Study of the Properties of CdS:Al (R = [Al3+]/[Cd2+] = 0.30, 0.40, 0.50) Thin Films Grown by the CBD Method in an Ammonia-Free System
Abstract
1. Introduction
2. Results and Discussion
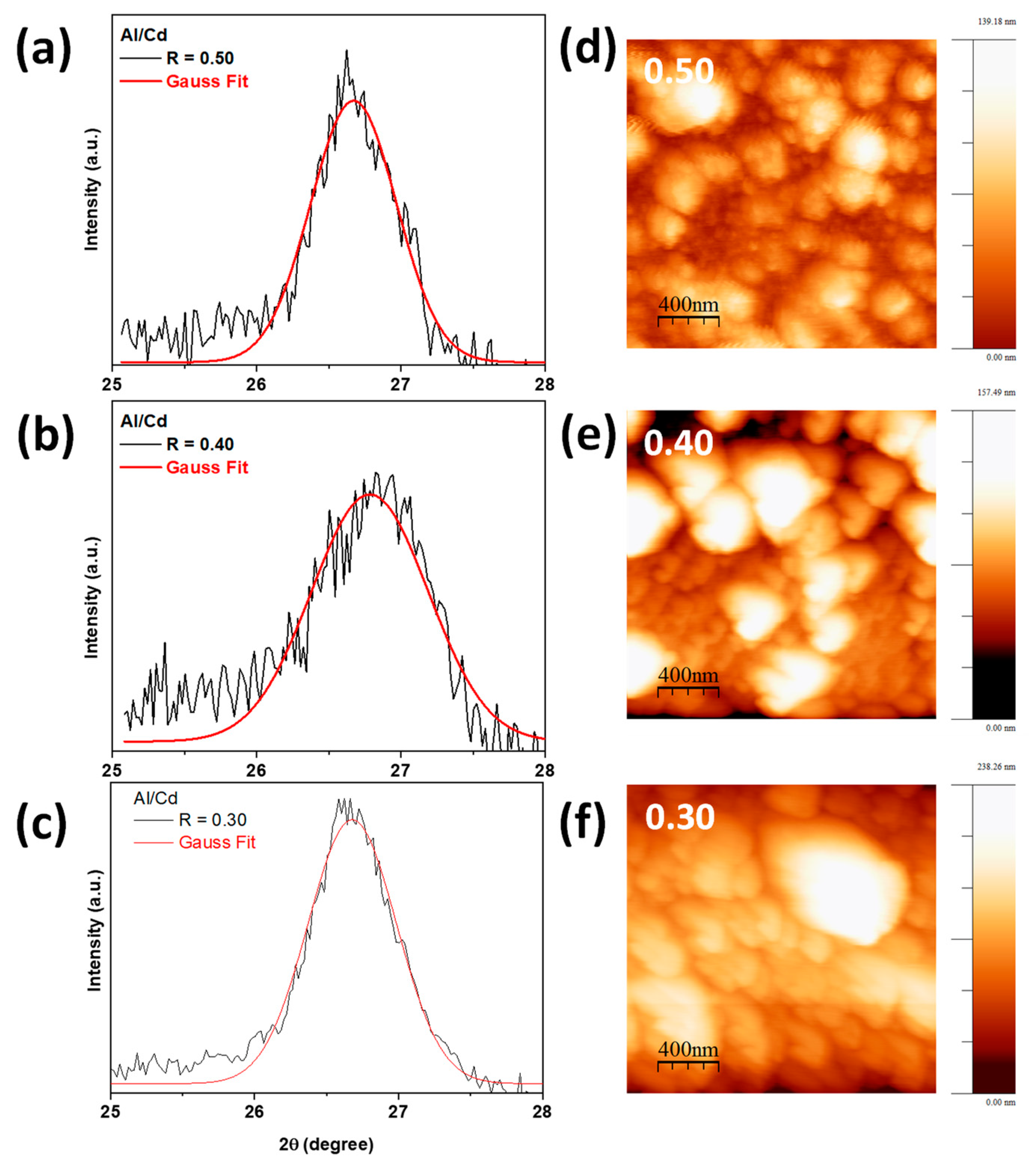
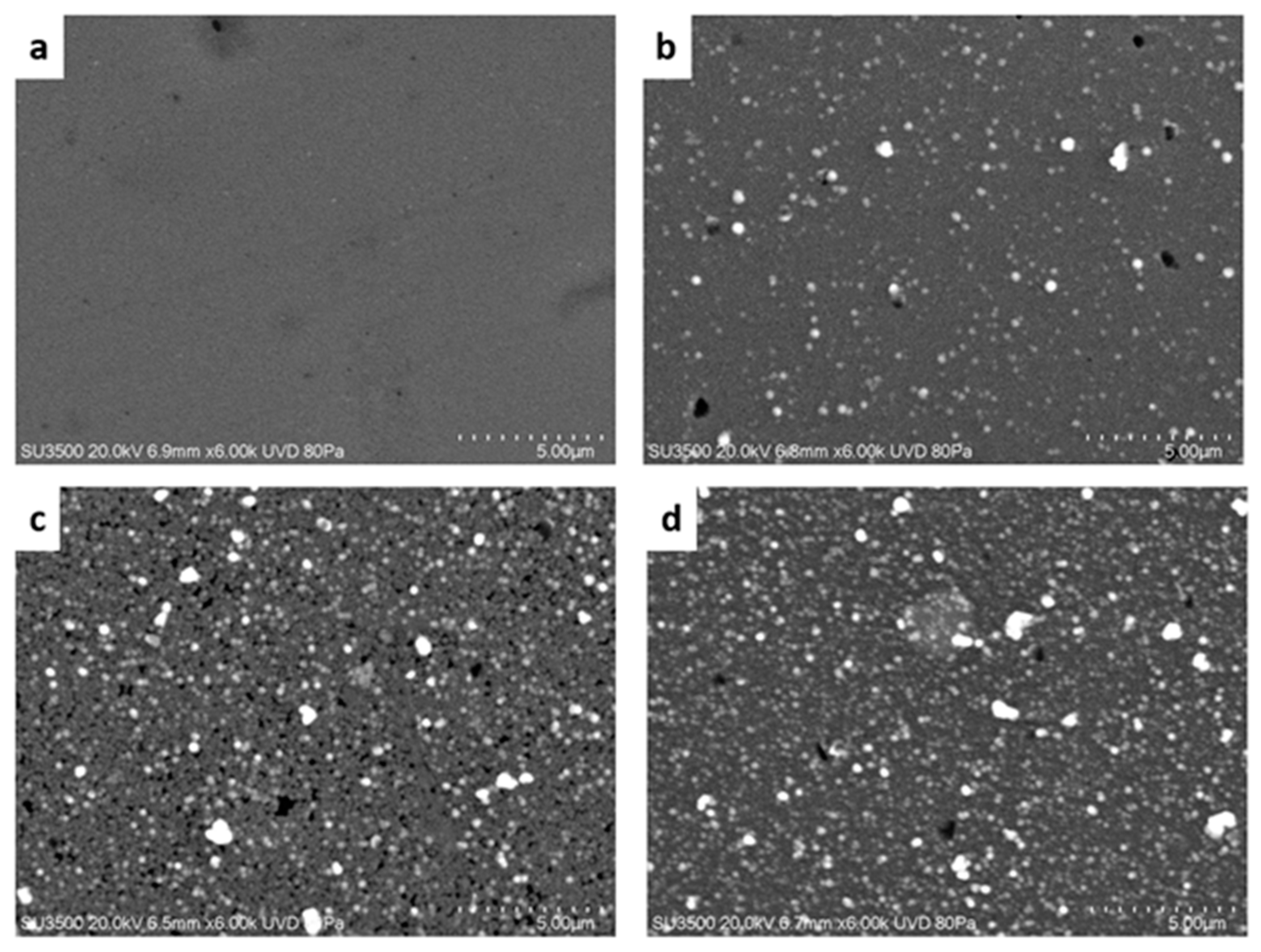
2.1. Morphological and Crystalline Characterization
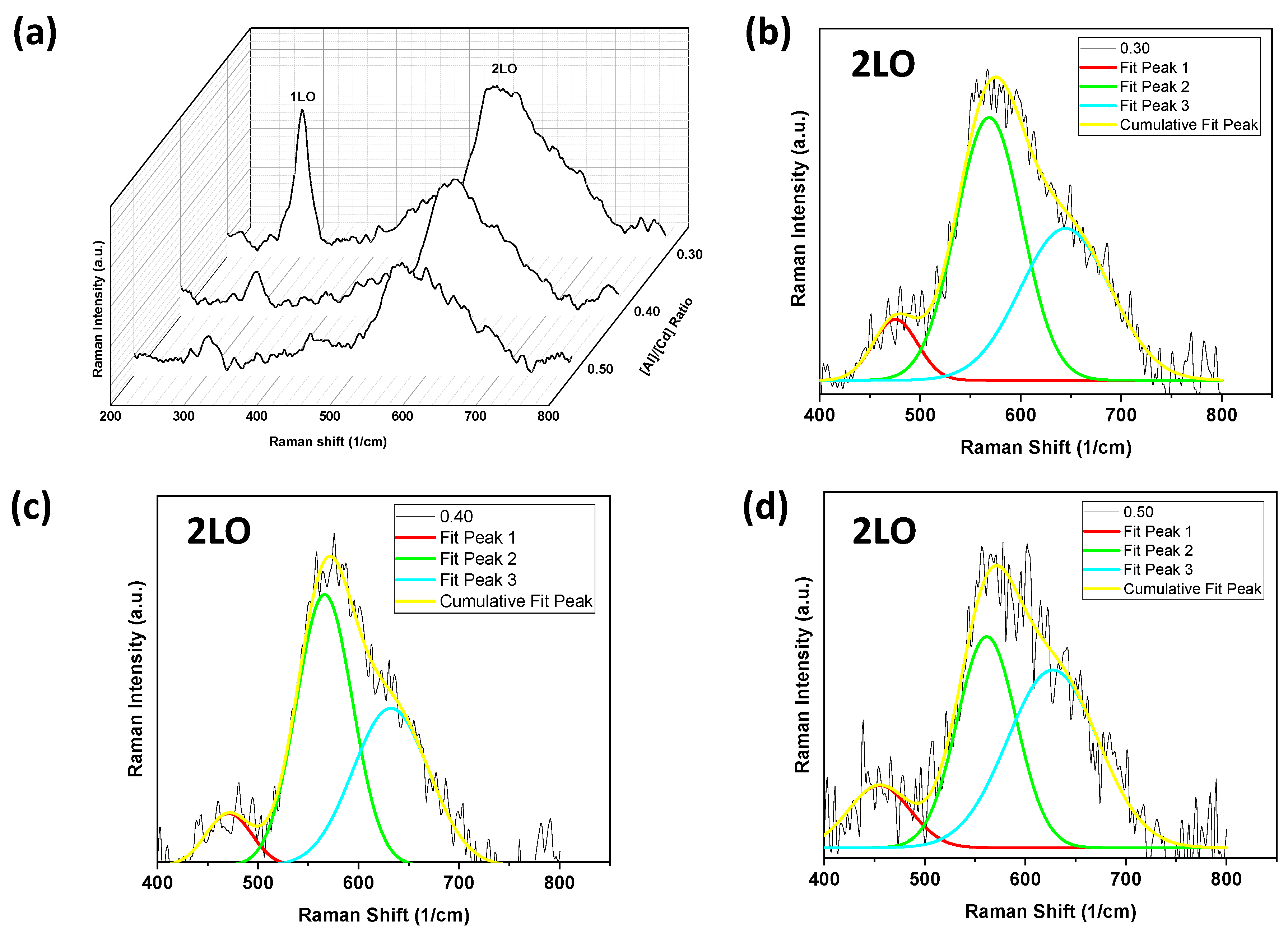
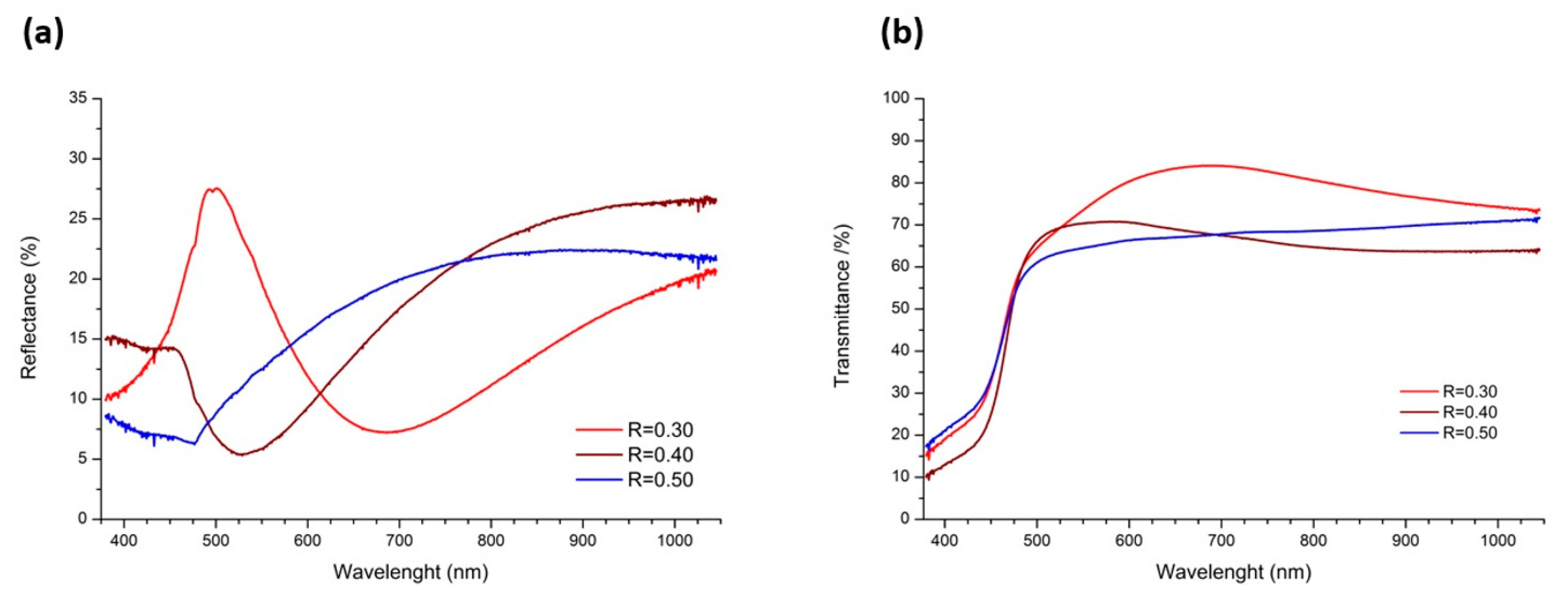
2.2. Spectroscopy Studies
3. Materials and Methods
3.1. Materials
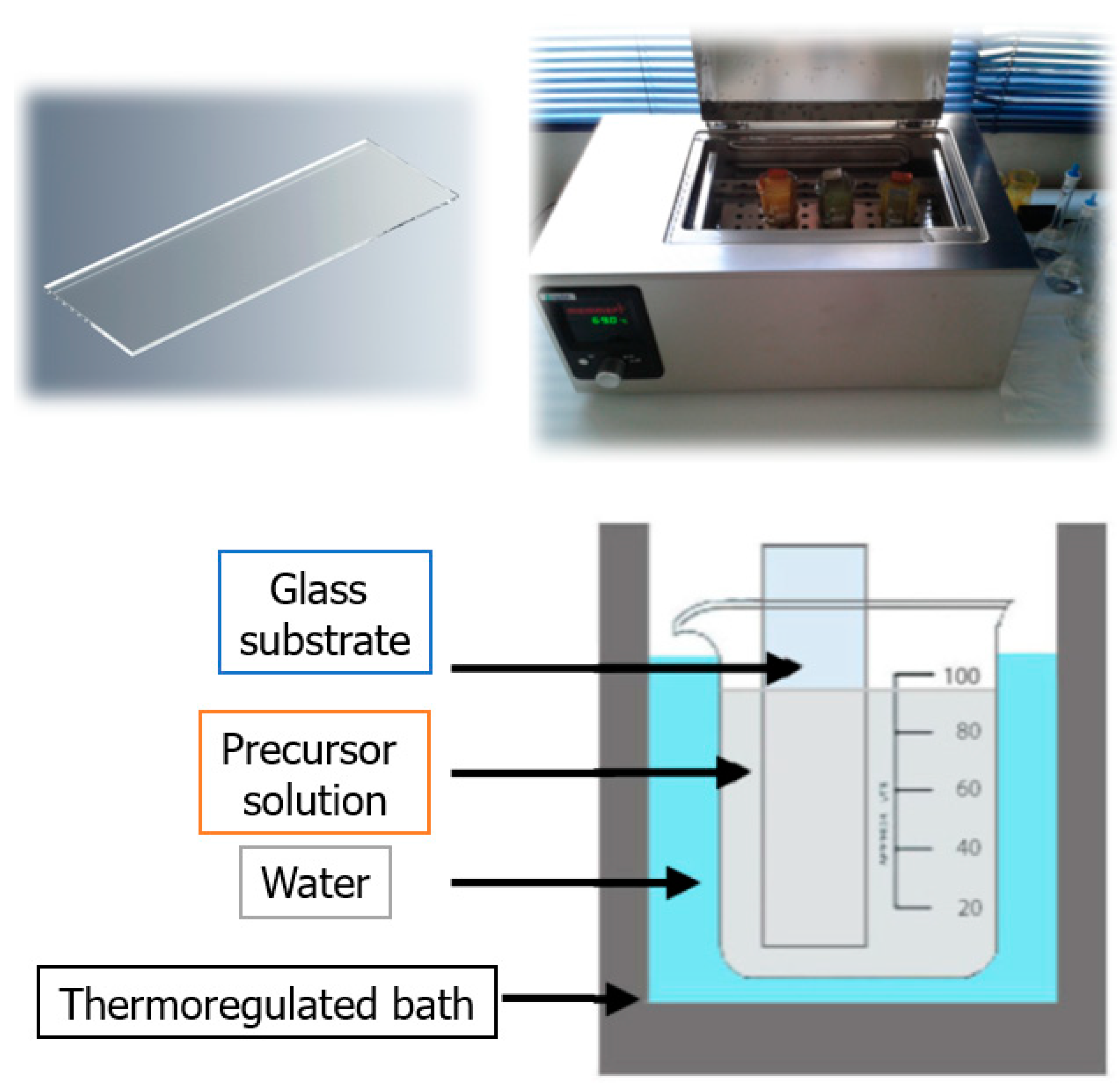
3.2. Sample Preparation
3.3. Characterization Techniques
3.3.1. X-ray Diffraction (XRD) and AFM Studies
3.3.2. Spectroscopy Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera-Molina, D.; Diosa, J.E.; Fernández-Pérez, A.; Mosquera-Vargas, E. Influence of aluminum doping on structural, morphological, vibrational, and optical properties of CdS thin films obtained by chemical bath deposition. Mater. Sci. Eng. B 2021, 273, 115451. [Google Scholar] [CrossRef]
- Contreras-Rascón, J.I.; Días-Reyes, J.; Flores-Pacheco, A.; Serrano-de la Rosa, L.E.; del Ángel-Vicente, P.; Lozada Morales, R.; Álvarez Ramos, M.E.; López-Salazar, P. Enhanced photoluminescence effects in nanostructured cubic CdS matrix doped with Cu2+ obtained by chemical bath deposition. J. Mater. Res. Technol. 2020, 9, 364–372. [Google Scholar] [CrossRef]
- Aboud, A.A.; Mukherjee, A.; Revaprasadu, N.; Mohamed, A.N. The effect of Cu-doping on CdS thin films deposited by the spray pyrolysis technique. J. Mater. Res. Technol. 2019, 8, 2021–2030. [Google Scholar] [CrossRef]
