Utilization of Phytic Acid as a Selective Depressant for Quartz Activated by Zinc Ions in Smithsonite Flotation
Abstract
:1. Introduction
2. Results and Discussion
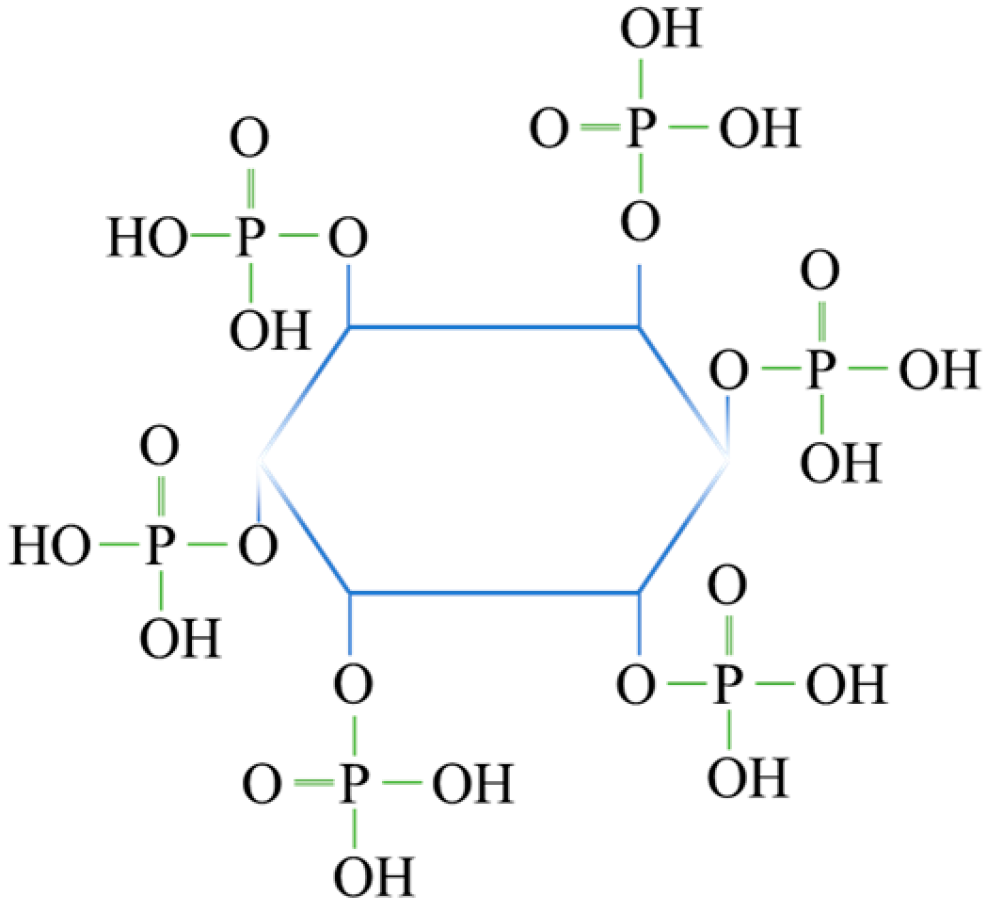
