Abstract
Four new dammarane triterpenoid saponins cypaliurusides Z1–Z4 (1–4) and eight known analogs (5–12) were isolated from the leaves of Cyclocarya paliurus. The structures of the isolated compounds were determined using a comprehensive analysis of 1D and 2D NMR and HRESIMS data. The docking study demonstrated that compound 10 strongly bonded with PTP1B (a potential drug target for the treatment of type-II diabetes and obesity), hydrogen bonds, and hydrophobic interactions, verifying the importance of sugar unit. The effects of the isolates on insulin-stimulated glucose uptake in 3T3-L1 adipocytes were evaluated and three dammarane triterpenoid saponins (6, 7 and 10) were found to enhance insulin-stimulated glucose uptake in 3T3-L1 adipocytes. Furthermore, compounds 6, 7, and 10 exhibited potent abilities to promote insulin-stimulated glucose uptake in 3T3-L1 adipocytes in a dose-dependent manner. Thus, the abundant dammarane triterpenoid saponins from C. paliurus leaves exhibited stimulatory effects on glucose uptake with application potential as a antidiabetic treatment.
1. Introduction
Triterpenoids, with a wide variety of structural types and extensive biological activity, are important compounds for the prevention and treatment of various kinds of diseases, such as diabetes mellitus and metabolic syndrome, cancer, hyperlipidemia, cardiovascular and cerebrovascular disease, neurodegenerative disease, bone disease, liver disease, kidney disease, aging, gastrointestinal disease, mental illness involving depression, and skin aging [1,2,3,4,5]. Among triterpenoids, dammarane triterpenoids exhibit the most significant hypoglycemic activity [6,7,8].
Cyclocarya paliurus, an endemic plant that grows in southern China, has been widely used as an herbal tea and is commonly known as the ‘sweet tea tree’. The leaves of this plant have been used in Chinese folk medicine to prevent diabetes, hypertension, inflammation, and heart disease [9,10]. There have been many reports on the significant antidiabetic effects of the extract of C. paliurus leaves [11,12,13,14,15,16,17]. An antidiabetic prescription containing the leaves of C. paliurus reduces blood glucose and improves glucose tolerance [18,19]. The leaves of C. paliurus were approved as a novel raw food by the National Health Commission of the People’s Republic of China in 2013 [20]. Currently, C. paliurus is a crop for rural revitalization and an important medical and functional raw food. A variety of triterpenoids, flavonoids, and other compounds can be isolated from the leaves of this plant [9,10]. Among these constituents, dammarane triterpenoids are characteristic indicators and the key functional and active constituents of C. paliurus [9,10,21,22]. Several dammarane triterpenoids from this plant demonstrated to moderate the activity of PTP1B (a potential drug target for the treatment of type-II diabetes and obesity) and enhance insulin-stimulated glucose uptake in 3T3-L1 adipocytes [23,24,25,26]. To further investigate potential constituents for promoting glucose uptake activity in 3T3-L1 adipocytes, the active ingredients were separated from the leaves of C. paliurus to afford four novel dammarane saponins (1−4) and eight known analogs (5−12) (Figure 1). Furthermore, stimulation of glucose consumption in 3T3-L1 adipocytes by the triterpenoids isolated from C. paliurus leaves was evaluated and possible hypoglycemic mechanisms of the active compounds were studied. Herein, the isolation, purification, and determination of these isolates, as well as the assays used to determine the glucose uptake activity in 3T3-L1 adipocytes of the constituents and predictions of their mechanisms, are described.
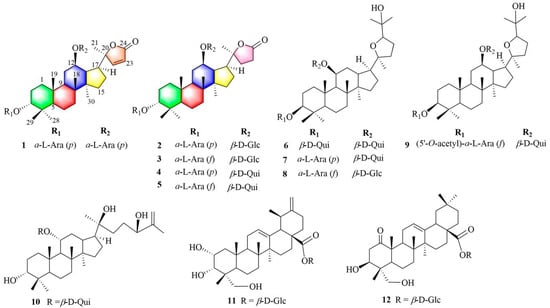
Figure 1.
Structures of compounds 1–12 isolated from C. paliurus.
2. Results and Discussion
2.1. Elucidation of the Chemical Structure of Cypaliurusides Z1–Z4 (1−4)
Cypaliuruside Z1 (1) was deduced to have the molecular formula C37H58O12 (Figure S1) based on its NMR (1H, 13C, pyridine-d5) data and the positive-ion peak [M+Na]+ at m/z 717.3811 (calculated for C37H58O12Na, 717.3826) in its HRESIMS spectrum. Comparisons between the MS and NMR signals of compound 1 and those of cyclocarioside L (5) [27] indicated that 1 and 5 have almost identical NMR data (Table 1; Figures S2 and S3). There were only a few differences in the 1H-and 13C-NMR data (pyridine-d5) between 1 and 5 at the positions 20, 22, 23, and 24 and two sugar units. The 1H NMR signals of H-22 and 23 shifted from δH 1.97 (m, H-22), 1.82 (m, H-22), 2.64 (m, H-23), and 2.49 (m, H-23) for 5 to δH 7.64 (d, 5.7, H-22) and 6.26 (d, 5.7, H-23) for 1, respectively (Table 1; Figure S2). The 13C NMR (pyridine-d5, Table 1; Figure S3) signals of C-20, C-22, C-23, and C-24 shifted from δC 89.6, 32.5, 29.7, and 176.9 for 5 to δC 92.6, 161.2, 121.9, and 173.4 for 1, respectively (Table 1); this indicates that there is a double bond at the positions 22 and 23 in 1. Moreover, the 13C NMR data of the two sugar units in 1 contained ten carbon signals is compared to eleven corresponding carbon signals for 5. In the 1H-1H COSY spectrum of 1, the correlations of H-1/H-2/H-3, H-5/H-6/H-7 and H-9/H-11/H-12/H-13/H-15/H-16/H-17 substantiated the existence of three fragments: –CH2CH2CH–, –CH2CH2CH–, and –CHCH2CHCHCHCH CH2– (Figure 2A and Figure S6). In the HMBC spectrum of 1 (Figure 2A and Figure S7, pyridine-d5), the cross peaks from H-19-CH3 (δH 1.29, s) to C-1 (δC 36.1), C-5 (δC 51.3) and C-9 (δC 54.3), H-18-CH3 (δH 0.90, s) to C-9 (δC 54.3) and C-14 (δC 41.9), and H-30-CH3 (δH 0.55, s) to C-8 (δC 50.7) and C-15 (δC 31.7) indicated that CH3-19 was linked to C-10, CH3-18 was located at C-8, and CH3-30 was connected to C-14, respectively. The HMBC correlations from H-21 (δH 1.36, s) to C-17 (δC 47.8) and C-22 (δC 121.9) suggested that CH3-21 was situated at C-20. Furthermore, the diagnostic ROESY correlations of H-3 and H-11 to H-19 suggested that H-3, H-11, and H-19 are β-oriented, biosynthetic characteristics of dammarane trierpenoids [28], further indicating that H-19 just located at β-oriented. Similarly, the diagnostic ROESY peaks of H-17 and H-30 indicated that H-17 and H-30 are α-oriented (Figure 2B). The HMBC correlations from the anomeric protons H-1′ (δH 4.73, d, J = 6.8 Hz) to C-3 (δC 82.2) and H-1″ (δH 4.87, d, J = 6.5 Hz) to C-12 (δC 76.8) suggest that the two L-arabinoses are situated at C-3 and C-12 respectively (Figure 2A). The sugar unit was determined to be an α-L-arabinosyl moiety based on the hydrolysis of 1 with 1 M HCl and HPLC (Table 2). Therefore, 1 was deduced to have the structure (20S,24)-lactone-22-en-dammarane-(3α,12β)-12-O-α-L-arabinopyranosyl-3-O-α-L-arabinopyranoside and, therefore, is named cypaliuruside Z1 (Figure 1).

Table 1.
1H and 13C NMR spectral data of compounds 1–2 (600 MHz, Pyridine-d5, δ in ppm).