- Dhamodharan, P.; Chen, J.; Manoharan, C. Fabrication of In doped ZnO thin films by spray pyrolysis as photoanode in DSSCs. Surf. Interfaces 2021, 23, 100965. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Navarrete, C.; Muñoz, R.; Baradit, E.; Saavedra, M.; Cabello-Guzmán, G.; Gacitúa, W. Modification of the junction parameters via Al doping in Ag/CdS:Al thin films Schottky diodes for microwave sensors. Mater. Res. Express 2021, 8, 016408. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Muñoz, R.; Vargas, F.; Oviedo, C.; Cabello, G.; Zárate, R.A. Effect of Al-doping on the efficiency of chemically-deposited CdS/PbS thin films solar cells. Chalcogenide Lett. 2021, 18, 367–373. [Google Scholar] [CrossRef]
- Fernández-Pérez, A.; Navarrete, C.; Valenzuela, P.; Gacitúa, W.; Mosquera, E.; Fernández, H. Characterization of chemically deposited aluminum-doped CdS thin films with post-deposition thermal annealing. Thin Solid Films 2017, 623, 127–134. [Google Scholar] [CrossRef]
- Fernández-Perez, A.; Sandoval-Paz, M.G.; Saavedra, R. Photoacoustic study on the optical properties of Aluminium-doped Cadmium Sulphide thin-films. Chalcogenide Lett. 2016, 13, 507–514. [Google Scholar]
- Wilson, K.C.; Basheer Ahamed, M. Influence of bath temperature on surface modification and optoelectronic properties of chemical bath deposited CdS thin film nanostructures. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2019, 251, 114444. [Google Scholar] [CrossRef]
- Nobari, N.; Behboudnia, M.; Maleki, R. Systematic in morphological, structural and optoelectrical properties of nanocrystalline CdS thin films grown by electrodeposition method. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2017, 224, 181–189. [Google Scholar] [CrossRef]
- Willars-Rodríguez, F.J.; Chávez-Urbiola, I.R.; Ramírez-Bon, R.; Vorobiev, P.; Vorobiev, Y.V. Effects of aluminum doping in CdS thin films prepared by CBD and the performance on Schottky diodes TCO/CdS:Al/C. J. Alloy. Compd. 2020, 817, 152740. [Google Scholar] [CrossRef]
