Removal of Fluoride from Aqueous Solution Using Shrimp Shell Residue as a Biosorbent after Astaxanthin Recovery
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of ETSSs
2.1.1. Component Analysis
2.1.2. XRD Analysis
2.1.3. SEM Analysis
2.2. Fluoride Removal Using ETSSs
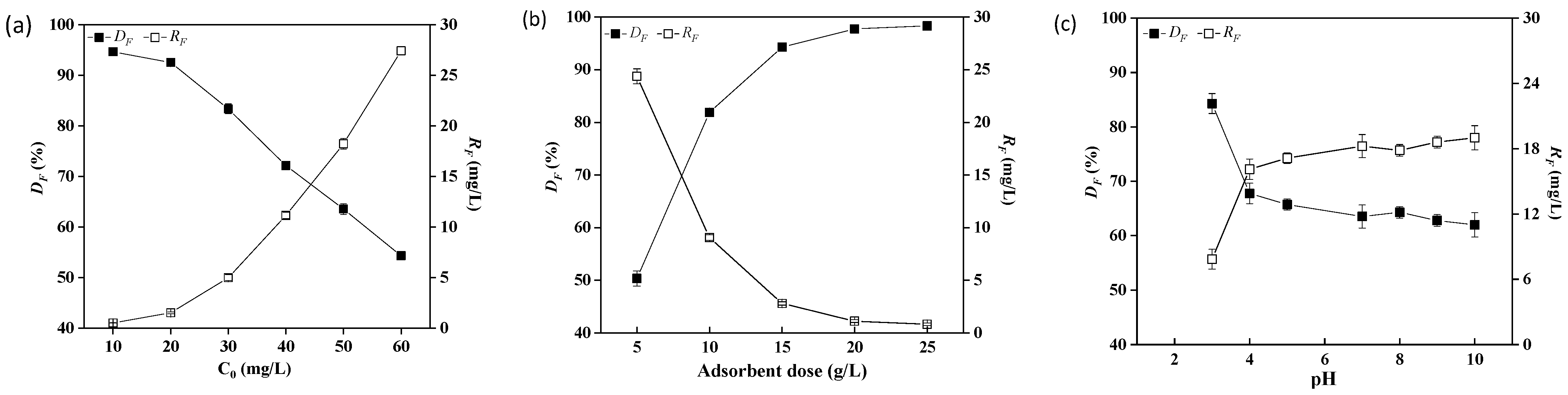
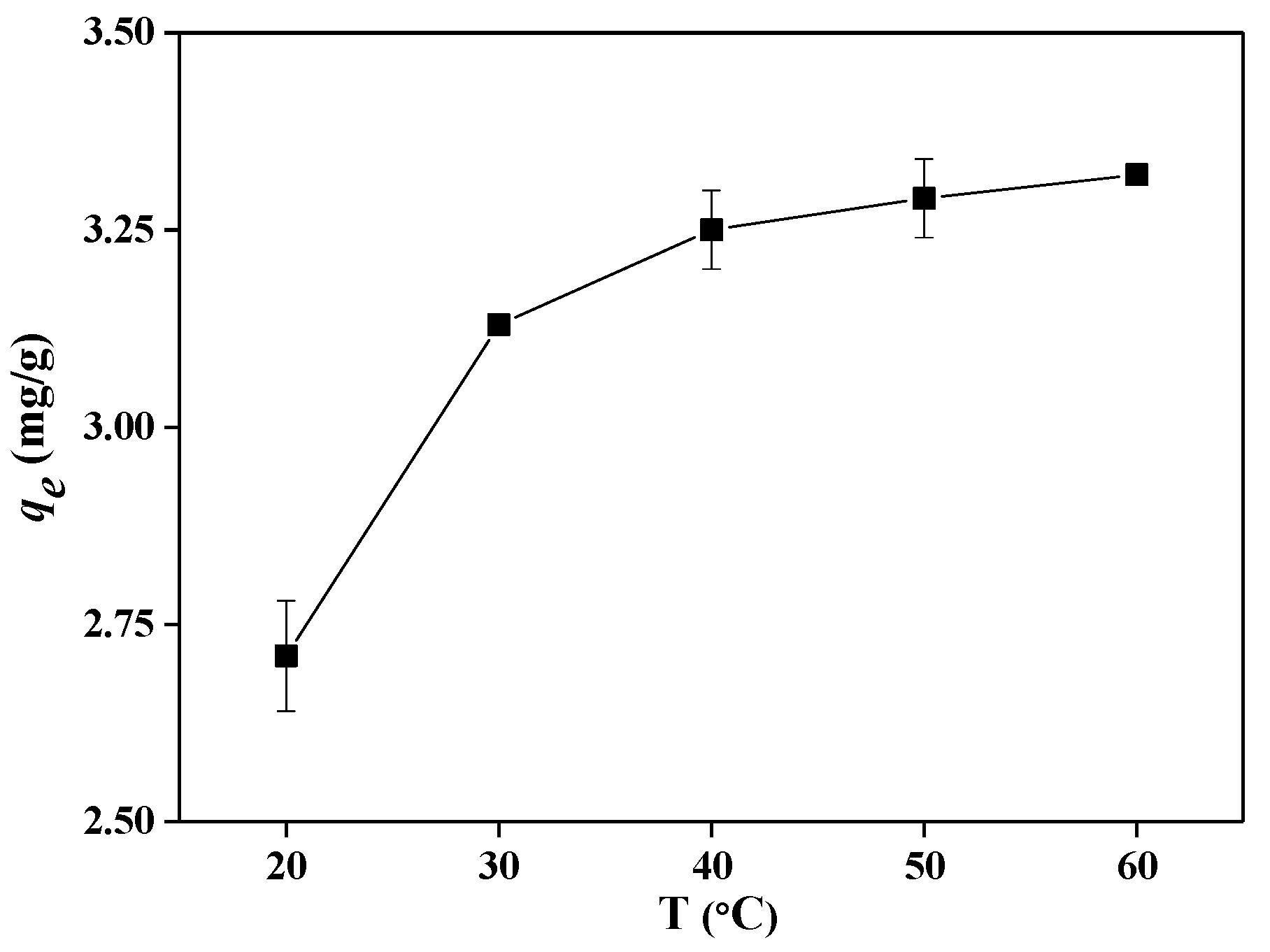
2.3. Optimization of Adsorption Conditions
2.3.1. Effect of Initial Fluoride Concentration
2.3.2. Effect of Adsorbent Dose
2.3.3. Effect of pH
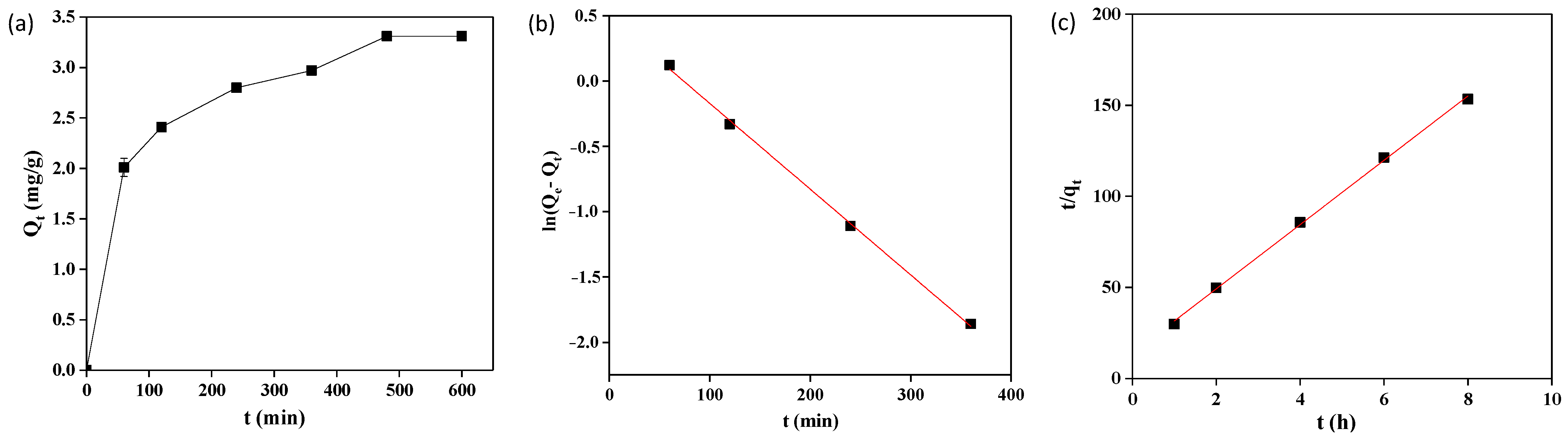
2.4. Adsorption Kinetics
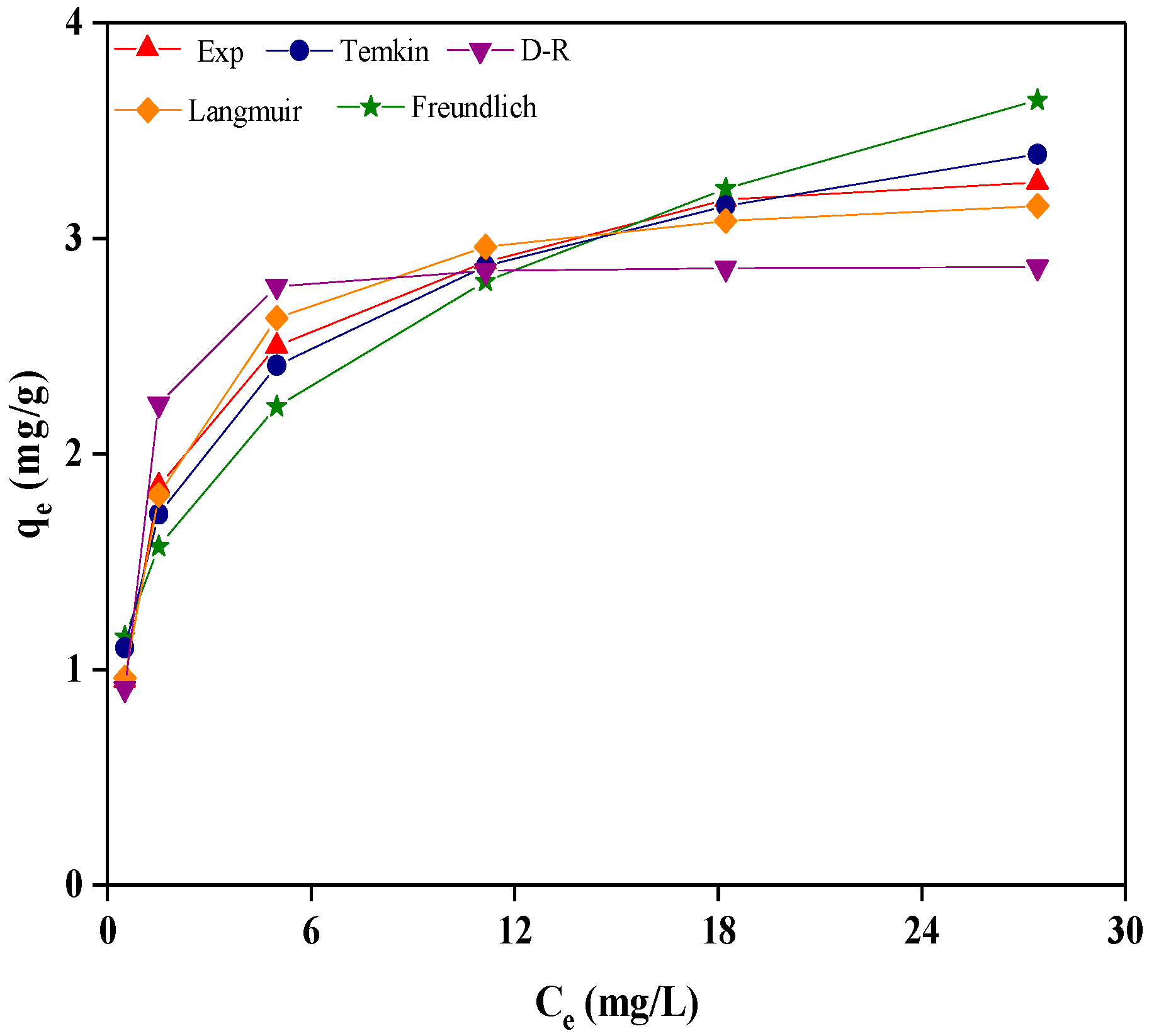
2.5. Adsorption Isotherm
2.6. Thermodynamic Parameters
2.7. Adsorption Mechanism
3. Materials and Methods
3.1. Materials
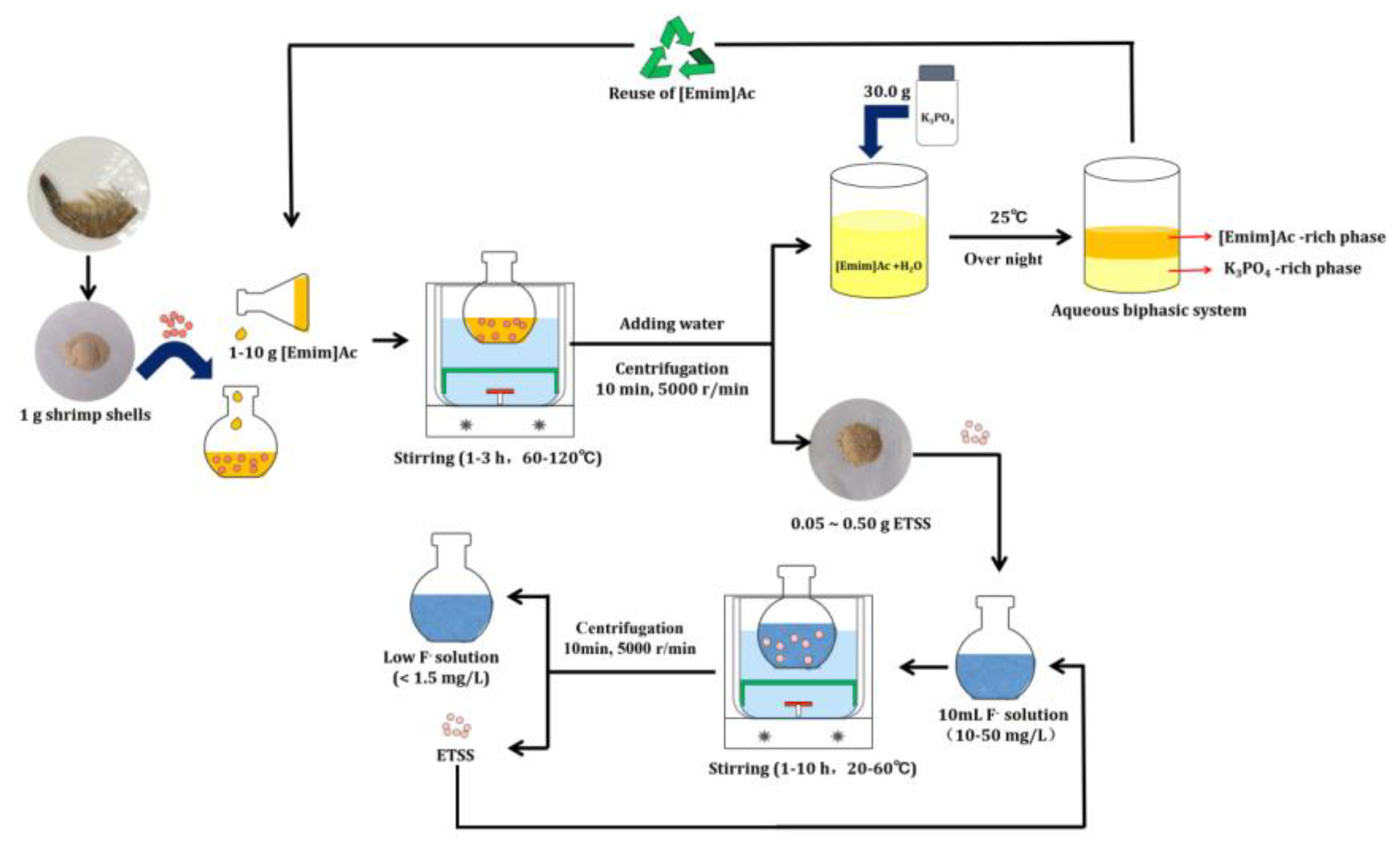
3.2. Bioadsorbent Preparation
3.3. Characterization
3.4. Adsorption Experiment
3.5. Recovery of IL
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, C.H.; Xu, Z.M.; Zhu, H.; Lv, X.J.; Liu, Q.S. Insights into the mechanism of fluoride adsorption over different crystal phase alumina surfaces. J. Hazard. Mater. 2022, 423, 127109. [Google Scholar] [CrossRef] [PubMed]
- Solanki, Y.S.; Agarwal, M.; Gupta, A.B.; Gupta, S.; Shukla, P. Fluoride occurrences, health problems, detection, and remediation methods for drinking water: A comprehensive review. Sci. Total Environ. 2022, 807, 150601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.K.; Tan, X.J.; Ding, W.H.; Jiang, Z.Y.; He, K.; Zhao, B.; Takeuchi, H.; Huang, Y.X. Aluminum-based electrocoagulation for residual fluoride removal during per- and polyfluoroalkyl substances (PFASs) wastewater treatment. Sep. Purif. Technol. 2023, 308, 122989. [Google Scholar] [CrossRef]
- Raghav, S.; Nair, M.; Kumar, D. Tetragonal prism shaped Ni-Al bimetallic adsorbent for study of adsorptive removal of fluoride and role of ion-exchange. Appl. Surf. Sci. 2019, 498, 143785. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Bolisetty, S.; Cao, Y.P.; Handschin, S.; Adamcik, J.; Peng, Q.M.; Mezzenga, R. Selective and efficient removal of fluoride from water: In situ engineered amyloid Fibril/ZrO2 hybrid membranes. Angew. Chem. Int. Ed. 2019, 58, 6012–6016. [Google Scholar] [CrossRef]
