Potential Effects of Geraniol on Cancer and Inflammation-Related Diseases: A Review of the Recent Research Findings
Abstract
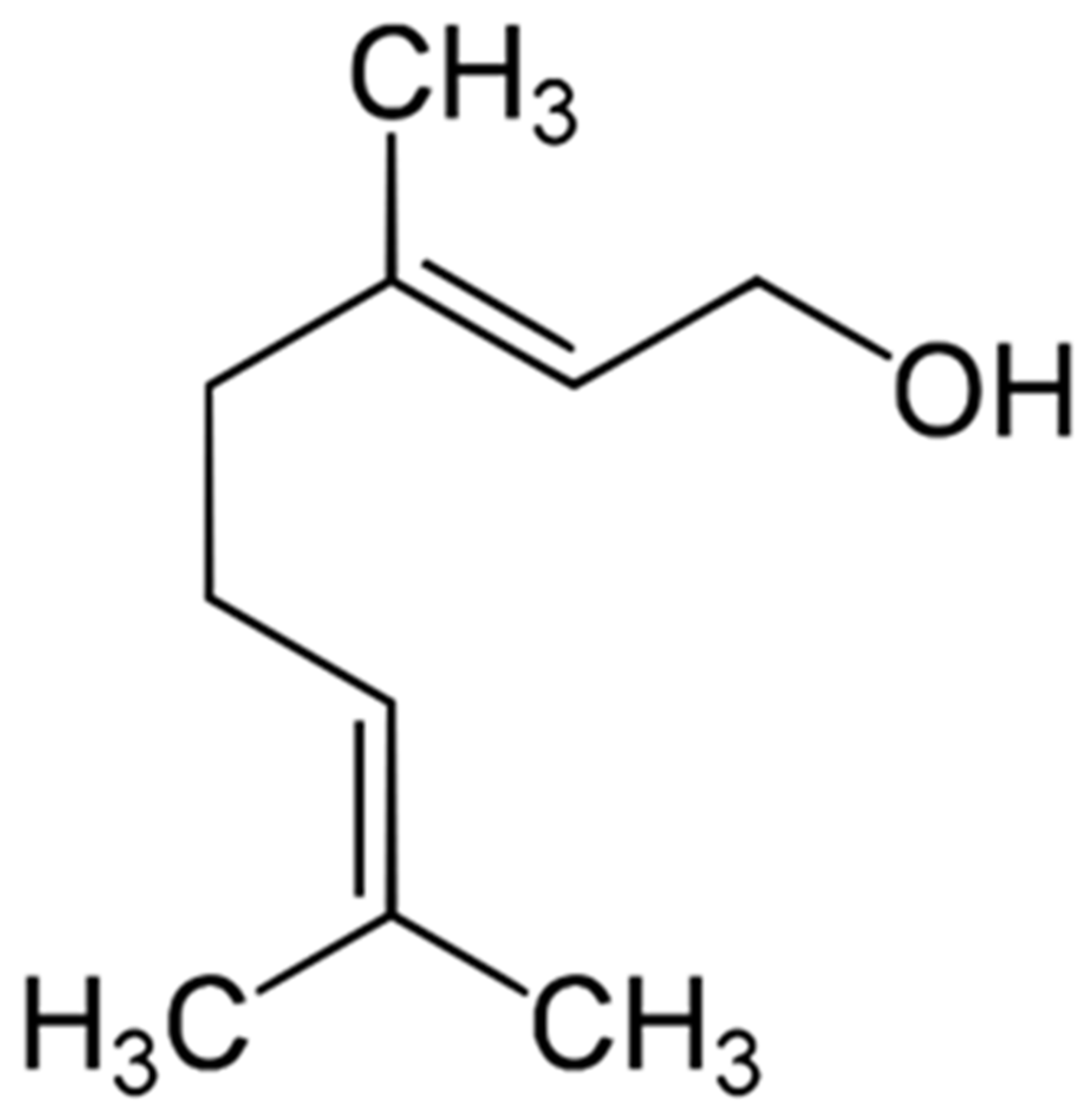
1. Introduction
2. Research Methodology
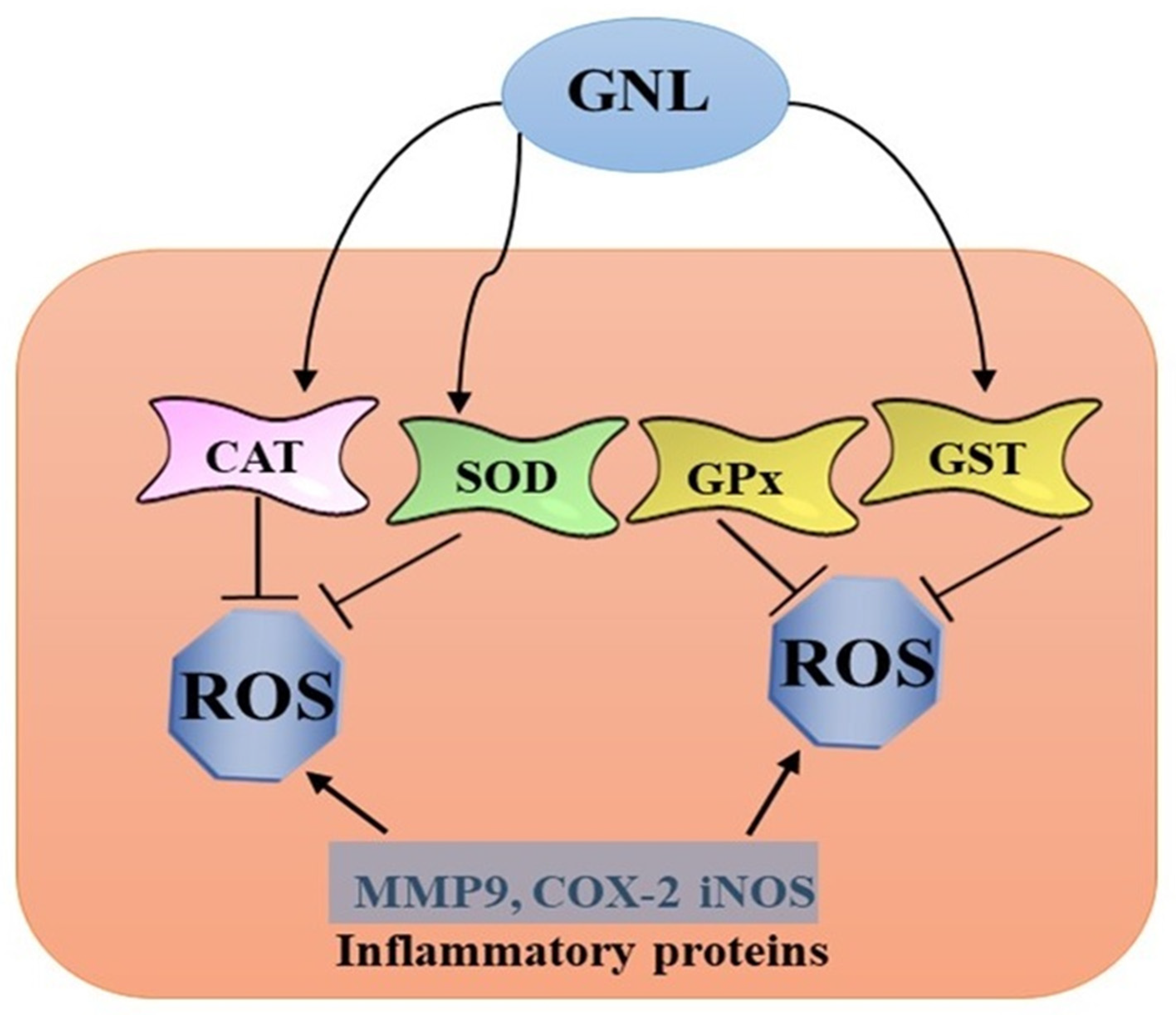
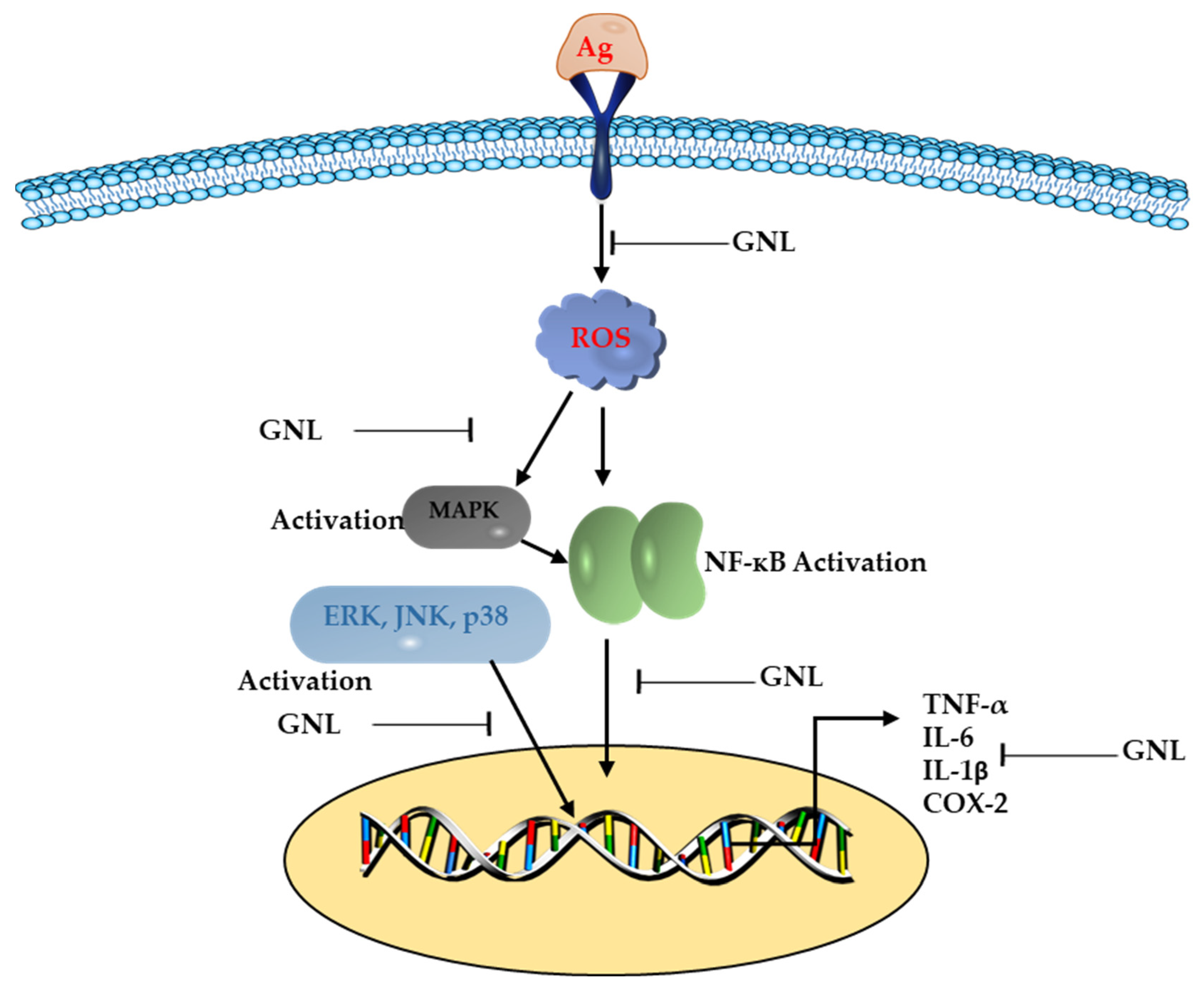
3. Anti-Inflammatory and Antioxidant Properties of GNL
4. Anti-Free Radical Properties of GNL
5. Effects of GNL on Autoimmune Diseases
6. Glutathione Modulation by GNL
7. Transcriptional Effects of GNL on Inflammation
8. Anticancer and Chemo Preventive Effects of GNL
9. Effect of GNL on Cell Proliferation
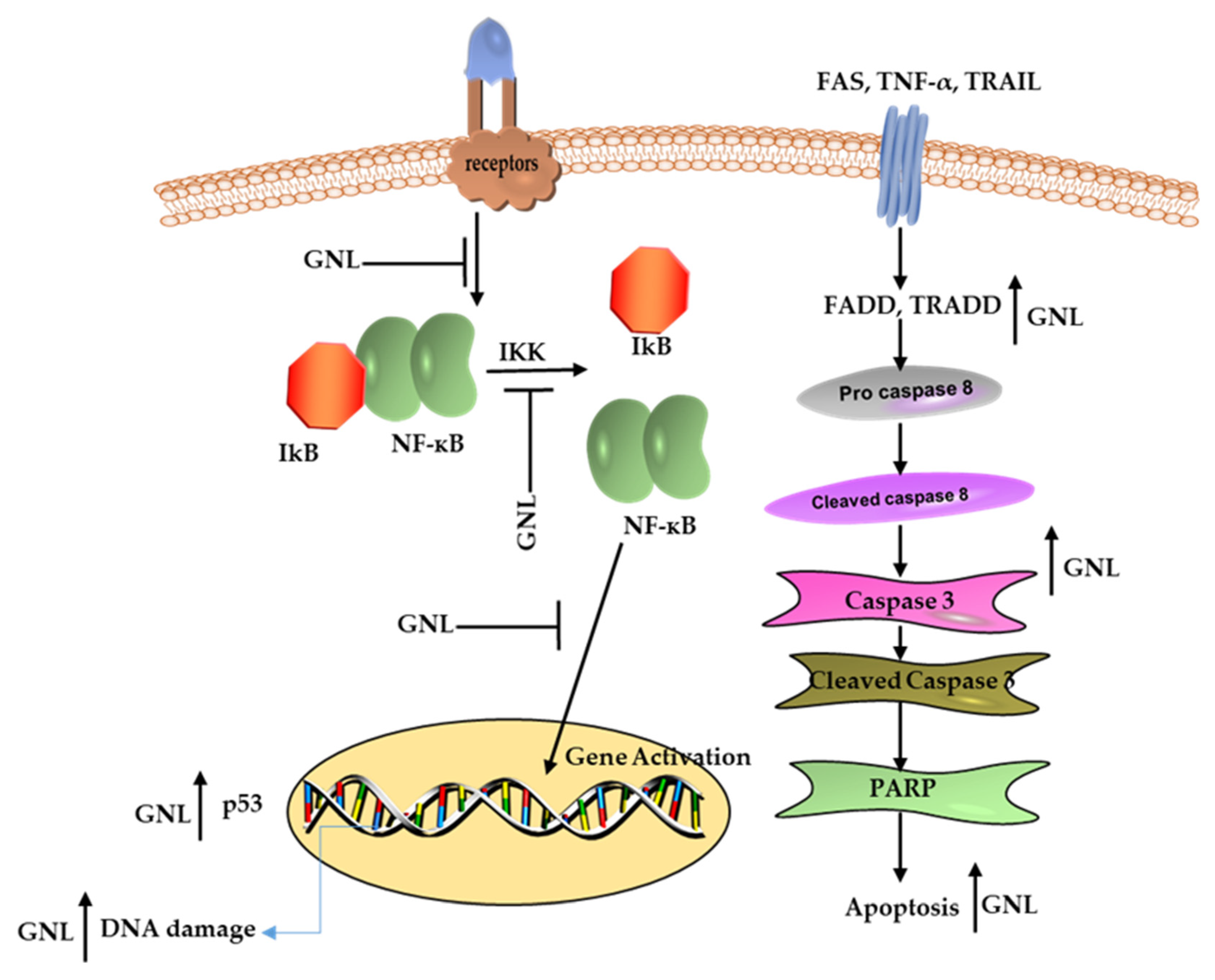
10. Cancer Cells Respond to GNL in A Pro-Apoptotic Way
11. Effect of GNL on Metastasis and Angiogenesis Inhibition
12. An Overview of GNL Bioavailability
13. Conclusions and Future Prospects
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lei, Y.; Fu, P.; Jun, X.; Cheng, P. Pharmacological Properties of Geraniol—A Review. Planta Med. 2019, 85, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Hegde, M.M.; Lakshman, K. Role of Polyphenols and Flavonoids as Anti-Cancer Drug Candidates: A Review. Pharmacognosy Res. 2023, 15, 206–216. [Google Scholar] [CrossRef]
- Chimento, A.; De Luca, A.; D’Amico, M.; De Amicis, F.; Pezzi, V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1680. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhou, L.; Li, L.; Zhou, P.; Shen, Z. Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Aloo, S.O.; Ofosu, F.K.; Kim, N.H.; Kilonzi, S.M.; Oh, D.H. Insights on Dietary Polyphenols as Agents against Metabolic Disorders: Obesity as a Target Disease. Antioxidants 2023, 12, 416. [Google Scholar] [CrossRef]
- Gupta, N.; Singh, S.; Chauhan, D.; Srivastava, R.; Singh, V.K. Exploring the Anticancer Potentials of Polyphenols: A Comprehensive Review of Patents in the Last Five Years. Recent Pat. Anticancer Drug Discov. 2023, 18, 3–10. [Google Scholar]
- Sahiner, M.; Yilmaz, A.S.; Gungor, B.; Sahiner, N. A Review on Phyto-Therapeutic Approaches in Alzheimer’s Disease. J. Funct. Biomater. 2023, 14, 50. [Google Scholar] [CrossRef]
- Yang, J.; Zhong, C.; Yu, J. Natural Monoterpenes as Potential Therapeutic Agents against Atherosclerosis. Int. J. Mol. Sci. 2023, 24, 2429. [Google Scholar] [CrossRef]
- Sajid, A.; Manzoor, Q.; Sajid, A.; Nazir, A.; Mumtaz, M.A.; Fatima, N.; Alshawwa, S.Z.; Iqbal, M.; Younas, U. Downregulation of NF-κB activation pathways using essential oil derived from Citrus pseudolimon: Anticancer and anti-inflammatory potential. Biocatal. Agric. Biotechnol. 2023, 47, 102599. [Google Scholar] [CrossRef]
- Abdul Ghani, M.A.; Ugusman, A.; Latip, J.; Zainalabidin, S. Role of Terpenophenolics in Modulating Inflammation and Apoptosis in Cardiovascular Diseases: A Review. Int. J. Mol. Sci. 2023, 24, 5339. [Google Scholar] [CrossRef]
- Abdul-Hameed, Z.H.; Bawakid, N.O.; Alorfi, H.S.; Sobahi, T.R.; Alburae, N.A.; Abdel-Lateff, A.; Elbehairi, S.E.I.; Alfaifi, M.Y.; Alhakamy, N.A.; Alarif, W.M. Monoterpene Indole Alkaloids from the Aerial Parts of Rhazya stricta Induce Cytotoxicity and Apoptosis in Human Adenocarcinoma Cells. Molecules 2022, 27, 1422. [Google Scholar] [CrossRef] [PubMed]
- Shoff, S.M.; Grummer, M.; Yatvin, M.B.; Elson, C.E. Concentration-dependent increase of murine P388 and B16 population doubling time by the acyclic monoterpene geraniol. Cancer Res. 1991, 51, 37–42. [Google Scholar] [PubMed]
