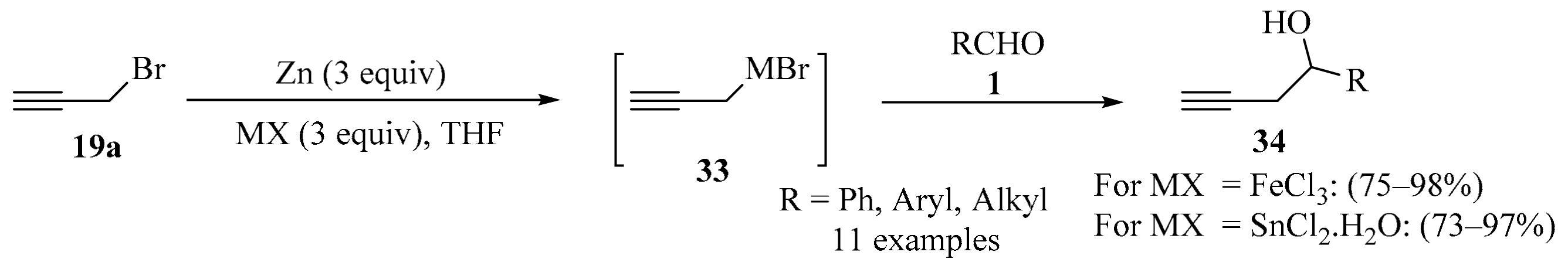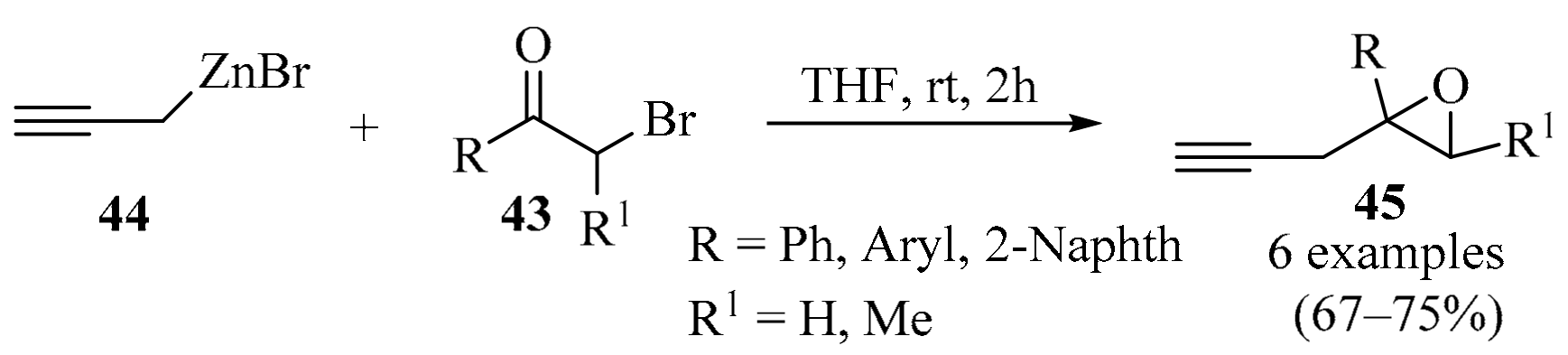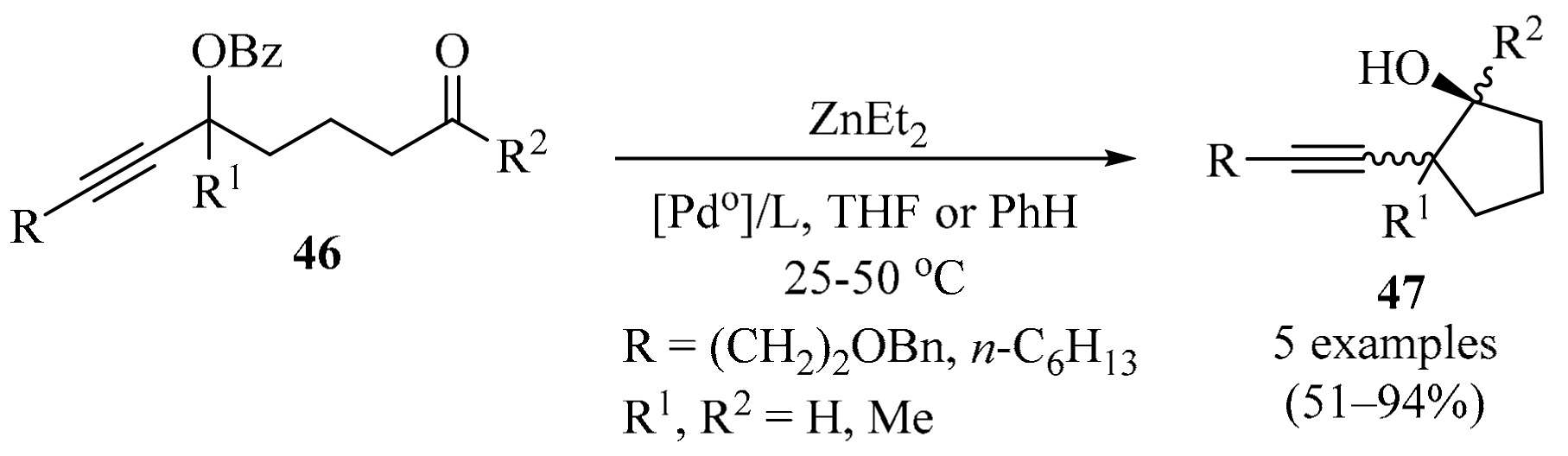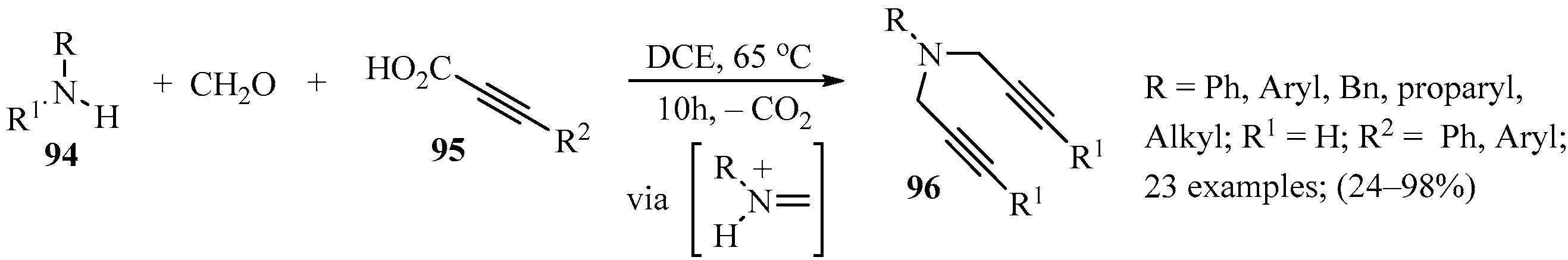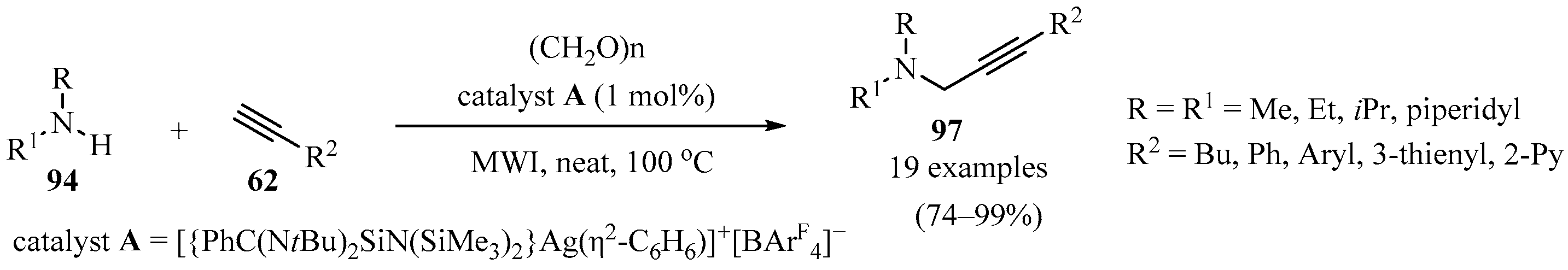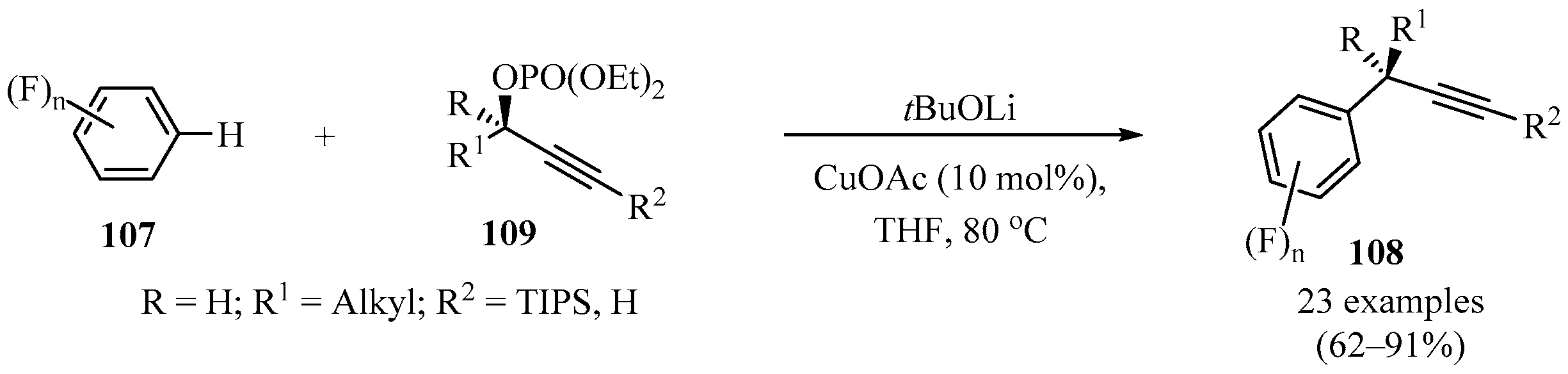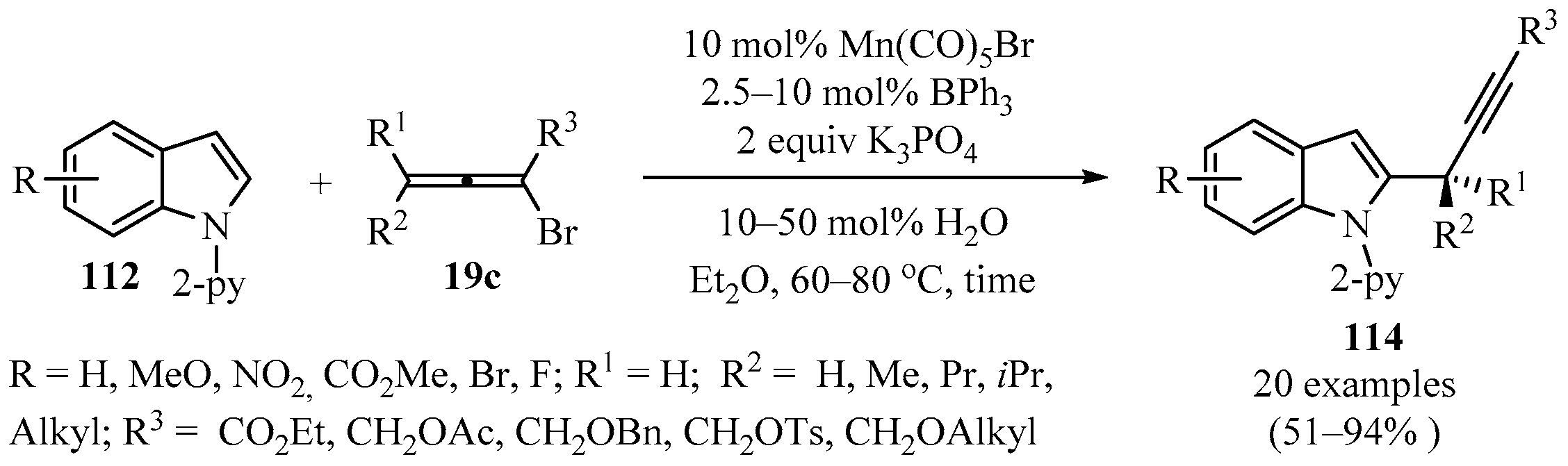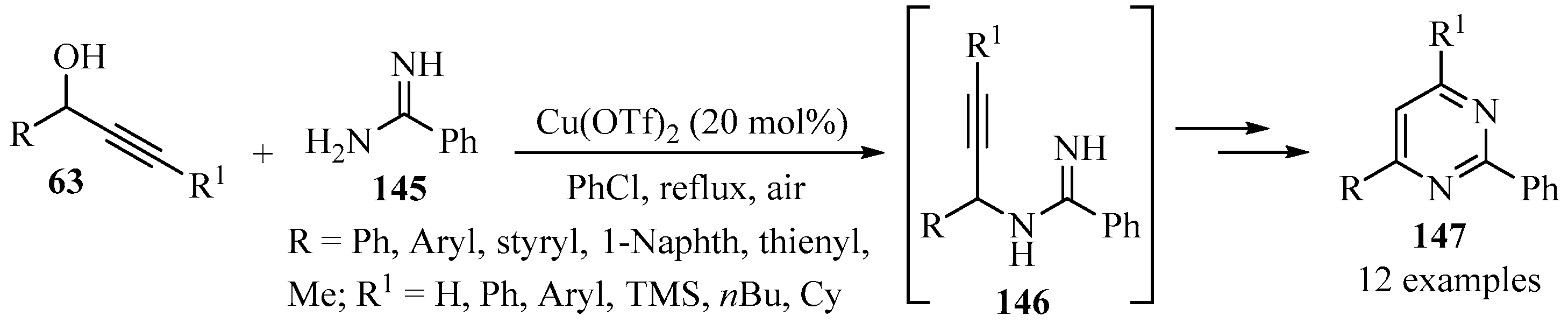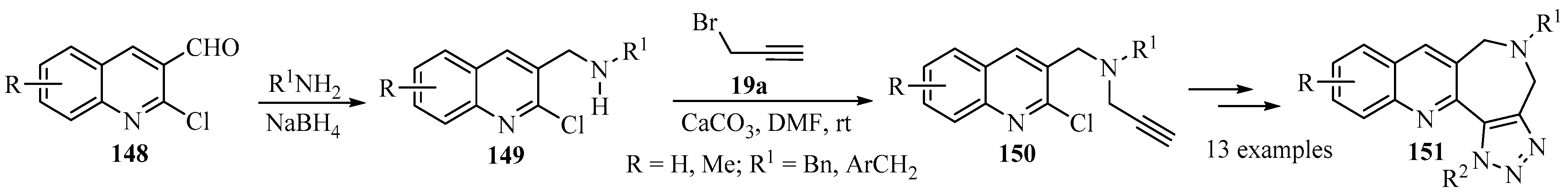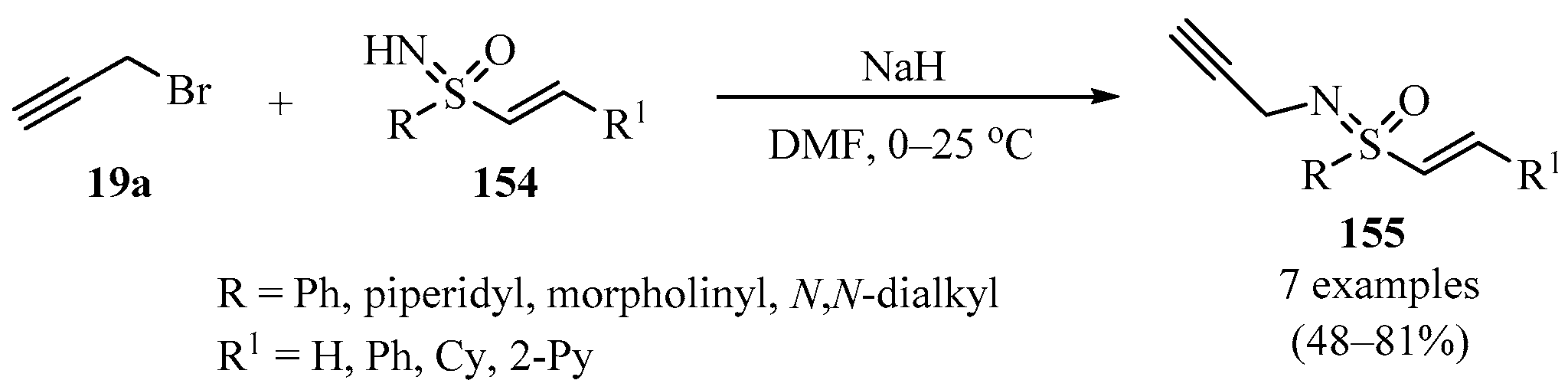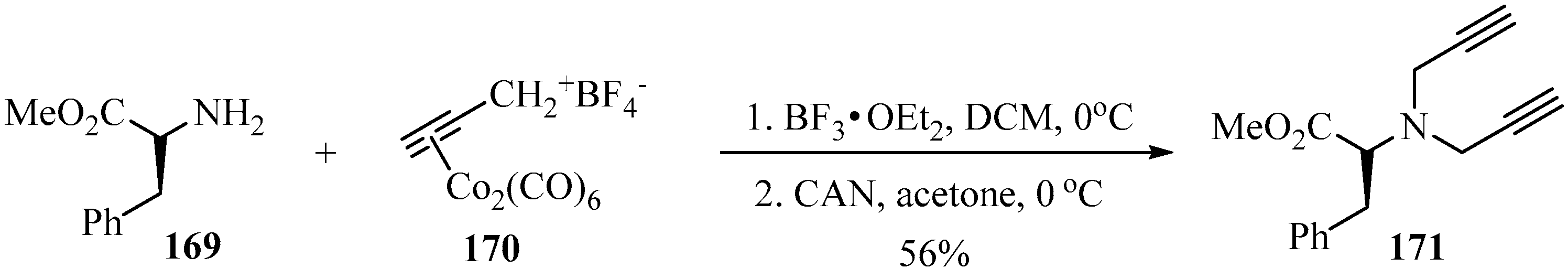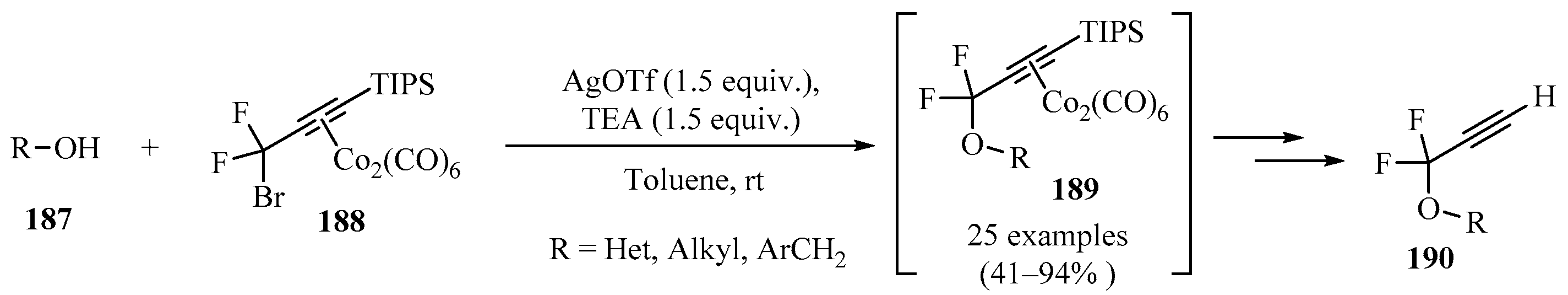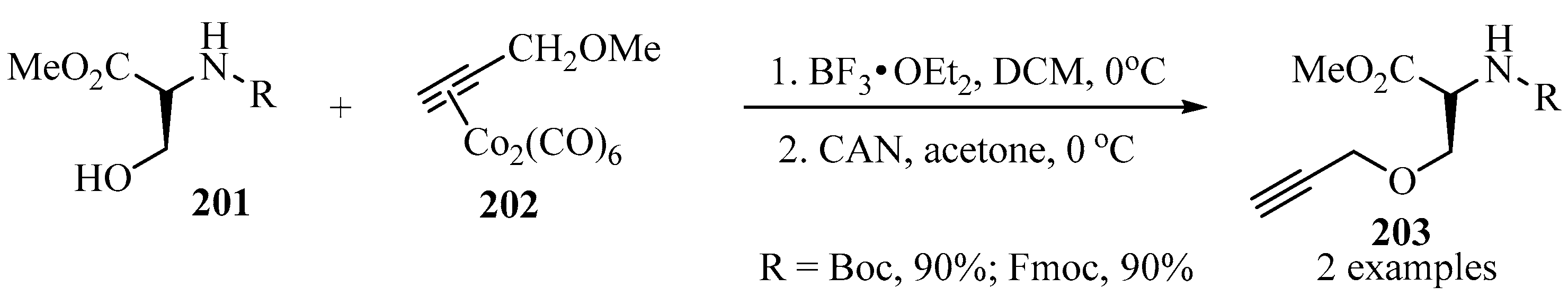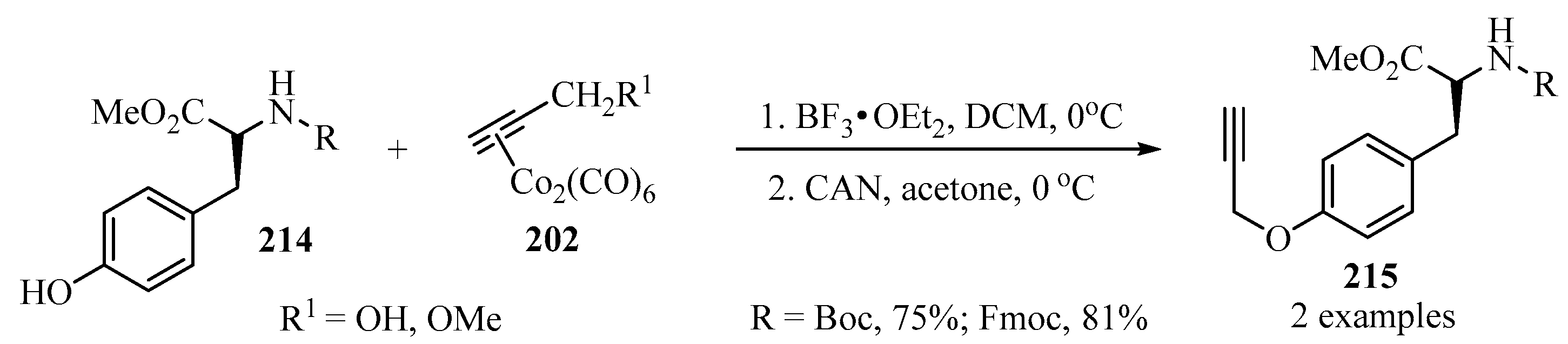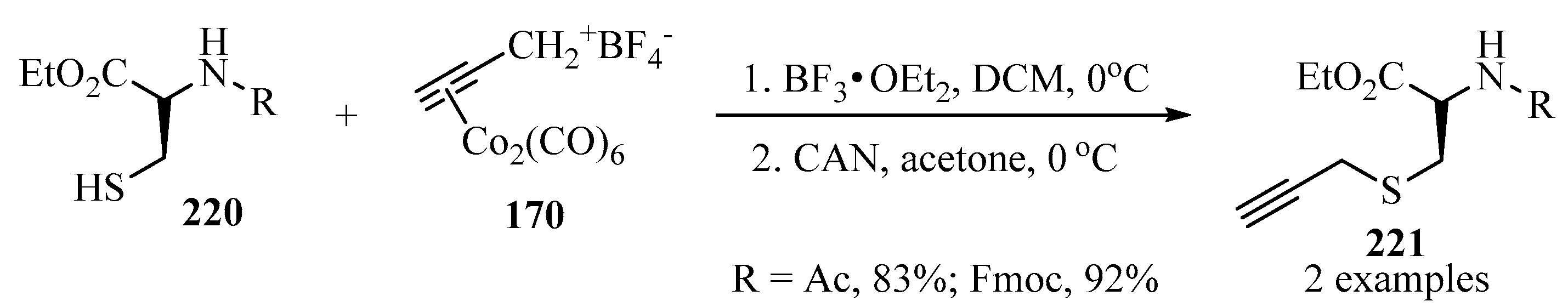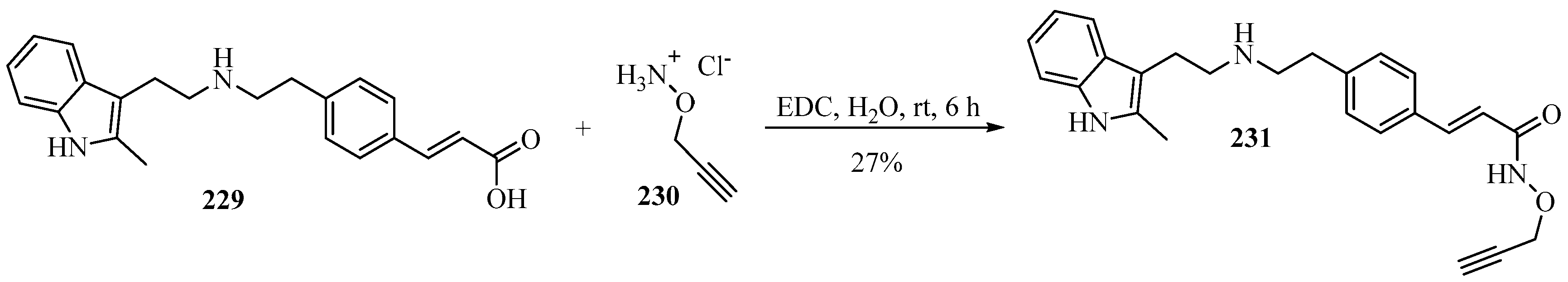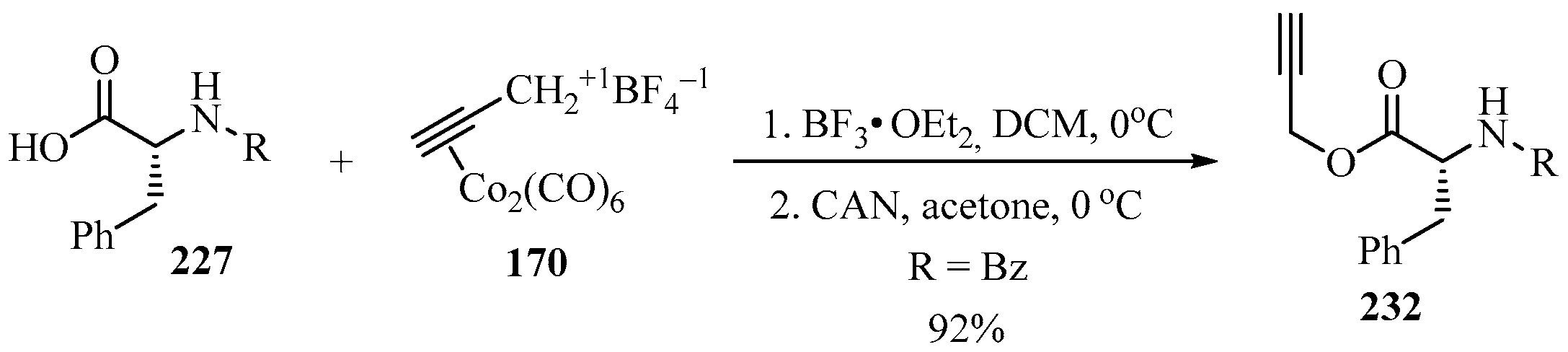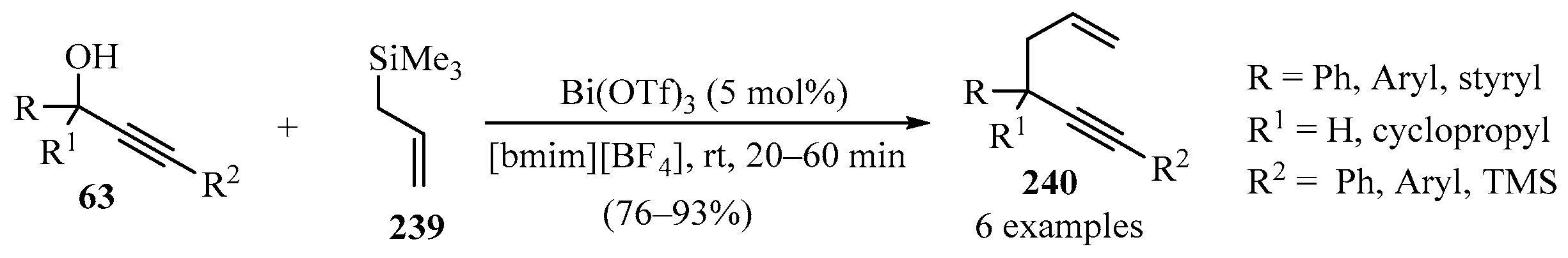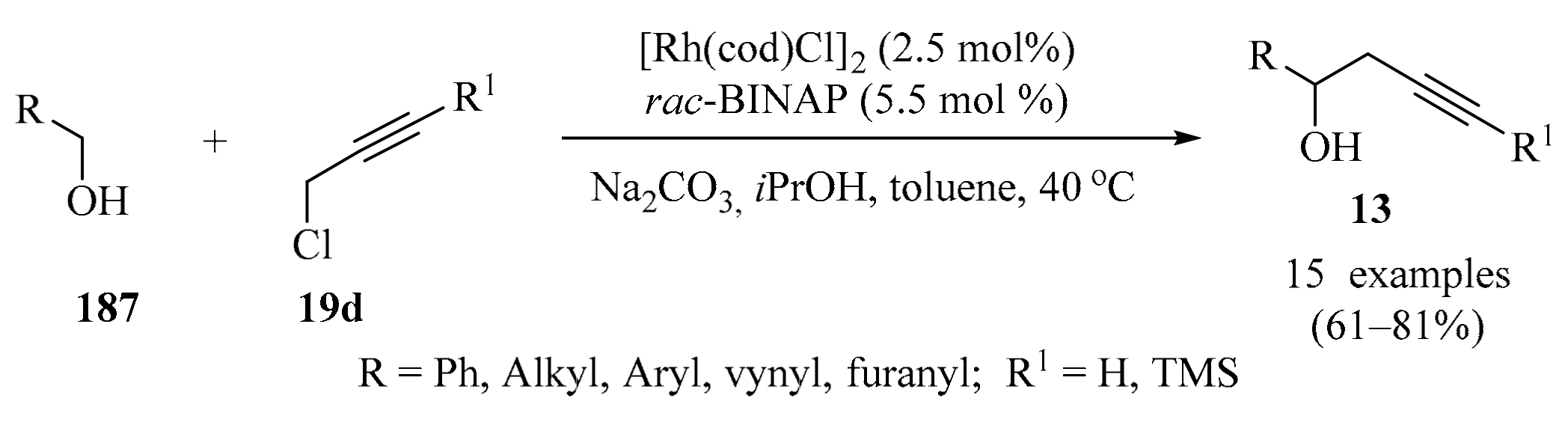Abstract
The propargyl group is a highly versatile moiety whose introduction into small-molecule building blocks opens up new synthetic pathways for further elaboration. The last decade has witnessed remarkable progress in both the synthesis of propargylation agents and their application in the synthesis and functionalization of more elaborate/complex building blocks and intermediates. The goal of this review is to highlight these exciting advances and to underscore their impact.
1. Introduction
The present review covers relevant literature published from 2010 to present. According to the consulted reports, whereas in the majority of cases the target compounds result from direct introduction of the propargyl moiety, in many examples, the propargylation reaction serves as a strategic step in a reaction sequence that results in the formation of more elaborate/complex structures. In such cases, this review emphasizes the propargylation methodologies rather than the subsequent steps en route to more complex synthetic targets. It is noteworthy that tautomerization between the propargyl (I) and allenyl (II) moieties (Scheme 1) greatly expands the scope of propargylation, since either one may function as a propargylation agent [1,2]. Indeed, in many examples discussed in this review, allenyl derivatives and propargyl derivatives can be employed interchangeably to obtain the same propargylated derivative, or be applied to different substrates, all leading to the propargylated analogs.
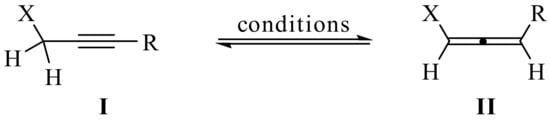
Scheme 1.
Propargyl–allenyl tautomerization process.
As depicted in Table 1, this review is organized based on the type of substrate/functional group reacting with various classes of propargylating reagents (propargyl and/or allenyl derivatives), while also highlighting the catalysts/catalytic systems employed, including complex catalytic systems formed via catalyst/ligand interactions applied to asymmetric propargylation.

Table 1.
Summary of the types of substrates, propargylating agents, and catalysts/catalytic systems.
2. Types of Substrates
2.1. (a) Aldehydes and Ketones and (b) Hemiacetals
A propargylation reaction in carbonyl derivatives (aldehydes and ketones) whereby the propargylation reagent acts as a nucleophile toward the C=O functionality is a convenient method for the synthesis of chiral and achiral secondary or tertiary homopropargylic alcohols from aldehydes or ketones, respectively [3]. Significant progress has been made in the development of chiral propargylation reagents and diastereoselective additions of propargylic anion equivalents to chiral aldehydes and ketones [4].
Homopropargylic alcohols are present as fundamental structural entities in many bioactive compounds [5,6], and have also attracted significant interest as useful building blocks for complex molecule synthesis [7,8,9]. In this regard, several synthetic strategies and propargylation reagents have been employed for the synthesis of this interesting family of alcohols, as summarized below.
- (a)
- Aldehyde and ketones
2.1.1. With Boron-Based Propargyl Reagents
Propargyl–/allenyl–boron-based compounds are a family of propargylation reagents with easy availability and relatively low costs, and for this reason, they are widely used in the propargylation processes of diverse organic substrates, as summarized in Table 2 and Scheme 2, Scheme 3 and Scheme 4.

Table 2.
Propargylation of diversely substituted aldehydes/ketones 1 with propargyl-/allenyl borolanes 2.
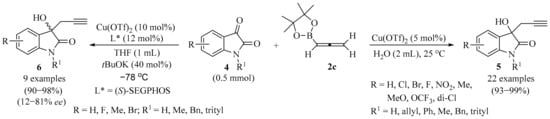
Scheme 2.
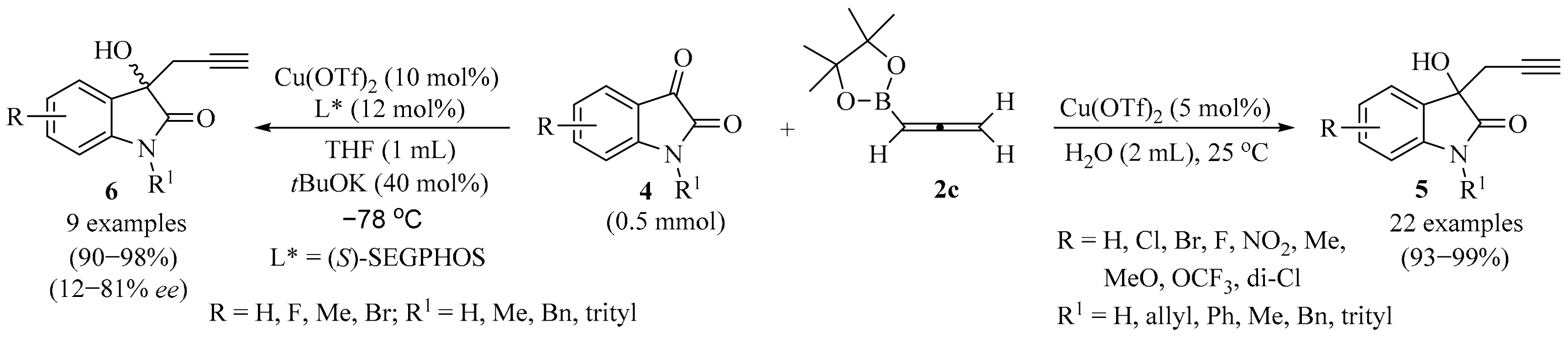
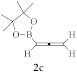
Synthesis of homopropargyl alcohols 5/6 from isatin derivatives 4 and allenylboronic ester 2c.

Scheme 3.
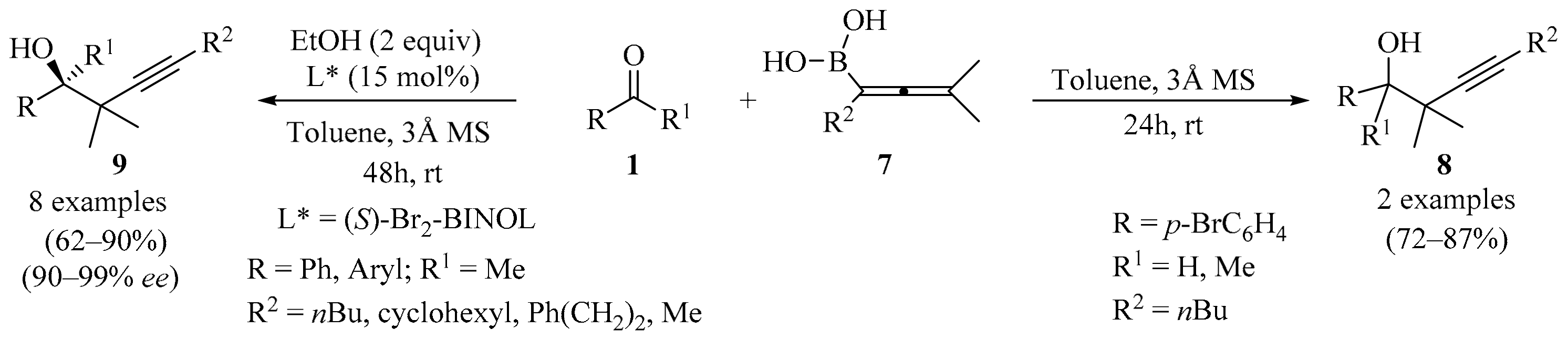
Synthesis of homopropargyl alcohols 8/9 from ketones 1 and allenylboronic acid 7.
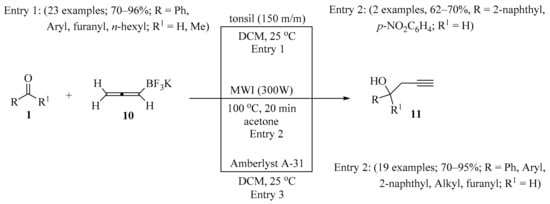
Scheme 4.
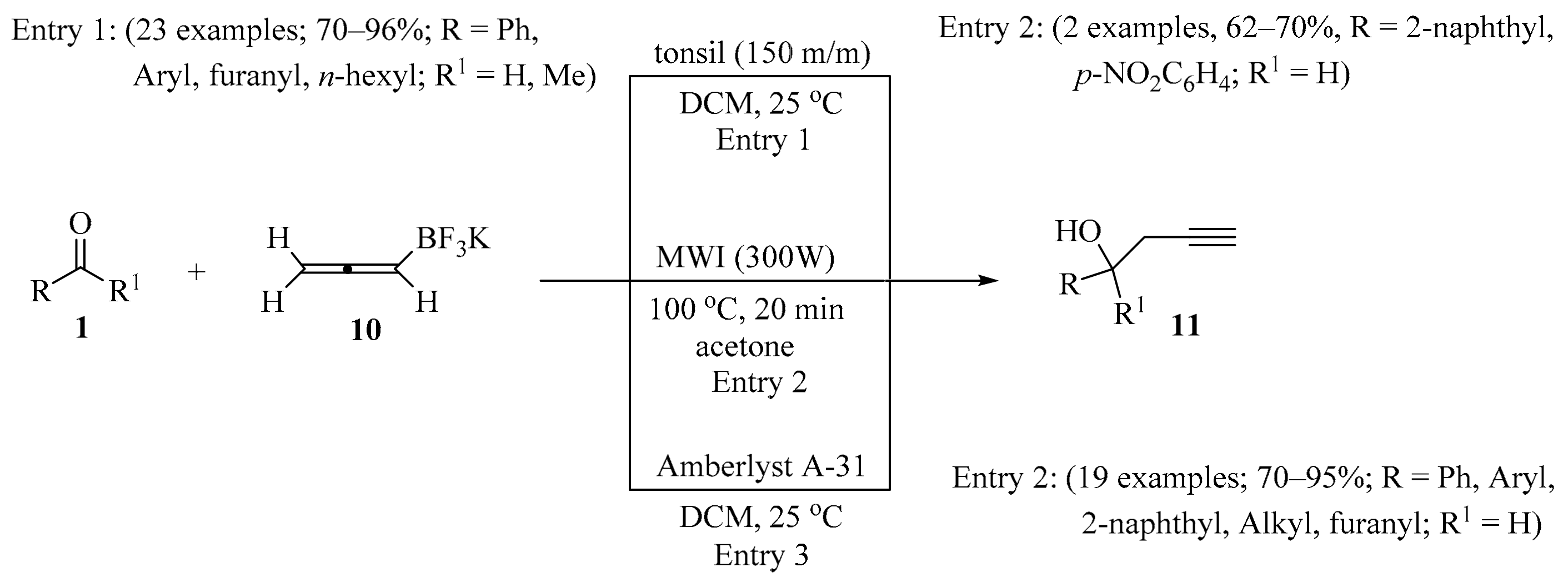
Synthesis of homopropargyl alcohols 11 from aldehydes/ketones 1 and allenyltrifluoroborate 10.
Following the discovery of the highly enantioselective and site-selective copper alkoxide-catalyzed propargylation of aldehydes 1 (R1 = H) with a propargyl borolane 2a (Table 2, entry 1), a catalytic cycle based on a Cu-alkoxide-mediated B/Cu exchange with propargyl borolane 2a was proposed, with an allenyl Cu intermediate as a key species. Additional experiments demonstrated the proposed catalytic cycle [10]. Table 2 also summarizes several other synthetic approaches to the propargylation reaction of diverse aldehydes and ketones 1 through propargyl/allenyl borolane reagents 2, producing a variety of chiral and achiral secondary and tertiary homopropargylic alcohols 3.
A simple protocol for the synthesis of homopropargyl alcohols 5, starting with isatin derivatives 4 under mild reaction conditions, was reported (Scheme 2) [22]. Reactions were performed in the presence of copper triflate as a Lewis acid catalyst, with allenylboronic acid pinacol ester 2c as a nucleophile, in aqueous media, producing excellent product 5 yields. The enantioselective synthesis of chiral propargyl alcohols 6 was also explored. The best regioselectivity was achieved when (S)-SEGPHOS was used as a chiral ligand, resulting in enantiomeric ratios up to 12:88. Gram-scale synthesis, performed to check the efficiency of the protocol, showed retention in selectivity [22].
The synthesis of tri- and tetrasubstituted allenylboronic acids was established via a versatile copper-catalyzed methodology (Scheme 3) [23]. Subsequently, the obtained allenylboronic acids 7 were subjected to propargylboration reactions with ketones 1 without any additives, producing homopropargyl alcohols 8 (Scheme 3). Additionally, catalytic asymmetric propargylboration of the ketones 1 with high stereoselectivity was achieved when (S)-Br2-BINOL was used as chiral ligand, allowing for the synthesis of highly enantioenriched tertiary homopropargyl alcohols 9 (Scheme 3). The reaction was suitable for the kinetic resolution of racemic allenylboronic acids, producing alkynes with adjacent quaternary stereocenters [23].
The propargylation of aldehydes/ketones 1 using potassium allenyltrifluoroborate 10 promoted by tonsil, an inexpensive and readily available clay, in a chemo- and regioselective manner was described, leading to homopropargyl alcohols 11 in good to moderate yields (Scheme 4, entry 1) [24]. The described method is simple and avoids the use of air- and moisture-sensitive organometallics. In the same way, alcohols 11 were synthesized under MW irradiation (Scheme 4, entry 2) [17] or by using Amberlyst A-31 (Scheme 4, entry 3) [25].
2.1.2. With Propargyl Silanes
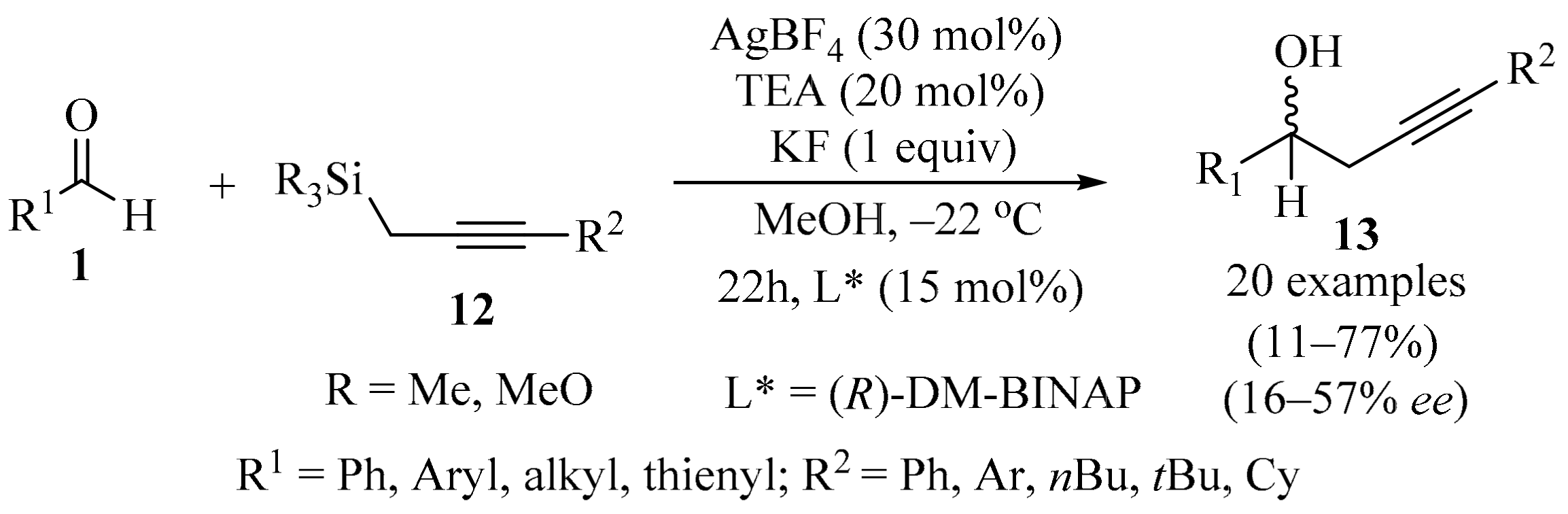
In the context of silane-mediated transformations promoted by chiral Lewis base catalysis, it has been shown that the coupling of a Lewis base with a silane reagent can promote several synthetically useful reactions, opening up the possibility for further studies [26]. In a recently developed catalytic asymmetric addition process (Scheme 5), optically active homopropargylic alcohols 13 were synthesized by reacting propargylic silanes 12 with aldehydes 1 (R = H), using a chiral organosilver species as a pre-catalyst. The catalyst was formed in situ via an (R)-DM-BINAP⋅AgBF4 complex. The other additives were TEA (base pre-catalyst), along with KF and MeOH [27].
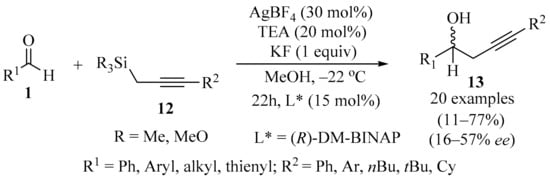
Scheme 5.
Organosilver-catalyzed asymmetric synthesis of homopropargylic alcohols 13 from aldehydes 1 and propargylic silane reagents 12.
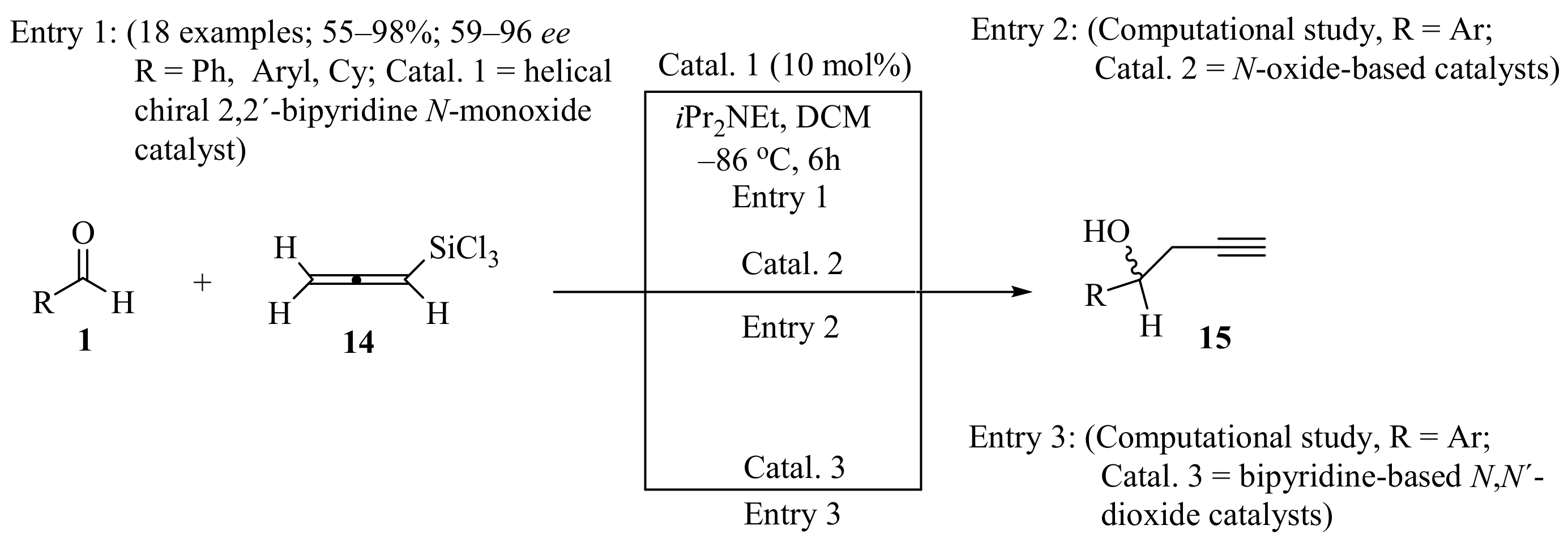
Allenyltrichlorosilane is an attractive candidate as a nucleophilic partner in C=O and C=N propargylation reactions because of its mildness, regiospecificity, and low toxicity [28]. It was reported that a new bidentate helical chiral 2,2′-bipyridine N-monoxide Lewis base can efficiently catalyze the addition of allenyltrichlorosilane 14 to aromatic aldehydes 1 (R = H), producing homopropargylic alcohols 15 with high levels of enantioselectivity and high yields (Scheme 6, entry 1) [29]. Additionally, extensive computational studies have made it possible to predict stereoselectivities for the synthesis of alcohols 15 using axially chiral bipyridine N,N’-dioxides as catalysts (Scheme 6, entries 2 and 3). It was found that the stereoselectivity of these bidentate catalysts is controlled by well-defined rigid transition-state structures. It was suggested that N,N’-dioxides are superior platforms for rational catalyst development for asymmetric propargylation [30,31].