- Veerathangam, K.; Pandian, M.S.; Ramasamy, P. Photovoltaic performance of Pb-doped CdS quantum dots for solar cell application. Mater. Lett. 2018, 220, 74–77. [Google Scholar] [CrossRef]
- Manthrammel, M.A.; Shkir, M.; Anis, M.; Shaikh, S.S.; Ali, H.E.; AlFaify, S. Facile spray pyrolysis fabrication of Al:CdS thin films and their key linear and third order nonlinear optical analysis for optoelectronic applications. Opt. Mater. 2020, 100, 109696. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Mohammed, R.Y. The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique. Photonics 2022, 9, 161. [Google Scholar] [CrossRef]
- El Madani, A.; Essajai, R.; Qachaou, A.; Raidou, A.; Fahoume, M.; Lharch, M. The temperature effect on the physical properties of PbS thin films produced by the chemical bath deposition (CBD) technique. Adv. Mater. Process. Technol. 2022, 8, 3413–3424. [Google Scholar] [CrossRef]
- Sengupta, S.; Aggarwal, R.; Raula, M. A review on chemical bath deposition of metal chalcogenide thin films for heterojunction solar cells. J. Mater. Res. 2023, 38, 142–153. [Google Scholar] [CrossRef]
- Najm, A.S.; Naeem, H.S.; Alwarid, D.A.R.M.; Aljuhani, A.; Hasbullah, S.A.; Hasan, H.A.; Sopian, K.; Bais, B.; Al-Iessa, H.J.; Majdi, H.S.; et al. Mechanism of Chemical Bath Deposition of CdS Thin Films: Influence of Sulphur Precursor Concentration on Microstructural and Optoelectronic Characterizations. Coatings 2022, 12, 1400. [Google Scholar] [CrossRef]
- Hodes, G. Chemical Solution Deposition of Semiconductor Films; Marcel Dekker, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Hernández-Contreras, H.; Mejía-García, C.; Contreras-Puente, G. Optical study of the influence of CdCl2 in large area CdS thin films grown by RF-sputtering. Thin Solid Films 2004, 451–452, 203–206. [Google Scholar] [CrossRef]