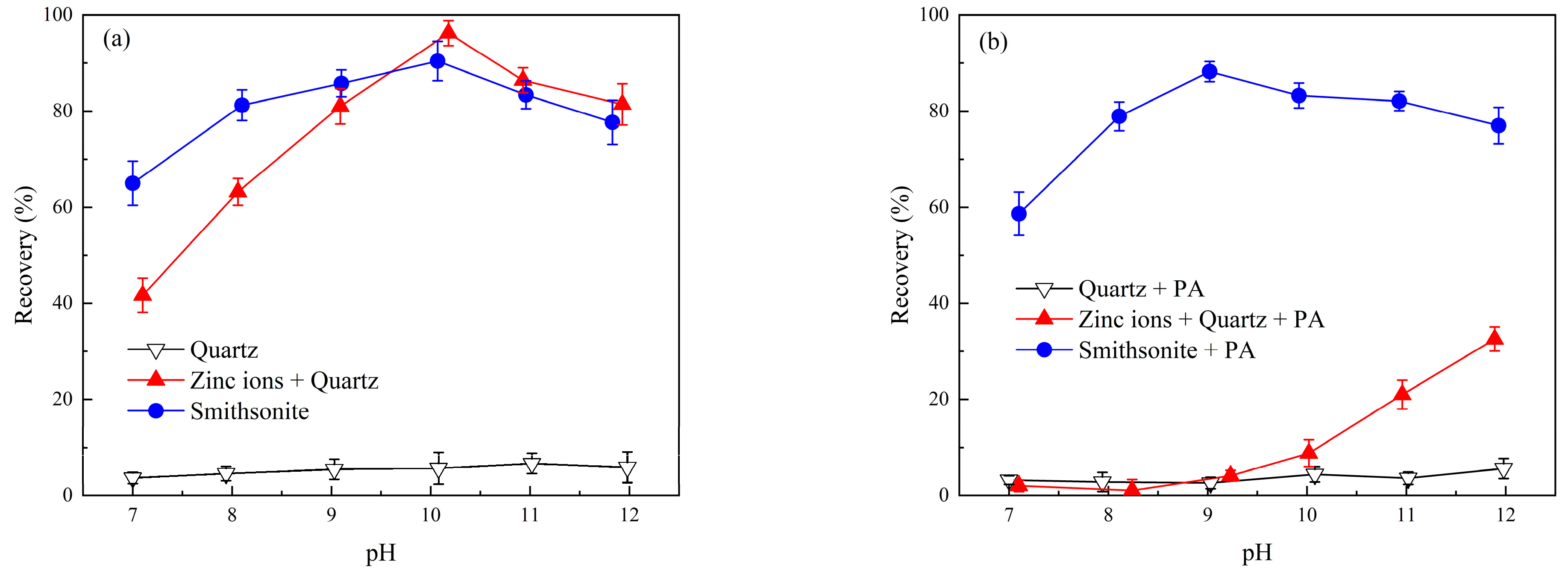
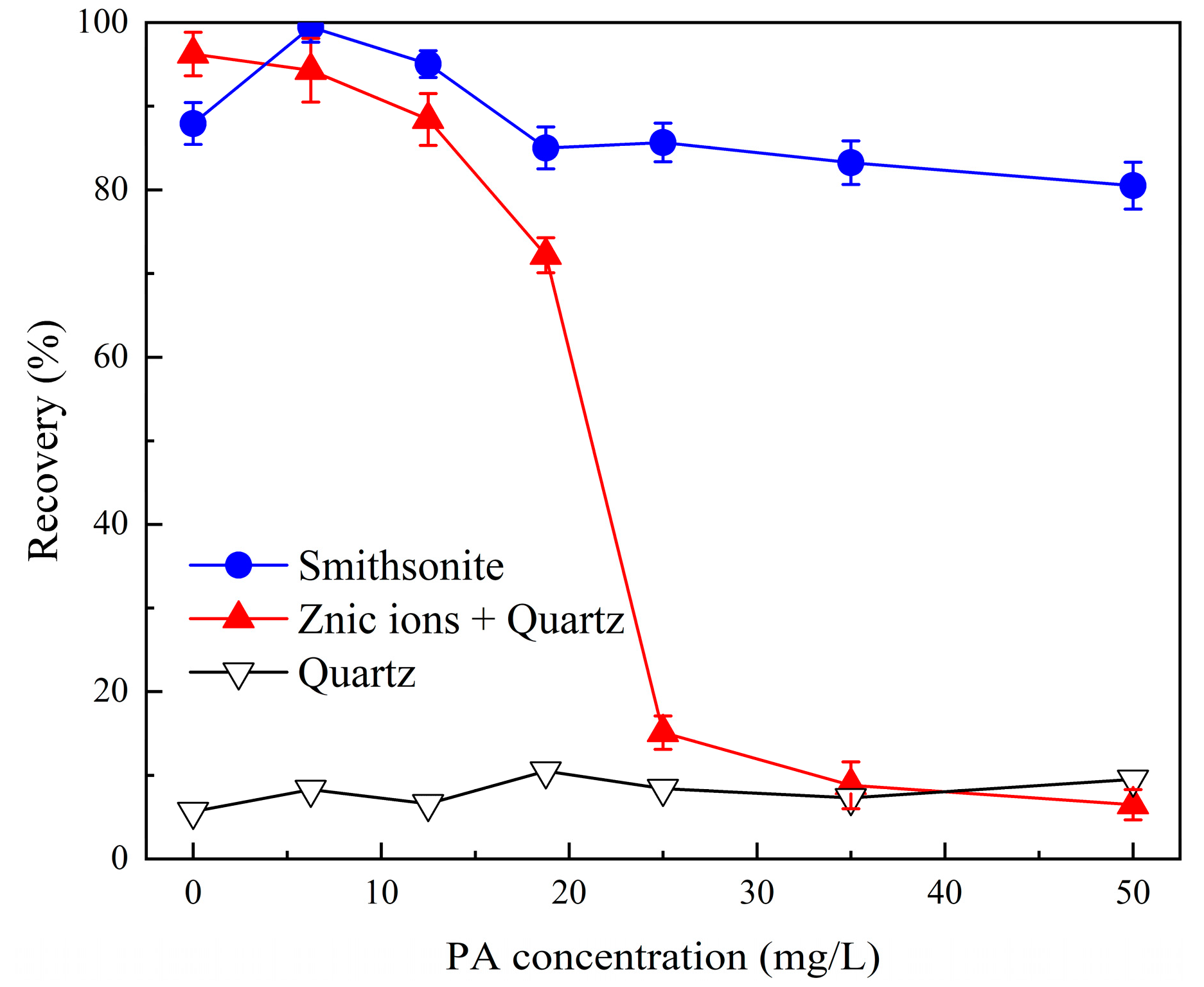
2.1. Effect of Phytic Acid on the Microflotation of Zinc-Ion-Activated Quartz and Smithsonite