Scheme 6.
Asymmetric synthesis of homopropargylic alcohols 15 from allenyltrichlorosilane 14 and aromatic aldehydes 1.
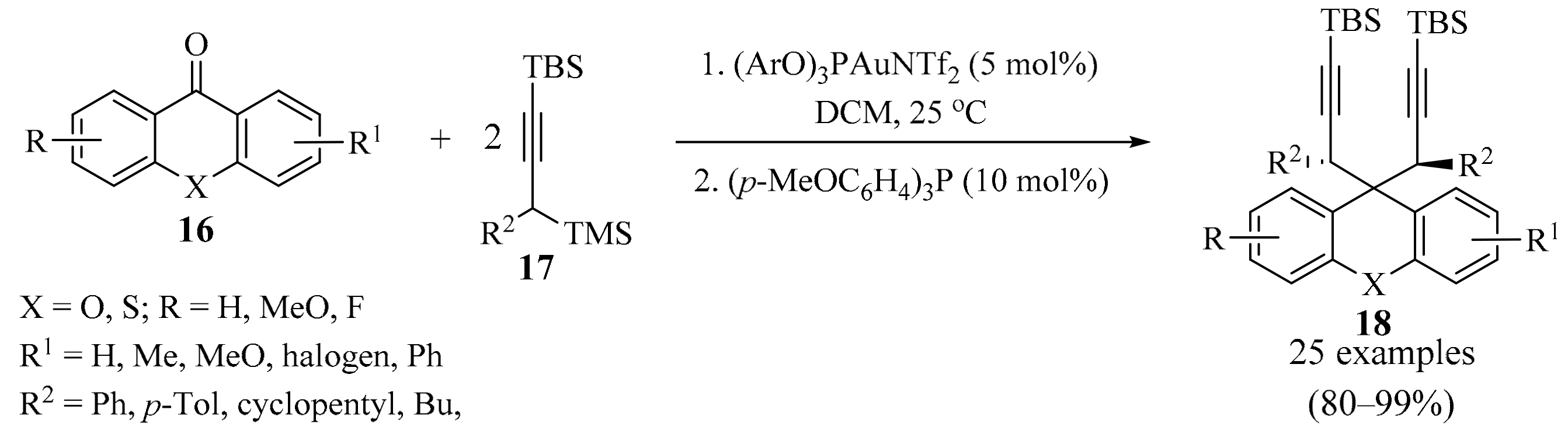
Xanthones, thioxanthones, and xanthenes are naturally occurring molecules and have interesting properties due to their special structures [32,33]. With this in mind, gold-catalyzed bispropargylation of xanthones and thioxanthones 16 (X = O, S, respectively) was devised (Scheme 7) [34]. In this approach, the use of propargylsilanes 17 permitted deoxygenative bispropargylation through the double catalytic addition of the corresponding allenylgold intermediate to the synergistically activated carbonyl moiety. This methodology worked in a diastereoselective manner, with either xanthone or thioxanthone derivatives 16, producing the corresponding 9,9-bispropargylxanthenes and thioxanthenes 18 (X = O, S, respectively) in high yields.
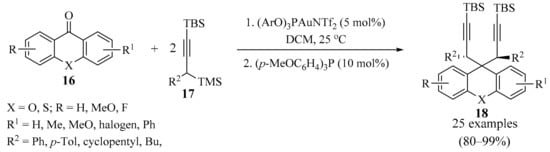
Scheme 7.
Gold-catalyzed bispropargylation of xanthones and thioxanthones 16.
2.1.3. With Propargyl Halides
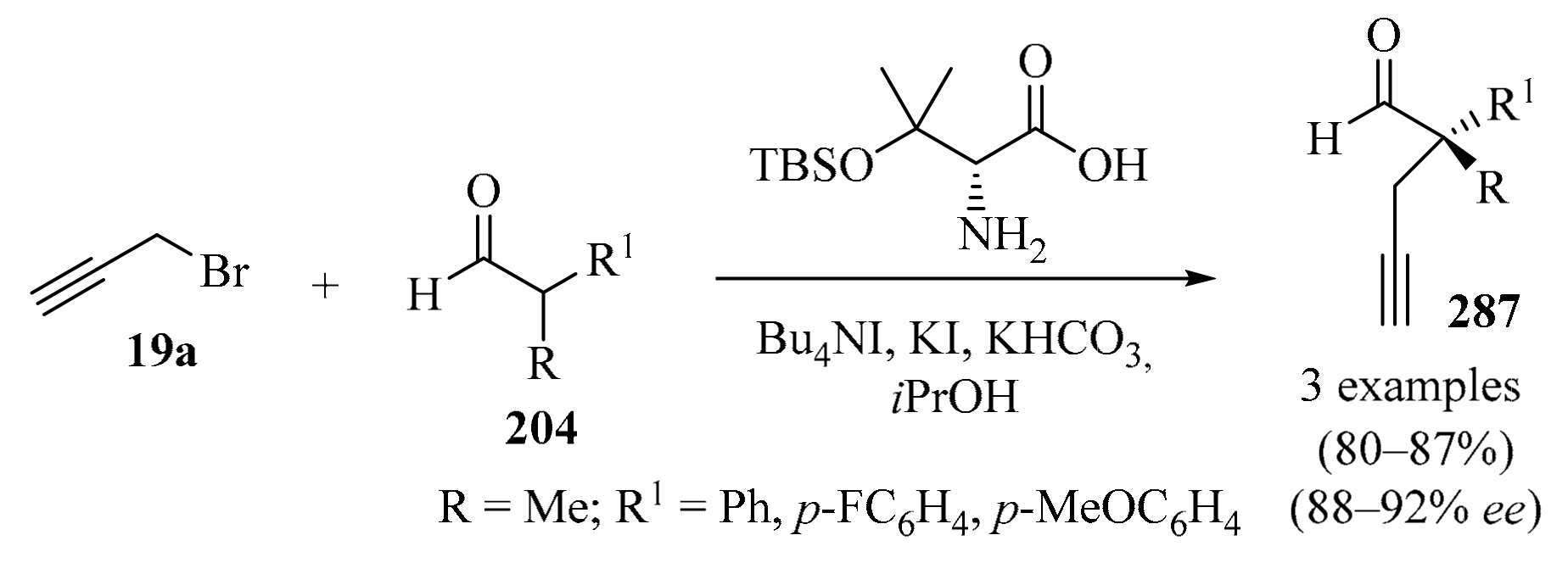
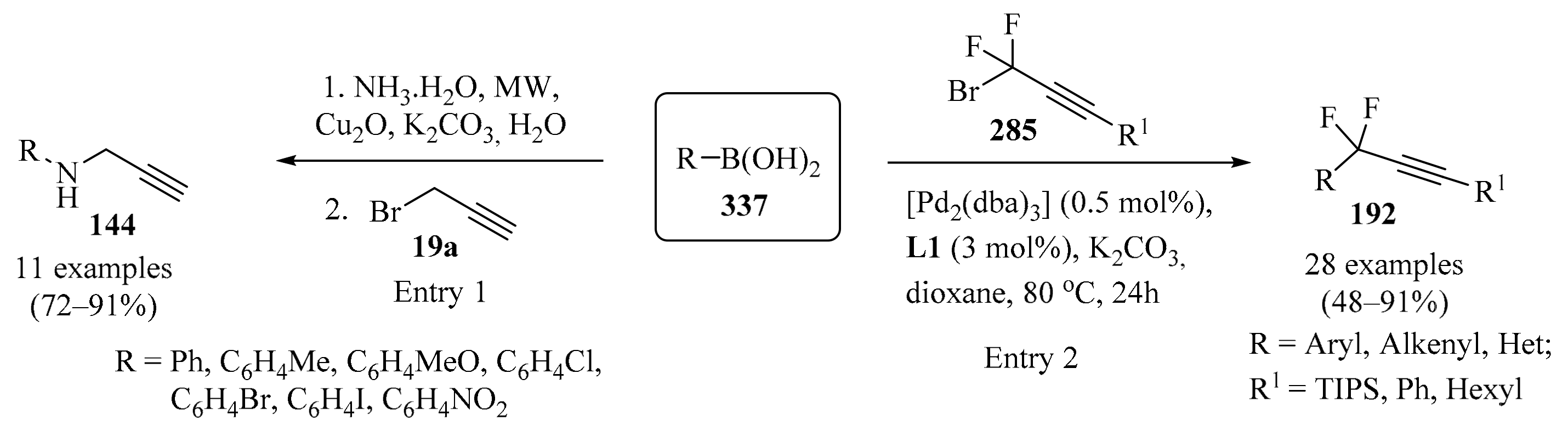
The addition of organochromium reagents to carbonyl compounds is considered an important tool in contemporary organic synthesis because of a number of unique features, such as mild reaction conditions, high chemoselectivity, and compatibility with a wide range of functional groups [35]. Chiral homopropargyl alcohols 3 were envisioned among the products potentially accessible using this methodology. Most of the asymmetric methods that provide access to these compounds involve the use of chiral allenyl reagents, for which catalytic enantioselective NH propargylation was considered a suitable alternative, owing to the ready availability of propargyl halides 19 as sources of propargyl moieties.
Following the development of a tethered bis-(8-quinolinato) (TBOx) chromium complex [36], it was successfully used as a highly stereoselective catalyst for several asymmetric reactions [37,38,39,40]. Its application as a catalyst was extended to the asymmetric NH propargylation of aldehydes. Thus, a highly enantioselective catalytic system for the NH propargylation of aldehydes 1 (R = H) via a Barbier-type reaction [41] employing low Mn catalyst loading was developed (Table 3, entry 1). High enantioselectivities, not previously achievable for aromatic, heteroaromatic, and α,β-unsaturated aldehydes using NH chemistry, were reported for a range of substrates 1 [42].

Table 3.
Propargylation of diversely substituted aldehydes/ketones 1 with propargyl-/allenyl halides 19.
Several other approaches to the synthesis of diversely substituted chiral and achiral homopropargyl alcohols 3, starting with carbonyl compounds 1 and employing halogen-based propargylation reagents 19, in the presence of a variety of catalytic systems, are outlined in Table 3 and Scheme 8.
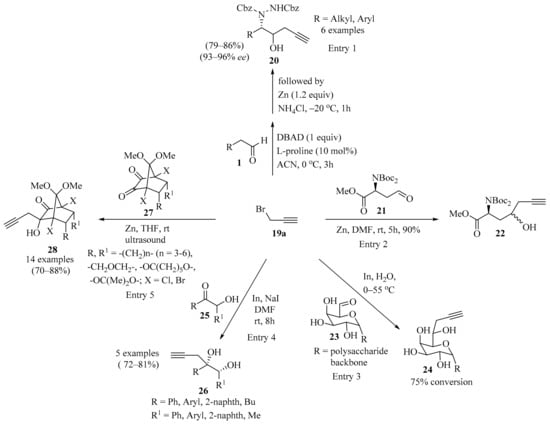
Scheme 8.
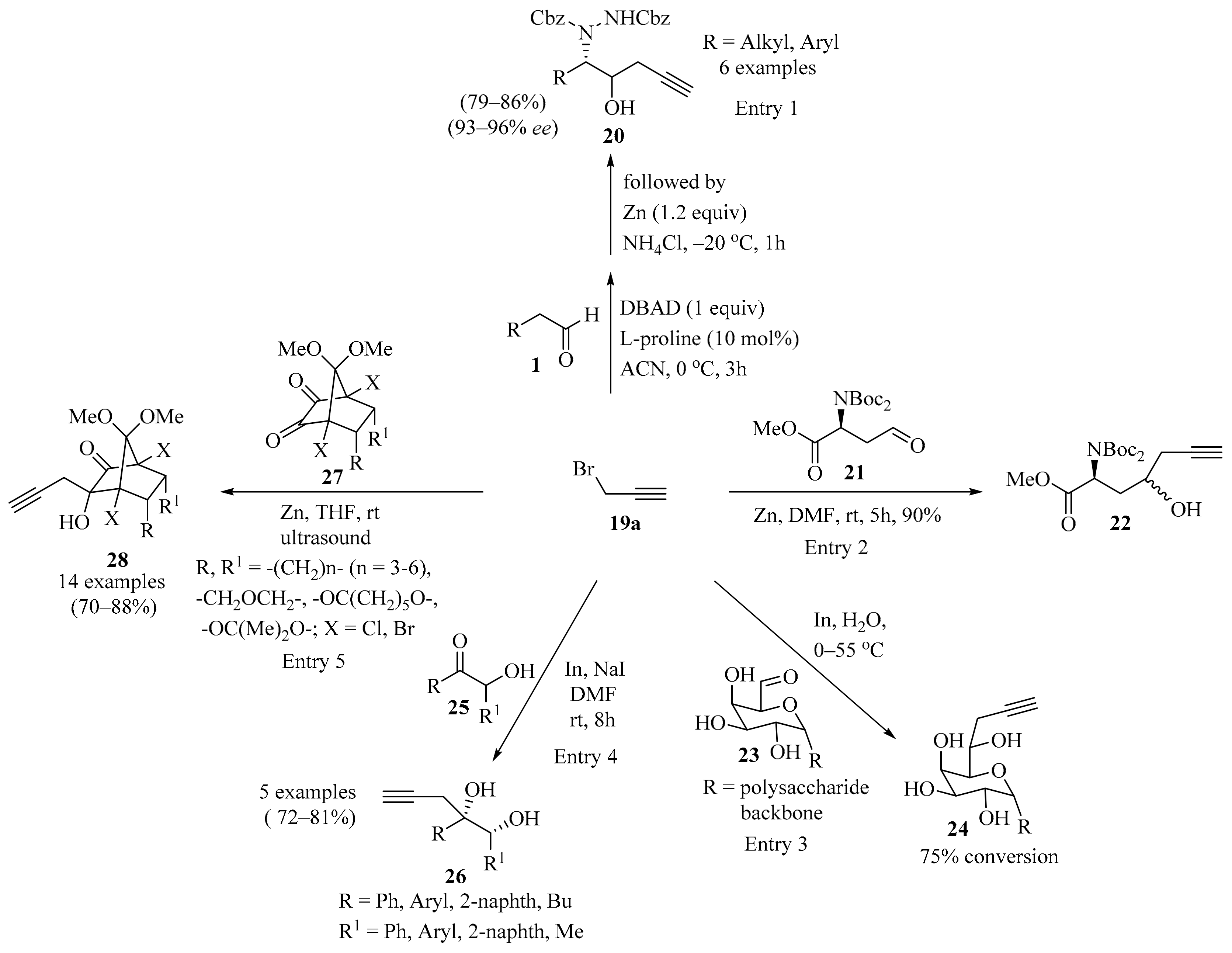
Propargylation of carbonyl compounds 1, 21, 23, 25, and 27 with propargyl bromide 19a.
A protocol for the total synthesis of (–)-epiquinamide involving the L-proline-catalyzed one-pot sequential α-amination/propargylation of aldehyde 1 (R = H) was established (Scheme 8). The synthesis was accomplished in nine steps, with the formation of homopropargyl alcohol 20 as a strategic step (entry 1) [48]. In the same way, six-step asymmetric total synthesis of the natural pyrrole lactone longanlactone was designed. The reaction involved the formation of propargyl alcohol 22 through the Zn-catalyzed Barbier propargylation of the aldehyde 21 as one of the key steps in this process (Scheme 8, entry 2) [49].
A chemo-enzymatic process was established as a useful method for the derivatization of galactose unit of spruce galactoglucomannan (GGM) and other galactose-containing polysaccharides. In this approach, a series of GGMs were selectively formylated at the C-6 position via enzymatic oxidation by galactose oxidase. The formed aldehydes 23 were further derivatized via an indium-mediated Barbier–Grignard-type reaction using propargyl bromide 19a, resulting in the formation of homoallylic alcohols 24 (Scheme 8). All the reaction steps were performed in water in a one-pot reaction. The formation of the propargylated products was identified via MALDI-TOF–MS. The polysaccharide products were isolated and further characterized via GC–MS or NMR spectroscopy. The derivatized polysaccharides 24 were considered potential platforms for further functionalization (entry 3) [50].
A stereospecific Barbier-type reaction of α-hydroxyketones 25 with propargyl bromide 19a in the presence of indium metal provided (1RS,2SR)-1,2-diarylpent-4-yne-1,2-diols 26 in good yields as single diastereomers (Scheme 8). The observed high diastereoselectivity (>99%) in 1,2-diols 26 was consistent with the Cram’s chelation model [51]. The 1,2-diols 26 were successfully used as precursors for furan synthesis through iodine-mediated 5-exo-trig cyclization, dehydration, and reductive deiodination (entry 4) [52].
Another study described diastereoselective Zn-mediated propargylation for non-enolizable norbornyl α-diketones 27. In this approach, the treatment of 27 with zinc and propargyl bromide 19a in anhydrous THF, using the Barbier procedure under ultrasound, produced the corresponding norbornyl homopropargyl alcohols 28 in good yields (Scheme 8). An analysis of the crude reaction mixtures revealed that 28 was obtained in a diastereomerically pure form, along with small amounts of allene derivatives as byproducts. Moreover, the stereochemistry of 28 was confirmed via X-ray crystal structure analysis. Subsequently, homopropargyl alcohols 28 were used as precursors for an AgI-catalyzed cycloisomerization toward diversely substituted spirocyclic dihydrofuran derivatives and produced acceptable to good yields (entry 5) [53].
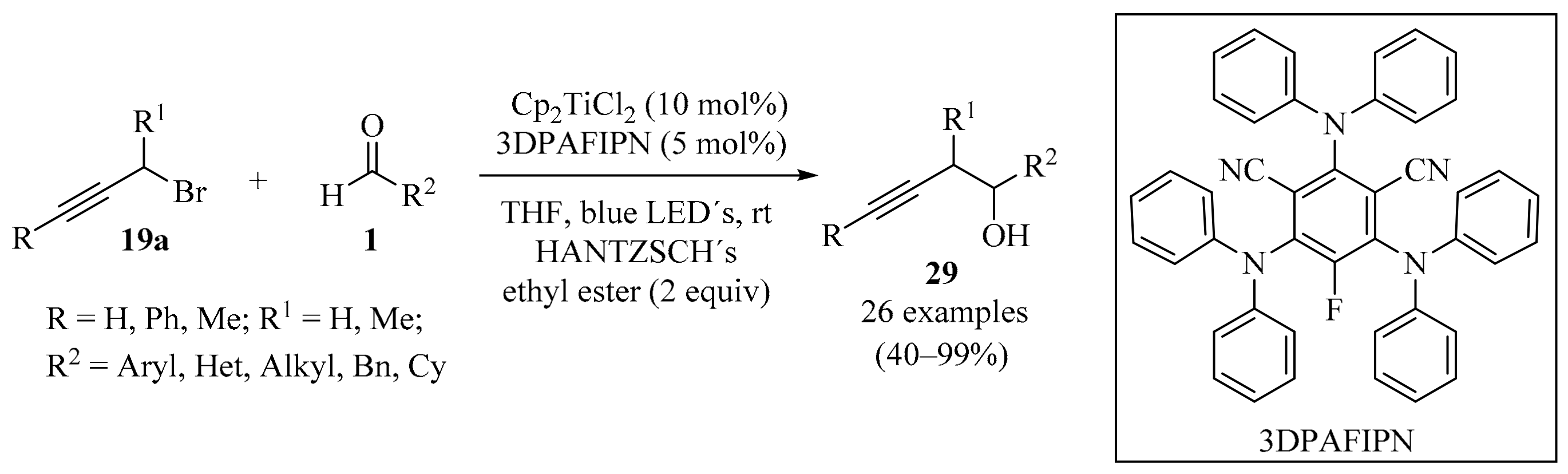
Based on the dual photoredox catalytic strategy [54,55], practical and effective photoredox propargylation of aldehydes 1 (R = H) promoted by [Cp2TiCl2] was developed (Scheme 9). The reaction did not require stoichiometric metals or scavengers, and employed a catalytic amount of [Cp2TiCl2], along with the organic dye 3DPAFIPN (as a reductant for titanium). The reaction displayed a broad scope, producing the desired homopropargylic alcohols 29 in good yields with both aromatic and aliphatic aldehydes [56].
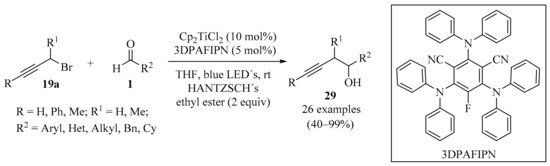
Scheme 9.
Dual photoredox-mediated catalysis with titanium for the propargylation of aldehydes 1 with propargyl bromides 19a.
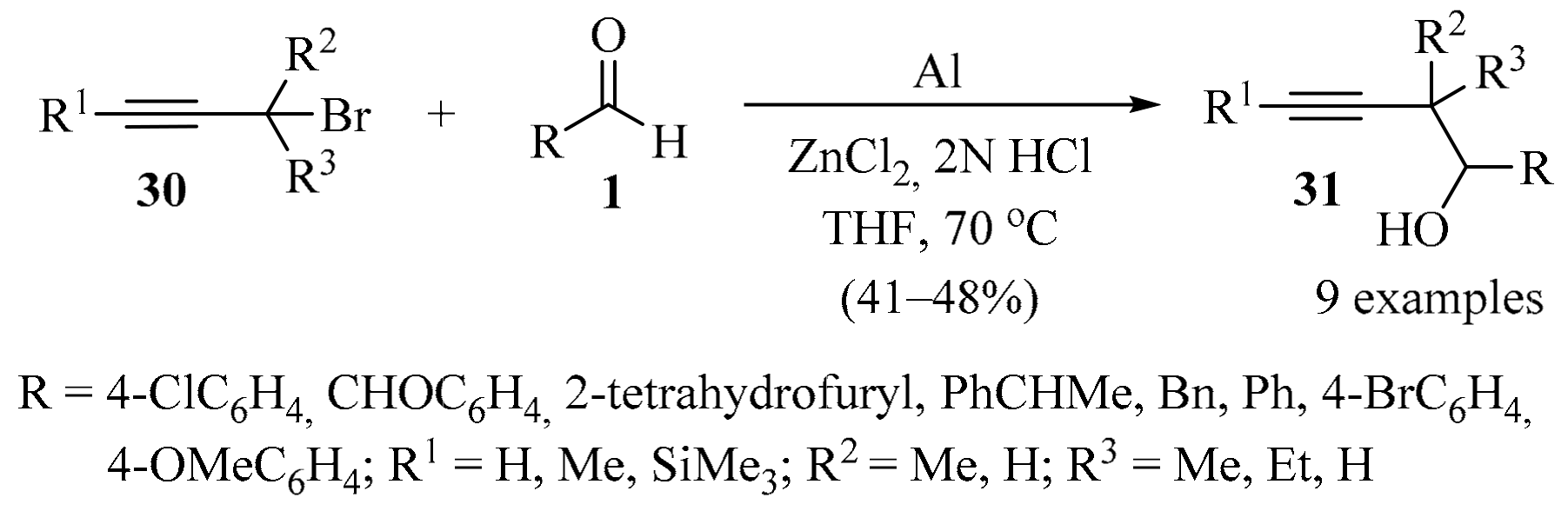
The synthesis of homopropargyl alcohol 31 with a two-carbon extension was achieved through the propargylation of aldehydes 1, mediated by zinc(0). This reagent was generated in situ from the redox coupling of Al and ZnCl2 in 2N HCl and THF, producing products 31 in acceptable to good yields (Scheme 10) [57].
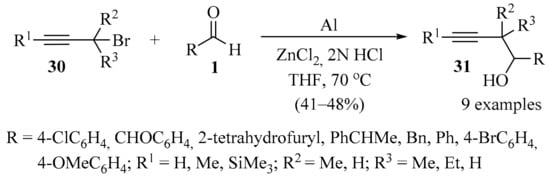
Scheme 10.
Zinc(0)-mediated synthesis of homopropargyl alcohols 31.
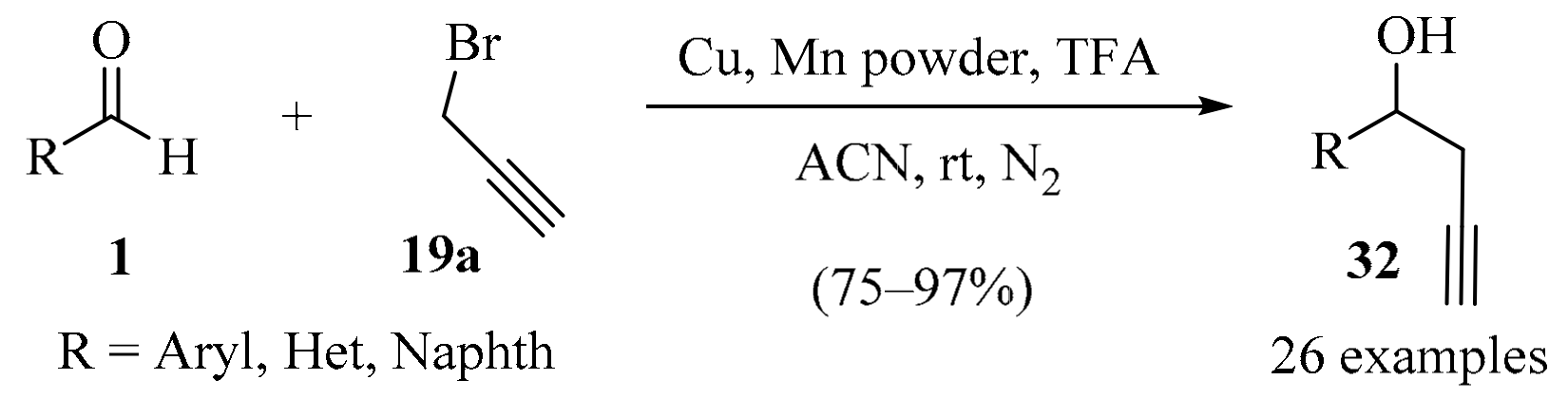
Aldehydes 1 were transformed into their corresponding homopropargyl alcohols 32 via a reaction with propargyl bromide 19a, with CuCl and Mn powder employed in the presence of TFA in ACN solvent (Scheme 11). This method proved compatible with a variety of substrates, leading to diversely substituted products 32 in high yields. A large-scale reaction was also performed, demonstrating the potential synthetic applications of this transformation [58].

Scheme 11.
Cu-Catalyzed/Mn-mediated chemo-selective synthesis of homopropargyl alcohols 32.
2.1.4. With Organometallic Propargyl Reagents
The Barbier type nucleophilic addition of functionalized halides to carbonyls mediated by metals or metal compounds constitutes an important strategy for carbon–carbon bond formation in organic synthesis [59,60,61]. In this context, an operationally simple procedure for the propargylation of aldehydes 1 in moist solvent (distilled THF) was developed through the direct addition of propargyl bromide 19a to the aldehyde substrates 1, mediated by low-valent iron or tin (Scheme 12). The metals were prepared in situ using a bimetal redox strategy. Using different aldehydes 1 as substrates, both metals proved applicable, producing homopropargyl alcohols 34 in good yields and with high chemoselectivity in most cases. Due to its efficacy, operational simplicity, performance in moist solvent, and its use of inexpensive metal/metal salts, the procedure was claimed to be practically viable and potentially scalable [62].
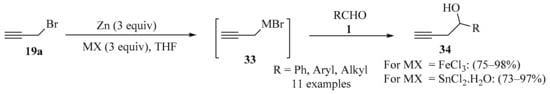
Scheme 12.
Bimetal redox synthesis of homopropargyl alcohols 34 from aldehydes 1 and propargyl bromide 19a.
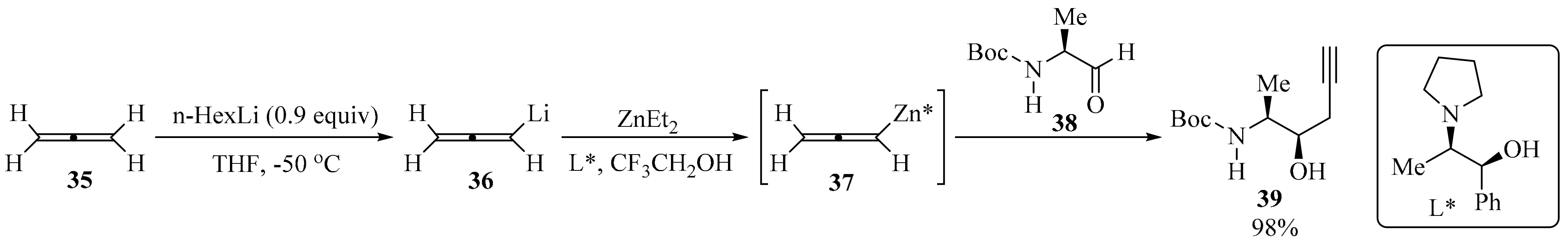
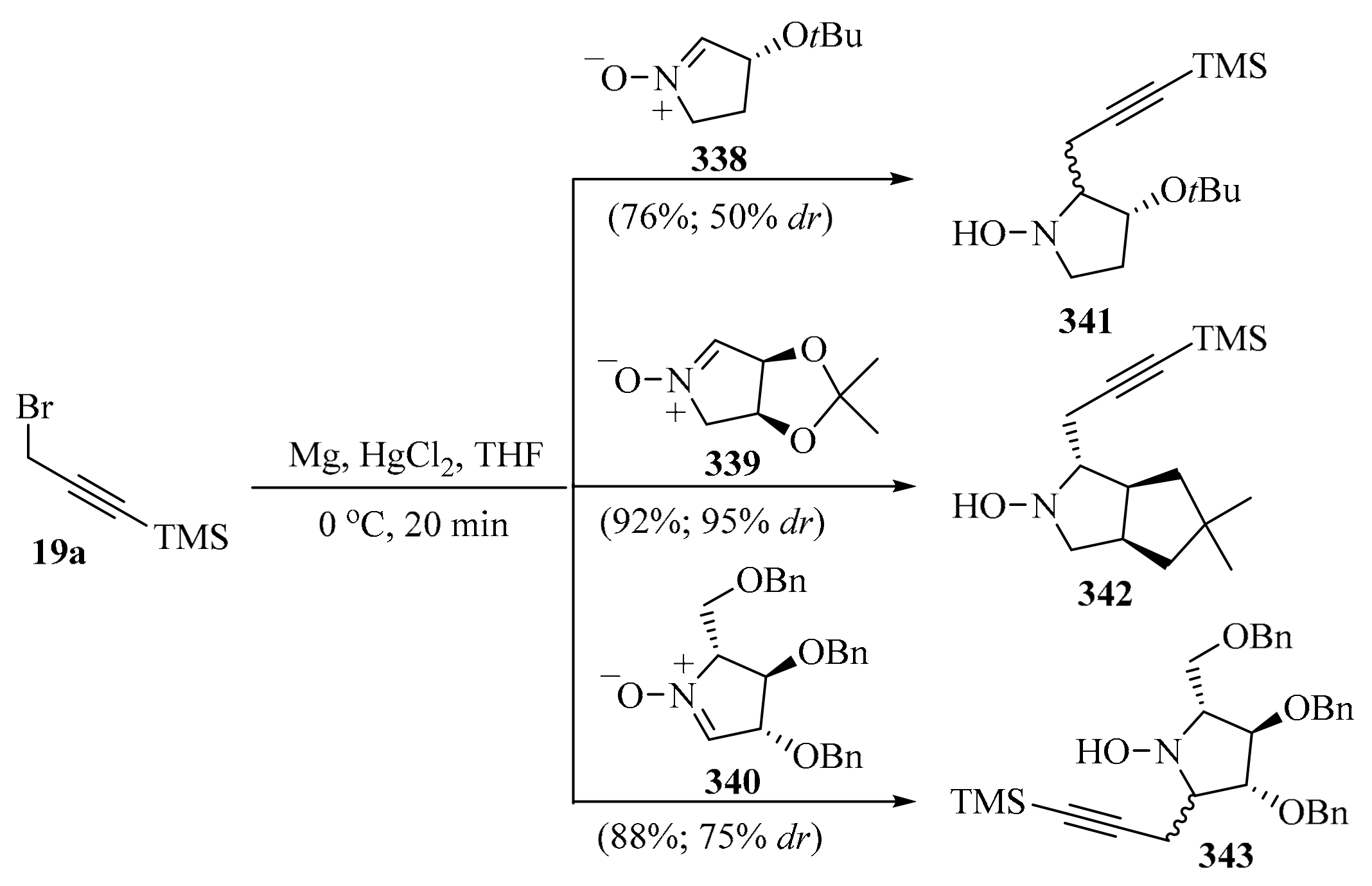
Allenyl boronic acids are widely used as propargylation reagents. These compounds are usually prepared via the Hg-catalyzed magnesiation of propargyl bromide [63]. However, the use of mercury, the corrosiveness of propargyl bromide, and the pyrophoric nature of allenyl boronic acid raise environmental and safety concerns, particularly when using these reagents for large-scale applications. To circumvent these limitations, the development of a mercury-free flow chemistry process for the asymmetric propargylation of aldehydes using allene gas 35 as a reagent was reported (Scheme 13). The connected continuous processes of allene dissolution, lithiation, Li-Zn transmetalation, and the asymmetric propargylation of the chiral aldehyde 38 provided a homopropargyl β-amino alcohol 39 with high regio- and diastereoselectivity in high yield. This flow process represents a practical use for an unstable allenyllithium intermediate 36, using the commercially available and recyclable (1S,2R)-N-pyrrolidinyl norephedrine (L*) as a ligand to promote the diastereoselective propargylation of 38 [64].
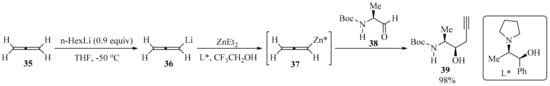
Scheme 13.
Zn-Mediated asymmetric propargylation of aldehydes 38 with allene gas 35 as reagent.
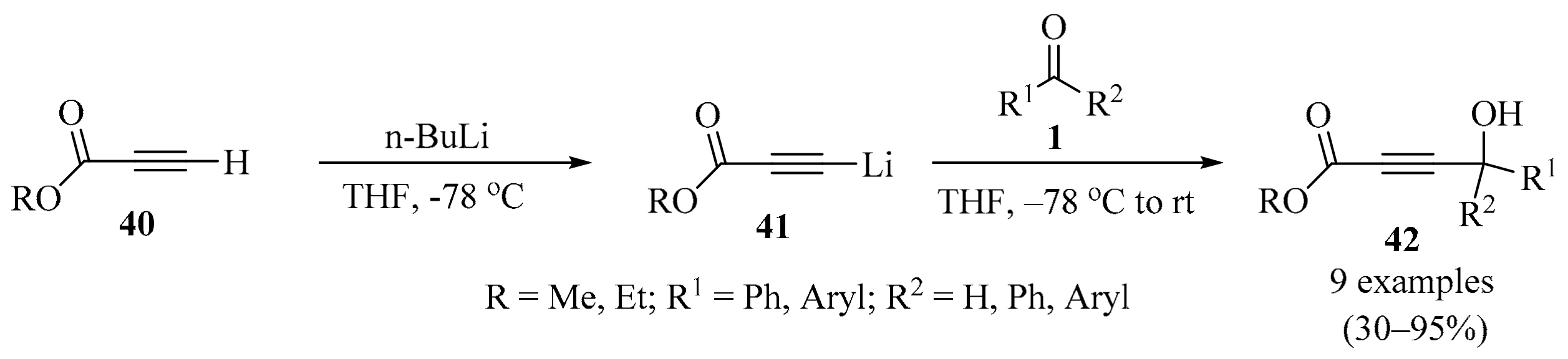
The esters of 4-hydroxybut-2-ynoic acid (alkyl 4-hydroxybut-2-ynoates) 42 are promising building blocks for organic synthesis. The presence of three important functional groups, namely the acetylene bond conjugated with the ester moiety, and the hydroxyl group of the propargyl unit in the structure of these compounds, make them highly versatile and applicable to many useful synthetic transformations [65,66,67,68,69,70]. With this in mind and based on previous works on the superelectrophilic activation of acetylene compounds [71], a series of 4-aryl(or 4,4-diaryl)-4-hydroxybut-2-ynoates 42 were obtained for further studies on their transformations under the action of various acids. The treatment of propynoates 40 with a solution of BuLi in hexanes produced lithiated intermediates in situ 41. Then, carbonyl compounds 1 were added at low temperature to form the target alkyls 4-hydroxybut-2-ynoates 42 in acceptable to excellent yields (Scheme 14) [72].

Scheme 14.
Synthesis of 4-hydroxybut-2-ynoates 42 from carbonyl compounds and lithiated propynoates 41.
Epoxides serve as both building blocks and synthetic intermediates in various organic transformations [73,74]. The conjugation of a propargyl group to an epoxide creates a highly functional small-molecule building block. A series of substituted propargyl epoxides 45 were prepared via the propargylation of α-bromoketones 43 with an organozinc reagent 44 (Scheme 15). This method complements existing synthetic methods due to the advantageous properties of the organozinc reagents, such as their availability, selectivity, operational simplicity, and low toxicity [75].
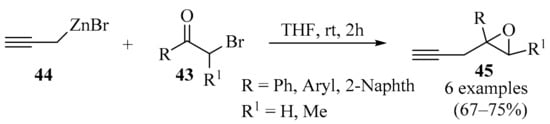
Scheme 15.
Synthesis of propargyl epoxides 45 via propargylation of α-bromoketones 43 with the propargylic organozinc reagent 44.
2.1.5. With Propargylic Ethers, Acids, and Esters
The intramolecular propargylation of aldehydes and ketones enables their entry into cyclic compounds containing a homopropargyl alcohol unit, a structural motif that is present in a variety of biologically active compounds and is highly useful for synthetic transformations [76,77]. Due to their ready availability, propargylic esters 46 [78] are logical starting points in these transformations. It has been shown that carbonyl-tethered propargylic benzoates 46 undergo intramolecular carbonyl propargylation upon treatment with Et2Zn in the presence of a catalytic amount of Pd0 to form 2-alkynylcyclopentanol products 47 (Scheme 16). Diastereoselectivity for the formation of simple homopropargylcycloalkanols 47, generated through the use of Pd0/Et2Zn, was examined as a function of the palladium phosphine ligand in the absence of further structural constraints imposed by additional substituents or rings. In this approach, a ligand/solvent effect on the cis/trans selectivity (referring to the relative positions of the alkynyl and OH groups) of ring-closure was found. In a non-coordinating solvent (benzene), increasing the electron-donating ability of the phosphine ligand (while decreasing its dissociation ability) led to an increased tendency towards the trans product, while the combination of a coordinating solvent (THF) and PPh3 resulted in the exclusive formation of cis products. The experimental and computational results were compatible with the divergent behavior of an allenyl-ethylpalladium intermediate that partitions between competitive carbonyl-addition and transmetalation pathways, each leading to a different diastereoisomers. The results also suggested that the dissociating ability of the phosphine acted as a regulating factor for this behavior [79].
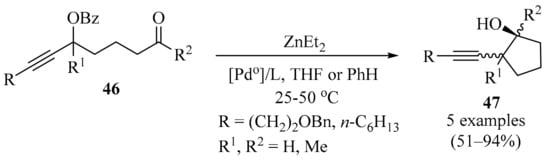
Scheme 16.
Pd0/Et2Zn-mediated synthesis of 2-alkynylcyclopentanols 47 from carbonyl-tethered propargylic benzoates 46.
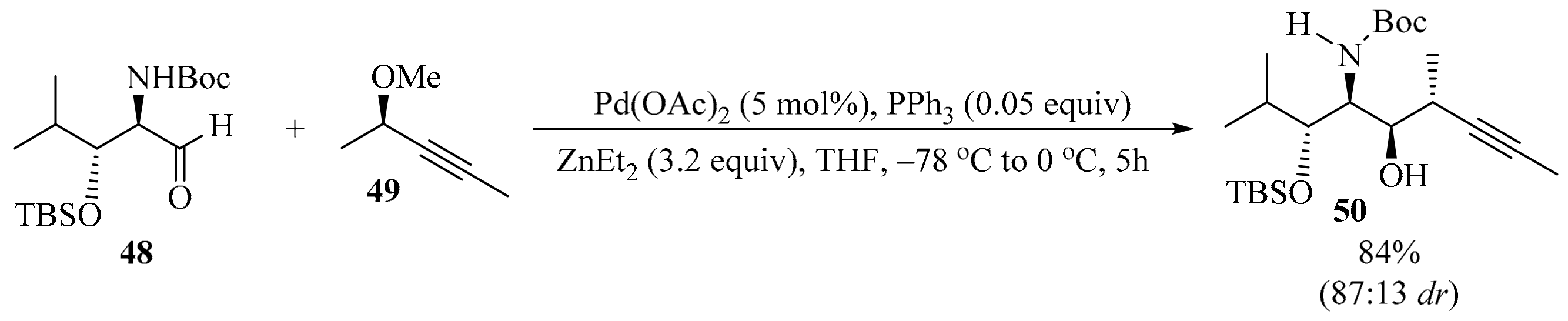
Isolated in 2008 from the marine sponge Siliquariaspongia mirabilis, mirabalin [80] was found to inhibit the growth of the tumor cell line HCT-116, with an IC50 value of 0.27 μM. This compound belongs to the chondropsin family of macrolide lactams, which comprises chondropsins A−D, 73-deoxychondropsin A, and poecillastrins A−C [81]. Alcohol 50 is a key intermediate in the convergent and flexible stereoselective synthesis of one isomer of the C44−C65 fragment of mirabalin [82]. To synthesize alcohol 50, aldehyde 48 was subjected to stereoselective Marshall allenylation [83] through the addition of a chiral allenylzinc reagent, prepared in situ via palladozincation of the (S)-propargylic mesylate 49. This method delivered propargyl alcohol 50 with good diastereoselectivity in favor of the anti,syn,anti-isomer (Scheme 17). The two diastereomers were separated via flash chromatography on silica gel.
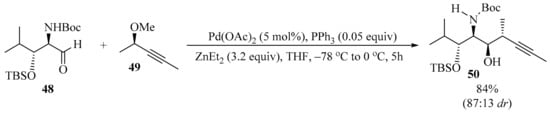
Scheme 17.
Pd-mediated stereoselective Marshall allenylation of aldehyde 48 with (S)-propargylic mesylate 49.
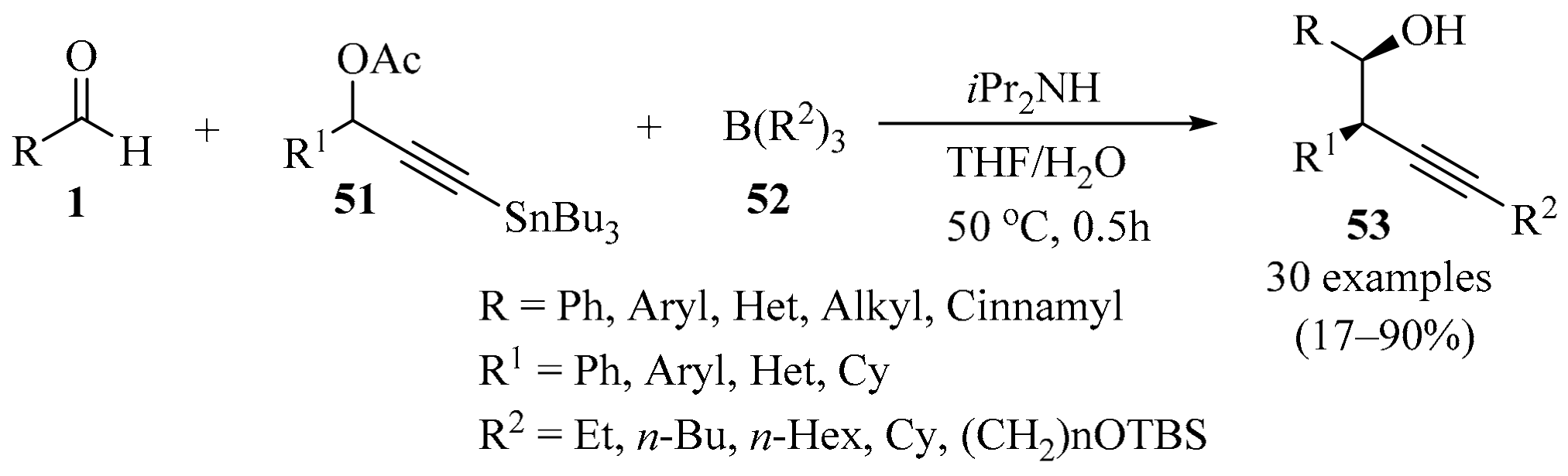
The transition metal-catalyzed carbonyl propargylation protocol is an elegant approach to the diastereo- and enantioselective construction of homopropargylic alcohols. Addition reactions of propargyl metal or metalloid to aldehydes have been widely used as general synthetic methods. Nevertheless, some limitations exist in this strategy because of its ambident nucleophile characteristics as propargyl/allenyl organometallic reagents, which open up new reaction channels and widen their synthetic scope [84,85]. To circumvent these limitations, researchers have focused on transition metal-free carbonyl propargylation for the synthesis of 1,2,4-substituted homopropargylic alcohols.
In this regard, a transition metal-free three-component process was developed by combining aldehydes 1, 3-(tributylstannyl)propargyl acetates 51 formed in situ from readily available propargyl acetates, and trialkylboranes 52, providing access to a range of 1,2,4-trisubstituted homopropargylic alcohols 53 (Scheme 18). It was found that the addition of diisopropylamine played a crucial role in the selective formation of homopropargylic alcohols 53. Importantly, this methodology could be extended to a single-flask reaction sequence starting with propargyl acetates [86].