- He, C.; Lin, H.L.; Dai, L.L.; Qiu, R.L.; Tang, Y.T.; Wang, Y.P.; Duan, P.G.; Ok, Y.S. Waste shrimp shell-derived hydrochar as an emergent material for methyl orange removal in aqueous solutions. Environ. Int. 2020, 134, 105340. [Google Scholar] [CrossRef]
- Wan, K.L.; Huang, L.; Yan, J.; Ma, B.Y.; Huang, X.J.; Luo, Z.X.; Zhang, H.G.; Xiao, T.F. Removal of fluoride from industrial wastewater by using different adsorbents: A review. Sci. Total Environ. 2021, 773, 145535. [Google Scholar] [CrossRef]
- Bhomick, P.C.; Supong, A.; Karmaker, R.; Baruah, M.; Pongener, C.; Sinha, D. Activated carbon synthesized from biomass material using single-step KOH activation for adsorption of fluoride: Experimental and theoretical investigation. Korean J. Chem. Eng. 2019, 36, 551–562. [Google Scholar] [CrossRef]
- Markeb, A.A.; Alonso, A.; Sanchez, A.; Font, X. Adsorption process of fluoride from drinking water with magnetic core-shell Ce-Ti@Fe3O4 and Ce-Ti oxide nanoparticles. Sci. Total Environ. 2017, 598, 949–958. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Jia, Y. Preparation of porous alumina hollow spheres as an adsorbent for fluoride removal from water with low aluminum residual. Ceram. Int. 2016, 42, 17472–17481. [Google Scholar] [CrossRef]
- Rahman, A.; Yoshida, K.; Islam, M.M.; Kobayashi, G. Investigation of efficient adsorption of toxic heavy metals (chromium, lead, cadmium) from aquatic environment using orange peel cellulose as adsorbent. Sustainability 2023, 15, 4470. [Google Scholar] [CrossRef]
- Qin, L.; Feng, L.H.; Li, C.; Fan, Z.; Zhang, G.L.; Shen, C.; Meng, Q. Amination/oxidization dual-modification of waste ginkgo shells as bio-adsorbents for copper ion removal. J. Clean. Prod. 2019, 228, 112–123. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, S.J.; Kim, Y.; Jeong, C.H.; Hong, Y.P.; Ryoo, K.S. Removal of heavy metals from water using chicken egg shell powder as a bio-adsorbent. Bull. Korean Chem. Soc. 2019, 40, 1156–1161. [Google Scholar] [CrossRef]
- Gao, J.; Fang, C.; Lin, Y.; Nie, F.; Ji, H.; Liu, S. Enhanced extraction of astaxanthin using aqueous biphasic systems composed of ionic liquids and potassium phosphate. Food Chem. 2020, 309, 125672. [Google Scholar] [CrossRef]