- de Cássia da Silveira e Sá, R.; Andrade, L.N.; de Sousa, D.P. A review on antiinflammatory activity of monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef] [PubMed]
- Solorzano-Santos, F.; Miranda-Novales, M.G. Essential oils from aromatic herbs as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 136–141. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Bras-Goncalves, R.; Bradaia, A.; Zeisel, M.; Gosse, F.; Poupon, M.F.; Raul, F. Geraniol, a component of plant essential oils, modulates DNA synthesis and potentiates 5-fluorouracil efficacy on human colon tumor xenografts. Cancer Lett. 2004, 215, 53–59. [Google Scholar] [CrossRef]
- Pavan, B.; Dalpiaz, A.; Marani, L.; Beggiato, S.; Ferraro, L.; Canistro, D.; Paolini, M.; Vivarelli, F.; Valerii, M.C.; Comparone, A. Geraniol pharmacokinetics, bioavailability and its multiple effects on the liver antioxidant and xenobiotic-metabolizing enzymes. Front. Pharmacol. 2018, 9, 18. [Google Scholar] [CrossRef]
- Turina, A.d.V.; Nolan, M.; Zygadlo, J.; Perillo, M. Natural terpenes: Self-assembly and membrane partitioning. Biophys. Chem. 2006, 122, 101–113. [Google Scholar] [CrossRef]
- Chen, W.; Viljoen, A.M. Geraniol—A review of a commercially important fragrance material. S. Afr. J. Bot. 2010, 76, 643–651. [Google Scholar] [CrossRef]
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.-H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways. Int. J. Oncol. 2016, 48, 1772–1782. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Mamur, S. Geraniol, a natural monoterpene, identifications of cytotoxic and genotoxic effects in vitro. J. Essent. Oil Res. 2022, 34, 54–64. [Google Scholar] [CrossRef]
- Polo, M.; Crespo, R.; De Bravo, M. Geraniol and simvastatin show a synergistic effect on a human hepatocarcinoma cell line. Cell Biochem. Funct. 2011, 29, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Burke, Y.D.; Stark, M.J.; Roach, S.L.; Sen, S.E.; Crowell, P.L. Inhibition of pancreatic cancer growth by the dietary isoprenoids farnesol and geraniol. Lipids 1997, 32, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.G.; Hildebrandt, L.A.; Elson, C.E. Geraniol, an inhibitor of mevalonate biosynthesis, suppresses the growth of hepatomas and melanomas transplanted to rats and mice. J. Nutr. 1995, 125, 2763–2767. [Google Scholar]
- Mączka, W.; Wińska, K.; Grabarczyk, M. One Hundred Faces of Geraniol. Molecules 2020, 25, 3303. [Google Scholar] [CrossRef]
- Ji, P.; Si, M.-S.; Podnos, Y.; Imagawa, D.K. Monoterpene geraniol prevents acute allograft rejection. Transplant. Proc. 2002, 34, 1418–1419. [Google Scholar] [CrossRef]
- e Silva, G.d.S.; de Jesus Marques, J.N.; Linhares, E.P.M.; Bonora, C.M.; Costa, É.T.; Saraiva, M.F. Review of anticancer activity of monoterpenoids: Geraniol, nerol, geranial and neral. Chem.-Biol. Interact. 2022, 362, 109994. [Google Scholar] [CrossRef]
- Crespo, R.; Rodenak-Kladniew, B.E.; Castro, M.A.; Soberón, M.V.; Lavarías, S.M.L. Induction of oxidative stress as a possible mechanism by which geraniol affects the proliferation of human A549 and HepG2 tumor cells. Chem.-Biol. Interact. 2020, 320, 109029. [Google Scholar] [CrossRef]
