Thermal Rearrangement of 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridines
Abstract
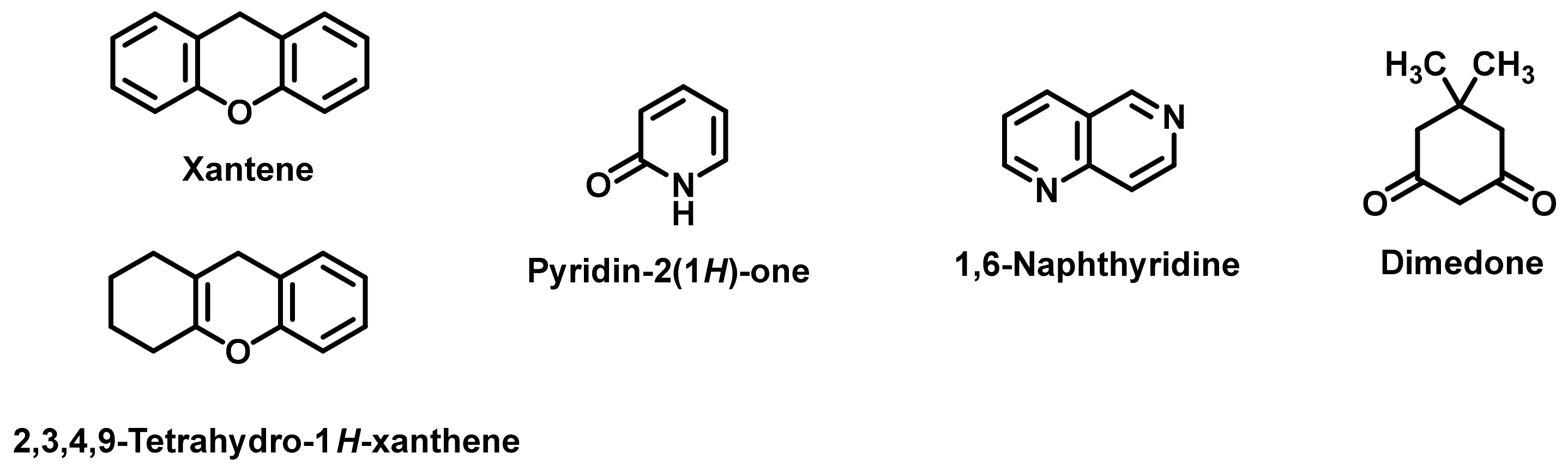
1. Introduction
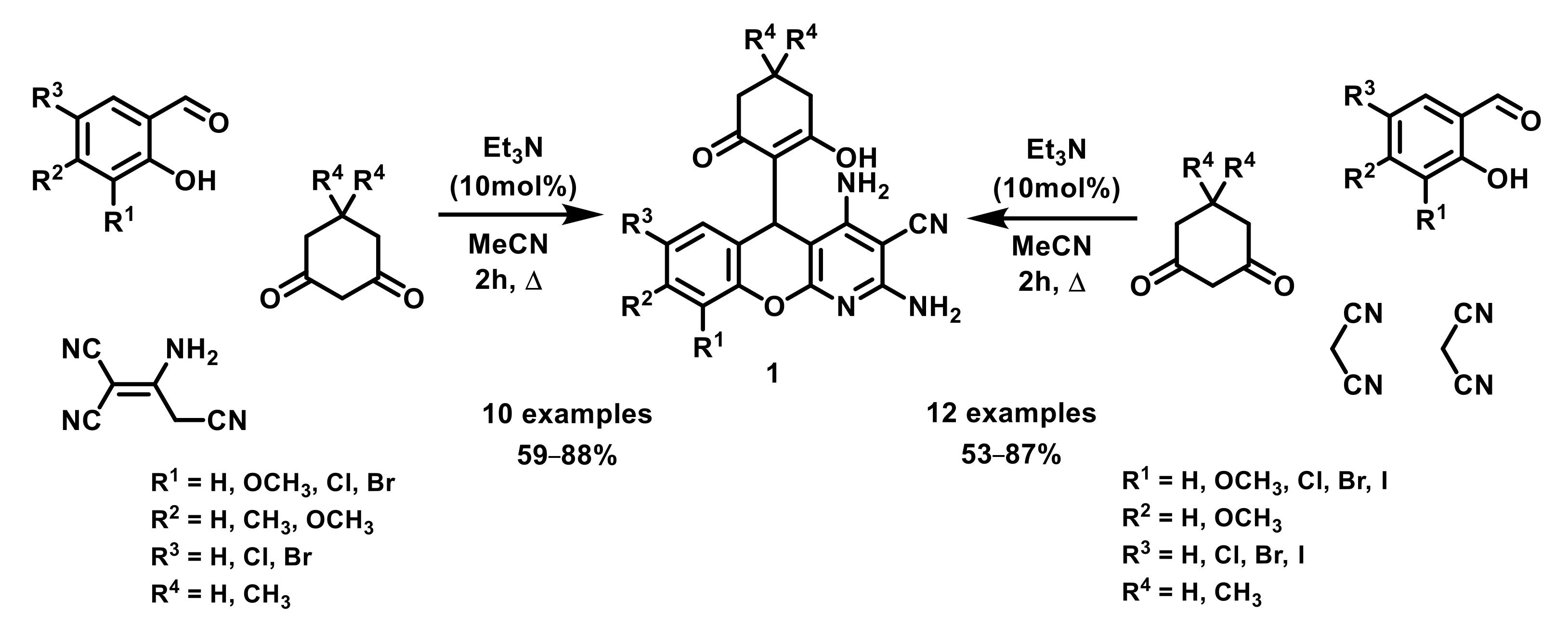
2. Results and Discussion
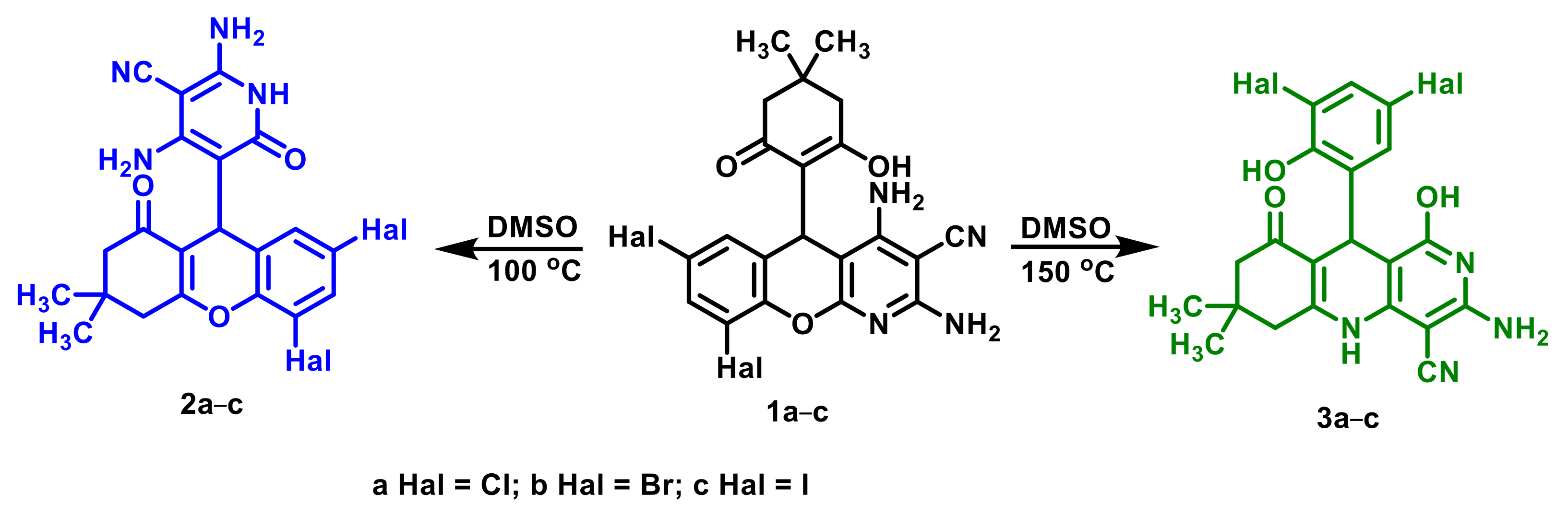
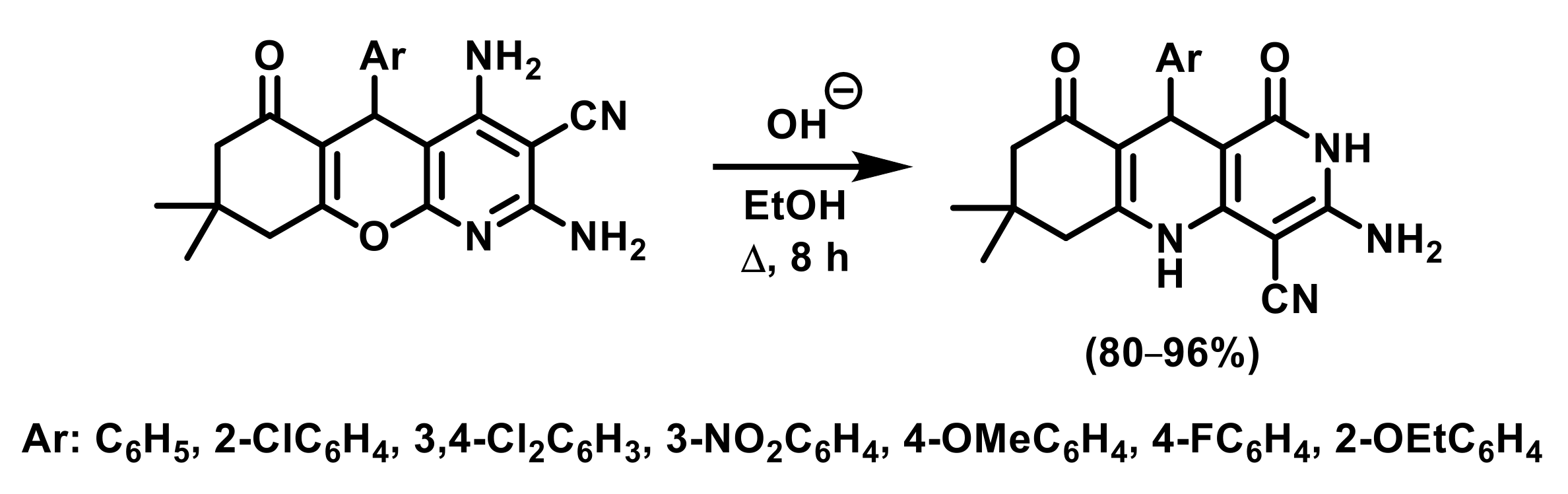
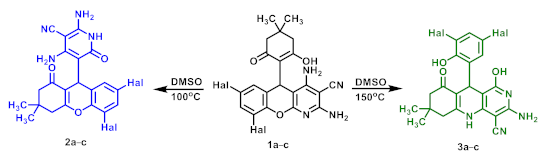
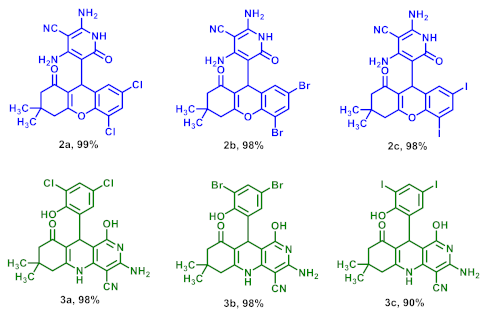
2.1. Thermal Rearrangement of 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridines
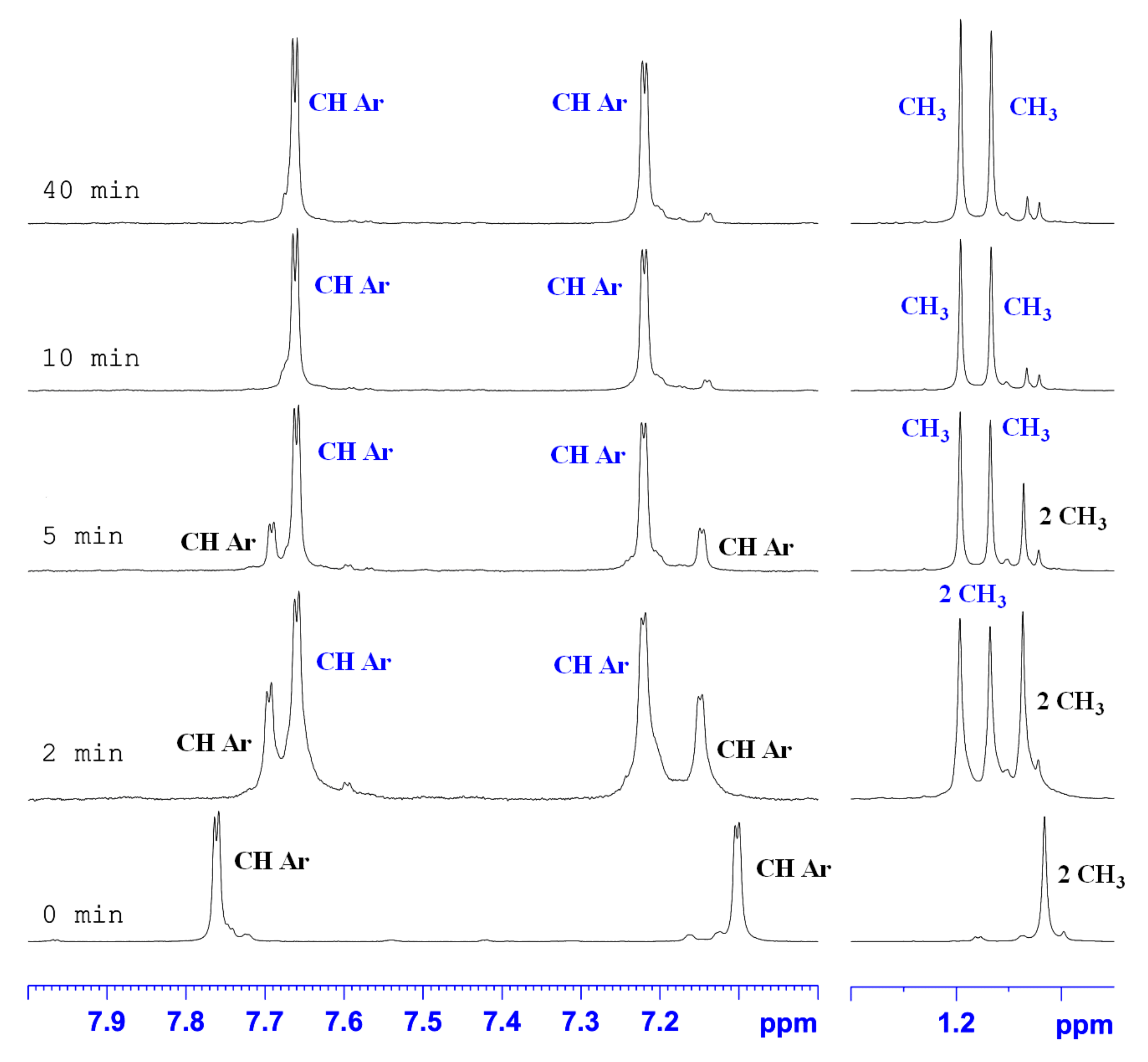
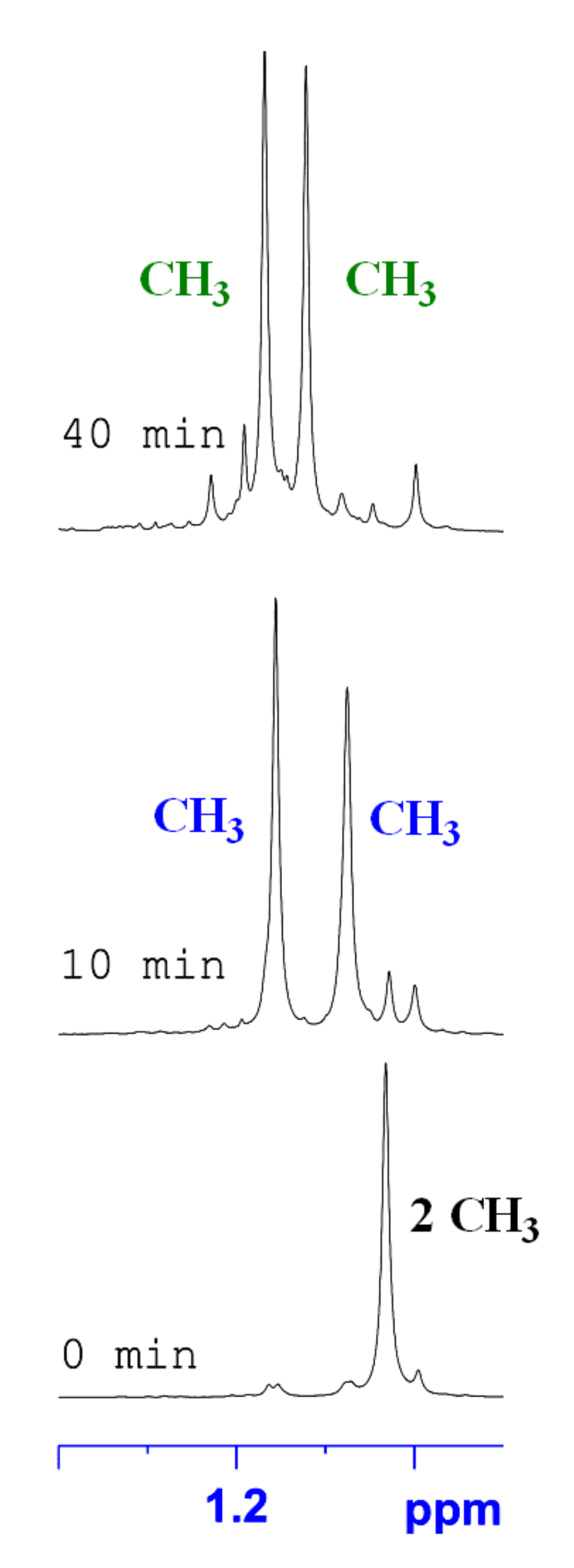
2.2. The 1H-NMR Monitoring of Thermal Rearrangement
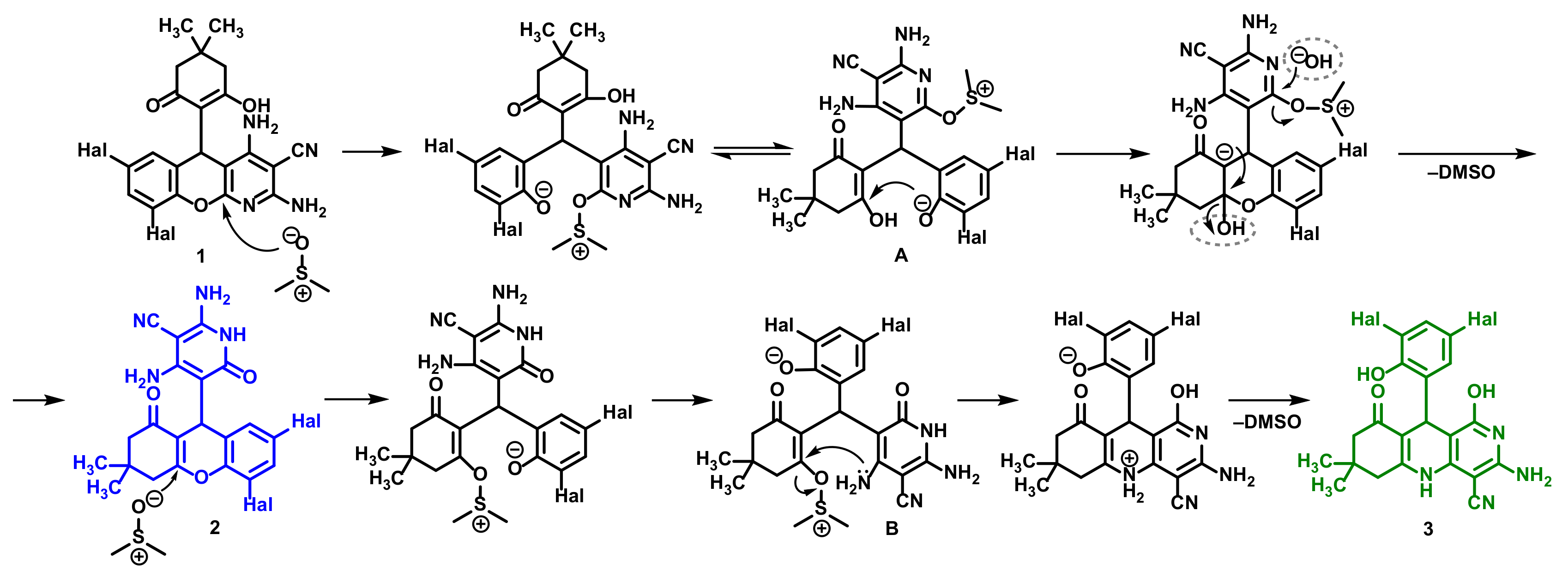
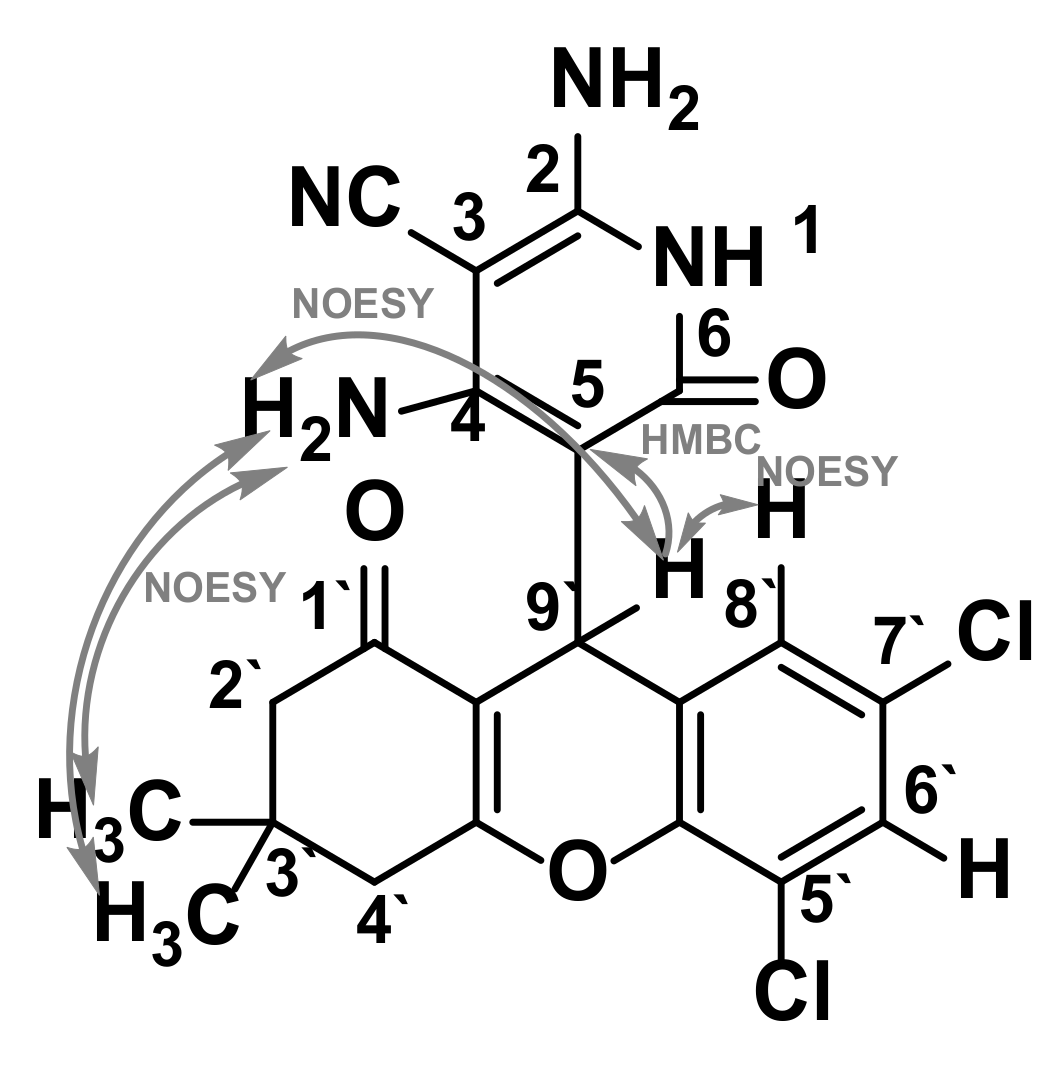
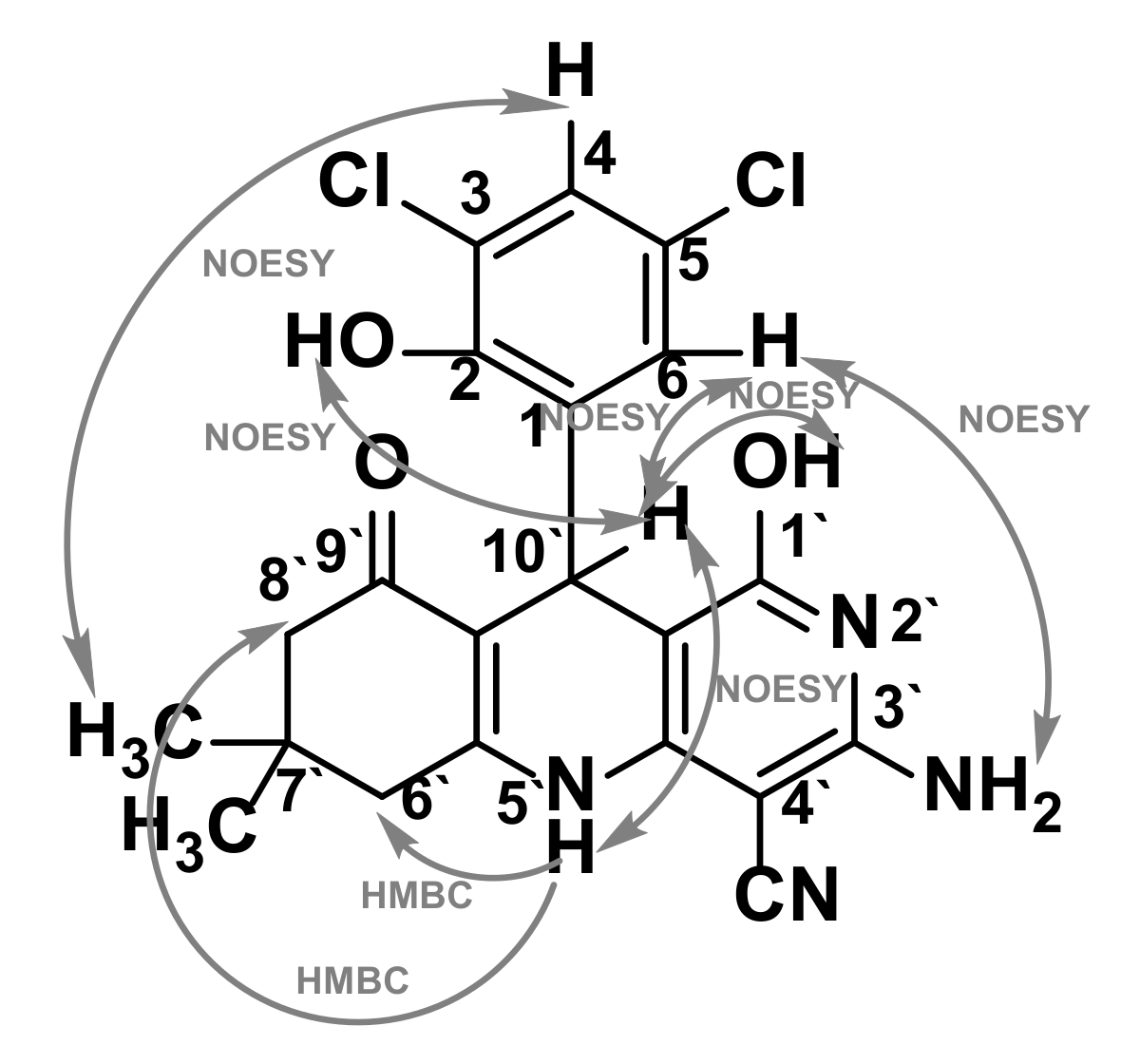
2.3. Confirmation of the Structure of the Synthesized 2,3,4,9-Tetrahydro-1H-xanthenes 2 and 5,6,7,8,9,10-Hexahydrobenzo[b][1,6]naphthyridines 3
3. Materials and Methods
3.1. General Methods
3.2. Thermal Rearrangement of 7,9-Dihalogenated 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridine-3-carbonitriles 1 at 100 °C
3.3. Thermal Rearrangement of 7,9-Dihalogenated 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridine-3-carbonitriles 1 at 150 °C
3.4. Thermal Rearrangement of 5,7-Dihalogenated 5-(1-oxo-2,3,4,9-tetrahydro-1H-xanthen-9-yl)-6-oxo-1,6-dihydropyridine-3-carbonitriles 2 at 150 °C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, X.; Ma, Z.; Feng, T.; Zhu, C. Radical-mediated rearrangements: Past, present, and future. Chem. Soc. Rev. 2021, 50, 11577–11613. [Google Scholar] [CrossRef]
- Zhang, X.M.; Li, B.S.; Wang, S.H.; Zhang, K.; Zhang, F.M.; Tu, Y.Q. Recent development and applications of semipinacol rearrangement reactions. Chem. Sci. 2021, 12, 9262–9274. [Google Scholar] [CrossRef] [PubMed]
- Biellmann, J.F.; Jung, M.J. The mechanism of isomerization of an olefin and its possible relation to the mechanism of the catalytic hydrogenation with tris(triphenylphosphine)rhodium chloride. J. Am. Chem. Soc. 1968, 90, 1673–1674. [Google Scholar] [CrossRef]
