In Vitro α-Amylase and α-Glucosidase Inhibitory Effects, Antioxidant Activities, and Lutein Content of Nine Different Cultivars of Marigold Flowers (Tagetes spp.)
Abstract
1. Introduction
2. Results
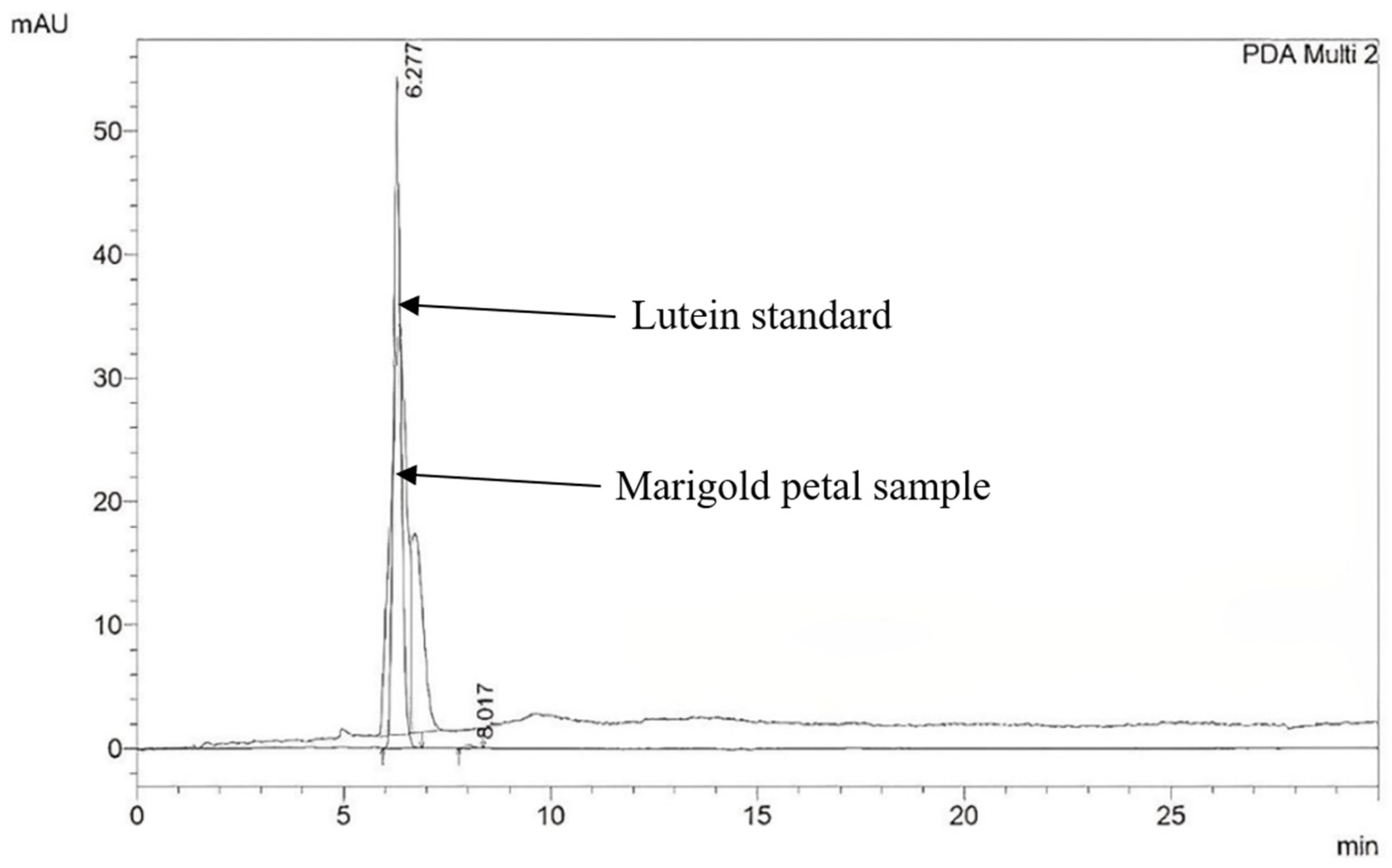
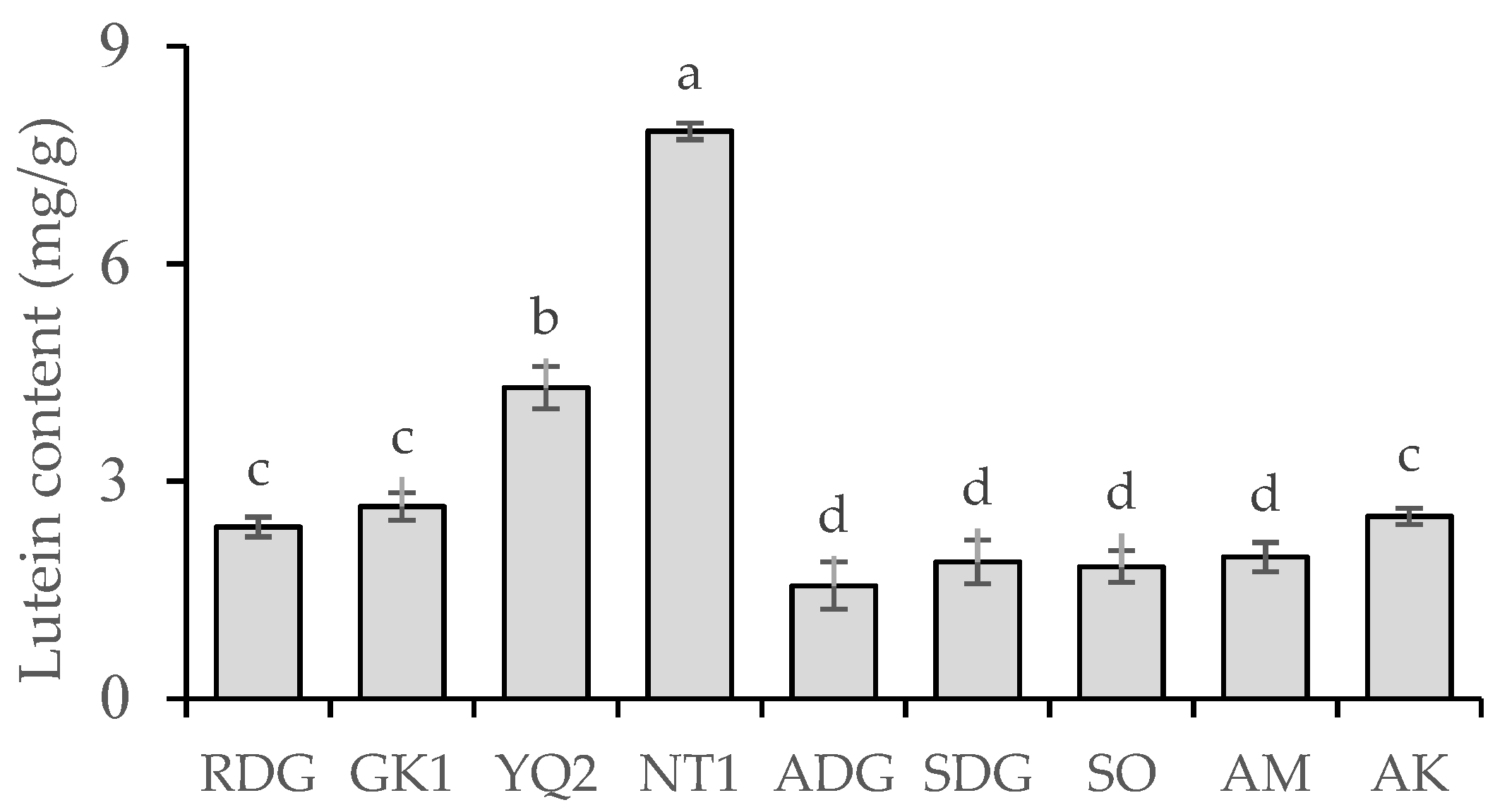
2.1. Bioactive Compound Contents in Nine Marigold Cultivars
2.2. Antioxidant Activities of Nine Marigold Cultivars
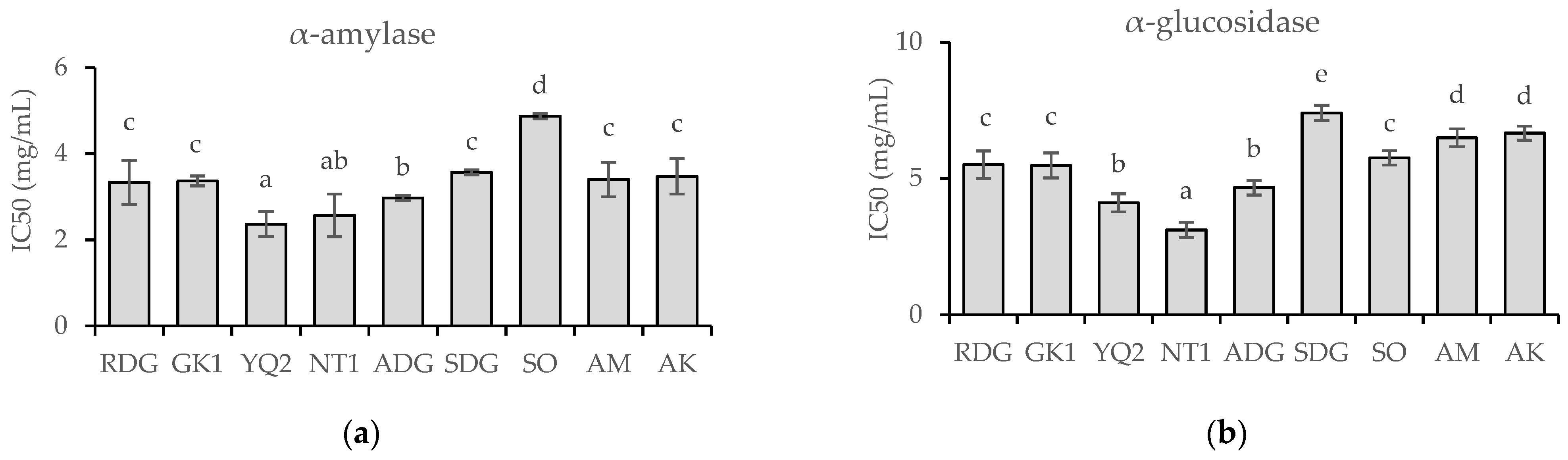
2.3. In Vitro α-Amylase and α-Glucosidase Inhibitory Effects of Nine Marigold Cultivars
2.4. Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Preparation of Marigold Petals
4.2. Determination of Phytochemical Contents
4.3. Determination of Antioxidant Activities
4.4. In Vitro Antidiabetic Activity
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nanditha, A.; Ma, R.C.W.; Ramachandran, A.; Snehalatha, C.; Chan, J.C.N.; Chia, K.S.; Shaw, J.E.; Zimmet, P.Z. Diabetes in Asia and the Pacific: Implications for the global epidemic. Diabetes Care 2016, 39, 472–485. [Google Scholar] [CrossRef]
- Nolan, C.J.; Prentki, M. Insulin resistance and insulin hypersecretion in the metabolic syndrome and type 2 diabetes: Time for a conceptual framework shift. Diab. Vasc. Dis. Res. 2019, 16, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2010, 51, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Jahangir, M.; Hussan, S.S.; Choudhary, M.I. Inhibition of α-glucosidase by oleanolic acid and its synthetic derivatives. Phytochemistry 2002, 60, 295–299. [Google Scholar] [CrossRef]
- Cheplick, S.; Kwon, Y.; Bhowmik, P.; Shetty, K. Phenolic-linked variation in strawberry cultivars for potential dietary management of hyperglycemia and related complications of hypertension. Bioresour. Technol. 2010, 101, 404–413. [Google Scholar] [CrossRef]
- Ortiz-Andrade, R.R.; García-Jiménez, S.; Castillo-España, P.; Ramírez-Avila, G.; Villalobos-Molina, R.; Estrada-Soto, S. Alpha-glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: An anti-hyperglycemic agent. J. Ethnopharmacol. 2007, 109, 48–53. [Google Scholar] [CrossRef]
