Full Regio- and Stereoselective Protocol for the Synthesis of New Nicotinoids via Cycloaddition Processes with the Participation of Trans-Substituted Nitroethenes: Comprehensive Experimental and MEDT Study
Abstract
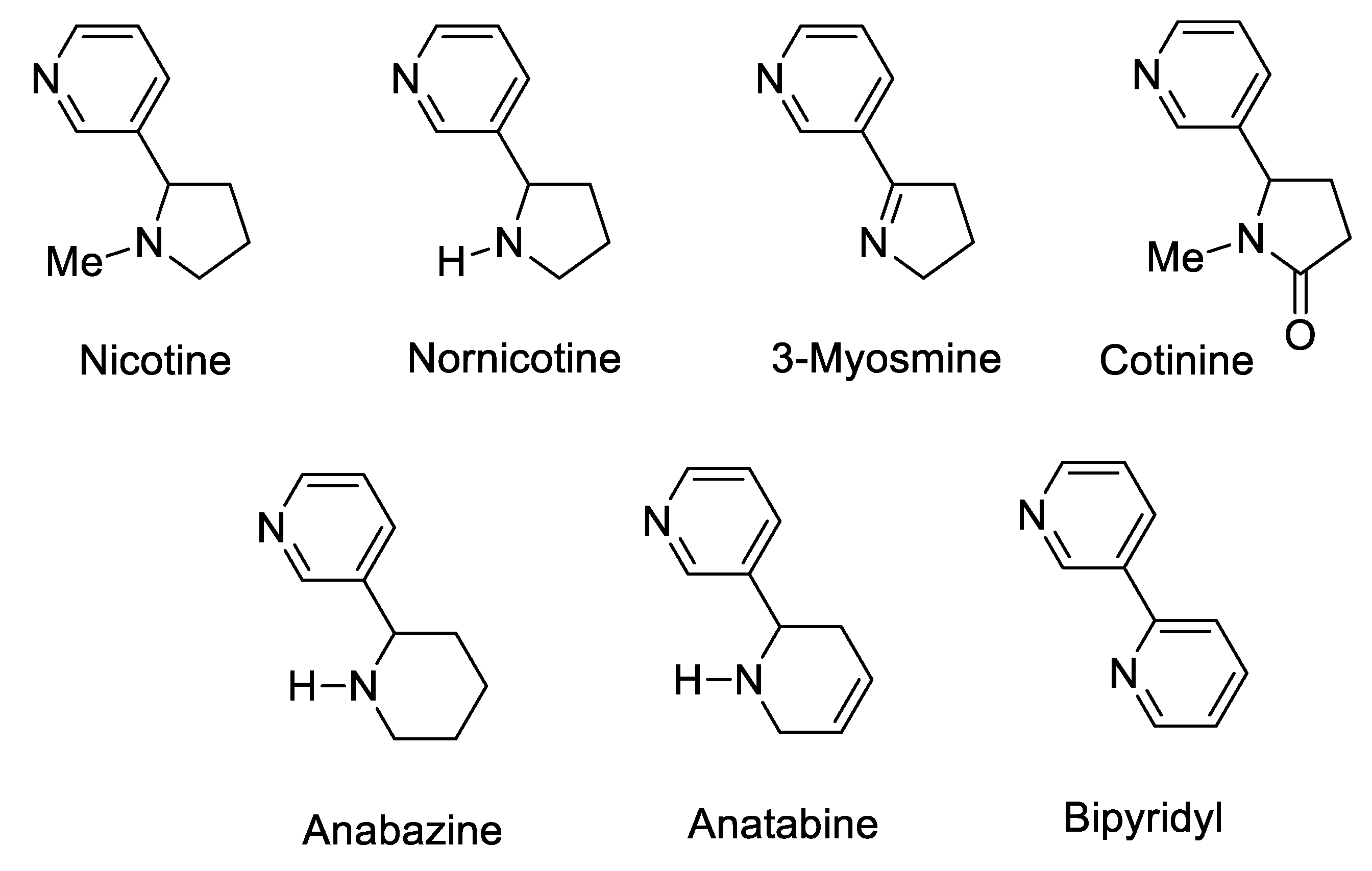
1. Introduction
2. Results
2.1. Analysis of the Conceptual DFT Reactivity Descriptors
2.2. BET Mechanistic Investigations
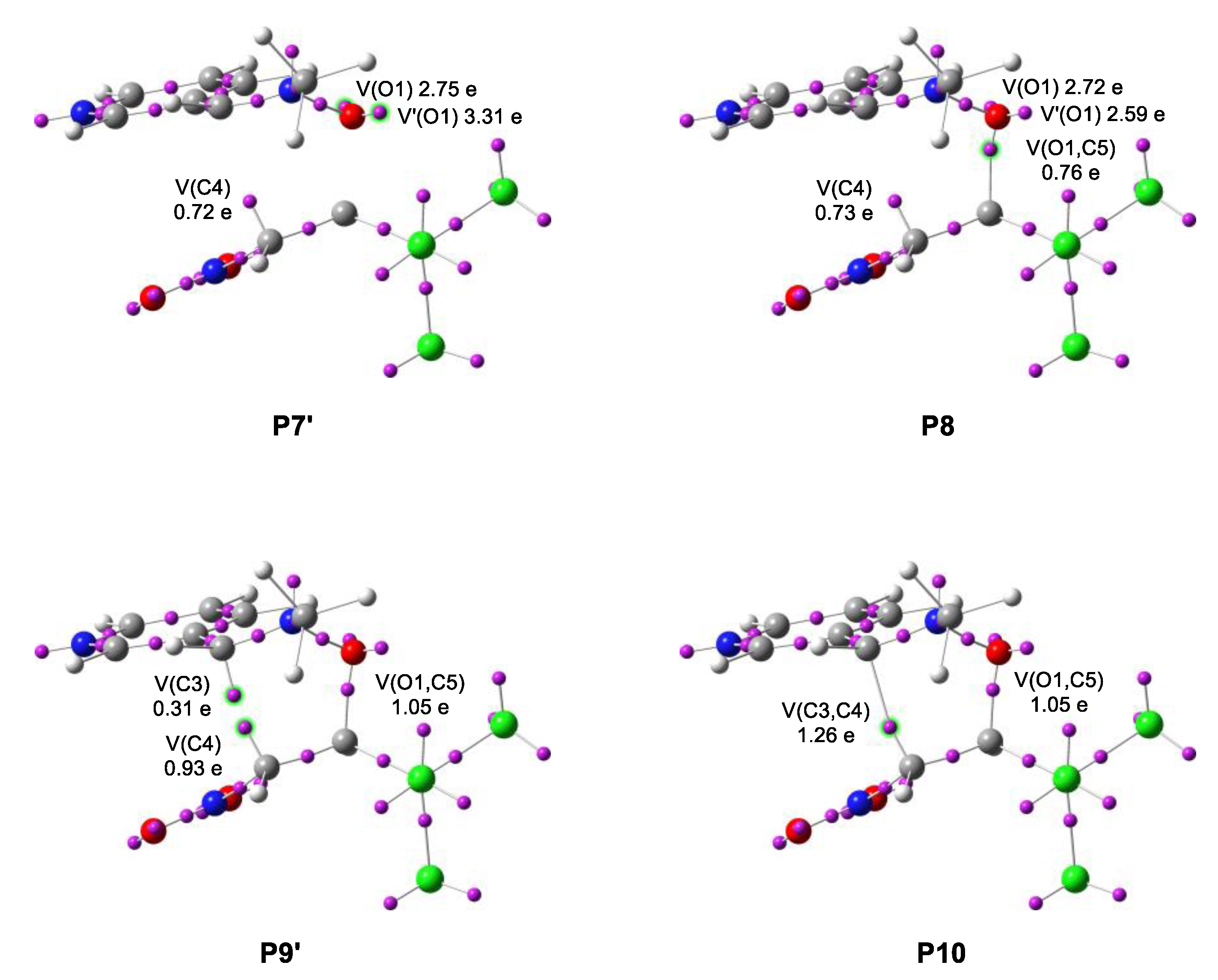
- From phase I to phase IV, the topological changes leading to the creation of the pseudoradical center [33] on C10 are taking place. In structures P1 and P2, the V(N13) and V’(N13) monosynaptic basins respectively vanish, increasing the population in the V(C4,N13) disynaptic basin. Then, the V’(C4,C5) disynaptic basin of the double bond merges with V(C4,C5), increasing its integration in P3. At the same time, the N2-O1 bond becomes depopulated, transferring 0.12 e to the V(C3,N2) disynaptic basin. These changes contribute most to the energetic cost, 11.0 kcal∙mol−1, which is only 1.1 kcal∙mol−1 lower than the TS (coincidentally being the P7 structure);
- At the start of phase V, the pseudoradical center at C4 is created from the electron density of the C4-C5 bond, with an initial population of 0.44 e, after which the V(C4,C5) disynaptic basin is further depopulated, increasing the V(C4) monosynaptic basins integration;
- Then, the V(N2) monosynaptic basin representing nonbonding electron density is created at P5 with an initial population of 0.90 e originating from the V(C3,N2) basin. The V(N2) starts with only 39% of its final population, to which the V(C3,N2) disynaptic basin contributes further in the leading phases, becoming an underpopulated double bond;