- Delaude, L.; Noels, A.F. Metathesis. In Kirk-Othmer Encyclopedia of Chemical Technology; Othmer, D., Kirk., R.E., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Tebbe, F.N.; Parshall, G.W.; Ovenall, D.W. Titanium-catalyzed olefin metathesis. J. Am. Chem. Soc. 1979, 101, 5074–5075. [Google Scholar] [CrossRef]
- Weatherhead, G.S.; Cortez, G.A.; Schrock, R.R.; Hoveyda, A.H. Mo-catalyzed asymmetric olefin metathesis in target-oriented synthesis: Enantioselective synthesis of (+)-africanol. Proc. Natl. Acad. Sci. USA 2004, 101, 5805–5809. [Google Scholar] [CrossRef] [PubMed]
- Wolf, J.; Stüer, W.; Grünwald, C.; Werner, H.; Schwab, P.; Schulz, M. Ruthenium Trichloride, Tricyclohexyl- phosphane, 1-Alkynes, Magnesium, Hydrogen, and Water-Ingredients of an Efficient One-Pot Synthesis of Ruthenium Catalysts for Olefin Metathesis. Angew. Chem. Int. Ed. Engl. 1998, 37, 1124–1126. [Google Scholar] [CrossRef]
- Birladeanu, L. The Story of the Wagner-Meerwein Rearrangement. J. Chem. Educ. 2000, 77, 858. [Google Scholar] [CrossRef]
- Bartlett, P.D.; Pöckel, I. The Wagner—Meerwein Rearrangement. A Kinetic Reinvestigation of the Isomerization of Camphene Hydrochloride. J. Am. Chem. Soc. 1938, 60, 1585–1590. [Google Scholar] [CrossRef]
- Hanson, J.R. 3.1-Wagner–Meerwein Rearrangements. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 3, pp. 705–719. [Google Scholar] [CrossRef]
- Salvador, J.A.; Pinto, R.M.; Santos, R.C.; Le Roux, C.; Beja, A.M.; Paixão, J.A. Bismuth triflate-catalyzed Wagner-Meerwein rearrangement in terpenes. Application to the synthesis of the 18alpha-oleanane core and A-neo-18alpha-oleanene compounds from lupanes. Org. Biomol. Chem. 2009, 7, 508–517. [Google Scholar] [CrossRef]
- Romanov-Michailidis, F.; Guénée, L.; Alexakis, A. Enantioselective Organocatalytic Fluorination-Induced Wagner–Meerwein Rearrangement. Angew. Chem. Int. Ed. 2013, 52, 9266–9270. [Google Scholar] [CrossRef]