- Shirwaikar, A.; Rajendran, K.; Punitha, I.S. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J. Ethnopharmacol. 2005, 97, 369–374. [Google Scholar] [CrossRef]
- Pires, T.C.; Dias, M.I.; Barros, L.; Calhelha, R.C.; Alves, M.J.; Oliveira, M.B.; Santos-Buelga, C.; Ferreira, I.C. Edible flowers as sources of phenolic compounds with bioactive potential. Food Res. Int. 2018, 105, 580–588. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edibles flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Mokrani, A.; Krisa, S.; Cluzet, S.; Da Costa, G.; Temsamani, H.; Renouf, E.; Mérillon, J.M.; Madani, K.; Mesnil, M.; Monvoisin, A.; et al. Phenolic contents and bioactive potential of peach fruit extracts. Food Chem. 2016, 202, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Banwo, K.; Sanni, A.; Sarkar, D.; Ale, O.; Shetty, K. Phenolics-Linked Antioxidant and Anti-hyperglycemic Properties of Edible Roselle (Hibiscus sabdariffa Linn.) Calyces Targeting Type 2 Diabetes Nutraceutical Benefits in vitro. Front. Sustain. Food Syst. 2022, 6, 1–13. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Piccaglia, R.; Marotti, M.; Grandi, S. Lutein and lutein ester content in different types of Tagetes patula and T. erecta. Ind. Crops Prod. 1998, 8, 45–51. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.H.; Xu, H.G.; Yuan, F.; Gao, Y.X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, Y.; Zhao, J.; Wang, Q. Phenolic, flavonoid, and lutein ester content and antioxidant activity of 11 cultivars of Chinese marigold. J. Agric. Food Chem. 2007, 55, 8478–8484. [Google Scholar] [CrossRef]
- Hojnik, M.; Škerget, M.; Knez, Ž. Extraction of lutein from marigold flower petals-experimental kinetics and modelling. LWT-Food Sci. Technol. 2008, 41, 2008–2016. [Google Scholar] [CrossRef]
- Kusmiati; Caesarianto, W.; Afiati, F.; Hutabarat, R. Effect lutein of marigold flower (Tagetes erecta L.) on decreasing glucose and malondialdehyde levels in Alloxan-induced blood mice. AIP Conf. Proc. 2019, 2120, 070009. [Google Scholar] [CrossRef]