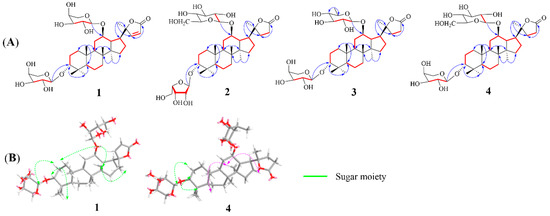
Figure 2.
(A) Key HMBC (red arrows) and COSY (blue bold bonds) correlations of compounds 1–4. (B) ROESY correlations of compounds 1 and 4.

Table 2.
The HPLC retention time of sugar moieties of compounds 1–4.
Cypaliuruside Z2 (2) was deduced to have the molecular formula C38H62O13 by HRESIMS m/z 749.4075 [M + Na]+ (calculated for C38H62O13Na, 749.4088) (Figure S9), suggesting eight degrees of unsaturation. Detailed analyses show that the NMR data of 2 (Table 1) were highly similar to those of 5 [27], suggesting that 2 includes a basic (20S,24)-lactone-22-en-dammarane triterpenoid skeleton and differed from those of cyclocarioside L only in the signals of the two sugar units (Table 1; Figure S10). The 1H NMR spectrum indicated two anomeric protons at δH 5.11 (d, J = 7.7 Hz, H-1″) and 4.74 (d, J = 6.5 Hz, H-1′) and six methyl protons at δH 1.38 (s, H-19), 1.26 (s, H-29), 0.98 (s, H-18), 0.96 (s, H-28), 1.28 (s, H-21), and 0.62 (s, H-30) (Table 1). The 13C NMR of 38 carbon resonances confirmed the aforementioned moieties (Table 1). The 13C-NMR (pyridine-d5, Table 1, Figure S11) and DEPT (distortionless enhancement by polarization transfer, pyridine-d5) spectra indicated the presence of six methyl carbon signals at δC 17.5 (C-18), 17.4 (C-19), 24.9 (C-21), 23.8 (C-28), 30.0 (C-29), and 16.8 (C-30) and two glycosyl anomeric carbon signals at δC 101.8 (C-1′) and δC 102.3 (C-1″), respectively. In the 1H-1H COSY spectrum of 2, the correlations of H-1/H-2/H-3, H-5/H-6/H-7 and H-9/H-11/H-12/H-13/H-15/H-16/H-17 substantiated the existence of three fragments: –CH2CH2CH–, –CH2CH2CH–, and –CHCH2CHCHCHCH2CH2– (Figure 2A and Figure S6). The sugar unit was determined to be α-L-arabinopyranose and β-D-glucopyranose based on the hydrolysis of 2 with 1 M HCl and HPLC (Table 2). Therefore, 2 was deduced to have the structure (20S,24)-lactone-dammarane-(3α,12β)-12- O-β-D-glucopyranosyl-3-O-α-L-arabinopyranoside and is, thus, named cypaliuruside Z2 (Figure 1).
The molecular formula of cypaliuruside Z3 (3) was deduced to be C38H62O13 due to the positive sodium adduct ion HRESIMS peak at m/z 749.4096 [M + Na]+ (calculated for C38H62O13Na, 749.4088) (Figure S17), which is the same as that of 2. The 1H- and 13C-NMR data of 3 (pyridine-d5, Table 3) were found to be identical to those of 2. A small difference between the 1H- and 13C-NMR data of 3 and 2 (pyridine-d5) was related to a sugar unit linked to C-3. That is, the 1H-NMR data of the sugar unit linked to C-3 shifted from δH 4.74 (d, J = 6.5 Hz, H-1′), 4.45 (t, J = 8.9 Hz, H-2′), 4.27 (dd, J = 8.4, 3.5 Hz, H-3′), 4.42 (m, H-4′), 4.34 (dd, J = 12.1, 3.7 Hz, H-5′), and 3.80 (m, H-5′) for 2 (pyridine-d5, Table 2) to δH 5.55 (d, J = 6.5 Hz, H-1′), 4.89 (t, J = 5.6 Hz, H-2′), 4.85 (m, H-3′), 4.76 (dd, J = 6.0, 5.0 Hz, H-4′), 4.40 (m, H-5′), and 4.29 (d, J = 9.4 Hz, H-5′) for 3 (pyridine-d5, Table 2). Moreover, the 13C-NMR data of the sugar unit linked to C-3 changed from δC 102.3 (C-1′), 72.1 (C-2′), 75.3 (C-3′), 69.7 (C-4′), and 66.9 (C-5′) for 2 to δC 106.9 (C-1′), 84.6 (C-2′), 79.9 (C-3′), 86.0 (C-4′), and 63.4 (C-5′) for 3, respectively (pyridine-d5, Table 2). Comparing the 1H- and 13C-NMR (Table 2) and HRESIMS data between 3 and 2 revealed the presence of an arabinofuranose linked to C-3 in 3 instead of an arabinopyranose linked to C-3 in 2. α-L-Arabinofuranose and β-D-glucopyranose were confirmed by the acid hydrolysis solution of 3 in conjunction with comparison to authentic sugars in the HPLC assay (Table 2). Accordingly, 3 was deduced to have the structure (20S,24)-lactone-dammarane-(3α,12β)-12-O-β-D-glucopyranosyl-3-O-α-L-arabino-furanoside and is, thus, named cypaliuruside Z3 (3) (Figure 1).

Table 3.
1H and 13C NMR spectral data of compounds 3–4 (600 MHz, pyridine-d5, δ in ppm).
Cypaliuruside Z4 (4) was deduced to have the molecular formula C38H62O12 based on the positive sodium adduct ion HRESIMS peak at m/z 733.4146 [M + Na]+ (calculated for C38H62O12Na, 733.4139) (Figure S25), which is 16 Da lower than that of 2. A detailed analysis of the 1H- and 13C-NMR data (pyridine-d5, Table 3) showed that 4 is very similar to 2, except that the glucopyranosyl moiety is replaced by a quinovopyranosyl moiety at C-12 in 4. Moreover, the hydrolysate of compound 4 was determined to contain L-arabinose and quinovose by comparison with an authentic sample in the HPLC test (Table 2). Accordingly, 4 was deduced to have the structure (20S,24)-lactone-dammarane-(3α,12β)-12-O-β-D-quinovopyranosyl-3-O-α-L-arabinopyranoside and is, thus, named cypaliuruside Z4 (Figure 1).
By comparing the measured NMR (1H and 13C) and MS data to those reported in the literature, the known dammarane triterpenoid saponins were identified as cyclocarioside L (5) [27], cypaliuruside M (6) [29], cyclocarioside J (7) [30], cyclocarioside Z18 (8) [25], cyclocarioside Z10 (9) [25], cyclocarioside H (10) [31], 2α,3α,23-trihydroxy-12,20(30)-dien-28-ursolic acid 28-O-β-D-glucopyranoside (11) [32], 1-oxo-3β,23-dihydroxy olean-12-en-28-oic acid 28-O-β-D-glucopyranoside (12) [33].
2.2. Predicted Binding Modes of Compounds and PTP1B Using Molecular Docking Analysis
PTP1B is a potential drug target for the treatment of type-II diabetes and obesity. To to investigate the interactions of PTP1B with dammarane triterpenoid saponins, an independent docking run was performed and the compounds ligand with the lowest binding affinity mode was selected for analysis.
The results showed that 10 was well docked into the active site in PTP1B and the binding energy was −8.9 kcal·mol−1, which is superior to that of the oleanolic acid (positive control, −7.2 kcal·mol−1). The catalytic and allosteric sites of docking compound 10 with PTP1B were further simulated. The results showed that compound 10 depicted multiple important interactions, such as Pi-sigma, alkyl and Pi-alkyl, conventional hydrogen bonds, and van der Waals interactions within the active pocket of PTP1B (Figure 3). In visualization of the catalytic site docking results (Figure 3A,C), various amino acid residues, such as Asp548, Ser618, Lys616, Lys620, Asp681, Gly718, Ile719, Ala717, Asp715, Gly720, Ser716, Arg721, Gln762, Phe682, Tyr546, and Val549, surrounded the active pocket of PTP1B. Hydroxyls of compound 10 mainly formed hydrogen bonds with the PTP1B residues Gly720, Asp715, Ile719, ASP681, and Gln762. Moreover, Phe628 revealed Pi-sigma and Pi-alkyl interaction with the methyl of C-6′ and C-30, respectively. In visualization of the allosteric site docking results, the sugar unit of compound 10 formed hydrogen bonding with the PTP1B residue Asp789, whereas van der Waals interactions were noticed with Ser786 and Gln788. In addition, the hydrophobic part of the ligand revealed Alkyl and Pi-alkyl interaction with the Phe780 and Lys792 (Figure 3B,D).