- Shaha, N.A.; Nazirb, A.; Mahmooda, W.; Syedc, W.A.A.; Buttb, S.; Ali, Z.; Maqsood, A. Physical properties and characterization of Ag doped CdS thin films. J. Alloy. Comp. 2012, 512, 27–32. [Google Scholar] [CrossRef]
- Abdallah, B.; Ismail, A.; Kashoua, H.; Zetoun, W. Effects of Deposition Time on the Morphology, Structure, and Optical Properties of PbS Thin Films Prepared by Chemical Bath Deposition. J. Nanomater. 2018, 2018, 1826959. [Google Scholar] [CrossRef]
- Niesen, T.P.; Calwer, H.; Stetter, W.; Probst, V. Industrial Application of Chemical Bath Deposition: CdS Buffer Layers in Cu(In,Ga)(S,Se)2 Solar Cells; Shell Solar GmbH: Munchen, Germany, 2003; pp. 14–21. [Google Scholar]
- Repins, I.; Contreras, B.; Egaas, C.; Dehart, J.; Scharf, C.; Perkins, B.; Noufi, B. 19.9%-efficient ZnO/CdS/CuInGaSe2 Solar Cell with 81.2% Fill Factor. Prog. Photovolt. Res. Appl. 2008, 16, 235. [Google Scholar] [CrossRef]
- Kumar, A.; Pednekar, D.; Mukherjee, S.; Choubey, R.K. Effect of deposition time and complexing agents on hierarchical nanoflake-structured CdS thin films. J. Mater. Sci. Mater. Electron. 2020, 31, 17055–17066. [Google Scholar] [CrossRef]
- Carrillo-Castillo, A.; Lázaro, A.; Lira Ojeda, E.M.; Martínez-Pérez, C.A.; Quevedo-López, M.A.; Aguirre-Tostado, F.S. Characterization of CdS thin films deposited by chemical bath deposition using novel complexing agents. Chalcogenide Lett. 2013, 10, 421–425. [Google Scholar]
- Khallaf, H.; Oladeji, I.O.; Chow, L. Optimization of chemical bath deposited CdS thin films using nitrilotriacetic acid as a complexing agent. Thin Solid Film. 2008, 516, 5967–5973. [Google Scholar] [CrossRef]