- At P6, the V(C5) monosynaptic basin is created from the population of disynaptic basin V(C4,C5), only integrating 0.07 e for a duration of a short phase VII, after which it disappears. At P8, a new bond is created, at an O-C distance of 1.658 Å, by the donation of nonbonding electron density of O1. The new V(O1,C5) disynaptic basin starts with a population of 0.76 e, ultimately increasing to 1.29 e at the cost of the nonbonding electron density of O1 and the V(N2,O1) disynaptic basins integration. The N2-O1 single bond becomes strongly underpopulated with its final integration of 0.94 e;
- Phase X starts with the creation of a pseudoradical center at C3 with a population of 0.12 e originating from the disynaptic basin V(C3,N2). Then, at P10, the C3-C4 single bond is created by the coupling of C3 and C4 pseudoradical centers, integrating 0.31 and 0.93 e, respectively, just before the event.
- The formation of the second C3-C4 single bond begins while the first O1-C5 single bond has reached 81% of its final population. Therefore, the mechanism of 32CA of nitrone 1 and nitroalkene 2a proceeds by a two-stage one-step mechanism [34].
3. Materials and Methods
3.1. Materials
3.2. Analytical Techniques
3.3. Cycloaddition between Z-C-(3-pyridyl)-N-methylnitrone and Nitroalkenes–General Procedure
3.4. ELF Computational Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lapczuk, A. The [3 + 2] cycloaddition reaction as an attractive way for the preparation of nicotine analogs. Chem. Heterocycl. Compd. 2023, 59, 109. [Google Scholar]
- Kavita, G.; Rashmi, K. Nicotine. Carcinogenicity and Effects on Response to Cancer Treatment–A Review. Asian Pac. J. Cancer Prev. 2015, 16, 5987–5992. [Google Scholar]