- Rajendran, P.; Chen, Y.F.; Chen, Y.F.; Chung, L.C.; Tamilselvi, S.; Shen, C.Y.; Day, C.H.; Chen, R.J.; Viswanadha, V.P.; Kuo, W.W. The multifaceted link between inflammation and human diseases. J. Cell. Physiol. 2018, 233, 6458–6471. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Farokhcheh, M.; Hejazian, L.; Akbarnejad, Z.; Pourabdolhossein, F.; Hosseini, S.M.; Mehraei, T.M.; Soltanpour, N. Geraniol improved memory impairment and neurotoxicity induced by zinc oxide nanoparticles in male wistar rats through its antioxidant effect. Life Sci. 2021, 282, 119823. [Google Scholar] [CrossRef] [PubMed]
- Elsayad, J.; Adeshina, N. Antitumor effects of geraniol on oral cancer. STEMedicine 2023, 4, e155. [Google Scholar] [CrossRef]
- Younis, N.S.; Elsewedy, H.S.; Soliman, W.E.; Shehata, T.M.; Mohamed, M.E. Geraniol isolated from lemon grass to mitigate doxorubicin-induced cardiotoxicity through Nrf2 and NF-κB signaling. Chem.-Biol. Interact. 2021, 347, 109599. [Google Scholar] [CrossRef]
- El Azab, E.F.; Abdulmalek, S. Amelioration of Age-Related Multiple Neuronal Impairments and Inflammation in High-Fat Diet-Fed Rats: The Prospective Multitargets of Geraniol. Oxid. Med. Cell. Longev. 2022, 2022, 4812993. [Google Scholar] [CrossRef] [PubMed]
- AlAsmari, A.F.; Ali, N.; Alharbi, M.; Alqahtani, F.; Alasmari, F.; Almoqbel, D.; AlSwayyed, M.; Alshammari, A.; Alanazi, M.M.; Alhoshani, A. Geraniol Ameliorates Doxorubicin-Mediated Kidney Injury through Alteration of Antioxidant Status, Inflammation, and Apoptosis: Potential Roles of NF-κB and Nrf2/Ho-1. Nutrients 2022, 14, 1620. [Google Scholar] [CrossRef]
- Su, Y.-W.; Chao, S.-H.; Lee, M.-H.; Ou, T.-Y.; Tsai, Y.-C. Inhibitory effects of citronellol and geraniol on nitric oxide and prostaglandin E2 production in macrophages. Planta Med. 2010, 76, 1666–1671. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, N.; Jiang, Y. Geraniol protects against lipopolysaccharide and D-galactosamine-induced fulminant hepatic failure by activating PPARγ. Microb. Pathog. 2019, 128, 7–12. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Elmorsy, M.A.; Younis, N.S. Renal Ischemia/Reperfusion Mitigation via Geraniol: The Role of Nrf-2/HO-1/NQO-1 and TLR2,4/MYD88/NFκB Pathway. Antioxidants 2022, 11, 1568. [Google Scholar] [CrossRef]
- Mohammed, M.J.; Tadros, M.G.; Michel, H.E. Geraniol protects against cyclophosphamide-induced hepatotoxicity in rats: Possible role of MAPK and PPAR-γ signaling pathways. Food Chem. Toxicol. 2020, 139, 111251. [Google Scholar] [CrossRef]
- Xue, Z.; Zhang, X.-G.; Wu, J.; Xu, W.-C.; Li, L.-Q.; Liu, F.; Yu, J.-E. Effect of treatment with geraniol on ovalbumin-induced allergic asthma in mice. Ann. Allergy Asthma Immunol. 2016, 116, 506–513. [Google Scholar] [CrossRef] [PubMed]
- El-Bassossy, H.M.; Elberry, A.A.; Ghareib, S.A. Geraniol improves the impaired vascular reactivity in diabetes and metabolic syndrome through calcium channel blocking effect. J. Diabetes Complicat. 2016, 30, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, Z.; Fu, X.; Lin, Z.; Yu, K. Geraniol-mediated osteoarthritis improvement by down-regulating PI3K/Akt/NF-κB and MAPK signals: In vivo and in vitro studies. Int. Immunopharmacol. 2020, 86, 106713. [Google Scholar] [CrossRef] [PubMed]
