Facile Attachment of Halides and Pseudohalides to Dodecaborate(2-) via Pd-catalyzed Cross-Coupling
Abstract
1. Introduction
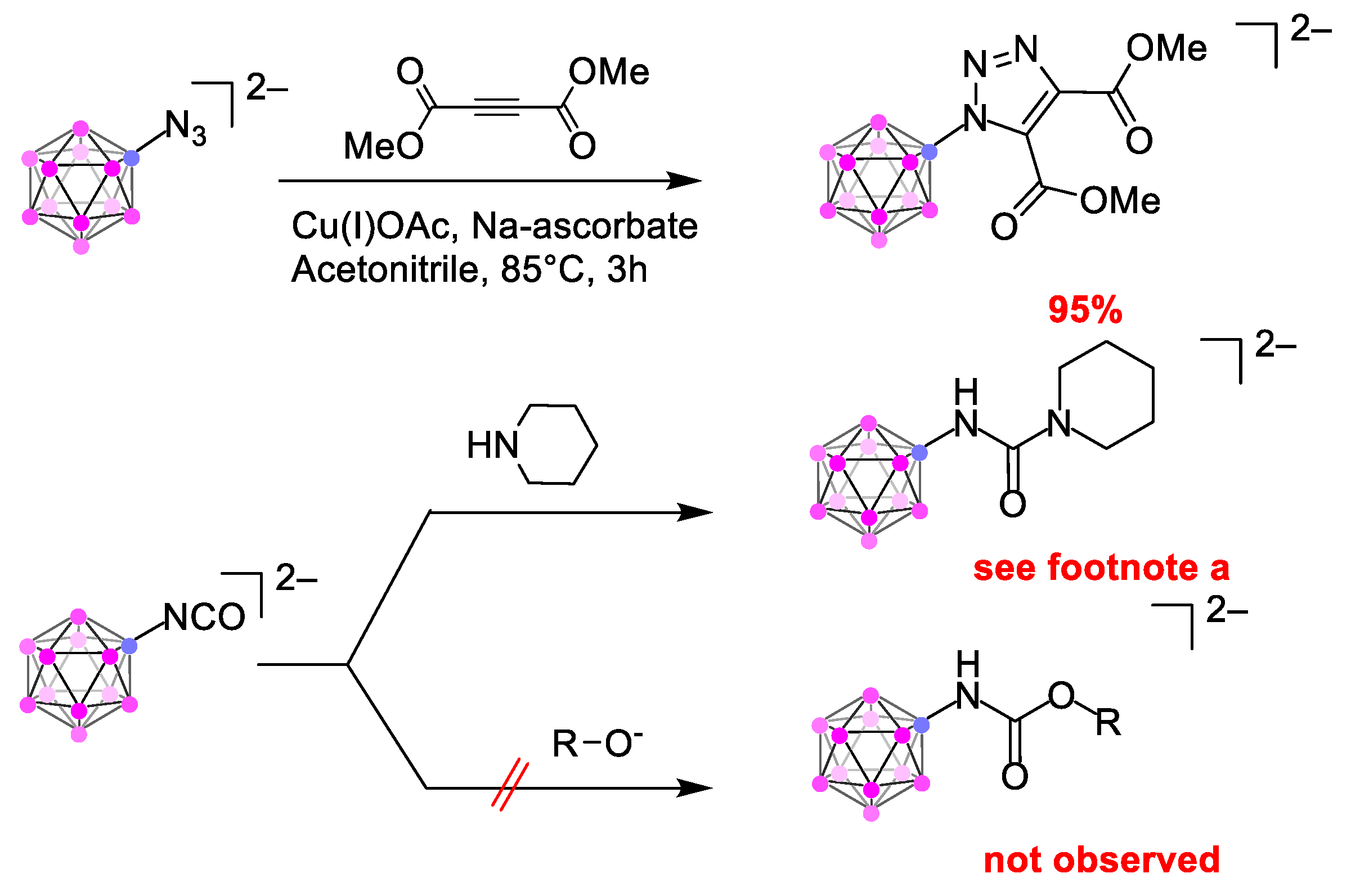
2. Results
3. Discussion
4. Materials and Methods
4.1. General Procedure
4.2. Click Reaction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marei, T.; Al-Joumhawy, M.K.; Alnajjar, M.A.; Nau, W.M.; Assaf, K.I.; Gabel, D. Binding affinity of aniline-substituted dodecaborates to cyclodextrins. Chem. Commun. 2022, 58, 2363–2366. [Google Scholar] [CrossRef] [PubMed]
- Assaf, K.I.; Suckova, O.; Al Danaf, N.; von Glasenapp, V.; Gabel, D.; Nau, W.M. Dodecaborate-Functionalized Anchor Dyes for Cyclodextrin-Based Indicator Displacement Applications. Org. Lett. 2016, 18, 932–935. [Google Scholar] [CrossRef]
- Assaf, K.I.; Gabel, D.; Zimmermann, W.; Nau, W.M. High-affinity host-guest chemistry of large-ring cyclodextrins. Org. Biomol. Chem. 2016, 14, 7702–7706. [Google Scholar] [CrossRef]
- Assaf, K.I.; Ural, M.S.; Pan, F.; Georgiev, T.; Simova, S.; Rissanen, K.; Gabel, D.; Nau, W.M. Water Structure Recovery in Chaotropic Anion Recognition: High-Affinity Binding of Dodecaborate Clusters to gamma-Cyclodextrin. Angew. Chem. Int. Ed. 2015, 54, 6852–6856. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, X.; Cao, J.; Liu, J.; Qi, B.; Zhou, X.; Zhang, S.; Gabel, D.; Nau, W.M.; Assaf, K.I.; et al. The chaotropic effect as an orthogonal assembly motif for multi-responsive dodecaborate-cucurbituril supramolecular networks. Chem. Commun. 2018, 54, 2098–2101. [Google Scholar] [CrossRef]
