Effect of Drying Kinetics, Volatile Components, Flavor Changes and Final Quality Attributes of Moslae herba during the Hot Air Thin-Layer Drying Process
Abstract
1. Introduction
2. Results and Discussion
2.1. Drying Kinetics of Moslae herba
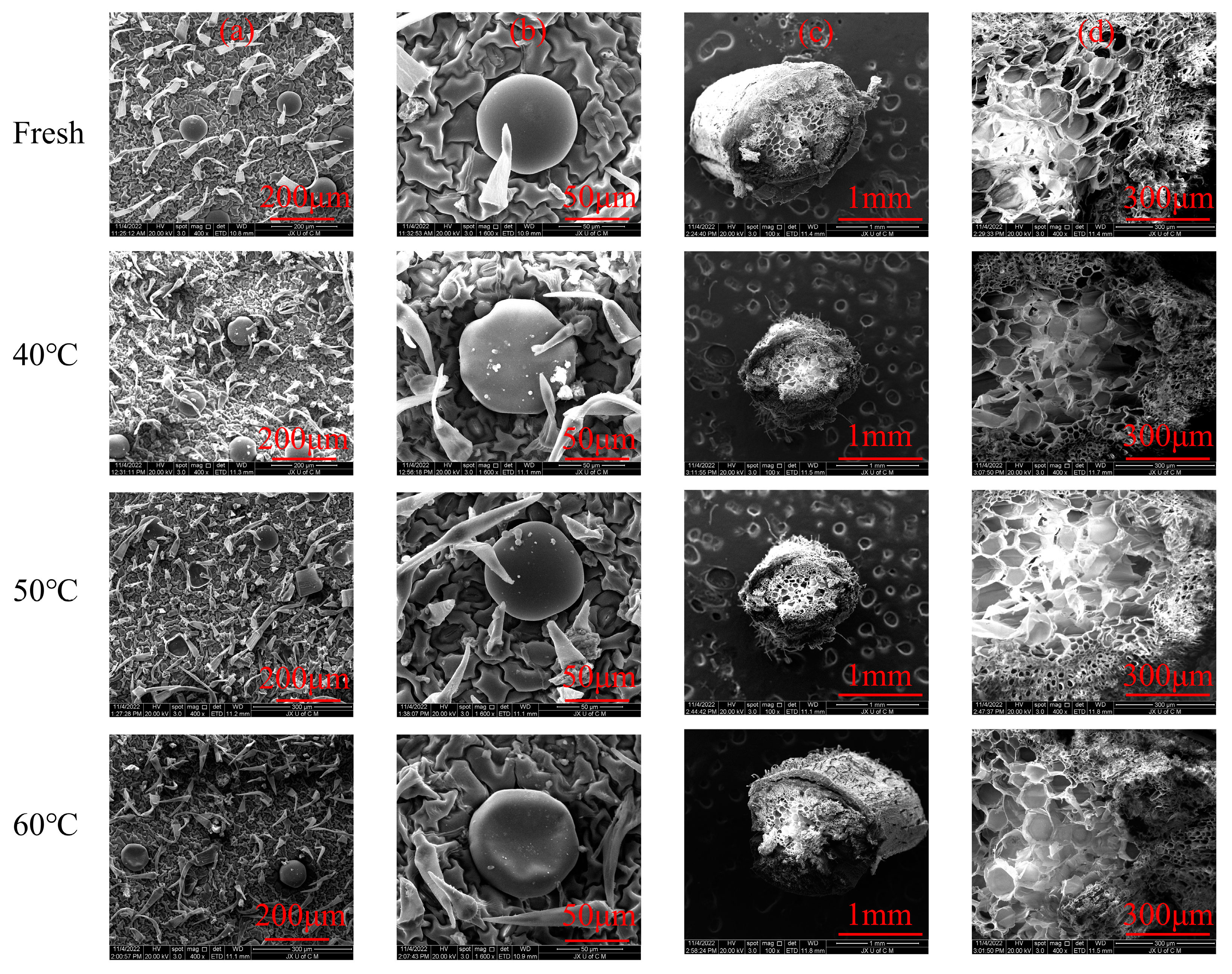
2.2. Effects of the Drying Process on the Microstructure of Moslae herba
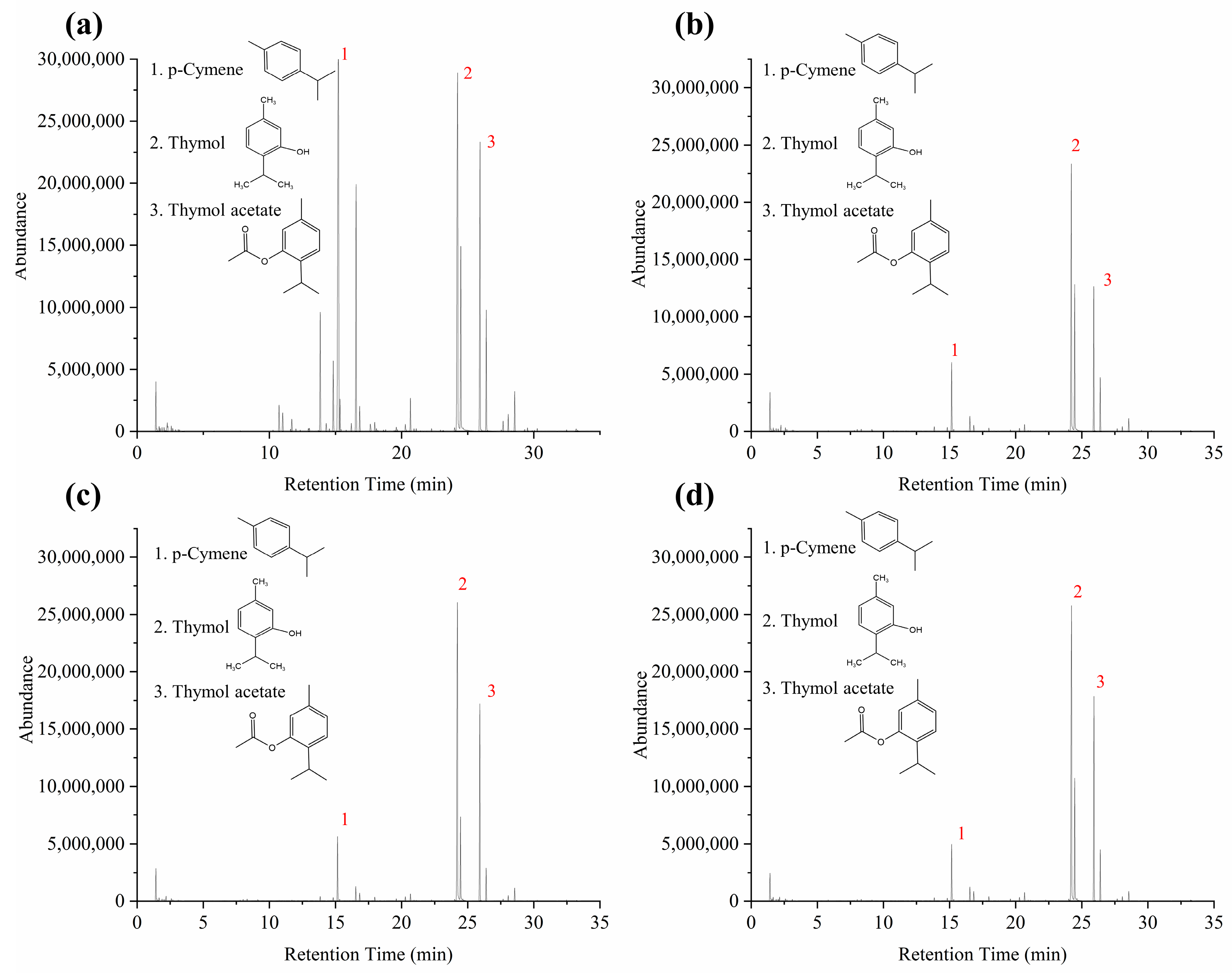
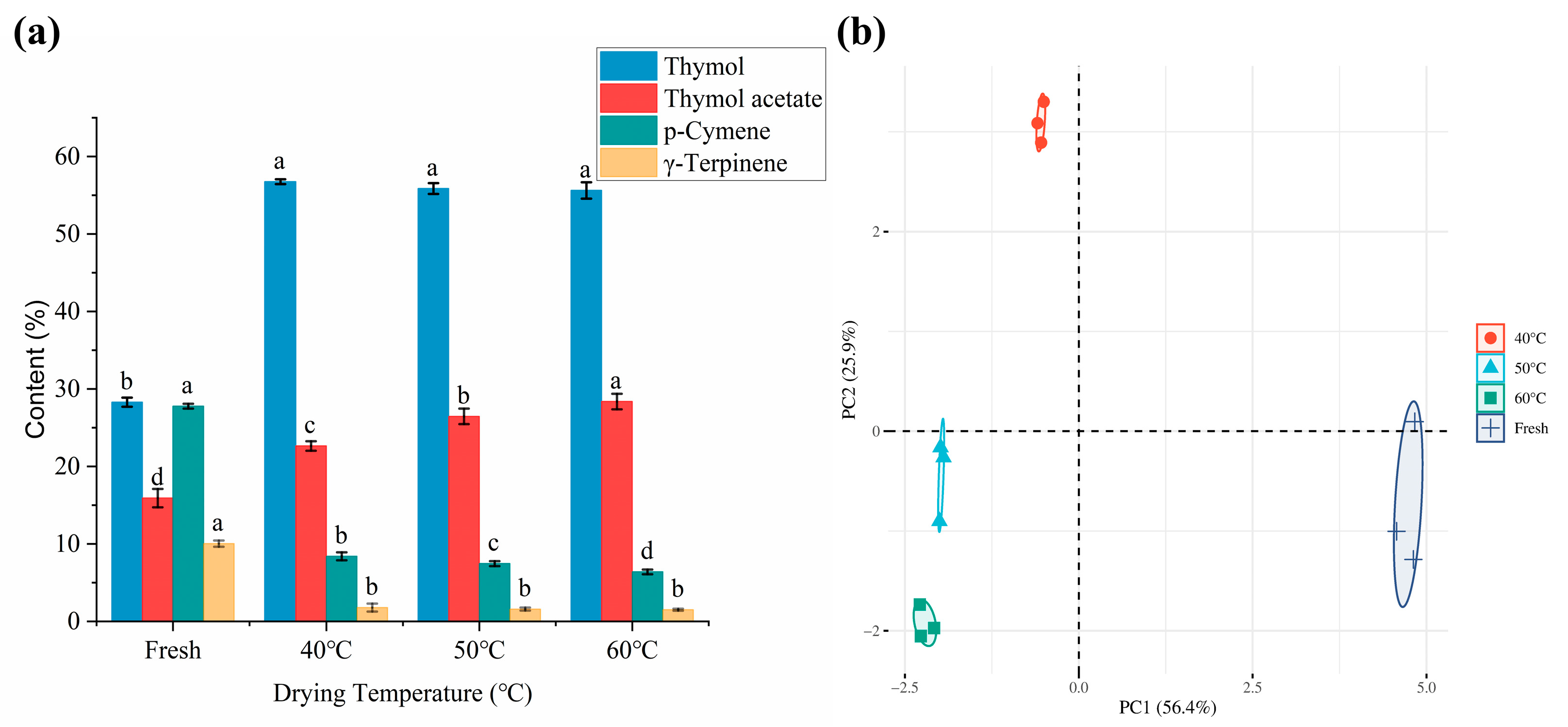
2.3. Effects of the Drying Process on the Volatile Compounds of Moslae herba
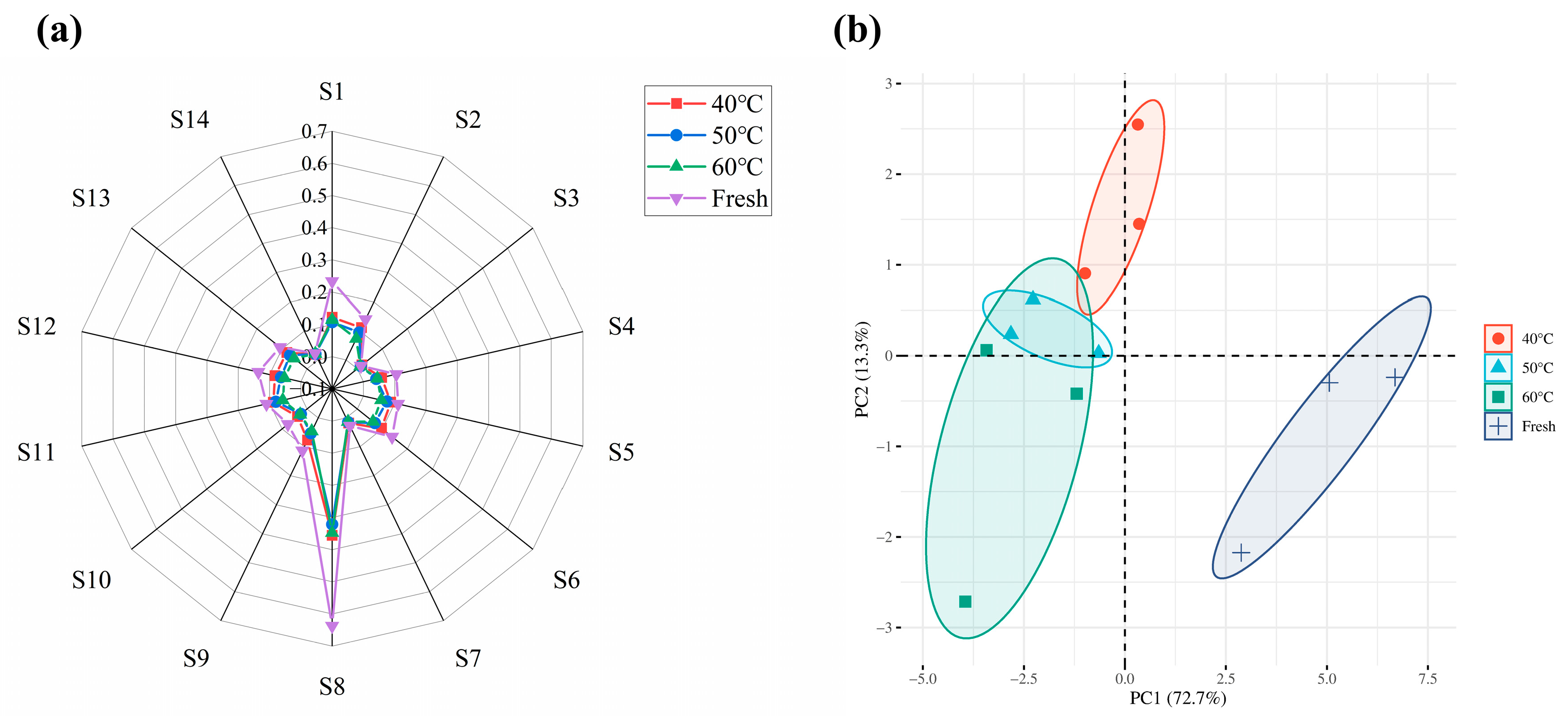
2.4. Effect of the Drying Process on the Aroma Profile of Dried Moslae herba
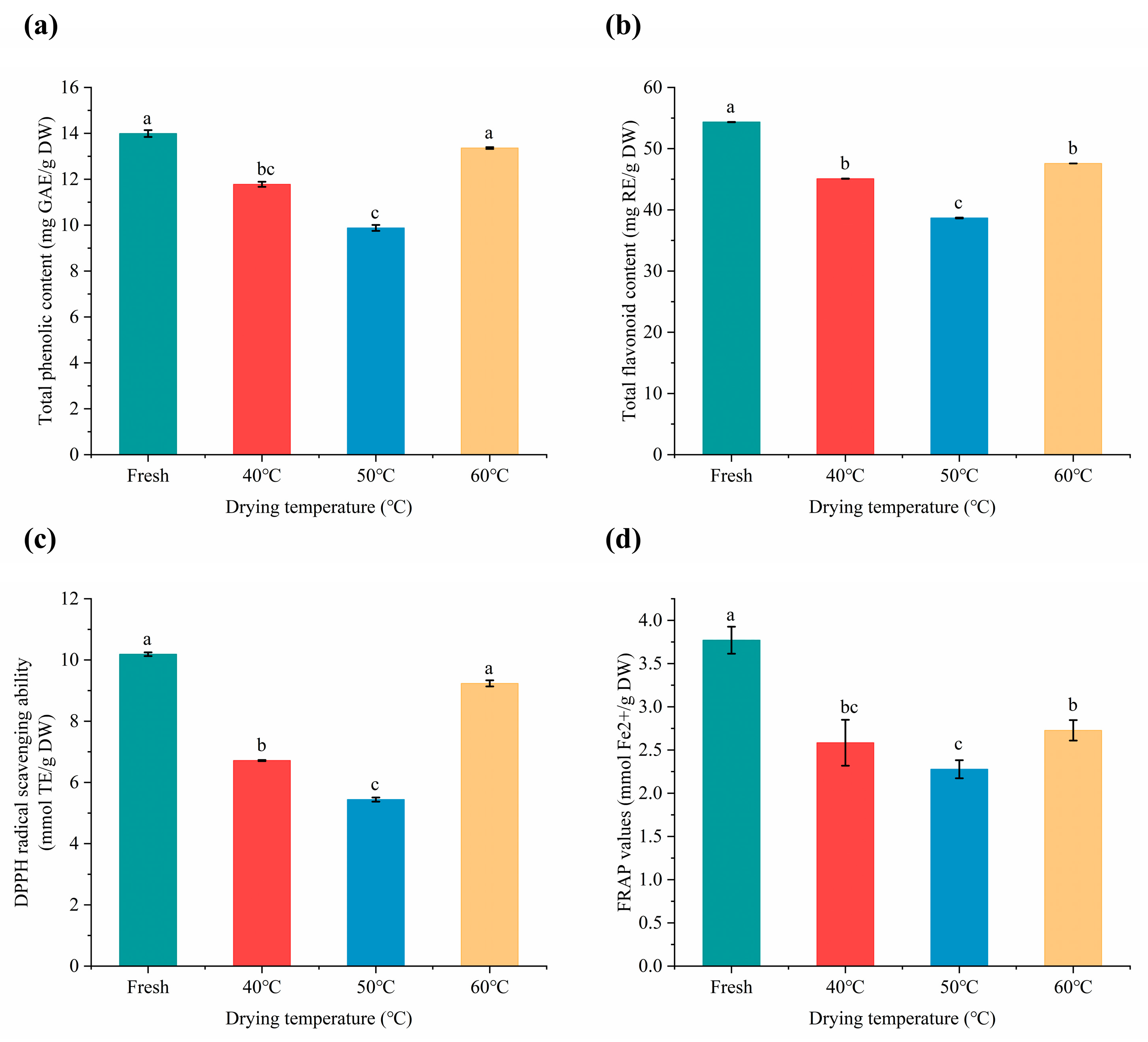
2.5. Total Phenolic and Flavonoid Contents
2.6. Antioxidant Activities
3. Materials and Methods
3.1. Raw Material and Chemical Regents
3.2. Hot Air-Drying of Moslae herba
3.3. Determination of the Moisture Content
3.4. Determining the Behavior of the Hot Air-Drying Process
3.4.1. Determination of the Moisture Ratio
3.4.2. Determination of the Drying Rate
3.4.3. Analysis of Drying Behaviors of Moslae herba
3.4.4. Effective Diffusion Coefficient (Deff)
3.4.5. Activation Energy (Ea)
3.5. Scanning Electron Microscopy
3.6. Headspace Gas Chromatography–Mass Spectrometry (HS-GC–MS) Analysis
3.7. Determination of the Flavor Characteristics of Moslae herba
3.8. Determination of the Bioactive Ingredients in Moslae herba
3.8.1. Preparation of the Extracts
3.8.2. Determination of the Total Phenolic Content (TPC)
3.8.3. Determination of the Total Flavonoid Content (TFC)
3.9. Antioxidant Ability
3.9.1. DPPH Radical Scavenging Assay
3.9.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xue, X.; Guo, Z.; Zhang, H.; Liu, X.; Luo, J.; Li, D.; Li, J. Chemical composition, in vitro antioxidant activity and O±-glucosidase inhibitory effects of the essential oil and methanolic extract of Elsholtzia densa Benth. Nat. Prod. Res. 2016, 30, 2707–2711. [Google Scholar]
- Li, J.-E.; Nie, S.-P.; Xie, M.-Y.; Li, C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods 2014, 6, 410–418. [Google Scholar] [CrossRef]