- Rakhshani, A.; Al-Azab, A. Characterization of CdS films prepared by chemical-bath deposition. J. Phys. Condens. Matter 2000, 12, 8745–8755. [Google Scholar] [CrossRef]
- Bairy, R.; Jayarama, A.; Shivakumar, G.K.; Kulkarni, S.D.; Maidur, S.R.; Patil, P.S. Effect of aluminum doping on photoluminescence and third-order nonlinear optical properties of nanostructured CdS thin films for photonic device applications. Phys. B Condens. Matter 2019, 555, 145–151. [Google Scholar] [CrossRef]
- Ijhmayies, S.J.; Ahmad-Bitar, R.N. Effects of processing on the electrical and structural properties of spray deposited CdS:In thin films. Phys. B Condens. Matter 2009, 404, 2419–2424. [Google Scholar] [CrossRef]
- Khallaf, H.; Chai, G.; Lupan, O.; Chow, L.; Park, S.; Schulte, A. Characterization of gallium-doped CdS thin films grown by chemical bath deposition. Appl. Surf. Sci. 2009, 255, 4129–4134. [Google Scholar] [CrossRef]
- Saleem, M.F.; Zhang, H.; Deng, Y.; Wang, D. Resonant Raman scattering in nanocrystalline thin CdS film. J. Raman Spectrosc. 2017, 48, 224–229. [Google Scholar] [CrossRef]
- Khallaf, H.; Chai, G.; Lupan, O.; Chow, L.; Park, S.; Schulte, A. Investigation of aluminum and indium in situ doping of chemical bath deposited CdS thin films. J. Phys. D Appl. Phys. 2008, 41, 185304. [Google Scholar] [CrossRef]
- Zhao, J.; Bardecker, J.A.; Munro, A.M.; Liu, M.S.; Niu, Y.; Ding, I.; Luo, J.; Chen, B.; Jen, A.K.-Y.; Ginger, D.S. Efficient CdSe/CdS Quantum Dot Light-Emitting Diodes Using a Thermally Polymerized Hole Transport Layer. Nano Lett. 2006, 6, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.A.M.; Yahia, I.S.; Fadel, M. Electrical and photovoltaic characteristics of Al/n-CdS Schottky diodes. Int. J. Hydrogen Energy 2009, 34, 4906–4913. [Google Scholar] [CrossRef]