- Jozwiak, T.; Filipkowska, U.; Bakula, T.; Bralewska-Piotrowicz, B.; Karczmarczyk, K.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Szyrynska, N.; Lewczuk, B. The Use of Chitin from the Molts of Mealworm (Tenebrio molitor) for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Materials 2023, 16, 545. [Google Scholar] [CrossRef]
- Rathinam, K.; Jayaram, P.; Sankaran, M. Synthesis and characterization of magnetic chitin composite and its application towards the uptake of Pb(II) and Cd(II) ions from aqueous solution. Environ. Prog. Sustain. Energy 2019, 38, S288–S297. [Google Scholar] [CrossRef]
- Rahman, A.; Haque, M.A.; Ghosh, S.; Shinu, P.; Attimarad, M.; Kobayashi, G. Modified shrimp-based chitosan as an emerging adsorbent removing heavy metals (chromium, nickel, arsenic, and cobalt) from polluted water. Sustainability 2023, 15, 2431. [Google Scholar] [CrossRef]
- Khanday, W.A.; Ahmed, M.J.; Okoye, P.U.; Hummadi, E.H.; Hameed, B.H. Single-step pyrolysis of phosphoric acid-activated chitin for efficient adsorption of cephalexin antibiotic. Bioresour. Technol. 2019, 280, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.J.; Li, L.Z.; Huang, H.H.; Wu, J.L.; Zhou, X.X.; Yan, X.L.; Jia, J.B.; Yue, T.T.; Chu, Y.H.; Yan, B. Biosafety-inspired structural optimization of triazolium ionic liquids based on structure-toxicity relationships. J. Hazard. Mater. 2022, 424, 127521. [Google Scholar] [CrossRef]
- Khan, H.W.; Elgharbawy, A.A.M.; Bustam, M.A.; Goto, M.; Moniruzzaman, M. Ionic liquid-based green emulsion liquid membrane for the extraction of the poorly soluble drug ibuprofen. Molecules 2023, 28, 2345. [Google Scholar] [CrossRef]
- Husson, E.; Hadad, C.; Huet, G.; Laclef, S.; Lesur, D.; Lambertyn, V.; Jamali, A.; Gottis, S.; Sarazin, C.; Nhien, A.N.V. The effect of room temperature ionic liquids on the selective biocatalytic hydrolysis of chitin via sequential or simultaneous strategies. Green Chem. 2017, 19, 4122–4131. [Google Scholar] [CrossRef]
- Parjikolaei, B.R.; Errico, M.; El-Houri, R.B.; Mantell, C.; Frette, X.C.; Christensen, K.V. Process design and economic evaluation of green extraction methods for recovery of astaxanthin from shrimp waste. Chem. Eng. Res. Des. 2017, 117, 73–82. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.M.; Sun, N.D.; Rogers, R. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Ketnawa, S.; Martinez-Alvarez, O.; Gomez-Estaca, J.; Gomez-Guillen, M.D.C.; Benjakul, S.; Rawdkuen, S. Obtaining of functional components from cooked shrimp (Penaeus vannamei) by enzymatic hydrolysis. Food Biosci. 2016, 15, 55–63. [Google Scholar] [CrossRef]