- Li, J.-E.; Fan, S.-T.; Qiu, Z.-H.; Li, C.; Nie, S.-P. Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. Cv. Jiangxiangru. LWT—Food Sci. Technol. 2015, 64, 1022–1027. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crops Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Liaudanskas, M.; Jekabsone, A.; Sadauskiene, I.; Bernatoniene, J. Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities. Molecules 2020, 25, 1153. [Google Scholar] [CrossRef] [PubMed]
- Li, J.E.; Cui, S.W.; Nie, S.P.; Xie, M.Y. Structure and biological activities of a pectic polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru. Carbohydr. Polym. 2014, 105, 276–284. [Google Scholar] [CrossRef]
- Zhang, Q.; Porto, N.M.; Guilhon, C.C.; Giorno, T.; Alviano, D.S.; Agra, M.; Boylan, F. Pharmacognostic Study on Elsholtzia ciliata (Thumb.) Hyl: Anatomy. Phytochemistry and Pharmacological Activities. Pharmaceuticals 2021, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Dincer, I.; Hussain, M.M.; Yilbas, B.S.; Sahin, A.Z. Development of a new drying correlation for practical applications. Int. J. Energy Res. 2002, 26, 245–251. [Google Scholar] [CrossRef]
- Naidu, M.M.; Vedashree, M.; Satapathy, P.; Khanum, H.; Ramsamy, R.; Hebbar, H.U. Effect of drying methods on the quality characteristics of dill (Anethum graveolens) greens. Food Chem. 2016, 192, 849–856. [Google Scholar] [CrossRef]
- An, N.N.; Sun, W.H.; Li, B.Z.; Wang, Y.; Shang, N.; Lv, W.Q.; Wang, L.J. Effect of different drying techniques on drying kinetics. nutritional components. antioxidant capacity. physical properties and microstructure of edamame. Food Chem. 2022, 373, 131412. [Google Scholar] [CrossRef]
- Ma, L.J.; Ma, N.; Cao, J.L.; Wan, J.B. Characterizing the influence of different drying methods on chemical components of Panax notoginseng leaves by heart-cutting two-dimensional liquid chromatography coupled to orbitrap high-resolution mass spectrometry. Food Chem. 2022, 369, 130965. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.W.; Rao, J.Q.; Wang, D.; Xie, L.; Xiao, H.W.; Liu, Y.H.; Gao, Z.J. Process-Based Drying Temperature and Humidity Integration Control Enhances Drying Kinetics of Apricot Halves. Dry. Technol. 2015, 33, 365–376. [Google Scholar] [CrossRef]