2.2. Effect of Phytic Acid on the Zeta Potentials of Minerals
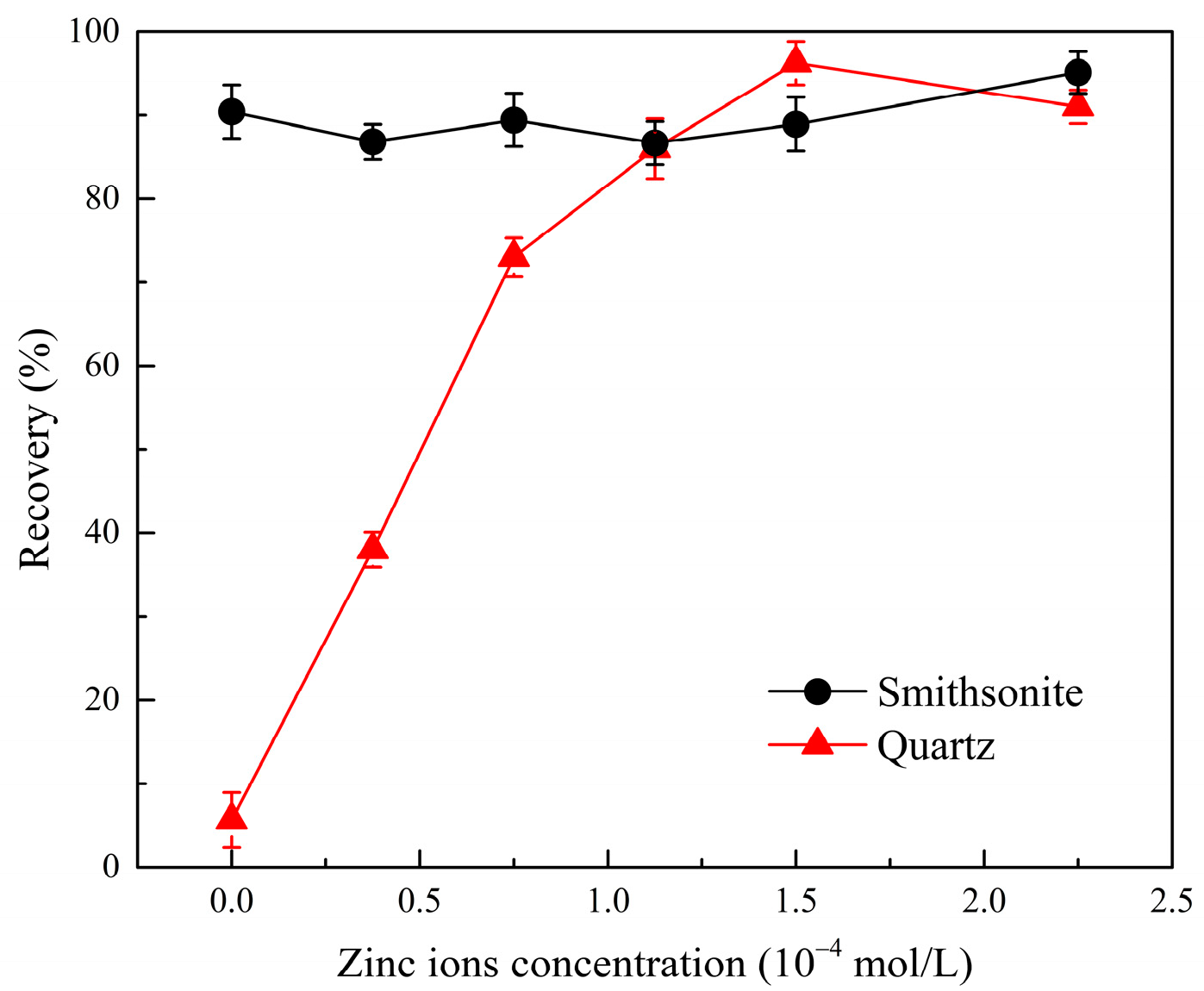
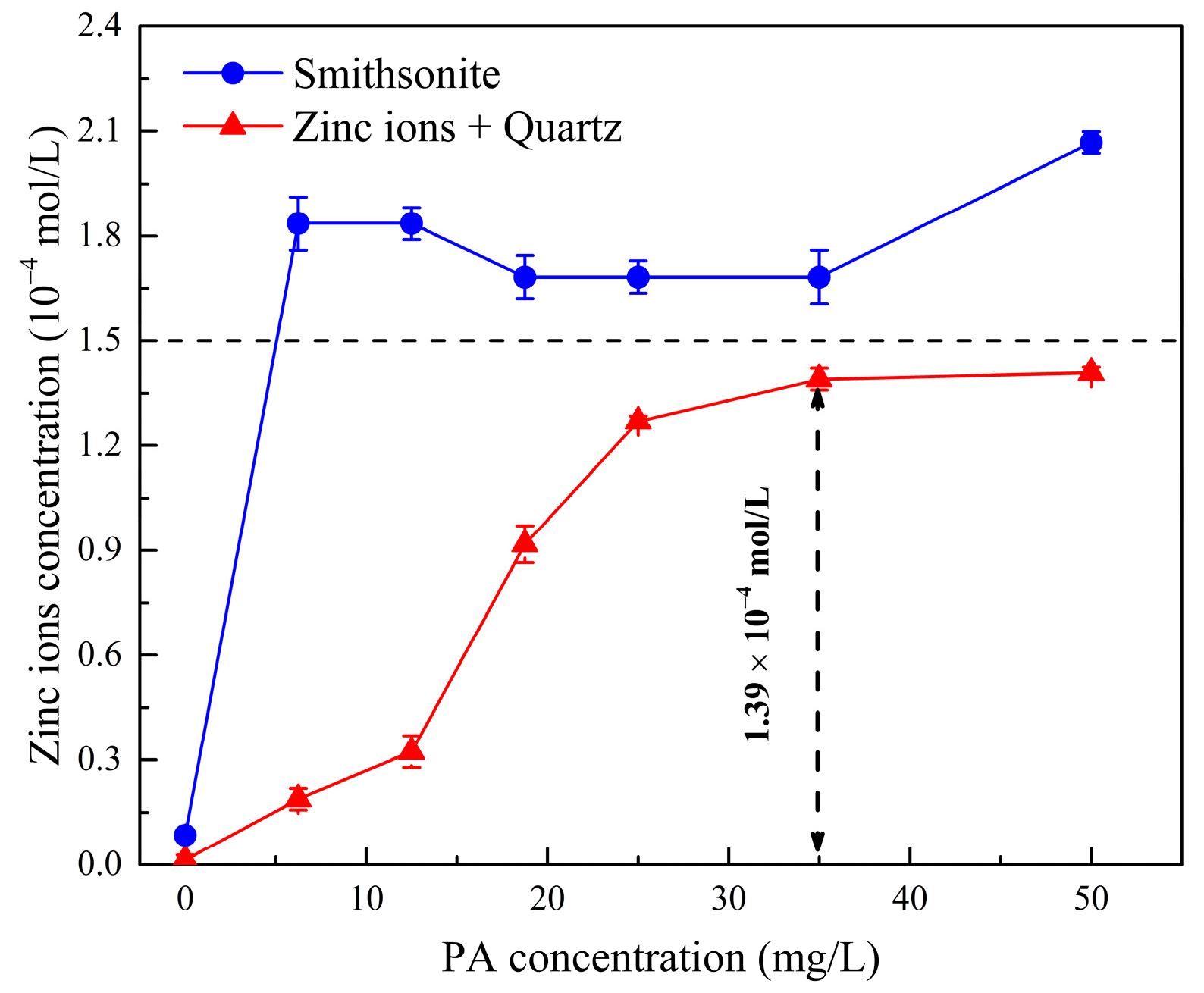
2.3. Effect of Phytic Acid on