Scheme 18.
Three-component synthesis of homopropargylic alcohols 53 mediated by 3-(tributylstannyl)propargyl acetates 51 as propargylation reagents.
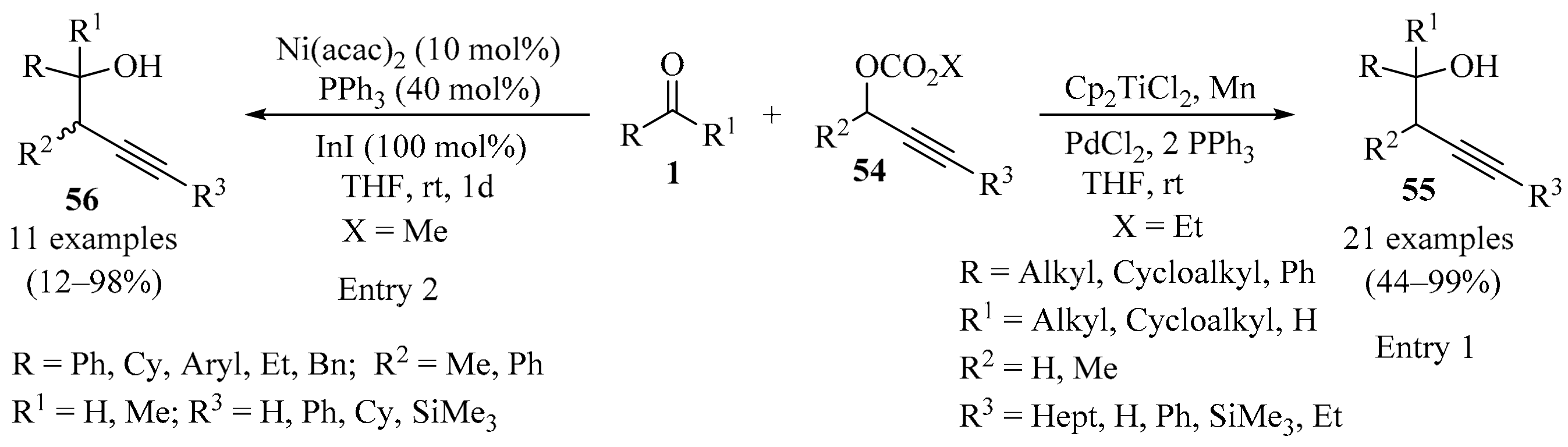
Although propargylic carbonates are readily available compounds that could potentially be used instead of the corresponding propargylic halides in the carbonyl propargylation process, they are inert under classical Barbier conditions. Whereas notable examples of the use of propargyl carbonates have been described, their applications were typically limited to aldehydes as electrophiles [78,87]. To circumvent this limitation, an efficient protocol for the synthesis of homopropargylic alcohols 55 in moderate to good yields was reported that utilized propargylic carbonates 54 as pronucleophiles (Scheme 19). This reaction is based on a combination of transition metal (palladium) and radical (titanium) chemistry, in which allenyl titanocenes and transient propargylic radicals are formed in situ as key species for the success of this multimetallic protocol. The reaction took place with excellent regioselectivity, tolerating a variety of terminal and internal alkyne functionalities of the starting propargylic carbonates 54 with different substitution patterns, as well as diverse carbonyl compounds 1 (aldehydes and ketones), thus providing a useful method for application in synthetic organic chemistry (entry 1) [88].
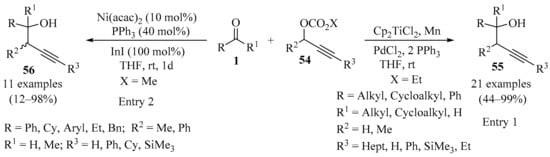
Scheme 19.
Multimetallic protocols for the synthesis of homopropargylic alcohols 55/56 from propargylic carbonates 54.
In a similar way, low-valent indium(I)-mediated nickel-catalyzed propargylation of aldehydes 1 with propargylic carbonates 54 was established. In this approach, the nickel/indium(I)-mediated reaction of the starting materials 54, which possessed different substitution patterns, produced syn-homopropargylic alcohols 56 in acceptable to high yields upon coupling with a variety of carbonyl compounds 1 (Scheme 19). Both the nickel catalyst and the phosphane ligands were found to play a crucial role in this transformation. Diastereoselectivity was also strongly dependent on the ligand employed. Moreover, a mechanistic sequence involving an umpolung of propargylnickel intermediates under the influence of low-valent indium was proposed, to account for the dependence of the stereochemical characteristics of the phosphane ligands (entry 2) [89].
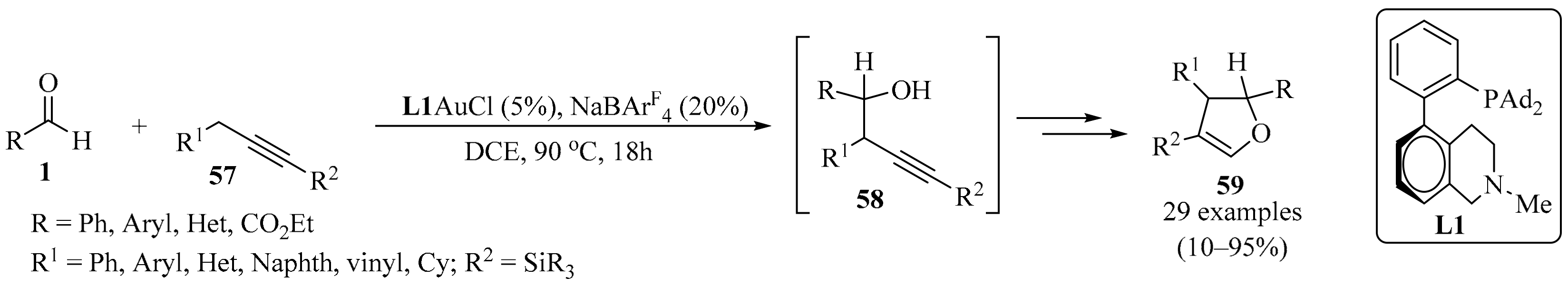
2.1.6. With Methylene-Active Propargyl Compounds
Despite extensive studies on gold catalysis, σ-allenylgold species have not been invoked as catalytic intermediates and their reactivities remain to be studied. In a recent study, the formation of an in situ-generated σ-allenylgold was proposed via soft propargylic deprotonation of the methylene-active derivatives 57, mediated by the isomerization of an alkyne to an allene. The σ-allenylgold species formed from 57 underwent nucleophilic addition to the activated aldehydes 1 in bifunctional biphenyl-2-ylphosphine (L1) ligand-enabled gold catalysis. This development revealed a broad range of opportunities to achieve the propargylic C−H functionalization of 57 under catalytic and mild conditions, producing homopropargyl alcohol intermediates 58 (Scheme 20). Subsequently, the resulting homopropargyl alcohols 58 underwent ligand-enabled cycloisomerization, involving an unexpected silyl migration process, to deliver dihydrofurans 59 as isolated products [90].
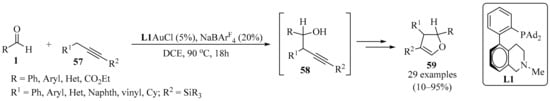
Scheme 20.
Gold-catalyzed synthesis of homopropargyl alcohol intermediates 58 from propargyl methylene-active derivatives 57 and aldehydes 1.
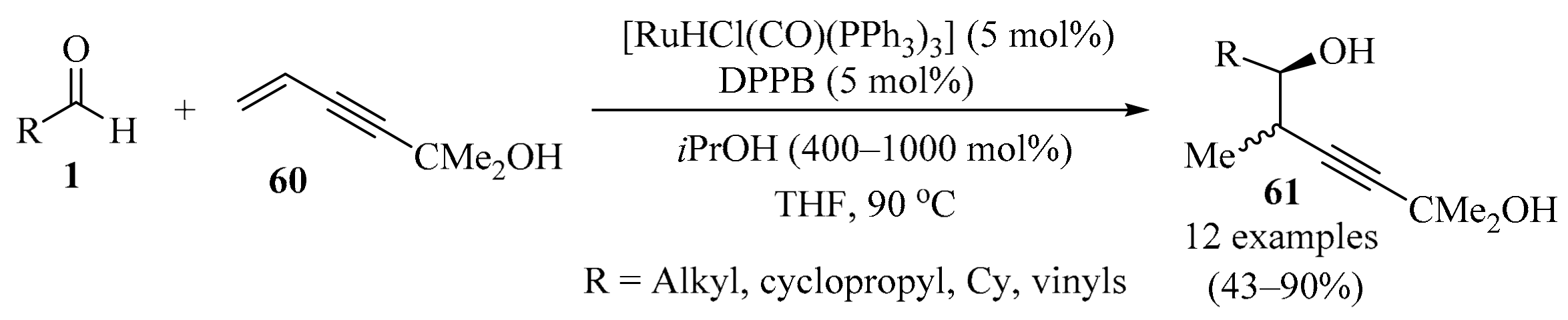
2.1.7. With 1,3-Enynes
While most methods for enantioselective carbonyl propargylation promote the formation of the parent α-unsubstituted homopropargylic alcohols, less attention has been devoted to the development of diastereo- and enantioselective propargylation protocols that generate useful (α-methyl)homopropargyl alcohols [91]. Under the conditions of ruthenium-catalyzed transfer hydrogenation, employing isopropanol as a source of hydrogen, unprotected isopropoxy-substituted enyne 60 and aldehydes 1 engaged in reductive coupling to provide propargylation product (α-methyl)homopropargyl alcohols 61 with good to complete levels of anti-diastereoselectivity (Scheme 21). Remarkably, it was found that the unprotected tertiary hydroxy moiety of isopropoxy enyne 60 is required in order to enforce diastereoselectivity. Moreover, deuterium-labeling studies corroborated reversible enyne hydrometalation in advance of carbonyl addition. Additionally, it was demonstrated that the isopropoxy group of products 61 could be readily cleaved upon exposure to aqueous sodium hydroxide to reveal the terminal alkyne functionality [92].

Scheme 21.
Ru-catalyzed synthesis of (α-methyl)homopropargyl alcohols 61 from enyne 60 and aldehydes 1.
2.1.8. With Aryl-Acetylenes
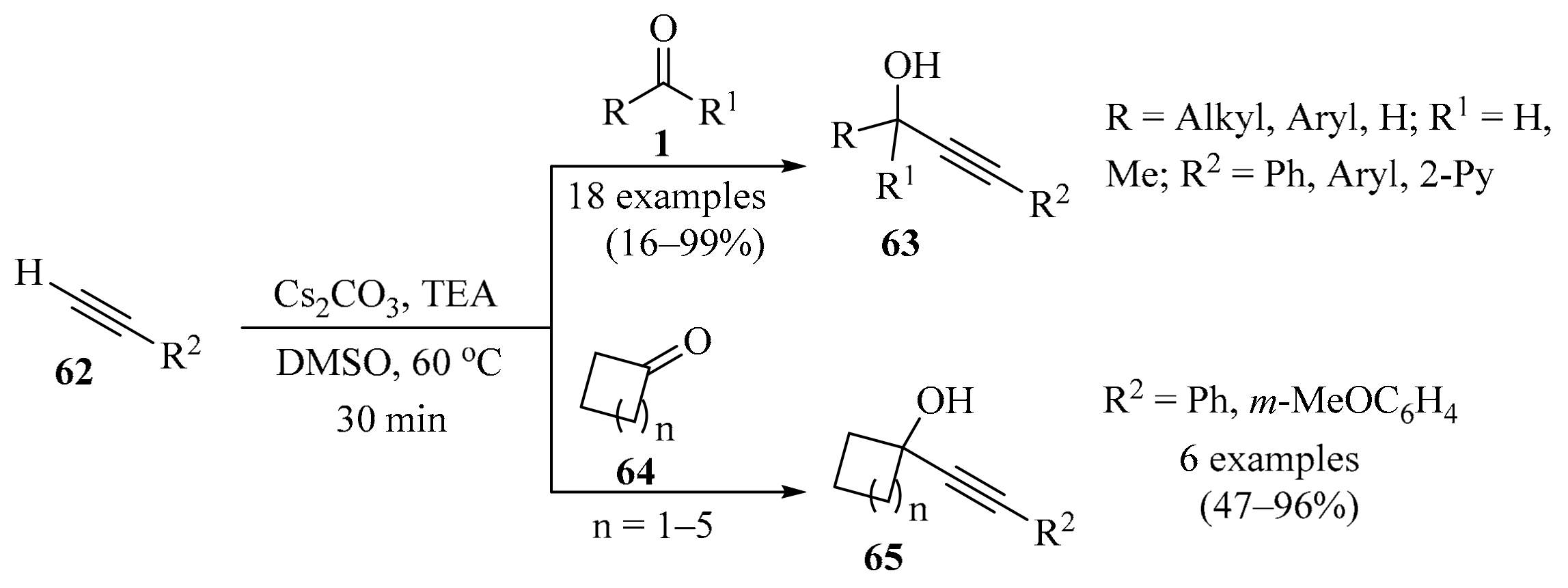
The Favorskii reaction, which involves the nucleophilic addition of alkynes to aldehydes in the presence of a strong base, has been recognized as an efficient synthetic strategy to produce propargyl alcohols and α,β-unsaturated ketones [93]. Direct propargylation/alkenylation via the allenol-enone isomerization sequence through the activation of the C-H bond in terminal alkynes, without a transition metal and employing a weak base, represents a challenging research area. In response to this, a fast and efficient transition metal-free, modified Favorskii-type direct alkynylation protocol for the synthesis of propargyl alcohols 63/65 was developed using a combination of Cs2CO3 and TEA as weak bases (Scheme 22). Aliphatic aldehydes 1 (R1 = H) produced propargyl alcohols 63, while cyclic ketones 64 furnished propargyl alcohols 65. The operationally simple protocol, wide substrate scope, and gram-scale synthesis represent key aspects of this methodology. A plausible mechanism for this transformation involving the weak base-assisted propargylation of carbonyl compounds 1 was suggested [94].

Scheme 22.
Favorskii-type direct propargylation of carbonyl compounds 1 for the synthesis of propargyl alcohols 63/65 using a combination of Cs2CO3 and TEA as weak bases.
- (b)
- Hemiacetals
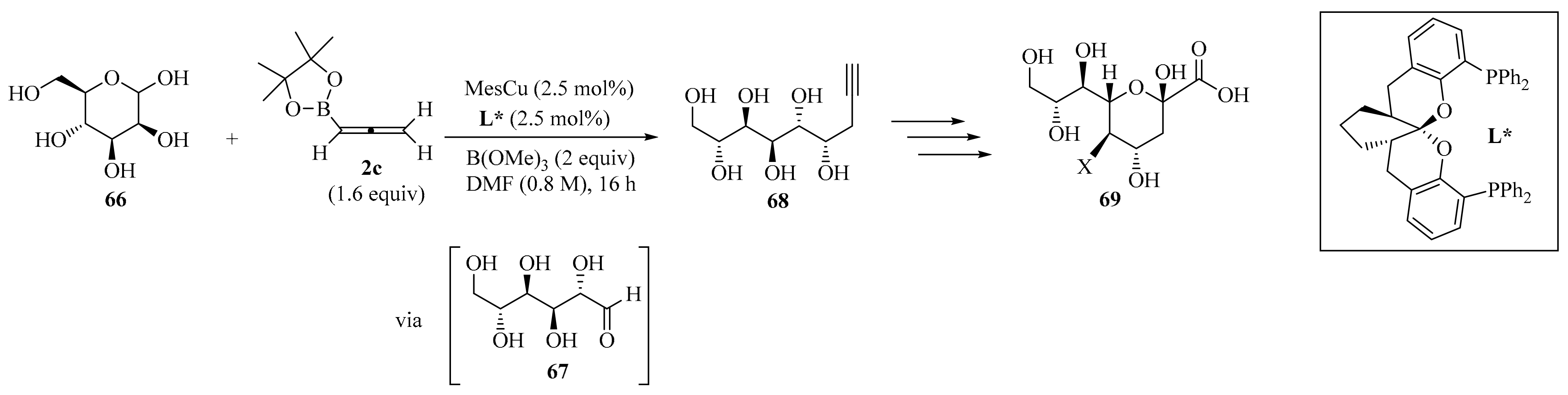
The development of copper(I)-catalyzed stereodivergent anomeric propargylation of unprotected aldose 66 was established as a facile synthetic pathway to a broad variety of sialic acid derivatives 69, via a key propargylation intermediate 68 (Scheme 23). The reaction proceeded with the in situ formation of a soft allenylcopper(I) species, catalytically generated from the stable allenylboronic acid pinacolate 2c. It was also observed that the addition of B(OMe)3 facilitated the ring-opening of the non-electrophilic cyclic hemiacetal form of aldose 66 to reach its corresponding open-chain reactive aldehyde form 67, subsequently leading to the formation of the key intermediate 68. This synthetic method, which required no protecting groups, could be performed at the gram-scale, offering general and practical access to various sialic acid derivatives from unprotected-type aldoses 66 [95].
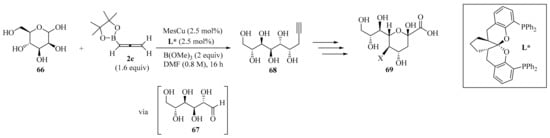
Scheme 23.
Copper(I)-catalyzed stereodivergent anomeric propargylation of unprotected aldose 66 with allenylboronic acid pinacolate 2c.
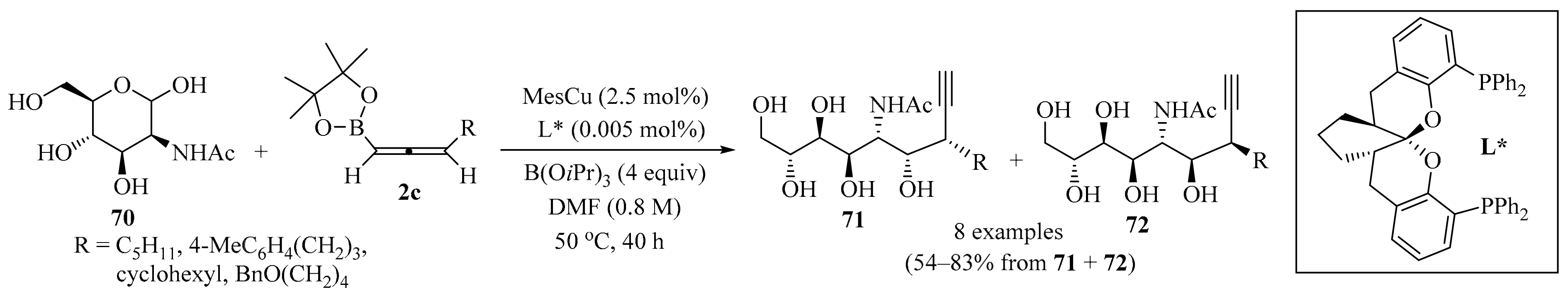
In a similar way, copper(I)-catalyzed stereodivergent nucleophilic propargylation at the anomeric carbon of unprotected N-acetyl mannosamine 70 was devised using 3-substituted allenylboronates 2c as nucleophiles (Scheme 24). The homopropargylic alcohol products 71 and 72 containing two contiguous stereocenters, and two stereoisomers out of the four possible isomers, were selectively obtained in a catalyst-controlled manner by applying either basic conditions (a MesCu/(R,R,R)-Ph-SKP catalyst with a B(OiPr)3 additive) or acidic conditions (a CuBF4/(S,S,S)-Ph-SKP catalyst with an MeB(OiPr)2 additive). In the following two steps, the propargylation products 71 and 72 were transformed into C3-substituted sialic acids without the use of protecting groups [96].
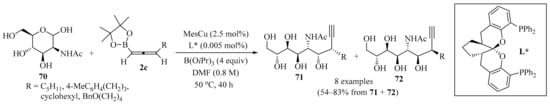
Scheme 24.
Copper(I)-catalyzed stereodivergent nucleophilic propargylation of the unprotected N-acetyl mannosamine 70 using 3-substituted allenylboronates 2c as nucleophiles.
2.2. (a) Imines, (b) Iminium, and (c) Azo Compounds
- (a)
- Imines
The addition of organometallic reagents to imines is one of the most useful and versatile methodologies for creating both a new carbon–carbon bond and new amine functionality [97]. When a propargyl organometallic reagent is used [98], via diverse synthetic strategies, the process offers the possibility for further transformation of the unsaturation to form more carbon–carbon or carbon–heteroatom bonds [99], thus giving practical use to this synthetic approach.
2.2.1. With Propargyl Halide/Metal Reagents
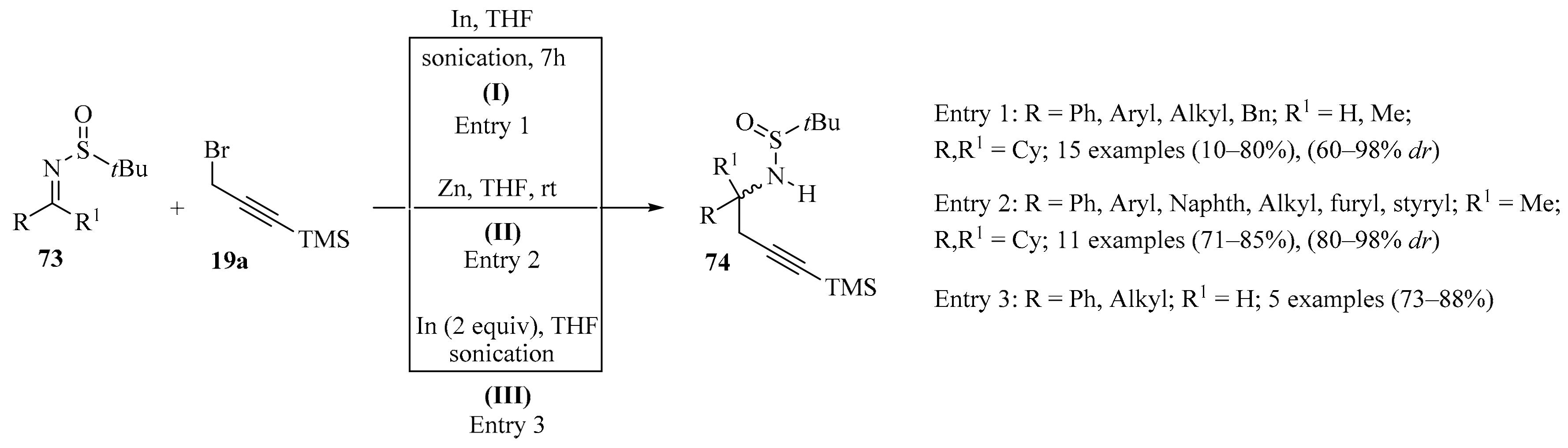
The enantio- and/or diastereoselective version of the propargylation of imines is of additional interest because at least one new stereogenic center is created [100]. Moreover, α- or γ-substitution in the imine reagent could also induce chemoselectivity in this process because the propargyl moiety could be selectively added to the structure of the product [101]. Using this approach, the diastereoselective Barbier-type addition of allyl halides to chiral sulfinylimines 73, promoted by indium metal [102], resulted in the formation of chiral N-protected homoallylic amines in good yields and % dr. More specifically, the reaction of different chiral imines 73, derived from aldehydes or ketones, with the silylated propargyl bromide 19a under sonication, in the presence of indium metal, led mainly or exclusively to the formation of protected homopropargylamines 74 in a diastereoselective manner (Scheme 25, entry 1). Of special interest in this process are the ketimine derivatives 73 (derived from ketones) because the new stereocenter has a quaternary configuration. Further, selective deprotection of the two protecting groups (TMS and sulfinyl moieties) was accomplished using conventional methods [103].

Scheme 25.
Diverse synthetic approaches of homopropargylamines 74 to the reaction of chiral sulfinylimines 73 and the silylated propargyl bromide 19a.
In another approach, a highly efficient method for the asymmetric synthesis of a wide range of quaternary carbon-containing homopropargylic amines 74 via the Zn-mediated asymmetric propargylation of N-tert-butanesulfinyl ketimines 73 was reported (Scheme 25, entry 2). In this approach, the ketimines 73 were readily prepared according to known procedures [104], producing products 74 in good yields and with high diastereoselectivities [105].
A series of enantioenriched homopropargylic amines 74 were obtained in good yields and with excellent diastereomeric ratios via the indium-mediated N-propargylation of chiral N-tert-butanesulfinyl ketimines 73 using trimethylsilylpropargyl bromide 19a, in the presence of indium metal, under sonication (Scheme 25, entry 3). Further, the chiral amines 74 were used as starting materials to obtain access to 3-substituted 1,2,3,4-tetrahydroisoquinoline derivatives in their enantioenriched form [106].
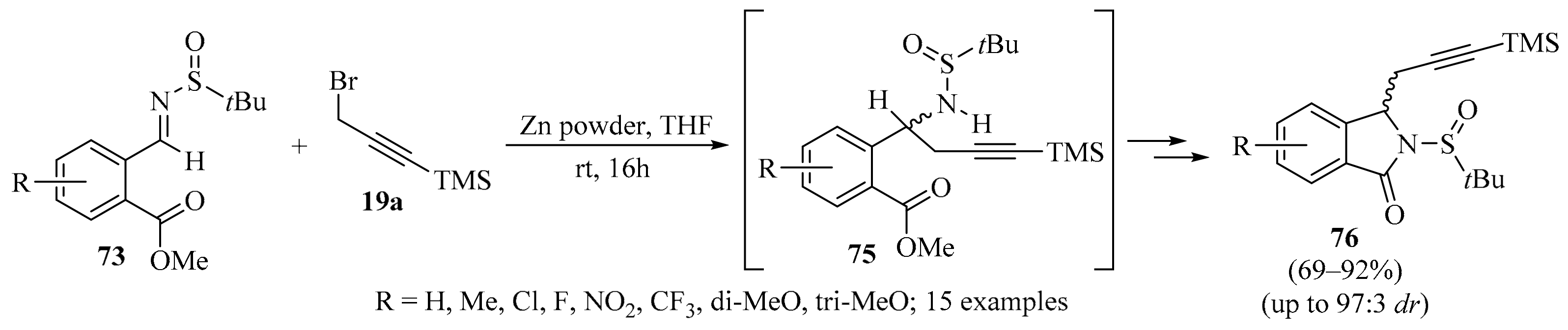
A Zn-mediated propargylation/lactamization cascade reaction with chiral 2-formylbenzoate-derived N-tert-butanesulfinyl imines 73 (R = aryl, R1 = H) was realized, as described in Scheme 26. In this strategy, sulfinyl amines 75 were obtained as intermediates, providing a practical and efficient method for the synthesis of chiral isoindolinones 76. Moreover, high diastereoselectivities and good reaction yields were observed for the majority of the examined cases [107].
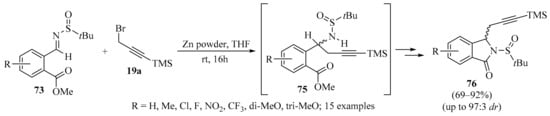
Scheme 26.
Zn-mediated propargylation/lactamization cascade reaction of chiral 2-formylbenzoate-derived N-tert-butanesulfinyl imines 73 and silylated propargyl bromide 19a.
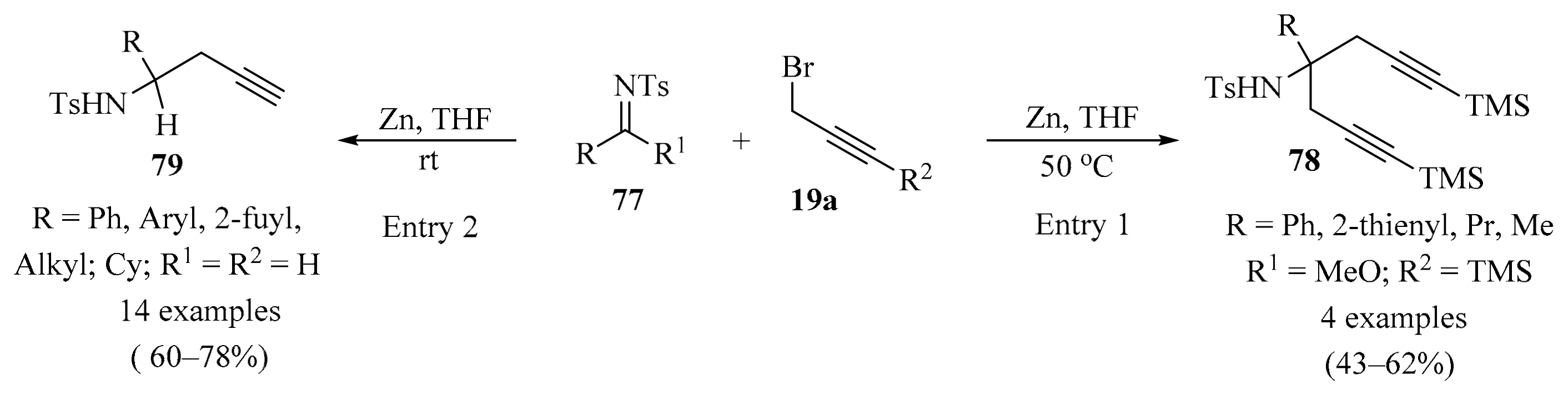
An efficient approach to the synthesis of α,α-bispropargyl-substituted amines 78 in acceptable yields was achieved via Zn-promoted aza-Barbier-type reactions of N-sulfonyl imidates 77 with various propargyl reagents 19a (Scheme 27, entry 1). The synthetic utility of this approach was demonstrated via the rapid construction of pyrrolidine derivatives [108]. In a similar way, a one-pot method for the synthesis of homopropargylic N-sulfonylamines 79 from aldehydes catalyzed by zinc powder was described. The imine derivatives 77 were obtained in situ as intermediates from a reaction between the corresponding aldehydes 1 and TsNH2 in the presence of BnBr and Zn. This procedure offers simplicity, good yields, and was shown to be applicable to a variety of aldehydes (Scheme 27, entry 2) [109].
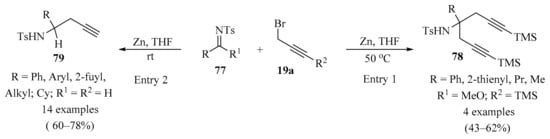
Scheme 27.
Zn-promoted synthesis of mono and α,α-bispropargyl-substituted amines 79/78 from N-sulfonyl imidates 77 and various propargyl reagents 19a.
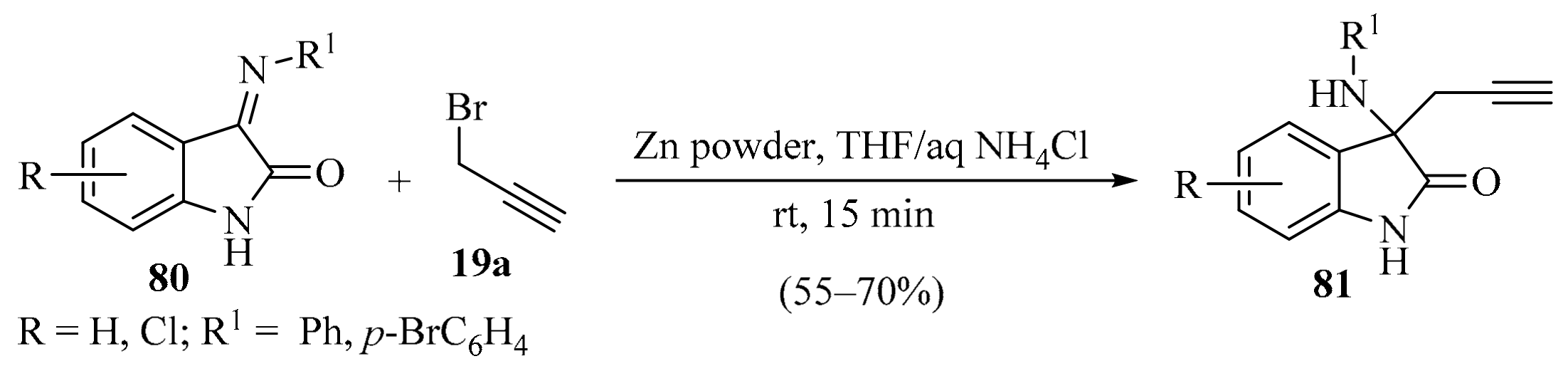
The synthesis of 3-propargylated 3-aminooxindoles 81 was carried out via the zinc-mediated propargylation of isatin-derived imines 80 (Scheme 28). This approach avoided the use of catalysts, severe reaction conditions, multistep procedures, and reaction additives. To demonstrate its synthetic utility, different isatin-derived imines 80 and propargyl bromide 19a were used to obtain products 81 in good yields [110].
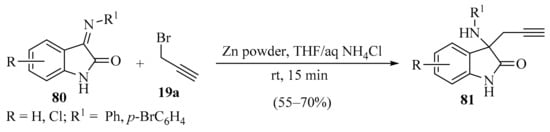
Scheme 28.
Zinc-mediated propargylation of isatin-derived imines 80 using propargyl bromide 19a as propargylation reagent.
2.2.2. With Propargyl/Allenyl Boron Reagents
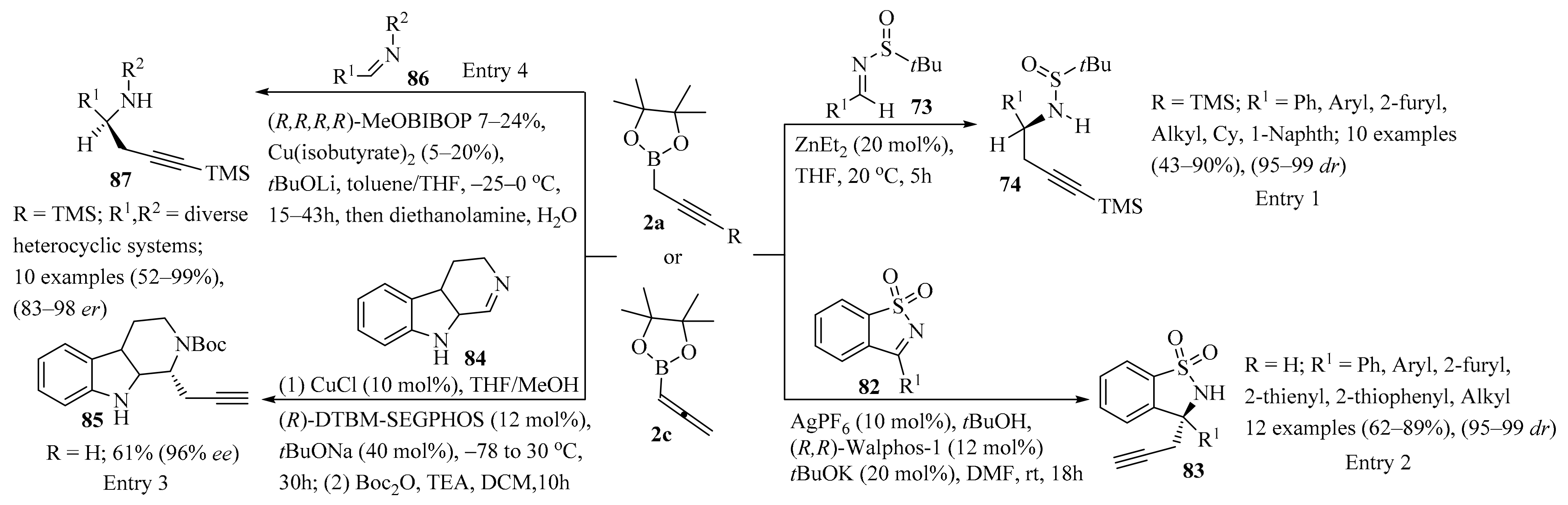
Expanding the available methods for the synthesis of homopropargylic amines, zinc-catalyzed diastereoselective propargylation of tert-butanesulfinyl imines 73 using propargyl borolanes 2a was reported (Scheme 29, entry 1). This method produced both aliphatic and aryl homopropargylic amines 74 in acceptable to good yields and with good stereoselectivity. The utility of the homopropargylic amines 74 was demonstrated in the synthesis of a cis-substituted pyrido-indole through diastereoselective Pictet-Spengler cyclization [111].
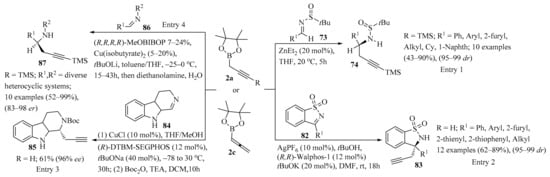
Scheme 29.
Propargyl-/allenylboron-mediated synthesis of diverse propargyl derivatives 74/83/85/87 from imine substrates 73/82/84/86. In entries 2 and 3, the synthetic equivalent allenyl-Bpin 2c was used instead propargyl-Bpin 2a.
Allenylborolane 2c (instead of propargyl borolane 2a) was employed in the enantioselective Ag-catalyzed propargylation of N-sulfonylketimines 82 (Scheme 29, entry 2). The reaction was compatible with a wide variety of diaryl- and alkylketimines 82, producing their respective homopropargylic sulfonamides 83 in high yields and in excellent enantiomeric ratios. It was also found that both propargyl and allenylborolane reagents (2a and 2c) could be used to obtain homopropargylic products 83, and a mechanism involving transmetalation of the borolane reagent 2c with a silver catalyst was proposed. Further, the homopropargylic products 83 were used as starting materials to elaborate diverse products of higher complexity with high stereochemical fidelity, including enyne ring-closing metathesis, Sonogashira cross-coupling, and reduction reactions [112].
The catalytic asymmetric propargylation of 3,4-dihydro-β-carboline 84 with allenylborolane 2c (instead of propargyl borolane 2a) was investigated (Scheme 29, entry 3). Optimization of the reaction conditions in the presence of CuCl and (R)-DTBM-SEGPHOS ligands gave chiral scaffolds 85 with reproducible results, good yields, and high ee values. Further transformations of 85 via designed Au(I)/Ag(I)-mediated 6-endo-dig cyclization directly delivered the indolenine-fused methanoquinolizidine core of the akuammiline alkaloid strictamine in its native oxidation state [113].
The copper-catalyzed asymmetric propargylation of cyclic aldimines 86 was also reported. Asymmetric propargylation of a diverse series of N-alkyl and N-aryl aldimines 86 with propargyl borolanes 2a was achieved, producing the corresponding chiral propargylamine scaffolds 87 with good to high asymmetric induction (Scheme 29, entry 4). The utility of products 87 was further demonstrated via titanium-catalyzed hydroamination and reduction to generate the chiral indolizidines (−)-crispine A and (−)-harmicine alkaloids. Moreover, the influence of the trimers of imines 86 on inhibiting the reaction was identified, and equilibrium constants between the monomers 86 and their trimers were determined for general classes of imines [114].
2.2.3. With Propargyl/Allenyl-MX reagents
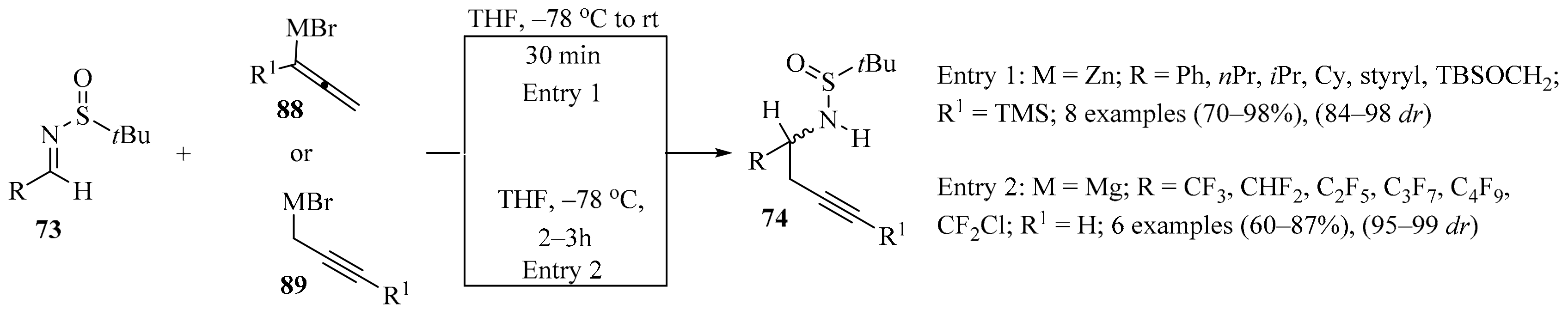
The diastereoselective synthesis of enantiopure homopropargylic amines 74 via the propargylation of various N-tert-butylsulfinylimines 73 with 1-trimethylsilyl allenylzinc bromides 88 was achieved (Scheme 30, entry 1). In this approach, the full conversion of imines 73 was observed when two equivalents of Zn derivatives 88 were used, giving homopropargylic amines 74 as single isomers in very good isolated yields [115].
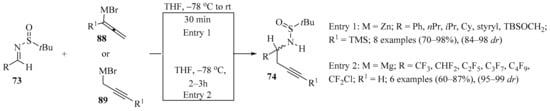
Scheme 30.
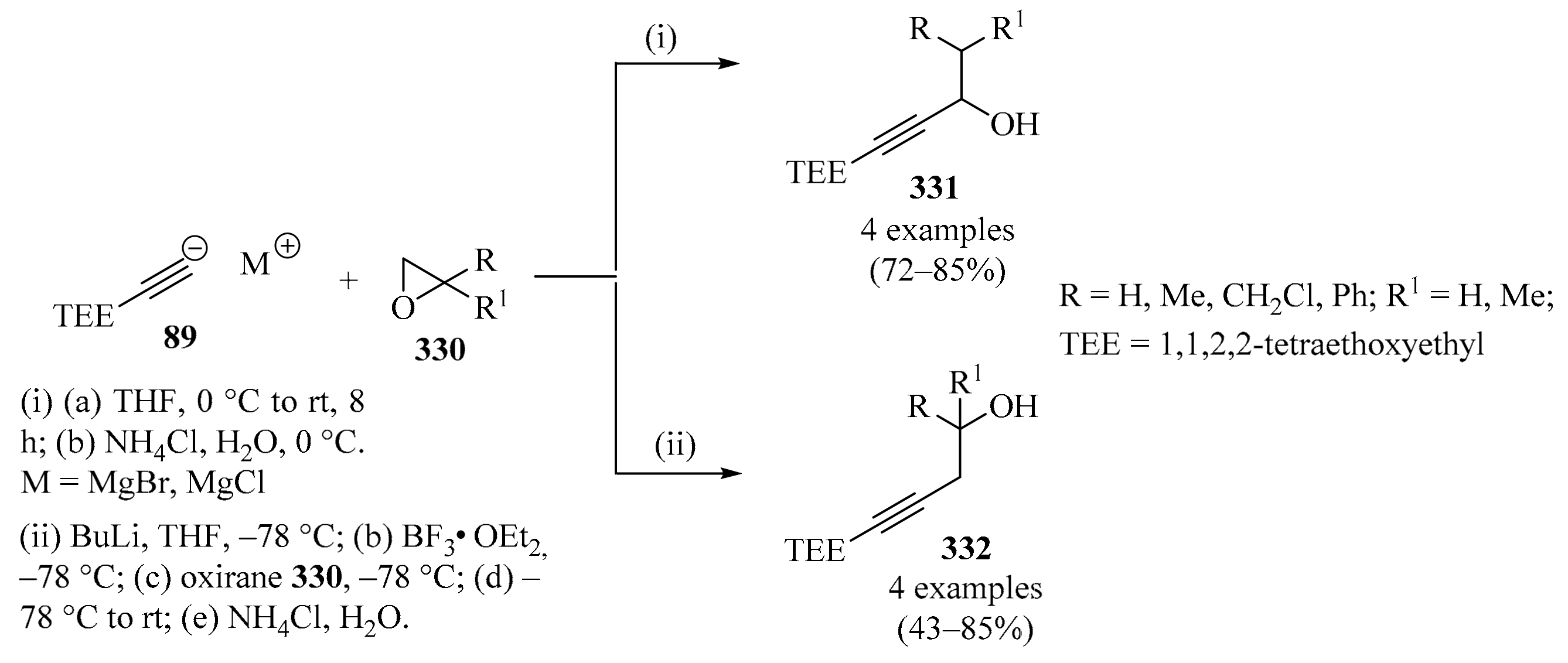
Diastereoselective synthesis of enantiopure homopropargylic amines 74 via propargylation of sulfinylimines 73 with allenylzinc/propargylmagnesium bromides 88/89.
The fluorinated analogs of tert-butanesulfinyl imines 73 were considered convenient precursors for a synthetic route to obtaining enantioenriched fluorinated monoterpenic alkaloid analogues via a Pauson–Khand cyclization reaction [116]. In this approach, diastereoselective propargylation of 73 was implemented as the key step to introducing the chiral information necessary for the rest of the synthetic sequence to be performed. In the first assay, the addition of propargyl magnesium bromide 89 to sulfinyl imine 73 (R = CF3) in DCM resulted in the formation of homopropargylamine 74 (R = CF3) with low diastereoselectivity. When DCM was replaced with THF, not only was the diastereoselectivity vastly improved, but the major diastereoisomer was actually the opposite of the one observed in DCM. Following the latter reaction conditions, sulfinyl amines 74 were obtained in good yields with high diastereoselectivity (Scheme 30, entry 2).
The dramatic effect of the solvent in this type of transformation was attributed to differing transition states depending on the nature of the solvent, but it was also suspected that the strong electron-withdrawing characteristics of the fluorinated groups of substrates 73 played a role in increasing the reactivity of the imines 73 and decreasing the difference in energy between the two transition states in non-coordinating solvents such as DCM [116].
2.2.4. With Imino-Masked Propargyl Reagents
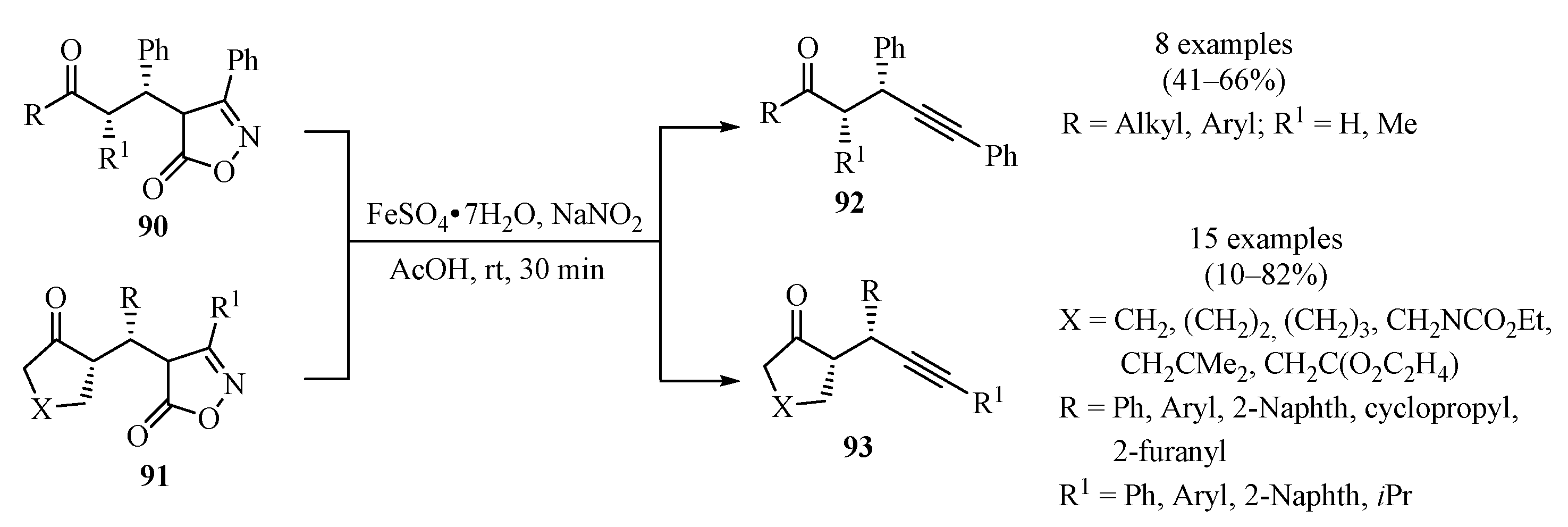
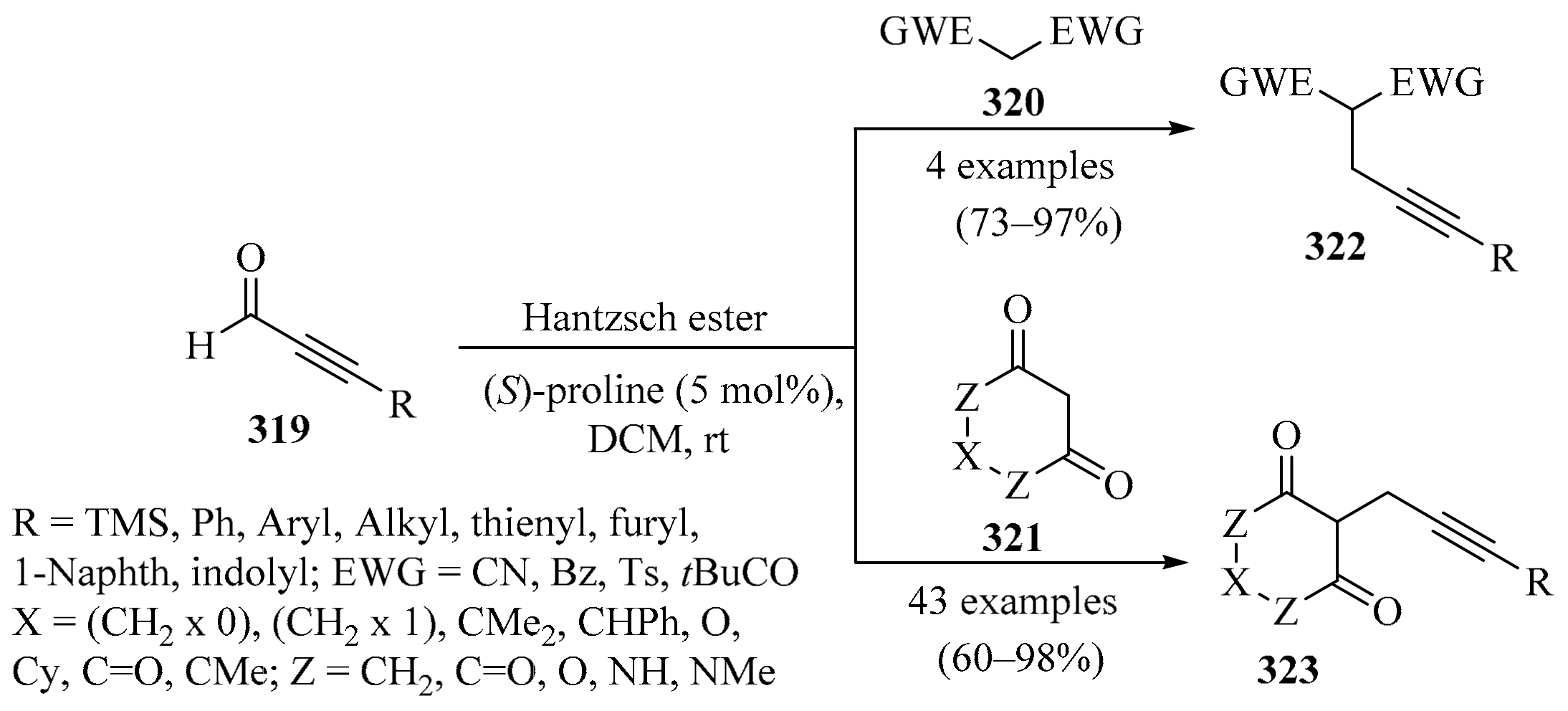
Whereas the development of methods for the α-alkylation of carbonyl compounds has advanced tremendously in recent years, catalytic enantioselective α-propargylation is relatively less developed [117,118]. In response to this, a two-step reaction sequence for the asymmetric formal α-propargylation of ketones was introduced (Scheme 31). This approach took advantage of the amino-catalyzed conjugate addition of ketones to alkylidene isoxazol-5-ones, producing intermediates 90/91, which, through a controlled nitrosative degradation event, produced α-propargyl ketones 92/93 in moderate to good yields, with perfect diastereocontrol, good to excellent enantioselectivity, and broad structural scope [119].
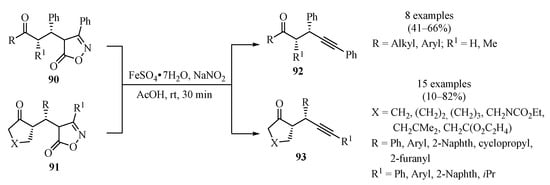
Scheme 31.
Fe-catalyzed enantioselective synthesis of α-propargyl ketones 92/93 via controlled nitrosative degradation of the alkylidene isoxazol-5-ones 90/91.
- (b)
- Iminium Compounds
2.2.5. With Propiolic Acids
Thermal-induced transition metal-catalyzed decarboxylative coupling reactions are recognized as a powerful tool in organic synthesis and medicinal chemistry as they require simple operation and produce CO2 as a byproduct [120,121,122]. Based on previous works in which dipropargylic amines were obtained as side products mediated by isobutylboronic acid reagents [123], the expansion of this chemistry led to the development of a more flexible approach for the synthesis of dipropargylic amines from primary amines, formaldehyde, and propiolic acids under metal-free conditions. After assaying different reaction conditions, a method in which a mixture of amine 94 (R1 = H), formaldehyde, and propiolic acid 95 in DCE was heated in a sealed tube produced optimal yields of the target dipropargylic amines 96 (Scheme 32). The method exhibited a broad range of functional group compatibility for primary amines 94 and propiolic acids 95, and produced the corresponding products 96 in low to excellent yields [124].
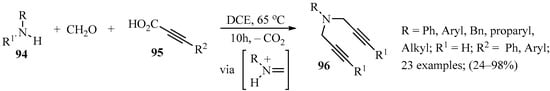
Scheme 32.
Three-component synthesis of dipropargylic amines 96 mediated by a thermally induced metal-free decarboxylative transition process.
2.2.6. With Acetylene Derivatives
A series of N-heterocyclic silylene-stabilized monocoordinated Ag(I) cationic complexes weakly bound to free arene rings (C6H6, C6Me6, and C7H8) were synthesized, and the efficacy of these electrophilic Ag(I) complexes as catalysts was investigated toward A3-coupling reactions, producing a series of propargylamines 97 in good to excellent yields in a tricomponent reaction of amines 94, acetylenes 62, and polyformaldehyde (Scheme 33). The process was accompanied by the in situ formation of an iminium species from 94 and polyformaldehyde. The best results were obtained when catalyst A was used, with low catalyst loading under solvent-free conditions [125].
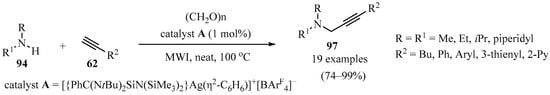
Scheme 33.
Synthesis of propargylamines 97 mediated by N-heterocyclic silylene-stabilized monocoordinated Ag(I) cationic complexed under solvent-free conditions.
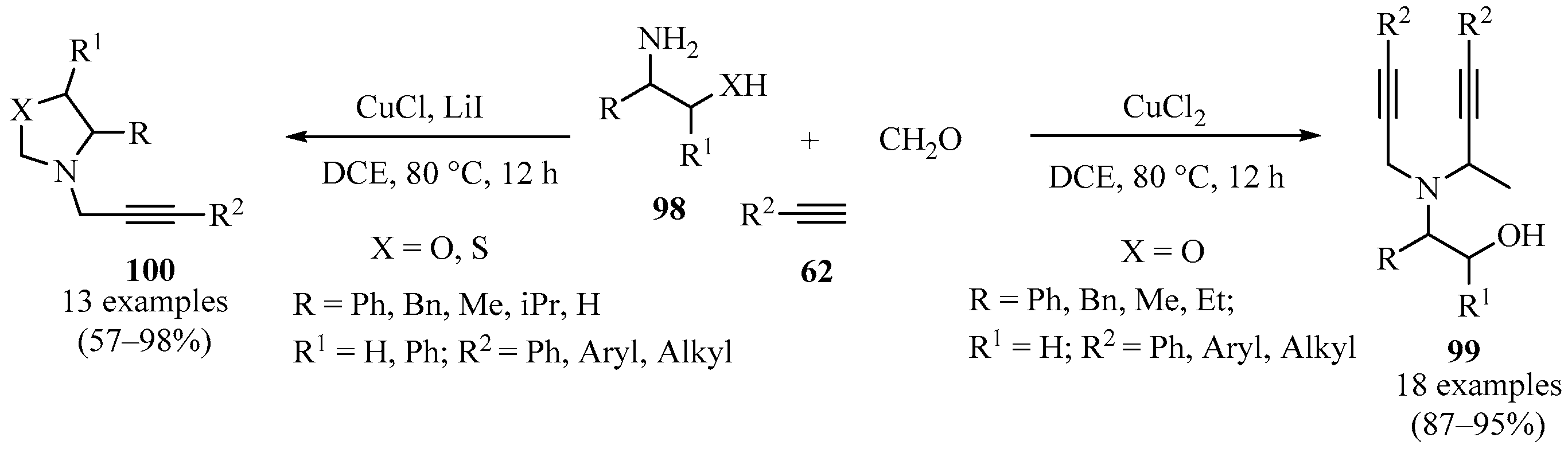
A library of N-propargyl oxazolidines and N,N-dipropargyl vicinal amino alcohols was prepared through a multicomponent reaction of formaldehyde, β-aminoalcohols 98, and acetylenes 62 using a copper-catalyzed A3-type-coupling process (Scheme 34). Whereas the presence of bromide and chloride ions accelerated the process toward open-ring alkynylation, producing dipropargylated products 99, the presence of the catalytic system Cu/I favored the formation of propargyl oxazolidines 100 [126].
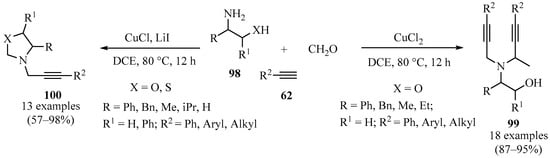
Scheme 34.
Synthesis of N,N-dipropargyl aminoalcohols 99 and N-propargyl oxazolidines100 via copper-catalyzed A3-type-coupling.
- (c)
- Azo compounds
2.2.7. With Propargyl Halides
The addition of propargylic or allenylic metal reagents to azo compounds is a convenient method for the preparation of propargylic hydrazines [127,128]. Expanding on earlier studies, the Barbier-type propargylation of azo compounds 101 with propargylic halides 19 that utilizes reactive barium as a low-valent metal in THF as solvent was reported (Scheme 35), providing diverse propargylic hydrazines 102 regioselectively in moderate to high yields. The corresponding α-adducts 102 were exclusively formed not only from azobenzenes (diaryldiazenes) but also from dialkyl azodicarboxylates. The method was also applicable to γ-alkylated and γ-phenylated propargylic bromides 19. Notably, the ester moieties of dialkyl azodicarboxylates remained unaffected by the barium reagent, thus providing the corresponding propargylated compounds 102 as unique products [129].