- Yao, L.; Fan, L.; Duan, Z. Effect of different pretreatments followed by hot-air and far-infrared drying on the bioactive compounds, physicochemical property and microstructure of mango slices. Food Chem. 2020, 305, 125477. [Google Scholar] [CrossRef] [PubMed]
- Senadeera, W.; Adiletta, G.; Onal, B.; Di Matteo, M.; Russo, P. Influence of Different Hot Air Drying Temperatures on Drying Kinetics. Shrinkage. and Colour of Persimmon Slices. Foods 2020, 9, 101. [Google Scholar] [CrossRef]
- Qu, C.; Wang, X.; Wang, Z.; Yu, S.; Wang, D. Effect of Drying Temperatures on the Peanut Quality during Hot Air Drying. J. Oleo Sci. 2020, 69, 403–412. [Google Scholar] [CrossRef]
- Akpinar, E.K.; Bicer, Y.; Yildiz, C. Thin layer drying of red pepper. J. Food Eng. 2003, 59, 99–104. [Google Scholar] [CrossRef]
- Sarsavadia, P.N.; Sawhney, R.L.; Pangavhane, D.R. Drying behaviour of brined onion slices. J. Food Eng. 1999, 40, 219–226. [Google Scholar] [CrossRef]
- Kian-Pour, N.; Akdeniz, E.; Toker, O.S. Influence of coating-blanching in starch solutions, on the drying kinetics, transport properties, quality parameters, and microstructure of celery root chips. LWT 2022, 160, 113262. [Google Scholar] [CrossRef]
- Xin, Y.; Huang, X.B.; Hu, Y.; Ran, X. Thin-layer drying characteristics and modeling of longan pulp undergoing microwave treatment. Food Sci. Technol. 2012, 37, 67–71. [Google Scholar]
- Shen, F.; Peng, L.; Zhang, Y.; Wu, J.; Zhang, X.; Gang, Y. Thin-layer drying kinetics and quality changes of sweet sorghum stalk for ethanol production as affected by drying temperature. Ind. Crops Prod. 2011, 34, 1588–1594. [Google Scholar] [CrossRef]
- Díaz-Maroto, M.C.; Pérez-Coello, M.S.; Gonzalez Vinas, M.A.; Cabezudo, M.D. Influence of drying on the flavor quality of spearmint (Mentha spicata L.). J. Agric. Food Chem. 2003, 51, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Neeragunda Shivaraj, Y.; Plancot, B.; Ramdani, Y.; Gügi, B.; Kambalagere, Y.; Jogaiah, S.; Ramasandra Govind, S. Physiological and biochemical responses involved in vegetative desiccation tolerance of resurrection plant Selaginella brachystachya. 3 Biotech 2021, 11, 1–17. [Google Scholar]