- Boric, M.; Puliyalil, H.; Novak, U.; Likozar, B. An intensified atmospheric plasma-based process for the isolation of the chitin biopolymer from waste crustacean biomass. Green Chem. 2018, 20, 1199–1204. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.C.; Gao, L.; Sun, J.; Liu, Z.; Mao, X. Efficient enzymatic hydrolysis of ionic liquid pretreated chitin and its dissolution mechanism. Carbohydr. Polym. 2019, 211, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Al-Sawalmih, A.; Li, C.H.; Siegel, S.; Fabritius, H.; Yi, S.B.; Raabe, D.; Fratzl, P.; Paris, O. Microtexture and chitin/calcite orientation relationship in the mineralized exoskeleton of the american lobster. Adv. Funct. Mater. 2010, 18, 3307–3314. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Rajeevgandhi, C.; Rajan, D.K.; Seedevi, P. Extraction and characterization of chitin from sea snail Conus inscriptus (Reeve, 1843). Int. J. Biol. Macromol. 2019, 126, 555–560. [Google Scholar] [CrossRef]
- Yitbarek, M.; Abdeta, K.; Beyene, A.; Astatkie, H.; Dadi, D.; Desalew, G.; Van, D.; Bruggen, B. Experimental evaluation of sorptive removal of fluoride from drinking water using natural and brewery waste diatomite. Process Saf. Environ. Prot. 2019, 128, 95–106. [Google Scholar] [CrossRef]
- Vinati, A.; Mahanty, B.; Behera, S.K. Clay and clay minerals for fluoride removal from water: A state-of-the-art review. Appl. Clay Sci. 2015, 114, 340–348. [Google Scholar] [CrossRef]
- Wagutu, A.W.; Machunda, R.; Jande, Y.A.C. Crustacean derived calcium phosphate systems: Application in defluoridation of drinking water in East African rift valley. J. Hazard. Mater. 2018, 347, 95–105. [Google Scholar] [CrossRef]
- Huang, L.; Luo, Z.X.; Huang, X.X.; Wang, Y.; Yan, J.; Liu, W.; Guo, Y.F.; Arulmani, S.R.B.; Shao, M.H.; Zhang, H.G. Applications of biomass-based materials to remove fluoride from wastewater: A review. Chemosphere 2022, 301, 134679. [Google Scholar] [CrossRef]