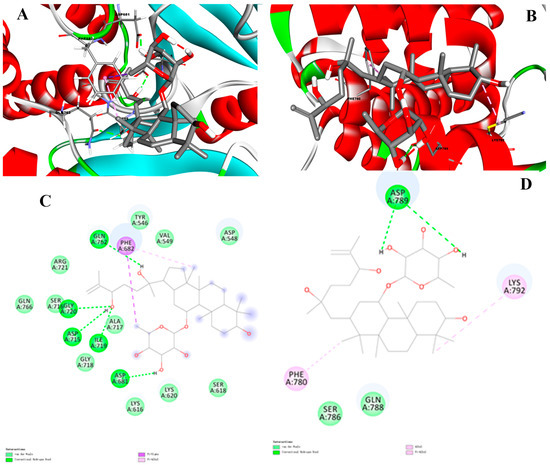
Figure 3.
Catalytic site of three-dimensional (A) and two-dimensional (C) visualization of compound 10 against PTP1B; Allosteric sites of three-dimensional (B) and two-dimensional (D) visualization of compound 10 against PTP1B. Conventional hydrogen bond as green, Pi-sigma as purple, Pi-alkyl and alkyl interactions as light pink, and van der Waals as light green.
2.3. EtOAc Extract and Dammarane Saponins Enhance Glucose Uptake
The 3T3-L1 preadipocyte is one of the most commonly used in vitro models for screening antidiabetic compounds [34,35]. In the present study, the enhancement of glucose uptake by the EtOAc extract (GAE) and triterpenoid saponins (1–12) of C. paliurus was investigated using a 2-(N-(7-nitrobenz-2-oxa-1,3-diazol-4-yl)amino)-2-deoxy glucose (2-NBDG) uptake model.
Firstly, the cell viability was measured using the MTT assay [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]. The 3T3-L1 adipocyte cell viability test showed the extract at a glucose: 2-NBDG. Isolated compounds and rosiglitazone (ROS, the positive control) were treated to differentiate concentrations of 25 μg/mL; the triterpenoid saponins (1–12) at a concentration of 10 μM were not cytotoxic and the cells had a survival rate of up to 90%.
Thus, the extract of C. paliurus leaves (25 μg/mL) and isolated triterpenoid saponins (1–12, 10 μM) were added to differentiated 3T3-L1 adipocytes with 2-NBDG. As shown in Figure 4A, all isolated triterpenoid saponins (1–12) exhibited potency to enhance glucose uptake in 3T3-L1 adipocytes. Among the isolates, compounds 6–7 and 10 at 10 μM increased insulin-stimulated glucose uptake by approximately 37%, 35%, and 46% in 3T3-L1 adipocytes, respectively. The positive control rosiglitazone (ROS, 10 μM) increased glucose uptake in the 3T3-L1 adipocytes by approximately 55%. These triterpene saponins enhance glucose uptake in 3T3-L1 adipocytes more effectively than those of the extract of C. paliurus leaves. The active 6–7 and 10 at various concentrations (2.5, 5, and 10 μM) were further investigated for their effect on glucose uptake. As shown in Figure 4B, 6–7 and 10 significantly increase glucose uptake in the 3T3-L1 adipocytes compared with the vehicle control, which indicates that these compounds may enhance glucose uptake by improving insulin sensitivity in a concentration-dependent manner.
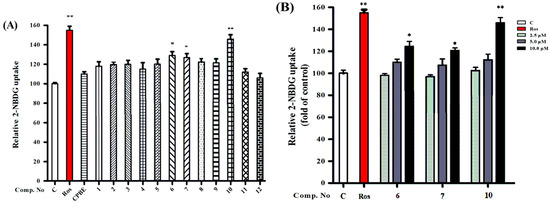
Figure 4.
(A) The glucose uptake effects of compounds 1–12 on 3T3-L1 adipocytes using a fluorescent derivate of cells at 10 μM. (B) Compounds 6–7 and 10 increased glucose uptake in 3T3-L1 adipocytes. The differentiated 3T3-L1 adipocytes were treated with compounds 6–7 and 10 at various concentrations (2.5, 5.0, and 10 μM) for 1 h. These results are expressed as the mean ± SD (n = 3) of triplicate analysis; * p < 0.05 and ** p < 0.01.
3. Materials and Methods
3.1. General Experimental Procedures
Silica gel (200–300 mesh, Qingdao Marine Chemical Co., Ltd., Qingdao, China), reversed-phase C18 (50 μm, Merck, Rahway, NJ, USA), and Sephadex LH-20 (Amersham Pharmacia Biotech, Amersham, UK) columns were used for chromatographic separations. L-Arabinose, D-arabinose, L-glucose, D-glucose, L-quinovose and D-quinovose were used as sugar standards (Sigma, Munich, Germany). All solvents were of HPLC or analytical grade. Agilent 1260 semipreparative HPLC (ODS-A, 8 μm, 250 ×10.0 mm, YMC, Kyoto, Japan).
3.2. Plant Material
Dried leaves (10 kg) of C. paliurus were obtained from Xiushui County, Jiangxi, China, in July 2020 and identified by Associate Professor Qiang Xie (Guangxi Normal University). A voucher specimen (No. ID-202070910) was deposited at the State Key Laboratory of Guangxi Normal University (GXNU), China.
3.3. Computational Analysis of Molecular Docking Simulation
Molecular docking was used to investigate the interactions between triterpenoids and PTP1B. The structure of PTP1B (PDB ID: 1EEO [36]) was obtained from the Online Protein Data Bank and the structures of the ligands were downloaded from Pubchem. AutoDock tools (ADT, version: 1.5.6) to explore the binding mode between PTP1B and the ligands. AutoDock vina (version 1.2.3) calculated scoring function and predicted binding affinity (kcal/mol), while Pymol (version 2.2.0) was used to analyze the visualization of the docking results.
3.4. Extraction and Separation of Dammarane Saponins
The leaves of C. paliurus (10 kg) were purchased from Liuhe medicine market of Guilin city in October 2020. The leaves were extracted 3 times with a 75% ethanol–H2O mixture (3:1, v/v, 20 L) under reflux and concentrated under vacuum to remove the ethanol, obtaining a crude extract (2.5 kg). The crude extract (2.5 kg) was suspended in distilled water and partitioned with polyethylene (PE), ethyl acetate (EtOAc), and n-butanol (n-BuOH) to obtain a PE extract (480 g), EtOAc extract (450 g), and n-BuOH extract (1090 g). The glucose uptake activity of the sub-extracts in 3T3-L1 adipocytes was assessed.
The active n-BuOH fraction (1090 g) was chromatographed on a silica gel column eluted with a gradient of increasing methanol content (0–100%) in a mixture with dichloromethane to yield 8 fractions (Fr. I to Fr. VIII). The active Fr. III (220 g) was subjected to C18 column chromatography (H2O/MeOH 50:50–10:90) to provide nine fractions (Fr. III-1 to Fr. III-9). Fr. III-2 (2.3 g) was fractioned by an HW-40F column (H2O/MeOH, 100:0−0:100) to obtain four subfractions (Fr. III-2-1 to Fr. III-2-7). Fr. III-2-1 (1000 mg) was isolated with a C18 column, followed by C18 semipreparative HPLC (H2O-MeCN, 34:66, v/v, 3.0 mL/min), to yield 1 (tR 28.43 min, 10.7 mg), 2 (tR 18.59 min, 3.2 mg), and 3 (tR 22.68 min, 5.1 mg). Fr. III-3 (4.0 g) was separated by silica gel (CH2Cl2-MeOH, 100:0–0:100) to obtain nine subfractions (Fr. III-3-1 to Fr. III-3-9). Fr. III-3-6 was purified by Sephadex LH-20 (MeOH) and C18 semipreparative HPLC (H2O-MeCN, 58:42, v/v, 3.0 mL/min) to obtain 4 (tR 15.10 min, 3.5 mg), 11 (tR 35.46 min, 6.8 mg) and 12 (tR 45.12 min, 2.7 mg). Fr. III-6 (3.8 g) was further chromatographed by silica gel CC eluted with CH2Cl2-MeOH (12:1, 10:1, 6:1) to 100% to afford three subfractions (Fr. III-6-1 to Fr. III-6-3). Fr. III-6-2 was subjected to Sephadex LH-20 CC, eluted with MeOH, and then further purified by C18 preparative HPLC (H2O-MeOH, 15:85, v/v, 3.0 mL/min) to afford compounds 7 (tR 25.35 min, 3.0 mg), 9 (tR 13.05 min, 3.7 mg), and 10 (tR 16.89 min, 2.0 mg). Fr. III-6-3 was isolated by Sephadex LH-20, eluted with MeOH, and then purified by C18 preparative HPLC (H2O-MeOH, 15:85, v/v, 3.0 mL/min) to obtain compounds 5 (tR 16.89 min, 2.0 mg), 6 (tR 16.78 min, 3.5 mg), and 8 (tR 22.23 min, 10.3 mg).
