New Insight into the Reactivity of S,S-Bis-ylide
Abstract
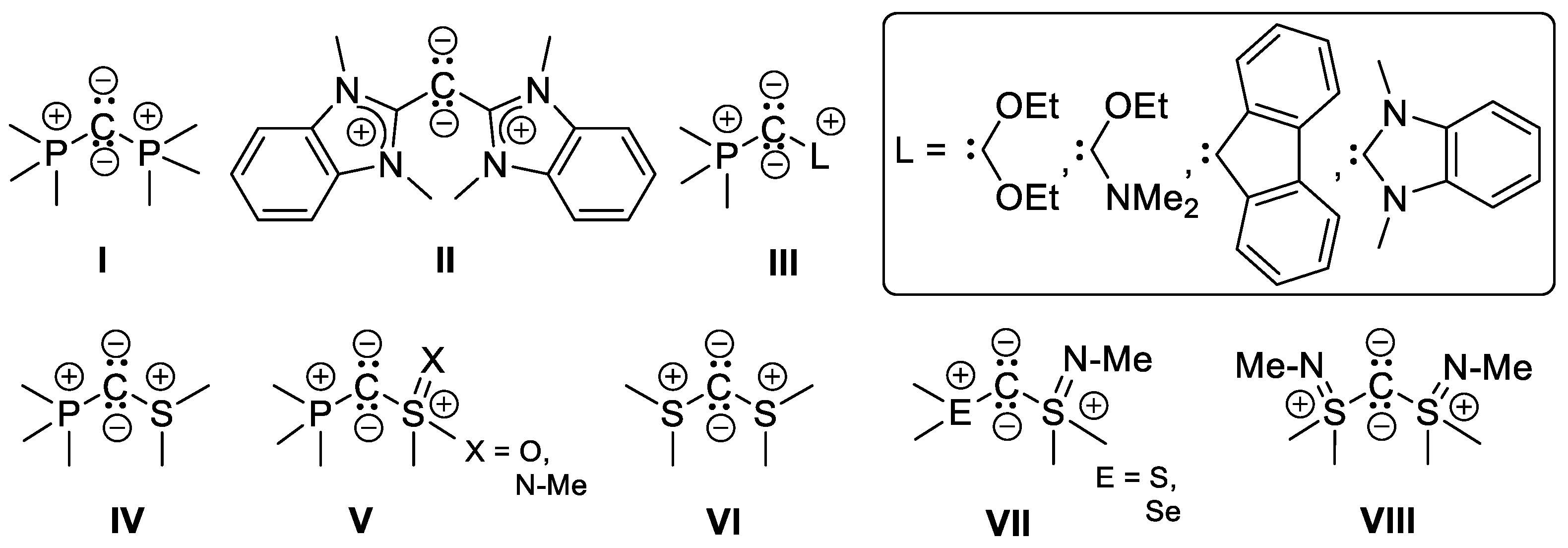
1. Introduction
2. Results and Discussion
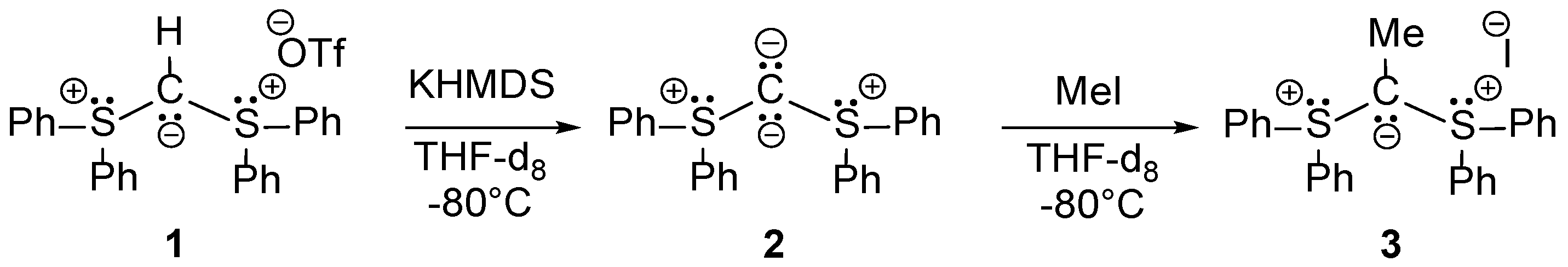
2.1. Synthesis
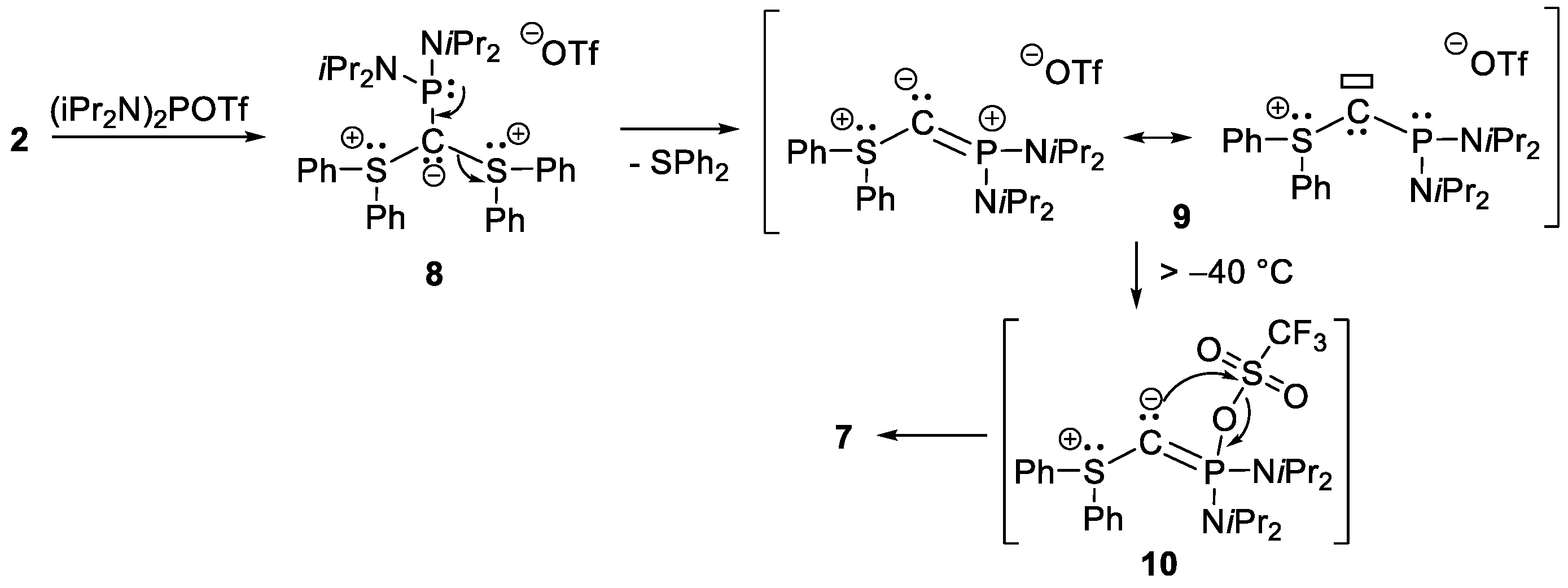
2.2. Reactivity
3. Materials and Methods
3.1. General Comments
3.2. Synthesis
3.3. X-ray Data
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Sample Availability
References and Notes
- Staudinger, H.; Meyer, J. New organic compounds of phosphorus. II. Phosphazines. Helv. Chim. Acta 1919, 2, 619–635. [Google Scholar] [CrossRef]
- Staudinger, H.; Braunholtz, B.H. New organic phosphorus compounds. V. The action of carbonylene derivatives on phosphazines. Helv. Chim. Acta 1921, 4, 897–900. [Google Scholar] [CrossRef]
- Clark, S.J. (Ed.) Nitrogen, Oxygen and Sulfur Ylide Chemistry; Oxford University Press: Oxford, UK, 2002. [Google Scholar]
- Wittig, G.; Rieber, M. Metallizability (“Metallierbarkeit”) of quaternary ammonium and phosphonium salts. Lieb. Ann. Chem. 1949, 562, 177–187. [Google Scholar] [CrossRef]
- Wittig, G.; Geissler, G. Course of reactions of pentaphenylphosphorus and certain derivatives. Lieb. Ann. Chem. 1953, 580, 44–57. [Google Scholar] [CrossRef]
- Abell, A.D.; Edmonds, M.K. The Wittig and related Reactions. In Organophosphorus Reagents; Murphy, P.J., Ed.; Oxford University Press: Oxford, UK, 2004; pp. 99–127. [Google Scholar]
- Schobert, R. Applications of the Wittig reaction in the synthesis of heterocyclic and carbocyclic compounds. In Organophosphorus Reagents; Murphy, P.J., Ed.; Oxford University Press: Oxford, UK, 2004; pp. 128–148. [Google Scholar]
- Edmonds, M.; Abell, A. Modern Carbonyl Olefination; Takeda, T., Ed.; WileyVCH: Weinheim, Germany, 2004; Chapter 1. [Google Scholar]