Scheme 35.
Barium-induced Barbier-type propargylation of azo compounds 101 with propargylic halides 19.
2.3. Aryl and Heterocyclic Derivatives
- (a)
- Aryl derivatives
2.3.1. With Propargyl-TMS
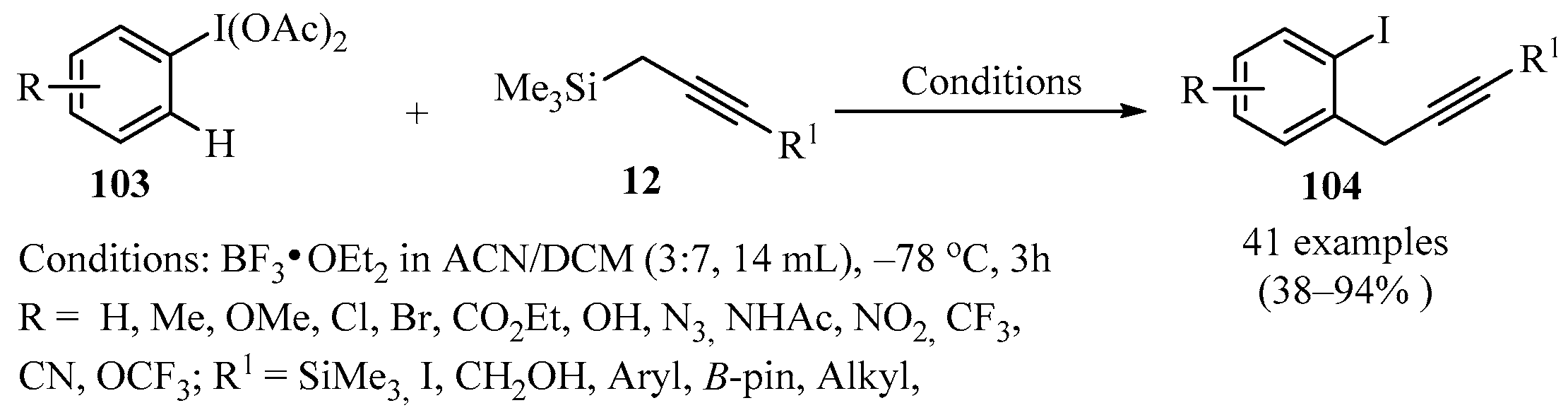
Haloarenes are of great synthetic interest, since they are used as structural scaffolds of different compounds employed in catalytic chemistry, medical chemistry, and agrochemistry. Due to this, new strategies have emerged to obtain various halogenated aromatics, for example, the insertion of a substituent in the ortho-position with respect to a pre-existing halogen group. In this context, the synthesis of ortho-propargyl iodobenzenes 104 represents a desirable goal. A viable procedure to synthesize these derivatives involves reacting (diacetoxyiodo)arenes 103, previously activated with BF3, with a propargyl metalate 12 using an ACN/DCM mixture as solvent, to furnish ortho-propargyl iodobenzenes 104 in moderate to high yields (Scheme 36), as described in [130]. A striking feature of this protocol is that it generates a singly propargylated product 104 for each substrate 103 bearing a single type of ortho-CH site. The regioselectivity is affected by the electronic environment of the iodoarene nucleus 103, and the method is applicable to electron-deficient iodoarenes 103.
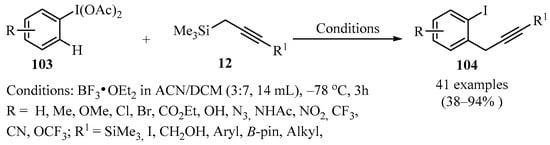
Scheme 36.
BF3-catalyzed synthesis of ortho-propargyl iodobenzenes 104 from (diacetoxyiodo)arenes 103 and propargyl metalates 12.
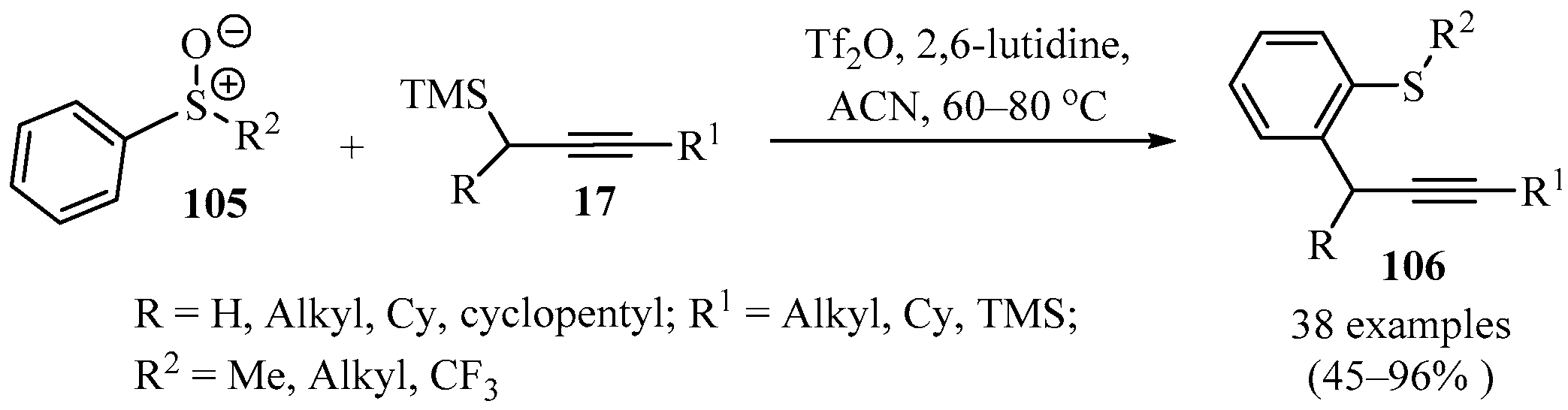
Synthetic access to ortho-propargylated arylsulfides, as in compounds 106, is also of great interest, since a variety of synthetic derivatives with a wide catalog of applications can be produced from these types of structures. Compounds 106 have been synthesized in good to excellent yields via a cross-coupling reaction between aryl-sulfoxide 105 and propargylsilanes 17, using Tf2O as an electrophilic activator and 2,6-lutidine as base in ACN (Scheme 37). The addition of 2,6-lutidine improved their reaction yields and prevented the formation of undesirable products via acid-mediated cyclization. A plausible mechanism for this metal-free cross-coupling process involves an interrupted Pummerer/allenyl thio-Claisen rearrangement, where the formation of classic Pummerer products did not occur, even in the presence of electron-scavenging alkyl chains on sulfur. Hence, this methodology allows for the formation of sp2-sp3 C-C bonds in products 106 in an efficient and regioselective manner [131].

Scheme 37.
Synthesis of o-propargylated arylsulfide derivatives 106 via sulfoxide-directed, metal-free ortho-propargylation of sulfoxides 105.
2.3.2. With Propargyl Alcohols
The nucleophilic substitution of the -OH group in propargyl alcohols is an efficient methodology for the preparation of synthetic precursors, which, due to its versatility, could be further implemented in synthetic schemes via alkyne functionality and the possible addition of acetylides to different carbonyls. However, this type of substitution is challenging in aryl-propargyl alcohols due to the low reactivity of the hydroxyl as a leaving group and the formation of unwanted side products, as well as polymers originating from unstable/highly reactive carbocationic intermediates. The viable alternative methods for the preparation of propargyl derivatives, such as 108, via the nucleophilic substitution of aryl-propargyl alcohols 63 are highlighted in Scheme 38.
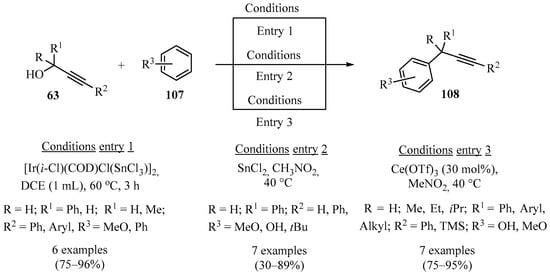
Scheme 38.
Metal- and heterobimetallic-catalyzed synthesis of diverse aryl-propargylated products 108 from propargyl alcohols 63.
There is currently considerable interest in multi-metallic catalysis since it allows for the design of specifically homogeneous hetero-bimetallic catalysts that can facilitate the activation of different electrophiles through the stereoelectronic characteristics of two metals present in a single compound, thus promoting selective binding to a substrate. In this sense, the use of hetero-bimetallic catalysts constitutes an alternative method for the functionalization of propargyl alcohols. For example, using an IrIII-SnIV catalyst in 1,2-dichloroethane (DCE) as a solvent enabled the activation of propargyl alcohols 63 (electrophiles), which reacted with a series of aromatic nucleophiles (Nu-H) 107 regioselectively, to furnish aryl-propargylated derivatives 108 with high turnover frequency (TOF) and with moderate to good yields (Scheme 38, entry 1) [132]. Furthermore, the direct propargylation of arenes 107 with propargyl alcohols 63 was promoted by SnCl2 or Ce(OTf)3 in MeNO2 as a solvent. These transformations resulted in high selectivity toward the propargylated products 108 (Scheme 38, entry 2 and entry 3) [133,134].
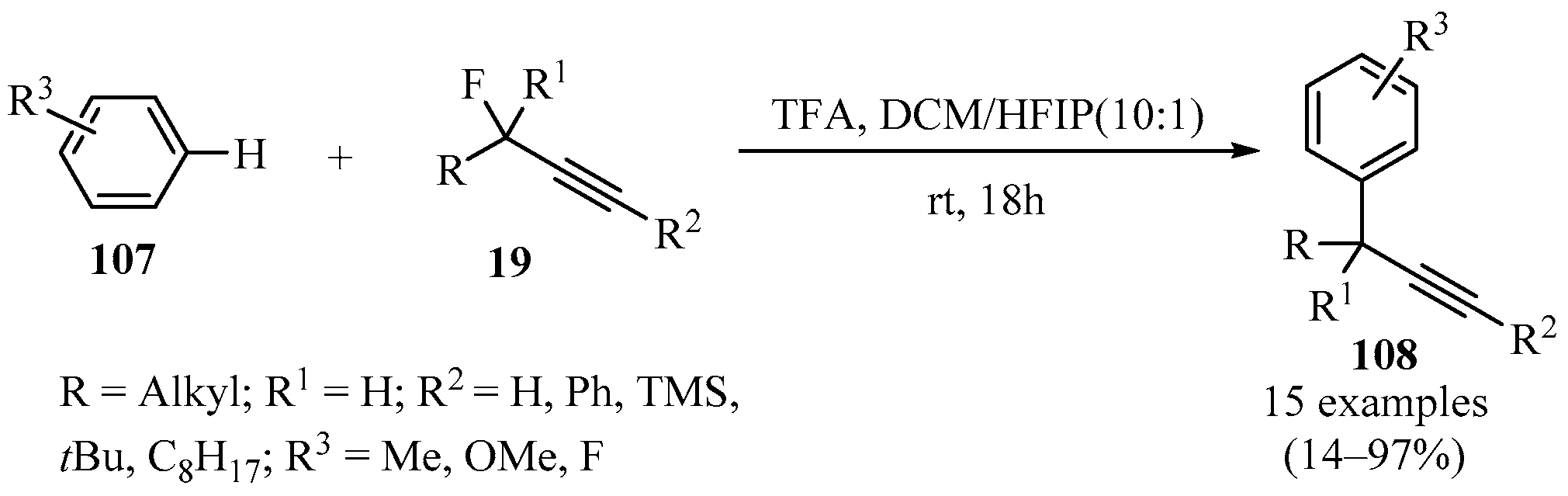
2.3.3. With Propargyl Fluorides
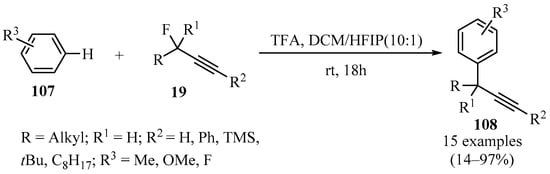
The Nicholas reaction has been employed as an alternative to circumvent the challenges involved in the propargylation of arenes, but this method has drawbacks because it uses Co2(CO6), requires several steps, and gives low yields with electron-poor arenes. The ionization of propargyl fluorides 19 (X = F) in trifluoroacetic acid (TFA) in a mixture of DCM/HFIP as solvents produced products 108 in acceptable to excellent yields (Scheme 39), thus providing a viable method to directly obtain a variety of substituted aryl-propargyl derivatives 108 in a Friedel–Crafts-type propargylation reaction [135].
Scheme 39.
TFA-catalyzed synthesis of diverse aryl-propargyl derivatives 108 from the reaction of propargyl fluorides 19 with arenes 107 in DCM/HFIP solvent.
2.3.4. With Propargyl Phosphates
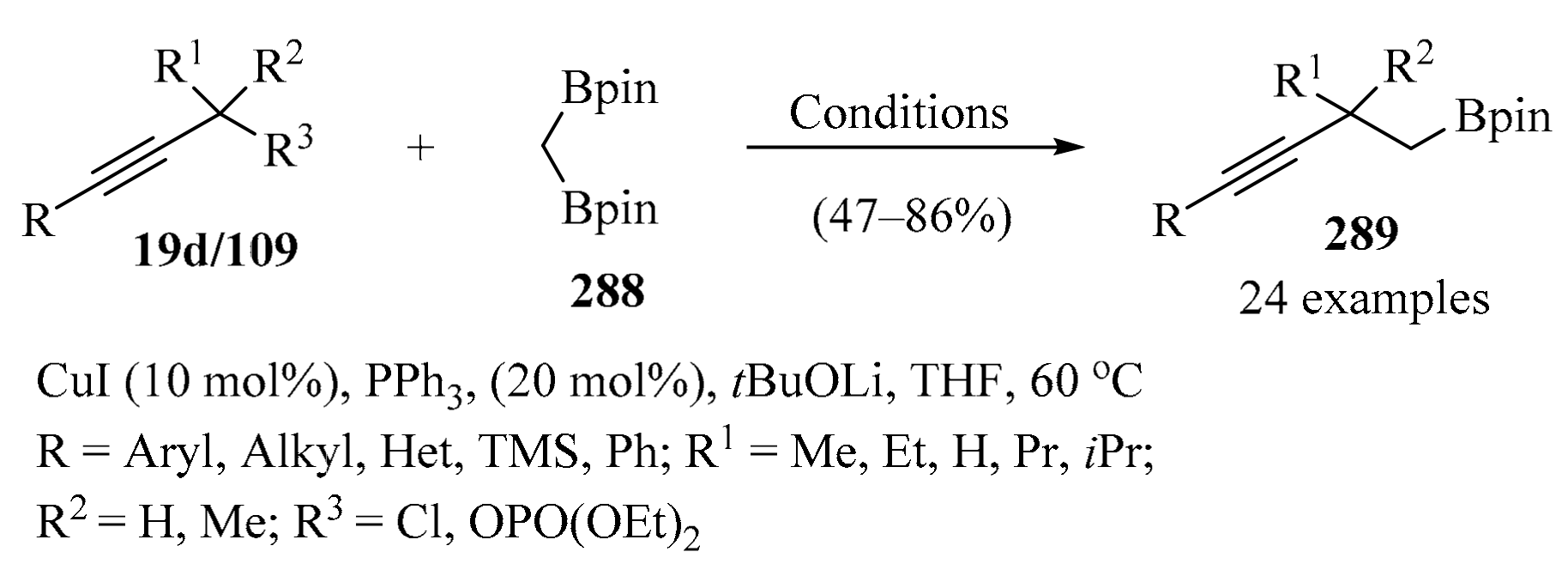
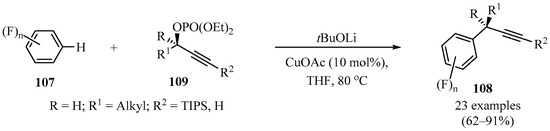
The copper-catalyzed direct propargylation of polyfluoroarenes 107 (n = 4 and 5) with secondary propargyl phosphates 109 that uses a strong base, such as, tBuOLi or THF, as a solvent has been described. Using this method, a series of propargylated polyfluoroarenes 108 were synthesized in moderate to good yields, with high chemo- and regioselectivity (Scheme 40). Furthermore, this reaction could also be extended to triethylsilyl- and tert-butyl substituted alkynes [136].
Scheme 40.
Synthesis of propargylated polyfluoroarenes 108 from secondary propargyl phosphates 109 in the presence of tBuOLi/CuOAc.
2.3.5. With Propargyl Cation Equivalents
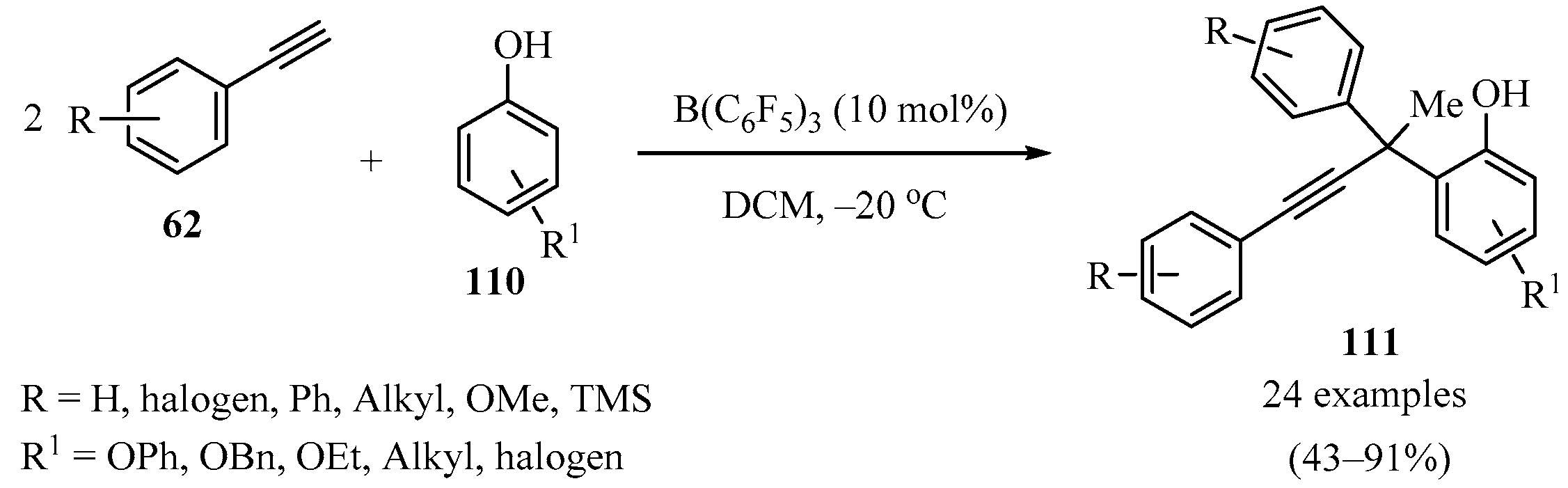
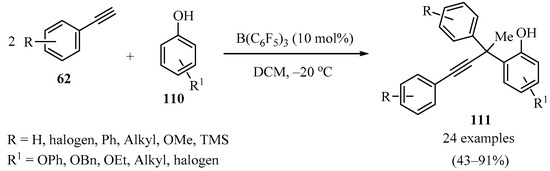
Given the prevalence of the phenol motif in bioactive molecules, pharmaceuticals, and functional materials [137], a series of ortho-propargyl phenols 111 were synthesized via a boron-catalyzed sequential procedure through the addition of terminal alkynes 62 (R2 = Aryl) to substituted phenols 110, bearing congested quaternary carbons (Scheme 41). Control experiments combined with DFT calculations suggested that the reaction proceeds via a sequential phenol alkenylation/hydroalkynylation process [138].
Scheme 41.
Boron-catalyzed sequential procedure for the synthesis of congested o-propargyl phenols 111.
- (b)
- Heterocyclic derivatives
- (i)
- Indoles
2.3.6. With Propargyl Alcohols, Ethers, and Esters
N-Heterocyclic systems are important as building blocks of natural products, drugs, and functional organic materials, and the development of mild and selective methods for the direct introduction of propargyl groups into heterocyclic rings is highly desirable in order to access important and novel organic precursors.
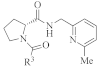
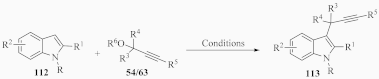
Focusing on indoles, Table 4 provides a summary of available methods for the synthesis of propargyl–indole hybrids 113 via the reaction of indole derivatives 112 with diversely substituted propargyl derivatives 54/63, employing various Lewis acids, zeolites, and superacids, in molecular solvents, as well in ionic liquids (entries 1-7) [134,139,140,141,142,143,144].

Table 4.
Diverse methodologies for the synthesis of propargyl–indole hybrids 113 from substituted propargyl derivatives 54/63 and indoles 112.
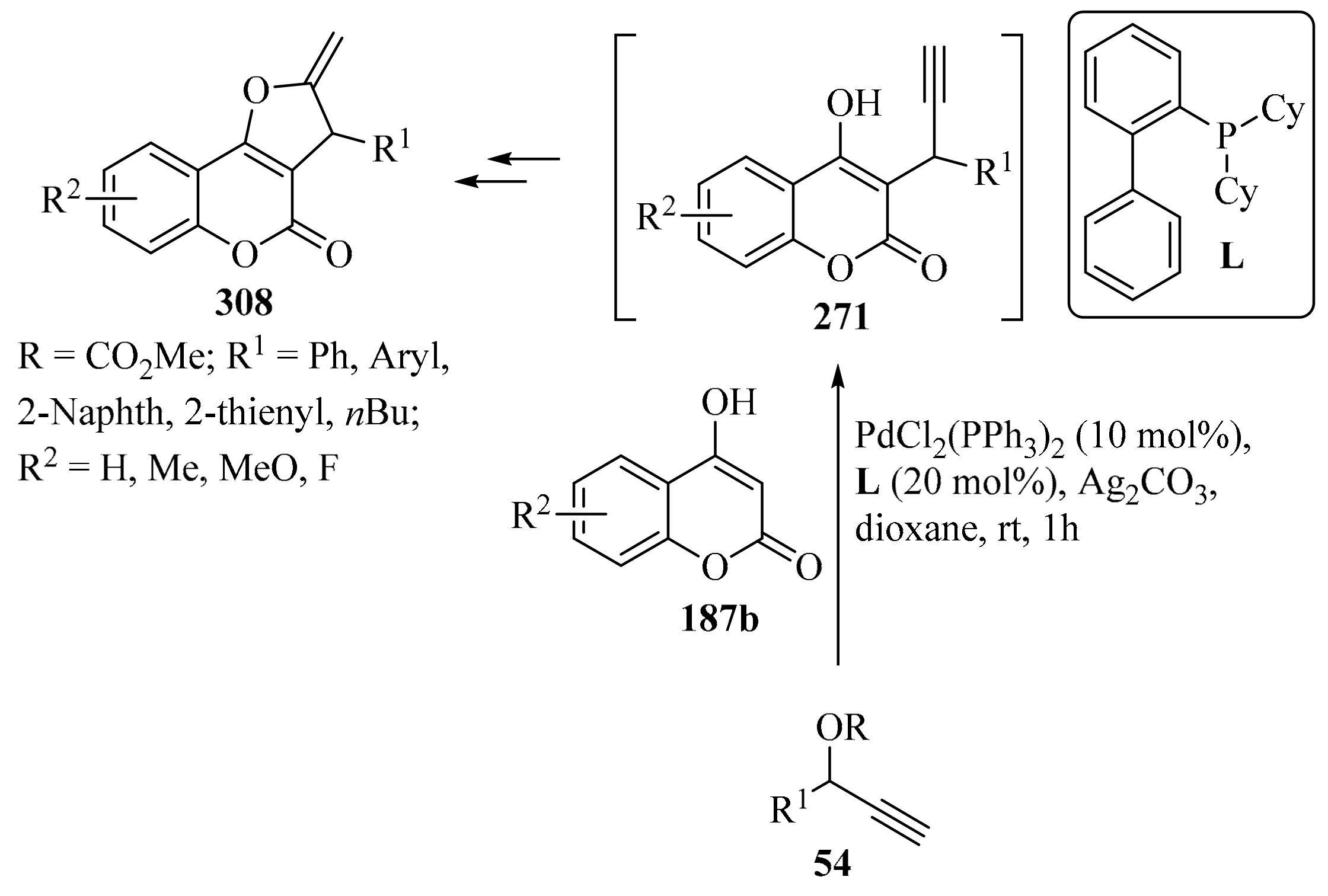
Enantioselective propargylation between indoles 112 and propargyl esters 54, catalyzed by the transition metal CuOTf•1/2C6H6, was reported in the presence of a chiral ligand ((4S,5R)-diPh-Pybox) in 4-methylmorpholine and MeOH, leading to products 113 in moderate to high yields, (Table 4, entry 8) [145]. Likewise, an asymmetric procedure was described, consisting of Friedel–Crafts alkylation between substituted indoles 112 and propargyl carbonates 54, in the presence of Ni(cod)2 and the chiral ligand (R)-BINAP and a base, in toluene, forming propargyl–indole derivatives 113 with high enantioselectivity and regioselectively and in moderate to good yields (entry 9) [146].
2.3.7. With Allenyl Bromides
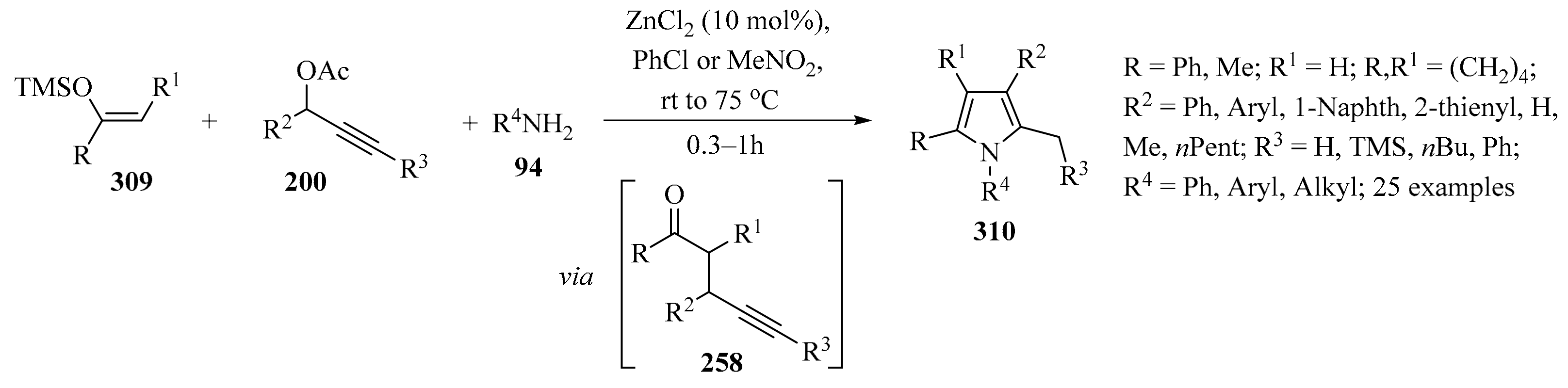
A direct method for a C-H propargylation reaction of indole derivatives 112 using bromoallenes 19c (X = Br) was reported, which employed Mn(I)/Lewis acid as cocatalyst [147]. The presence of BPh3 not only promoted reactivity, but also enhanced selectivity. Using this method, secondary, tertiary, and even quaternary carbon centers in the propargylic position could be directly constructed, leading to diversely substituted propargyl–indoles 114 in moderate to high yields (Scheme 42) [147].

Scheme 42.
Direct Mn(I)/BPh3 co-catalyzed synthesis of propargyl–indoles 114 using bromoallenes 19c as propargylating reagents.
- (ii)
- Other heterocyclic substrates
2.3.8. With Propargyl-TMS
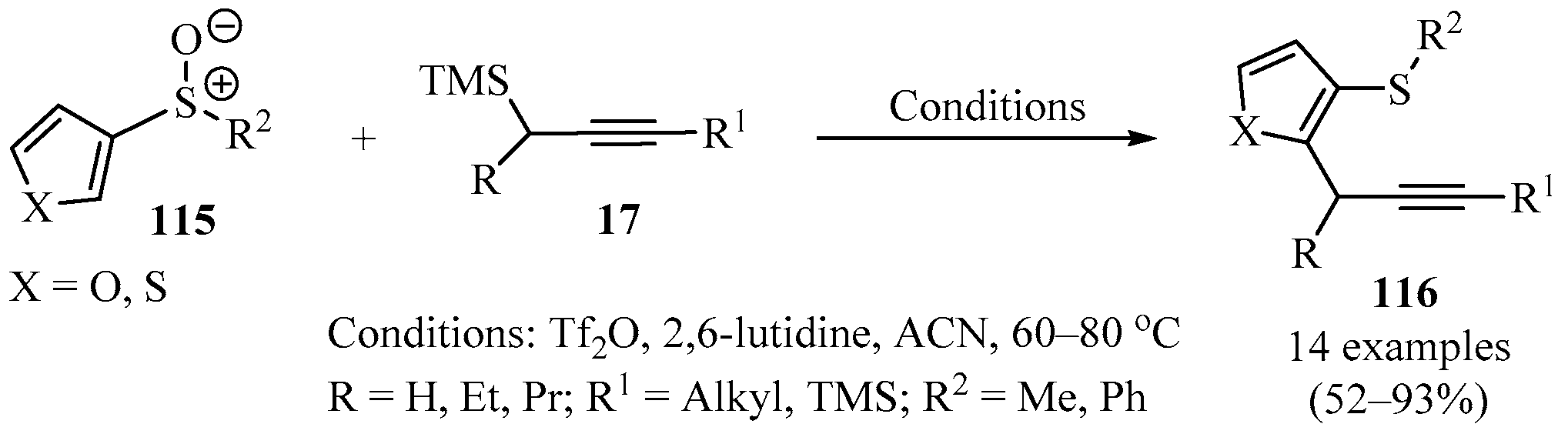
The same approach as that described in Scheme 37 was adopted for the direct metal-free ortho-propargylation of heteroaromatics 115 to produce o-propargylated heteroaromatic sulfides 116. Thus, mixtures of thiophenyl or furanyl sulfoxide 115, propargyl-TMS derivatives 17, and Tf2O were reacted in ACN as a solvent to produce products 116 regioselectively and in good to excellent yields (Scheme 43) [131].

Scheme 43.
Synthesis of o-propargylated heteroaromatic sulfides 116 via sulfoxide-directed, metal-free ortho-propargylation of heteroaromatic sulfoxides 115.
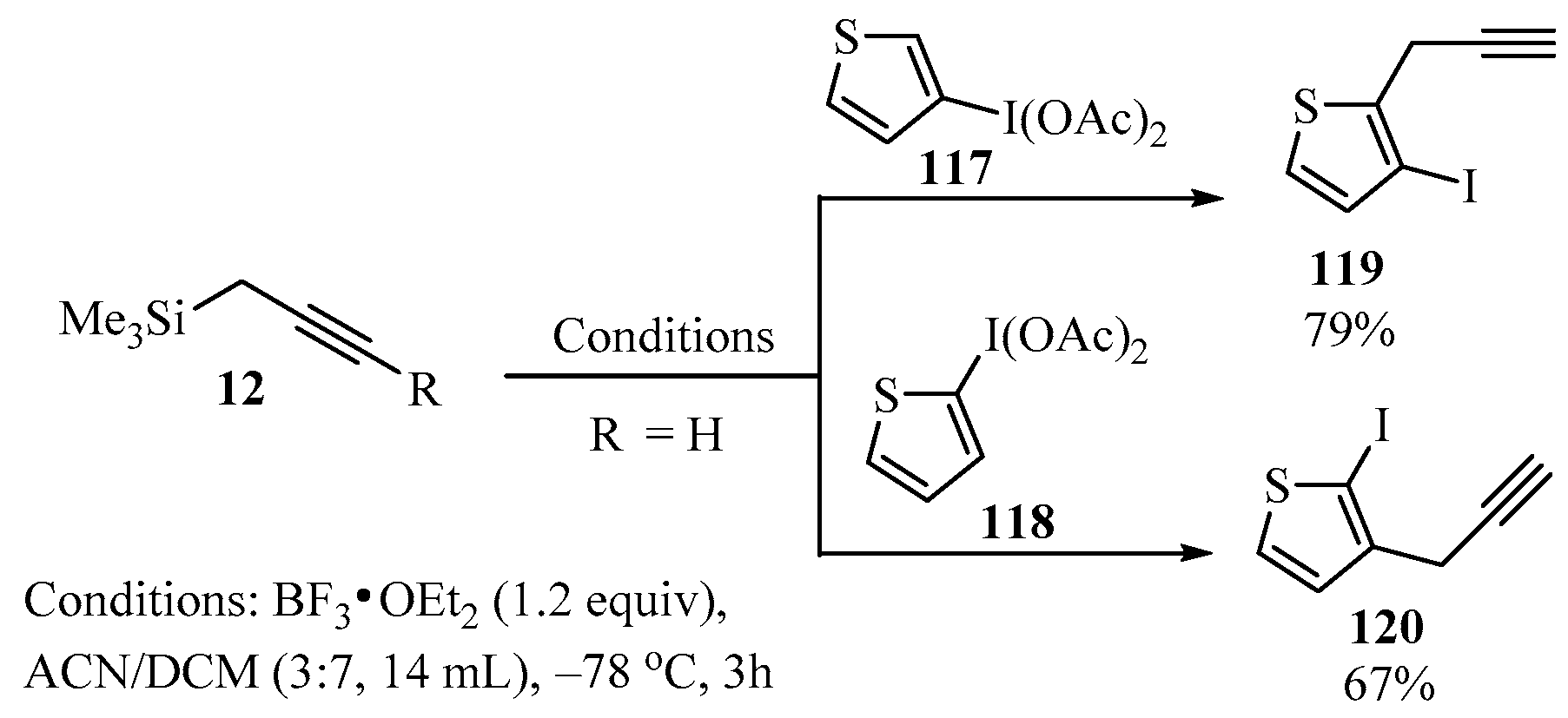
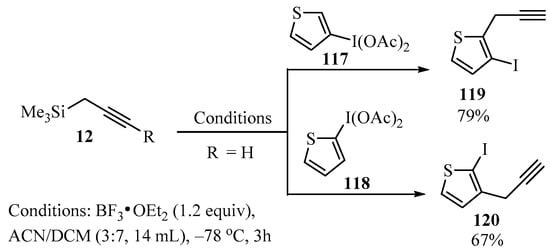
Following the approach described in Scheme 36, a method for the synthesis of ortho-propargyl iodothiophenes 119/120 was described [130]. In this case, a mixture of propargyl-TMS derivative 12, thiophenyliodine diacetates 117/118, and BF3•OEt2 in ACN/DCM as a solvent was allowed to react at low temperature to produce products 119/120 regioselectively, and in good yields (Scheme 44) [130].
Scheme 44.
BF3-catalyzed synthesis of ortho-propargyl iodothiophenes 119/120 from thiophenyliodine diacetates 117/118 and propargyl metalates 12.
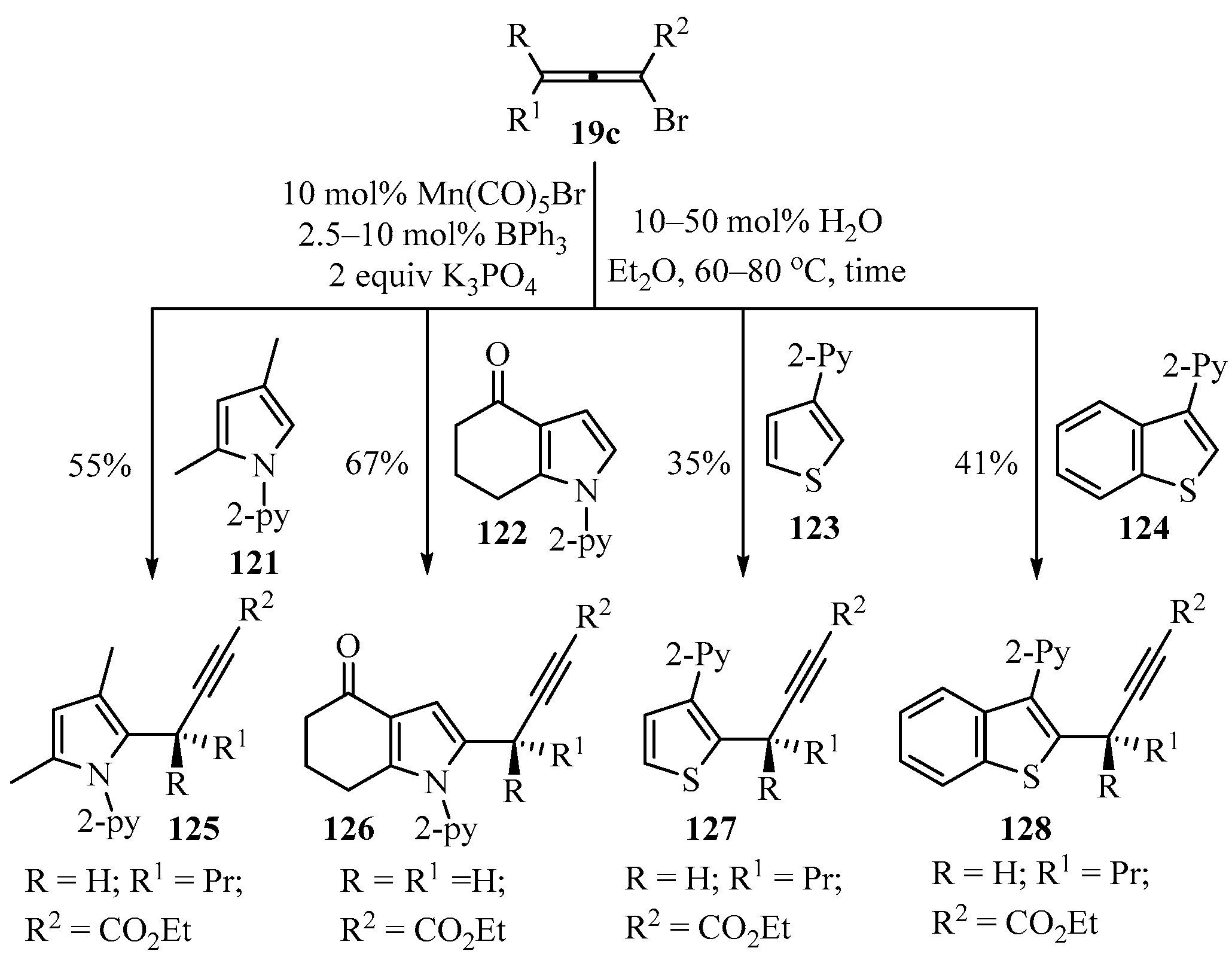
2.3.9. With Allenyl Bromide
Following the procedure described in Scheme 42, propargylated pyrrole and thiphene derivatives 125–128 were obtained in acceptable to good yields from bromoallenes 19c (X = Br), and the corresponding heteroaromatic precursors 121–124 are shown in Scheme 45 [147].