- Rodda, R.; Avvari, S.K.; Chidrawar, V.R.; Reddy, T.R. Pharmacological screening of synergistic antidiabetic efficacy of Tagetes erecta and Foeniculum vulgare. Int. J. Phytopharm. 2013, 4, 223–229. [Google Scholar]
- Santhosh, N.; Tejaswini; Shivashankar, K.S.; Seetharamu, G.K.; Archana, G. Genetic diversity for morphological characters and biochemical components in African marigold. Int. J. Chem. Stud. 2018, 6, 624–2627. [Google Scholar]
- Jung, H.Y.; Ok, H.M.; Park, M.Y.; Kim, J.Y.; Kwon, O. Bioavailability of carotenoids from chlorella powder in healthy subjects: A comparison with marigold petal extract. J. Funct. Foods 2016, 21, 27–35. [Google Scholar] [CrossRef]
- Ingkasupart, P.; Manochai, B.; Song, W.T.; Hong, J.H. Antioxidant activities and lutein content of 11 marigold cultivars (Tagetes spp.) grown in Thailand. Food Sci. Technol. 2015, 35, 380–385. [Google Scholar] [CrossRef]
- Akshaya, H.R.; Namita, K.P.S.; Saha, S.; Panwar, S.; Bharadwaj, C. Determination and correlation of carotenoid pigments and their antioxidant activities in marigold (Tagetes sp.) flowers. Indian J. Agric. Sci. 2017, 87, 390–396. [Google Scholar] [CrossRef]
- Gregory, G.K.; Chen, T.S.; Philip, T. 1986. Quantitative analysis of lutein esters in marigold flowers (Tagetes erecta) by high performance liquid chromatography. J. Food Sci. 1986, 51, 1093–1104. [Google Scholar] [CrossRef]
- Kasemsap, S.; Suthevaree, P.; Warunyanond, W.; Pethsom, A. Determination of xanthophyll and carotene in marigold petal for dye purposes. Kasetsart J. Nat. Sci. 1990, 24, 408–416. [Google Scholar]
- González-Barrio, R.; Periago, M.J.; Luna-Recio, C.; Javier, G.A.F.; Navarro-González, I. Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem. 2018, 252, 373–380. [Google Scholar] [CrossRef]
- Vanegas-Espinoza, P.E.; Ramos-Viveros, V.; Jiménez-Aparicio, A.R.; López-Villegas, O.; Heredia-Mira, F.J.; Meléndez-Martínez, A.J.; Quintero-Gutiérrez, A.G.; Paredes-López, O.; Del Villar-Martínez, A.A. Plastid analysis of pigmented undifferentiated cells of marigold Tagetes erecta L. by transmission electron microscopy. In Vitro Cell Dev. Plant 2011, 47, 596–603. [Google Scholar] [CrossRef]