- Fan, P.; Neumann, J.; Stolte, S.; Arning, J.; Ferreira, D.; Edwards, K.; Gabel, D. Interaction of dodecaborate cluster compounds on hydrophilic column materials in water. J. Chromatogr. A 2012, 1256, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Justus, E.; Rischka, K.; Wishart, J.F.; Werner, K.; Gabel, D. Trialkylammoniododecaborates: Anions for Ionic Liquids with Potassium, Lithium and Protons as Cations. Chem. Eur. J. 2008, 14, 1918–1923. [Google Scholar] [CrossRef]
- Karki, K.; Gabel, D.; Roccatano, D. Structure and dynamics of dodecaborate clusters in water. Inorg. Chem. 2012, 51, 4894–4896. [Google Scholar] [CrossRef]
- Assaf, K.I.; Nau, W.M. The Chaotropic Effect as an Assembly Motif in Chemistry. Angew. Chem. Int. Ed. 2018, 57, 13968–13981. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Sivaev, I.B.; Bregadze, V.I. Cyclic oxonium derivatives of polyhedral boron hydrides and their synthetic applications. Dalton Trans. 2008, 977–992. [Google Scholar] [CrossRef]
- Peymann, T.; Lork, E.; Gabel, D. Hydroxoundecahydro-closo-dodecaborate(2-) as a nucleophile. Preparation and structural characterization of O-alkyl and O-acyl derivatives of hydroxoundecahydro-closo-dodecaborate(2-). Inorg. Chem. 1996, 35, 1355–1360. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Justus, E.; Ratajski, M.; Lork, E.; Gabel, D. B12H11-containing guanidinium derivatives by reaction of carbodiimides with H3N–B12H11(1−). A new method for connecting boron clusters to organic compounds. J. Organomet. Chem. 2005, 690, 2757–2760. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Zhang, Y.; Liu, J.; van der Veen, S.; Duttwyler, S. The closo-Dodecaborate Dianion Fused with Oxazoles Provides 3D Diboraheterocycles with Selective Antimicrobial Activity. Chem. Eur. J. 2018, 24, 10364–10371. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bruskin, A.B.; Nesterov, V.V.; Antipin, M.Y.; Bregadze, V.I.; Sjöberg, S. Synthesis of Schiff bases derived from the ammoniaundecahydro-closo- dodecaborate (1–) anion, [B12H11NH=CHR]−, and their reduction into monosubstituted amines [B12H11NH2CH2R]−: A new route to water soluble agents for BNCT. Inorg. Chem. 1999, 38, 5887–5893. [Google Scholar] [CrossRef]
- Gabel, D.; Moller, D.; Harfst, S.; Roesler, J.; Ketz, H. Synthesis of S-alkyl and S-acyl derivatives of mercaptoundecahydrododecaborate, a possible boron carrier for neutron capture therapy. Inorg. Chem. 1993, 32, 2276–2278. [Google Scholar] [CrossRef]
- Al-Joumhawy, M.K.; Marei, T.; Shmalko, A.; Cendoya, P.; La Borde, J.; Gabel, D. B–N bond formation through palladium-catalyzed, microwave-assisted cross-coupling of nitrogen compounds with iodo-dodecaborate. Chem. Commun. 2021, 57, 10007–10010. [Google Scholar] [CrossRef]
- Peymann, T.; Knobler, C.B.; Hawthorne, M.F. Synthesis of Alkyl and Aryl Derivatives of closo-B12H122− by the Palladium-Catalyzed Coupling of closo-B12H11I2− with Grignard Reagents. Inorg. Chem. 1998, 37, 1544–1548. [Google Scholar] [CrossRef]