- Babukumar, S.; Vinothkumar, V.; Sankaranarayanan, C.; Srinivasan, S. Geraniol, a natural monoterpene, ameliorates hyperglycemia by attenuating the key enzymes of carbohydrate metabolism in streptozotocin-induced diabetic rats. Pharm. Biol. 2017, 55, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, S.Z.; Soubh, A.A.; Al-Mokaddem, A.K.; Abo El-Ella, D.M. Geraniol activates Nrf-2/HO-1 signaling pathway mediating protection against oxidative stress-induced apoptosis in hepatic ischemia-reperfusion injury. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- El Azab, E.F.; Mostafa, H.S. Geraniol ameliorates the progression of high fat-diet/streptozotocin-induced type 2 diabetes mellitus in rats via regulation of caspase-3, Bcl-2, and Bax expression. J. Food Biochem. 2022, 46, e14142. [Google Scholar] [CrossRef]
- Prasad, S.N. Protective effects of geraniol (a monoterpene) in a diabetic neuropathy rat model: Attenuation of behavioral impairments and biochemical perturbations. J. Neurosci. Res. 2014, 92, 1205–1216. [Google Scholar] [CrossRef]
- El-Said, Y.A.M.; Sallam, N.A.A.; Ain-Shoka, A.A.-M.; Abdel-Latif, H.A.-T. Geraniol ameliorates diabetic nephropathy via interference with miRNA-21/PTEN/Akt/mTORC1 pathway in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 2325–2337. [Google Scholar] [CrossRef]
- Coelho, M.; Sabino, K.; Dalmau, S. Immunomodulatory effects of sucupira (Pterodon pubescens) seed infusion on collagen-induced arthritis. Clin. Exp. Rheumatol. 2004, 22, 213–218. [Google Scholar]
- Zhi, H.; Cui, J.; Yang, H.; Zhang, Y.; Zhu, M. Research progress of geraniol in tumor therapy. Proc. Anticancer Res. 2021, 5, 26–30. [Google Scholar] [CrossRef]
- Van Aller, R.; Nes, W. The phosphorylation of geraniol in germinating peas. Phytochemistry 1968, 7, 85–88. [Google Scholar] [CrossRef]
- Stobiecka, A. Comparative study on the free radical scavenging mechanism exerted by geraniol and geranylacetone using the combined experimental and theoretical approach. Flavour Fragr. J. 2015, 30, 399–409. [Google Scholar] [CrossRef]
- Tiwari, M.; Kakkar, P. Plant derived antioxidants–geraniol and camphene protect rat alveolar macrophages against t-BHP induced oxidative stress. Toxicol. In Vitro 2009, 23, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.M.; Allam, E.A.; Alghriany, A.A. Role of Psyllium Husk (Plantago ovata) on Liver Function Alterations Induced by Carbon Tetrachloride (CCl4) in Adult Male Albino Rats. Egypt. Acad. J. Biol. Sci. 2022, 14, 47–60. [Google Scholar] [CrossRef]
- Younis, N.S.; Abduldaium, M.S.; Mohamed, M.E. Protective effect of geraniol on oxidative, inflammatory and apoptotic alterations in isoproterenol-induced cardiotoxicity: Role of the Keap1/Nrf2/HO-1 and PI3K/Akt/mTOR pathways. Antioxidants 2020, 9, 977. [Google Scholar] [CrossRef]
- Ozkaya, A.; Sahin, Z.; Gorgulu, A.O.; Yuce, A.; Celik, S. Geraniol attenuates hydrogen peroxide-induced liver fatty acid alterations in male rats. J. Intercult. Ethnopharmacol. 2017, 6, 29. [Google Scholar] [CrossRef]
- Ozherelkov, S.; Timofeev, A.; Novikova, G.; Deeva, A.; Narovlianskiĭ, A.; Sanin, A.; Pronin, A. Protective effect of a new antiviral preparation of phosprenyl in experimental tick-borne encephalitis. Vopr. Virusol. 2000, 45, 33–37. [Google Scholar]
