Liu, R.; Cheng, C.; Shuai, S.; Hu, Y.; Guo, Z.; Yu, X.; He, G.; et al. Flotation performance of a novel Gemini collector for kaolinite at low temperature. Int. J. Min. Sci. Techno. 2021, 31, 115–152. [Google Scholar] [CrossRef]
- Churaev, N.V.; Derjaguin, B.V. Inclusion of structural forces in the theory of stability of colloids and films. J. Colloids Interf. Sci. 1985, 103, 542–553. [Google Scholar] [CrossRef]
- Fu, J.; Han, H.; Wei, Z.; Sun, W.; Yue, T. Recovery of ultrafine scheelite particles by magnetic seeding flocculation and its mechanism. Colloids Surf. A 2021, 628, 127266. [Google Scholar] [CrossRef]
- Piñeres, J.; Barraza, J. Energy barrier of aggregates coal particle–bubble through the extended DLVO theory. Int. J. Miner. Process. 2011, 100, 14–20. [Google Scholar] [CrossRef]
- Yoon, R.H.; Flinn, D.H.; Rabinovich, Y.I. Hydrophobic interactions between dissimilar surfaces. J. Colloids Interf. Sci. 1997, 185, 363–370. [Google Scholar] [CrossRef] [PubMed]


| Hydroxamic Acid | Concentration/(mol/L) | T2/min | d2 − d1/μm | r/(μm/min) |
|---|---|---|---|---|
| C8 | 1 × 10−3 | 40 | 44.2–35.6 | 0.25 |
| 1.5 × 10−3 | 20 | 53.5–38.3 | 1.02 | |
| C10 | 1 × 10−4 | 40 | 45.1–35.7 | 0.27 |
| 2 × 10−4 | 20 | 52.5–33.6 | 1.26 | |
| C12 | 2 × 10−5 | 40 | 47.4–34.2 | 0.38 |
| 4 × 10−5 | 15 | 54.9–31.9 | 2.30 | |
| 8 × 10−5 | 15 | 64.3–31.5 | 2.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, S.; Shi, Q.; Ou, L. Hydrophobic Flocculation of Fine Cassiterite Using Alkyl Hydroxamic Acids with Different Carbon Chain Lengths as Collectors. Molecules 2023, 28, 3911. https://doi.org/10.3390/molecules28093911
Jin S, Shi Q, Ou L. Hydrophobic Flocculation of Fine Cassiterite Using Alkyl Hydroxamic Acids with Different Carbon Chain Lengths as Collectors. Molecules. 2023; 28(9):3911. https://doi.org/10.3390/molecules28093911
Chicago/Turabian StyleJin, Saizhen, Qing Shi, and Leming Ou. 2023. "Hydrophobic Flocculation of Fine Cassiterite Using Alkyl Hydroxamic Acids with Different Carbon Chain Lengths as Collectors" Molecules 28, no. 9: 3911. https://doi.org/10.3390/molecules28093911
APA StyleJin, S., Shi, Q., & Ou, L. (2023). Hydrophobic Flocculation of Fine Cassiterite Using Alkyl Hydroxamic Acids with Different Carbon Chain Lengths as Collectors. Molecules, 28(9), 3911. https://doi.org/10.3390/molecules28093911