- Kishimoto, S.; Maoka, T.; Sumitomo, K.; Ohmiya, A. Analysis of Carotenoid Composition in Petals of Calendula (Calendula ocinalis L.). Biosci. Biotechnol. Biochem. 2005, 69, 2122–2128. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Oszmia’nski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamorpun, S. Potential health enhancing properties of edible flowers from Thailand. Int. Food Res. J. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The antioxidants change in ornamental flowers during development and senescence. Antioxidants 2013, 2, 132–155. [Google Scholar] [CrossRef]
- Munira, S. Antioxidant activities of methanolic extract of Tagetes erecta flower growing in Bangladesh. Am.-Eurasian J. Sci. Res. 2014, 9, 182–195. [Google Scholar] [CrossRef]
- Pratheesh, V.B.; Benny, N.; Sujatha, C.H. Isolation, Stabilization and Characterization of Xanthophyll from marigold flower- Tagetes eracta L. Mod. Appl. Sci. 2009, 3, 19–27. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A. Anti-Hyperglycemic and Anticholinergic Effects of Natural Antioxidant Contents in Edible Flowers. Antioxidants 2019, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Kulprachakarn, K.; Pangjit, K.; Paradee, N.; Srichairatanakool, S.; Rerkasem, K.; Ounjaijean, S. Antioxidant Properties and Cytotoxicity of White Mugwort (Artemisia lactiflora) Leaf Extract in Human Hepatocellular Carcinoma Cell Line. Walailak J. Sci. Tech. 2019, 16, 185–192. [Google Scholar] [CrossRef]
- Sembiring, E.N.; Elya, B.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content, and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacog. J. 2018, 10, 123–127. [Google Scholar] [CrossRef]