- Trost, B.M.; Yasukata, T. A Catalytic Asymmetric Wagner−Meerwein Shift. J. Am. Chem. Soc. 2001, 123, 7162–7163. [Google Scholar] [CrossRef]
- Fittig, R. 41. Ueber einige Derivate des Acetons. Justus Liebigs Ann. Chem. 1860, 114, 54–63. [Google Scholar] [CrossRef]
- Fattori, D.; Henry, S.; Vogel, P. The Demjanov and Tiffeneau-Demjanov one-carbon ring enlargements of 2-aminomethyl-7-oxabicyclo[2.2.1]heptane derivatives. The stereo- and regioselective additions of 8-oxabicyclo[3.2.1]oct-6-en-2-one to soft electrophiles. Tetrahedron 1993, 49, 1649–1664. [Google Scholar] [CrossRef]
- Beckmann, E. Zur Kenntniss der Isonitrosoverbindungen. Ber. Dtsch. Chem. Ges. 1886, 19, 988–993. [Google Scholar] [CrossRef]
- Krow, G.R. The Baeyer–Villiger Oxidation of Ketones and Aldehydes. In Organic Reactions; Denmark, S.E., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Claisen, L. Über Umlagerung von Phenol-allyläthern in C-Allyl-phenole. Ber. Dtsch. Chem. Ges. 1912, 45, 3157–3166. [Google Scholar] [CrossRef]
- Bryon Gill, G. 3.9-The Wolff Rearrangement. In Comprehensive Organic Synthesis; Trost, B.M., Fleming, I., Eds.; Pergamon: Oxford, UK, 1991; Volume 3, pp. 887–912. [Google Scholar] [CrossRef]
- Van der Plas, H.C. The SN(ANRORC) mechanism: A new mechanism for nucleophilic substitution. Acc. Chem. Res. 1978, 11, 462–468. [Google Scholar] [CrossRef]
- Wang, Z. ANRORC Rearrangement. In Comprehensive Organic Name Reactions and Reagents; Wang, Z., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2010; pp. 87–90. [Google Scholar] [CrossRef]
- Van der Plas, H.C. Chapter III SN(ANRORC) Reactions in Azaheterocycles Containing an “Inside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 87–151. [Google Scholar] [CrossRef]
- Babaev, E.V. Chapter 2-2-Aminoimidazoles: Synthesis by Ring Transformation Reactions. Stud. Nat. Prod. Chem. 2017, 52, 69–113. [Google Scholar] [CrossRef]
- Gallardo-Fuentes, S.; Contreras, R. Mechanistic insights into the ANRORC-like rearrangement between methylhydrazine and 1,2,4-oxadiazole derivatives. Org. Biomol. Chem. 2015, 13, 9439–9444. [Google Scholar] [CrossRef] [PubMed]