- Kandeil, M.A.; Mahmoud, M.O.; Abdel-Razik, A.-R.H.; Gomaa, S.B. Thymoquinone and geraniol alleviate cisplatin-induced neurotoxicity in rats through downregulating the p38 MAPK/STAT-1 pathway and oxidative stress. Life Sci. 2019, 228, 145–151. [Google Scholar] [CrossRef]
- Nennig, S.; Schank, J. The role of NFkB in drug addiction: Beyond inflammation. Alcohol Alcohol. 2017, 52, 172–179. [Google Scholar] [CrossRef]
- Hunter, C.J.; De Plaen, I.G. Inflammatory signaling in NEC: Role of NFKB and cytokines. Pathophysiol. Off. J. Int. Soc. Pathophysiol. ISP 2014, 21, 55. [Google Scholar]
- Jiang, K.; Zhang, T.; Yin, N.; Ma, X.; Zhao, G.; Wu, H.; Qiu, C.; Deng, G. Geraniol alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis. Oncotarget 2017, 8, 71038. [Google Scholar] [CrossRef] [PubMed]
- Ben Ammar, R.; Mohamed, M.E.; Alfwuaires, M.; Abdulaziz Alamer, S.; Bani Ismail, M.; Veeraraghavan, V.P.; Sekar, A.K.; Ksouri, R.; Rajendran, P. Anti-Inflammatory Activity of Geraniol Isolated from Lemon Grass on Ox-LDL-Stimulated Endothelial Cells by Upregulation of Heme Oxygenase-1 via PI3K/Akt and Nrf-2 Signaling Pathways. Nutrients 2022, 14, 4817. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, H.S.; Kim, D.J.; Noh, Y.H.; Yuk, D.Y.; Hong, J.T. Inhibitory effect of citral on NO production by suppression of iNOS expression and NF-κB activation in RAW264. 7 cells. Arch. Pharm. Res. 2008, 31, 342–349. [Google Scholar] [CrossRef]
- Sharma, R.; Rao, R.; Kumar, S.; Mahant, S.; Khatkar, S. Therapeutic potential of citronella essential oil: A review. Curr. Drug Discov. Technol. 2019, 16, 330–339. [Google Scholar] [CrossRef]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Kannaiyan, R.; Goh, J.N.; Wong, K.F.; Wang, W.; Khin, E.; Tergaonkar, V.; Kumar, A.P. Celastrol Suppresses Growth and Induces Apoptosis of Human Hepatocellular Carcinoma through the Modulation of STAT3/JAK2 Signaling Cascade In Vitro and In Vivo Celastrol Inhibits STAT3 Signaling in HCC. Cancer Prev. Res. 2012, 5, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Shanmugam, M.K.; Vali, S.; Abbasi, T.; Kapoor, S.; Ahn, K.S.; Kumar, A.P.; Sethi, G. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. J. Cell. Physiol. 2012, 227, 2184–2195. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, P.; Li, F.; Manu, K.A.; Shanmugam, M.K.; Loo, S.Y.; Kumar, A.P.; Sethi, G. γ-Tocotrienol is a novel inhibitor of constitutive and inducible STAT3 signalling pathway in human hepatocellular carcinoma: Potential role as an antiproliferative, pro-apoptotic and chemosensitizing agent. Br. J. Pharmacol. 2011, 163, 283–298. [Google Scholar] [CrossRef]
- Kuzu, B.; Cüce, G.; Ayan, İ.Ç.; Gültekin, B.; Canbaz, H.T.; Dursun, H.G.; Şahin, Z.; Keskin, İ.; Kalkan, S.S. Evaluation of apoptosis pathway of geraniol on Ishikawa cells. Nutr. Cancer 2021, 73, 2532–2537. [Google Scholar] [CrossRef]
- Sundin, T.; Peffley, D.M.; Gauthier, D.; Hentosh, P. The isoprenoid perillyl alcohol inhibits telomerase activity in prostate cancer cells. Biochimie 2012, 94, 2639–2648. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Zhao, X. Targeting Bcl-2 for cancer therapy. Biochim. Biophys. Acta Rev. Cancer BBA-REV CANCER 2021, 1876, 188569. [Google Scholar] [CrossRef]