- Yamamoto, I.; Casida, J.E. Nicotinoid Insecticides and the Nicotinic Acetylcholine Receptor; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Casida, J.E. Neonicotinoids and Other Insect Nicotinic Receptor Competitive Modulators: Progress and Prospects. Annu. Rev. 2018, 63, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Fatima, E.O.; Mohamed, A.D.; Mohammed, B. Synthesis, characterization, molecular docking and in-vitro anticancer evaluation of new thiourea derivatives. Mon. Für Chem. Chem. Mon. 2022, 153, 1083–1093. [Google Scholar]
- Ahmed, A.; Fatima, Z.C.; Abdelmalek, L. Microwave-assisted synthesis of some new thiophene and pyrazole derivatives and their molecular docking study. Mon. Für Chem. Chem. Mon. 2022, 153, 687–697. [Google Scholar]
- Karima, B.; Hajar, T.; Mohammed, B. Synthesis, characterization, and computational study of some new indole derivatives as potential anticancer agents. Heterocycl. Commun. 2022, 22, 17–25. [Google Scholar]
- Jasiński, R. On the Question of Stepwise [4 + 2] Cycloaddition Reactions and Their Stereochemical Aspects. Symmetry 2021, 13, 1911. [Google Scholar] [CrossRef]
- Siadati, S.A.; Rezazadeh, S. The extraordinary gravity of three atom 4π-components and 1,3-dienes to C20-nXn fullerenes; a new gate to the future of Nano technology. Sci. Radices 2022, 1, 46–68. [Google Scholar] [CrossRef]
- Rios-Gutierez, M.; Domingo, L.R.; Jasinski, R. Unveiling the High Reactivity of Experimental Pseudodiradical Azomethine Ylides within Molecular Electron Density Theory. Phys. Chem. Chem. Phys. 2023, 25, 314. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Barakat, A.; Domingo, L.R. A Molecular Electron Density Theory Study of the [3 + 2] Cycloaddition Reaction of Pseudo(mono)radical Azomethine Ylides with Phenyl Vinyl Sulphone. Organics 2022, 3, 122–136. [Google Scholar] [CrossRef]
- Kacka-Zych, A.; Jasinski, R. Mechanistic aspects of the synthesis of seven-membered internal nitronates via stepwise [4 + 3] cycloaddition involving conjugated nitroalkenes: Molecular Electron Density Theory computational study. J. Comput. Chem. 2022, 43, 1221. [Google Scholar] [CrossRef] [PubMed]