- Kulprachakarn, K.; Chaipoot, S.; Phongphisutthinant, R.; Paradee, N.; Prommaban, A.; Ounjaijean, S.; Rerkasem, K.; Parklak, W.; Prakit, K.; Saengsitthisak, B.; et al. Antioxidant Potential and Cytotoxic Effect of Isoflavones Extract from Thai Fermented Soybean (Thua-Nao). Molecules 2021, 26, 7432. [Google Scholar] [CrossRef] [PubMed]
| Cultivar Name | Total Phenolic Content (mg GAE/g) | Total Flavonoid Content (mg QE/g) | Total Carotenoid Content (mg/100 g) |
|---|---|---|---|
| RDG | 118.95 ± 7.24 cd | 13.72 ± 1.39 c | 212.21 ± 5.22 d |
| GK1 | 134.59 ± 5.99 b | 16.49 ± 1.05 b | 133.26 ± 4.84 f |
| YQ2 | 131.14 ± 2.07 bc | 17.92 ± 1.38 b | 53.80 ± 1.56 i |
| NT1 | 161.17 ± 9.77 a | 20.05 ± 0.93 a | 58.57 ± 1.16 h |
| ADG | 116.14 ± 7.68 de | 16.32 ± 0.88 b | 91.46 ± 1.75 g |
| SDG | 94.58 ± 4.48 f | 13.73 ± 0.61 c | 225.49 ± 4.10 c |
| SO | 103.73 ± 10.62 ef | 14.36 ± 1.31 c | 431.63 ± 11.23 a |
| AM | 121.21 ± 3.91 cd | 12.67 ± 0.61 c | 267.80 ± 5.24 b |
| AK | 112.98 ± 7.06 de | 13.59 ± 0.93 c | 145.90 ± 2.32 e |
| Cultivar Name | DPPH (% Inhibition) | ABTS (% Inhibition) | FRAP (µmol Trolox/g) |
|---|---|---|---|
| RDG | 55.55 ± 0.96 c | 69.48 ± 2.66 de | 330.75 ± 0.65 d |
| GK1 | 59.11 ± 1.22 b | 67.01 ± 1.73 e | 350.86 ± 0.86 c |
| YQ2 | 67.23 ± 1.39 a | 89.57 ± 1.19 a | 361.91 ± 3.33 b |
| NT1 | 67.01 ± 1.52 a | 90.41 ± 0.92 a | 392.30 ± 3.75 a |
| ADG | 59.53 ± 1.13 b | 75.26 ± 1.38 bc | 359.29 ± 3.83 b |
| SDG | 53.14 ± 1.81 d | 73.66 ± 2.61 bc | 330.31 ± 1.74 d |
| SO | 56.85 ± 1.37 c | 77.04 ± 1.33 b | 349.27 ± 4.98 c |
| AM | 53.30 ± 0.98 d | 72.07 ± 2.56 cd | 335.03 ± 4.25 d |
| AK | 61.05 ± 0.22 b | 68.69 ± 1.69 de | 333.69 ± 4.66 d |
| Bioactive Compound Contents | DPPH | ABTS | FRAP | α-Amylase Inhibitory Potential | α-Glucosidase Inhibitory Potential |
|---|---|---|---|---|---|
| Total phenolic content | 0.652 ** | 0.498 ** | 0.759 ** | 0.612 ** | 0.766 ** |
| Total flavonoid content | 0.800 ** | 0.733 ** | 0.872 ** | 0.523 ** | 0.796 ** |
| Total carotenoid content | −0.685 ** | −0.365 | −0.500 ** | −0.866 ** | −0.554 ** |
| Lutein content | 0.757 ** | 0.744 ** | 0.810 ** | 0.547 ** | 0.747 ** |
| Trade Name | Common Name | Scientific Name | Flower Color | Flowering Time |