- Swain, S.K.; Patel, S.B.; Panda, A.P.; Patnaik, T.; Dey, R.K. Pea (Pisum sativum L.) peel waste carbon loaded with zirconium: Study of kinetics, thermodynamics and mechanism of fluoride adsorption. Sep. Sci. Technol. 2019, 54, 2194–2211. [Google Scholar] [CrossRef]
- Dan, S.; Chattree, A. Sorption of fuoride using chemically modifed Moringa oleifera leaves. Appl. Water Sci. 2018, 8, 76. [Google Scholar] [CrossRef]
- Dobaradaran, S.; Nabipour, I.; Mahvi, A.H.; Keshtkar, M.; Elmi, F.; Amanollahzade, F.; Khorsand, M. Fluoride removal from aqueous solutions using shrimp shell waste as a cheap biosorbent. Fluoride 2014, 47, 253–257. [Google Scholar]
- Shah, K.H.; Yameen, M.A.; Yousaf, T.; Waseem, M.; Fahad, M.; Sherazi, T.A.; Ahmad, H. Adsorption of bacteria by highly efficient, economic and biodegradable magnetic coated chitosan adsorbent. J. Solution Chem. 2020, 49, 1304–1318. [Google Scholar] [CrossRef]
- Razmi, F.A.; Ngadi, N.; Wong, S.; Inuwa, I.M.; Opotu, L.A. Kinetics, thermodynamics, isotherm and regeneration analysis of chitosan modified pandan adsorbent. J. Clean. Prod. 2019, 231, 98–109. [Google Scholar] [CrossRef]
- George, A.M.; Tembhurkar, A.R. Analysis of equilibrium, kinetic, and thermodynamic parameters for biosorption of fluoride from water onto coconut (Cocos nucifera Linn.) root developed adsorbent. Chin. J. Chem. Eng. 2019, 27, 92–99. [Google Scholar] [CrossRef]
- George, A.M.; Tembhurkar, A.R. Taguchi experimental design for adsorptive removal of fluoride from water using novel Ficus Glomerata Bark-developed biosorbent. Int. J. Environ. Sci. Technol. 2020, 17, 4829–4840. [Google Scholar] [CrossRef]
- Getachew, T.; Hussen, A.; Rao, V.M. Defluoridation of water by activated carbon prepared from banana (Musa paradisiaca) peel and coffee (Coffea arabica) husk. Int. J. Environ. Sci. Technol. 2015, 12, 1857–1866. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Khan, A.A.; Singh, K.; Tripathi, A.; Hasda, R.; Halder, G. Insight into Optimization, isotherm, kinetics, and thermodynamics of fluoride adsorption onto activated alumina. Environ. Prog. Sustain. Energy 2018, 37, 766–776. [Google Scholar] [CrossRef]
- Gok, G.; Kocyigit, H.; Gok, O.; Celebi, H. The use of raw shrimp shells in the adsorption of highly polluted waters with Co2+. Chem. Eng. Res. Des. 2022, 186, 229–240. [Google Scholar] [CrossRef]