- Alessandra, P.; Maria, M.C.; Silvia, B. Synthesis, Characterization, and Molecular Docking of Novel Pyridyl Derivatives as COX-2 Inhibitors. Pure Appl. Chem. 2020, 92, 1511–1525. [Google Scholar]
- Kacka-Zych, A. Understanding the uniqueness of the stepwise [4 + 1] cycloaddition reaction between conjugated nitroalkenes and electrophilic carbene systems with a molecular electron density theory perspective. Int. J. Quantum Chem. 2021, 121, e26440. [Google Scholar] [CrossRef]
- Silvi, B.S.A. Nature of the Chemical Bond and the Structure of Molecular Orbitals from a Density Functional Theory Viewpoint. Molecules 1994, 371, 683–686. [Google Scholar]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Natural Atomic Orbitals and Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Liu, S. Conceptual Density Functional Theory: Towards a New Chemical Reactivity Theory; WILEY: Hoboken, NJ, USA, 2022; Volume 2. [Google Scholar]
- Sadowski, M.; Utnicka, J.; Wójtowicz, A.; Kula, K. The global and local Reactivity of C,N-diarylnitryle imines in [3 + 2] cycloaddition processes with trans-β-nitrostyrene according to Molecular Electron Density Theory: A computational study. Curr. Chem. Lett. 2023, 12, 421–430. [Google Scholar] [CrossRef]
- Woliński, P.; Kącka-Zych, A.; Mirosław, B.; Wielgus, E.; Olszewska, A.; Jasiński, R. Green, one-pot synthesis of 1,2-oxazine-type herbicides via non catalyzed Hetero Diels-Alder reactions comprising (2E)-3-aryl-2-nitroprop-2-enenitriles. J. Clean. Prod. 2022, 356, 131878. [Google Scholar] [CrossRef]
- Kula, K.; Kącka-Zych, A.; Łapczuk-Krygier, A.; Jasiński, R. Analysis of the possibility and molecular mechanism of carbon dioxide consumption in the Diels-Alder processes. Pure Appl. Chem. 2021, 93, 427–446. [Google Scholar] [CrossRef]