- Kolodiazhny, O.I. Phosphorus Ylides: Chemistry and Application in Organic Synthesis; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Chauvin, R.; Canac, Y. (Eds.) Topics in Organometallic Chemistry; Springer: Berlin, Germany, 2010. [Google Scholar]
- Fustier-Boutignon, M.; Mezailles, N. Stable geminal dianions as precursors for Gem-diorganometallic and carbene complexes. Top. Organomet. Chem. 2014, 47, 63–127. [Google Scholar]
- Gessner, V.H.; Becker, J.; Feichtner, K.-S. Carbene Complexes Based on Dilithium Methandiides. Eur. J. Inorg. Chem. 2015, 4, 1841–1859. [Google Scholar] [CrossRef]
- Scharf, L.T.; Gessner, V.H. Metalated Ylides: A New Class of Strong Donor Ligands with Unique Electronic Properties. Inorg. Chem. 2017, 56, 8599–8607. [Google Scholar] [CrossRef]
- Fustier-Boutignon, M.; Nebra, N.; Mézailles, N. Geminal Dianions Stabilized by Main Group Elements. Chem. Rev. 2019, 119, 8555–8700. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chai, C.; Petz, W.; Frenking, G. Carbones and Carbon Atom as Ligands in Transition Metal Complexes. Molecules 2020, 25, 4943. [Google Scholar] [CrossRef]
- Wang, Y.; Robinson, G.H. Counterintuitive Chemistry: Carbene Stabilization of Zero-Oxidation State Main Group Species. J. Am. Chem. Soc. 2023, 145, 5592–5612. [Google Scholar] [CrossRef]
- Ramirez, F.; Desai, N.B.; Hansen, B.; McKelvie, N.N. Hexaphenylcarbodiphosphorane, (C6H5)3PCP(C6H5)3. J. Am. Chem. Soc. 1961, 83, 3539–3540. [Google Scholar] [CrossRef]
- Dyker, C.A.; Lavallo, V.; Donnadieu, B.; Bertrand, G. Synthesis of an extremely bent acyclic allene (a “carbodicarbene”): A strong donor ligand. Angew. Chem. Int. Ed. 2008, 47, 3206–3209. [Google Scholar] [CrossRef]
- Tonner, R.; Frenking, G. C(NHC)2: Divalent carbon(0) compounds with N-heterocyclic carbene ligands-theoretical evidence for a class of molecules with promising chemical properties. Angew. Chem. Int. Ed. 2007, 46, 8695–8698. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, C.W.; Anoop, A.; Thiel, W.; Fürstner, A.; Alcarazo, M. Coordination chemistry at carbon. Nat. Chem. 2009, 1, 295–301. [Google Scholar]