- Himmelspach, A.; Finze, M.; Vöge, A.; Gabel, D. Cesium and Tetrabutylammonium Salt of the Ethynyl-closo-dodecaborate Dianion. Z. Anorg. Allg. Chem. 2012, 638, 512–519. [Google Scholar] [CrossRef]
- Al-Joumhawy, M.; Cendoya, P.; Shmalko, A.; Marei, T.; Gabel, D. Improved synthesis of halo- and oxonium derivatives of dodecahydrido-closo-dodecaborate(2-). J. Organomet. Chem. 2021, 949, 121967. [Google Scholar] [CrossRef]
- Semioshkin, A.A.; Petrovskii, P.V.; Sivaev, I.B.; Balandina, E.G.; Bregadze, V.I. Synthesis and NMR spectra of the hydroxyundecahydro-closo-dodecaborate [B12H11OH]2− and its acylated derivatives. Russ. Chem. Bull. 1996, 45, 683–686. [Google Scholar] [CrossRef]
- Alam, F.; Soloway, A.H.; Barth, R.F.; Mafune, N.; Adams, D.M.; Knoth, W.H. Boron neutron capture therapy: Linkage of a boronated macromolecule to monoclonal antibodies directed against tumor-associated antigens. J. Med. Chem. 1989, 32, 2326–2330. [Google Scholar] [CrossRef]
- Evano, G.; Nitelet, A.; Thilmany, P.; Dewez, D.F. Metal-Mediated Halogen Exchange in Aryl and Vinyl Halides: A Review. Front. Chem. 2018, 6, 114. [Google Scholar] [CrossRef]
- Himmelspach, A.; Reiss, G.J.; Finze, M. Microwave-assisted Kumada-type cross-coupling reactions of iodinated carba-closo-dodecaborate anions. Inorg. Chem. 2012, 51, 2679–2688. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I.; Sjöberg, S. A mild synthesis of the [B12H11CO]− anion. Russ. Chem. Bull. 1998, 47, 193–194. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Kulikova, N.Y.; Nizhnik, E.A.; Vichuzhanin, M.V.; Starikova, Z.A.; Semioshkin, A.A.; Bregadze, V.I. Practical synthesis of 1,4-dioxane derivative of the closo-dodecaborate anion and its ring opening with acetylenic alkoxides. J. Organomet. Chem. 2008, 693, 519–525. [Google Scholar] [CrossRef]