- Shekhawat, A.; Kahu, S.S.; Saravanan, D.; Jugade, R.M. Assimilation of chitin with tin for defluoridation of water. RSC Adv. 2016, 6, 18936–18945. [Google Scholar] [CrossRef]
- Kamble, S.P.; Jagtap, S.; Labhsetwar, N.K.; Thakare, D.; Godfrey, S.; Devotta, S.; Rayalu, S.S. Defluoridation of drinking water using chitin, chitosan and lanthanum-modified chitosan. Chem. Eng. J. 2007, 129, 173–180. [Google Scholar] [CrossRef]
- Tang, D.D.; Zhao, Y.W.; Wang, Y.X.; Yang, Y.J.; Li, D.Y.; Peng, T.W.; Mao, X.H. Defluorination from aqueous solution by Ti(IV)-modified granular activated carbon. Desalin. Water Treat. 2015, 54, 3432–3443. [Google Scholar] [CrossRef]
- Alagumuthu, G.; Rajan, M. Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem. Eng. J. 2010, 158, 451–457. [Google Scholar] [CrossRef]
- Gautam, C.; Islam, S.K.M.; Sadistap, S.; Sarma, U. Effect of microwave heat on the nutritional properties of infected red kidney beans. IEEE Access 2018, 6, 57137–57143. [Google Scholar] [CrossRef]
- Gao, J.; Chen, L.; Yan, Z.C.; Wang, L. Effect of ionic liquid treatment on the composition, structure and biogas production of water hyacinth (Eichhornia crassipes). Bioresour. Technol. 2013, 132, 361–364. [Google Scholar] [CrossRef]
- George, A.M.; Tembhurkar, A.R. Biosorptive removal of fluoride from aqueous solution onto newly developed biosorbent from Ficus benghalensis leaf: Evaluation of equilibrium, kinetics, and thermodynamics. Sustain. Chem. Pharm. 2018, 10, 125–133. [Google Scholar] [CrossRef]
- Araga, R.; Kali, S.; Sharma, C.S. Coconut-shell-derived carbon/carbon nanotube composite for fluoride adsorption from aqueous solution. Clean-Soil Air Water 2019, 47, 1800286. [Google Scholar] [CrossRef]
- Niyitegeka, H.; Kassahun, S.K.; Nyangi, M.J. Removal of fluoride from water using aluminum-modified activated carbon prepared from khat (Catha edulis) stems. Remediat. J. Environ. Cleanup Costs Technol. Tech. 2023, 33, 119–133. [Google Scholar] [CrossRef]