|---|---|---|---|---|
| Rocco Deep Gold (RDG) | Marigold | Tagetes erecta L. | Orange | Apr–Jun |
| Golden King 001 (GK1) | Marigold | Tagetes erecta L. | Deep yellow | Apr–Jun |
| Yellow Queen 002 (YQ2) | Marigold | Tagetes erecta L. | Yellow | Apr–Jun |
| Nata 001 (NT1) | Marigold | Tagetes erecta L. | Yellow | Apr–Jun |
| Amari Deep Gold (ADG) | Marigold | Tagetes erecta L. | Deep yellow | Apr–Jun |
| Sara Deep Gold (SDG) | Marigold | Tagetes erecta L. | Deep yellow | Apr–Jun |
| Sara Orange (SO) | Marigold | Tagetes erecta L. | Deep orange | Apr–Jun |
| Amari (AM) | Marigold | Tagetes erecta L. | Orange | Apr–Jun |
| Angka (AK) | Marigold | Tagetes erecta L. | Deep yellow | Apr–Jun |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parklak, W.; Ounjaijean, S.; Kulprachakarn, K.; Boonyapranai, K. In Vitro α-Amylase and α-Glucosidase Inhibitory Effects, Antioxidant Activities, and Lutein Content of Nine Different Cultivars of Marigold Flowers (Tagetes spp.). Molecules 2023, 28, 3314. https://doi.org/10.3390/molecules28083314
Parklak W, Ounjaijean S, Kulprachakarn K, Boonyapranai K. In Vitro α-Amylase and α-Glucosidase Inhibitory Effects, Antioxidant Activities, and Lutein Content of Nine Different Cultivars of Marigold Flowers (Tagetes spp.). Molecules. 2023; 28(8):3314. https://doi.org/10.3390/molecules28083314
Chicago/Turabian StyleParklak, Wason, Sakaewan Ounjaijean, Kanokwan Kulprachakarn, and Kongsak Boonyapranai. 2023. "In Vitro α-Amylase and α-Glucosidase Inhibitory Effects, Antioxidant Activities, and Lutein Content of Nine Different Cultivars of Marigold Flowers (Tagetes spp.)" Molecules 28, no. 8: 3314. https://doi.org/10.3390/molecules28083314
APA StyleParklak, W., Ounjaijean, S., Kulprachakarn, K., & Boonyapranai, K. (2023). In Vitro α-Amylase and α-Glucosidase Inhibitory Effects, Antioxidant Activities, and Lutein Content of Nine Different Cultivars of Marigold Flowers (Tagetes spp.). Molecules, 28(8), 3314. https://doi.org/10.3390/molecules28083314