3.5. Characterization of the Isolates
Cypaliuruside Z1 (1): white amorphous powder; = −18.72 (c 0.30, MeOH); HRESIMS m/z 717.3811 [M + Na]+ (calculated for C37H58O12Na, 717.3826); for the 1H (pyridine-d5, 500 MHz) and 13C NMR (pyridine-d5, 125 MHz) data, see Table 1. All significant data are given in the electronic supporting information materials (Figures S1–S8).
Cypaliuruside Z2 (2): white amorphous powder;
= −20.03 (c 0.22, MeOH); HRESIMS m/z 749.4075 [M + Na]+ (calculated for C38H62O13Na, 749.4088); for the 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) data, see Table 1. All significant data are given in the electronic supporting information materials (Figures S9–S16).
Cypaliuruside Z3 (3): white amorphous powder; = −20.94 (c 0.22, MeOH); HRESIMS m/z 749.4096 [M + Na]+ (calculated for C38H62O13Na, 749.4088); for the 1H (pyridine-d5, 500 MHz) and 13C NMR (pyridine-d5, 125 MHz) data, see Table 2. All significant data are given in the electronic supporting information materials (Figures S17–S24).
Cypaliuruside Z4 (4): white amorphous powder; = −19.89 (c 0.25, MeOH); HRESIMS m/z 733.4146 [M + Na]+ (calculated for C38H62O12Na, 733.4139); for the 1H (pyridine-d5, 600 MHz) and 13C NMR (pyridine-d5, 150 MHz) data, see Table 2. All significant data are given in the electronic supporting information materials (Figures S25–S32).
Cyclocarioside L (5): white amorphous powder. 1H NMR (pyridine-d5, 600 MHz) δH 2.98 (1H, m, H-1a), 2.01 (1H, m, H-1b), 2.01 (1H, m, H-2a), 1.89 (1H, m, H-2b), 3.50 (1H, m, H-3), 1.53 (1H, d, J = 11.0 Hz, H-5), 1.63 (1H, m, H-6a), 1.45 (1H, m, H-6b), 1.39 (1H, m, H-7a), 1.08 (1H, d, J = 9.2 Hz, H-7b), 4.16 (1H, m, H-12), 0.92 (3H, s, H-18), 1.35 (3H, s, H-19), 1.45 (3H, s, H-21), 0.93 (3H, s, H-28), 1.23 (3H, s, H-29), 0.65 (3H, s, H-30), 5.45 (1H, d, J = 7.0 Hz, H-1′), 4.75 (1H, dd, J = 3.1, 8.5 Hz, H-4′), 5.10 (1H, d, J = 7.5 Hz, H-1″), 1.65 (3H, d, J = 7.3 Hz, H-6″). 13C NMR (pyridine-d5, 150 MHz) δC 37.1 (C-1), 22.4 (C-2), 80.5 (C-3), 38.9 (C-4), 50.7 (C-5), 19.3 (C-6), 36.0 (C-7), 50.6 (C-8), 57.7 (C-9), 40.3 (C-10), 34.2 (C-11), 76.1 (C-12), 41.6 (C-13), 40.5 (C-14), 31.7 (C-15), 25.7 (C-16), 50.1 (C-17), 17.0 (C-18), 17.1 (C-19), 89.6 (C-20), 21.4 (C-21), 32.1 (C-22), 30.3 (C-23), 176.2 (C-24), 23.6 (C-28), 30.4 (C-29), 16.8 (C-30), 102.4 (C-1′), 72.3 (C-2′), 75.4 (C-3′), 69.4 (C-4′), 69.8 (C-5′), 101.5 (C-1″), 75.1 (C-2″), 78.7 (C-3″), 78.1 (C-4″), 72.1 (C-5″), 18.9 (C-6″).
Cypaliuruside M (6): white amorphous powder. 1H NMR (pyridine-d5, 600 MHz) δH 3.12 (1H, m, H-1a), 2.11 (1H, m, H-1b), 1.97 (1H, m, H-2a), 1.59 (1H, m, H-2b), 3.64 (1H, t, J = 2.7 Hz, H-3), 1.68 (1H, m, H-5), 1.53 (1H, m, H-6a), 1.45 (1H, m, H-6b), 1.49 (1H, m, H-7a), 1.28 (1H, m, H-7b), 4.26 (1H, dt, J = 4.7, 10.5 Hz, H-11), 1.12 (3H, s, H-18), 1.45 (3H, s, H-19), 1.15 (3H, s, H-21), 1.45 (3H, s, H-26), 1.43 (3H, s, H-27), 1.03 (3H, s, H-28), 1.33 (3H, s, H-29), 0.65 (3H, s, H-30), 4.65 (1H, d, J = 7.5 Hz, H-1′), 4.21 (1H, dd, J = 2.1, 8.5 Hz, H-3′), 4.25 (1H, dd, J = 5.1, 9.5 Hz, H-4′), 4.35 (1H, dd, J = 5.1, 11.5 Hz, H-5′), 1.59 (3H, d, J = 6.7 Hz, H-6′), 5.05 (1H, d, J = 7.7 Hz, H-1″), 4.05 (1H, d, J = 5.3 Hz, H-2″), 4.10 (1H, dd, J = 5.1, 11.5 Hz, H-3″), 1.68 (3H, d, J = 5.3 Hz, H-6″). 13C NMR (pyridine-d5, 150 MHz) δC 36.1 (C-1), 21.4 (C-2), 81.5 (C-3), 38.9 (C-4), 50.9 (C-5), 18.3 (C-6), 36.7 (C-7), 50.6 (C-8), 54.7 (C-9), 40.6 (C-10), 76.2 (C-11), 34.1 (C-12), 41.6 (C-13), 40.8 (C-14), 31.4 (C-15), 26.7 (C-16), 50.1 (C-17), 17.2 (C-18), 17.0 (C-19), 86.2 (C-20), 24.0 (C-21), 34.1 (C-22), 26.3 (C-23), 84.2 (C-24), 71.2 (C-25), 26.1 (C-26), 28.4 (C-27), 23.6 (C-28), 30.4 (C-29), 16.8 (C-30), 101.4 (C-1′), 75.3 (C-2′), 78.4 (C-3′), 77.4 (C-4′), 72.8 (C-5′), 18.8 (C-6′), 102.5 (C-1″), 75.3 (C-2″), 78.7 (C-3″), 77.6 (C-4″), 73.1 (C-5″), 18.9 (C-6″).