the Migration of Active Zinc Ions
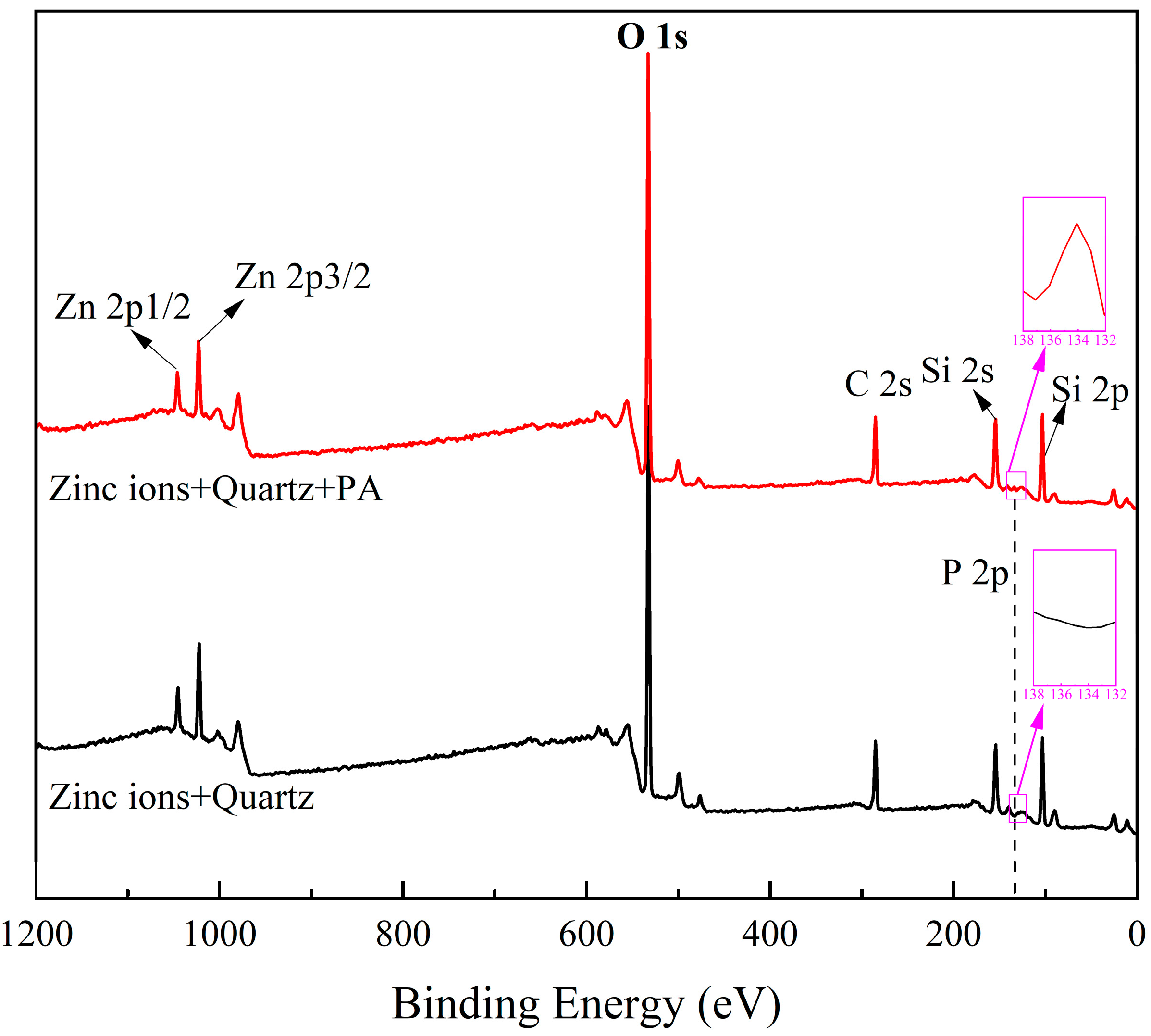
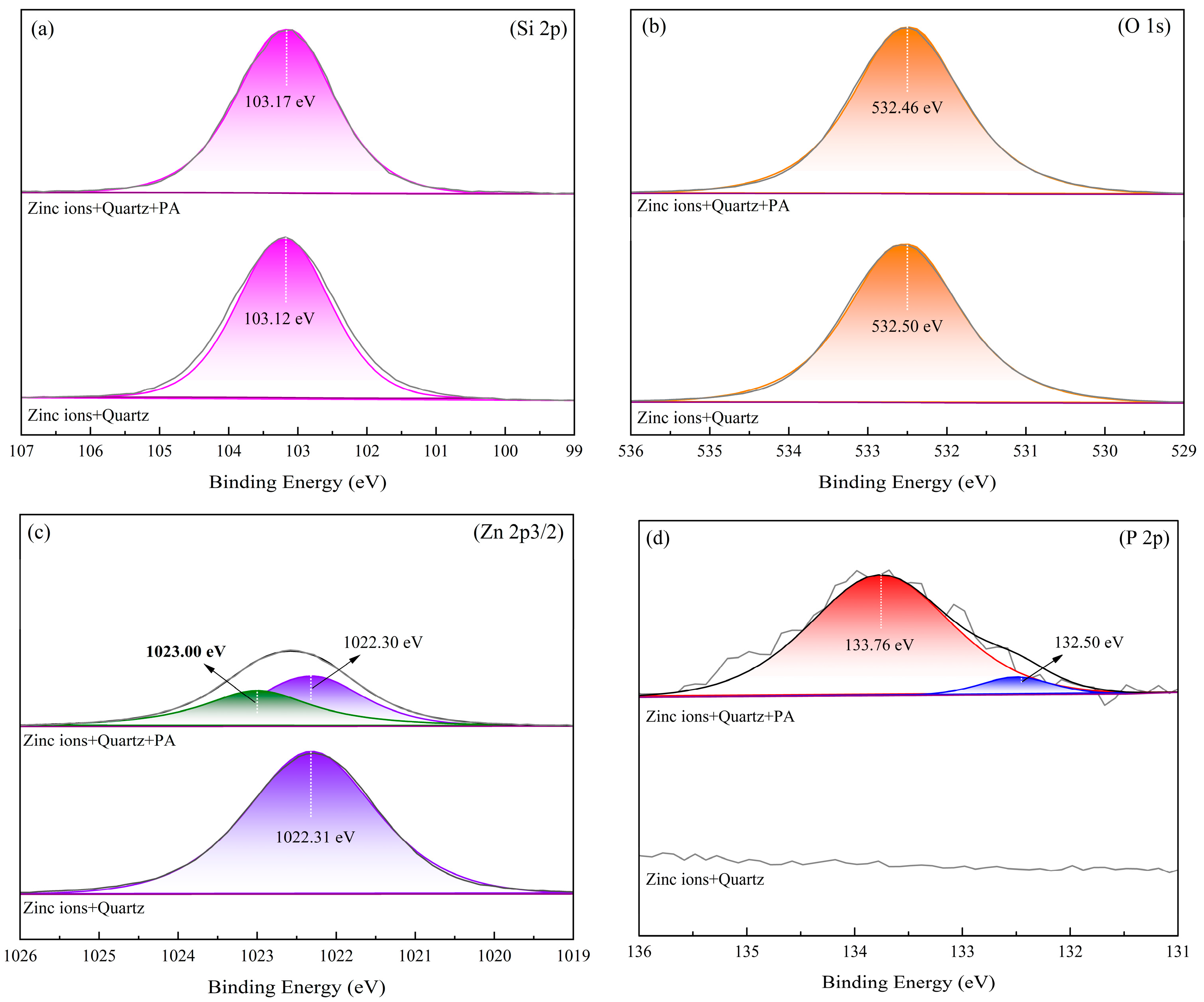
2.4. XPS Analysis Results
3. Materials and Methods
3.1. Single Smithsonite and Quartz Sample and Reagents
3.2. Microflotation Tests
3.3. Zeta Potential Measurements
3.4. Zinc Ions Dissolution Tests and SEM-EDS Analysis
3.5. XPS Analysis
4. Conclusions
- The addition of 35 mg/L phytic acid selectively inhibited the flotation of Zn2+-activated quartz without affecting the flotation of smithsonite, enabling the separation of these minerals.