- Tomakin, M.; Altunba, M.; Bacaks, E. The influence of substrate temperature on electrical properties of Cu/CdS/SnO2 Schottky diode. Phys. B Condens. Matter 2011, 406, 4355–4360. [Google Scholar] [CrossRef]
- Halge, D.I.; Narwade, V.N.; Khanzode, P.M.; Begum, S.; Banerjee, I.; Dadge, J.W.; Kovac, J.; Rana, A.S.; Bogle, K.A. Development of highly sensitive and ultra-fast visible-light photodetector using nano-CdS thin film. Appl. Phys. A 2021, 127, 446. [Google Scholar] [CrossRef]
- Mosquera, E.; Rojas-Michea, C.; Morel, M.; Gracia, F.; Fuenzalida, V.; Zárate, R.A. Zinc oxide nanoparticles with incorporated silver: Structural, morphological, optical and vibrational properties. Appl. Surf. Sci. 2015, 347, 561–568. [Google Scholar] [CrossRef]
- Yilmaz, S.; Atasoy, Y.; Tomakin, M.; Bacaks, E. Comparative studies of CdS, CdS:Al, CdS:Na, and CdS:(Al-Na) thin films prepared by spray pyrolysis. Supperlattices Microstruct. 2015, 88, 299–307. [Google Scholar] [CrossRef]
- Song, G.L.; Guo, S.; Wang, X.X.; Li, Z.S.; Zou, B.S.; Fan, H.M.; Liu, R.B. Temperature dependent Raman and photoluminescence of an individual Sn-doped CdS branched nanostructure. New J. Phys. 2015, 17, 063024. [Google Scholar] [CrossRef]
- Murali, G.; Amaranatha Reddy, D.; Giribabu, G.; Vijayalakshmi, R.P.; Venugapal, R. Room temperature ferromagnetism in Mn doped CdS nanowires. J. Alloy. Compd. 2013, 581, 849–855. [Google Scholar] [CrossRef]
- Kulkarni, R.; Pawbake, A.; Waykar, R.; Jadhawar, A.; Borate, H.; Aher, R.; Bhorde, A.; Nair, S.; Sharma, P.; Jadkar, S. Single crystal, high band gap CdS thin films grown by RF magnetron sputtering in argon atmosphere for solar cell applications. J. Nano Electron. Phys. 2018, 10, 03005. [Google Scholar] [CrossRef]