- Owen, G.R.; Vilar, R.; White, A.J.P.; Williams, D.J. Studies on the Reactivity of Isocyanates and Isothiocyanates with Palladium−Imidoyl Complexes. Organometallics 2003, 22, 4511–4521. [Google Scholar] [CrossRef]
- Srebny, H.G.; Preetz, W. Darstellung und Charakterisierung von Thiocyanatderivaten der Hydroboratanionen B10H102− und B12H122−. Z. Anorg. Allg. Chem. 1984, 513, 7–14. (In German) [Google Scholar] [CrossRef]
- Anderson, K.W.; Ikawa, T.; Tundel, R.E.; Buchwald, S.L. The Selective Reaction of Aryl Halides with KOH: Synthesis of Phenols, Aromatic Ethers, and Benzofurans. J. Am. Chem. Soc. 2006, 128, 10694–10695. [Google Scholar] [CrossRef] [PubMed]
- Kamin, A.A.; Juhasz, M.A. Exhaustive Cyanation of the Dodecaborate Dianion: Synthesis, Characterization, and X-ray Crystal Structure of [B12(CN)12]2−. Inorg. Chem. 2020, 59, 189–192. [Google Scholar] [CrossRef]
- Mu, X.; Hopp, M.; Dziedzic, R.M.; Waddington, M.A.; Rheingold, A.L.; Sletten, E.M.; Axtell, J.C.; Spokoyny, A.M. Expanding the Scope of Palladium-Catalyzed B–N Cross-Coupling Chemistry in Carboranes. Organometallics 2020, 39, 4380–4386. [Google Scholar] [CrossRef] [PubMed]
- Justus, E.; Vöge, A.; Gabel, D. N-alkylation of ammonioundecahydro-closo-dodecaborate(1-) for the preparation of anions for ionic liquids. Eur. J. Inorg. Chem. 2008, 2008, 5245–5250. [Google Scholar] [CrossRef]