Scheme 45.
Direct Mn(I)/BPh3 co-catalyzed synthesis of propargyl-heterocycles 125–128 using bromoallenes 19c as propargylating reagents.
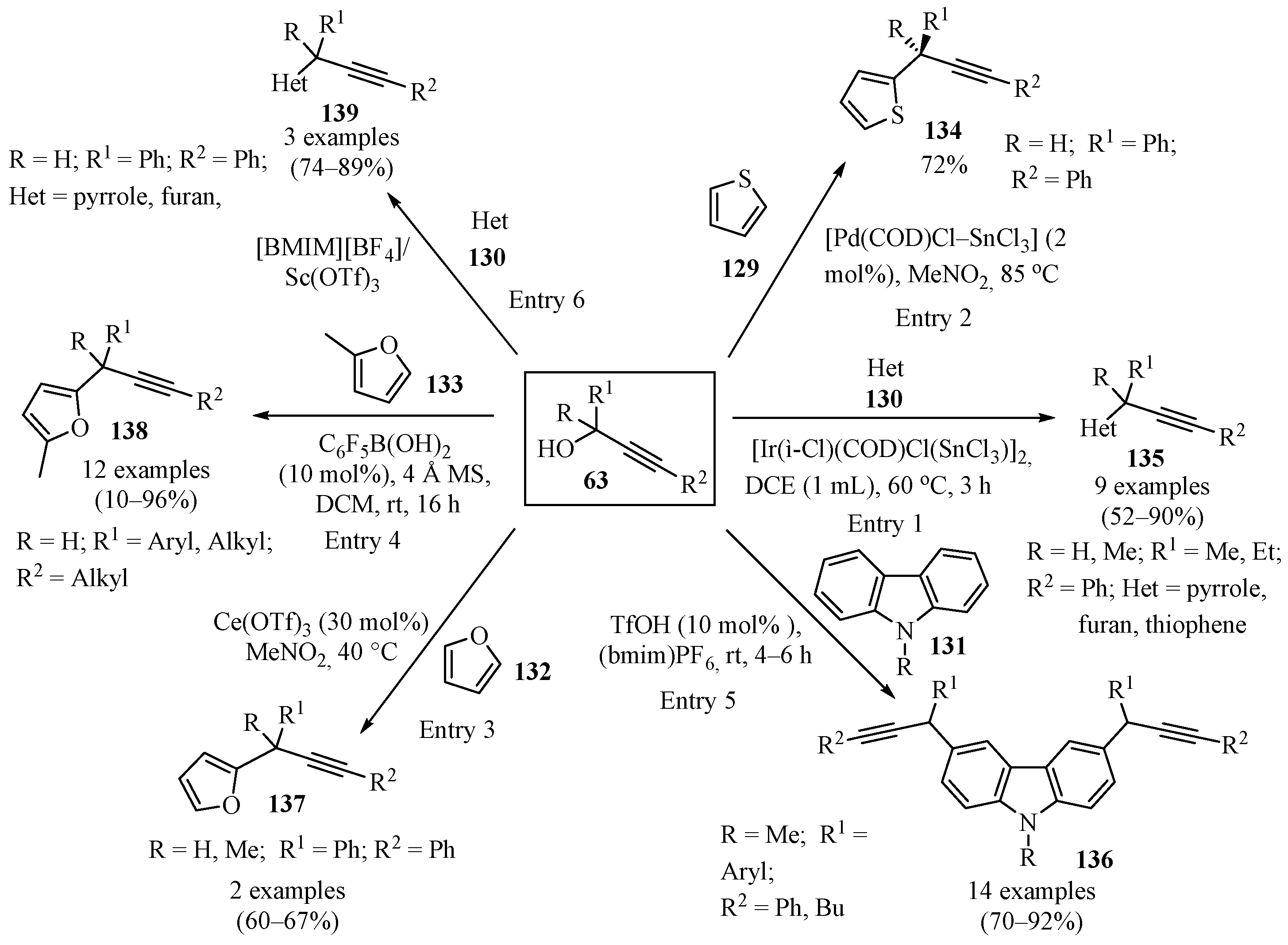
2.3.10. With Propargyl Alcohols
Scheme 46 gives an overview of the reported methods for the synthesis of propargylated heterocycles 134–139 using propargyl alcohols 63. A wide variety of catalytic systems have been employed, including hetero-bimetallic catalysts of IrIII-SnIV (entry 1) [132], Pd-Sn bimetallic catalysts (entry 2) [148], Ce(OTf)3 (entry 3) [134], and boron Lewis acids (entry 4) [149]. Doubly propargylated N-methylcarbazoles 136 were synthesized in [BMIM][PF6]/TfOH (entry 5) [150], and [BMIM][BF4]/Sc(OTf)3 proved effective for the propargylation of various classes of heterocycles under mild reaction conditions (entry 6) [151].
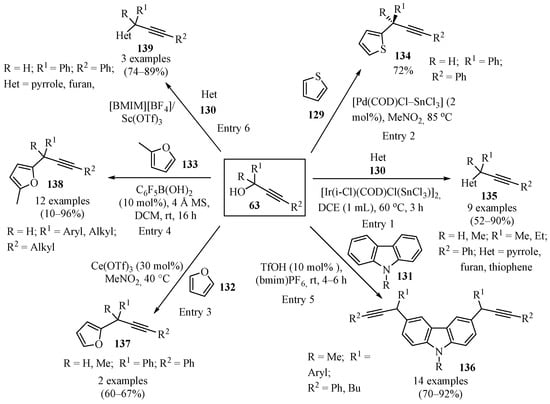
Scheme 46.
Different synthetic approaches to propargylated heterocycles 134–139 using propargyl alcohols 63.
2.4. Acyl Halides
With Propargyl-Organolithium Reagent
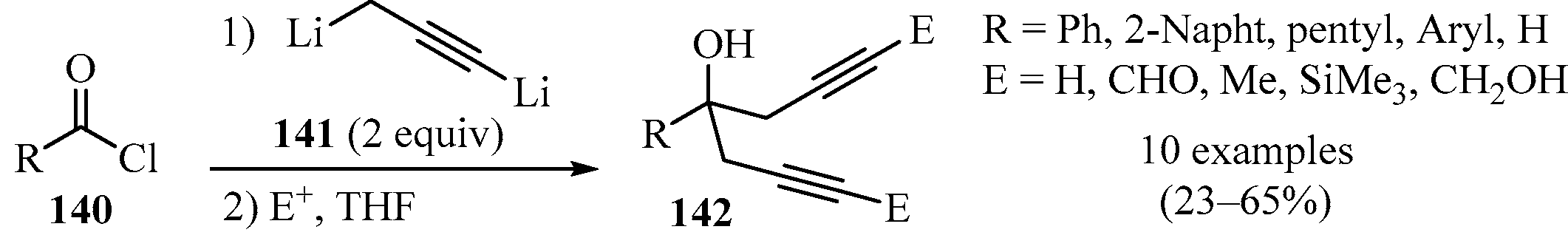
Homopropargyl and bis-homopropargyl alcohols are convenient intermediates in organic synthesis [152]. Previous studies have established that the controlled lithiation of allenes forms operational equivalents of propargyl dianions (C3H2Li2, 1,3-dilithiopropyne) 143 [153,154]. In this vein, controlled dilithiation of propargyl bromide with two equivalents of n-butyllithium, in the presence of TMEDA, was reported to be a productive method for the synthesis of bis-homopropargylic alcohols 142 (Scheme 47). In this approach, dianion 141 underwent in situ reactions with acid chlorides 140 to produce alcohols 142 in moderate yields with high regioselectivity [155].
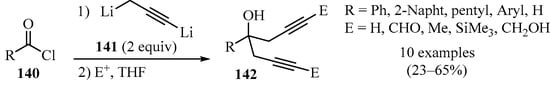
Scheme 47.
Synthesis of bis-homopropargylic alcohols 142 from 1,3-dilithiopropyne 141 and acid chlorides 140.
2.5. Amine/Amide Derivatives
2.5.1. With Propargyl Alcohols
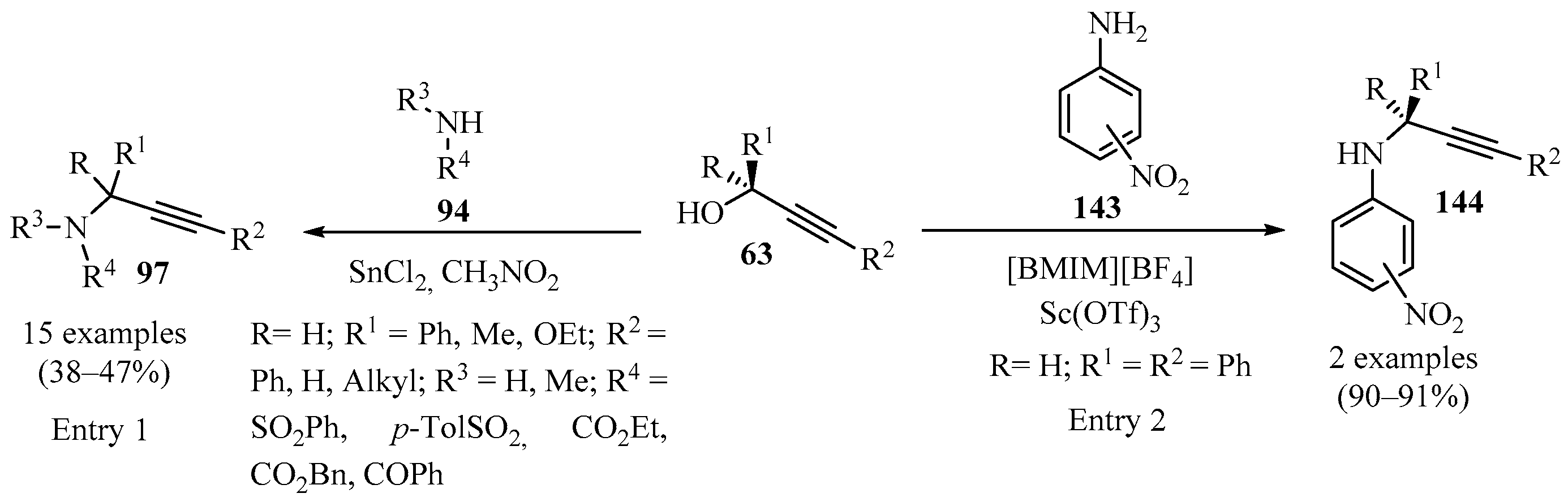
Scheme 48 gives an overview of the reported methods for the synthesis of N-propargylamines 97/144 from secondary propargyl alcohols 63, utilizing SnCl2 in CH3NO2 (entry 1) [133] and Sc(OTf)3 in [BMIM][BF4] (entry 2) [151] as catalysts.

Scheme 48.
Synthesis of N-propargylamines 97/144 from secondary propargyl alcohols 63.
Scheme 49 highlights an efficient tandem propargylation–cyclization–oxidation procedure for the synthesis of diversely substituted pyrimidines 147 via propargylamine intermediates 146, by reacting propargylic alcohols 63 with amidine 145 using copper(II) triflate as a catalyst [156].
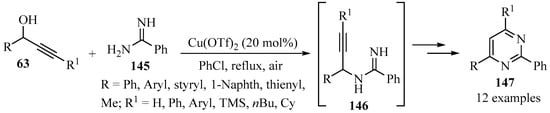
Scheme 49.
Cu-catalyzed synthesis of propargylamine intermediates 146 from propargylic alcohols 63 and amidine 145.
2.5.2. With Propargyl Bromide
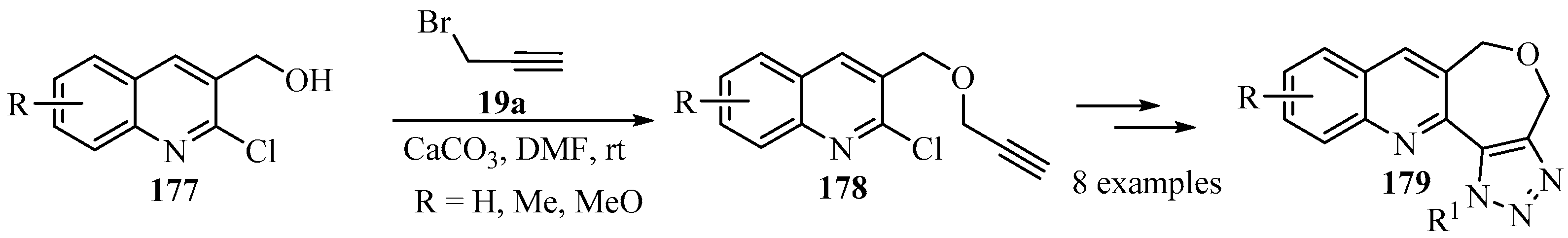
Among the nitrogen-containing fused heterocycles, quinoline, azepine, and triazole moieties are considered privileged scaffolds, are present in numerous natural products, and are among the most widely exploited heterocyclic rings for the development of bioactive molecules [157,158,159]. The propargylation of secondary amines 149, prepared via the reductive amination of 2-chloro-3-formylquinolines 148, produced tertiary propargylamines 150 as key intermediates for the synthesis of fused-heterocyclic products 151, incorporating three active pharmacophores (quinoline, azepine and triazole) in a single molecular framework [160]; this illustrates the potential of the N-propargyl moiety in heterocyclic synthesis (Scheme 50).
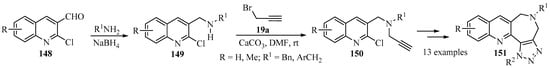
Scheme 50.
Synthesis of tertiary propargylamine intermediates 150 through propargylation of secondary amines 149 with propargyl bromide 19a in the presence of calcium carbonate.
Chiral N-tert-butanesulfinyl imines are important for the stereoselective synthesis of nitrogen-containing heterocyclic systems [161]. With the goal of synthesizing 3-substituted 1,2,3,4-tetrahydroisoquinolines 153 in an enantioenriched form, the N-propargylation of enantioenriched homopropargylic amines 74 was performed under basic conditions to give the corresponding 4-azaocta-1,7-diyne intermediates 152 in fair to good yields (Scheme 51). An oxidation step, followed by [2+2+2] cyclotrimerization promoted by a Wilkinson catalyst, produced the target structure 153 which contained substituents at the 3-, 6- and 7-positions in high yields [106]. This illustrative example highlights the efficacy of bis-homopropargylamine in heterocyclic synthesis.

Scheme 51.
Synthesis of 4-azaocta-1,7-diyne intermediates 152 through propargylation of homopropargylic amines 74 with propargyl bromide 19a.
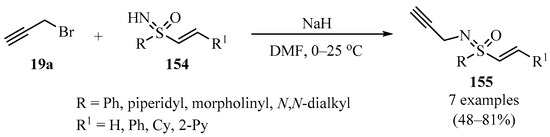
The N-propargylation of vinyl sulfoximines 154 with propargyl bromide 19a produced N-propargyl-sulfoximines 155 as highly functionalized biologically promising small molecules (Scheme 52) [162].
Scheme 52.
NaH-Catalyzed synthesis of N-propargyl-sulfoximines 155 via treatment of sulfoximines 154 with propargyl bromide 19a.
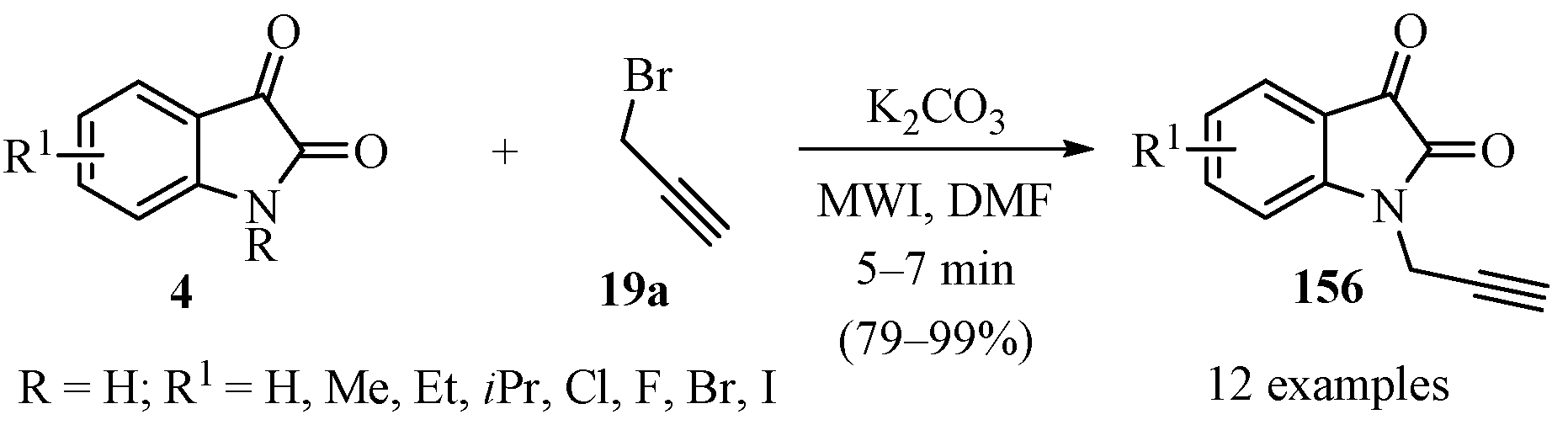
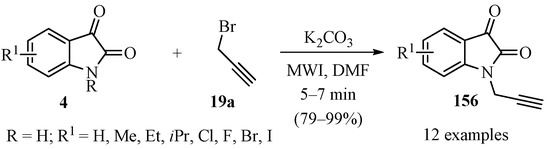
The N-propargylation of substituted isatins 4 (R = H) was accomplished via a microwave-assisted reaction using anhydrous K2CO3 as base in DMF solvent, according to Scheme 53, to produce a set of diversely substituted N-propargyl isatins 156 in good to excellent yields [163].
Scheme 53.
Microwave-assisted synthesis of substituted N-propargyl isatins 156.
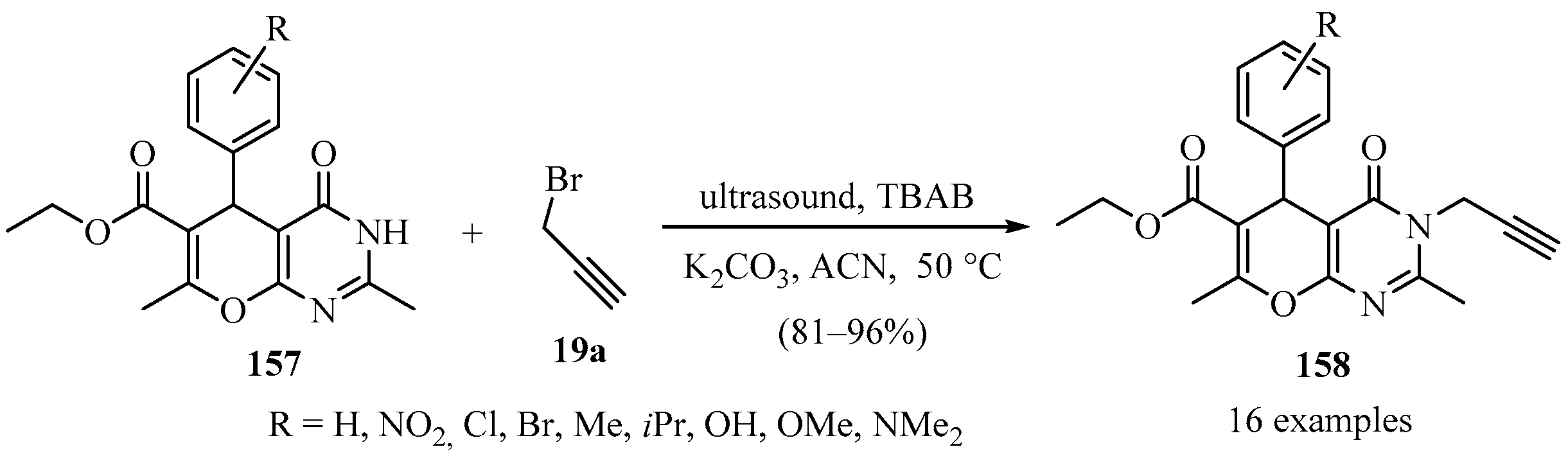
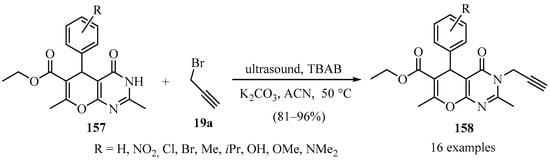
Similarly, a library of N-propargyl 4H-pyrano[2,3-d]pyrimidine derivatives 158 was prepared through the N-propargylation of pyrano derivatives 157, under ultrasound-assisted reaction conditions via phase transfer catalysis, according to Scheme 54 [164].
Scheme 54.
Ultrasound-assisted synthesis of N-propargyl 4H-pyrano[2,3-d]pyrimidine derivatives 158 using TBAB as phase-transfer catalyst.
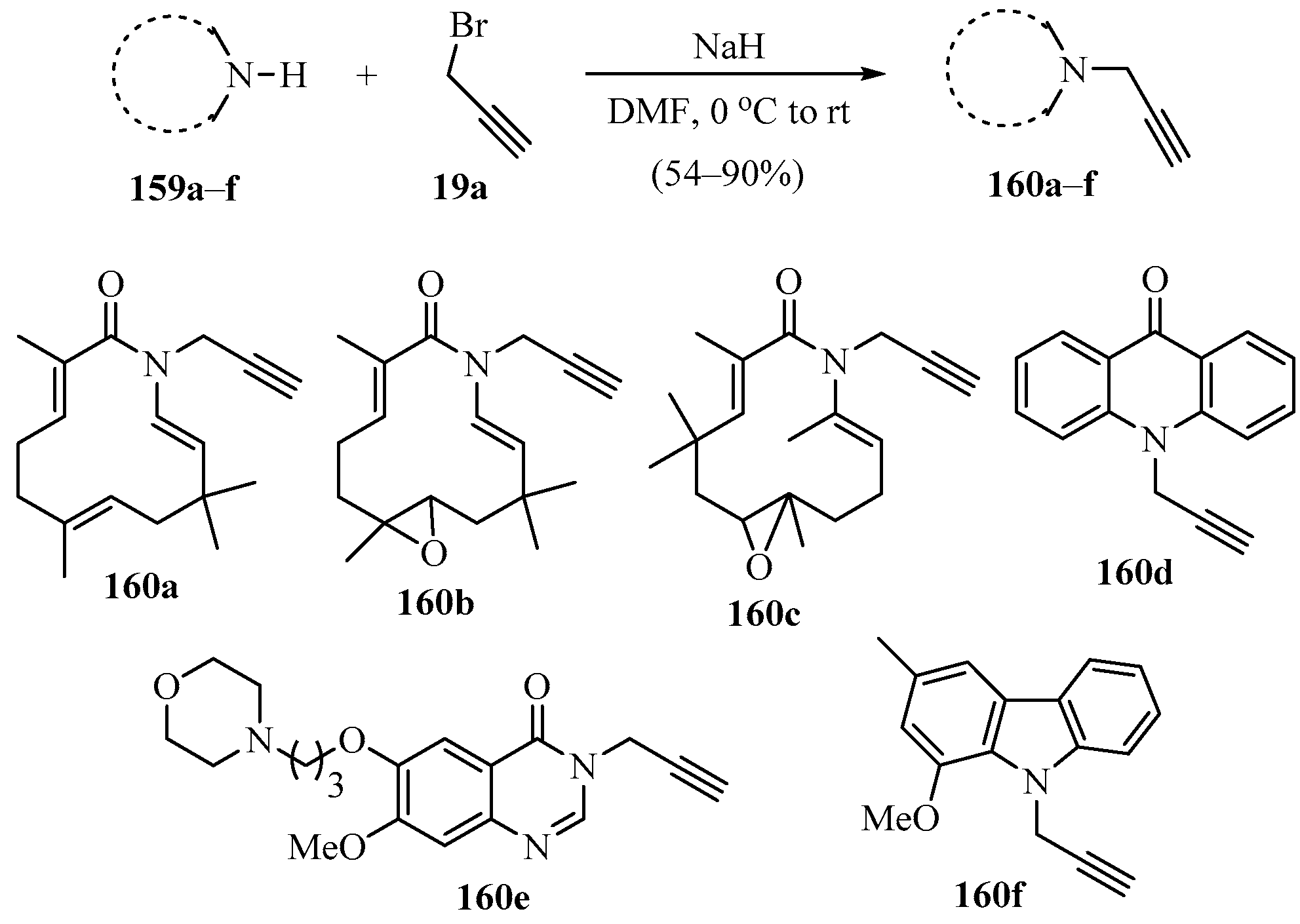
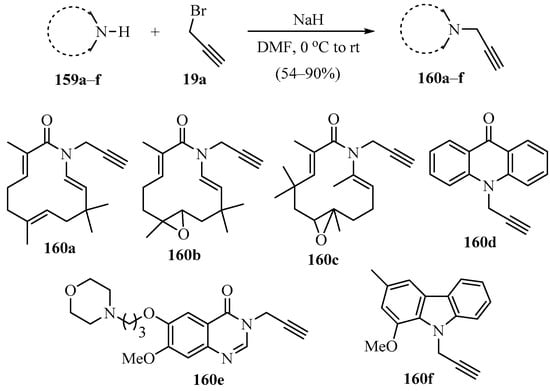
A procedure for the synthesis of a series of N-propargylated compounds 160a–f was conducted, according to Scheme 55 [165], using azazerumbone (159a), azazerumbone oxides (159b,c), acridin-9(10H)-one (159d), 7-methoxy-6-[3-(morpholin-4-yl)propoxy]quinazolin-4(3H)-one (159e), and murrayafoline A (159f) as substrates.
Scheme 55.
NaH-catalyzed synthesis of N-propargylated heterocyclic compounds 160 using propargyl bromide 19a as propargylating agent.
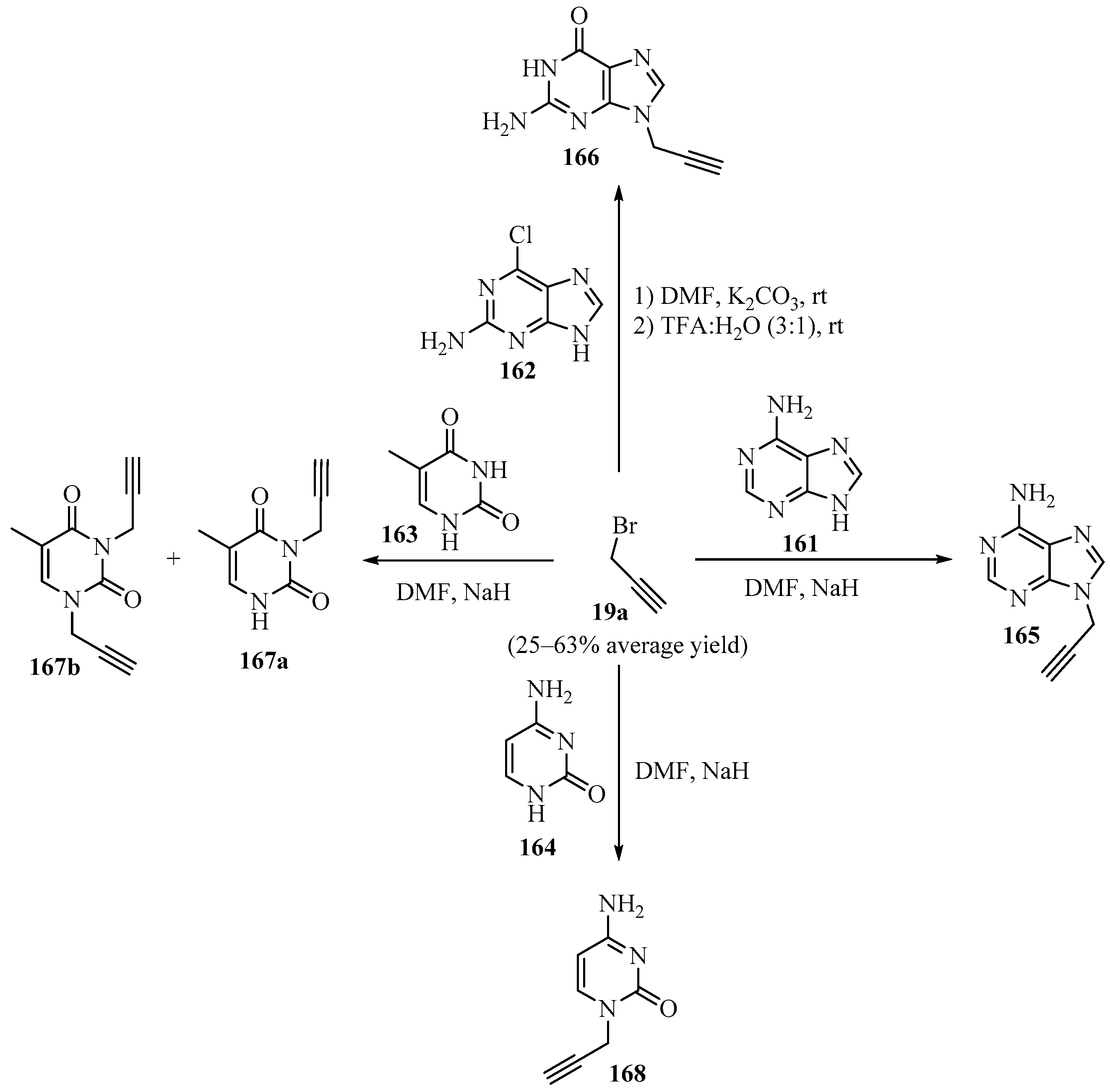
A series of nucleobase derivatives 165–168 were synthesized via the propargylation of DNA nucleobases 161–164 according to Scheme 56, with the goal of extending their functionality to obtain biofunctional materials. The in vitro biocompatibility of the native 161–164 and nucleobase derivatives 165–168 was assessed using primary human dermal fibroblasts (HF), showing that they were non-toxic, and hence, suitable for biomedical applications [166].

Scheme 56.
One-pot synthesis of nucleobase derivatives 165–168 via regioselective N-H functionalization of the DNA nucleobases 161–164 with propargyl bromide 19a.
2.5.3. With Propargylic Cation Intermediates
The nucleophilic addition of the primary amino-ester 169 to cobalt-stabilized propargylic carbocation 170—initially in the presence of BF3•OEt2, followed by CAN, as catalytic systems—generated the corresponding dipropargylamino-ester 171 according to Scheme 57 [167].
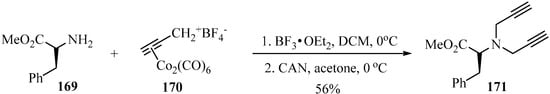
Scheme 57.
Synthesis of dipropargylamino-ester 171 using co-stabilized propargylic carbocation 170 as a propargylating agent, in the presence of BF3•OEt2/CAN as a catalytic system.
2.6. Vinylstananes
With Propargyl Bromide
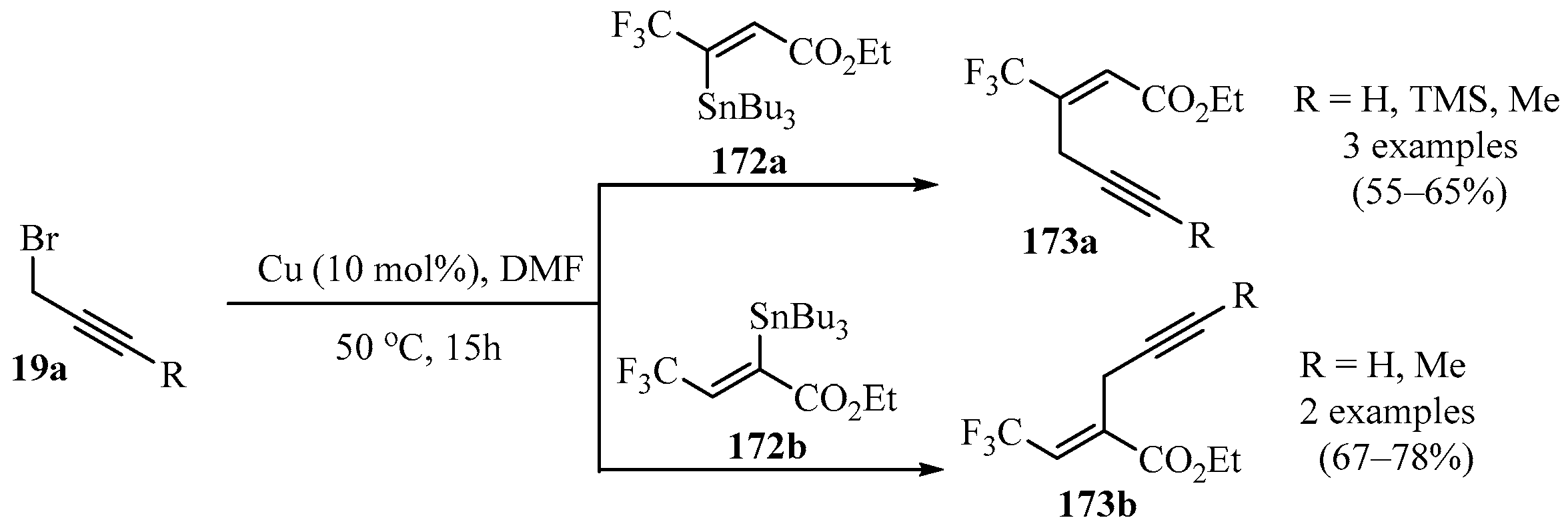
A methodology involving the coupling of vinyl-stannanes (β-trifluoromethyl (Z)-α- and (Z)-β-stannylacrylates) 172 to propargylic bromides 19a catalyzed by copper(I) provided access to the corresponding propargylated products 173 without allenic transposition (Scheme 58). This Pd-free cross-coupling process tolerated various R-groups, and occurred with retention of the configuration at the double bond; furthermore, homocoupling and allenic products were not detected [168].

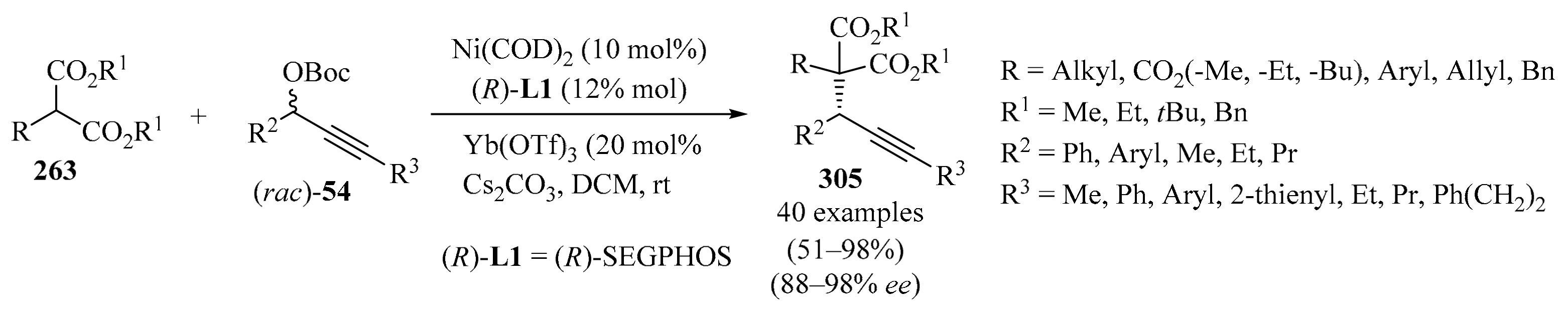
Scheme 58.
Copper(I)-catalyzed synthesis of propargylated products 173 from trifluoromethyl stannylacrylates 172 and propargylic bromides 19a.
2.7. (a) Alcohols, (b) Enol-Like Precursors, (c) Phenols, (d) Thiols, and (e) Carboxylic Acids
- (a)
- Alcohols
2.7.1. With Propargyl Bromides
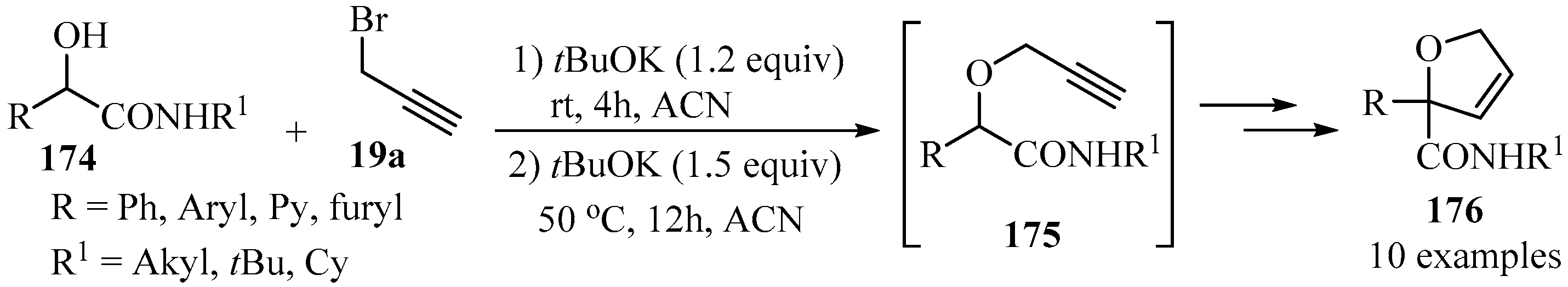
The propargylation of hydroxyl-amides 174, synthesized via a Passerini reaction mediated by boric acid, generated O-propargyloxyamides 175 as key intermediates (Scheme 59) [169], whose cyclization in the presence of potassium tert-butoxide via a 5-endo-dig process produced a series of 2,5-dihydrofurans 176 of synthetic interest [170,171,172,173].
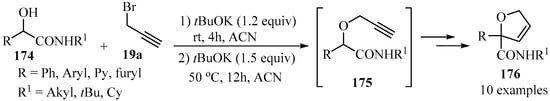
Scheme 59.
The synthesis of O-propargyloxyamide intermediates 175 from hydroxyl-amides 174 and propargyl bromide 19a in the presence of potassium tert-butoxide as a base.
Expanding on the strategy for the synthesis of quinoline/azepine pharmacophores fused to a triazole moiety (see Scheme 50), hetero-polycyclic products 179 were obtained from (2-chloroquinolin-3-yl)methanol derivatives 177 via the O-propargylation of 177 to give the key propargyl intermediates 178, followed by a click reaction and Pd-catalyzed C-H functionalization (Scheme 60) [160].

Scheme 60.
Synthesis of O-propargyl intermediates 178 from the propargylation of (2-chloroquinolin-3-yl)methanol derivatives 177 with propargyl bromide 19a in the presence of calcium carbonate as a base.
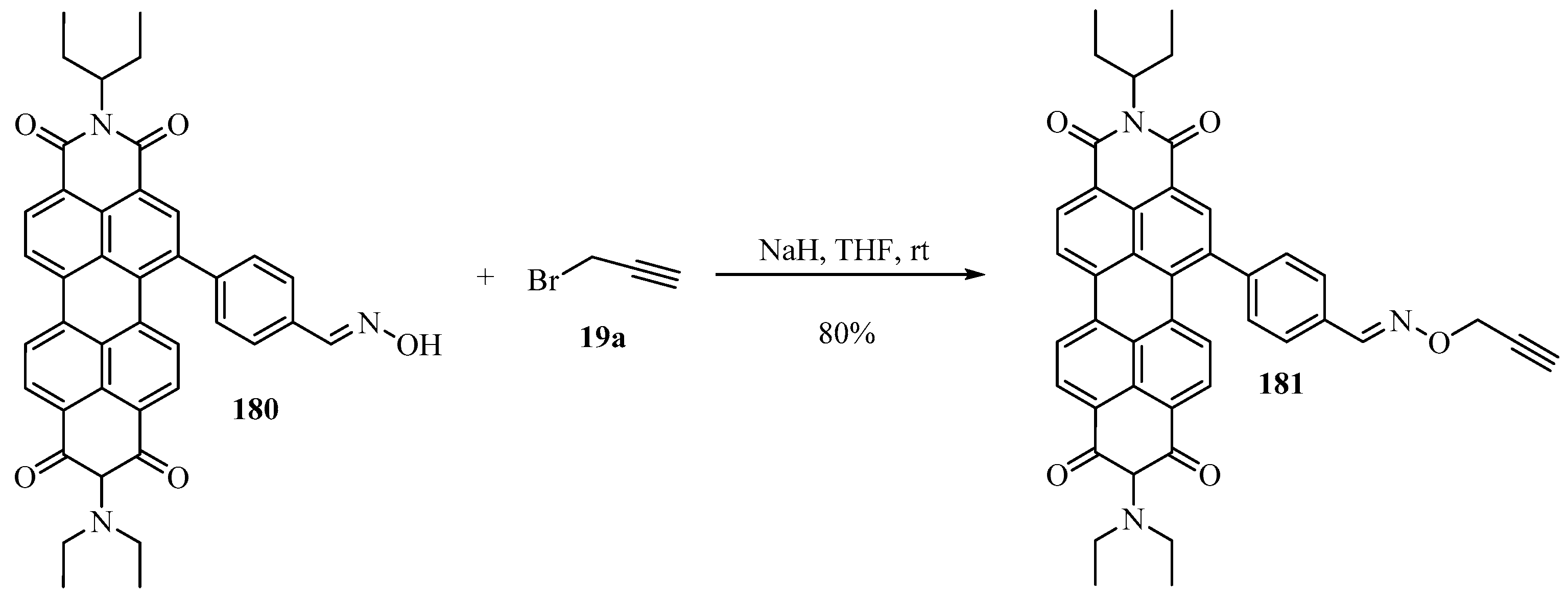
The O-Propargylation of oxime 180 with propargyl bromide 19a, according to Scheme 61, provided facile access to the perylenediimide compound 181, whose main characteristic was its capability to detect Cu2+ and Pd+2 ions in water [174].

Scheme 61.
NaH-mediated synthesis of propargyl-perylenediimide 181 from the reaction of oxime 180 with propargyl bromide 19a.
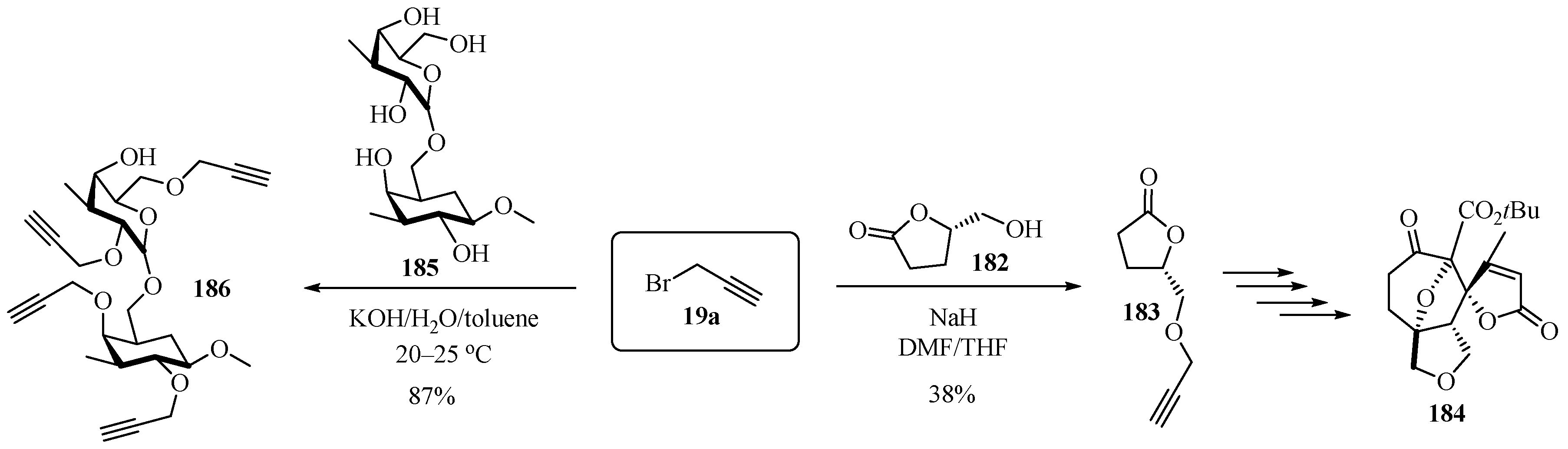
Scheme 62 highlights two synthetic strategies for access to propargylated ethers 183 and 186. The first process involves the cyclization of L-glutamic acid to obtain the lactone 182, which was reacted with propargyl bromide 19a in alkaline medium in a mixture of polar aprotic solvents to obtain the propargylated lactone 183 in moderate yields [175]. Compound 183 was then used as a starting point for multistep synthesis, leading to polycyclic compound 184. The goal of the second etherification process was to generate propargylated disaccharides. In this case, glycoside 185 was reacted with propargyl bromide 19a to produce the tetra-propargylated arabino-3,6-galactane 186 in good yields [176].
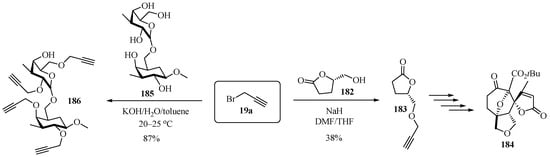
Scheme 62.
Alternative routes to propargylated ethers 183 and 186 via hydroxyderivatives 182 and 185.
Scheme 63 highlights a method for the synthesis of terminal gem-difluoropropargyl ethers 190 from gem-difluoropropargyl bromide dicobalt complex 188 in the presence of silver triflate and TEA in toluene. Complex 188 reacted selectively with aliphatic alcohols 187, even if the substrates 187 contained other nucleophilic functional groups, producing propargyl ether complexes 189. Decomplexation of the resulting dicobalt complexes 189 using cerium ammonium nitrate (CAN) or N,N,N′-trimethylethylenediamine, followed by desilylation by TBAF, produced compound 190 [177].

Scheme 63.
AgOTf-mediated synthesis of propargyl and both dicobalt complexes 189 from the reaction of gem-difluoropropargyl bromide dicobalt complex 188 with diversely substituted alcohols 187.
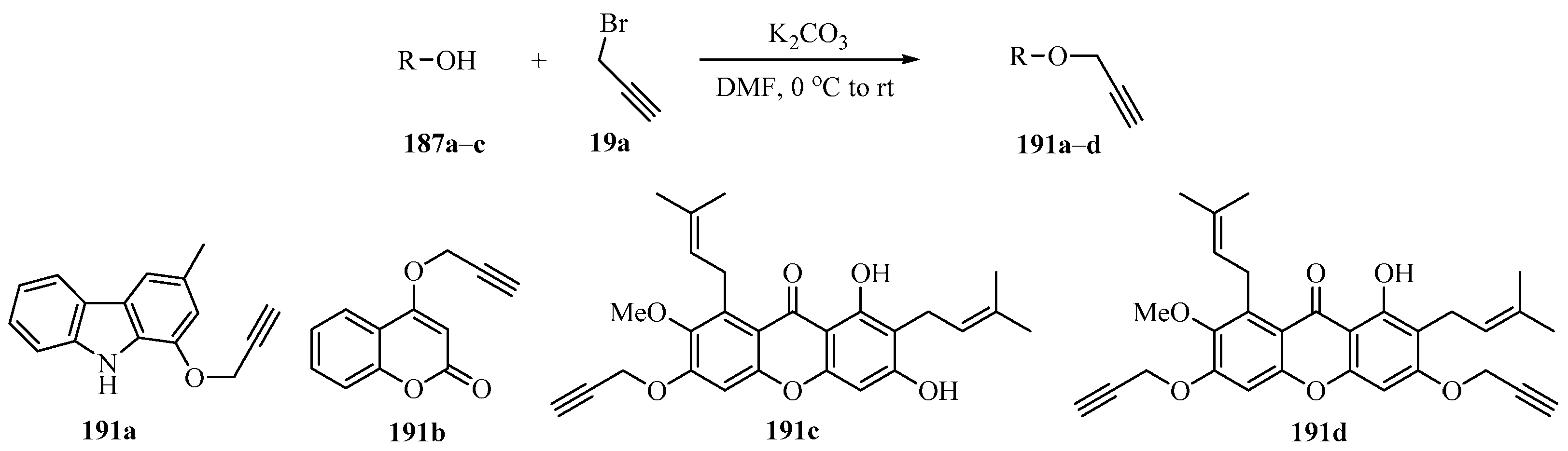
Implementing the strategy outlined in Scheme 55, a series of O-propargylated compounds 191a-d bearing one or two propargyl groups in their structures were synthesized using 3-methyl-9H-carbazol-1-ol (187a), 4-hydroxycoumarin (187b), and α-mangostin (187c) as substrates (Scheme 64). These compounds were evaluated for their in vitro cytotoxicity against three human cancer cell lines, the HepG2, LU-1, and Hela cell lines. Compound 191c proved most active, showing IC50 values of 1.02, 2.19, and 2.55 μg/mL, respectively [165].
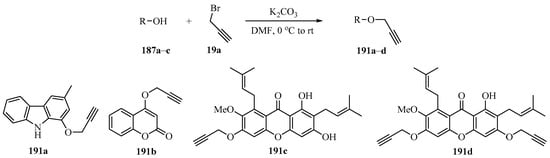
Scheme 64.
K2CO3-catalyzed synthesis of O-propargylated compounds 191 from propargyl bromide 19a and hydroxy derivatives 187.
2.7.2. With Propargyl Esters
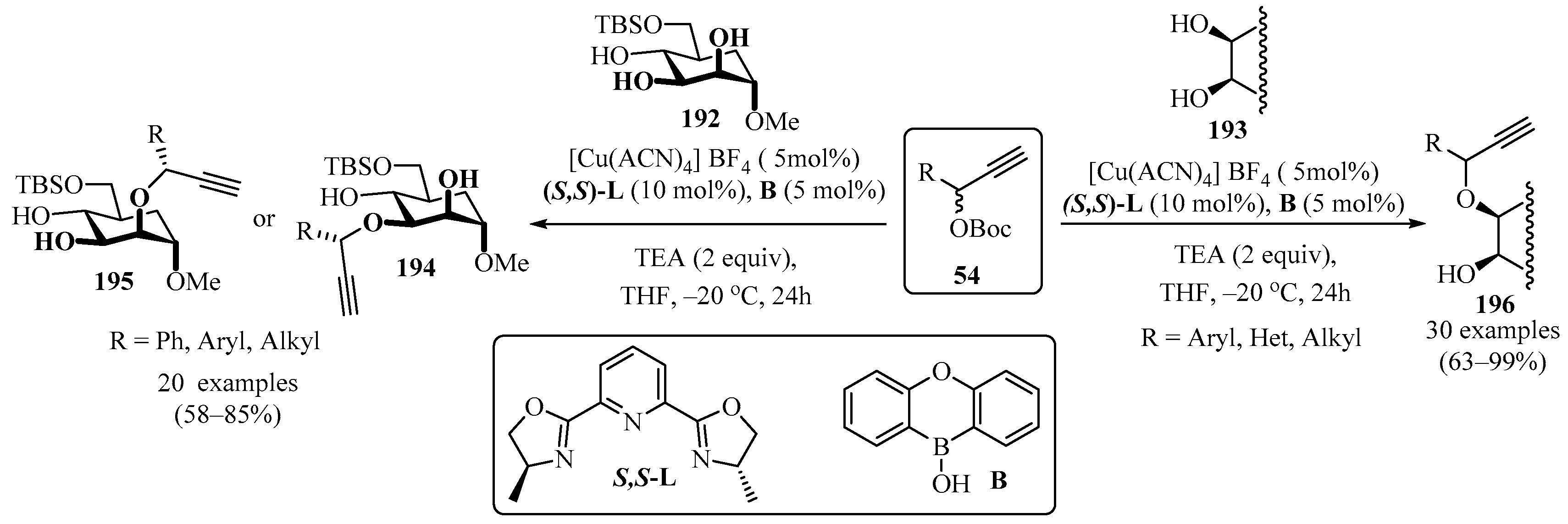
Compounds 194/195 and 196 were synthesized via O-propargylation of the monosaccharide 194 and hydroxylic precursors 193 with propargyl esters 54, employing dual catalysis between [Cu(ACN)4]BF4 and boronic acid (B), and using a chiral ligand ((S,S)-L) in the presence of a weak base (TEA) in THF (Scheme 65). A notable feature of this approach is the formation of several stereocenters in a chemo- and stereoselective manner [178,179].
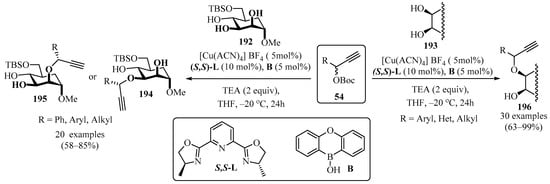
Scheme 65.
Propargylation of the monosaccharides 192 and the hydroxylic precursors 193 from their reactions with propargyl esters 54.
2.7.3. With Propargyl Alcohol/Ethers
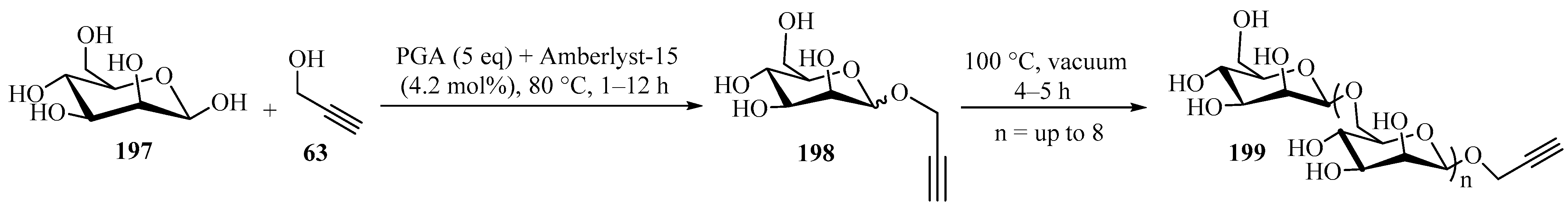
An efficient method for the synthesis of end-functionalized oligosaccharides from unprotected monosaccharides using a one-pot/two-step approach was developed (Scheme 66) [180]. In the first step, mannose 197 was functionalized with propargyl alcohol 63 (R = R1 = H) at the anomeric position through Fisher glycosylation using Amberlyst-15, producing a propargyl monosaccharide 198. In a second step, the reaction mixture was heated under vacuum at 100 °C in order to increase the degree of polymerization of 198, leading to a fully functionalized propargylated glycoside 199, with a degree of polymerization (n) up to 8 [180].
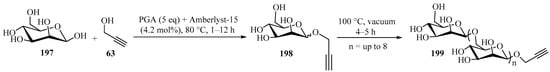
Scheme 66.
Amberlyst-15-mediated synthesis of end-propargylated glycosides 199.
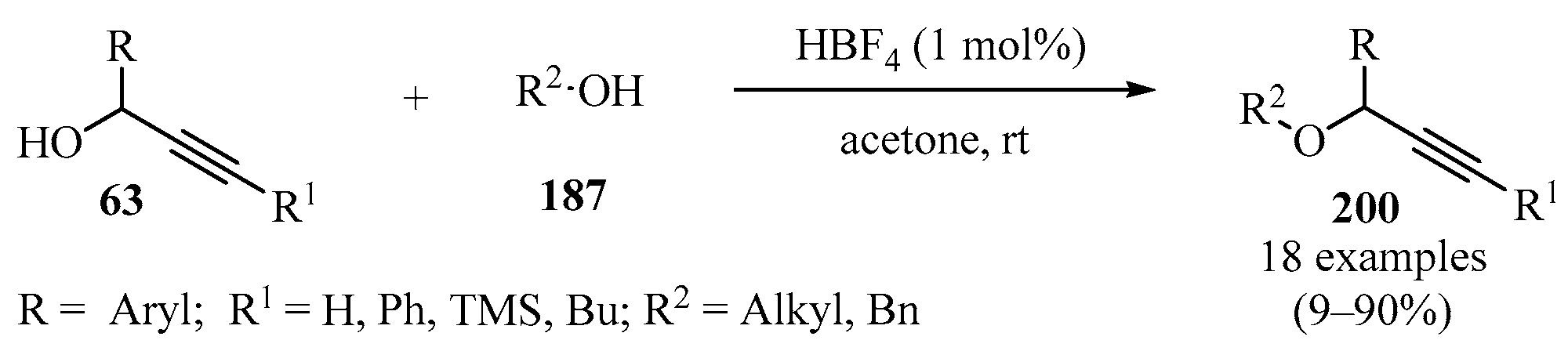
Propargyl ethers 200 were synthesized by reacting propargylic alcohols 63 and different primary and secondary alcohols 187 in the presence of catalytic amounts of aqueous HBF4 as a catalyst (Scheme 67) [181].
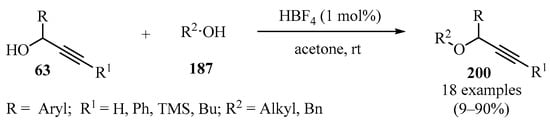
Scheme 67.
HBF4-catalyzed synthesis of propargyl ethers 200 using propargylic alcohols 63 as propargylating agents.
Implementing the procedure described in Scheme 57, the corresponding propargylated amino-ethers 203 were synthesized via a reaction of dicobalt hexacarbonyl-complexed (Co2(CO)6)-propargyl methyl ether 202 with aminoalcohols 201 in the presence of BF3•OEt2 and CAN as catalytic systems (Scheme 68) [167].