- El Azab, E.F.; Saleh, A.M.; Yousif, S.O.; Mazhari, B.B.Z.; Abu Alrub, H.; Elfaki, E.M.; Hamza, A.; Abdulmalek, S. New insights into geraniol’s antihemolytic, anti-inflammatory, antioxidant, and anticoagulant potentials using a combined biological and in silico screening strategy. Inflammopharmacology 2022, 30, 1811–1833. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by D-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.T.; Arjumand, W.; Seth, A.; Nafees, S.; Rashid, S.; Ali, N.; Sultana, S. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicology 2011, 290, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Veena, M.S.P.; Mourisha, P.; Nithya, G.; Dhivya, S.; Ilakkia, A.; Sakthisekaran, D. Biochemical Studies on the Cytoprotective Efficacy of Geraniol in Benzo(a)pyrene Induced Experimental Lung Cancer. Am. J. PharmTech Res. 2012, 2, 360–371. [Google Scholar]
- Rajendran, P.; Abdelsalam, S.A.; Renu, K.; Veeraraghavan, V.; Ben Ammar, R.; Ahmed, E.A. Polyphenols as Potent Epigenetics Agents for Cancer. Int. J. Mol. Sci. 2022, 23, 11712. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, P.; Mehta, A.; Verma, R.S. Essential oils: From prevention to treatment of skin cancer. Drug Discov. Today 2019, 24, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Faraji, P.; Araj-Khodaei, M.; Ghaffari, M.; Dolatabadi, J.E.N. Anticancer Effects of Melissa officinalis: A Traditional Medicine. Pharm. Sci. 2021, 28, 355–364. [Google Scholar] [CrossRef]
- Magalhaes, M.; Manadas, B.; Efferth, T.; Cabral, C. Chemoprevention and therapeutic role of essential oils and phenolic compounds: Modeling tumor microenvironment in glioblastoma. Pharmacol. Res. 2021, 169, 105638. [Google Scholar] [CrossRef]
- Machado, T.Q.; da Fonseca, A.C.; Duarte, A.; Robbs, B.K.; de Sousa, D.P. A Narrative Review of the Antitumor Activity of Monoterpenes from Essential Oils: An Update. Biomed. Res. Int. 2022, 2022, 6317201. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1934578X20903555. [Google Scholar] [CrossRef]
- Madankumar, A.; Jayakumar, S.; Gokuladhas, K.; Rajan, B.; Raghunandhakumar, S.; Asokkumar, S.; Devaki, T. Geraniol modulates tongue and hepatic phase I and phase II conjugation activities and may contribute directly to the chemopreventive activity against experimental oral carcinogenesis. Eur. J. Pharmacol. 2013, 705, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Vinothkumar, V.; Manoharan, S.; Sindhu, G.; Nirmal, M.R.; Vetrichelvi, V. Geraniol modulates cell proliferation, apoptosis, inflammation, and angiogenesis during 7, 12-dimethylbenz [a] anthracene-induced hamster buccal pouch carcinogenesis. Mol. Cell. Biochem. 2012, 369, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Xia, Y.; Tian, X.; Cui, J.; Zhang, X.; Yang, Q.; Zhao, T.; Lin, Y.; Zhang, F.; Zhang, X. A multi-bioresponsive self-assembled nano drug delivery system based on hyaluronic acid and geraniol against liver cancer. Carbohydr. Polym. 2023, 310, 120695. [Google Scholar] [CrossRef] [PubMed]
- Carnesecchi, S.; Bradaia, A.; Fischer, B.; Coelho, D.; Schöller-Guinard, M.; Gosse, F.; Raul, F. Perturbation by geraniol of cell membrane permeability and signal transduction pathways in human colon cancer cells. J. Pharmacol. Exp. Ther. 2002, 303, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, D.; Yang, T.; Qian, D.; Xu, R. Apoptosis triggering, an important way for natural products from herbal medicines to treat pancreatic cancers. Front. Pharmacol. 2022, 12, 3835. [Google Scholar] [CrossRef]