- Chen, W.-C.; Shen, J.-S.; Jurca, T.; Peng, C.-J.; Lin, Y.-H.; Wang, Y.-P.; Shih, W.-C.; Yap, G.P.A.; Ong, T.-G. Expanding the Ligand Framework Diversity of Carbodicarbenes and Direct Detection of Boron Activation in the Methylation of Amines with CO2. Angew. Chem. Int. Ed. 2015, 54, 15207–15212. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-K.; Chen, W.-C.; Yap, G.P.A.; Ong, T.-G. Synthesis of Carbophosphinocarbene and Their Donating Ability: Expansion of the Carbone Class. Organometallics 2020, 39, 4395–4401. [Google Scholar] [CrossRef]
- Dellus, N.; Kato, T.; Bagan, X.; Saffon-Merceron, N.; Branchadell, V.; Baceiredo, A. An isolable mixed P,S-bis(ylide) as an asymmetric carbon atom source. Angew. Chem. Int. Ed. 2010, 49, 6798–6801. [Google Scholar] [CrossRef]
- Lozano Gonzalez, M.; Bousquet, L.; Hameury, S.; Alvarez Toledano, C.; Saffon-Merceron, N.; Branchadell, V.; Maerten, E.; Baceiredo, A. Phosphine/Sulfoxide-Supported Carbon(0) Complex. Chem. Eur. J. 2018, 24, 2570–2574. [Google Scholar] [CrossRef] [PubMed]
- Authesserre, U.; Hameury, S.; Dajnak, A.; Saffon-Merceron, N.; Baceiredo, A.; Madec, D.; Maerten, E. Complexes of Dichlorogermylene with Phosphine/Sulfoxide-Supported Carbone as Ligand. Molecules 2021, 26, 2005. [Google Scholar] [CrossRef]
- Ikeda, T.; Mikami, T.; Suzuki, T.; Yoshimura, T.; Fujii, T. Synthesis and structure of (MeN)Ph2S=C=SPh2(NMe). Angew. Chem. Int. Ed. 2002, 41, 2576–2578. [Google Scholar]
- Morosaki, T.; Suzuki, T.; Wang, W.-W.; Nagase, S.; Fujii, T. Syntheses, Structures, and Reactivities of Two Chalcogen-Stabilized Carbones. Angew. Chem. Int. Ed. 2014, 53, 9569–9571. [Google Scholar] [CrossRef]