Scheme 68.
Synthesis of the propargylated amino-ethers 203 from aminoalcohols 201 with (Co2(CO)6)-propargyl ether complex 202 as propargylating agent.
- (b)
- Enolic substrates
2.7.4. With Propargyl Bromides
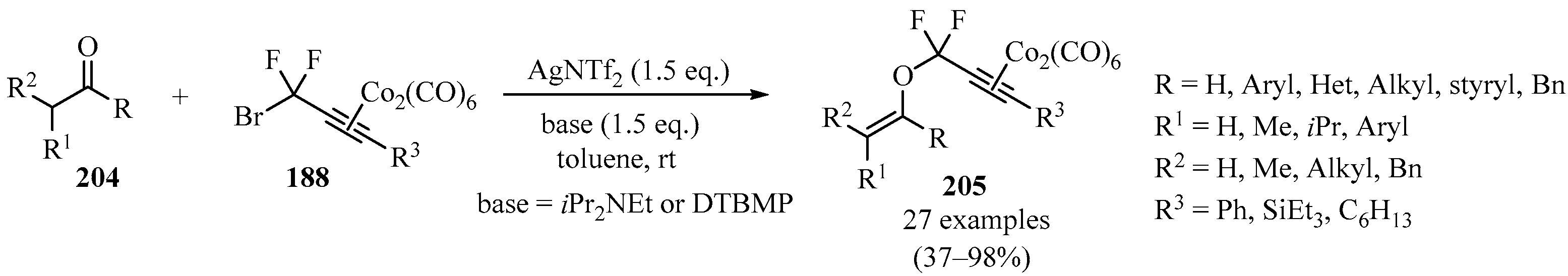
The reaction of difluoropropargyl–bromide–dicobalt complexes 188 with enolizable ketones and aldehydes 204, in the presence of AgNTf2 and with iPr2NEt or DTBMP as a base, led to the synthesis of difluoropropargyl vinyl ether–dicobalt complexes 205 bearing diverse substituents (Scheme 69). These compounds were then utilized as convenient precursors for the synthesis of difluorodienone and difluoroallene derivatives [182].
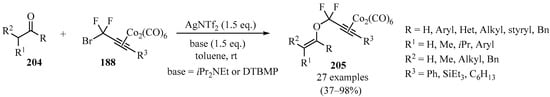
Scheme 69.
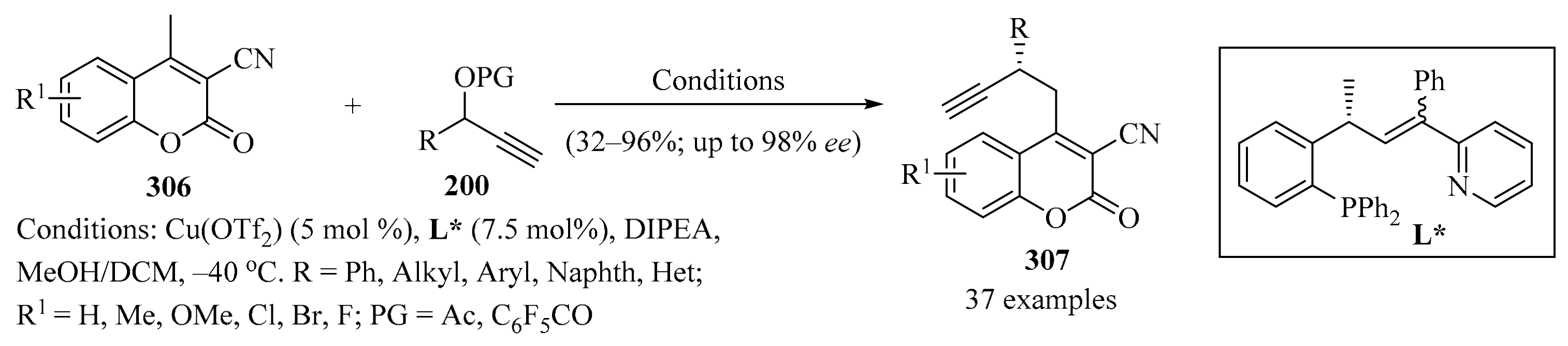
Synthesis of difluoropropargyl vinyl ether–dicobalt complexes 205 from carbonyl compounds 188 mediated by AgNTf2 and iPr2NEt or DTBMP bases.
- (c)
- Phenolic substrates
2.7.5. With Propargyl Bromides
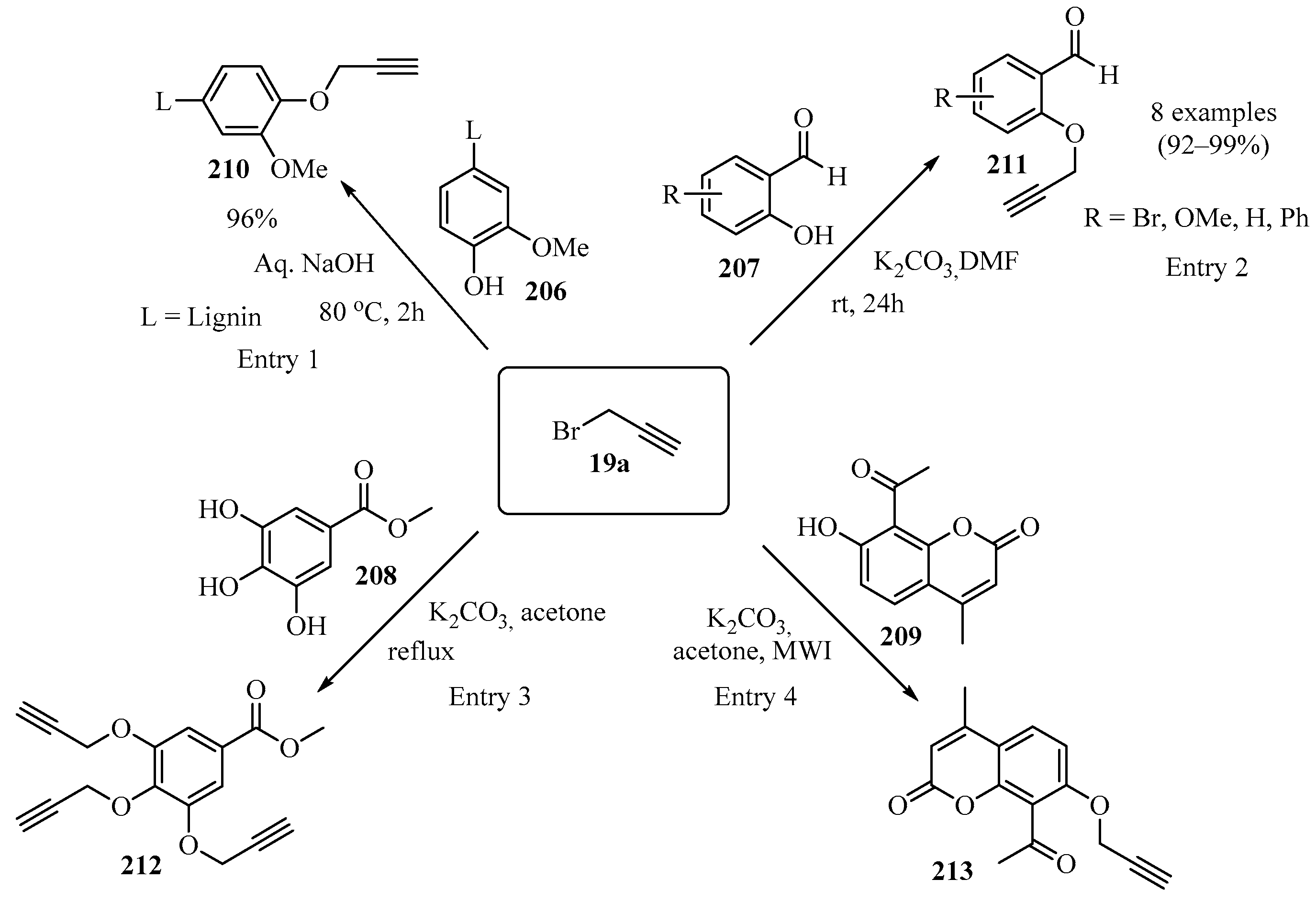
The propargylation of phenolic hydroxyl groups is important because of its potential as starting material for the preparation of high-molecular-weight synthetic and natural polymers. The reaction of propargyl bromide 19a with the phenolic OHs of the lignin derivative 206, in the presence of an aqueous base, yielded a propargylated-lignin product 210 (entry 1) [183]. In other studies, the propargylation of phenols 207, 208, and 209, in the presence of K2CO3 as catalysts in acetone or DMF and under MW irradiation, produced the corresponding propargylated ethers 211 (entry 2) [184], 212 (entry 3) [185], and 213 (entry 4) [186] (Scheme 70). These compounds were further functionalized via “click” chemistry.

Scheme 70.
Propargylation of phenolic hydroxyl groups in precursors 206–209 using propargyl bromide 19a as propargylating agent.
2.7.6. With Propargyl Alcohols/Ethers
Following the procedure described in Scheme 57, propargylated tyrosine derivatives 215, were prepared starting from with dicobalt complexes 202 as propargylating agents, according to Scheme 71, and employing BF3•OEt2 and CAN as catalytic systems [167].

Scheme 71.
Synthesis of the propargylated tyrosine derivatives 215 from tyrosine analogues 214 and (Co2(CO)6)-propargylated complexes 202 as propargylating agents.
- (d)
- Thiolic substrates
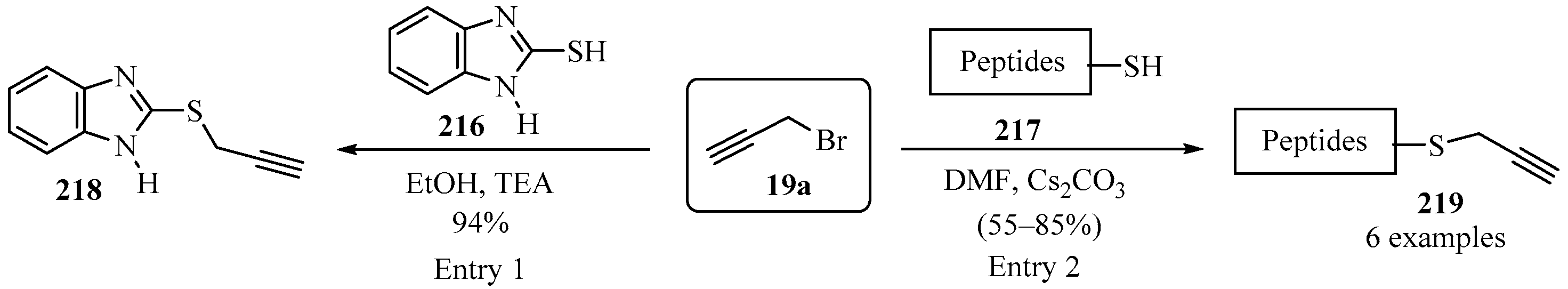
2.7.7. With Propargyl Bromide
Thiobenzimidazole- 216 and cysteine-containing peptides 217 were S-propargylated using a mild base, according to Scheme 72, to produce propargylated thiobenzimidazole 218 (entry 1) [187] and propargylated peptides 219 (entry 2) [188].
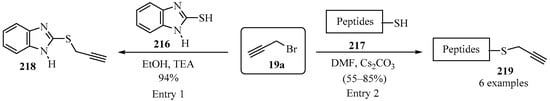
Scheme 72.
Propargylation reactions of thiobenzimidazole- 216 and cysteine-containing peptides 217 with propargyl bromide 19a as propargylating agent.
2.7.8. Propargylic Cation Intermediates
S-propargylated cysteine ethyl ester derivatives 221 were prepared according to the conditions established in Scheme 57, starting with propargyl–dicobalt complexes 170 in the presence of BF3•OEt2 and CAN as catalytic systems (Scheme 73) [167].
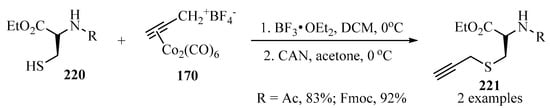
Scheme 73.
Synthesis of the propargylated cysteine ethyl ester derivatives 221 from cysteine analogues 220 and the (Co2(CO)6)-propargylated complex 170 as propargylating agent.
- (e)
- Carboxylic acids
2.7.9. With Propargyl Bromide and Propargylamine
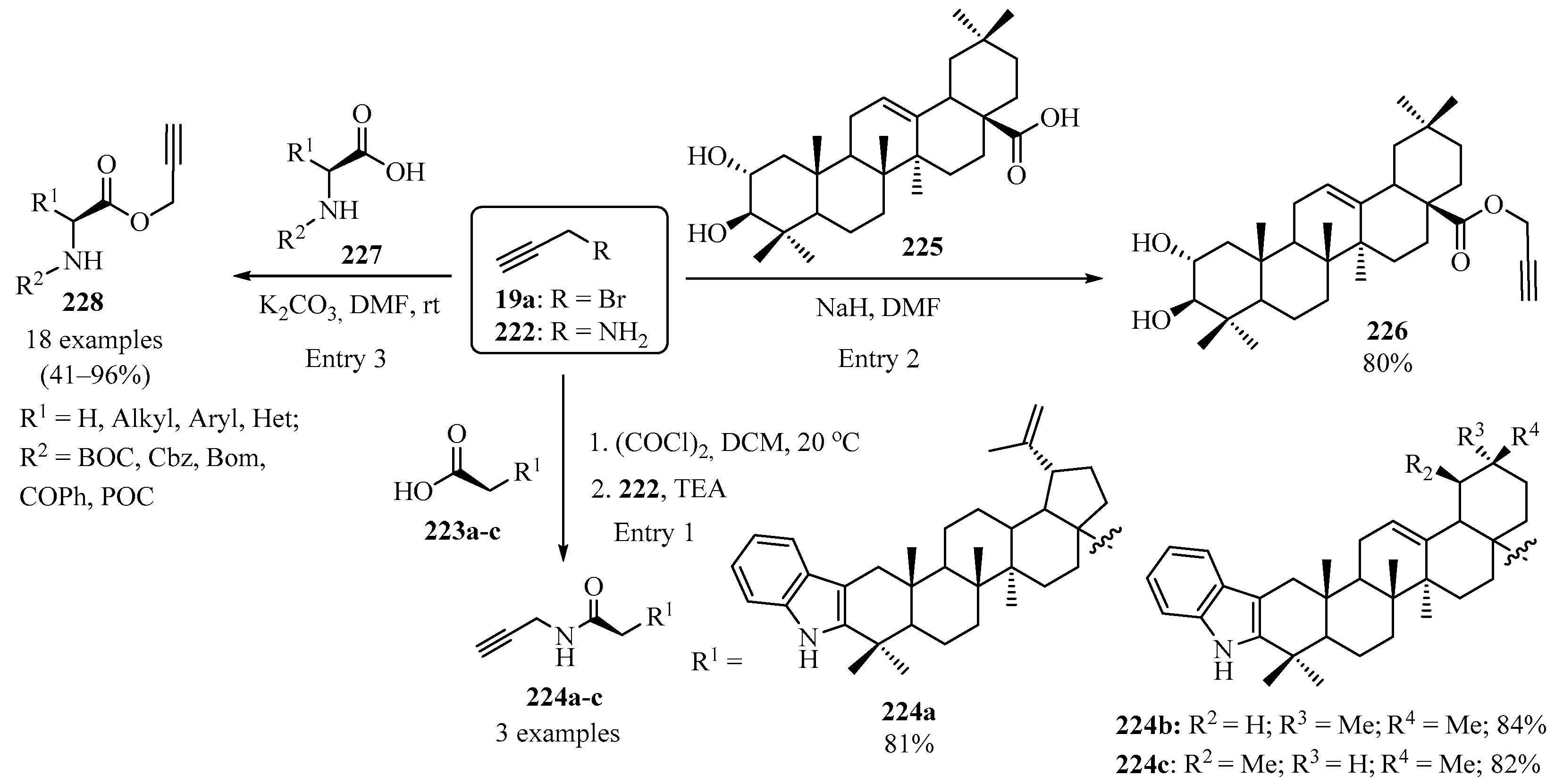
The propargylamides 224 were synthesized through a reaction between indoloacids 224 with propargylamine 222 (R = NH2) via an acyl chloride intermediate (generated in situ by reacting 223 with oxalyl chloride) (Scheme 74, entry 1) [189]. Using the same approach, propargylation of natural maslinic acid 225 with propargyl bromide 19a (R = Br) produced the desired propargyl derivative 226 (entry 2) [190].

Scheme 74.
Propargylation of the hydroxyl groups in carboxylic acids 223, 225, and 227 using propargyl bromide 19a and propargylamine 222.
The preparation of C-propargylic esters 228 was carried out via a reaction between N-protected amino acids 227 and propargyl bromide 19a (R = Br) in DMF in the presence of anhydrous potassium carbonate (Scheme 74, entry 3) [191].
2.7.10. With O-Propargylated Hydroxylamine
A novel bio-orthogonal prodrug 231 of the HDACi panobinostat was developed that was harmless to cells and could be converted back into the cytotoxic panobinostat via Au catalysis. The key propargylated product 231 was obtained from O-propargylated hydroxylamine 230 with β-substituted-acrylic acid 229 using N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) in H2O, according to Scheme 75 [192].
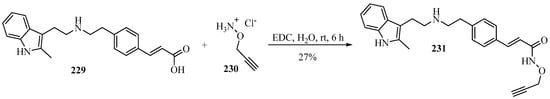
Scheme 75.
EDC-catalyzed synthesis of the propargylated prodrug 231 from O-propargylated hydroxylamine 230 and β-substituted-acrylic acid 229.
2.7.11. With Propargylic Cation Intermediates
Following a similar procedure to that described in Scheme 57, the propargylated N-Bz-D-phenylalanine 232 was synthesized through its carboxyl–CO2H functionality, by reacting the propargyl–dicobalt complex 170 with a phenylalanine derivative 227 (R1 = Bn) in the presence of BF3•OEt2 and CAN (Scheme 76) [167].

Scheme 76.
Synthesis of the propargylated N-Bz-D-phenylalanine 232 from the phenylalanine derivative 227 and propargyl–dicobalt complex 170.
2.8. (a) Alkenes, (b) Allenes, and (c) Enynes
- (a)
- Alkenes
2.8.1. With Propargyl-/Allenylboron
Catalytic enantioselective allylic substitution is a widely used strategy in organic synthesis, because it transforms an alkenyl substrate into a new unsaturated compound bearing an allylic stereogenic center [193].
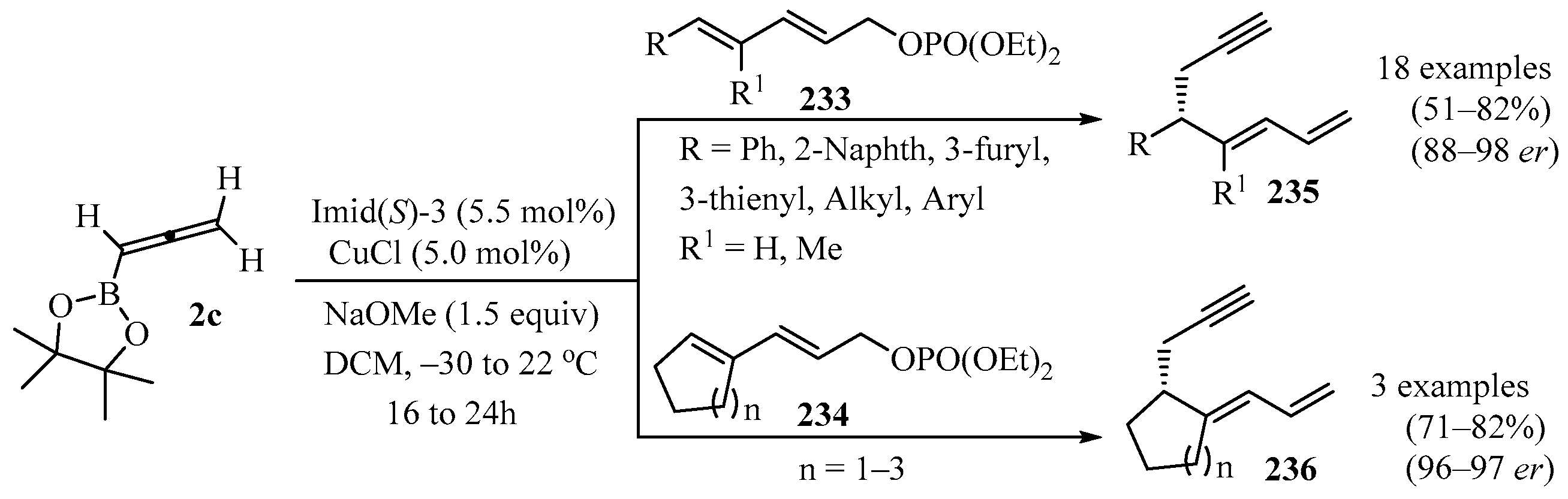
Transformations of acyclic, or aryl-, heteroaryl-, and alkyl-substituted penta-2,4-dienyl phosphates 233, as well as cyclic dienyl phosphates 234, were carried out in the presence of commercially available allenyl-B-(pinacolato) 2c, mediated by a sulfonate-containing NHC-Cu complex (NHC = imidazolyl carbene). Products 235/236 were obtained that contained, in addition to a 1,3-dienyl group, a readily functionalizable propargyl moiety (Scheme 77). The positive attributes of this reaction were high yields, high E:Z ratios, and impressive enantiomeric ratios (er). Kinetic isotope effect measurements and DFT computations provided mechanistic insights into this catalytic process [194].
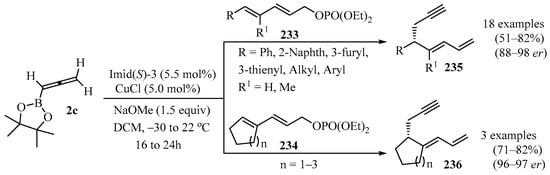
Scheme 77.
Synthesis of propargyl-containing 1,3-dienyl derivatives 235/236 from dienyl phosphates 233/234 and allenyl-B-(pinacolato) 2c mediated by a sulfonate-containing NHC-Cu complex.
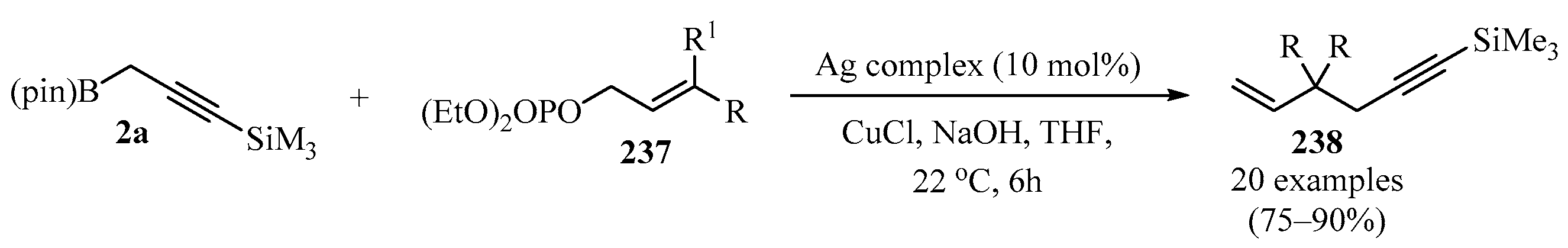
Focusing on allylic substitution, in another study, 1,5-enynes 238 were synthesized via a silver-catalyzed allylic substitution by reacting a propargylic organoboron compound 2a with allylic phosphates 237, using a chiral N-heterocyclic carbene (NHC) ligand and a silver catalyst complexed to a copper chloride salt (Scheme 78) [195]. In all cases, the incorporation of the propargylic group was favored over allenyl addition.

Scheme 78.
Ag-Catalyzed synthesis of the 1,5-enynes 238 from the reaction of allylic phosphates 237 with propargyl organoboron compound 2a.
2.8.2. With Propargyl Alcohols
The 1,5-enynes 240 were synthesized via the reaction of allyltrimethylsilane 239 with propargylic alcohols 63 in the presence of Bi(OTf)3 in [bmim][BF4] ionic liquid (IL) (Scheme 79). The reaction exhibited a broad substrate scope, with the possibility for the recovery/reuse of the IL solvent with a minimal decrease in isolated yields, after six cycles [196].

Scheme 79.
Synthesis of the 1,5-enynes 240 from allyltrimethylsilane 239 and propargylic alcohols 63 in the presence of Bi(OTf)3/[bmim][BF4] catalytic system.
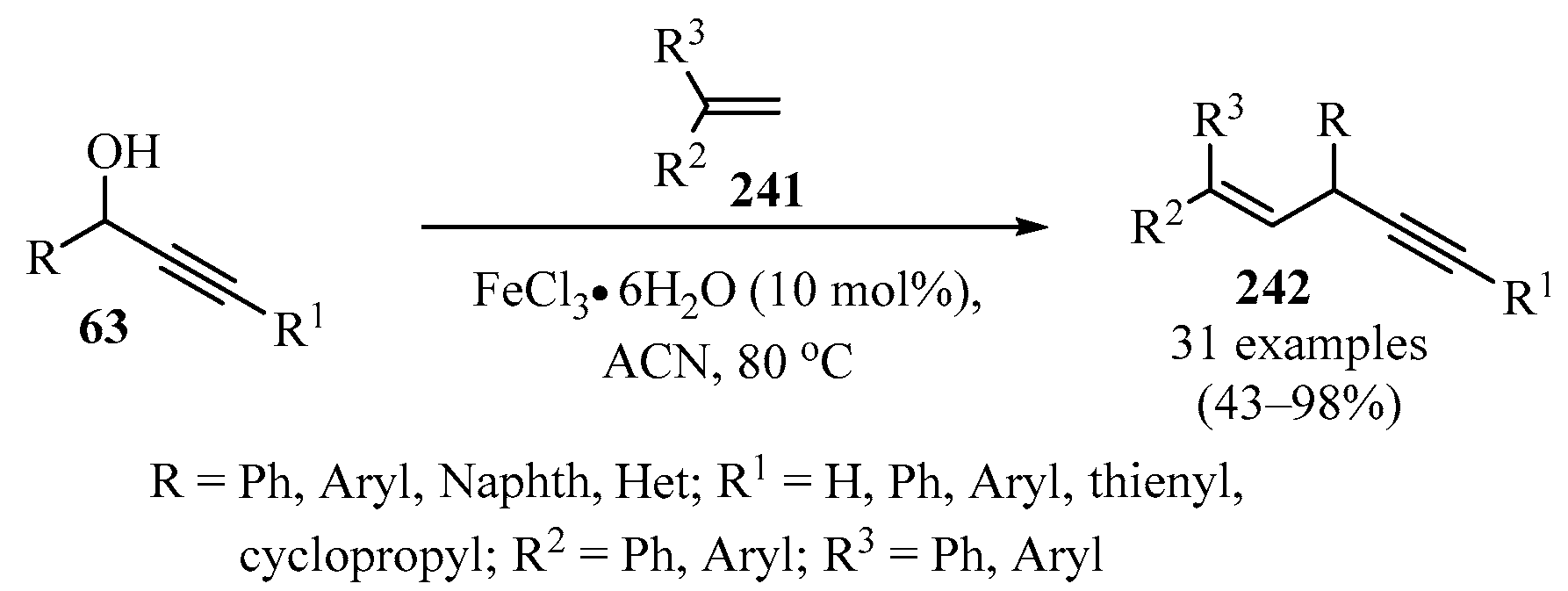
In another approach, diarylalkenyl propargylic frameworks 242 were synthesized via an Fe-catalyzed reaction of propargylic alcohols 63 with various symmetric and asymmetric 1,1-diarylethylenes 241 (Scheme 80). The reaction worked well for a wide range of ethylenes 241 bearing electron-donating or electron-withdrawing groups (as R2 or R3 substituents) [197].
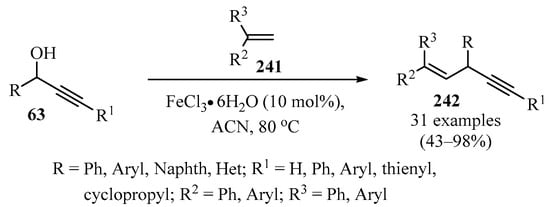
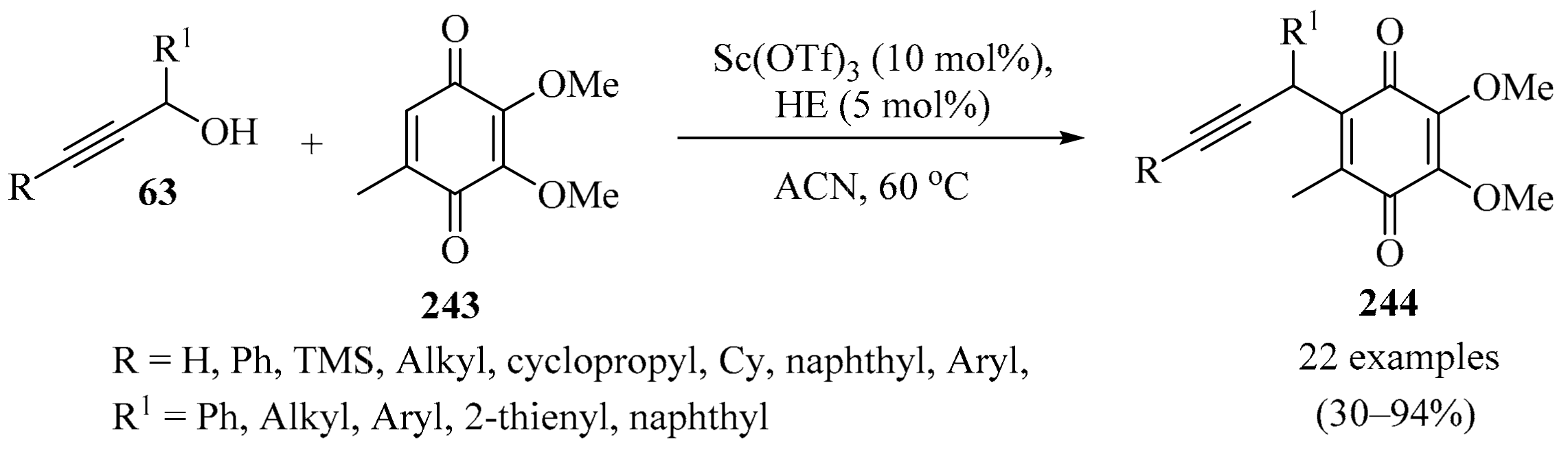
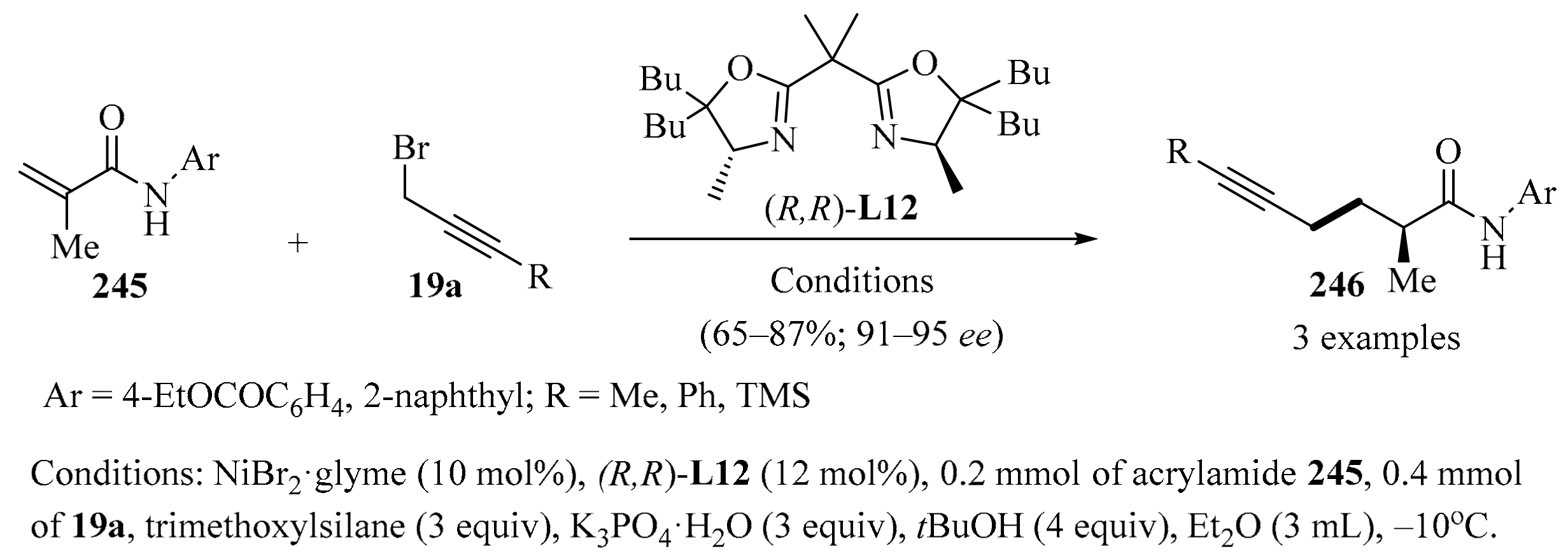
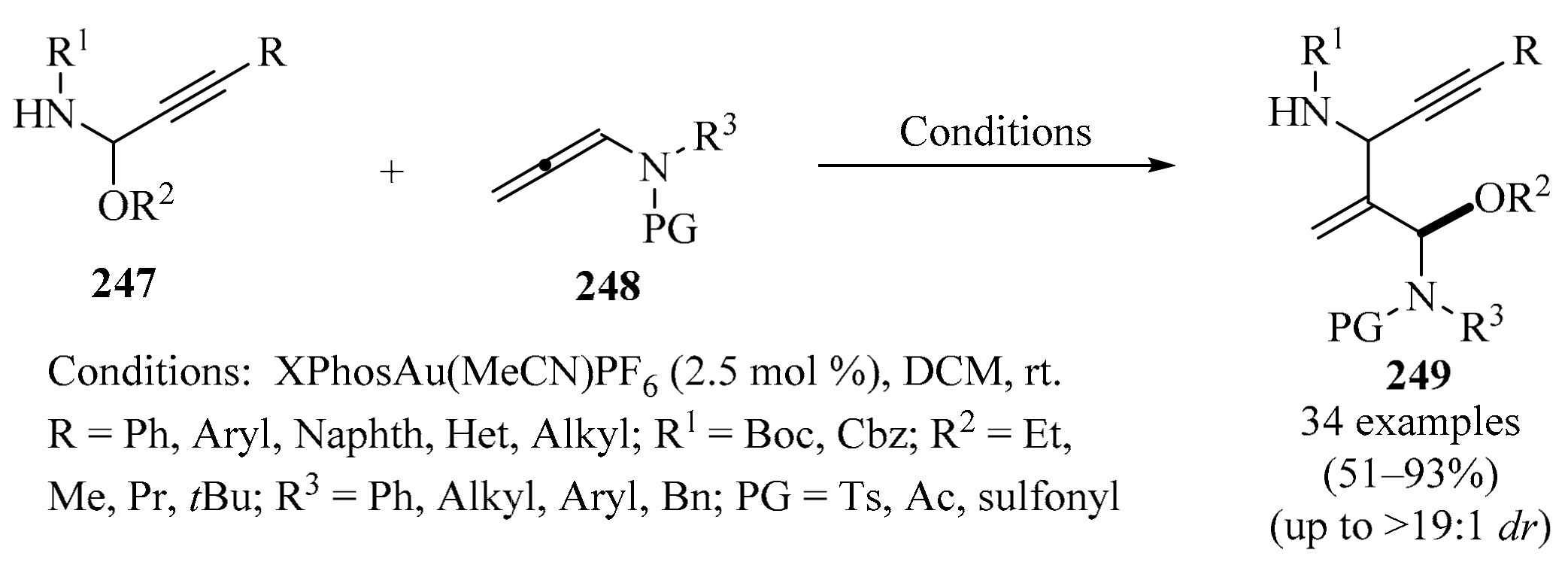
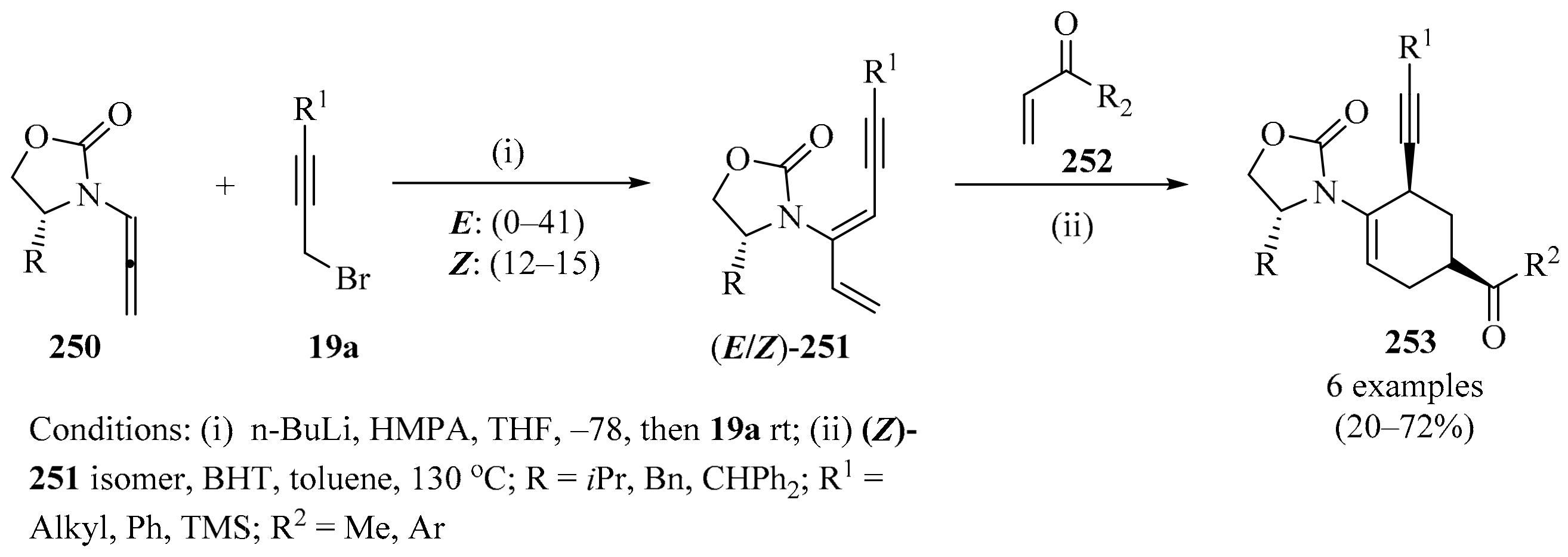
Scheme 80.
FeCl3•6H2O-catalyzed synthesis of diarylalkenyl propargylic derivatives 242 using propargylic alcohols 63 as propargylating agents.
An efficient catalytic method for the propargylation of quinones 243 that benefits from the cooperative effect of Sc(OTf)3 and Hantzsch ester (HE) has been reported, yielding the corresponding propargylated quinone derivatives 244 (Scheme 81). Using this approach, a broad range of propargylic alcohols 63 were converted into the appropriate propargyl derivatives 244 in acceptable to excellent yields [198].

Scheme 81.
Cooperative catalytic propargylation of quinones 243 mediated by Sc(OTf)3 and Hantzsch ester (HE).
2.8.3. With Propargyl Bromides
The development of enantioselective alkyl–alkyl cross-couplings with the formation of a stereogenic center is significant and highly desirable. In this context, the regio- and enantioselective Ni-catalyzed hydropropargylation of acrylamides 245 yielded propargylamides 246 bearing a tertiary stereogenic carbon center (Scheme 82). This protocol was carried out using propargyl bromides with alkyl, aryl, and silyl substituents 19a in the presence of a NiBr2 glyme, an (R,R)-L12 chiral ligand, trimethoxylsilane, potassium phosphate monohydrate, and tert-butanol in diethyl ether, producing Csp3–Csp3 cross-coupling products 246 in good yields and with excellent enantioselectivities [199].
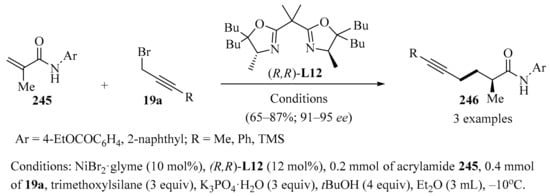
Scheme 82.
Regio- and enantioselective Ni-catalyzed synthesis of chiral propargylamides 246.
- (b)
- Alkenes
2.8.4. With Propargyl Ethers/Esters
Allenamides have received increasing attention in recent decades due to their diverse reactivity. In this context, highly diastereoselective oxy-propargylamination of allenamides 248 with C-alkynyl N-Boc-acetals as difunctionalization reagents 247 has been described, which employs XPhosAu-(MeCN)PF6 as a catalyst. This methodology provided highly functionalized propargyl-1,3-amino alcohol derivatives 249 in acceptable to good yields and with good to excellent diastereoselectivities (Scheme 83) [200].

Scheme 83.
Gold-catalyzed synthesis of propargyl-1,3-amino ether derivatives 249 from C-alkynyl N-Boc-acetals 248 and allenamides 247.
2.8.5. With Propargyl Bromides
A series of (E/Z)-3-amidodienynes 251 were synthesized via a tandem α-propargylation–1,3-H isomerization reaction of chiral allenamides 250 and propargyl bromides 19a with moderate E/Z ratios. Subsequently, the reactivities of these E/Z-isomers 251 were examined via thermal Diels–Alder cycloaddition reactions. The results showed that only the (Z)-3-amidodienynes (Z)-251 reacted to provide endo-II products 253 (Scheme 84) [201].
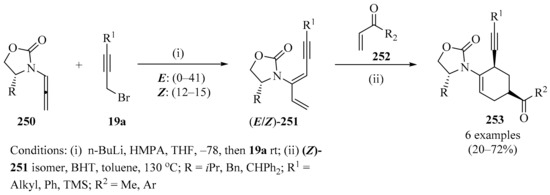
Scheme 84.
Synthesis of (E/Z)-3-amidodienynes 251 via tandem α-propargylation–1,3-H isomerization reaction of chiral allenamides 250 and propargyl bromides 19a and their Diels–Alder cycloadditions to produce cyclo-adducts 253.
- (c)
- Enynes
2.8.6. With Propargyl Alcohols
The chemoselectivity in the 1,4-carbooxygenations of 3-en-1-ynamides 254 with propargylic alcohols 63 was examined using a gold catalyst via non-Claisen pathways. The reactions were performed with electron-rich propargylic alcohols 63, using Ph3PAuCl/AgOTf as a catalytic system in toluene, producing 1,4-oxopropargylation products 255 in good yields and with high E-selectivity (Scheme 85) [202].
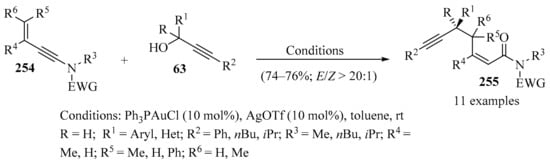
Scheme 85.
Synthesis of 1,4-oxopropargylated products 255 from propargylic alcohols 63 using Ph3PAuCl/AgOTf as catalytic system.
A chiral ruthenium-based complex was prepared from (TFA)2Ru(CO)(PPh3)2 and (R)-BINAP in order to catalyze the enantioselective C−C coupling of diverse-type primary alcohols 187 with conjugated enyne 60. This approach produced secondary homopropargyl alcohols 256 bearing gem-dimethyl groups in their structures (Scheme 86) [203].