- Mukarram, M.; Choudhary, S.; Khan, M.A.; Poltronieri, P.; Khan, M.M.A.; Ali, J.; Kurjak, D.; Shahid, M. Lemongrass essential oil components with antimicrobial and anticancer activities. Antioxidants 2021, 11, 20. [Google Scholar] [CrossRef]
- Pu, Z.; Yang, F.; Wang, L.; Diao, Y.; Chen, D. Advancements of compounds targeting Wnt and Notch signalling pathways in the treatment of inflammatory bowel disease and colon cancer. J. Drug Target. 2021, 29, 507–519. [Google Scholar] [CrossRef]
- Duncan, R.E.; Lau, D.; El-Sohemy, A.; Archer, M.C. Geraniol and β-ionone inhibit proliferation, cell cycle progression, and cyclin-dependent kinase 2 activity in MCF-7 breast cancer cells independent of effects on HMG-CoA reductase activity. Biochem. Pharmacol. 2004, 68, 1739–1747. [Google Scholar] [CrossRef]
- Zhuang, K.; Tang, H.; Guo, H.; Yuan, S. Geraniol prevents Helicobacterium pylori-induced human gastric cancer signalling by enhancing peroxiredoxin-1 expression in GES-1 cells. Microb. Pathog. 2023, 174, 105937. [Google Scholar] [CrossRef]
- Chaudhary, S.C.; Siddiqui, M.S.; Athar, M.; Alam, M.S. Geraniol inhibits murine skin tumorigenesis by modulating COX-2 expression, Ras-ERK1/2 signaling pathway and apoptosis. J. Appl. Toxicol. 2013, 33, 828–837. [Google Scholar] [CrossRef]
- Fatima, K.; Wani, Z.A.; Meena, A.; Luqman, S. Geraniol exerts its antiproliferative action by modulating molecular targets in lung and skin carcinoma cells. Phytother. Res. 2021, 35, 3861–3874. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, S.; Selvan, M.V. Chemopreventive potential of geraniol in 7, 12-dimethylbenz (a) anthracene (DMBA) induced skin carcinogenesis in Swiss albino mice. J. Environ. Biol. 2012, 33, 255. [Google Scholar] [PubMed]
- Jin, X.; Sun, J.; Miao, X.; Liu, G.; Zhong, D. Inhibitory effect of geraniol in combination with gemcitabine on proliferation of BXPC-3 human pancreatic cancer cells. Int. J. Med. Res. 2013, 41, 993–1001. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.R.; Kim, S.H.; Park, E.J.; Kang, M.J.; So, I.; Chun, J.N.; Jeon, J.H. Geraniol suppresses prostate cancer growth through down-regulation of E2F8. Cancer Med. 2016, 5, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON 2018, 23, 346–352. [Google Scholar] [PubMed]
- Thapa, D.; Richardson, A.J.; Zweifel, B.; Wallace, R.J.; Gratz, S.W. Genoprotective effects of essential oil compounds against oxidative and methylated DNA damage in human colon cancer cells. J. Food Sci. 2019, 84, 1979–1985. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Montero Villegas, S.; Abba, M.C.; de Bravo, M.G.; Polo, M.P. Transcriptional and posttranscriptional inhibition of HMGCR and PC biosynthesis by geraniol in 2 Hep-G2 cell proliferation linked pathways. Biochem. Cell Biol. 2013, 91, 131–139. [Google Scholar] [CrossRef]
- Queiroz, T.; Santos, G.; Ventura, S.; Hiruma-Lima, C.; Gaivã, I.; Maistro, E. Cytotoxic and genotoxic potential of geraniol in peripheral blood mononuclear cells and human hepatoma cell line (HepG2). Genet. Mol. Res. 2017, 16, gmr16039777. [Google Scholar] [CrossRef]
- Sawada, S.; Okano, J.-i.; Imamoto, R.; Yasunaka, Y.; Abe, R.; Koda, M.; Murawaki, Y.; Isomoto, H. Preventive effect of geraniol on diethylnitrosamine-induced hepatocarcinogenesis in rats. Yonago Acta Med. 2016, 59, 37. [Google Scholar]