- Morosaki, T.; Wang, W.-W.; Nagase, S.; Fujii, T. Synthesis, Structure, and Reactivities of Iminosulfane- and Phosphane-Stabilized Carbones Exhibiting Four-Electron Donor Ability. Chem. Eur. J. 2015, 21, 15405–15411. [Google Scholar] [CrossRef]
- Morosaki, T.; Fujii, T. Synthesis of phosphorus- and sulfur-stabilized carbone (Me)Ph2P→C←SPh2(=NMe). Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 159–162. [Google Scholar] [CrossRef]
- Morosaki, T.; Iijima, R.; Suzuki, T.; Wang, W.-W.; Nagase, S.; Fujii, T. Synthesis, Electronic Structure, and Reactivities of Two-Sulfur-Stabilized Carbones Exhibiting Four-Electron Donor Ability. Chem. Eur. J. 2017, 23, 8694–8702. [Google Scholar] [CrossRef]
- Pranckevicius, C.; Fan, L.; Stephan, D.W. Cyclic Bent Allene Hydrido-Carbonyl Complexes of Ruthenium: Highly Active Catalysts for Hydrogenation of Olefins. J. Am. Chem. Soc. 2015, 137, 5582–5589. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-C.; Shih, W.-C.; Jurca, T.; Zhao, L.; Andrada, D.M.; Peng, C.-J.; Chang, C.-C.; Liu, S.-K.; Wang, Y.-P.; Wen, Y.-S.; et al. Carbodicarbenes: Unexpected π-Accepting Ability during Reactivity with Small Molecules. J. Am. Chem. Soc. 2017, 139, 12830–12836. [Google Scholar] [CrossRef]
- Chan, Y.-C.; Bai, Y.; Chen, W.-C.; Chen, H.-Y.; Li, C.-Y.; Wu, Y.-Y.; Tseng, M.-C.; Yap, G.P.A.; Zhao, L.; Chen, H.-Y.; et al. Synergistic Catalysis by Brønsted Acid/Carbodicarbene Mimicking Frustrated Lewis Pair-Like Reactivity. Angew. Chem. Int. Ed. 2021, 60, 19949–19956. [Google Scholar] [CrossRef] [PubMed]
- Kamitani, M.; Nakayasu, B.; Fujimoto, H.; Yasui, K.; Kodama, T.; Tobisu, M. Single-carbon atom transfer to α,β-unsaturated amides from N-heterocyclic carbenes. Science 2023, 379, 484–488. [Google Scholar] [CrossRef]