- Lu, X.; Weng, H.; Li, C.; He, J.; Zhang, X.; Ma, Z. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crops Prod. 2020, 147, 112237. [Google Scholar] [CrossRef]
- Li, Z.; Wang, M.; Peng, L.; Pharmacy, S.O. Chemical Composition Analysis of Essential Oil from Mosla chinensis Maxim. cv. Jiangxiangru and Inhibitory Activity of the Oil and Its Major Constituents on Biofilm Formation of Staphylococcus aureus. Food Sci. 2016, 37, 138–143. [Google Scholar]
- Braun, T.; Voland, P.; Kunz, L.; Prinz, C.; Gratzl, M. Enterochromaffin cells of the human gut: Sensors for spices and odorants. Gastroenterology 2007, 132, 1890–1901. [Google Scholar] [CrossRef]
- Karapinar, M.; Aktuǧ, Ş.E. Inhibition of foodborne pathogens by thymol. eugenol. menthol and anethole. Int. J. Food Microbiol. 1987, 4, 161–166. [Google Scholar] [CrossRef]
- Ge, S.; Chen, Y.; Ding, S.; Zhou, H.; Jiang, L.; Yi, Y. Changes in volatile flavor compounds of peppers during hot air drying process based on headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS). J. Sci. Food Agric. 2020, 100, 3087–3098. [Google Scholar] [CrossRef]
- Poulose, A.; Croteau, R. Biosynthesis of aromatic monoterpenes: Conversion of γ-terpinene to p-cymene and thymol in Thymus vulgaris L. Arch. Biochem. Biophys. 1978, 187, 307–314. [Google Scholar] [CrossRef]
- Li, M.; Yang, R.; Zhang, H.; Wang, S.; Chen, D.; Lin, S. Development of a flavor fingerprint by HS-GC-IMS with PCA for volatile compounds of Tricholoma matsutake Singer. Food Chem. 2019, 290, 32–39. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and Advances in Electronic-Nose Technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Yang, W.; Yu, J.; Pei, F.; Mariga, A.M.; Ma, N.; Fang, Y.; Hu, Q. Effect of hot air drying on volatile compounds of Flammulina velutipes detected by HS-SPME-GC-MS and electronic nose. Food Chem. 2016, 196, 860–866. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Real-time aroma monitoring of mint (Mentha spicata L.) leaves during the drying process using electronic nose system. Measurement 2018, 124, 447–452. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Sao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on alpha-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Ahammed, G.J.; Li, X. Hormonal regulation of health-promoting compounds in tea (Camellia sinensis L.). Plant Physiol. Biochem. 2022, 185, 390–400. [Google Scholar] [CrossRef]
- Zhao, C.-C.; Ameer, K.; Eun, J.-B. Effects of various drying conditions and methods on drying kinetics and retention of bioactive compounds in sliced persimmon. LWT 2021, 143, 111149. [Google Scholar] [CrossRef]
- Akpinar, E.K. Drying of mint leaves in a solar dryer and under open sun: Modelling. performance analyses. Energy Convers. Manag. 2010, 51, 2407–2418. [Google Scholar] [CrossRef]
- Demirhan, E.; Özbek, B. Thin-layer drying characteristics and modelling of mint leaves undergoing microwave treatment. Chem. Eng. Commun. 2011, 198, 957–975. [Google Scholar] [CrossRef]
- Gunhan, T.; Demir, V.; Hancioglu, E.; Hepbasli, A. Mathematical modelling of drying of bay leaves. Energy Convers. Manag. 2005, 46, 1667–1679. [Google Scholar] [CrossRef]
- Vega, A.; Fito, P.; Andrés, A.; Lemus, R. Mathematical modeling of hot-air drying kinetics of red bell pepper (var. Lamuyo). J. Food Eng. 2007, 79, 1460–1466. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Khanlari, Y.; Aroujalian, A.; Fazel, S.; Fathizadeh, M. An Experimental Work and Mathematical Modeling on Kinetic Drying of Tomato Pulp Under Different Modified Atmosphere Conditions. Int. J. Food Prop. 2014, 17, 1–12. [Google Scholar] [CrossRef]
- Ertekin, O.; Yaldiz, C. Thin-layer solar drying of some different vegetables. Dry. Technol. 2001, 19, 583–596. [Google Scholar]
- Akpinar, E.K. Mathematical modelling of thin layer drying process under open sun of some aromatic plants. J. Food Eng. 2006, 77, 864–870. [Google Scholar] [CrossRef]
- Sharaf-Eldeen, Y.I.; Blaisdell, J.L.; Hamdy, M.Y. A Model for Ear Corn Drying. Trans. ASAE 1980, 23, 1261–1265. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. A Review of Thin Layer Drying of Foods: Theory. Modeling. and Experimental Results. Crit. Rev. Food Sci. Nutr. 2010, 50, 441–464. [Google Scholar] [CrossRef]