- Galle, M.; Crespo, R.; Rodenak Kladniew, B.; Montero Villegas, S.; Polo, M.; de Bravo, M.G. Suppression by geraniol of the growth of A549 human lung adenocarcinoma cells and inhibition of the mevalonate pathway in culture and in vivo: Potential use in cancer chemotherapy. Nutr. Cancer 2014, 66, 888–895. [Google Scholar] [CrossRef]
- Rodenak-Kladniew, B.; Gambaro, R.; Cisneros, J.S.; Huck-Iriart, C.; Padula, G.; Castro, G.R.; Chain, C.Y.; Islan, G.A. Enhanced anticancer activity of encapsulated geraniol into biocompatible lipid nanoparticles against A549 human lung cancer cells. J. Drug Deliv. Sci. Technol. 2023, 80, 104159. [Google Scholar] [CrossRef]
- De Fazio, L.; Spisni, E.; Cavazza, E.; Strillacci, A.; Candela, M.; Centanni, M.; Ricci, C.; Rizzello, F.; Campieri, M.; Valerii, M.C. Dietary geraniol by oral or enema administration strongly reduces dysbiosis and systemic inflammation in dextran sulfate sodium-treated mice. Front. Pharmacol. 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Wittig, C.; Scheuer, C.; Parakenings, J.; Menger, M.D.; Laschke, M.W. Geraniol suppresses angiogenesis by downregulating vascular endothelial growth factor (VEGF)/VEGFR-2 signaling. PLoS ONE 2015, 10, e0131946. [Google Scholar] [CrossRef] [PubMed]
- El-Ella, D.M.A. Autophagy/apoptosis induced by geraniol through HIF-1α/BNIP3/beclin-1 signaling pathway in A549 CoCl2 treated cells. Adv. Pharm. Bull. 2022, 12, 155. [Google Scholar] [CrossRef] [PubMed]
| Type of Cancer | Mode of Study | Cell Lines/Animal Model | Target | Effect | References |
|---|---|---|---|---|---|
| Skin cancer | In vivo/In vitro | Swiss albino mice PC-3, A431, and A549 cells Swiss albino mice | Ras/Raf/ERK1/2 ornithine decarboxylase, LOX-5, and hyaluronidase Phase II and Antioxidants | Apoptosis Anti-proliferation Chemoprevention | [90,91,92] |
| Pancreatic cancer | In vitro | BXPC-3 cells | DNA damage | Apoptosis, Anti-proliferative | [93,94] |
| Prostate cancer | In vitro | PC-3 | E2F8 expression | Anti-proliferative | [95] |
| Colon cancer | In vitro | HT-29 | DNA damage | Apoptosis | [96] |
| Liver cancer | In vitro/In vivo | HepG2/Rats | Mevalonate pathway, HMGCR, DNA damage and ERK, NFkB | Apoptosis; anti-proliferative | [83,97,98,99,100] |
| Lung Cancer | In vitro/In vivo | A549, Albino mice | Mevalonate pathway, DNA damage, Chemoprevention | Apoptosis; anti-proliferative | [101,102] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Ammar, R. Potential Effects of Geraniol on Cancer and Inflammation-Related Diseases: A Review of the Recent Research Findings. Molecules 2023, 28, 3669. https://doi.org/10.3390/molecules28093669
Ben Ammar R. Potential Effects of Geraniol on Cancer and Inflammation-Related Diseases: A Review of the Recent Research Findings. Molecules. 2023; 28(9):3669. https://doi.org/10.3390/molecules28093669
Chicago/Turabian StyleBen Ammar, Rebai. 2023. "Potential Effects of Geraniol on Cancer and Inflammation-Related Diseases: A Review of the Recent Research Findings" Molecules 28, no. 9: 3669. https://doi.org/10.3390/molecules28093669
APA StyleBen Ammar, R. (2023). Potential Effects of Geraniol on Cancer and Inflammation-Related Diseases: A Review of the Recent Research Findings. Molecules, 28(9), 3669. https://doi.org/10.3390/molecules28093669