- Palumbo Piccionello, A.; Guarcello, A.; Buscemi, S.; Vivona, N.; Pace, A. Synthesis of Amino-1,2,4-triazoles by Reductive ANRORC Rearrangements of 1,2,4-Oxadiazoles. J. Org. Chem. 2010, 75, 8724–8727. [Google Scholar] [CrossRef] [PubMed]
- Piccionello, A.P.; Pace, A.; Buscemi, S. Rearrangements of 1,2,4-Oxadiazole: “One Ring to Rule Them All. ” Chem. Heterocycl. Compd. 2017, 53, 936–947. [Google Scholar] [CrossRef]
- Piccionello, A.P.; Pace, A.; Buscemi, S.; Vivona, N. An ANRORC approach to the synthesis of perfluoroalkylated 1,2,4-triazole-carboxamides. ARKIVOC 2009, 2009, 235–244. [Google Scholar] [CrossRef]
- Voskressensky, L.G.; Festa, A.A.; Sokolova, E.A.; Khrustalev, V.N.; Varlamov, A. V Synthesis of Polycyclic Imidazo[1,4]thiazine Derivatives by an ANRORC Domino Reaction. Eur. J. Org. Chem. 2012, 2012, 6124–6126. [Google Scholar] [CrossRef]
- Ghoneim, A.A.; El-Farargy, A.F.; Abdelaziz, S. Synthesis and Antimicrobial Activities of New S-Nucleosides of Chromeno[2,3-B]Pyridine Derivatives and C-Nucleosides of [1,2,4]Triazolo[1,5-A]Quinoline Derivatives. Nucleosides Nucleotides Nucleic Acids 2014, 33, 583–596. [Google Scholar] [CrossRef]
- Ghoneim, A.A.; El-Farargy, F.A. Synthesis of Some New Chromeno[2,3-b]pyridine and [1,2,4]Triazolo[1,5-a]quinoline Nucleoside Analogues with Expected Biological Activity. Lett. Org. Chem. 2015, 12, 13–20. [Google Scholar] [CrossRef]
- Alekseeva, A.Y.; Bardasov, I.N.; Malyshkina, N.L.; Ershov, O.V. Rearrangement of 3-cyano-5H-chromeno[2,3-b]pyridines to 1,6-naphthyridine derivatives. Chem. Heterocycl. Comp. 2017, 53, 1050–1052. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Ryzhkov, F.V.; Fakhrutdinov, A.N.; Elinson, M.N. Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines, Their NMR Study and Computer Evaluation as Material for LED. Molecules 2022, 27, 4156. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, S.; Maurya, R.A.; Sarkar, J. Diversity oriented synthesis of benzoxanthene and benzochromene libraries via one-pot, three-component reactions and their anti-proliferative activity. J. Comb. Chem. 2010, 12, 20–24. [Google Scholar] [CrossRef]