- Doymaz, I. The kinetics of forced convective air-drying of pumpkin slices. J. Food Eng. 2007, 79, 243–248. [Google Scholar] [CrossRef]
- Özdemir, M.; Devres, Y.O. The thin layer drying characteristics of hazelnuts during roasting. J. Food Eng. 1999, 42, 225–233. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Wu, T.; Liu, R.; Loewen, S.; Tsao, R. Microwave-assisted extraction of phenolics with maximal antioxidant activities in tomatoes. Food Chem. 2012, 130, 928–936. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Zhu, H.; Hu, C.; Liu, R.; Young, J.C.; Tsao, R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012, 46, 250–259. [Google Scholar] [CrossRef]
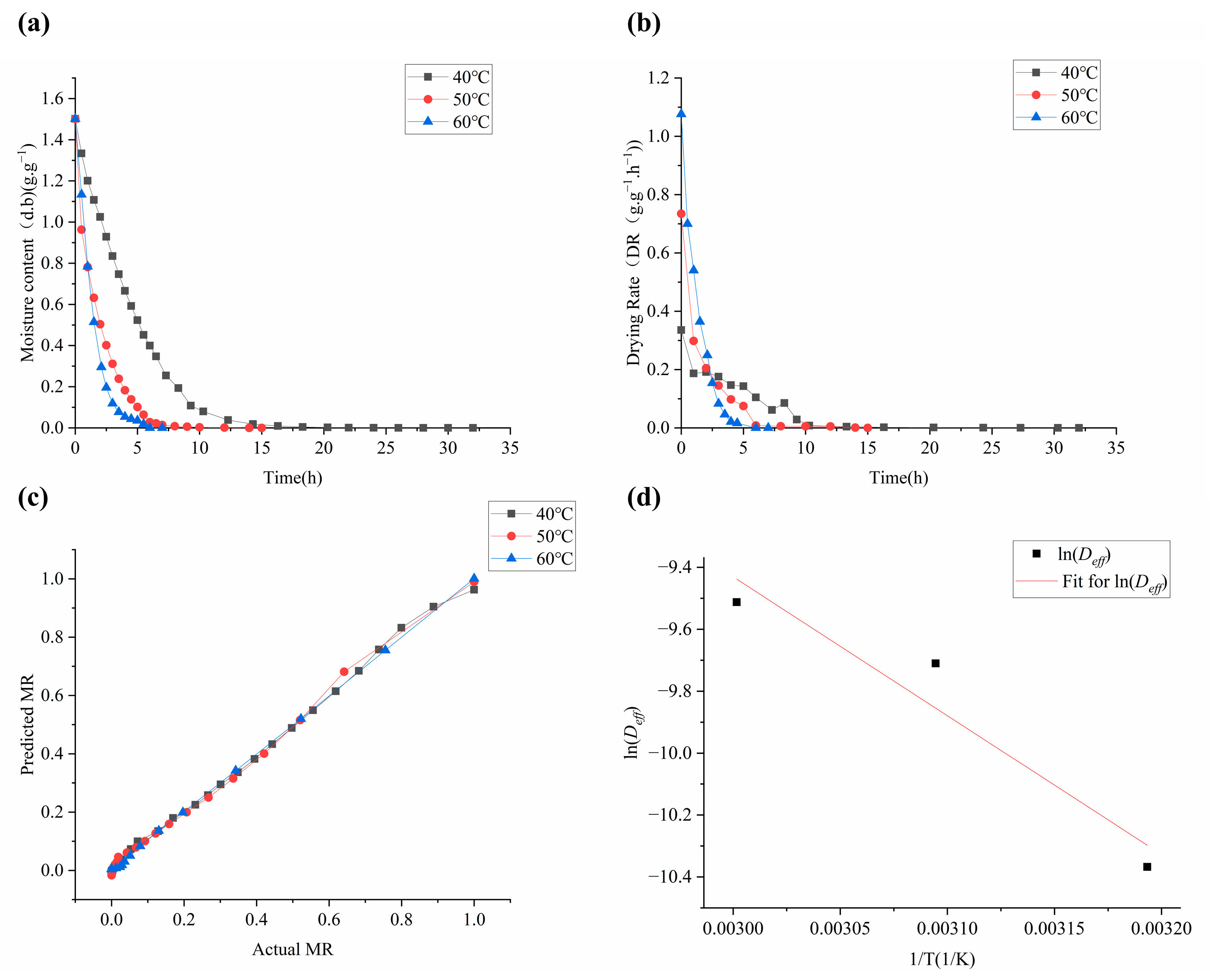

| Model | SP | 40 °C | 50 °C | 60 °C | MSP |
|---|---|---|---|---|---|
| 1 | R2 | 0.9977 | 0.9954 | 0.9996 | 0.9976 |
| χ2 | 0.0002 | 0.0003 | 0.0000 | 0.0002 | |
| RMSE | 0.0142 | 0.0164 | 0.0053 | 0.0120 | |
| 2 | R2 | 0.9969 | 0.9934 | 0.9996 | 0.9966 |
| χ2 | 0.0003 | 0.0005 | 0.0000 | 0.0003 | |
| RMSE | 0.0142 | 0.0209 | 0.0056 | 0.0136 | |
| 3 | R2 | 0.9977 | 0.9933 | 0.9996 | 0.9969 |
| χ2 | 0.0056 | 0.0080 | 0.0005 | 0.0047 | |
| RMSE | 0.0144 | 0.0205 | 0.0056 | 0.0135 | |
| 4 | R2 | 0.9969 | 0.9934 | 0.9996 | 0.9966 |
| χ2 | 0.0003 | 0.0005 | 0.0000 | 0.0003 | |
| RMSE | 0.0142 | 0.0209 | 0.0056 | 0.0136 | |
| 5 | R2 | 0.9972 | 0.9957 | 0.9997 | 0.9975 |
| χ2 | 0.0003 | 0.0003 | 0.0000 | 0.0002 | |
| RMSE | 0.0160 | 0.0169 | 0.0048 | 0.0126 | |
| 6 | R2 | 0.9926 | 0.9869 | 0.9934 | 0.9910 |
| χ2 | 0.0007 | 0.0010 | 0.0006 | 0.0008 | |
| RMSE | 0.0267 | 0.0303 | 0.0239 | 0.0270 | |
| 7 | R2 | 0.9947 | 0.9894 | 0.9949 | 0.9930 |
| χ2 | 0.0128 | 0.0126 | 0.0062 | 0.0105 | |
| RMSE | 0.0218 | 0.0258 | 0.0196 | 0.0224 |
| Temperature | 40 °C | 50 °C | 60 °C |
|---|---|---|---|
| a | 0.96247 | 0.98966 | 1.00079 |
| k | 0.14540 | 0.64514 | 0.65621 |
| y | 1.22734 | 0.79928 | 1.21805 |
| b | −2.4287 × 10−4 | −0.00339 | 4.19041 × 10−4 |
| No | tR (min) | Formula | Compounds | Common Name | Fresh | 40 °C | 50 °C | 60 °C |