- Kula, K.; Zawadzińska, K. Local nucleophile-electrophile interactions in [3 + 2] cycloaddition reactions between benzonitrile N-oxide and selected conjugated nitroalkenes in the light of MEDT computational study. Curr. Chem. Lett. 2021, 10, 9–16. [Google Scholar] [CrossRef]
- Kącka-Zych, A.; Jasiński, R. Molecular mechanism of Hetero Diels-Alder reactions between (E)-1,1,1-trifluoro-3-nitrobut-2-enes and enamine systems in the light of Molecular Electron Density Theory. J. Mol. Graph. Model. 2020, 101, 107714. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M. A Useful Classification of Organic Reactions Based on the Flux of the Electron Density. SciRad 2023, 2, 1–24. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A molecular electron density theory study of the participation of tetrazines in aza-Diels–Alder reactions. RSC Adv. 2020, 10, 15394–15405. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. Applications of the Conceptual Density Functional Theory Indices to Organic Chemistry Reactivity. Molecules 2016, 21, 748. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Perez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R.; Ghodsi, F. Unveiling the Different Reactivity of Bent and Linear Three-Atom-Components Participating in [3 + 2] Cycloaddition Reactions. Organics 2021, 2, 274–286. [Google Scholar] [CrossRef]
- Jasiński, R.; Ziółkowska, M.; Demchuk, O.M.; Maziarka, A. Regio- and stereoselectivity of polar [2 + 3] cycloaddition reactions between (Z)-C-(3,4,5-trimethoxyphenyl)-N-methylnitrone and selected (E)-2-substituted nitroethenes. Cent. Eur. J. Chem. 2014, 12, 586–593. [Google Scholar] [CrossRef]
- Jasiński, R. A stepwise, zwitterionic mechanism for the 1,3-dipolar cycloaddition between (Z)-C-4-methoxyphenyl-N-phenylnitrone and gem-chloronitroethene catalysed by 1-butyl-3-methylimidazolium ionic liquid cations. Tetrahedron Lett. 2015, 56, 532–535. [Google Scholar] [CrossRef]