- Rama, V.; Kanagaraj, K.; Pitchumani, K. A multicomponent, solvent-free, one-pot synthesis of benzoxanthenones catalyzed by HY zeolite: Their anti-microbial and cell imaging studies. Tetrahedron Lett. 2012, 53, 1018–1024. [Google Scholar] [CrossRef]
- Khurana, J.M.; Chaudhary, A.; Lumb, A.; Nand, B. Efficient one-pot syntheses of dibenzo[a,i]xanthene-diones and evaluation of their antioxidant activity. Can. J. Chem. 2012, 90, 739–746. [Google Scholar] [CrossRef]
- Sato, N.; Jitsuoka, M.; Shibata, T.; Hirohashi, T.; Nonoshita, K.; Moriya, M.; Haga, Y.; Sakuraba, A.; Ando, M.; Ohe, T.; et al. (9S)-9-(2-Hydroxy-4,4-dimethyl-6-oxo-1-cyclohexen-1-yl)-3,3-dimethyl-2,3,4,9-tetrahydro-1H-xanthen-1-one, a selective and orally active neuropeptide Y Y5 receptor antagonist. J. Med. Chem. 2008, 51, 4765–4770. [Google Scholar] [CrossRef]
- Shams, H.Z.; Mohareb, R.M.; Helal, M.H.; Mahmoud, A.E. Novel Synthesis and Antitumor Evaluation of Polyfunctionally Substituted Heterocyclic Compounds Derived from 2-Cyano-N-(3-cyano-4,5,6,7-tetrahydrobenzo[b]thiophen-2-yl)-acetamide. Molecules 2011, 16, 52–73. [Google Scholar] [CrossRef]
- Mekheimer, R.A.; Sayed, A.A.; Ahmed, E.A. Novel 1,2,4-triazolo[1,5-a]pyridines and their fused ring systems attenuate oxidative stress and prolong lifespan of Caenorhabiditis elegans . J. Med. Chem. 2012, 55, 4169–4177. [Google Scholar] [CrossRef] [PubMed]
- Ruchelman, A.L.; Singh, S.K.; Ray, A.; Wu, X.H.; Yang, J.M.; Li, T.K.; Liu, A.; Liu, L.F.; LaVoie, E.J. 5H-Dibenzo[c,h]1,6-naphthyridin-6-ones: Novel topoisomerase I-targeting anticancer agents with potent cytotoxic activity. Bioorg. Med. Chem. 2003, 11, 2061–2073. [Google Scholar] [CrossRef]