- Chi, T.T.K.; Gouadec, G.; Colomban, P.; Wang, G.; Mazerolles, L.; Liem, N.Q. Off-resonance Raman analysis of wurtzite CdS ground to the nanoscale: Structural ans size-related effects. J. Raman Spectrosc. 2011, 42, 1007–1015. [Google Scholar] [CrossRef]
- Fan, H.M.; Ni, Z.H.; Feng, Y.P.; Fan, X.F.; Kuo, J.L.; Shen, Z.X.; Zou, B.S. Anisotropy of electron -phonon coupling in single wurtzite CdS nanowires. Appl. Phys. Lett. 2007, 91, 171911. [Google Scholar] [CrossRef]
- Escobelo Morales, A.; Sánchez Mora, E.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. Fis. 2007, S53, 18–22. [Google Scholar]
- Khorsand Zak, A.; Razali, R.; Abd Majid, W.H.; Darroudi, M. Synthesis and characterization of a narrow size distribution of zinc oxide nanoparticles. Int. J. Nanomed. 2011, 6, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; Wang, Y.; Xue, C.; Liang, J.; Jiang, G.; Lu, W.; Zhu, C. Formation of Cu2ZnSnS4 thin film solar cell by CBD-annealing route: Comparision of Cu and CuS in stacked layer SnS/Cu(S)/ZnS. Solar Energy 2016, 129, 1–9. [Google Scholar] [CrossRef]
- Ahmad-Bitar, R.N. Photoluminescence spectra of doped and undoped CdS films prepared by spray pyrolysis. In Proceedings of the IEEE Tenth International Conference on Microelectronics (Cat. No.98EX186), Monastir, Tunisia, 16 December 1998; pp. 127–130. [Google Scholar] [CrossRef]

| Thin Film R = [Al]/[Cd] | Crystallite Size D ± 1 (nm) | Thickness (nm) | Strain ε ± 0.8 (× 10−4) | Refraction Index N | Optical Energy Bandgap Egopt (eV) | |
|---|---|---|---|---|---|---|
| K-M | 1st Derivative | |||||
| 0.30 | 12 | 173 | 31.3 | 2.0(7) | 2.5(6) | 2.6(4) |
| 0.40 | 11 | 213 | 40.5 | 1.7(8) | 2.5(8) | 2.6(7) |
| 0.50 | 14 | 193 | 29.7 | 1.4(4) | 2.5(5) | 2.6(5) |