- Angelin, A.; Kalpana, M.; Govindan, K.; Kavitha, S. Characterizations and fluoride adsorption performance of wattle humus biosorbent. Environ. Sci. Pollut. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, M.H.; Farhang, M.; Alimohammadi, M.; Afsharnia, M.; Mckay, G. Adsorptive removal of fluoride from water by activated carbon derived from CaCl2-modified Crocus sativus leaves: Equilibrium adsorption isotherms, optimization, and influence of anions. Chem. Eng. Commun. 2018, 205, 955–965. [Google Scholar] [CrossRef]
- Paudyal, H.; Inoue, K. Adsorptive removal of trace concentration of fluoride from water using gerium loaded dried orange juice residue. J. Inst. Sci. Technol. 2018, 23, 43–48. [Google Scholar] [CrossRef]

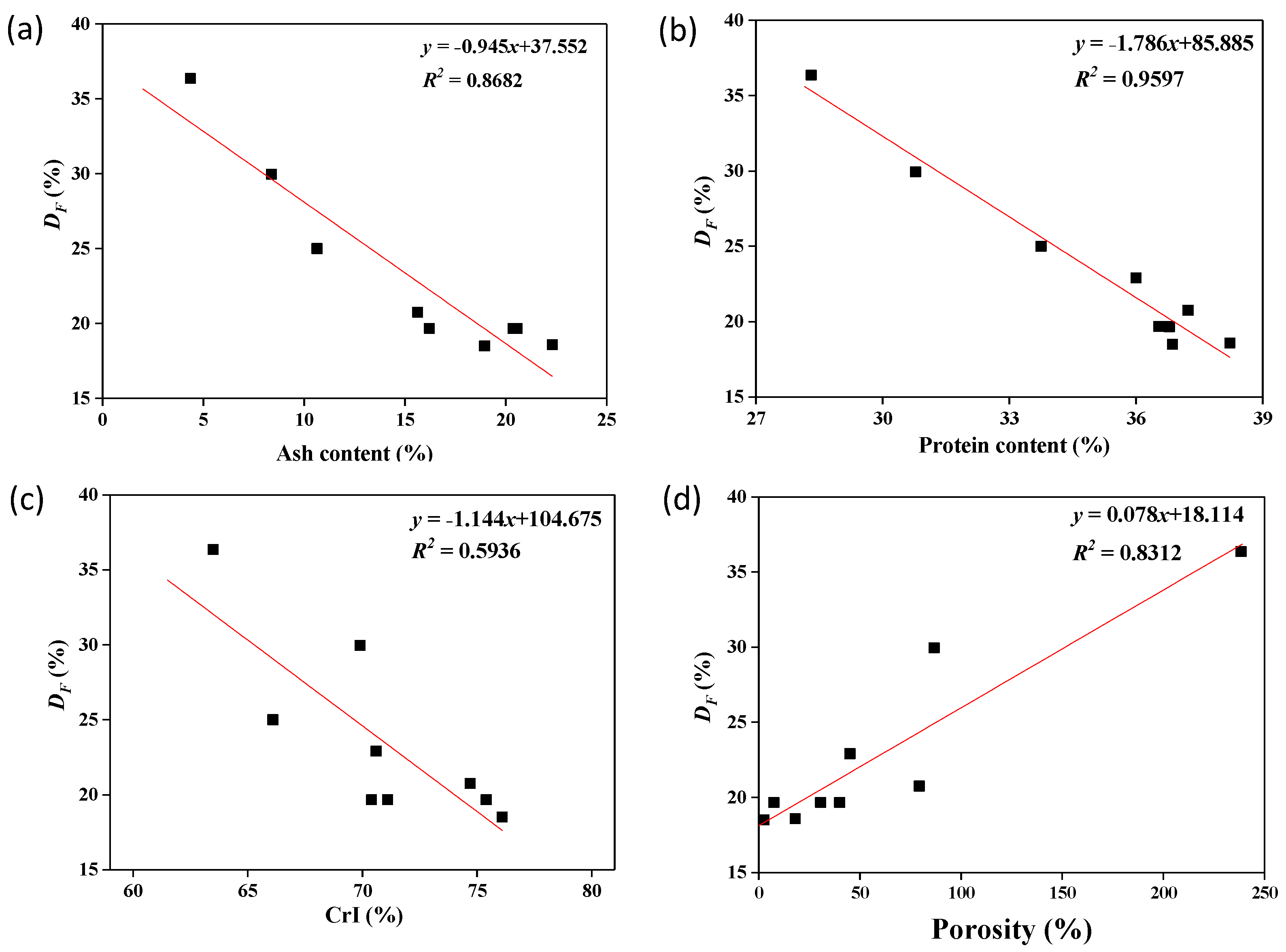
| Treatment Conditions | Chemical Compositions | Structure Properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solid–Liquid Ratio (w/v) | Time (h) | Particle Size (μm) | Temperature (°C) | Ash (%) | Protein (%) | Chitin (%) | MN a (30.76 μm2) | APD b (nm) | Porosity (%) | CrI (%) | DF c (%) |
| - | - | 180–250 | - | 28.50 ± 0.06 | 42.90 ± 0.35 | 22.74 ± 0.05 | - | - | - | 81.8 | 5.23 ± 0.02 |
| 1:10 | 1 | 180–250 | 60 | 18.95 ± 0.14 | 36.86 ± 0.33 | 9.70 ± 0.10 | 10 | 157.78 | 2.54 | 76.1 | 18.58 ± 0.05 |
| 1:10 | 2 | 180–250 | 60 | 14.38 ± 0.05 | 36.00 ± 0.36 | 39.70 ± 0.45 | 296 | 122.18 | 45.11 | 70.6 | 22.90 ± 1.82 |
| 1:10 | 3 | 180–250 | 60 | 16.21 ± 0.18 | 32.31 ± 0.32 | 43.57 ± 0.44 | 648 | 77.78 | 40.02 | 70.4 | 19.67 ± 1.85 |
| 1:10 | 2 | 180–250 | 100 | 4.36 ± 0.06 | 28.31 ± 0.32 | 57.10 ± 0.25 | 352 | 257.58 | 238.40 | 63.5 | 36.36 ± 0.10 |
| 1:10 | 2 | 180–250 | 120 | 8.38 ± 0.08 | 30.78 ± 0.26 | 51.02 ± 0.37 | 20 | 651.85 | 86.75 | 69.9 | 29.95 ± 1.67 |
| 1:10 | 2 | 120–150 | 60 | 22.31 ± 0.07 | 38.22 ± 0.20 | 30.56 ± 0.23 | 309 | 75.56 | 18.01 | 54.7 | 18.58 ± 0.26 |
| 1:10 | 2 | 150–180 | 60 | 20.36 ± 0.03 | 36.54 ± 0.32 | 38.77 ± 0.31 | 340 | 93.94 | 30.63 | 71.1 | 19.67 ± 1.89 |
| 1:5 | 2 | 180–250 | 60 | 15.63 ± 0.09 | 37.23 ± 0.34 | 38.16 ± 0.68 | 228 | 184.67 | 79.37 | 74.7 | 20.75 ± 1.85 |
| 1:1 | 2 | 180–250 | 60 | 20.57 ± 0.11 | 36.79 ± 0.25 | 36.22 ± 0.23 | 57 | 113.33 | 7.47 | 75.4 | 19.66 ± 1.80 |
| Experiment Value | Pseudo-First-Order | Pseudo-Second-Order |
|---|---|---|
| qe,exp = 3.13 mg/g | k1 = 0.39 min−1 | k2 = 0.006 g∙mg−1∙min |
| qe,cal = 1.62 mg/g | qe,cal = 3.40 mg/g | |
| R2 = 0.9985 | R2 = 0.9987 |