- Chan, L.; Jin, H.; Stefanac, T.; Lavallée, J.F.; Falardeau, G.; Wang, W.; Bédard, J.; May, S.; Yuen, L. Discovery of 1,6-naphthyridines as a novel class of potent and selective human cytomegalovirus inhibitors. J. Med. Chem. 1999, 42, 3023–3025. [Google Scholar] [CrossRef] [PubMed]
- Cywin, C.L.; Zhao, B.P.; McNeil, D.W.; Hrapchak, M.; Prokopowicz, A.S.; Goldberg, D.R.; Morwick, T.M.; Gao, A.; Jakes, S.; Kashem, M.; et al. Discovery and SAR of novel Naphthyridines as potent inhibitors of spleen tyrosine kinase (SYK). Bioorg. Med. Chem. Lett. 2003, 13, 1415–1418. [Google Scholar] [CrossRef] [PubMed]
- Austin, N.E.; Hadley, M.S.; Harling, J.D.; Harrington, F.P.; Macdonald, G.J.; Mitchell, D.J.; Riley, G.J.; Stean, T.O.; Stemp, G.; Stratton, S.C.; et al. The design of 8,8-dimethyl[1,6]naphthyridines as potential anticonvulsant agents. Bioorg. Med. Chem. Lett. 2003, 13, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Galatsis, P.; Yamagata, K.; Wendt, J.A.; Connolly, C.J.; Mickelson, J.W.; Milbank, J.B.; Bove, S.E.; Knauer, C.S.; Brooker, R.M.; Augelli-Szafran, C.E.; et al. Synthesis and SAR comparison of regioisomeric aryl naphthyridines as potent mGlu5 receptor antagonists. Bioorg. Med. Chem. Lett. 2007, 17, 6525–6528. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Abouzied, A.S.; Abbas, N.S. Synthesis and Biological Evaluation of Novel 4,5,6,7-Tetrahydrobenzo[D]-Thiazol-2-Yl Derivatives Derived from Dimedone with Anti-Tumor, C-Met, Tyrosine Kinase and Pim-1 Inhibitions. Anticancer Agents Med. Chem. 2019, 19, 1438–1453. [Google Scholar] [CrossRef]
- Rao, T.N.; Krishnarao, N.; Ahmed, F.; Alomar, S.Y.; Albalawi, F.; Mani, P.; Aljaafari, A.; Parvatamma, B.; Arshi, N.; Kumar, S. One-Pot Synthesis of 7,7-Dimethyl-4-Phenyl-2-Thioxo-2,3,4,6,7,8-Hexahydro-1H-Quinazoline-5-OnesUsing Zinc Ferrite Nanocatalyst and Its Bio Evaluation. Catalysts 2021, 11, 431. [Google Scholar] [CrossRef]
- Barakat, A.; Al-Majid, A.M.; Al-Qahtany, B.M.; Ali, M.; Teleb, M.; Al-Agamy, M.H.; Naz, S.; Ul-Haq, Z. Synthesis, antimicrobial activity, pharmacophore modeling and molecular docking studies of new pyrazole-dimedone hybrid architectures. Chem. Cent. J. 2018, 12, 29. [Google Scholar] [CrossRef]