|---|---|---|---|---|---|---|---|---|
| 1. | 1.424 | / | Unknown | / | 1.54 | 4.36 | 3.69 | 3.45 |
| 2. | 2.286 | C2H4O2 | Acetic acid | / | 0.34 | 0.62 | / | / |
| 3. | 10.733 | C10H16 | 5-Isopropyl-2-methylbicyclo[3.1.0]hex-2-ene | α-Thujene | 1.09 | / | / | / |
| 4. | 11.010 | C10H16 | 3,6,6-Trimethyl-bicyclo [3.1.1] hept-2-ene | / | 0.82 | / | / | / |
| 5. | 11.696 | C10H16 | Camphene | / | 0.53 | / | / | / |
| 6. | 13.841 | C10H16 | β-Myrcene | 7-Methyl-3-methyleneocta-1,6-diene | 4.59 | 0.58 | 0.53 | / |
| 7. | 14.302 | C10H16 | α-Phellandrene | / | 0.32 | / | / | / |
| 8. | 14.832 | C10H16 | 1-methyl-4-(1-methylethyl)-1,3-Cyclohexadiene | α-Terpinene | 3.14 | 0.49 | / | / |
| 9. | 15.157 | C10H14 | p-Cymene | / | 27.77 | 8.40 | 7.46 | 6.39 |
| 10. | 15.344 | C10H16 | (R)-1-methyl-5-(1- methylethenyl)-cyclohexe (R)-isocarvestrene | 1.07 | / | / | / | |
| 11. | 16.199 | C10H16 | β-Ocimene | / | 0.32 | / | / | / |
| 12. | 16.568 | C10H16 | γ-Terpinene | / | 10.04 | 1.78 | 1.59 | 1.51 |
| 13. | 17.114 | C10H18O | (1α,2α,5α)-2-methyl-5-(1-methylethyl)bicyclo[3.1.0]hexan-2-ol | (E)-sabinene hydrate | 1.15 | 1.16 | 0.91 | 1.06 |
| 14. | 17.970 | C10H16 | (+)-3-Carene | / | / | / | / | 0.50 |
| 15. | 20.281 | C10H18O | Borneol | / | 0.31 | 0.42 | 0.58 | 0.50 |
| 16. | 20.667 | C10H18O | Terpinen-4-ol | / | 1.07 | 0.77 | 0.78 | 0.88 |
| 17. | 24.211 | C10H14O | Thymol | / | 28.29 | 56.75 | 55.86 | 55.62 |
| 18. | 24.783 | C10H18O | (1Sendo)-1,7,7-trimethyl-bicyclo[2.2.1]heptan2-ol | (−)-Borneol | / | 0.44 | / | / |
| 19. | 26.170 | C12H16O2 | Phenol 5-methyl-2-(1-methylethyl) acetate | Thymol acetate | 15.91 | 22.64 | 26.46 | 28.38 |
| 20. | 27.679 | C15H24 | Caryophyllene | (−)-β-Caryophyllene | 0.33 | / | / | / |
| 21. | 28.065 | C15H24 | 2,6-Dimethyl-6-(4-methyl-3-pentenyl)-bicyclo[3.1.1]hept-2-ene | α-Bergamotene | 0.52 | 0.50 | 0.54 | 0.45 |
| 22. | 28.552 | C15H24 | Humulene | (1E,4E,8E)-α-humulene | 1.35 | 1.51 | 1.45 | 1.07 |
| 23. | 33.222 | / | Unknown | / | / | / | / | 0.47 |
| No | Model Name | Model Equation | References |
|---|---|---|---|
| 1 | Midilli | MR = a exp(−kty) + bt | [40] |