- Jasiński, R.; Żmigrodzka, M.; Dresler, E.; Kula, K. A Full Regioselective and Stereoselective Synthesis of 4-Nitroisoxazolidines via Stepwise [3 + 2] Cycloaddition Reactions between (Z)-C-(9-Anthryl)-N-arylnitrones and (E)-3,3,3-Trichloro-1-nitroprop-1-ene: Comprehensive Experimental and Theoretical Study. J. Heterocyclic Chem. 2017, 54, 3314–3320. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gadocha, Z.; Pabian, K.; Wróblewska, A.; Wielgus, E.; Jasiński, R. The First Examples of [3 + 2] Cycloadditions with the Participation of (E)-3,3,3-Tribromo-1-Nitroprop-1-Ene. Materials 2022, 15, 7584. [Google Scholar] [CrossRef]
- Domingo, L.R.; Saez, J.A. Understanding the Electronic Reorganization along the Nonpolar [3 + 2] Cycloaddition Reactions of Carbonyl Ylides. J. Org. Chem. 2011, 76, 373–379. [Google Scholar] [CrossRef]
- Domingo, L.R.; Saez, J.A.; Zaragoza, R.J.; Arno, M. Understanding Bond Formation in Polar One-Step Reactions. Topological Analyses of the Reaction between Nitrones and Lithium Ynolates. J. Org. Chem. 2008, 73, 8791–8799. [Google Scholar] [CrossRef]
- Domingo, L.R. A New C-C Bond Formation Model Based on the Quantum Chemical Topology of Electron Density. RSC Adv. 2014, 4, 32415–32428. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Bonding Evolution Theory Study of the Mechanism of [3 + 2] Cycloaddition Reactions of Nitrones with Electron-Deficient Ethylenes. RSC Adv. 2015, 5, 58464–58477. [Google Scholar]
- Jasiński, R.; Dresler, E.; Mikulska, M.; Polewski, D. [3 + 2] Cycloadditions of 1-halo-1-nitroethenes with (Z)-C-(3,4,5-trimethoxyphenyl)-N-methyl-nitrone as regio- and stereocontrolled source of novel bioactive compounds: Preliminary studies. Curr. Chem. Lett. 2016, 5, 123–128. [Google Scholar] [CrossRef]