- Maharvi, G.M.; Ali, S.; Riaz, N.; Afza, N.; Malik, A.; Ashraf, M.; Iqbal, L.; Lateef, M. Mild and efficient synthesis of new tetraketones as lipoxygenase inhibitors and antioxidants. J. Enzyme Inhib. Med. Chem. 2008, 23, 62–69. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Bushmarinov, I.S.; Zlotin, S.G.; Egorov, M.P. Pot, atom and step economic (PASE) synthesis of 5-isoxazolyl-5H-chromeno[2,3-b]pyridine scaffold. Mendeleev Commun. 2015, 25, 424–426. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Krymov, S.K.; Fakhrutdinov, A.N.; Egorov, M.P. Potassium fluoride catalysed multicomponent approach to medicinally privileged 5-[3-hydroxy-6-(hydroxymethyl)-4-oxo-4H-pyran-2-yl] substituted chromeno[2,3-b]pyridine scaffold. Arkivoc 2019, 2, 38–49. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Fakhrutdinov, A.N.; Goloveshkin, A.S.; Egorov, M.P. Pot-, Atom- and Step-Economic (PASE) Multicomponent approach to the 5-(Dialkylphosphonate)-Substituted 2,4-Diamino-5H-chromeno-[2,3-b]pyridine scaffold. Eur. J. Org. Chem. 2019, 2019, 4171–4178. [Google Scholar] [CrossRef]
- Ryzhkova, Y.E.; Elinson, M.N.; Maslov, O.I.; Fakhrutdinov, A.N. Multicomponent Synthesis of 2-(2,4-Diamino-3-cyano-5H-chromeno[2,3-b]pyridin-5-yl)malonic Acids in DMSO. Molecules 2021, 26, 6839. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Anisina, Y.E.; Egorov, M.P. Efficient Multicomponent Approach to the Medicinally Relevant 5-aryl-chromeno[2,3-b]pyridine Scaffold. Polycycl. Aromat. Compd. 2020, 40, 108–115. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Novikov, R.A.; Egorov, M.P. PASE Pseudo-Four-Component Synthesis and Docking Studies of New 5-C-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]pyridine-3-Carbonitriles. ChemistrySelect 2017, 2, 4593–4597. [Google Scholar] [CrossRef]

| Entry | Solvent, mL | Temperature, °C | Time, h | Yield of 2a, % | Yield of 3a, % |
|---|---|---|---|---|---|