| Isotherm Model | Langmuir | Freundlich | Temkin | D-R |
|---|---|---|---|---|
| Fitting parameter | KL = 0.805 L/mg | KF = 1.394 mg/g | KT = 0.576 | E = 1.924 kJ/mol |
| qm,cal = 3.290 mg/g | 1/n = 0.290 | f = 13.147 L/mg | qm,cal = 2.869 mg/g | |
| R2 | 0.9970 | 0.8964 | 0.9803 | 0.9317 |
| Bioadsorbent | Defluoridation Conditions | qe,max (mg/g) | Reference | |||
|---|---|---|---|---|---|---|
| Time (h) | Temperature (°C) | C0 (mg/L) | Dose (g/L) | |||
| Zirconium-modified pea peel waste carbon | 1.0 | 25 | 0–50 | - | 3.652 | [35] |
| Brewery waste diatomite | 0.5 | 25 | - | 60 | 0.617 | [31] |
| Ficus benghalensis leaf | 1.5 | 27 | 2–25 | 8 | 2.242 | [51] |
| Coconut-shell-derived carbon nanotube | 3.5 | 30 | 2.68–9.57 | 10 | 0.360 | [52] |
| Aluminum-modified activated carbon from Khat waste | 1.0 | Room temperature | 2–9 | 2.47 | 0.306 | [53] |
| Wattle humus biosorbent | - | 30 | 2–10 | - | 0.231 | [54] |
| Modified Moringa oleifera leaves | 2.5 | Room temperature | 0.5–2 | 2.5 | 1.14 | [36] |
| CaCl2-modified Crocus sativus leaves | - | 25 | 2–25 | 10 | 2.01 | [55] |
| Ce(IV)-modified orange juice residue | 24 | 30 | 0–12 | 0.67 | 1.22 | [56] |
| Shrimp shells treated using [Emim]Ac | 8 | 50 | 10–60 | 10 | 3.290 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, L.; Liao, M.; Huang, C.; Gao, J. Removal of Fluoride from Aqueous Solution Using Shrimp Shell Residue as a Biosorbent after Astaxanthin Recovery. Molecules 2023, 28, 3897. https://doi.org/10.3390/molecules28093897
Li Y, Zhang L, Liao M, Huang C, Gao J. Removal of Fluoride from Aqueous Solution Using Shrimp Shell Residue as a Biosorbent after Astaxanthin Recovery. Molecules. 2023; 28(9):3897. https://doi.org/10.3390/molecules28093897
Chicago/Turabian StyleLi, Yan, Lili Zhang, Minru Liao, Chao Huang, and Jing Gao. 2023. "Removal of Fluoride from Aqueous Solution Using Shrimp Shell Residue as a Biosorbent after Astaxanthin Recovery" Molecules 28, no. 9: 3897. https://doi.org/10.3390/molecules28093897
APA StyleLi, Y., Zhang, L., Liao, M., Huang, C., & Gao, J. (2023). Removal of Fluoride from Aqueous Solution Using Shrimp Shell Residue as a Biosorbent after Astaxanthin Recovery. Molecules, 28(9), 3897. https://doi.org/10.3390/molecules28093897