Cyclocarioside J (7): white amorphous powder. 1H NMR (pyridine-d5, 600 MHz) δH 3.11 (1H, m, H-1a), 1.98 (1H, m, H-1b), 1.87 (1H, m, H-2a), 1.69 (1H, m, H-2b), 3.65 (1H, t, J = 3.7 Hz, H-3), 1.65 (1H, m, H-5), 1.48 (1H, m, H-6a), 1.49 (1H, m, H-6b), 4.33 (1H, dt, J = 5.3, 10.8 Hz, H-11), 1.04 (3H, s, H-18), 1.42 (3H, s, H-19), 1.10 (3H, s, H-21), 1.55 (3H, s, H-26), 1.39 (3H, s, H-27), 0.98 (3H, s, H-28), 1.30 (3H, s, H-29), 0.67 (3H, s, H-30), 4.75 (1H, d, J = 6.8 Hz, H-1′), 4.42 (1H, t, J = 7.6 Hz, H-2′), 4.21 (1H, dd, J = 2.1, 8.5 Hz, H-3′), 4.25 (1H, dd, J = 5.1, 9.5 Hz, H-4′), 4.35 (1H, m, H-5a′), 3.78 (1H, dd, J = 11.0, 13.4 Hz, H-5b′), 5.01 (1H, d, J = 7.1 Hz, H-1″), 4.12 (1H, m, H-2″), 4.21 (1H, dd, J = 4.8, 10.9 Hz, H-3″), 1.65 (3H, d, J = 5.7 Hz, H-6″). 13C NMR (pyridine-d5, 150 MHz) δC 36.2 (C-1), 21.5 (C-2), 81.5 (C-3), 38.1 (C-4), 51.2 (C-5), 18.4 (C-6), 36.5 (C-7), 50.1 (C-8), 54.4 (C-9), 40.2 (C-10), 76.5 (C-11), 34.3 (C-12), 41.2 (C-13), 41.2 (C-14), 31.2 (C-15), 26.9 (C-16), 49.9 (C-17), 17.0 (C-18), 16.9 (C-19), 85.8 (C-20), 24.4 (C-21), 34.5 (C-22), 25.8 (C-23), 84.0 (C-24), 71.1 (C-25), 26.1 (C-26), 28.6 (C-27), 23.3 (C-28), 30.4 (C-29), 16.6 (C-30), 103.4 (C-1′), 72.3 (C-2′), 75.4 (C-3′), 70.0 (C-4′), 67.8 (C-5′), 102.2 (C-1″), 76.0 (C-2″), 78.7 (C-3″), 77.1 (C-4″), 73.0 (C-5″), 19.0 (C-6″).
Cyclocarioside Z18 (8): white amorphous powder. 1H NMR (pyridine-d5, 600 MHz) δH 3.05 (1H, m, H-1a), 1.84 (1H, m, H-1b), 1.77 (1H, m, H-2a), 1.67 (1H, m, H-2b), 3.55 (1H, br s, H-3), 1.56 (1H, m, H-5), 1.58 (1H, m, H-6a), 1.44 (1H, m, H-6b), 4.43 (1H, dt, J = 5.0, 10.5 Hz, H-11), 1.05 (3H, s, H-18), 1.32 (3H, s, H-19), 1.45 (3H, s, H-21), 1.35 (3H, s, H-26), 1.35 (3H, s, H-27), 0.98 (3H, s, H-28), 1.26 (3H, s, H-29), 0.80 (3H, s, H-30), 5.51 (1H, br s, H-1′), 4.45 (1H, m, H-2′), 4.63 (1H, m, H-3′), 4.75 (1H, m, H-4′), 4.61 (1H, m, H-5a′), 4.28 (1H, m, H-5b′), 5.11 (1H, d, J = 7.5 Hz, H-1″), 4.02 (1H, d, J = 8.5 Hz, H-2″), 4.26 (1H, m, H-3″), 4.03 (1H, dd, J = 4.1, 8.4 Hz, H-4″), 4.17 (1H, t, J = 8.9 Hz, H-5″), 4.65 (H, d, J = 11.2 Hz, H-6a″), 4.35 (H, m, H-6b″). 13C NMR (pyridine-d5, 150 MHz) δC 36.2 (C-1), 21.6 (C-2), 79.5 (C-3), 38.1 (C-4), 51.2 (C-5), 18.6 (C-6), 36.5 (C-7), 50.1 (C-8), 54.4 (C-9), 40.7 (C-10), 77.5 (C-11), 34.3 (C-12), 41.4 (C-13), 41.6 (C-14), 31.7 (C-15), 26.8 (C-16), 49.6 (C-17), 17.2 (C-18), 16.9 (C-19), 86.8 (C-20), 24.5 (C-21), 34.5 (C-22), 26.8 (C-23), 84.5 (C-24), 71.1 (C-25), 26.1 (C-26), 28.3 (C-27), 23.3 (C-28), 30.4 (C-29), 16.9 (C-30), 106.4 (C-1′), 84.5 (C-2′), 80.0 (C-3′), 81.2 (C-4′), 65.8 (C-5′), 102.2 (C-1″), 76.0 (C-2″), 78.9 (C-3″), 78.1 (C-4″), 73.0 (C-5″), 19.6 (C-6″).
Cyclocarioside Z10 (9): white amorphous powder. 1H NMR (pyridine-d5, 400 MHz) δH 3.08 (1H, dd, J = 2.7, 9.2 Hz, H-1a), 2.12 (1H, m, H-1b), 2.11 (1H, m, H-2a), 1.79 (1H, m, H-2b), 3.63 (1H, m, H-3), 1.83 (1H, m, H-5), 1.53 (1H, m, H-6a), 1.45 (1H, m, H-6b), 4.46 (1H, dt, J = 5.7, 9.9 Hz, H-11), 1.12 (3H, s, H-18), 1.38 (3H, s, H-19), 1.44 (3H, s, H-21), 5.30 (1H, s, H-26a), 4.95 (1H, s, H-26b), 1.90 (3H, s, H-27), 0.98 (3H, s, H-28), 1.23 (3H, s, H-29), 0.85 (3H, s, H-30), 4.95 (1H, d, J = 7.5 Hz, H-1″), 3.97 (1H, t, J = 8.5 Hz, H-2″), 2.00 (3H, s, CH3COO), 4.96 (1H, br s, H-1′), 4.05 (1H, m, H-2′), 3.89 (1H, dd, J = 6.3, 7.5 Hz, H-3′), 4.75 (1H, m, H-4′), 4.21 (1H, dd, J = 3.3, 11.5 Hz, H-5a′), 4.28 (1H, dd, J = 6.3, 11.3 Hz, H-5b′), 4.40 (1H, d, J = 7.7 Hz, H-1″), 3.10 (1H, dd, J = 7.7, 9.0 Hz, H-2″), 3.35 (1H, dd, J = 8.7,9.0 Hz, H-3″), 2.98 (1H, dd, J = 2.7, 8.7 Hz, H-4″), 3.24 (1H, m, H-5″), 1.24 (3H, d, J = 5.8 Hz, H-6″). 13C NMR (pyridine-d5, 100 MHz) δC 37.1 (C-1), 26.3 (C-2), 79.5 (C-3), 38.2 (C-4), 51.0 (C-5), 18.7 (C-6), 36.6 (C-7), 41.6 (C-8), 54.7 (C-9), 40.3 (C-10), 34.2 (C-11), 76.9 (C-12), 41.6 (C-13), 50.0 (C-14), 31.7 (C-15), 27.7 (C-16), 50.1 (C-17), 17.1 (C-18), 16.8 (C-19), 86.6 (C-20), 24.4 (C-21), 34.1 (C-22), 26.3 (C-23), 84.2 (C-24), 71.5 (C-25), 26.5 (C-26), 26.4 (C-27), 29.6 (C-28), 22.4 (C-29), 16.9 (C-30), 106.4 (C-1′), 83.3 (C-2′), 79.4 (C-3′), 82.0 (C-4′), 65.8 (C-5′), 170.9 (CH3COO), 100.9 (C-1″), 75.1 (C-2″), 78.2 (C-3″), 77.1 (C-4″), 72.1 (C-5″), 18.3 (C-6″).