| Raman Shift, vexp (cm– 1) | Symmetry | |||||
|---|---|---|---|---|---|---|
| Ref. [1] | Ref. [36] | Ref. [37] | R = 0.30 | 0.40 | 0.50 | |
| 304 | 301 | 306–308 | 302.8 | 303.7 | 302.8 | (1LO) |
| 473.7 | 470 | - | 485.5 | 477 | 458 | (2LO) multiphonon Raman scattering |
| 564 | - | 556–560 | 561.8 | 571.1 | 563 | |
| - | - | - | 599.2 | - | 602.7 | |
| 620.3 | 640 | - | 630.5 | 640 | 618 | |
| 685.7 | 670 | - | 688.3 | - | 635.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasanna-Kumari, R.; Herrera-Molina, D.; Fernández-Pérez, A.; Diosa, J.E.; Mosquera-Vargas, E. Study of the Properties of CdS:Al (R = [Al3+]/[Cd2+] = 0.30, 0.40, 0.50) Thin Films Grown by the CBD Method in an Ammonia-Free System. Molecules 2023, 28, 3626. https://doi.org/10.3390/molecules28083626
Prasanna-Kumari R, Herrera-Molina D, Fernández-Pérez A, Diosa JE, Mosquera-Vargas E. Study of the Properties of CdS:Al (R = [Al3+]/[Cd2+] = 0.30, 0.40, 0.50) Thin Films Grown by the CBD Method in an Ammonia-Free System. Molecules. 2023; 28(8):3626. https://doi.org/10.3390/molecules28083626
Chicago/Turabian StylePrasanna-Kumari, Raju, Daniela Herrera-Molina, Arturo Fernández-Pérez, Jesús E. Diosa, and Edgar Mosquera-Vargas. 2023. "Study of the Properties of CdS:Al (R = [Al3+]/[Cd2+] = 0.30, 0.40, 0.50) Thin Films Grown by the CBD Method in an Ammonia-Free System" Molecules 28, no. 8: 3626. https://doi.org/10.3390/molecules28083626
APA StylePrasanna-Kumari, R., Herrera-Molina, D., Fernández-Pérez, A., Diosa, J. E., & Mosquera-Vargas, E. (2023). Study of the Properties of CdS:Al (R = [Al3+]/[Cd2+] = 0.30, 0.40, 0.50) Thin Films Grown by the CBD Method in an Ammonia-Free System. Molecules, 28(8), 3626. https://doi.org/10.3390/molecules28083626