- A high dosage of phytic acid (75 mg/L) was required to achieve satisfactory separation of artificially mixed minerals.
- Phytic acid exhibited a chemisorption capability with the Zn sites on the surface of Zn2+-activated quartz, resulting in the desorption of Zn ions from the quartz surface into the solution.
- The significant desorption of Zn ions from the mineral surface reduced the adsorption sites available for sodium oleate, leading to a significant decrease in the flotation recovery of Zn2+-activated quartz.
- Phytic acid had minimal influence on the adsorption of sodium oleate and the flotation recovery of smithsonite. Therefore, a satisfactory flotation separation of smithsonite from Zn2+-activated quartz was achieved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- 2018 Minerals Yearbook—Zinc; USGS Science a Change World; US Geological Survey: Reston, VA, USA, 2018; pp. 67.1–67.13.
- Ejtemaei, M.; Gharabaghi, M.; Irannajad, M. A Review of Zinc Oxide Mineral Beneficiation Using Flotation Method. Adv. Colloid Interface Sci. 2014, 206, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Abkhoshk, E.; Jorjani, E.; Al-Harahsheh, M.S.; Rashchi, F.; Naazeri, M. Review of the Hydrometallurgical Processing of Non-Sulfide Zinc Ores. Hydrometallurgy 2014, 149, 153–167. [Google Scholar] [CrossRef]
- Li, W.; Gong, Z.; Yan, X.; Wang, D.; Liu, J.; Guo, X.; Zhang, Z.; Li, G. In Situ Engineered ZnS-FeS Heterostructures in N-Doped Carbon Nanocages Accelerating Polysulfide Redox Kinetics for Lithium Sulfur Batteries. J. Mater. Chem. A 2020, 8, 433–442. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Chen, Y.; Zhao, L. Effect of Surface Oxidization on Quartz Slime Coating in the Sulfidization-Amine Flotation of Smithsonite. Miner. Eng. 2022, 188, 107847. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Chen, Y.; Zhao, L. Effect of Dissolved Oxygen on the Sulfidization Flotation of Smithsonite. Miner. Eng. 2022, 186, 107741. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Influence of Important Factors on Flotation of Zinc Oxide Mineral Using Cationic, Anionic and Mixed (Cationic/Anionic) Collectors. Miner. Eng. 2011, 24, 1402–1408. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, G.; Song, S.; Li, H. Flotation Separation of Smithsonite from Quartz Using Calcium Lignosulphonate as a Depressant and Sodium Oleate as a Collector. Miner. Eng. 2019, 131, 385–391. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhao, L.; Chen, Y.; Liu, D.; Li, C. Application of Eco-Friendly Tetrasodium Iminodisuccinate for Sephytic acidration of Smithsonite from Zinc Ions Activated Quartz. Miner. Eng. 2022, 181, 107545. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Role of Dissolved Mineral Species in Selective Flotation of Smithsonite from Quartz Using Oleate as Collector. Int. J. Miner. Process. 2012, 114–117, 40–47. [Google Scholar] [CrossRef]
- Zhu, H.L.; Qin, W.Q.; Chen, C.; Chai, L.Y.; Li, L.S.; Liu, S.J.; Zhang, T. Selective Flotation of Smithsonite, Quartz and Calcite Using Alkyl Diamine Ether as Collector. Trans. Nonferrous Met. Soc. China 2018, 28, 163–168. [Google Scholar] [CrossRef]
- Kou, J.; Xu, S.; Sun, T.; Sun, C.; Guo, Y.; Wang, C. A Study of Sodium Oleate Adsorption on Ca2+ Activated Quartz Surface Using Quartz Crystal Microbalance with Dissiphytic acidtion. Int. J. Miner. Process. 2016, 154, 24–34. [Google Scholar] [CrossRef]
- Wang, Y.; Ahmed Khoso, S.; Luo, X.; Tian, M. Understanding the Depression Mechanism of Citric Acid in Sodium Oleate Flotation of Ca2+-Activated Quartz: Experimental and DFT Study. Miner. Eng. 2019, 140, 105878. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, G.; Zhu, Y. Rheological Investigations of the Improved Fine Scheelite Flotation Spiked with Agitation Medium. Int. J. Min. Sci. Technol. 2022, 32, 1379–1388. [Google Scholar] [CrossRef]