- Zawadzińska, K.; Gaurav, G.K.; Jasiński, R. Preparation of conjugated nitroalkenes: Short review. Sci. Radices 2022, 1, 69–83. [Google Scholar] [CrossRef]
- Jasinski, R.; Mróz, K. Kinetic aspects of [3 + 2] cycloaddition reactions between (E)-3,3,3-trichloro-1-nitroprop-1-ene and ketonitrones. React. Kinet. Mech. Catal. 2015, 116, 35–41. [Google Scholar] [CrossRef]
- Jasinski, R.; Mróz, K.; Kacka, A. Experimental and theoretical DFT study on synthesis of sterically crowded 2,3,3,(4)5-tetrasubstituted-4-nitroisoxazolidines via 1,3-dipolar cycloaddition reactions between ketonitrones and conjugated nitroalkenes. J. Heterocyclic Chem. 2016, 53, 1424–1429. [Google Scholar] [CrossRef]
- Chai, J.D.; Head-Gordon, M. Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys. Chem. Chem. Phys. 2008, 10, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Scalmani, G.; Frisch, M.G. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110. [Google Scholar] [CrossRef]
- Kula, K.; Sadowski, M. Regio- and stereoselectivity of [3 + 2] cycloaddition reactions between (Z)-C-(9-anthryl)-N-methylnitrone and analogues of trans- -nitrostyrene in the light of MEDT computational study. Chem. Heterocycl. Compd. 2023, 59, 138. [Google Scholar]
- Reed, A.E.; Weinstock, R.B.; Weinhold, F. Theoretical Study of the Reaction of (2, 2)-Dichloro (Ethyl) Arylphosphine with Bis (2, 2)-Dichloro (Ethyl) Arylphosphine by Hydrophosphination Regioselective by the DFT Method. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Noury, S.; Krokidis, X.; Fuster, F.; Silvi, B. How Malonaldehyde Bonds Change during Proton Transfer. Comput. Chem. 1999, 23, 597–604. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, 6th ed.; Semichem Inc.: Shawnee Mission, KS, USA, 2016. [Google Scholar]