Scheme 86.
Ruthenium-mediated synthesis of secondary homopropargyl alcohols 256 from conjugated enyne substrate 60.
2.9. Carbanionic-Like Nucleophiles
2.9.1. With Propargyl Alcohols
Propargylations of 1,3-diketones 257 were achieved with propargylic alcohols 63 mediated by Lewis and Brønsted acidic ILs in the presence of the metallic triflate Sc(OTf)3 or Bi(NO3)3 as catalysts, and produced products 258 (Scheme 87, entry 1). The scope of this condensation reaction was investigated using a variety of propargylic alcohols and a host of β-ketoesters 259 and cyclic dicarbonyl compounds 260, producing the corresponding adducts 261 and 262, respectively. The [BMIM][PF6]/Bi(NO3)3•5H2O catalytic system proved superior for propargylation reactions, and the IL solvent could be recycled and reused [204].
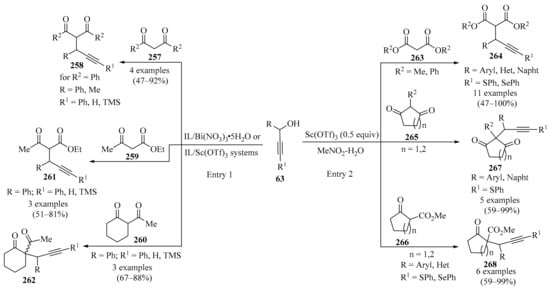
Scheme 87.
Propargylations of diverse dicarbonylic/dicarboxylic compounds 257/259/260/263/265/266 with propargylic alcohols 63 mediated by Lewis and Brønsted acidic ILs using Sc(OTf)3 or Bi(NO3)3•5H2O as catalysts.
Using Sc(OTf)3 as catalysts, alkynyl diesters 264 were synthesized via propargylations of 1,3-diesters 263 using 3-sulfanyl and 3-selanylpropargyl alcohols 63 (R1 = SPh, SePh) in MeNO2–H2O. Cyclic alkynyl diketones 265 and ketoesters 266 were similarly propargylated, (Scheme 87, entry 2). Further, under the action of bases such as Bu4NF, CsCO3, K2CO3 and NaH, some of the obtained propargylated derivatives 264, 267–268 underwent intramolecular cyclization to give diversely substituted tetrahydro-benzofurans [205].
Propargylic alcohols can be activated towards SN1-type reactions with nucleophiles using a variety of Lewis acids or Brønsted acids as catalysts [206]. In this process, the highly stereoselective organocatalytic alkylation of internal propargylic alcohols with aldehydes has been described, with water used as a solvent, using a mixture of In(OTf)3 and the MacMillan organocatalyst L*; these worked in a cooperative manner to produce propargyl aldehydes 270 regioselectively (Scheme 88). The reported method is versatile and tolerates diverse functional groups, allowing for the use of highly functionalized internal alkynes 63 and aldehydes 269 as precursors. According to the reaction conditions, the formation of 270 proceeds via an SN1-type reaction involving a stabilized propargylic cation species formed via the ionization of propargylic alcohols 63 [207].
Scheme 88.
Indium-mediated regioselective synthesis of propargyl aldehydes 270 from propargyl alcohols 63 using MacMillan reagent L* as chiral organocatalyst.
Expanding on propargylation reactions mediated by Lewis and Brønsted acidic ILs (in Scheme 87), a [BMIM][PF6]/Bi(NO3)3•5H2O catalytic system proved efficient for the propargylation of 4-hydroxycoumarins 187b, producing the corresponding propargylated 4-hydroxycoumarins 271 (Scheme 89) [204].

Scheme 89.
IL/Bi-mediated synthesis of C-propargylated 4-hydroxycoumarins 271 from propargyl alcohols 63.
2.9.2. With Propargyl Halides/Phosphoesters
With the goal of synthesizing the bicyclic fragment (i.e., AE rings) of the Daphniphyllum alkaloid yuzurine, the key intermediate 272 was synthesized via the diastereoselective propargylation of the α-position of lactone 271 with propargyl bromide 19a (X = Et) (Scheme 90, entry 1) [208]. In other approach, the propargylation of Ugi adducts 273 with propargyl bromide 19a (X = H), under the addition of excess sodium hydride in DMSO, led to the direct formation of pyrrolidinone enamides 275. Products 275 were produced via the intermediate formation of the propargyl derivatives 274, and cyclized in situ through the action of NaH (Scheme 90, entry 2). The latter compounds 275 were identified as useful precursors of iminium intermediates, and were applied to the formation of benzoindolizidine alkaloids via Ugi/propargylation/Pictet−Spengler cyclization [209].
Scheme 90.
Propargylation reactions of diverse methyne/methylene-active compounds 271/273/276/278/280 with propargyl bromides 19a.
1,3-diester 276 was propargylated with propargyl bromide 19a (X = H) using metallic zinc in DMF, producing the corresponding propargyl 1,3-diester 277 (Scheme 90, entry 3) [210]. In the context of multistep asymmetric total synthesis, the propargyl intermediate 279 was synthesized in a highly stereoselective fashion via LDA-mediated propargylation of the 1,3-dioxolanone 278 with propargyl bromide 19a (X = H), producing intermediate 279 (Scheme 90, entry 4) [211].
With the aim of evaluating the influence of ultrasound in association with a new phase-transfer catalyst (PTC) for synthetic purposes, 2,2-di(prop-2-ynyl)-1H-indene-1,3(2H)-dione 281 was synthesized via the propargylation of indene-1,3-dione 280 with propargyl bromide 19a (X = H) using aqueous potassium hydroxide under phase-transfer catalysis, employing N-benzyl-N-ethyl-N-isopropylpropan-2-ammonium bromide and ultrasonic irradiation in chlorobenzene (Scheme 90, entry 5). Based on a kinetic study, it was established that the overall reaction rate can be greatly enhanced with ultrasound irradiation [212].
Scheme 91 illustrates the reported synthesis of γ-ketoacetylene 284 via a condensation reaction between propargyl chloride 282 and β-keto ester 283 in the presence of sodium hydride [213]. This compound is a key intermediate in the biomimetic synthesis of plumarellide, a polycyclic diterpene [214].

Scheme 91.
Synthesis of the γ-ketoacetylene 284 via a condensation reaction between propargyl chloride 282 and β-keto ester 283 in the presence of sodium hydride.
1,4-Diynes are valuable and versatile synthons for natural products, organometallic complexes, and the synthesis of novel molecules [215]. Scheme 92 illustrates a reported method for the catalytic synthesis of difluorinated compounds 286, difluoromethylene (CF2)-skipped 1,4-diynes, via palladium-catalyzed cross-coupling between terminal alkynes 62 and gem-difluoropropargyl bromide 285 in toluene. The method exhibited high functional group tolerance and a broad substrate scope [216].
Scheme 92.
Pd-catalyzed synthesis of difluoromethylene (CF2)-skipped 1,4-diynes 286 from reaction of gem-difluoropropargyl bromide 285 with terminal alkynes 62.
Compounds bearing a quaternary carbon stereocenter are important building blocks in medicinal chemistry, and are found in biologically active compounds such as pharmaceuticals and agrochemicals. Scheme 93 illustrates an efficient enantioselective method for the asymmetric α-alkylation of α-branched aldehydes 204 with propargyl bromide 19a to generate products 287 bearing a chiral quaternary carbon stereocenter. The reaction proceeds through enamine-based organocatalysis using a chiral primary amino acid as a catalyst [217].

Scheme 93.
Asymmetric α-propargylation of α-branched aldehydes 204 mediated via primary amino acid catalyst.
Propargylated products 289 were synthesized via the Suzuki-type coupling of propargylic electrophiles 19d/109 with diborylmethane 288, using CuI/PPh3 as the catalytic system and tBuOLi as a base, under mild conditions with good functional group tolerance (Scheme 94) [218].
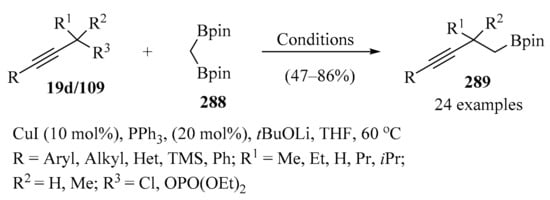
Scheme 94.
CuI/PPh3-mediated Suzuki–Miyaura-type cross-coupling reaction for the synthesis of propargylated products 289 from propargyl electrophiles 19d/109 and diborylmethane 288.
2.9.3. With Propargyl Ethers or Esters
The diastereo- and enantioselective synthesis of 2,2-disubstituted benzofuran-3(2H)-ones 291 was achieved via a “copper-pybox”-catalyzed reaction between 2-substituted benzofuran-3(2H)-ones 290 and propargyl acetates 200 (R = Ac), as outlined in Scheme 95, entry 1. The positive attributes of the method were good functional group tolerance and broad substrate scope. The utility of the method was demonstrated by further transformation of the terminal alkyne of 291 into a methyl ketone without loss of enantiomeric purity [219]. Using a similar approach, propargyl tricarboxylate derivatives 293 were synthesized via the copper-catalyzed enantioselective propargylation of triethylmethanetricarboxylate 292 with propargylic alcohol derivatives 200. The active catalyst “copper-pybox” was generated by combining the copper complex Cu(CH3CN)4BF4 with (S)-sec-butyl-Pybox (Ligand L1*) at low temperatures in methanol, with DIPEA as base, as outlined in Scheme 95, entry 2. The scope of the methodology was demonstrated using phenyl-substituted propargylic substrates 200 bearing electron-donating as well as electron-withdrawing groups at the para-position of the phenyl ring [220].
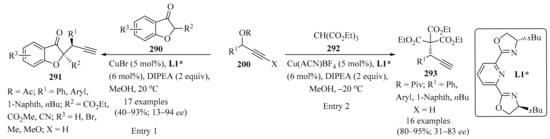
Scheme 95.
Copper-catalyzed diastereo- and enantioselective synthesis of propargylated compounds 291/293 using propargyl acetates 200 as propargylation reagents.
The efficacy of the copper–ligand complexes in stereoselective synthesis with propargyl esters are showcased here with the following examples, sketched in Scheme 96:

Scheme 96.
Efficacy of the copper–ligand complexes in stereoselective α-propargylation of diverse carbonylic/carboxylic compound 294/296/298/301/302 with propargyl esters 200.
- (i)
- The synthesis of a series of optically active 3,3-disubstituted oxindole skeletons 295 bearing vicinal tertiary and all-carbon quaternary stereocenters via the propargylation of 3-substituted oxindoles 294 with propargylic acetates 200, using Cu(ACN)4PF6 combined with a chiral tridentate ferrocenyl, P,N,N-ligand L1*, in methanol, entry 1 [221].
- (ii)
- The synthesis of a series of propargyl nitro derivatives 297 bearing two contiguous stereogenic centers by reacting propargylic carbonates 200 with α-substituted nitroacetates 296 using Cu−pybox as catalyst. The most striking features of these reactions are the observed high diastereo- and enantioselectivities. Products 297 were further employed as precursors of non-proteinogenic quaternary α-amino acids after the reduction of their nitro groups, entry 2 [222].
- (iii)
- The synthesis of highly functionalized chiral propargylated P-ylides 299 via the copper-catalyzed asymmetric propargylation of phosphonium salts 298 with racemic propargylic esters 200, in the presence of the chiral ligand L*, and further Wittig reactions of 299 with aliphatic aldehydes; this led to the synthesis of diversely substituted chiral propargylated alkene building blocks 300 (Scheme 96, entry 3), with a wide substrate scope and satisfactory functional group compatibility [223].
- (iv)
- The synthesis of terminal alkyne-containing products 303 and 304 bearing two vicinal stereocenters via an asymmetric propargylic substitution (APS) reaction of thiazolones 301 (A = S) and oxazolones 302 (A = O) with propargyl esters 200 (X = H) mediated by Cu/Zn and Cu/Ti dual metal catalytic systems (Scheme 96, entry 4). The resulting functional group-rich products exhibited good to excellent diastereo- and enantioselectivities [224].
- (v)
- The enantioselective synthesis of propargylic diesters 305 via a nickel/Lewis acid-catalyzed asymmetric propargyl substitution, by reacting achiral starting-type materials 263 and 54 under mild conditions. The introduction of a Lewis acid cocatalyst such as Yb(OTf)3 was crucial in transforming the mixture of 263 and 54 into products 305 (Scheme 97). Further, this asymmetric propargylic substitution reaction was investigated for the development of a range of structurally diverse natural products and seven biologically active compounds, namely, (−)-thiohexital, (+)-thiopental, (+)-pentobarbital, (−)-AMG 837, (+)-phenoxanol, (+)-citralis, and (−)-citralis, demonstrating the efficacy of this asymmetric strategy [225].
 Scheme 97. Nickel/Lewis acid-catalyzed asymmetric synthesis of propargylic diesters 305.
Scheme 97. Nickel/Lewis acid-catalyzed asymmetric synthesis of propargylic diesters 305. - (vi)
- Enantioselective copper-catalyzed vinylogous propargylic substitution with coumarin derivatives. In this approach, aromatic and aliphatic propargylic esters 200 reacted with substituted coumarins 306 under mild conditions to yield propargylated coumarin derivatives 307 with impressive enantioselectivities (Scheme 98). Further, biological studies on the compounds 307 led to the discovery of a novel class of autophagy inhibitors [226].
 Scheme 98. Copper-catalyzed synthesis of propargyl-substituted coumarins 307 from propargylic esters 200.
Scheme 98. Copper-catalyzed synthesis of propargyl-substituted coumarins 307 from propargylic esters 200.
A catalytic system based on bis(triphenylphosphine)palladium (II) dichloride, Ag2CO3, and phosphine-based ligand L was developed for the one-pot selective synthesis of diversely substituted dihydrofuro[3,2-c]coumarins 308. The synthetic strategy involved a propargylation reaction between propargylic carbonates 54 and 4-hydroxycoumarins 187b, mediated by the aforementioned catalytic system (Scheme 99). Mechanistic studies have suggested that 4-hydroxycoumarins 187b react with an η1–(propargyl)palladium complex, formed in situ, to generate the key terminal alkyne intermediate 271, which undergoes selective intramolecular 5-exo-dig cyclization to give the isolated products 308 in one pot [227].

Scheme 99.
Use of the bis(triphenylphosphine)palladium(II) dichloride-/Ag2CO3-/phosphine-based ligand L catalytic system for propargylation of 4-hydroxycoumarins 187b with propargylic carbonates 54.
A series of substituted pyrrole derivatives 310 were synthesized via a zinc(II) chloride-catalyzed regioselective propargylation/amination/cycloisomerization process by reacting enoxysilanes 309 with propargylic acetates 200 and primary amines 94. This method was applicable to a variety of aromatic and aliphatic propargylic acetates 200 without the necessity of isolating intermediates such as 258 (Scheme 100) [228].

Scheme 100.
Zinc(II) chloride-catalyzed three-component and regioselective propargylation of enoxysilanes 309 with propargylic acetates 200.
A series of diversely substituted propargyl ethers 311 were obtained via a Re(I)-catalyzed hydropropargylation reaction between silyl enol ethers 309 and propargyl ether 191 (Scheme 101). Mechanistic studies suggested that the reaction proceeded via the intermediacy of vinylidene–alkenyl metal intermediates undergoing a 1,5-hydride transfer to generate the isolated products 311 [229].

Scheme 101.
Re(I)- catalyzed synthesis of propargyl ethers 311 from hydropropargylation reaction between silyl enol ethers 309 and propargyl ether 191.
Fully substituted pyrroles are important bioactive motifs, and are widely presented in many biologically active compounds and natural products [230]. In this context, a copper-catalyzed and microwave-assisted tandem propargylation/alkyne azacyclization/isomerization sequence between propargyl acetates 200 and β-enamino compounds 312 was established (Scheme 102). Through this process, a series of pentasubstituted pyrroles 314 were synthesized. This transformation was characterized by a broad substrate scope that tolerated diverse substituents in its starting materials 200 and 312, and could be scaled up for further biomedical research. A mechanistic sequence in which an enyne-like structure 313 acts as a key intermediate in the catalytic cycle was proposed [231].
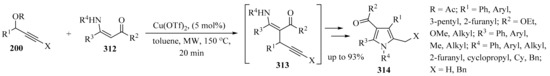
Scheme 102.
Copper-catalyzed and microwave-assisted synthesis of propargyl intermediates 313 via propargylation of β-enamino compounds 312 with propargyl acetates 200.
A highly diastereo- and enantioselective method for the synthesis of compounds 316/317 bearing vicinal tertiary stereocenters was devised by reacting propargylic acetates 200 with morpholine-derived cyclic enamine 315, in the presence of a copper catalyst, a chiral tridentate P,N,N-ligand ((R)-L*), and iPr2NEt in MeOH. This approach was compatible with a wide range of substrates 200, producing chiral propargylated cyclohexanones 316/317 in good yields and with excellent diastereoselectivity (Scheme 103) [232].
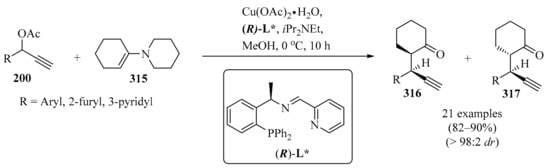
Scheme 103.
Copper-mediated diastereoselective synthesis of chiral propargylated cyclohexanones 316/317 from propargyl acetates 200 in the presence of the chiral tridentate P,N,N-ligand ((R)-L*).
2.9.4. With 1,3-Diarylpropynes
Direct C–C coupling from Csp3–H bonds with molecular oxygen as the terminal oxidant continues to be a challenging task. In this context, diversely substituted propargyl adducts 318 were synthesized via a coupling reaction between 1,3-dicarbonyl compounds 257/259 and 1,3-diarylpropynes 57 in the presence of molecular oxygen, DDQ, and sodium nitrite (Scheme 104). The addition of HCO2H dramatically increased the speed of the process [233].
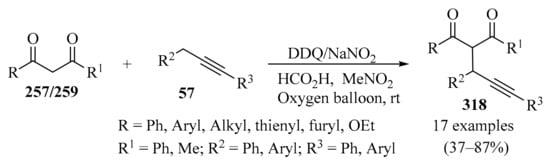
Scheme 104.
Synthesis of propargyl adducts 318 from a coupling reaction between 1,3-dicarbonyl compounds 257/259 and 1,3-diarylpropynes 57 in the presence of molecular oxygen, DDQ, and sodium nitrite.
2.9.5. With Propargyl Aldehydes
The metal-free, amino acid-catalyzed, three-component reductive coupling of propargyl aldehydes 319 and cyclic/acyclic methylene-active compounds 320/321, in the presence of Hantzsch ester and (S)-proline as catalysts, produced diversely substituted and gram-scalable propargylated cyclic/acyclic systems 322/323 (Scheme 105). To demonstrate the synthetic value of this protocol, in selected cases, adducts 322/323 were further transformed into dihydropyran derivatives through an annulative etherification reaction using AgOTf as a catalyst [234].
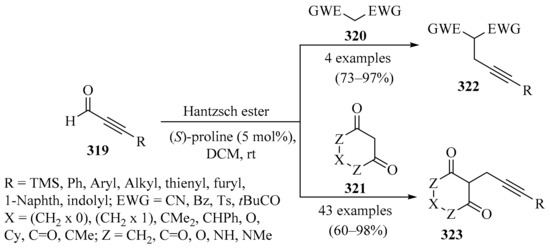
Scheme 105.
(S)-Proline-catalyzed three-component reductive coupling of propargyl aldehydes 319 with methylene-active compounds 320/321 in the presence of Hantzsch ester.
The propargylated alcohol 325 was synthesized via catalytic asymmetric propargylation of the highly enolizable β-keto-lactone 324 with propargyl aldehyde 319 (Scheme 106). The reaction was mediated by an Evans aldol type reaction [235], promoted by rigorously acid-free Sn(OTf)2. Notably, the synthesis of this compound was a key step in the total synthesis of leiodermatolide, a natural product derived from a deep-sea sponge with potent cytotoxic activity (Scheme 106) [236].
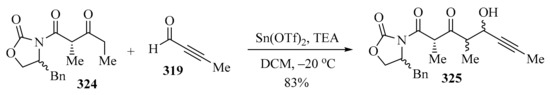
Scheme 106.
Synthesis of propargylated alcohol 325 via catalytic asymmetric propargylation of the enolizable β-keto-lactone 324 with propargyl aldehyde 319.
2.10. Carbocationic Electrophiles
With Propargyl Organometallic-Based Reagents
A series of diversely substituted o-propargylated phenols 327 were obtained through the transition metal-free alkynylation of substituted 2-(tosylmethyl)phenols 326 with bromo(alkynyl)zinc reagents 89, generated from the corresponding terminal alkyne with BuLi and ZnBr2, under N2 at room temperature. This efficient strategy exhibited good functional group compatibility (Scheme 107). The products were further used as intermediates for the synthesis of 2,3-disubstituted benzofurans [237].
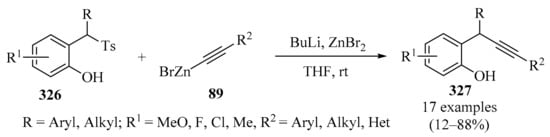
Scheme 107.
Bromo(alkynyl)zinc-mediated synthesis of o-propargylated phenols 327 from 2-(tosylmethyl)phenols 326.
A method for the synthesis of spiroketals 329 bearing a five-membered and a seven- or eight-membered ring was described. In this approach, initially, the alkyne 328 was treated with Co2(CO)8 in DCM at room temperature to form the corresponding alkyne–Co2(CO)6 complex intermediates, which were subsequently exposed to BF3•OEt2 at low temperature to produce the desired dioxaspiro[4.7]-compounds 329 (Scheme 108). This method was applicable to cyclopropanes possessing gem-disubstituents, as well as mono-aryl substituents [238].
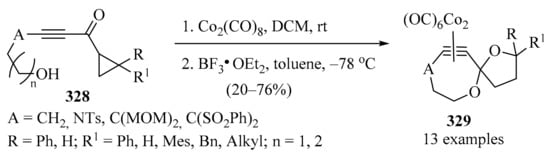
Scheme 108.
Synthesis of spiroketal derivatives 329 from propargyl derivatives 328 mediated by Co2(CO)6/BF3•OEt2 complex.
The synthesis of a series of propargylic and homopropargylic alcohols 331/332 was accomplished via the reaction of epoxides 330 with 3,3,4,4-tetraethoxybut-1-yne acetylide 89 (M = Mg). The use of a MgBr counterion in the acetylide proved superior for the selective formation of propargylic alcohol 331, while the use of a lithium acetylide and BF3, followed by hydrolysis, gave homopropargylic alcohols 332 (Scheme 109) [239].
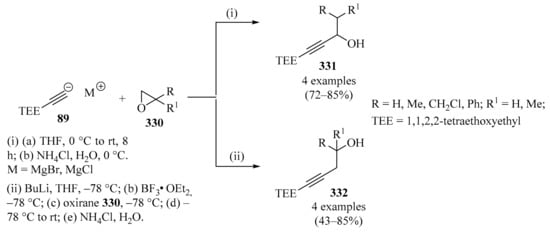
Scheme 109.
Synthesis of propargylic and homopropargylic alcohols 331/332 from the reaction of acetylide 89 with epoxides 330.
2.11. Free-Radical-like Precursors
2.11.1. With Propargyl Halides
Among the metal catalysts that promote alcohol C-H functionalization via C-X bond reductive cleavage pathways, rhodium-based catalysts were shown to be promising candidates [240]. In this sense, the carbinol C-propargylation of alcohols 187 with propargyl chlorides 19d in basic media, under rhodium-catalyzed transfer hydrogenation, enabled the direct conversion of primary alcohols 187 into propargylated alcohols 13. Interestingly, this methodology tolerated benzylic and heteroaromatic benzylic alcohols, as well as aliphatic and allylic alcohols 187, producing the expected homopropargyl alcohols 13 in good yields (Scheme 110) [241].
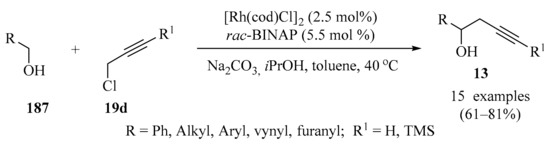
Scheme 110.
Synthesis of homopropargyl alcohols 13 via Rh-catalyzed C-C coupling of primary alcohols 187 with propargyl chlorides 19d.
A radical hydrodifluoropropargylation method in which alkenes 241 are reacted with silyl-protected bromodifluoropropyne 285 in DMF, at room temperature and under irradiation with blue LEDs, has been described [242]. The method employed diphenyldisulfide and benzothiazoline 333 as reductants, yielding silyl-protected difluoropropargylated products 334 in acceptable to good yields, with wide functional group tolerance (Scheme 111) [242].
Scheme 111.
Blue LED-catalyzed synthesis of difluoropropargylated products 334 from alkenes 241 and silyl-protected bromodifluoropropyne 285 as propargylating agent.
2.11.2. With 1,3-Enynes
The 1,3-enyne moiety has been recognized as an alternative pronucleophile for the carbonyl propargylation process [243]. Radical carbonyl propargylation via dual chromium/photoredox catalysis was recently reported [244]. Using this approach, a library of homopropargylic alcohols 336 bearing all-carbon quaternary centers was synthesized (Scheme 112) via the catalytic radical tricomponent coupling of 1,3-enynes 60 (R2 = Me, CH2OH), aldehydes 1, and suitable radical precursors (Hantzsch ester) 335 in the presence of an iridium-based photocatalyst (PC). This redox-neutral multi-component reaction occurred under mild conditions and showed high functional group tolerance, producing products 336 with acceptable diastereomeric ratios [244].
Scheme 112.
Enyne-mediated synthesis of homopropargylic alcohols 336 through radical carbonyl propargylation via dual chromium/photoredox catalysis.
2.12. Boronic Acids (ArB(OH)2)
With Propargyl Bromides
The efficient microwave-assisted (MW), two-step synthesis of N-aryl propargylamines 144 from aromatic boronic acids 337, aqueous ammonia, and propargyl bromide 19a was reported. The first step involved copper-catalyzed coupling of aromatic boronic acids 337 with aqueous ammonia, which reacted with propargyl bromide 19a in the second step to give a propargylamine derivative 144 (Scheme 113, entry 1) [245]. In another approach, gem-difluoropropargyl derivatives 190 were prepared via the difluoropropargylation of boronic acids 337 with gem-difluoropropargyl bromide 285, by employing [Pd2(dba)3]/P(o-Tol)3 (L1) as a catalyst in the presence of K2CO3 in dioxane (Scheme 113, entry 2) [246].