- Matthews, C.N.; Driscoll, J.S.; Birum, G.H. Mesomeric phosphonium inner salts. Chem. Comm. 1966, 20, 736–737. [Google Scholar] [CrossRef]
- Birum, G.H.; Matthews, C.N. Triphenylphosphoranylideneketene. Tet. Lett. 1966, 37, 5707–5710. [Google Scholar]
- Petz, F.; Öxler, F.; Brand, A.; Neumüller, B. Concerning the Reaction of the Betain-like Compound O2CC(PPh3)2 withTungsten-Carbonyl Derivatives; Preparation and Crystal Structures of [(CO)5W{η1-O2C2(PPh3)2}] and [(CO)4W{η2-O2C2(PPh3)2}]. Z. Anorg. Allg. Chem. 2006, 632, 588–592. [Google Scholar] [CrossRef]
- Petz, W.; Kutschera, C.; Heitbaum, M.; Frenking, G.; Tonner, R.; Neumüller, B. Experimental and Theoretical Studies of Carbodiphosphorane−CX2 Adducts with Unusual Bonding Situations: Preparation, Crystal Structures, and Bonding Analyses of S2CC(PPh3)2, O2CC(PPh3)2, and [(CO)4MS2CC(PPh3)2] (M = Cr, Mo, W). Inorg. Chem. 2005, 44, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, G.-X.; Zhang, W.-Z.; Lu, X.-B. CO2 Adducts of Phosphorus Ylides: Highly Active Organocatalysts for Carbon Dioxide Transformation. ACS Catal. 2015, 5, 6773–6779. [Google Scholar] [CrossRef]
- 5 was isolated as crystals from a THF/pentane solution and its structure was established by X-ray diffraction analysis (see supporting information). Unfortunately, treatment to isolate 5 leads to decomposition.
- Both iodine (6-I) and triflate (6-OTf) salts co-crystalized. Both structures are very similar and the complete data can be found in the supporting information.
- The same tendency was observed in the O-silylated salt 5.
- Dellus, N.; Kato, T.; Saffon-Merceron, N.; Branchadell, V.; Baceiredo, A. Synthesis and Characterization of a Stable Cyclic gem-Bis(phosphaylide): A 4π-Electron Three-Membered Heterocycle. Inorg. Chem. 2011, 50, 7949–7951. [Google Scholar] [CrossRef]
- If the temperature rise is too slow, the selectivity of the reaction is greatly reduced.