Cyclocarioside H (10): white amorphous powder. 1H NMR (pyridine-d5, 400 MHz) δH 2.58 (1H, dd, J = 3.7, 13.2 Hz, H-1a), 1.34 (1H, m, H-1b), 4.06 (1H, m, H-12), 1.10 (3H, s, H-18), 1.18 (3H, s, H-19), 1.14 (3H, s, H-21), 3.74 (1H, d, J = 7.5 Hz, H-24), 1.15 (3H, s, H-26), 1.19 (3H, s, H-27), 0.98 (3H, s, H-28), 0.90 (3H, s, H-29), 0.95 (3H, s, H-30), 4.95 (1H, d, J = 7.5 Hz, H-1″), 4.08 (1H, t, J = 8.5 Hz, H-2″), 4.08 (1H, d, J = 8.7 Hz, H-3″), 3.65 (1H, dd, J = 2.7, 8.7 Hz, H-4″), 3.60 (1H, d, J = 5.9 Hz, H-5″), 1.60 (3H, d, J = 5.8 Hz, H-6″). 13C NMR (pyridine-d5, 100 MHz) δC 36.1 (C-1), 26.4 (C-2), 75.5 (C-3), 38.9 (C-4), 50.9 (C-5), 19.0 (C-6), 36.0 (C-7), 50.6 (C-8), 54.7 (C-9), 40.3 (C-10), 77.2 (C-11), 35.0 (C-12), 41.6 (C-13), 42.0 (C-14), 31.7 (C-15), 27.7 (C-16), 50.1 (C-17), 17.5 (C-18), 17.1 (C-19), 74.6 (C-20), 27.4 (C-21), 38.1 (C-22), 31.3 (C-23), 76.2 (C-24), 150.1 (C-25), 110.5 (C-26), 18.9 (C-27), 23.6 (C-28), 30.4 (C-29), 16.9 (C-30), 101.9 (C-1″), 76.1 (C-2″), 78.7 (C-3″), 77.1 (C-4″), 73.1 (C-5″), 18.9 (C-6″).
2α,3α,23-trihydroxy-12,20(30)-dien-28-ursolic acid 28-O-β-D-glucopyranoside (11): 1H NMR (MeOD, 600 MHz) δH 5.28 (1H, t, J = 3.5 Hz, H-12), 4.71 (1H, br s, H-30a), 4.61 (1H, br s, H-30b), 3.78 (1H, m, H-23a), 3.70 (1H, dd, J = 11.6, 4.6 Hz, H-23b), 1.23 (s, 3H, H-26), 1.01 (3H, s, H-27), 0.99 (3H, s, H-25), 0.82 (3H, s, H-29), 0.80 (3H, s, H-24), 5.26 (1H, d, J = 8.4 Hz, H-1′), 3.85 (1H, m, H-6′a), 3.67 (1H, m, H-6′b). 13C NMR (MeOD, 150 MHz) δC 43.4 (C-1), 68.9 (C-2), 78.0 (C-3), 42.4 (C-4), 44.2 (C-5), 19.3 (C-6), 33.5 (C-7), 41.0 (C-8), 49.3 (C-9), 38.9 (C-10), 24.5 (C-11), 127.2 (C-12), 139.3 (C-13), 42.1 (C-14), 29.0 (C-15), 25.2 (C-16), 49.6 (C-17), 56.3 (C-18), 38.5 (C-19), 154.3 (C-20), 33.2 (C-21), 39.5 (C-22), 71.0 (C-23), 17.7 (C-24), 17.2 (C-25), 17.5 (C-26), 24.0 (C-27), 177.1 (C-28), 16.5 (C-29), 105.2 (C-30), 95.5 (C-1′), 74.1 (C-2′), 78.6 (C-3′), 71.3 (C-4′), 78.5 (C-5′), 62.0 (C-6′).
1-oxo-3β,23-dihydroxy-olean-12-en-28-oicacid-28-O-β-D-glucopyranosside (12): colorless amorphous power. 1H NMR (MeOD, 400 MHz) δH 5.15 (1H, t, J = 4.5 Hz, H-12), 3.72 (1H, m, H-3), 1.20 (3H, s, H-27), 0.93 (3H, s, H-30), 0.92 (3H, s, H-29), 0.85 (6H, s, H-24, 26), 5.33 (1H, d, J = 8.0 Hz, H-1′), 3.48–3.69 (6H, m, H-2′, H-3′, H-4′, H-5′, H-6′). 13C NMR (MeOD, 100 MHz) δC 215.0 (C-1), 44.6 (C-2), 73.4 (C-3), 44.1 (C-4), 47.4 (C-5), 18.3 (C-6), 33.2 (C-7), 40.7 (C-8), 40.3 (C-9), 53.1 (C-10),26.2 (C-11), 124.2 (C-12), 144.1 (C-13), 43.3 (C-14), 28.9 (C-15), 24.2 (C-16), 48.3 (C-17), 43.0 (C-18), 47.1 (C-19), 31.6 (C-20), 35.1 (C-21), 33.3 (C-22), 65.7 (C-23), 13.3 (C-24), 15.8 (C-25), 18.2 (C-26), 26.2 (C-27), 178.0 (C-28), 33.0 (C-29), 23.9 (C-30), 95.6 (C-1′), 78.3 (C-5′), 78.0 (C-3′), 73.7 (C-2′), 71.1 (C-4′), 62.5 (C-6’).
3.6. Hydrolyses of Novel Compounds to Determine the Linking Sugars
Each new isolate was respectively dissolved in 1 M hydrochloric acid (HCl) and refluxed for 2 h at 80 °C. The reaction solutions were then fractionated with ethyl acetate (EtOAc, 3 times). The remaining aqueous phase was concentrated under vacuum to remove water (H2O). Analytical HPLC was conducted on a Waters 2695 instrument (Waters Corporation, Milford, MA, USA) using Waters 2424 ELSD as a detector. A GH0525046C18AQ column (5 μm, 4.6 × 250 mm, Sil Green) was used, which used formic acid: water = 0.1: 100 (v/v) as mobile phase. The column temperature was 35 °C. The HPLC spectrum of the standards of L-arabinose, D-glucopyranose and D-quinovose are shown in the Supporting Information as Figure S33–S35. The existence of L-arabinose was determined by HPLC test with an authentic sugar in the aqueous phase of compound Z1 (1) (Figure S36), while D-glucopyranose and the L-arabinose were determined by HPLC test with two authentic sugars in the aqueous phase of compounds Z2 (2) and Z3 (3) (Figure S37). The presence of L-arabinose and D-quinovose was found by HPLC test with standard sugars in the water phase of compound Z4 (4) (Figure S38).
3.7. Assay for the Glucose Uptake Effects of the Fraction and Dammarane Saponins
3.7.1. Cell Viability Assay
The cell viability assay was determined by the 3-(4,5-dimethyl-2-thiazolyl) -2,5-diphenyl-2H-tetrazolium bromide (MTT) (Sigma-Aldrich). The 3T3-L1 adipocytes were treated at a density of 3000 cells/well in 96-well plates in DMEM with 10% FBS. After 24 h of incubation, the cells were exposed to the test extract (CPBE, 25 μg/mL) and compounds (10 μM), which were dissolved in serum-free DMEM for 24 h. Each well was filled with twenty microliters of 2 mg/mL MTT solution and incubated for 4 h at 37 ℃ in the dark. The reduction product, a formazan, was dissolved with DMSO and the absorbance was measured at 550 nm with a microplate reader.
3.7.2. Differentiation of 3T3-L1 Adipocytes
3T3-L1 cells were purchased from Cell Culture Center, Institute of Basic Medical Sciences, Institute of Basic Medicine, Chinese Academy of Medical Sciences (Batch number 1101MOU-PUMC 0001551). The cells were cultured in DMEM with 10% calf serum, 100 U/mL penicillin, and 100 mg/mL streptomycin (HyClone) in 5% CO2 at 37 ℃. The growth media was changed to DMEM with 10% fetal bovine serum (HyClone) containing 1 μM dexamethasone (Sigma-Aldrich, St. Louis, MO, USA), 520 μM 3-isobutyl-1-methylxanthine (Sigma-Aldrich), and 1 μg/mL insulin (Beijing, China, Roche, Germany) after two days. After 48 h, the cells were grown in DMEM with 10% FBS, 1 μg/mL insulin, 100 U/mL penicillin, and 100 mg/mL streptomycin for 2 days of incubation. Every two days, the medium was replenished with fresh DMEM supplemented with 10% FBS medium until adipogenesis was induced.