| 2 | Page | MR = exp(−kty) | [41] |
| 3 | Modified Page | MR = a exp[−(kty)] | [42] |
| 4 | Overhults | MR = exp[−(kt)y] | [43] |
| 5 | Two-term exponential | MR = a exp(−kt) + (1 − a) exp(−kat) | [44] |
| 6 | Newton | MR = exp(−kt) | [45] |
| 7 | Logarithmic | MR = a exp(−kt) + c | [46] |
| Sensors | Types of Flavor Detected |
|---|---|
| S1 | Aromatic compounds |
| S2 | Nitrogen oxides |
| S3 | Sulfides from vegetables |
| S4 | Organic acids and terpenoids |
| S5 | Biosynthetic compounds |
| S6 | Thionine |
| S7 | Combustible gas |
| S8 | Amines |
| S9 | Hydrogen |
| S10 | Hydrocarbons |
| S11 | Volatile organic compounds |
| S12 | Sulfides from the environment |
| S13 | Ethylene |
| S14 | Volatile gases from cooking |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, M.; Chen, Y.; Sun, Y.; Gao, Y.; Wu, Z.; Wu, R.; Li, R.; Hong, S.; Wang, M.; Zou, Y.; et al. Effect of Drying Kinetics, Volatile Components, Flavor Changes and Final Quality Attributes of Moslae herba during the Hot Air Thin-Layer Drying Process. Molecules 2023, 28, 3898. https://doi.org/10.3390/molecules28093898
Xie M, Chen Y, Sun Y, Gao Y, Wu Z, Wu R, Li R, Hong S, Wang M, Zou Y, et al. Effect of Drying Kinetics, Volatile Components, Flavor Changes and Final Quality Attributes of Moslae herba during the Hot Air Thin-Layer Drying Process. Molecules. 2023; 28(9):3898. https://doi.org/10.3390/molecules28093898
Chicago/Turabian StyleXie, Min, Ying Chen, Yong Sun, Yarou Gao, Zhenfeng Wu, Ruiyu Wu, Rui Li, Shixi Hong, Minyan Wang, Yiping Zou, and et al. 2023. "Effect of Drying Kinetics, Volatile Components, Flavor Changes and Final Quality Attributes of Moslae herba during the Hot Air Thin-Layer Drying Process" Molecules 28, no. 9: 3898. https://doi.org/10.3390/molecules28093898
APA StyleXie, M., Chen, Y., Sun, Y., Gao, Y., Wu, Z., Wu, R., Li, R., Hong, S., Wang, M., Zou, Y., Zhang, H., & Xiong, Y. (2023). Effect of Drying Kinetics, Volatile Components, Flavor Changes and Final Quality Attributes of Moslae herba during the Hot Air Thin-Layer Drying Process. Molecules, 28(9), 3898. https://doi.org/10.3390/molecules28093898