- Feng, Q.; Wen, S.; Zhao, W.; Chen, H. Interaction Mechanism of Magnesium Ions with Cassiterite and Quartz Surfaces and Its Response to Flotation Separation. Sep. Purif. Technol. 2018, 206, 239–246. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhao, L.; Chen, Y.; Liu, D. Utilization of 1-Hydroxyethylidene-1,1-Diphosphonic Acid to Selectively Sephytic acidrate Smithsonite from Zinc Ions Activated Quartz. Miner. Eng. 2022, 182, 107585. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Chen, Y. Using Phytic acid as a Depressant for the Selective Flotation Separation of Smithsonite from Calcite. Sep. Purif. Technol. 2022, 302, 122104. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Y.; Sun, W.; Zhai, J.; Yin, Z.; Guan, Q. Effect of Phytic acid on the Surface Properties of Scheelite and Fluorite for Their Selective Flotation. Colloids Surf. A Physicochem. Eng. Asp. 2019, 573, 80–87. [Google Scholar] [CrossRef]
- Shi, Q.; Feng, Q.; Zhang, G.; Deng, H. Electrokinetic Properties of Smithsonite and Its Floatability with Anionic Collector. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 178–183. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Song, S.; Li, H.; Liu, Y. Flotation Separation of Smithsonite from Calcite Using 2-Phosphonobutane-1,2,4-Tricarboxylic Acid as a Depressant. Powder Technol. 2019, 352, 11–15. [Google Scholar] [CrossRef]
- Shi, Q.; Zhang, G.; Feng, Q.; Ou, L.; Lu, Y. Effect of the Lattice Ions on the Calcite Flotation in Presence of Zn(II). Miner. Eng. 2013, 40, 24–29. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, H.; Zeng, L.; Feng, J.; Luo, H. An Environmental-Friendly Sphalerite Depressant (2-Hydroxyphosphonoacetic Acid) for the Selective Flotation Separation of Sphalerite from Galena. J. Mol. Liq. 2021, 343, 117614. [Google Scholar] [CrossRef]


| Phytic Acid Concentration (mg/L) | Product | Yield (%) | Zn Grade (%) | Zn Recovery (%) |
|---|---|---|---|---|
| 0 | Concentrate | 93.74 | 25.21 | 93.55 |
| Tailing | 6.26 | 26.03 | 6.45 | |
| Feed | 100.00 | 25.26 | 100.00 | |
| 35 | Concentrate | 85.12 | 27.11 | 91.18 |
| Tailing | 14.88 | 15.00 | 8.82 | |
| Feed | 100.00 | 25.31 | 100.00 | |
| 75 | Concentrate | 51.37 | 41.98 | 85.41 |
| Tailing | 48.63 | 7.58 | 14.59 | |
| Feed | 100.00 | 25.25 | 100.00 |
| Sample | Atomic Concentrations (%) | |||
|---|---|---|---|---|
| Si 2p | O 1s | Zn 2p | P 2p | |
| Zn2+-activated quartz | 29.41 | 65.45 | 5.14 | - |
| Zn2+-activated quartz + phytic acid | 31.85 | 66.28 | 1.09 | 0.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Jin, S. Utilization of Phytic Acid as a Selective Depressant for Quartz Activated by Zinc Ions in Smithsonite Flotation. Molecules 2023, 28, 5361. https://doi.org/10.3390/molecules28145361
Wang M, Jin S. Utilization of Phytic Acid as a Selective Depressant for Quartz Activated by Zinc Ions in Smithsonite Flotation. Molecules. 2023; 28(14):5361. https://doi.org/10.3390/molecules28145361
Chicago/Turabian StyleWang, Mengtao, and Saizhen Jin. 2023. "Utilization of Phytic Acid as a Selective Depressant for Quartz Activated by Zinc Ions in Smithsonite Flotation" Molecules 28, no. 14: 5361. https://doi.org/10.3390/molecules28145361
APA StyleWang, M., & Jin, S. (2023). Utilization of Phytic Acid as a Selective Depressant for Quartz Activated by Zinc Ions in Smithsonite Flotation. Molecules, 28(14), 5361. https://doi.org/10.3390/molecules28145361