- Goumri-Magnet, S.; Gornitzka, H.; Baceiredo, A.; Bertrand, G. Synthetic Utility of Stable Phosphanylcarbenes: Synthesis and Crystal Structure of an α-(Lithiomethylene)phosphorene. Angew. Chem. Int. Ed. 1999, 38, 678–680. [Google Scholar] [CrossRef]
- Birum, G.H.; Matthews, C.N. Mesomeric phosphonium inner salts. J. Am. Chem. Soc. 1966, 88, 4198–4203. [Google Scholar] [CrossRef]
- Mastryukova, T.A.; Aladzheva, I.M.; Leontéva, I.V.; Petrovski, P.V.; Fodin, I.; Kabachnik, M.I. Dyadic phosphorus-carbon tautomerism. Pure Appl. Chem. 1980, 52, 945–957. [Google Scholar] [CrossRef]
- SADABS, Program for data correction, Bruker−AXS.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]



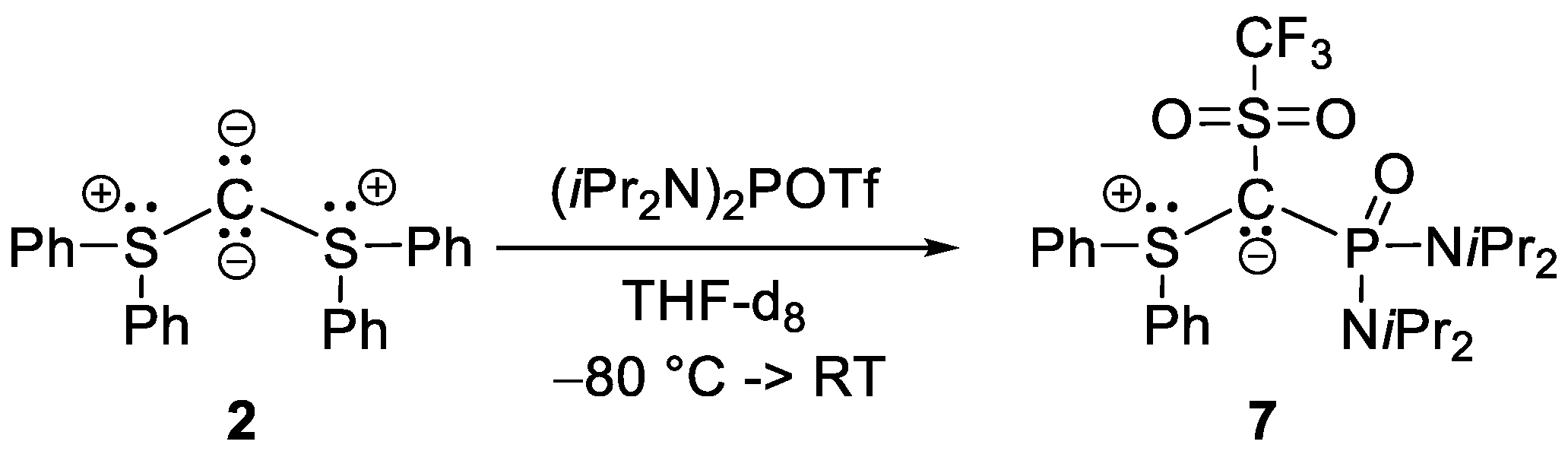

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Authesserre, U.; Swamy, V.S.V.S.N.; Saffon-Merceron, N.; Baceiredo, A.; Kato, T.; Maerten, E. New Insight into the Reactivity of S,S-Bis-ylide. Molecules 2023, 28, 3295. https://doi.org/10.3390/molecules28083295
Authesserre U, Swamy VSVSN, Saffon-Merceron N, Baceiredo A, Kato T, Maerten E. New Insight into the Reactivity of S,S-Bis-ylide. Molecules. 2023; 28(8):3295. https://doi.org/10.3390/molecules28083295
Chicago/Turabian StyleAuthesserre, Ugo, V. S. V. S. N. Swamy, Nathalie Saffon-Merceron, Antoine Baceiredo, Tsuyoshi Kato, and Eddy Maerten. 2023. "New Insight into the Reactivity of S,S-Bis-ylide" Molecules 28, no. 8: 3295. https://doi.org/10.3390/molecules28083295
APA StyleAuthesserre, U., Swamy, V. S. V. S. N., Saffon-Merceron, N., Baceiredo, A., Kato, T., & Maerten, E. (2023). New Insight into the Reactivity of S,S-Bis-ylide. Molecules, 28(8), 3295. https://doi.org/10.3390/molecules28083295