3.7.3. Measurement of Glucose Uptake Assay
The level of glucose uptake was assessed using a fluorescent derivative of glucose, 2-NBDG (Beijing, China, Invitrogen, OR, USA). The fully differentiated 3T3-L1 adipocytes were seeded on 96-well plates with glucose-free medium containing 10% FBS and 1 μg/mL insulin. After 24 h of incubation, the cells were treated with test extract (CPBE, 25 μg/mL) as well as compounds (10 μM) and rosiglitazone (ROS, 10 μM, as a positive control) in the presence or absence of 50 μM 2-NBDG. The cells were rinsed with phosphate-buffered saline (PBS) after 1 h of incubation, while cell lysates were treated with 70 μL of 1% Triton X-100 in PBS and 0.1 M K3PO4 for 10 min. To quantify 2-NBDG fluorescence, the fluorescence signal was measured at an excitation wavelength of 450 nm and an emission wavelength of 535 nm using a SpectraMax M5 microplate reader. The 2-NBDG signal was determined using a fluorescence microscope (Olympus ix70, Tokyo, Japan) to detect glucose uptake.
4. Conclusions
To identify compounds for promoting glucose uptake, the components of C. paliurus leaves were separated, resulting in the identification of twelve triterpenoid saponins (1–12), including four previously undescribed dammarane triterpenoid saponins. The docking study demonstrated that the most active compound strongly bonded with PTP1B, hydrogen bonds and hydrophobic interactions, verifying the importance of the sugar unit. Bioassays demonstrated that the dammarane triterpenoid saponins 6–7 and 10 strongly enhanced insulin-stimulated glucose uptake in 3T3-L1 adipocytes in a dose-dependent manner. Collectively, C. paliurus leaves contain abundant dammarane triterpenoid saponins that affect glucose uptake in 3T3-L1 adipocytes; this discovery could be meaningful for the development of new treatments for insulin resistance and hyperglycemia.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28083294/s1. Figure S1. HRESIMS spectrum of compound 1. Figure S2. 1H NMR spectrum of compound 1 (600 MHz, pyridine-d5). Figure S3. 13C NMR spectrum of compound 1 (150 MHz, pyridine-d5). Figure S4. DEPT spectrum of compound 1 in pyridine-d5. Figure S5. HSQC spectrum of compound 1 in pyridine-d5. Figure S6. 1H-1H COSY spectrum of compound 1 in pyridine-d5. Figure S7. HMBC spectrum of compound 1 in pyridine-d5. Figure S8. ROESY spectrum of compound 1 in pyridine-d5. Figure S9. HRESIMS spectrum of compound 2. Figure S10. 1H NMR spectrum of compound 2 (600 MHz, pyridine-d5). Figure S11. 13C NMR spectrum of compound 2 (150 MHz, pyridine-d5). Figure S12. DEPT spectrum of compound 2 in pyridine-d5. Figure S13. HSQC spectrum of compound 2 in pyridine-d5. Figure S14. 1H-1H COSY spectrum of compound 2 in pyridine-d5. Figure S15. HMBC spectrum of compound 2 in pyridine-d5. Figure S16. ROESY spectrum of compound 2 in pyridine-d5. Figure S17. HRESIMS spectrum of compound 3. Figure S18. 1H NMR spectrum of compound 3 (600 MHz, pyridine-d5). Figure S19. 13C NMR spectrum of compound 3 (150MHz, pyridine-d5). Figure S20. DEPT spectrum of compound 3 in pyridine-d5. Figure S21. HSQC spectrum of compound 3 in pyridine-d5. Figure S22. 1H-1H COSY spectrum of compound 3 in pyridine-d5. Figure S23. HMBC spectrum of compound 3 in pyridine-d5. Figure S25. HRESIMS spectrum of compound 4. Figure S26. 1H NMR spectrum of compound 4 (600 MHz, pyridine-d5). Figure S27. 13C NMR spectrum of compound 4 (150MHz, pyridine-d5). Figure S28. DEPT spectrum of compound 4 in pyridine-d5. Figure S29. HSQC spectrum of compound 4 in pyridine-d5. Figure S30. 1H-1H COSY spectrum of compound 4 in pyridine-d5. Figure S31. HMBC spectrum of compound 4 in pyridine-d5. Figure S32. ROESY spectrum of compound 4 in pyridine-d5. Figure S33. The HPLC spectrum of the standard of L-arabinose. Figure S34. The HPLC spectrum of the standard of D-glucopyranose. Figure S35. The HPLC spectrum of the standard of D-quinovose. Figure S36. The HPLC spectrum of the compound 1 of L-arabinose. Figure S37. The HPLC spectrum of the compound 2 and 3 of D-glucopyranose and L-arabinose. Figure S38. The HPLC spectrum of the compound 4 of L-arabinose and D-quinovose.
Author Contributions
J.L. conceived, designed and authored the article; W.X. and Y.H. analyzed the data; S.D. contributed the samples/reagents/materials/analysis tools; Data Curation, X.L., Y.C., P.H. and C.R.; writing—review and editing, R.Y. and R.H.; X.L., L.P. and K.L. performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was financially supported by National Natural Science Foundation (32060097, 3206020108); The Natural Science Foundation of Guangxi Province (2018GXNSFDA050007); Guanxi Key Laboratory of Traditional Chinese Medicine Quality Standards (grant number GZZK202001, GZZK201804); The Open Research Fund program of the Key Laboratory for the Chemistry and Molecular Engineering of Medicinal Resources (CMEMR2020-A04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of the article can be obtained from the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not available.
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.Q.; Niu, P.; Li, J.; Guan, X.L.; Zhang, Y.J.; Li, J. Discovery of atnti-inflammatory triterpenoid glucosides from the Heritiera littoralis dryand. Molecules 2023, 28, 1658. [Google Scholar] [CrossRef] [PubMed]
- Su, H.G.; Liang, H.F.; Hu, G.L.; Zhou, L.; Peng, X.R.; Bi, H.C.; Qiu, M.H. Applanoids A-E as the first examples of C-15/C-20 Michael adducts in Ganoderma triterpenoids and their PXR agonistic activity. Chin. J. Chem. 2022, 40, 2633–2641. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, B.; Wang, Z.L.; Qiao, X.; Ye, M. Terpenoids from the medicinal mushroom Antrodia camphorata: Chemistry and medicinal potential. Nat. Prod. Rep. 2021, 38, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Bruder, M.; Polo, G.; Trivella, D.B.B. Natural allosteric modulators and their biological targets: Molecular signatures and mechanisms. Nat. Prod. Rep. 2020, 37, 488–514. [Google Scholar] [CrossRef]
- Cao, J.Q.; Zhang, X.S.; Qu, F.Z.; Guo, Z.H.; Zhao, Y.Q. Dammarane triterpenoids for pharmaceutical use: A patent review (2005–2014). Expert Opin. Ther. Pat. 2015, 25, 805–817. [Google Scholar] [CrossRef]
- Fan, W.X.; Huang, Y.L.; Zheng, H.; Li, S.Q.; Li, Z.H.; Yuan, L.; Cheng, X.; He, C.S.; Sun, J.F. Ginsenosides for the treatment of metabolic syndrome and cardiovascular diseases: Pharmacology and mechanisms. Biomed. Pharmacother. 2020, 132, 110915. [Google Scholar] [CrossRef]