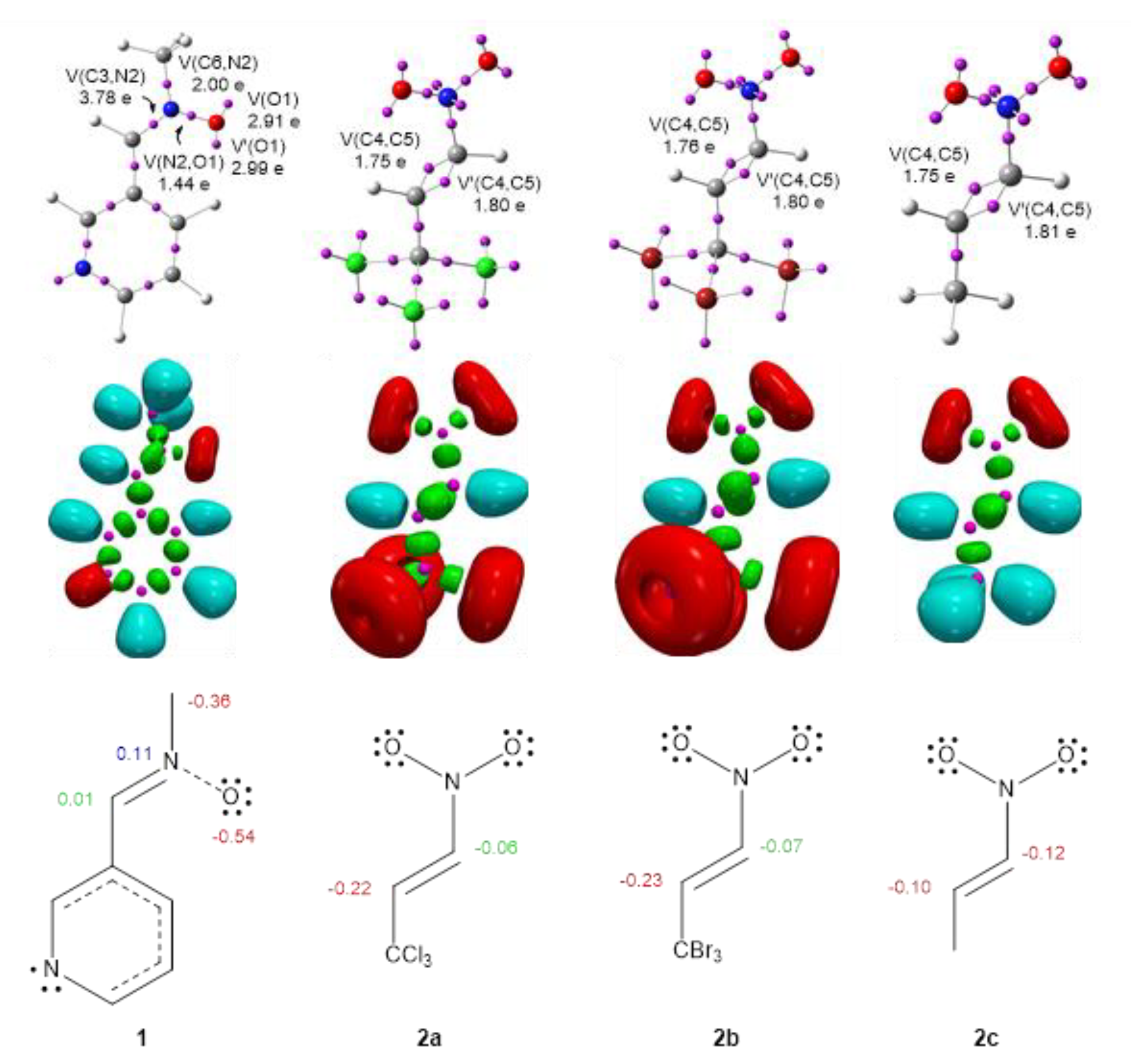
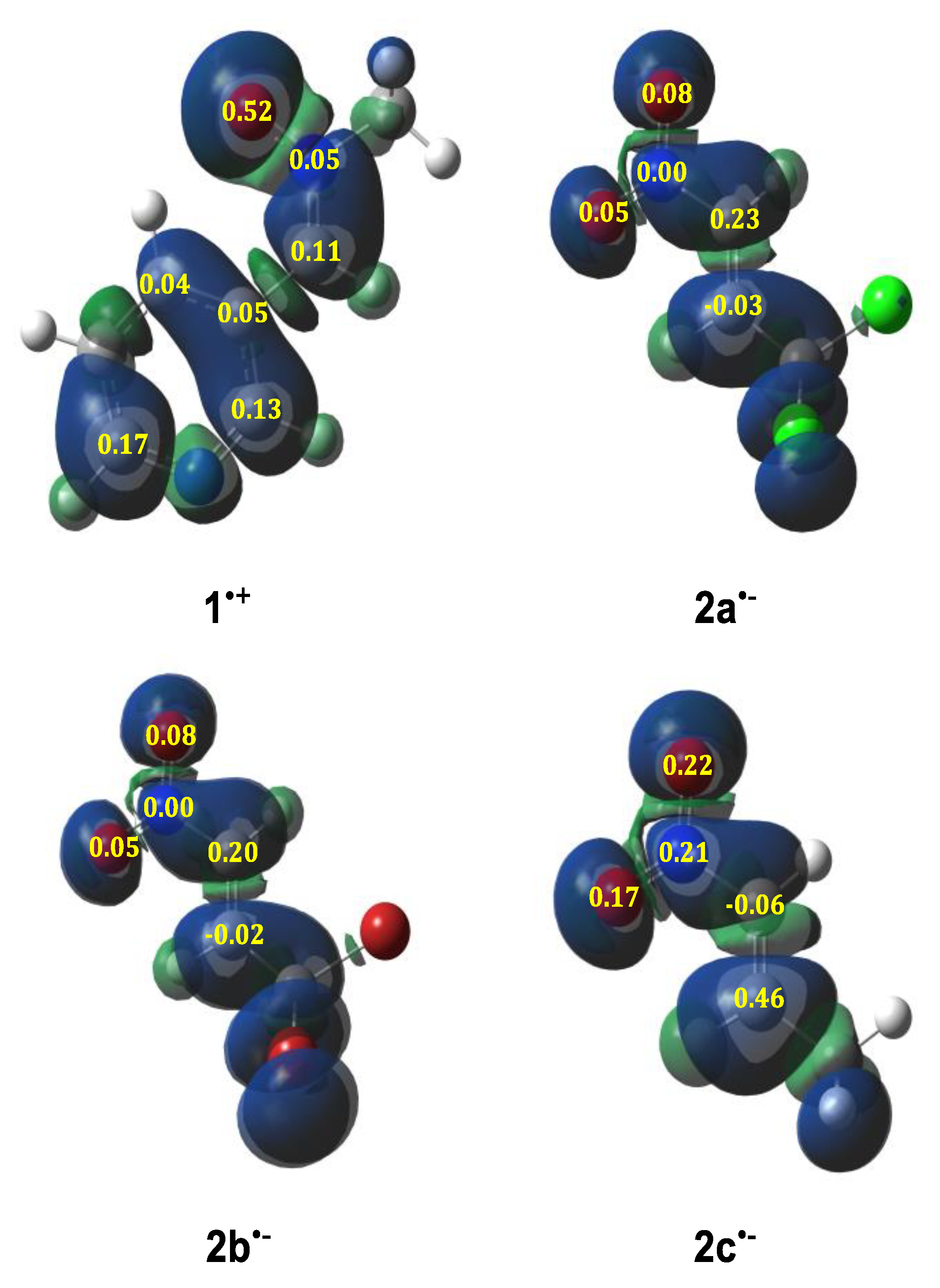

| μ | η | ω | N | |
|---|---|---|---|---|
| 3,3,3-trichloro-1-nitropropene 2a | −5.74 | 5.35 | 3.07 | 0.69 |
| 3,3,3-tribromo-1-nitropropene 2b | −5.50 | 5.03 | 3.00 | 1.09 |
| 1-nitropropene 2c | −5.00 | 5.59 | 2.23 | 1.32 |
| N-methyl-C-3-pyridylnitrone 1 | −3.65 | 4.33 | 1.53 | 3.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kras, J.; Woliński, P.; Nagatsky, R.; Demchuk, O.M.; Jasiński, R. Full Regio- and Stereoselective Protocol for the Synthesis of New Nicotinoids via Cycloaddition Processes with the Participation of Trans-Substituted Nitroethenes: Comprehensive Experimental and MEDT Study. Molecules 2023, 28, 3535. https://doi.org/10.3390/molecules28083535
Kras J, Woliński P, Nagatsky R, Demchuk OM, Jasiński R. Full Regio- and Stereoselective Protocol for the Synthesis of New Nicotinoids via Cycloaddition Processes with the Participation of Trans-Substituted Nitroethenes: Comprehensive Experimental and MEDT Study. Molecules. 2023; 28(8):3535. https://doi.org/10.3390/molecules28083535
Chicago/Turabian StyleKras, Jowita, Przemysław Woliński, Roman Nagatsky, Oleg M. Demchuk, and Radomir Jasiński. 2023. "Full Regio- and Stereoselective Protocol for the Synthesis of New Nicotinoids via Cycloaddition Processes with the Participation of Trans-Substituted Nitroethenes: Comprehensive Experimental and MEDT Study" Molecules 28, no. 8: 3535. https://doi.org/10.3390/molecules28083535
APA StyleKras, J., Woliński, P., Nagatsky, R., Demchuk, O. M., & Jasiński, R. (2023). Full Regio- and Stereoselective Protocol for the Synthesis of New Nicotinoids via Cycloaddition Processes with the Participation of Trans-Substituted Nitroethenes: Comprehensive Experimental and MEDT Study. Molecules, 28(8), 3535. https://doi.org/10.3390/molecules28083535