| 1 | DMSO, 0.5 | 100 | 1 | 99 2 | – |
| 2 | DMSO, 0.5 | 120 | 1 | 88 | 10 |
| 3 | DMSO, 0.5 | 150 | 1 | – | 98 2 |
| 4 | DMF, 0.5 | 100 | 1 | 95 2 | – |
| 5 | DMF, 0.5 | 150 | 1 | – | 94 2 |
| 6 | H2O, 2 | 100 | 1 | 6 | 5 |
| 7 | MeCN, 0.5 | 82 | 1 | 8 | – |
| 8 | n-PrOH, 0.5 | 97 | 1 | 25 | – |
| 9 | Dioxane, 0.5 | 101 | 1 | – | – |
| 10 | DMSO, 0.5 | 100 | 0.5 | 82 2 | – |
| 11 | DMSO, 0.5 | 150 | 0.5 | – | 80 2 |
 |
 |
| Entry | Chromeno[2,3-b]pyridine 1 | Heating in DMSO at 100 °C | Heating in DMSO at 150 °C | ||
|---|---|---|---|---|---|
| Yield of 2, % | Yield of 3, % | Yield of 2, % | Yield of 3, % | ||
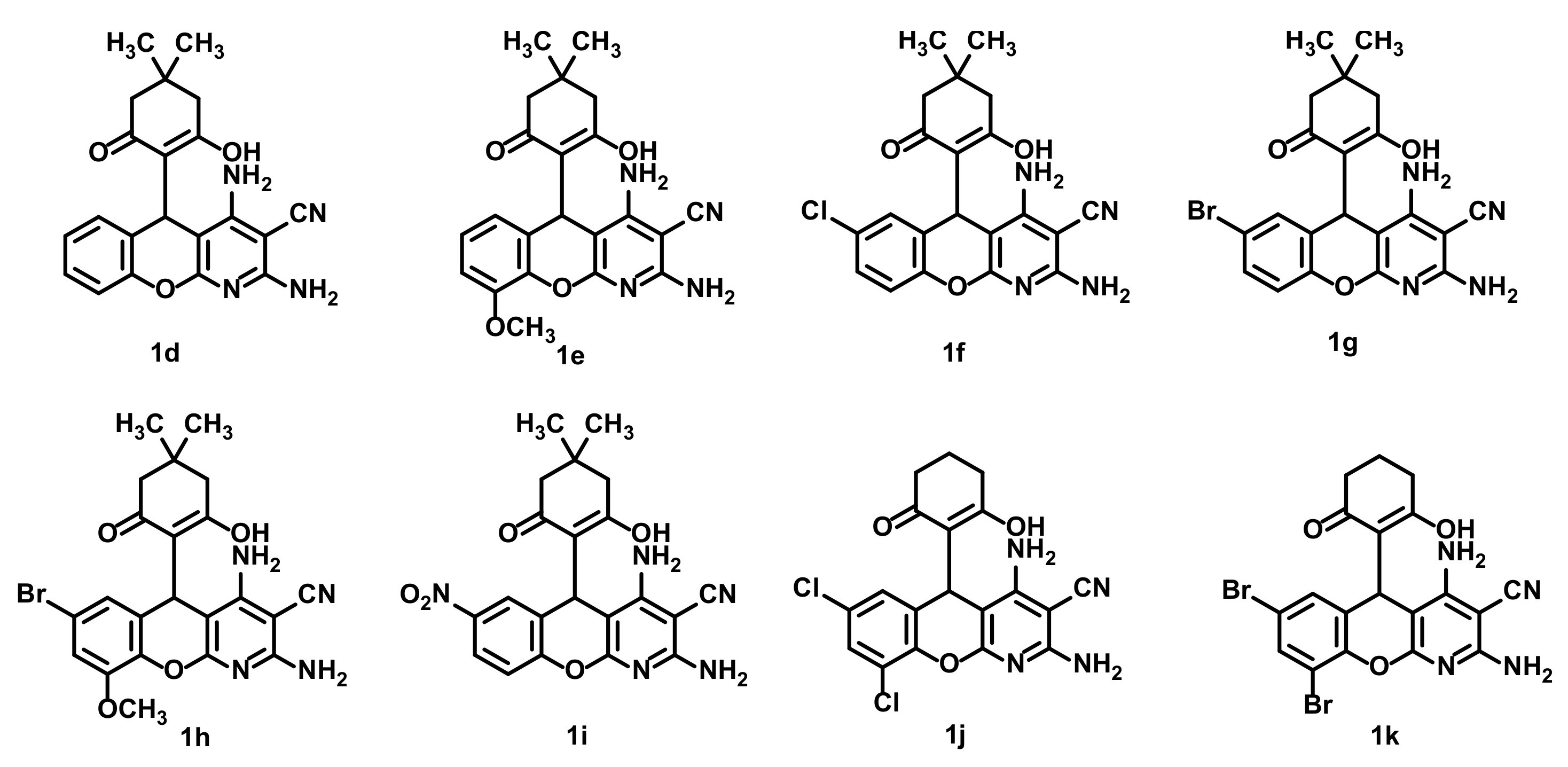
| 1 | 1d | – | – | Decomposition | |
| 2 | 1e | – | – | Decomposition | |
| 3 | 1f | 7 | – | 3 | 7 |
| 4 | 1g | 11 | – | 4 | 7 |
| 5 | 1h | – | – | Decomposition | |
| 6 | 1j | 80 | – | – | 78 |
| 7 | 1k | 82 | – | – | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Ryzhkov, F.V.; Elinson, M.N.; Vereshchagin, A.N.; Novikov, R.A.; Fakhrutdinov, A.N. Thermal Rearrangement of 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridines. Molecules 2023, 28, 3139. https://doi.org/10.3390/molecules28073139
Ryzhkova YE, Ryzhkov FV, Elinson MN, Vereshchagin AN, Novikov RA, Fakhrutdinov AN. Thermal Rearrangement of 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridines. Molecules. 2023; 28(7):3139. https://doi.org/10.3390/molecules28073139
Chicago/Turabian StyleRyzhkova, Yuliya E., Fedor V. Ryzhkov, Michail N. Elinson, Anatoly N. Vereshchagin, Roman A. Novikov, and Artem N. Fakhrutdinov. 2023. "Thermal Rearrangement of 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridines" Molecules 28, no. 7: 3139. https://doi.org/10.3390/molecules28073139
APA StyleRyzhkova, Y. E., Ryzhkov, F. V., Elinson, M. N., Vereshchagin, A. N., Novikov, R. A., & Fakhrutdinov, A. N. (2023). Thermal Rearrangement of 5-(2-Hydroxy-6-oxocyclohexyl)-5H-chromeno[2,3-b]pyridines. Molecules, 28(7), 3139. https://doi.org/10.3390/molecules28073139