- Xu, B.L.; Li, Z.L.; Zeng, T.; Zhan, J.F.; Wang, S.Z.; Ho, C.T.; Li, S.M. Bioactives of Momordica charantia as potential anti-diabetic/hypoglycemic agents. Molecules 2022, 27, 2175. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.Q.; Chang, Y.L.; Huang, Y.; Pan, L.W. Review on the constituents and pharmacological activities of Cyclocarya paliurus. J. Guangxi Normal Univ. (Nat. Sci. Ed.) 2022, 40, 227–252. [Google Scholar] [CrossRef]
- Chen, Z.L.; Jian, Y.Q.; Wu, Q.; Wu, J.; Sheng, W.B.; Jiang, S.; Shehla, N.; Aman, S.; Wang, W. Cyclocarya paliurus (Batalin) Iljinskaja: Botany, ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2022, 285, 114912. [Google Scholar] [CrossRef]
- Jiang, C.H.; Wang, Y.T.; Jin, Q.M.; Zhang, D.J.; Gao, M.; Yao, N.; Yin, Z.Q.; Zhang, J.; Ma, S.P. Cyclocarya paliurus triterpenoids improve diabetes-induced hepatic inflammation via the Rho-kinase-dependent pathway. Front. Pharmacol. 2019, 10, 811. [Google Scholar] [CrossRef]
- Li, J.; Zeng, W.W.; Wu, Y.X.; Zhang, Q.; Luo, M.; Zhu, Y.H.; Yang, X.L.; Guo, A.Y. Integrating transcriptome and experiments reveals the antidiabetic mechanism of Cyclocarya paliurus formula. Mol. Ther. Nucl. Acids. 2018, 13, 419–430. [Google Scholar] [CrossRef]
- Li, J.; Yang, X.L.; Zhu, Y.H.; Luo, M.; Guo, A.Y.; Zhang, Q. Investigating the molecular mechanism of aqueous extract of Cyclocarya paliurus on ameliorating diabetes by transcriptome profiling. Front. Pharmacol. 2018, 9, 912. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.N.; Fang, S.Z.; Wang, T.L.; Yin, Z.Q.; Shang, X.L.; Hu, M.H. Antidiabetic effect of Cyclocarya paliurus leaves depends on the contents of antihyperglycemic flavonoids and antihyperlipidemic triterpenoids. Molecules 2018, 23, 1042. [Google Scholar] [CrossRef]
- Zhai, L.X.; Ning, Z.W.; Huang, T.; Wen, B.; Liao, C.H.; Lin, C.Y.; Zhao, L.; Xiao, H.T.; Bian, Z.X. Cyclocarya paliurus leaves tea improves dyslipidemia in diabetic mice: A lipidomics-based network pharmacology study. Front. Pharmacol. 2018, 9, 973. [Google Scholar] [CrossRef]
- Sun, H.H.; Tan, J.; Lv, W.Y.; Li, J.; Wu, J.P.; Xu, J.L.; Zhu, H.; Yang, Z.C.; Wang, W.X.; Zou, Z.X.; et al. Hypoglycemic triterpenoid glycosides from Cyclocarya paliurus (sweet tea tree). Bioorg. Chem. 2020, 95, 103493. [Google Scholar] [CrossRef]
- Jiang, X.M.; Lin, Q.X.; He, G.D.; Hou, X.H.; Shan, Z.X.; Du, Y.M. Therapeutic effect of QQL prescription on type 2 diabetic rats. Chin. J. Pathophysiol. 2017, 33, 1794–1800. [Google Scholar]
- Ning, Z.W.; Zhai, L.X.; Huang, T.; Peng, J.; Hu, D.; Xiao, H.T.; Wen, B.; Lin, C.Y.; Zhao, L.; Bian, Z.X. Identification of alpha-glucosidase inhibitors from cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019, 10, 1893–1902. [Google Scholar] [CrossRef]
- Yao, J.K.; Wang, J.X.; Gao, X.M.; Fu, L.; Gao, F.; Wang, Y.J. Effects of Cyclocarya Paliurus leaves aqueous extract on pancreatic β cell’s apoptosis in type 2 diabetic rats. J. Beijing Univ. Trad. Chin. Med. 2018, 41, 663–669. [Google Scholar]
- The National Health Commission of the People’s Republic of China. Eight new raw materials for foods, such as Euglena gracilis. Bull. Natl. Health Comm. People’s Repub. China 2013, 124, 4. [Google Scholar]
- Yang, D.J.; Zhong, Z.C.; Xie, Z.M. Studies on the sweet principles from the leaves of Cyclocarya paliurus (Batal.) Iljinsk. Acta Pharm. Sin. 1992, 27, 841–844. [Google Scholar]
- Shu, R.G.; Xu, C.R.; Li, L.N. Sweet principles from the leaves of Cyclocarya paliurus (Batal.) Iljinsk. Acta Pharm. Sin. 1995, 30, 757–761. [Google Scholar]
- Fang, Z.J.; Shen, S.N.; Wang, J.M.; Wu, Y.J.; Zhou, C.X.; Mo, J.X.; Lin, L.G.; Gan, L.S. Triterpenoids from Cyclocarya paliurus that enhance glucose uptake in 3T3-L1 adipocytes. Molecules 2019, 24, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.N.; Jiang, C.H.; Tian, Y.S.; Xiao, N.; Wu, Z.F.; Ma, Y.L.; Lin, Z.; Fang, S.Z.; Shang, X.L.; Liu, K. Two triterpeniods from Cyclocarya paliurus (Batal) Iljinsk (Juglandaceae) promote glucose uptake in 3T3-L1 adipocytes: The relationship to AMPK activation. Phytomedicine 2015, 22, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Li, C.G.; Deng, S.P.; Liu, W.; Zhou, D.X.; Huang, Y.; Hao, L.L.; Zhang, G.R.; Su, S.S.; Xu, X.; Yang, R.Y.; et al. α-Glucosidase inhibitory and anti-inflammatory activities of dammarane triterpenoids from the leaves of Cyclocarya paliurus. Bioorg. Chem. 2021, 111, 104847. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, H.M.; Park, E.J.; Lee, B.W.; Nghiem, D.T.; Pham, H.T.T.; Pan, C.H.; Oh, W.K. Triterpenoid saponins from the leaves and stems of Pericampylus glaucus and their insulin mimetic activities. Bioorg. Chem. 2021, 117, 105445. [Google Scholar] [CrossRef]
- Wu, Z.F.; Meng, F.C.; Cao, L.J.; Jiang, C.H.; Zhao, M.G.; Shang, X.L.; Fang, S.Z.; Ye, W.C.; Zhang, Q.W.; Zhang, J.; et al. Triterpenoids from Cyclocarya paliurus and their inhibitory effect on the secretion of apoliprotein B48 in Caco-2 cells. Phytochemistry 2017, 142, 76–84. [Google Scholar] [CrossRef]
- Fei, Y.H.; Lou, H.X. Natural Medicinal Chemistry, 7th ed.; People’s Publishing Agence: Beijing, China, 2016. [Google Scholar]
- Zhou, X.L.; Li, S.B.; Yan, M.Q.; Luo, Q.; Wang, L.S.; Shen, L.L.; Liao, M.L.; Lu, C.H.; Liu, X.Y.; Liang, C.Q. Bioactive dammarane triterpenoid saponins from the leaves of Cyclocarya paliurus. Phytochemistry 2021, 183, 112618. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.Q.; Li, X.L.; Xu, T.H.; Xie, S.X.; Xu, Y.J.; Xu, D.M. Study on chemical constituents of Cyclocarya paliurus. J. Asian Nat. Prod. Res. 2014, 16, 206–209. [Google Scholar] [CrossRef]
- Li, S.; Cui, B.S.; Liu, Q.; Tang, L.; Yang, Y.C.; Jin, X.J.; Shen, Z.F. New triterpenoids from the leaves of Cyclocarya paliurus. Planta Med. 2012, 78, 290–296. [Google Scholar] [CrossRef]
- Yang, H.M.; Yin, Z.Q.; Zha, M.G.; Jiang, C.H.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]
- Yang, H.J.; Jeong, E.J.; Kim, J.W.; Sung, S.H.; Kim, Y.C. Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC-T6 cells. J. Nat. Prod. 2011, 74, 751–775. [Google Scholar] [CrossRef]
- Jakab, J.; Miskic, B.; Miksic, S.; Juranic, B.; Cosic, V.; Schwarz, D.; Vcev, A. Adipogenesis as a potential anti-obesity target: A review of pharmacological treatment and natural products. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 67–83. [Google Scholar] [CrossRef]
- Song, Q.Q.; Rao, Y.; Tang, G.H.; Sun, Z.H.; Zhang, J.S.; Huang, Z.S.; Yin, S. Tigliane diterpenoids as a new type of antiadipogenic agents inhibit GR alpha-dexras1 axis in adipocytes. J. Med. Chem. 2019, 62, 2060–2075. [Google Scholar] [CrossRef]
- Sarmiento, M.; Puius, Y.A.; Vetter, S.W.; Keng, Y.F.; Wu, L.; Zhao, Y.; Lawrence, D.S.; Almo, S.C.; Zhang, Z.Y. Structural basis of plasticity in protein tyrosine phosphatase 1B substrate recognition. Biochemistry 2000, 39, 8171–8179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).