Scheme 113.
Synthesis of propargyl derivatives 190 and 144 from coupling reactions of propargyl bromides 285 and 19a with boronic acid reagents 337.
2.13. Nitrones
With Propargyl Bromide
The propargylation of chiral nonracemic mono- and poly-hydroxylated cyclic nitrone derivatives 338–340 with Grignard reagents (generated in situ) was established as an efficient method for preparing building blocks containing an alkyne moiety 341–343. These compounds were then employed in copper-catalyzed azide alkyne cycloaddition click chemistry [247]. The synthesis of 341–343 was accompanied, in most cases, by the formation of diastereomeric mixtures, and also required the use of (trimethylsilyl)propargyl bromide 19a as a precursor for the formation of the Grignard reagent, in order to avoid the formation of undesired allene derivatives (Scheme 114).
Scheme 114.
Propargylation of chiral nonracemic mono- and poly-hydroxylated cyclic nitrones 338–340 with propargylated Grignard reagents (generated in situ) from TMS-propargyl bromide 19a.
3. Conclusions and Outlook
This review has underscored the importance of the propargyl moiety as a highly versatile and powerful building block in organic synthesis. Propargylic and homopropargylic reagents have been synthesized from a variety of precursors and applied to a highly diverse array of substrates to synthesize propargylated derivatives. Judicious selections of catalysts, co-catalysts, and chiral ligands have resulted in the development the stereo- and enantio-selective synthesis of numerous functional small molecules, with applications in natural products and medicinal chemistry. The progress in this area during the last decade has been nothing short of astonishing. Clearly, this is a highly dynamic and continuously evolving research area, and we are confident that it will continue to advance in the coming decade.
Author Contributions
K.K.L. conceived the project and worked with R.A. and D.I. through various stages of manuscript, including organization/development, writing/rewriting, reviewing, and editing. R.A. constructed the project, organized the material, and wrote various drafts of the manuscript with D.I., R.A. and D.I. performed the literature searches, assembled the references, and prepared the graphics and tables. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
D.I. thanks the Universidad del Norte for their partial financial support of this work. R.A. thanks Minciencias, the Universidad del Valle, and CIBioFi for their partial financial support. We are thankful to the reviewer of this paper for bringing to our attention references to shorter, more focused reviews within the topic, which we have cited as [248,249,250].
Conflicts of Interest
The authors declare no conflict of interest.
References
- Xing, Y.; Wei, Y.; Zhou, H. Applications of the in situ propargyl-allenyl isomerization in organic synthesis. Curr. Org. Chem. 2012, 16, 1594–1608. [Google Scholar] [CrossRef]
- Kobychev, V.B.; Vitkovskaya, N.M.; Klyba, N.S.; Trofimov, B.A. Acetylene-allene rearrangement of propargyl systems X-CH2-C≡CH (X = H, Me, NMe2, OMe, F, SMe): An ab initio study. Russ. Chem. Bull. Int. Ed. 2002, 51, 774–782. [Google Scholar] [CrossRef]
- Fandrick, D.R.; Fandrick, K.R.; Reeves, J.T.; Tan, Z.; Johnson, C.S.; Lee, H.; Song, J.J.; Yee, N.K.; Senanayake, C.H. Zinc catalyzed and mediated propargylations with propargyl boronates. Org. Lett. 2010, 12, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A. Chiral allylic and allenic metal reagents for organic synthesis. J. Org. Chem. 2007, 72, 8153–8166. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Usanov, D.L. Comprehensive Organic Synthesis II, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 2, pp. 209–242. [Google Scholar]
- Yu, S.; Ma, S. Allenes in catalytic asymmetric synthesis and natural product syntheses. Angew. Chem. Int. Ed. 2012, 51, 3074–3112. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Palovich, M.R. Synthesis of stereopentad subunits of zincophorin and rifamycin-S through use of chiral allenyltin reagents. J. Org. Chem. 1998, 63, 3701–3705. [Google Scholar] [CrossRef]
- Francais, A.; Leyva, A.; Etxebarria-Jardi, G.; Ley, S.V. Total synthesis of the anti-apoptotic agents iso- and bongkrekic acids. Org. Lett. 2010, 12, 340–343. [Google Scholar] [CrossRef]
- Qian, H.; Huang, D.; Bi, Y.; Yan, G. 2-Propargyl alcohols in organic synthesis. Adv. Synth. Catal. 2019, 361, 3240–3280. [Google Scholar] [CrossRef]
- Fandrick, D.R.; Fandrick, K.R.; Reeves, J.T.; Tan, Z.; Tang, W.; Capacci, A.G.; Rodriguez, S.; Song, J.J.; Lee, H.; Yee, N.K.; et al. Copper catalyzed asymmetric propargylation of aldehydes. J. Am. Chem. Soc. 2010, 132, 7600–7601. [Google Scholar] [CrossRef]
- Shi, S.-L.; Xu, L.-W.; Oisaki, K.; Kanai, M.; Shibasaki, M. Identification of modular chiral bisphosphines effective for Cu(I)-catalyzed asymmetric allylation and propargylation of ketones. J. Am. Chem. Soc. 2010, 132, 6638–6639. [Google Scholar] [CrossRef]
- Barnett, D.S.; Schaus, S.E. Asymmetric propargylation of ketones using allenylboronates catalyzed by chiral biphenols. Org. Lett. 2011, 13, 4020–4023. [Google Scholar] [CrossRef]
- Fandrick, K.R.; Fandrick, D.R.; Reeves, J.T.; Gao, J.; Ma, S.; Li, W.; Lee, H.; Grinberg, N.; Lu, B.; Senanayake, C.H. A general copper-BINAP-catalyzed asymmetric propargylation of ketones with propargyl boronates. J. Am. Chem. Soc. 2011, 133, 10332–10335. [Google Scholar] [CrossRef] [PubMed]
- Kohn, B.L.; Ichiishi, N.; Jarvo, E.R. Silver-catalyzed allenylation and enantioselective propargylation reactions of ketones. Angew. Chem. Int. Ed. 2013, 52, 4414–4417. [Google Scholar] [CrossRef] [PubMed]
- Fandrick, D.R.; Reeves, J.T.; Bakonyi, J.M.; Nyalapatla, P.R.; Tan, Z.; Niemeier, O.; Akalay, D.; Fandrick, K.R.; Wohlleben, W.; Ollenberger, S.; et al. Zinc catalyzed and mediated asymmetric propargylation of trifluoromethyl ketones with a propargyl boronate. J. Org. Chem. 2013, 78, 3592–3615. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, R.; Wang, X.; Wang, Z.; Dinga, K. Synthesis of chiral tertiary α,α-difluoromethyl carbinols by Cu-catalyzed asymmetric propargylation. Chem. Eur. J. 2019, 25, 16425–16434. [Google Scholar] [CrossRef] [PubMed]
- Freitas, J.J.R.; Freitas, Q.P.S.B.; Andrade, S.R.C.P.; Freitas, J.C.R.; Oliveira, R.A.; Menezes, P.H. Efficient method for propargylation of aldehydes promoted by allenylboron compounds under microwave irradiation. Beilstein J. Org. Chem. 2020, 16, 168–174. [Google Scholar] [CrossRef]
- Wang, H.; Jain, P.; Antilla, J.C.; Houk, K.N. Origins of stereoselectivities in chiral phosphoric acid catalyzed, allylborations and propargylations of aldehydes. J. Org. Chem. 2013, 78, 1208–1215. [Google Scholar] [CrossRef]
- Grayson, M.N.; Goodman, J.M. Understanding the mechanism of the asymmetric propargylation of aldehydes promoted by 1,1′-bi-2-naphthol-derived catalysts. J. Am. Chem. Soc. 2013, 135, 6142–6148. [Google Scholar] [CrossRef]
- Grayson, M.N.; Goodman, J.M. Lewis acid catalysis and ligand exchange in the asymmetric binaphthol-catalyzed propargylation of ketones. J. Org. Chem. 2013, 78, 8796–8801. [Google Scholar] [CrossRef]
- Zou, Y.; Gutierrez, O.; Sader, A.C.; Patel, N.D.; Fandrick, D.R.; Busacca, C.A.; Fandrick, K.R.; Kozlowski, M.; Senanayake, C.H. A computational investigation of the ligand-controlled Cu-catalyzed site-selective propargylation and allenylation of carbonyl compounds. Org. Lett. 2017, 19, 6064–6067. [Google Scholar] [CrossRef]
- Gupta, N.; Tak, R.; Nazish, M.; Jakhar, A.; Khan, N.H.; Kureshy, R.I. Copper(II) triflate catalyzed regioselective and enantioselective propargylation of isatin derivatives by using allenylboronic acid pinacol ester. Eur. J. Org. Chem. 2018, 2018, 1384–1392. [Google Scholar] [CrossRef]
- Zhao, J.; Jonker, S.J.T.; Meyer, D.N.; Schulz, G.; Tran, C.D.; Eriksson, L.; Szabó, K.J. Copper-catalyzed synthesis of allenylboronic acids. Access to sterically encumbered homopropargylic alcohols and amines by propargylboration. Chem. Sci. 2018, 9, 3305–3312. [Google Scholar] [CrossRef]
- Freitas, J.J.R.; Couto, T.R.; Cavalcanti, I.H.; Freitas, J.C.R.; Barbosa, Q.P.S.; Oliveira, R.A. Regioselective propargylation of aldehydes using potassium allenyltrifluoroborate promoted by tonsil. Tetrahedron Lett. 2016, 57, 760–765. [Google Scholar] [CrossRef]
- Couto, T.R.; Freitas, J.J.R.; Freitas, J.C.R.; Cavalcanti, I.H.; Menezes, P.H.; Oliveira, R.A. Propargylation of aldehydes using potassium allenyltrifluoroborate. Synthesis 2015, 47, 71–78. [Google Scholar] [CrossRef]
- Denmark, S.E.; Beutner, G.L. Lewis base catalysis in organic synthesis. Angew. Chem. Int. Ed. 2008, 47, 1560–1638. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, A.; Bamba, K.; Kawada, A. Asymmetric addition of propargylic silanes to aldehydes catalyzed by chiral phosphine-silver alkoxide complex. Chem. Select 2018, 3, 13777–13781. [Google Scholar] [CrossRef]
- Schneider, U.; Sugiura, M.; Kobayashi, S. Highly selective preparation of allenic and homopropargylic hydrazides through regiospecific addition of propargyltrichlorosilane and allenyltrichlorosilane to various types of N-acylhydrazones. Adv. Synth. Catal. 2006, 348, 323–329. [Google Scholar] [CrossRef]
- Chen, J.; Captain, B.; Takenaka, N. Helical chiral 2,2′-bipyridine N-monoxides as catalysts in the enantioselective propargylation of aldehydes with allenyltrichlorosilane. Org. Lett. 2011, 13, 1654–1657. [Google Scholar] [CrossRef]
- Lu, T.; Porterfield, M.A.; Wheeler, S.E. Explaining the disparate stereoselectivities of N-oxide catalyzed allylations and propargylations of aldehydes. Org. Lett. 2012, 14, 5310–5313. [Google Scholar] [CrossRef]
- Rooks, B.J.; Haas, M.R.; Sepulveda, D.; Lu, T.; Wheeler, S.E. Prospects for the computational design of bipyridine N,N′-dioxide catalysts for asymmetric propargylation reactions. ACS Catal. 2015, 5, 272–280. [Google Scholar] [CrossRef]
- Negi, J.S.; Bisht, V.K.; Singh, P.; Rawat, M.S.M.; Joshi, G.P. Naturally occurring xanthones: Chemistry and biology. J. Appl. Chem. 2013, 2013, 621459. [Google Scholar] [CrossRef]
- Grzelakowska, A.; Kolinska, J.; Zaklos-Szyda, M.; Sokolowska, J. Novel fluorescent probes for L-cysteine based on the xanthone skeleton. J. Photochem. Photobiol. 2020, 387, 112153. [Google Scholar] [CrossRef]
- Fernández, S.; Allegue, D.; Santamaría, J.; Ballesteros, A. Diastereoselective and synergistic gold-catalyzed bispropargylation of xanthones and thioxanthones: An access to xanthene derivatives. Adv. Synth. Catal. 2022, 364, 2620–2628. [Google Scholar] [CrossRef]
- Hiyama, T.; Okude, Y.; Kimura, K.; Nozaki, H. Highly selective carbon-carbon bond forming reactions mediated by chromium (II) reagents. Bull. Chem. Soc. Jpn. 1982, 55, 561–568. [Google Scholar] [CrossRef]
- Abell, J.P.; Yamamoto, H. Development and applications of tethered bis-(8-quinolinolato) metal complexes (TBOxM). Chem. Soc. Rev. 2010, 39, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, N.; Xia, G.; Yamamoto, H. Catalytic, highly enantio-and diastereoselective pinacol coupling reaction with a new tethered bis(8-quinolinolato) ligand. J. Am. Chem. Soc. 2004, 126, 13198–13199. [Google Scholar] [CrossRef]
- Xia, G.; Yamamoto, H. Catalytic enantioselective Nozaki−Hiyama allylation reaction with tethered bis(8-quinolinolato)(TBOx) chromium complex. J. Am. Chem. Soc. 2006, 128, 2554–2555. [Google Scholar] [CrossRef]
- Xia, G.; Yamamoto, H. Catalytic enantioselective allenylation reactions of aldehydes with tethered bis(8-quinolinolato)(TBOx) chromium complex. J. Am. Chem. Soc. 2007, 129, 496–497. [Google Scholar] [CrossRef]
- Naodovic, M.; Xia, G.; Yamamoto, H. TBOxCrIIICl-Catalyzed enantioselective synthesis of 1,3-butadien-2-ylcarbinols. Org. Lett. 2008, 10, 4053–4055. [Google Scholar] [CrossRef]
- Barbier, P. Synthèse du diéthylhepténol. Compt. Rend. 1899, 128, 110. [Google Scholar]
- Usanov, D.L.; Yamamoto, H. Asymmetric Nozaki–Hiyama propargylation of aldehydes: Enhancement of enantioselectivity by cobalt Co-catalysis. Angew. Chem. Int. Ed. 2010, 49, 8169–8172. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Ngai, M.-Y.; Dong, G. Ligand-accelerated enantioselective propargylation of aldehydes via allenylzinc reagents. Org. Lett. 2011, 13, 1900–1903. [Google Scholar] [CrossRef] [PubMed]
- Harper, K.C.; Sigman, M.S. Three-dimensional correlation of steric and electronic free energy relationships guides asymmetric propargylation. Science 2011, 333, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bascón, J.; Sancho-Sanz, I.; Álvarez-Manzaneda, E.; Rosales, A.; Oltra, J.E. Highly selective Barbier-type propargylations and allenylations catalyzed by titanocene(III). Chem. Eur. J. 2012, 18, 14479–14486. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Mandal, S.P.; Kundu, M.; Adhikari, U.; Roy, U.K. Synthesis and characterization of nano-zinc wire using a self-designed unit galvanic cell in aqueous medium and its reactivity in propargylation of aldehydes. Tetrahedron 2019, 75, 4669–4675. [Google Scholar] [CrossRef]
- Harper, K.C.; Vilardi, S.C.; Sigman, M.S. Prediction of catalyst and substrate performance in the enantioselective propargylation of aliphatic ketones by a multidimensional model of steric effects. J. Am. Chem. Soc. 2013, 135, 2482–2485. [Google Scholar] [CrossRef]
- Ahuja, B.B.; Emmanuvel, L.; Sudalai, A. A formal enantioselective synthesis of (–)-epiquinamide by proline-catalyzed one-pot sequential α-amination/propargylation of aldehyde and asymmetric dihydroxylation of olefin. Synlett 2016, 27, 1699–1702. [Google Scholar] [CrossRef]
- Reddy, C.R.; Reddy, M.D.; Dilipkumar, U. Total synthesis of a pyrrole lactone alkaloid, Longanlactone. Eur. J. Org. Chem. 2014, 2014, 6310–6313. [Google Scholar] [CrossRef]
- Leppänena, A.-S.; Xua, C.; Parikka, K.; Eklunde, P.; Sjöholme, R.; Brumer, H.; Tenkanend, M.; Willför, S. Targeted allylation and propargylation of galactose-containing polysaccharides in water. Carbohydr. Polym. 2014, 100, 46–54. [Google Scholar] [CrossRef]
- Cram, D.J.; Kopecky, K.R. Studies in stereochemistry. XXX. Models for steric control of asymmetric induction. J. Am. Chem. Soc. 1959, 81, 2748–2755. [Google Scholar] [CrossRef]
- Rao, H.S.P.; Satish, V.; Kanniyappan, S.; Kumari, P. Studies towards iodine-catalyzed dehydrative-cycloisomerization of pent-4-yne-1,2-diols to di- and tri-substituted furans. Tetrahedron 2018, 74, 6047–6056. [Google Scholar] [CrossRef]
- Mahadevegowda, S.H.; Khan, F.A. Diastereoselective synthesis of spirocyclic dihydrofurans and 1-oxaspiro[4.5]decan-6-one derivatives from norbornyl α-diketones. Eur. J. Org. Chem. 2015, 2015, 858–870. [Google Scholar] [CrossRef]
- Twilton, J.; Le, C.C.; Zhang, P.; Shaw, M.H.; Evans, R.W.; MacMillan, D.W.C. The merger of transition metal and photocatalysis. Nat. Rev. Chem. 2017, 1, 0052. [Google Scholar] [CrossRef]
- Zhang, H.H.; Chen, H.; Zhu, C.; Yu, S. A review of enantioselective dual transition metal/photoredox catalysis. Sci. China Chem. 2020, 63, 637–647. [Google Scholar] [CrossRef]
- Calogero, F.; Gualandi, A.; Di Matteo, M.; Potenti, S.; Fermi, A.; Bergamini, G.; Cozzi, P.G. Photoredox propargylation of aldehydes catalytic in titanium. J. Org. Chem. 2021, 86, 7002–7009. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Adhikari, U.; Hajra, P.P.; Roy, U.K. Allylation and propargylation of aldehydes mediated by in situ situgenerated zinc from the redox couple of Al and ZnCl2 in 2N HCl. New J. Chem. 2021, 45, 7163–7173. [Google Scholar] [CrossRef]
- Zhang, R.; Xia, Y.; Yan, Y.; Ouyang, L. Cu-Catalyzed, Mn-mediated propargylation and allenylation of aldehydes with propargyl bromides. BMC Chem. 2022, 16, 14. [Google Scholar] [CrossRef] [PubMed]
- Loh, T.P.; Lin, M.J.; Tan, K.L. Indium-mediated propargylation of aldehydes: Regioselectivity and enantioselectivity studies. Tetrahedron Lett. 2003, 44, 507–509. [Google Scholar] [CrossRef]
- Haddad, T.D.; Hirayama, L.C.; Buckley, J.J.; Singaram, B. Indium-mediated asymmetric Barbier-type propargylations: Additions to aldehydes and ketones and mechanistic investigation of the organoindium reagents. J. Org. Chem. 2012, 77, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Chan, T.S. Indium-mediated allenylation and propargylation of allenic alcohols with prop-2-ynyl bromides. Synthesis 2003, 34, 0785–0789. [Google Scholar] [CrossRef]
- Ghosh, P.; Chattopadhyay, A. A practical procedure of propargylation of aldehydes. Tetrahedron Lett. 2012, 53, 5202–5205. [Google Scholar] [CrossRef]
- Hopf, H.; Bohm, I.; Kleinschroth, J. Diels-Alder reaction of 1,2,4,5-hexatetraene: Tetramethyl[2.2]paracyclophane-4,5,12,13-tetracarboxylate. Org. Synth. 1981, 60, 41–48. [Google Scholar] [CrossRef]
- Li, H.; Sheeran, J.W.; Clausen, A.M.; Fang, Y.Q.; Bio, M.M.; Bader, S. Flow asymmetric propargylation: Development of continuous processes for the preparation of a chiral β-amino alcohol. Angew. Chem. Int. Ed. 2017, 56, 9425–99426. [Google Scholar] [CrossRef] [PubMed]
- Tsui, G.C.; Vielleneuve, K.; Carlson, E.; Tam, W. Ruthenium-catalyzed [2 + 2] cycloadditions between norbornene and propargylic alcohols or their derivatives. Organometallics 2014, 33, 3847–3856. [Google Scholar] [CrossRef]
- Xan, X. A practical and expedient synthesis of 2-heterocycle (C–N bond) substituted 4-oxo-4-arylbutanoates. Tetrahedron Lett. 2007, 48, 2845–2849. [Google Scholar] [CrossRef]
- Sonye, J.P.; Koide, K. Sodium bicarbonate-catalyzed stereoselective isomerizations of electron-deficient propargylic alcohols to (Z)-enones. J. Org. Chem. 2007, 72, 1846–1848. [Google Scholar] [CrossRef]
- Rao, K.S.; Reddy, D.S.; Pal, M.; Mukkanti, K.; Iqbal, J. Synthesis of γ-N-acylamino-β-keto esters and ethyl 5-oxazoleacetates via Ritter reaction and hydration of γ-hydroxy-α,β-alkynoic esters. Tetrahedron Lett. 2006, 47, 4385–4388. [Google Scholar] [CrossRef]
- Yaragola, S.; Dada, R.; Pareek, A.; Singh, G. A calcium catalysed regioselective (5-exo-dig) tandem process for the synthesis of fully substituted furans. RSC Adv. 2016, 6, 28865–28870. [Google Scholar] [CrossRef]
- Ramon, R.S.; Pottier, C.; Gomez-Suarez, A.; Nolan, S.P. Gold(I)-catalyzed tandem alkoxylation/lactonization of γ-hydroxy-α,β-acetylenic esters. Adv. Synth. Catal. 2011, 353, 1575–1583. [Google Scholar] [CrossRef]
- Vasilyev, A.V. Superelectrophilic activation of alkynes, alkenes, and allenes. Adv. Org. Synth. 2018, 8, 81–120. [Google Scholar] [CrossRef]
- Devleshova, N.A.; Lozovskiy, S.V.; Vasilyev, A.V. Reactions of alkyl 4-hydroxybut-2-ynoates with arenes under superelectrophilic activation with triflic acid or HUSY zeolite: Alternative propargylation or allenylation of arenes, and synthesis of furan-2-ones. Tetrahedron 2019, 75, 130517. [Google Scholar] [CrossRef]
- Karame, I.; Tommasino, M.L.; Lemaire, M. Iridium-catalyzed alternative of the Meinwald rearrangement. Tetrahedron Lett. 2003, 44, 7687–7689. [Google Scholar] [CrossRef]
- Robinson, M.W.C.; Pillinger, K.S.; Graham, A.E. Highly efficient Meinwald rearrangement reactions of epoxides catalyzed by copper tetrafluoroborate. Tetrahedron Lett. 2006, 47, 5919–5921. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, M.; Zhang, S. Efficient synthetic method for the preparation of allyl- and propargyl-epoxides by allylation and propargylation of α-haloketones with organozinc reagents. Org. Biomol. Chem. 2012, 10, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.-H.; Hou, X.-L. Catalytic asymmetric propargylation. Chem. Rev. 2011, 111, 1914–1937. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A. Synthesis and reactions of allylic, allenic, vinylic, and arylmetal reagents from halides and esters via transient organopalladium intermediates. Chem. Rev. 2000, 100, 3163–3186. [Google Scholar] [CrossRef]
- Aurrecoechea, J.M.; Fañanás, R.; Arrate, M.; Gorgojo, J.M.; Aurrekoetxea, N. SmI2/Pd(0)-Mediated intramolecular coupling between propargylic esters and tethered aldehydes or ketones. J. Org. Chem. 1999, 64, 1893–1901. [Google Scholar] [CrossRef]
- Arrate, M.; Durana, A.; Lorenzo, P.; de Lera, A.R.; Álvarez, R.; Aurrecoechea, J.M. Catalyst- and solvent-dependent stereodivergence in the intramolecular Et2Zn/Pd0-promoted carbonyl propargylation: Mechanistic implications. Chem. Eur. J. 2013, 19, 13893–13900. [Google Scholar] [CrossRef]
- Plaza, A.; Baker, H.L.; Bewley, C.A. Mirabalin, an antitumor macrolide lactam from the marine sponge Siliquariaspongia mirabilis. J. Nat. Prod. 2009, 72, 324. [Google Scholar] [CrossRef]
- Bowman, E.J.; Gustafson, K.R.; Bowman, B.J.; Boyd, M.R. Identification of a new chondropsin class of antitumor compound that selectively inhibits V-ATPases. J. Biol. Chem. 2003, 278, 44147–44152. [Google Scholar] [CrossRef]
- Echeverria, P.-G.; Prévost, S.; Cornil, J.; Férard, C.; Reymond, S.; Guérinot, A.; Cossy, J.; Ratovelomanana-Vidal, V.; Phansavath, P. Synthetic strategy toward the C44−C65 fragment of Mirabalin. Org. Lett. 2014, 16, 2390–2393. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.A.; Mulhearn, J.J. Synthesis of a C20−C26 segment of superstolide A by addition of a chiral allenylzinc reagent to (R)-N-Boc alaninal. Org. Lett. 2005, 7, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, H.M.; Jarvo, E.R. Correction to enantioselective propargylation and allenylation reactions of ketones and imines. J. Org. Chem. 2014, 79, 8505. [Google Scholar] [CrossRef]
- Thaima, T.; Zamani, F.; Hyland, C.; Pyne, S. Allenylation and propargylation reactions of ketones, aldehydes, imines, and iminium ions using organoboronates and related derivatives. Synthesis 2017, 49, 1461–1480. [Google Scholar] [CrossRef]
- Horino, Y.; Murakami, M.; Ishibashi, M.; Lee, J.H.; Watanabe, A.; Matsumoto, R.; Abe, H. Trialkylborane-mediated propargylation of aldehydes using γ-stannylated propargyl acetates. Org. Lett. 2019, 21, 9564–9568. [Google Scholar] [CrossRef] [PubMed]
- Yatsumonji, Y.; Sugita, T.; Tsubouchi, A.; Takeda, T. Preparation of syn-tertiary homoallylic alcohols utilizing allenyltitanocenes generated by reductive titanation of γ-trimethylsilylpropargylic carbonates. Org. Lett. 2010, 12, 1968–1971. [Google Scholar] [CrossRef]
- Millán, A.; Álvarez de Cienfuegos, L.; Martín-Lasanta, A.; Campaña, A.G.; Cuerva, J.M. Titanium/palladium-mediated regioselective propargylation of ketones using propargylic carbonates as pronucleophiles. Adv. Synth. Catal. 2011, 353, 73–78. [Google Scholar] [CrossRef]
- Hirashita, T.; Suzuki, Y.; Tsuji, H.; Sato, Y.; Naito, K.; Araki, S. Nickel-catalyzed indium(I)-mediated syn-selective propargylation of aldehydes. Eur. J. Org. Chem. 2012, 2012, 5668–5672. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L. Bifunctional biphenyl-2-ylphosphine ligand enables tandem gold-catalyzed propargylation of aldehyde and unexpected cycloisomerization. J. Am. Chem. Soc. 2018, 140, 17439–17443. [Google Scholar] [CrossRef]
- Gagnepain, J.; Moulin, E.; Fürstner, A. Gram-scale synthesis of iejimalide B. Chem. Eur. J. 2011, 17, 6964–6972. [Google Scholar] [CrossRef]
- Geary, L.M.; Leung, J.C.; Krische, M.J. Ruthenium-catalyzed reductive coupling of 1,3-enynes and aldehydes by transfer hydrogenation: Anti-diastereoselective carbonyl propargylation. Chem. Eur. J. 2012, 18, 16823–16827. [Google Scholar] [CrossRef] [PubMed]
- Voronin, V.V.; Ledovskaya, M.S.; Bogachenkov, A.S.; Rodygin, K.S.; Ananikov, V.P. Acetylene in organic synthesis: Recent progress and new uses. Molecules 2018, 23, 2442. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.K.; Yadav, L.; Shyamlal, B.R.K.; Chaudhary, S. Weak bases-mediated modified Favorskii reaction type direct alkynylation/(E)-alkenylation: A unified rapid access to α,β-unsaturated ketones and propargyl alcohols. Asian J. Org. Chem. 2019, 8, 2257–2268. [Google Scholar] [CrossRef]
- Wei, X.F.; Shimizu, Y.; Kanai, M. An expeditious synthesis of sialic acid derivatives by copper(I)-catalyzed stereodivergent propargylation of unprotected aldoses. ACS Cent. Sci. 2016, 2, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Majima, S.; Wei, X.-F.; Mitsunuma, H.; Shimizu, Y.; Kanai, M. Copper(I)-catalyzed stereodivergent propargylation of N-acetyl mannosamine for protecting group minimal synthesis of C3-substituted sialic acids. J. Org. Chem. 2019, 84, 10615–10628. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry, 6th ed.; John Wiley & Sons: New York, NY, USA, 2007; Chapter 16. [Google Scholar]
- Yus, M.; González-Gómez, J.C.; Foubelo, F. Catalytic enantioselective allylation of carbonyl compounds and imines. Chem. Rev. 2011, 111, 7774–7854. [Google Scholar] [CrossRef]
- Nolan, S.P. The development and catalytic uses of N-heterocyclic carbene gold complexes. Acc. Chem. Res. 2011, 44, 91–100. [Google Scholar] [CrossRef]
- Ramón, D.J.; Yus, M. Alkylation of carbonyl and imino groups. In Science of Synthesis: Stereoselective Synthesis 2; Molander, G.A., Ed.; Georg Thieme Verlag: Stuttgart, Germany, 2011; Volume 2, pp. 349–400. [Google Scholar]
- Alcaide, B.; Almendros, P.; Rodríguez-Acebes, R. Metal-mediated entry to functionalized 3-substituted 3-hydroxyindolin-2-ones via regiocontrolled carbonylallylation, bromoallylation, 1,3-butadien-2-ylation, propargylation, or allenylation reactions of isatins in aqueous media. J. Org. Chem. 2005, 70, 3198–3204. [Google Scholar] [CrossRef]
- Sirvent, J.A.; Foubelo, F.; Yus, M. Diastereoselective indium-mediated allylation of N-tert-butanesulfinyl ketimines: Easy access to asymmetric quaternary stereocenters bearing nitrogen atoms. Chem. Commun. 2012, 48, 2543–2545. [Google Scholar] [CrossRef]
- García-Muñoz, M.J.; Zacconi, F.; Foubelo, F.; Yus, M. Indium-promoted diastereo- and regioselective propargylation of chiral sulfinylimines. Eur. J. Org. Chem. 2013, 2013, 1287–1295. [Google Scholar] [CrossRef]
- Liu, G.; Cogan, D.A.; Owens, T.D.; Tang, T.P.; Ellman, J.A. Synthesis of enantiomerically pure N-tert-butanesulfinyl imines (tert-butanesulfinimines) by the direct condensation of tert-butanesulfinamide with aldehydes and ketones. J. Org. Chem. 1999, 64, 1278–1284. [Google Scholar] [CrossRef]
- Guo, T.; Song, R.; Yuan, B.-H.; Chen, X.-Y.; Sun, X.-W.; Lin, G.-Q. Highly efficient asymmetric construction of quaternary carbon-containing homoallylic and homopropargylic amines. Chem. Commun. 2013, 49, 5402–5404. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, A.; García-Muñoz, M.J.; Yus, M.; Foubelo, F. Stereoselective synthesis of tetrahydroisoquinolines from chiral 4-azaocta-1,7-diynes and 4-azaocta-1,7-enynes. Eur. J. Org. Chem. 2020, 2020, 113–126. [Google Scholar] [CrossRef]
- Meng, J.-L.; Jiao, T.-Q.; Chen, Y.-H.; Fu, R.; Zhang, S.-S.; Zhao, Q.; Feng, C.-G.; Lin, G.-Q. Synthesis of chiral isoindolinones via asymmetric propargylation/lactamization cascade. Tetrahedron Lett. 2018, 59, 1564–1567. [Google Scholar] [CrossRef]
- Guo, T.; Liu, Y.-C.; Li, B.; Liu, H.-M. Convenient synthesis of α,α-(bisallyl and bispropargyl)-substituted amines via aza-Barbier-type reaction. Tetrahedron Lett. 2015, 56, 2469–2471. [Google Scholar] [CrossRef]
- Wu, C.; Huang, W.; He, W.; Xiang, J. One-pot preparation of homopropargylic N-sulfonylamines catalyzed by zinc powder. Chem. Lett. 2013, 42, 1233–1234. [Google Scholar] [CrossRef]
- Karpe, S.A.; Singh, M.; Chowhan, L.R. Aqueous single step synthesis and structural characterization of allylated, propargylated, and benzylated 3-substituted 3-aminooxindoles. Synth. Commun. 2017, 47, 1737–1746. [Google Scholar] [CrossRef]
- Fandrick, D.R.; Johnson, C.S.; Fandrick, K.R.; Reeves, J.T.; Tan, Z.; Lee, H.; Song, J.J.; Yee, N.K.; Senanayake, C.H. Highly diastereoselective zinc-catalyzed propargylation of tert-butanesulfinyl imines. Org. Lett. 2010, 12, 748–751. [Google Scholar] [CrossRef]
- Osborne, C.A.; Endean, T.B.D.; Jarvo, E.R. Silver-catalyzed enantioselective propargylation reactions of N-sulfonylketimines. Org. Lett. 2015, 17, 5340–5343. [Google Scholar] [CrossRef]
- Smith, M.W.; Zhou, Z.; Gao, A.X.; Shimbayashi, T.; Snyder, S.A. A 7-step formal asymmetric total synthesis of strictamine via an asymmetric propargylation and metal-mediated cyclization. Org. Lett. 2017, 19, 1004–1007. [Google Scholar] [CrossRef]
- Fandrick, D.R.; Hart, C.A.; Okafor, I.S.; Mercadante, M.A.; Sanyal, S.; Masters, J.T.; Sarvestani, M.; Fandrick, K.R.; Stockdill, J.L.; Grinberg, N.; et al. Copper-catalyzed asymmetric propargylation of cyclic aldimines. Org. Lett. 2016, 18, 6192–6195. [Google Scholar] [CrossRef] [PubMed]
- Cyklinsky, M.; Botuha, C.; Chemla, F.; Ferreira, F.; Pérez-Luna, A. Diastereoselective synthesis of enantiopure homopropargylic N-tert-butylsulfinylamines. Synlett 2011, 2011, 2681–2684. [Google Scholar] [CrossRef]
- Llobat, A.; Sedgwick, D.M.; Cabré, A.; Román, R.; Mateu, N.; Escorihuela, J.; Medio-Simón, M.; Soloshonok, V.; Han, J.; Riera, A.; et al. Asymmetric synthesis of fluorinated monoterpenic alkaloid derivatives from chiral fluoroalkyl aldimines via the Pauson-Khand reaction. Adv. Synth. Catal. 2020, 362, 1378–1384. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, L.; Xu, C.; Luo, S. Chiral primary amine/palladium dual catalysis for asymmetric allylic alkylation of β-ketocarbonyl compounds with allylic alcohols. Angew. Chem. Int. Ed. 2015, 54, 12645–12648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-L.; Zou, Y.; Zhang, D.-Y.; Wang, Y.-H.; Hu, X.-H.; Chen, S.; Xu, J.; Hu, X.-P. Enantioselective copper-catalyzed decarboxylative propargylic alkylation of propargyl β-ketoesters with a chiral ketimine P,N,N-ligand. Angew. Chem. Int. Ed. 2014, 53, 1410–1414. [Google Scholar] [CrossRef]
- Jurberg, I.D. An aminocatalyzed stereoselective strategy for the formal α-propargylation of ketones. Chem. Eur. J. 2017, 23, 9716–9720. [Google Scholar] [CrossRef]
- Xia, Y.-Y.; Chen, L.-Y.; Lv, S.; Sun, Z.-H.; Wang, B. Microwave-assisted or Cu–NHC-catalyzed cycloaddition of azido-disubstituted alkynes: Bifurcation of reaction pathways. J. Org. Chem. 2014, 79, 9818–9825. [Google Scholar] [CrossRef]
- Lim, J.; Park, K.; Byeun, A.; Lee, S. Copper-catalyzed decarboxylative coupling reactions for the synthesis of propargyl amines. Tetrahedron Lett. 2014, 55, 4875–4878. [Google Scholar] [CrossRef]
- Park, K.; Lee, S. Transition metal-catalyzed decarboxylative coupling reactions of alkynyl carboxylic acids. RSC Adv. 2013, 3, 14165–14182. [Google Scholar] [CrossRef]
- Feng, H.-D.; Jia, H.-H.; Sun, Z.-H. Mild and catalyst-free Petasis/decarboxylative domino reaction: Chemoselective synthesis of N-benzyl propargylamines. J. Org. Chem. 2014, 79, 11812–11818. [Google Scholar] [CrossRef]
- Jia, H.; Feng, H.; Sun, Z. Decarboxylative dipropargylation of primary amines with propiolic acids and formaldehyde via metal-free coupling. Tetrahedron 2015, 71, 2724–2728. [Google Scholar] [CrossRef]
- Parvin, N.; Sen, N.; Tothadi, S.; Muhammed, S.; Parameswaran, P.; Khan, S. Synthesis and application of silylene-stabilized low-coordinate Ag(I)−arene cationic complexes. Organometallics 2021, 40, 1626–1632. [Google Scholar] [CrossRef]
- Feng, H.; Wang, F.; Cao, L.; Van der Eycken, E.V.; Yin, X. Switchable mono- and dipropargylation of amino alcohols: A unique property of the iodide anion in controlling ring-opening alkynylation. Eur. J. Org. Chem. 2021, 2021, 3676–3680. [Google Scholar] [CrossRef]
- Waser, J.; González-Gómez, J.C.; Nambu, H.; Huber, P.; Carreira, E.M. Cobalt-catalyzed hydrohydrazination of dienes and enynes: Access to allylic and propargylic hydrazides. Org. Lett. 2005, 7, 4249–4252. [Google Scholar] [CrossRef]
- An, D.K.; Hirakawa, K.; Okamoto, S.; Sato, F. Electrophilic amination of racemic and non-racemic allenyltitaniums. One-pot synthesis of α-hydrazinoalkynes from propargylic alcohol derivatives. Tetrahedron Lett. 1999, 40, 3737–3740. [Google Scholar] [CrossRef]
- Yanagisawa, A.; Koide, T.; Yoshida, K. Selective propargylation of azo compounds with barium reagents. Synlett 2010, 2010, 1515–1518. [Google Scholar] [CrossRef]
- Izquierdo, S.; Bouvet, S.; Wu, Y.; Molina, S.; Shafir, A. The coming of age in iodane-guided ortho-C−H propargylation: From insight to synthetic potential. Chem. Eur. J. 2018, 24, 15517–15521. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, A.J.; Shrives, H.J.; Álvarez, E.; Carrër, A.; Zhang, Y.; Procter, D.J. Sulfoxide-directed metal-free ortho-propargylation of aromatics and heteroaromatics. Chem. Eur. J. 2015, 21, 7428–7434. [Google Scholar] [CrossRef]
- Chatterjee, P.N.; Roy, S. Propargylic activation across a heterobimetallic Ir-Sn catalyst: Nucleophilic substitution and indene formation with propargylic alcohols. J. Org. Chem. 2010, 75, 4413–4423. [Google Scholar] [CrossRef]
- Masuyama, Y.; Hayashi, M.; Suzuki, N. SnCl2-Catalyzed propargylic substitution of propargylic alcohols with carbon and nitrogen nucleophiles: Propargylic substitution of propargylic alcohols. Eur. J. Org. Chem. 2013, 14, 2914–2921. [Google Scholar] [CrossRef]
- Silveira, C.C.; Mendes, S.R.; Martins, G.M. Propargylation of aromatic compounds using Ce(OTf)3 as catalyst. Tetrahedron Lett. 2012, 53, 1567–1570. [Google Scholar] [CrossRef]
- Hamel, J.D.; Beaudoin, M.; Cloutier, M.; Paquin, J.F. Hydrogen-bond-promoted Friedel-Crafts reaction of secondary propargylic fluorides: Preparation of 1-alkyl-1-aryl-2-alkynes. Synlett 2017, 28, 2823–2828. [Google Scholar] [CrossRef]
- Yu, Y.B.; Luo, Z.J.; Zhang, X. Copper-catalyzed direct propargylation of polyfluoroarenes with secondary propargyl phosphates. Org. Lett. 2016, 18, 3302–3305. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Chen, H.; Yang, M.; Wang, G.; Gao, L.; Ni, Z.; Zou, J.; Li, S. B(C6F5)3-Catalyzed sequential additions of terminal alkynes to para-substituted phenols: Selective construction of congested phenol-substituted quaternary carbons. Org. Lett. 2021, 23, 5533–5538. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.C.; Mendes, S.R.; Wolf, L.; Martins, G.M. Anhydrous CeCl3 catalyzed C3-selective propargylation of indoles with tertiary alcohols. Tetrahedron Lett. 2010, 51, 4560–4562. [Google Scholar] [CrossRef]
- Raji Reddy, C.; Rani Valleti, R.; Dilipkumar, U. One-pot sequential propargylation/cycloisomerization: A facile [4+2]-benzannulation approach to carbazoles. Chem. Eur. J. 2016, 22, 2501–2506. [Google Scholar] [CrossRef]
- Gohain, M.; Marais, C.; Bezuidenhoudt, B.C.B. An Al(OTf)3 catalyzed environmentally benign process for the propargylation of indoles. Tetrahedron Lett. 2012, 53, 4704–4707. [Google Scholar] [CrossRef]
- Kumar, G.G.K.S.N.; Aridoss, G.; Laali, K.K. Condensation of propargylic alcohols with indoles and carbazole in [bmim][PF6]/Bi(NO3) 3·5H2O: A simple high yielding propargylation method with recycling and reuse of the ionic liquid. Tetrahedron Lett. 2012, 53, 3066–3069. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, S.H.; Kim, J.; Kim, J.N. Synthesis of benzo[a]carbazoles from 2-arylindoles via a sequential propargylation, propargyl-allenyl isomerization, and 6π-electrocyclization. Bull. Korean Chem. Soc. 2015, 36, 1351–1359. [Google Scholar] [CrossRef]
- Raji Reddy, C.; Subbarao, M.; Sathish, P.; Kolgave, D.H.; Donthiri, R.R. One-pot assembly of 3-hydroxycarbazoles via uninterrupted propargylation/hydroxylative benzannulation reactions. Org. Lett. 2020, 22, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, K.; Senda, Y.; Nakajima, K.; Nishibayashi, Y. Construction of chiral tri- and tetra-arylmethanes bearing quaternary carbon centers: Copper-catalyzed enantioselective propargylation of indoles with propargylic esters. Angew. Chem. Int. Ed. 2016, 55, 9728–9732. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Zhou, B.; Tsuji, H.; Kawatsura, M. Nickel-catalyzed asymmetric Friedel-Crafts propargylation of 3-substituted indoles with propargylic carbonates bearing an internal alkyne group. Org. Lett. 2020, 22, 2049–2053. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Schwarz, J.L.; Cembellín, S.; Greßies, S.; Glorius, F. Highly selective manganese(I)/Lewis acid cocatalyzed direct C−H propargylation using bromoallenes. Angew. Chem. Int. Ed. 2018, 57, 437–441. [Google Scholar] [CrossRef]
- Das, D.; Pratihar, S.; Roy, U.K.; Mal, D.; Roy, S. First example of a heterobimetallic ‘Pd–Sn’ catalyst for direct activation of alcohol: Efficient allylation, benzylation and propargylation of arenes, heteroarenes, active methylenes and allyl-Si nucleophiles. Org. Biomol. Chem. 2012, 10, 4537. [Google Scholar] [CrossRef]
- McCubbin, J.; Nassar, C.; Krokhin, O. Waste-free catalytic propargylation/allenylation of aryl and heteroaryl nucleophiles and synthesis of naphthopyrans. Synthesis 2011, 19, 3152–3160. [Google Scholar] [CrossRef]
- Kumar, G.G.K.S.N.; Laali, K.K. Condensation of propargylic alcohols with N-methylcarbazole and carbazole in [Bmim]PF6 ionic liquid; synthesis of novel dipropargylic carbazoles using TfOH or Bi(NO3)3·5H2O as catalyst. Tetrahedron Lett. 2013, 54, 965–969. [Google Scholar] [CrossRef]
- Aridoss, G.; Sarca, V.D.; Ponder, J.F., Jr.; Crowe, J.; Laali, K.K. Electrophilic chemistry of propargylic alcohols in imidazolium ionic liquids: Propargylation of arenes and synthesis of propargylic ethers catalyzed by metallic triflates [Bi(OTf)3, Sc(OTf)3, Yb(OTf)3], TfOH, or B(C6F5)3. Org. Biomol. Chem. 2011, 9, 2518–2529. [Google Scholar] [CrossRef]
- Hanazawa, T.; Sasaki, K.; Takayama, Y.; Sato, F. Efficient and practical method for synthesizing optically active indan-2-ols by the Ti(O-i-Pr)4/2 i-PrMgCl-mediated metalative Reppe reaction. J. Org. Chem. 2003, 68, 4980–4983. [Google Scholar] [CrossRef]
- Hooz, J.; Cabezas, J.; Musmanni, S.; Calzada, J. Propargylation of alkyl halides: (E)-6,10-dimethyl-5,9-undecadien-1-yne and (E)-7,11-dimethyl-6,10-dodecadien-2-yn-1-ol. Org. Synth. 1990, 69, 120. [Google Scholar] [CrossRef]
- Pereira, A.R.; Cabezas, J.A. A new method for the preparation of 1,5-diynes. Synthesis of (4E,6Z,10Z)-4,6,10-hexadecatrien-1-ol, the pheromone component of the cocoa pod borer moth Conopomorpha cramerella. J. Org. Chem. 2005, 70, 2594–2597. [Google Scholar] [CrossRef] [PubMed]
- Vásquez, S.; Cabezas, J.A. A facile method for the preparation of bishomopropargylic alcohols from acyl chlorides. Tetrahedron Lett. 2014, 55, 1894–1897. [Google Scholar] [CrossRef]
- Lin, M.; Chen, Q.-Z.; Zhu, Y.; Chen, X.-L.; Cai, J.-J.; Pan, Y.-M.; Zhan, Z.-P. Copper(II)-catalyzed synthesis of pyrimidines from propargylic alcohols and amidine: A propargylation–cyclization–oxidation tandem reaction. Synlett 2011, 2011, 1179–1183. [Google Scholar] [CrossRef]
- Shaikh, S.K.J.; Kamble, R.R.; Somagond, S.M.; Devarajegowda, H.C.; Dixit, S.R.; Joshi, S.D. Tetrazolylmethyl quinolines: Design, docking studies, synthesis, anticancer and antifungal analyses. Eur. J. Med. Chem. 2017, 128, 258–273. [Google Scholar] [CrossRef]
- Reddy, R.J.; Waheed, M.; Karthik, T.; Shankar, A. An efficient synthesis of 4,5-disubstituted-2H-1,2,3-triazoles from nitroallylic derivatives via a cycloaddition–denitration process. New J. Chem. 2018, 42, 980–987. [Google Scholar] [CrossRef]
- Min, L.; Pan, B.; Gu, Y. Synthesis of quinoline-fused 1-benzazepines through a Mannich-type reaction of a C,N-bisnucleophile generated from 2-aminobenzaldehyde and 2-methylindole. Org. Lett. 2016, 18, 364–367. [Google Scholar] [CrossRef]
- Mahesh, K.; Ravi, K.; Rathod, P.K.; Leelavathi, P. A convenient synthesis of quinoline fused triazolo-azepine/oxepine derivatives through Pd-catalyzed C-H functionalisation of triazoles. New J. Chem. 2020, 44, 2367–2373. [Google Scholar] [CrossRef]
- Lahosa, A.; Yus, M.; Foubelo, F. Enantiodivergent approach to the synthesis of cis-2,6-disubstituted piperidin-4-ones. J. Org. Chem. 2019, 84, 7331–7341. [Google Scholar] [CrossRef]
- Craven, G.B.; Briggs, E.L.; Zammit, C.M.; McDermott, A.; Greed, S.; Affron, D.P.; Leinfellner, C.; Cudmore, H.R.; Tweedy, R.R.; Luisi, R.; et al. Synthesis and configurational assignment of vinyl sulfoximines and sulfonimidamides. J. Org. Chem. 2021, 86, 7403–7424. [Google Scholar] [CrossRef]
- Tri, N.M.; Thanh, N.D.; Ha, L.N.; Anh, D.T.T.; Toan, V.N.; Giang, N.T.K. Study on synthesis of some substituted N-propargyl isatins by propargylation reaction of corresponding isatins using potassium carbonate as base under ultrasound- and microwave-assisted conditions. Chem. Pap. 2021, 75, 4793–4801. [Google Scholar] [CrossRef]
- Hai, D.S.; Ha, N.T.T.; Tung, D.T.; Le, C.T.; Anh, H.H.; Toan, V.N.; Van, H.T.K.; Toan, D.N.; Giang, N.T.K.; Huong, N.T.T.; et al. N-Propargylation reaction of substituted 4H-pyrano[2,3-d]pyrimidine derivatives under conventional, ultrasound- and microwave-assisted conditions. Chem. Pap. 2022, 76, 5281–5292. [Google Scholar] [CrossRef]
- Tai, N.; Quan, P.M.; Ha, V.T.; Luyen, N.D.; Chi, H.K.; Cuong, L.H.; Phong, L.; Chinh, L. Synthesis of propargyl compounds and their cytotoxic activity. Russ. J. Org. Chem. 2021, 57, 462–468. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Machado, C.M.; Sousa, V.; Sousa, C.F.V.; Silva, V.L.M.; Silva, A.M.S.; Borges, J.; Mano, J.F. Customizable and regioselective one-pot N−H functionalization of DNA nucleobases to create a library of nucleobase derivatives for biomedical applications. Eur. J. Org. Chem. 2021, 2021, 4423–4433. [Google Scholar] [CrossRef]
- Wells, S.M.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Alkyne ligation handles: Propargylation of hydroxyl, sulfhydryl, amino, and carboxyl groups via the Nicholas reaction. Org. Lett. 2016, 18, 4566–4569. [Google Scholar] [CrossRef] [PubMed]
- Carcenac, Y.; Zine, K.; Kizirian, J.-C.; Thibonnet, J.; Duchêne, A.; Parrain, J.-L.; Abarbri, M. α- and β-Stannyl trifluoromethylbutenoates: Regioselective preparation and use in copper(I)-catalyzed allylation and propargylation reactions. Adv. Synth. Catal. 2010, 352, 949–954. [Google Scholar] [CrossRef]
- Gaied, L.B.; Fincias, N.; Garrec, J.; El Kaïm, L. 5-endo-dig Cyclization of O-propargyl mandelic acid amides towards 2,5-dihydrofurans. Eur. J. Org. Chem. 2019, 2019, 7656–7665. [Google Scholar] [CrossRef]
- Seo, K.; Kim, Y.J.; Rhee, Y.H. Ru-Catalyzed chemoselective olefin migration reaction of cyclic allylic acetals to enol acetals. Org. Lett. 2018, 20, 979–982. [Google Scholar] [CrossRef]
- Ukale, D.U.; Lönnberg, T. 2,6-Dimercuriphenol as a bifacial dinuclear organometallic nucleobase. Angew. Chem. Int. Ed. 2018, 57, 16171–16175. [Google Scholar] [CrossRef]
- Sharipov, B.T.; Davidova, A.N.; Valeev, F.A. Aromatization of 2,2,5-trialkyl-substituted 2,5-dihydrofurans and factors affecting their stabilization. Chem. Heterocycl. Compd. 2018, 54, 403–410. [Google Scholar] [CrossRef]
- Datta, R.; Dixon, R.J.; Ghosh, S. A convenient access to the tricyclic core structure of hippolachnin A. Tetrahedron Lett. 2016, 57, 29–31. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, S.; Kaur, S.; Singh, P. Near-IR oxime-based solvatochromic perylene diimide probe as a chemosensor for Pd species and Cu2+ ions in water and live cells. Photochem. Photobiol. Sci. 2020, 19, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Rodier, F.; Parrain, J.-L.; Chouraqui, G.; Commeiras, L. First studies directed towards the diastereoselective synthesis of the BCD tricyclic core of brownin F. Org. Biomol. Chem. 2013, 11, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Grischenko, L.A.; Parshina, L.N.; Kanitskaya, L.V.; Larina, L.I.; Novikova, L.N.; Trofimov, B.A. Propargylation of arabinogalactan with propargyl halides a facile route to new functionalized biopolymers. Carbohydr. Res. 2013, 376, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Okamura, T.; Egoshi, S.; Dodo, K.; Sodeoka, M.; Iwabuchi, Y.; Kanoh, N. Highly chemoselective gem-difluoropropargylation of aliphatic alcohols. Chem. Eur. J. 2019, 25, 16002–16006. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Z.; Tang, H.; Yang, K.R.; Wan, L.-Q.; Zhang, X.; Liu, J.; Fu, Z.; Niu, D. Enantioselective propargylation of polyols and desymmetrization of meso 1,2-diols by copper/borinic acid dual catalysis. Angew. Chem. Int. Ed. 2017, 56, 7213–7217. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Z.; Tang, H.; Wan, L.; Zhang, X.; Fu, Z.; Liu, J.; Yang, S.; Jia, D.; Niu, D. Site-divergent delivery of terminal propargyls to carbohydrates by synergistic catalysis. Chem 2017, 3, 834–845. [Google Scholar] [CrossRef]
- Spitzer, L.; Lecommandoux, S.; Cramail, H.; Jérôme, F. Sequential acid-catalyzed alkyl glycosylation and oligomerization of unprotected carbohydrates. Green Chem. 2021, 23, 1361–1369. [Google Scholar] [CrossRef]
- Barreiro, E.; Sanz-Vidal, A.; Tan, E.; Lau, S.H.; Sheppard, T.D.; Díez-González, S. HBF4-Catalysed nucleophilic substitutions of propargylic alcohols. Eur. J. Org. Chem. 2015, 34, 7544–7549. [Google Scholar] [CrossRef]
- Okamura, T.; Koyamada, K.; Kanazawa, J.; Miyamoto, K.; Iwabuchi, Y.; Uchiyama, M.; Kanoh, N. Synthetic access to gem-difluoropropargyl vinyl ethers and their application to propargyl Claisen rearrangement. J. Org. Chem. 2021, 86, 1911–1924. [Google Scholar] [CrossRef]
- Sen, S.; Sadeghifar, H.; Argyropoulos, D.S. Kraft lignin chain extension chemistry via propargylation, oxidative coupling, and claisen rearrangement. Biomacromolecules 2013, 14, 3399–3408. [Google Scholar] [CrossRef]
- Keskin, S.; Balci, M. Intramolecular heterocyclization of O-propargylated aromatic hydroxyaldehydes as an expedient route to substituted chromenopyridines under metal-free conditions. Org. Lett. 2015, 17, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.-F.; Luo, S.-H.; Huo, J.-P.; Liu, J.-Y.; Chen, S.-X.; Wang, Z.-Y. Design, synthesis, and characterization of 1,3,5-tri(1H-benzo[d]imidazol-2-yl)benzene-based fluorescent supramolecular columnar liquid crystals with a broad mesomorphic range. J. Org. Chem. 2014, 79, 8366–8373. [Google Scholar] [CrossRef] [PubMed]
- Dongamanti, A.; Bommidi, V.L.; Arram, G.; Sidda, R. Microwave-assisted synthesis of (E)-7-[(1-benzyl-1H-1,2,3-triazol-4-yl)methoxy]-8-(3-arylacryloyl)-4-methyl-2H-chromen-2-ones and their antimicrobial activity. Heterocycl. Commun. 2014, 20, 293–298. [Google Scholar] [CrossRef]
- Lamandé-Langle, S.; Collet, C.; Hensienne, R.; Vala, C.; Chrétien, F.; Chapleur, Y.; Mohamadi, A.; Lacolley, P.; Regnault, V. Click glycosylation of peptides through cysteine propargylation and CuAAC. Bioorg. Med. Chem. 2014, 22, 6672–6683. [Google Scholar] [CrossRef]
- Al-blewi, F.F.; Almehmadi, M.A.; Aouad, M.R.; Bardaweel, S.K.; Sahu, P.K.; Messali, M.; Rezki, N.; El Ashry, E.S.H. Design, synthesis, ADME prediction and pharmacological evaluation of novel benzimidazole-1,2,3-triazole-sulfonamide hybrids as antimicrobial and antiproliferative agents. Chem. Cent. J. 2018, 12, 110. [Google Scholar] [CrossRef]
- Khusnutdinova, E.F.; Petrova, A.V.; Kukovinets, O.S.; Kazakova, O.B. Synthesis and cytotoxicity of 28-N-propargylaminoalkylated 2,3-indolotriterpenic acids. Nat. Prod. Commun. 2018, 13, 665–668. [Google Scholar] [CrossRef]
- Ben Nejma, A.; Znati, M.; Daich, A.; Othman, M.; Lawson, A.M.; Ben Jannet, H. Design and semisynthesis of new herbicide as 1,2,3-triazole derivatives of the natural maslinic acid. Steroids 2018, 138, 102–107. [Google Scholar] [CrossRef]
- Bew, S.P.; Hiatt-Gipson, G.D. Synthesis of C-propargylic esters of N-protected amino acids and peptides. J. Org. Chem. 2010, 75, 3897–3899. [Google Scholar] [CrossRef]
- Rubio-Ruiz, B.; Pérez-López, A.M.; Sebastián, V.; Unciti-Broceta, A. A minimally-masked inactive prodrug of panobinostat that is bioorthogonally activated by gold chemistry. Bioorg. Med. Chem. 2021, 41, 116217. [Google Scholar] [CrossRef]
- Shintani, R. Recent progress in copper-catalyzed asymmetric allylic substitution reactions using organoboron nucleophiles. Synthesis 2016, 48, 1087–1100. [Google Scholar] [CrossRef]
- Zhou, Y.; Shi, Y.; Torker, S.; Hoveyda, A.H. SN2′′-Selective and enantioselective substitution with unsaturated organoboron compounds and catalyzed by a sulfonate-containing NHC-Cu complex. J. Am. Chem. Soc. 2018, 140, 16842–16854. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Jung, B.; Torker, S.; Hoveyda, A.H. N-heterocyclic carbene–copper-catalyzed group-, site-, and enantioselective allylic substitution with a readily accessible propargyl(pinacolato)boron reagent: Utility in stereoselective synthesis and mechanistic attributes. J. Am. Chem. Soc. 2015, 137, 8948–8964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.G.K.S.N.; Laali, K.K. Facile coupling of propargylic, allylic and benzylic alcohols with allylsilane and alkynylsilane, and their deoxygenation with Et3SiH, catalyzed by Bi(OTf)3 in [BMIM][BF4] ionic liquid (IL), with recycling and reuse of the IL. Org. Biomol. Chem. 2012, 10, 7347–7355. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wang, L.; Wang, J. Iron-catalyzed ene-type propargylation of diarylethylenes with propargyl alcohols. Org. Biomol. Chem. 2012, 10, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, R.; Stodulski, M.; Mlynarski, J. Propargylation of CoQ0 through the redox chain reaction. J. Org. Chem. 2022, 87, 683–692. [Google Scholar] [CrossRef]
- Shi, L.; Xing, L.L.; Hu, W.B.; Shu, W. Regio- and enantioselective Ni-catalyzed formal hydroalkylation, hydrobenzylation, and hydropropargylation of acrylamides to α-tertiary amides. Angew. Chem. Int. Ed. 2021, 60, 1599–1604. [Google Scholar] [CrossRef]
- Chen, L.; Yu, J.; Tang, S.; Shao, Y.; Sun, J. Gold-catalyzed highly diastereoselective oxy-propargylamination of allenamides with C-alkynyl N-Boc N,O-acetals. Org. Lett. 2019, 21, 9050–9054. [Google Scholar] [CrossRef]
- Ma, Z.-X.; Fang, L.-C.; Haugen, B.; Bruckbauer, D.; Feltenberger, J.; Hsung, R. Facile synthesis of 3-amido-dienynes via a tandem α-propargylation–isomerization of chiral allenamides and their applications in Diels–Alder cycloadditions. Synlett 2017, 28, 2906–2912. [Google Scholar] [CrossRef]
- Giri, S.S.; Lin, L.-H.; Jadhav, P.D.; Liu, R.-S. Gold-catalyzed 1,4-carbooxygenation of 3-en-1-ynamides with allylic and propargylic alcohols via non-Claisen pathways. Adv. Synth. Catal. 2017, 359, 590–596. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Herkommer, D.; Krische, M.J. Ruthenium-BINAP catalyzed alcohol C-H tert-prenylation via 1,3-enyne transfer hydrogenation: Beyond stoichiometric carbanions in enantioselective carbonyl propargylation. J. Am. Chem. Soc. 2016, 138, 5238–5241. [Google Scholar] [CrossRef]
- Aridoss, G.; Laali, K.K. Condensation of propargylic alcohols with 1,3-dicarbonyl compounds and 4-hydroxycoumarins in ionic liquids (ILs). Tetrahedron Lett. 2011, 52, 6859–6864. [Google Scholar] [CrossRef]
- Ohta, K.; Kobayashi, T.; Tanabe, G.; Muraoka, O.; Yoshimatsu, M. Scandium-catalyzed propargylation of 1,3-diketones with propargyl alcohols bearing sulfur or selenium functional groups: Useful transformation to furans and pyrans. Chem. Pharm. Bull. 2010, 58, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Emer, E.; Sinisi, R.; Capdevila, M.G.; Petruzziello, D.; De Vincetiis, F.; Cozzi, P.G. Direct nucleophilic SN1-type reactions of alcohols. Eur. J. Org. Chem. 2011, 2011, 647–666. [Google Scholar] [CrossRef]
- Sinisi, R.; Vita, M.V.; Gualandi, A.; Emer, E.; Cozzi, P.G. SN1-Type reactions in the presence of water: Indium(III)-promoted highly enantioselective organocatalytic propargylation of aldehydes. Chem. Eur. J. 2011, 17, 7404–7408. [Google Scholar] [CrossRef]
- Liu, Y.M.; Li, F.; Wang, Q.; Yang, J. Synthesis of the AE bicyclic of Daphniphyllum alkaloid yuzurine. Tetrahedron 2017, 73, 6381–6385. [Google Scholar] [CrossRef]
- Zidan, A.; Cordier, M.; El-Naggar, A.M.; El-Sattar, N.E.A.A.; Hassan, M.A.; Ali, A.K.; El Kaïm, L. Propargylation of Ugi amide dianion: An entry into pyrrolidinone and benzoindolizidine alkaloid analogues. Org. Lett. 2018, 20, 2568–2571. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Mishra, A.K. Zinc-mediated alkylation and acylation of 1,3-dicarbonyl compounds. Chem. Lett. 2010, 39, 280–281. [Google Scholar] [CrossRef]
- Wu, H.-H.; Hsu, S.-C.; Hsu, F.-L.; Uang, B.-J. Asymmetric synthesis of (–)-pterosin N from a chiral 1,3-dioxolanone. Eur. J. Org. Chem. 2014, 2014, 4351–4355. [Google Scholar] [CrossRef]
- Selvaraj, V.; Rajendran, V. Propargylation of indene-1,3-dione under a new phase-transfer catalyst combined with ultrasonication–A kinetic study. Ultras. Sonochem. 2014, 21, 612–619. [Google Scholar] [CrossRef]
- Li, Y.; Palframan, M.J.; Pattenden, G.; Winne, J.M. A strategy towards the synthesis of plumarellide based on biosynthesis speculation, featuring a transannular 4 + 2 type cyclisation from a cembranoid furanoxonium ion intermediate. Tetrahedron 2014, 70, 7229–7240. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kapustina, I.I.; Kalinovsky, A.I.; Dmitrenok, P.S.; Grebnev, B.B. New diterpenoids from the far-eastern gorgonian coral Plumarella sp. Tetrahedron Lett. 2002, 43, 315–317. [Google Scholar] [CrossRef]
- Tedeschi, C.; Saccavini, C.; Maurette, L.; Soleilhavoup, M.; Chauvin, R. 1,4-Diynes from alkynyl–propargyl coupling reactions. J. Organomet. Chem. 2003, 670, 151–169. [Google Scholar] [CrossRef]
- Guo, W.-H.; Luo, Z.-J.; Zeng, W.; Zhang, X. Access to difluoromethylene-skipped 1,4-diynes with gem-difluoropropargyl bromide. ACS Catal. 2017, 7, 896–901. [Google Scholar] [CrossRef]
- Yoshida, M. Organocatalytic asymmetric α-allylation and propargylation of α-branched aldehydes with alkyl Halides. J. Org. Chem. 2021, 86, 10921–10927. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zhang, Z.-Q.; Lu, X.; Xiao, B.; Fu, Y. Copper-catalyzed propargylation of diborylmethane. Chem. Commun. 2017, 53, 3551–3554. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, G.; Guo, B.; Xu, L.; Chen, J.; Cao, W.; Zhao, G.; Wu, X. Diastereo- and enantioselective propargylation of benzofuranones catalyzed by pybox-copper complex. Org. Lett. 2014, 16, 5584–5587. [Google Scholar] [CrossRef]
- Huang, G.; Cheng, C.; Ge, L.; Guo, B.; Zhao, L.; Wu, X. Trialkyl methanetricarboxylate as dialkyl malonate surrogate in copper-catalyzed enantioselective propargylic substitution. Org. Lett. 2015, 17, 4894–4897. [Google Scholar] [CrossRef]
- Xia, J.-T.; Hu, X.-P. Copper-catalyzed asymmetric propargylic alkylation with oxindoles: Diastereo- and enantioselective construction of vicinal tertiary and all-carbon quaternary stereocenters. Org. Lett. 2020, 22, 1102–1107. [Google Scholar] [CrossRef]
- Zhu, Q.; Meng, B.; Gu, C.; Xu, Y.; Chen, J.; Lei, C.; Wu, X. Diastereo- and enantioselective synthesis of quaternary α-amino acid precursors by copper-catalyzed propargylation. Org. Lett. 2019, 21, 9985–9989. [Google Scholar] [CrossRef]
- Zhang, K.; Lu, L.-Q.; Yao, S.; Chen, J.-R.; Shi, D.-Q. Enantioconvergent copper catalysis: In situ generation of the chiral phosphorus ylide and its Wittig reactions. J. Am. Chem. Soc. 2017, 139, 12847–12854. [Google Scholar] [CrossRef]
- Fu, Z.; Deng, N.; Su, S.-N.; Li, H.; Li, R.-Z.; Zhang, X.; Liu, J.; Niu, D. Diastereo- and enantioselective propargylation of 5H-thiazol-4-ones and 5H-oxazol-4-ones as enabled by Cu/Zn and Cu/Ti catalysis. Angew. Chem. Int. Ed. 2018, 57, 15217–15221. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Zhang, J.; Peng, L.; Guo, C. Collective synthesis of acetylenic pharmaceuticals via enantioselective Nickel/Lewis acid-catalyzed propargylic alkylation. Nat. Commun. 2021, 12, 299. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Laraia, L.; Schneider, L.; Louven, K.; Strohmann, C.; Antonchick, A.P.; Waldmann, H. Highly enantioselective catalytic vinylogous propargylation of coumarins yields a class of autophagy inhibitors. Angew. Chem. Int. Ed. 2017, 56, 11232–11236. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, C.; Miyadera, Y.; Hayashi, Y.; Yakushiji, F. One–pot selective synthesis of 2,3-dihydro-4H-furo[3,2-c]coumarins by palladium-catalyzed silver-assisted propargylation/intramolecular 5-exo–dig cyclization. Chem. Select 2017, 2, 3794–3798. [Google Scholar] [CrossRef]
- Liu, X.-t.; Hao, L.; Lin, M.; Chen, L.; Zhan, Z.-p. One-pot highly efficient synthesis of substituted pyrroles and N-bridgehead pyrroles by zinc-catalyzed multicomponent reaction. Org. Biomol. Chem. 2010, 8, 3064–3072. [Google Scholar] [CrossRef]
- Iwasawa, N.; Watanabe, S.; Ario, A.; Sogo, H. Re(I)-Catalyzed hydropropargylation of silyl enol ethers utilizing dynamic interconversion of vinylidene–alkenylmetal intermediates via 1,5-hydride transfer. J. Am. Chem. Soc. 2018, 140, 7769–7772. [Google Scholar] [CrossRef]
- Bass, P.D.; Gubler, D.A.; Judd, T.C.; Williams, R.M. Mitomycinoid alkaloids: Mechanism of action, biosynthesis, total syntheses, and synthetic approaches. Chem. Rev. 2013, 113, 6816–6863. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Yang, Z.-W.; Chen, Z.; Wang, J.; Yang, D.-L.; Shen, Z.; Hu, L.-L.; Xie, J.-W.; Zhang, J.; Cui, H.-L. Tandem copper-catalyzed propargylation/alkyne azacyclization/isomerization reaction under microwave irradiation: Synthesis of fully substituted pyrroles. J. Org. Chem. 2016, 81, 1778–1785. [Google Scholar] [CrossRef]
- Zhang, C.; Hui, Y.-Z.; Zhang, D.-Y.; Hu, X.-P. Highly diastereo-/enantioselective Cu-catalyzed propargylic alkylations of propargyl acetates with cyclic enamines. RSC Adv. 2016, 6, 14763–14767. [Google Scholar] [CrossRef]
- Cheng, D.; Zhou, X.; Xu, X.; Yan, J. Propargylation of 1,3-dicarbonyl compounds catalyzed by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone and sodium nitrite in the presence of molecular oxygen and formic acid. RSC Adv. 2016, 6, 52459–52463. [Google Scholar] [CrossRef]
- Pasha, M.A.; Krishna, A.V.; Ashok, E.; Ramachary, D.B. Organocatalytic reductive propargylation: Scope and applications. J. Org. Chem. 2019, 84, 15399–15416. [Google Scholar] [CrossRef]
- Evans, D.A.; Ng, H.P.; Clark, J.S.; Rieger, D.L. Diastereoselective anti aldol reactions of chiral ethyl ketones. Enantioselective processes for the synthesis of polypropionate natural products. Tetrahedron 1992, 48, 2127–2142. [Google Scholar] [CrossRef]
- Mailhol, D.; Willwacher, J.; Kausch-Busies, N.; Rubitski, E.E.; Sobol, Z.; Schuler, M.; Lam, M.-H.; Musto, S.; Loganzo, F.; Maderna, A.; et al. Synthesis, molecular editing, and biological assessment of the potent cytotoxin Leiodermatolide. J. Am. Chem. Soc. 2014, 136, 15719–15729. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Song, J.; Wang, L.; Yin, W.; Miao, M.; Ren, H. Direct propargylation of ortho-quinone methides with alkynyl zinc reagents: An application to the one-pot synthesis of 2,3-disubstituted benzofurans. Synlett 2020, 31, 818–822. [Google Scholar] [CrossRef]
- Mukai, C.; Kojima, T.; Kawamura, T.; Inagaki, F. Cyclopropanes in Nicholas reaction: Formation of spiroketals with a five-membered and a seven- or an eight-membered ring. Tetrahedron 2013, 69, 7659–7669. [Google Scholar] [CrossRef]
- Holmelid, B.; Sydnes, L. Reactions of epoxides with 3,3,4,4-tetraethoxybut-1-yne acetylide: Selective formation of propargylic and homopropargylic alcohols. Synthesis 2013, 45, 2567–2570. [Google Scholar] [CrossRef]
- Suzuki, T. Organic synthesis involving iridium-catalyzed oxidation. Chem. Rev. 2011, 111, 1825–1845. [Google Scholar] [CrossRef]
- Liang, T.; Woo, S.K.; Krische, M.J. C-Propargylation overrides O-propargylation in reactions of propargyl chloride with primary alcohols: Rhodium-catalyzed transfer hydrogenation. Angew. Chem. Int. Ed. 2016, 55, 9207–9211. [Google Scholar] [CrossRef]
- Chen, J.; Huang, W.; Li, Y.; Cheng, X. Visible-light-induced difluoropropargylation reaction with benzothiazoline as a reductant. Adv. Synth. Catal. 2018, 360, 1466–1472. [Google Scholar] [CrossRef]
- Ambler, B.R.; Woo, S.K.; Krische, M.J. Catalytic enantioselective carbonyl propargylation beyond preformed carbanions: Reductive coupling and hydrogen auto-transfer. ChemCatChem 2019, 11, 324–332. [Google Scholar] [CrossRef]
- Huang, H.M.; Bellotti, P.; Daniliuc, C.; Glorius, F. Radical carbonyl propargylation by dual catalysis. Angew. Chem. Int. Ed. 2021, 60, 2464–2471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-B.; Zhang, W.-S.; Cheng, H.-L.; Liu, Y.-Q.; Yang, R. One-pot synthesis of N-aryl propargylamine from aromatic boronic acid, aqueous ammonia, and propargyl bromide under microwave-assisted conditions. Chin. Chem. Lett. 2014, 25, 779–782. [Google Scholar] [CrossRef]
- Yu, Y.-B.; He, G.-Z.; Zhang, X. Synthesis of α,α-difluoromethylene alkynes by palladium-catalyzed gem -difluoropropargylation of aryl and alkenyl boron reagents. Angew. Chem. Int. Ed. 2014, 53, 10457–10461. [Google Scholar] [CrossRef] [PubMed]
- García-Viñuales, S.; Delso, I.; Merino, P.; Tejero, T. Stereoselective ethynylation and propargylation of chiral cyclic nitrones: Application to the synthesis of glycomimetics. Synthesis 2016, 48, 3339–3351. [Google Scholar] [CrossRef]
- Guo, L.-N.; Duan, X.-H.; Liang, Y.-M. Palladium-catalyzed cyclization of propargylic compounds. Acc. Chem. Res. 2011, 44, 111–122. [Google Scholar] [CrossRef]
- Zhang, D.-Y.; Hu, X.-P. Recent advances in copper-catalyzed propargylic substitution. Tetrahedron Lett. 2015, 56, 283–295. [Google Scholar] [CrossRef]
- Hua, R.; Nizami, T.A. Synthesis of heterocycles by using propargyl compounds as versatile synthons. Mini-Rev. Org. Chem. 2018, 15, 198–207. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).