- Barba-Bon, A.; Salluce, G.; Lostalé-Seijo, I.; Assaf, K.I.; Hennig, A.; Montenegro, J.; Nau, W.M. Boron clusters as broadband membrane carriers. Nature 2022, 603, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Wang, L.; Duttwyler, S.; Xing, H. A Microporous Metal-Organic Framework Supramolecularly Assembled from a CuII Dodecaborate Cluster Complex for Selective Gas Separation. Angew. Chem. Int. Ed. 2019, 58, 8145–8150. [Google Scholar] [CrossRef] [PubMed]
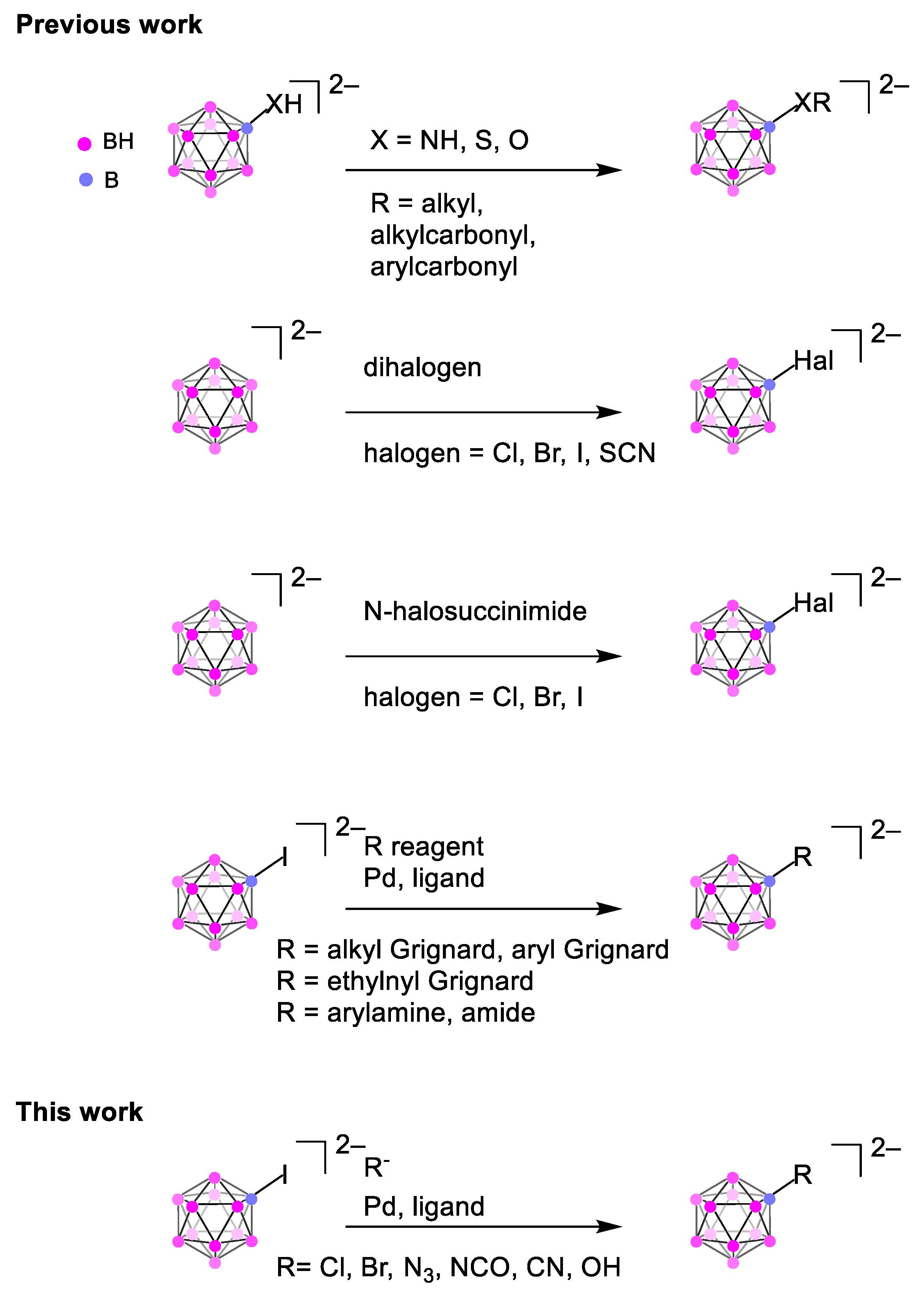
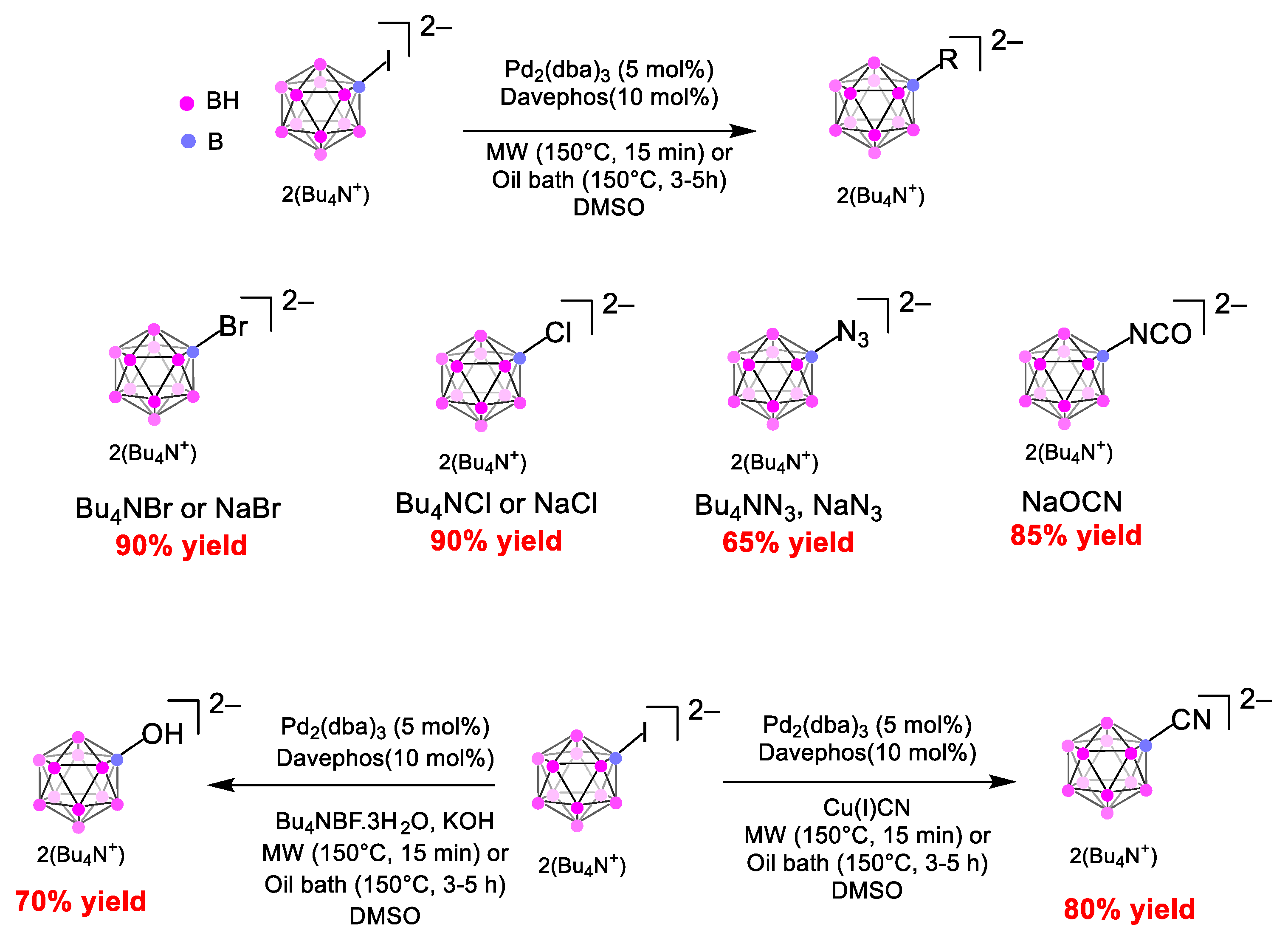
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Joumhawy, M.K.; Chang, J.-C.; Sabzi, F.; Gabel, D. Facile Attachment of Halides and Pseudohalides to Dodecaborate(2-) via Pd-catalyzed Cross-Coupling. Molecules 2023, 28, 3245. https://doi.org/10.3390/molecules28073245
Al-Joumhawy MK, Chang J-C, Sabzi F, Gabel D. Facile Attachment of Halides and Pseudohalides to Dodecaborate(2-) via Pd-catalyzed Cross-Coupling. Molecules. 2023; 28(7):3245. https://doi.org/10.3390/molecules28073245
Chicago/Turabian StyleAl-Joumhawy, Mahmoud K., Jui-Chi Chang, Fariba Sabzi, and Detlef Gabel. 2023. "Facile Attachment of Halides and Pseudohalides to Dodecaborate(2-) via Pd-catalyzed Cross-Coupling" Molecules 28, no. 7: 3245. https://doi.org/10.3390/molecules28073245
APA StyleAl-Joumhawy, M. K., Chang, J.-C., Sabzi, F., & Gabel, D. (2023). Facile Attachment of Halides and Pseudohalides to Dodecaborate(2-) via Pd-catalyzed Cross-Coupling. Molecules, 28(7), 3245. https://doi.org/10.3390/molecules28073245





