Polyhedral Dicobaltadithiaboranes and Dicobaltdiselenaboranes as Examples of Bimetallic Nido Structures without Bridging Hydrogens
Abstract
1. Introduction
2. Results and Discussion
2.1. The 11-Vertex Systems Cp2Co2E2B7H7 (E = S, Se)
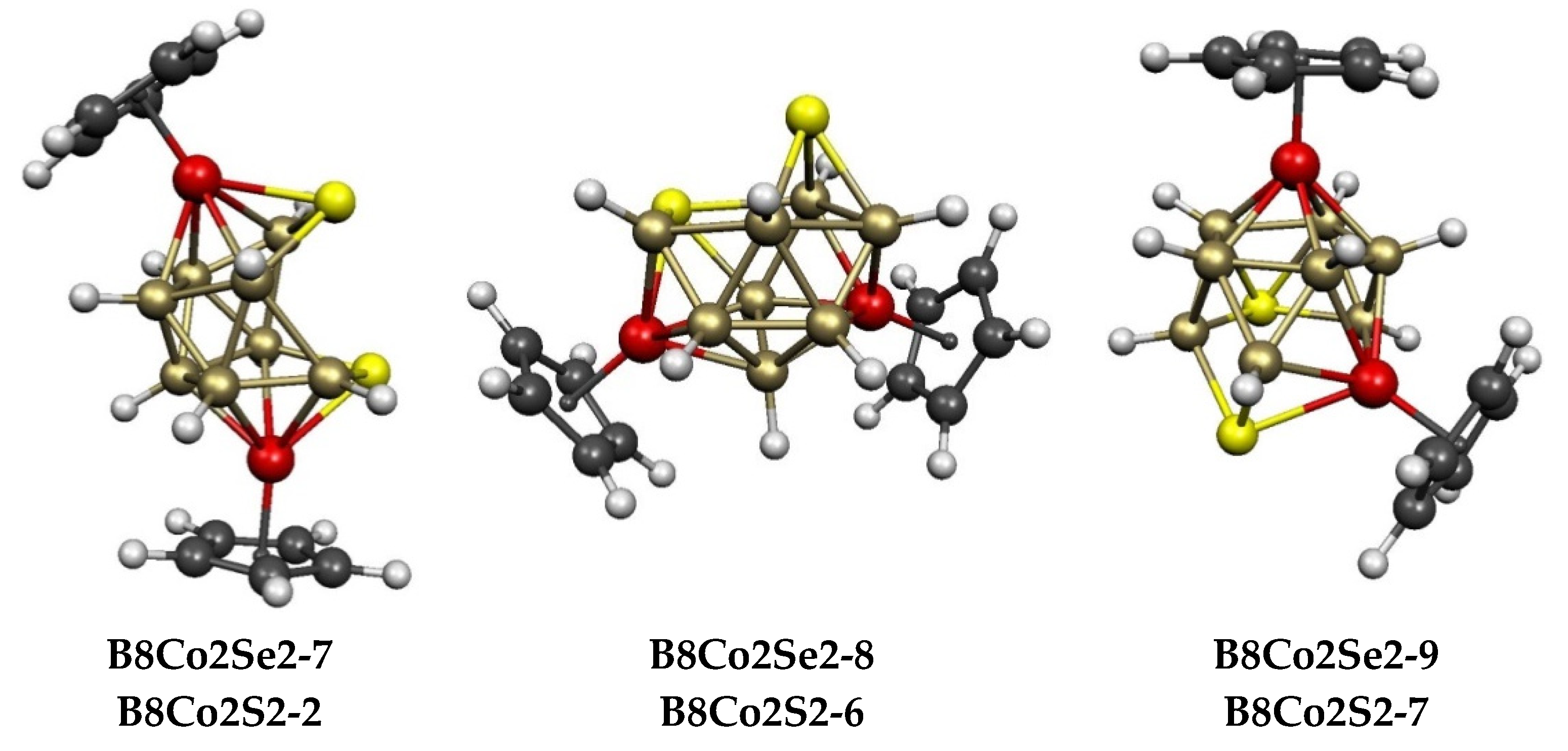
2.2. The 12-Vertex Systems Cp2Co2E2B8H8 (E = S, Se)
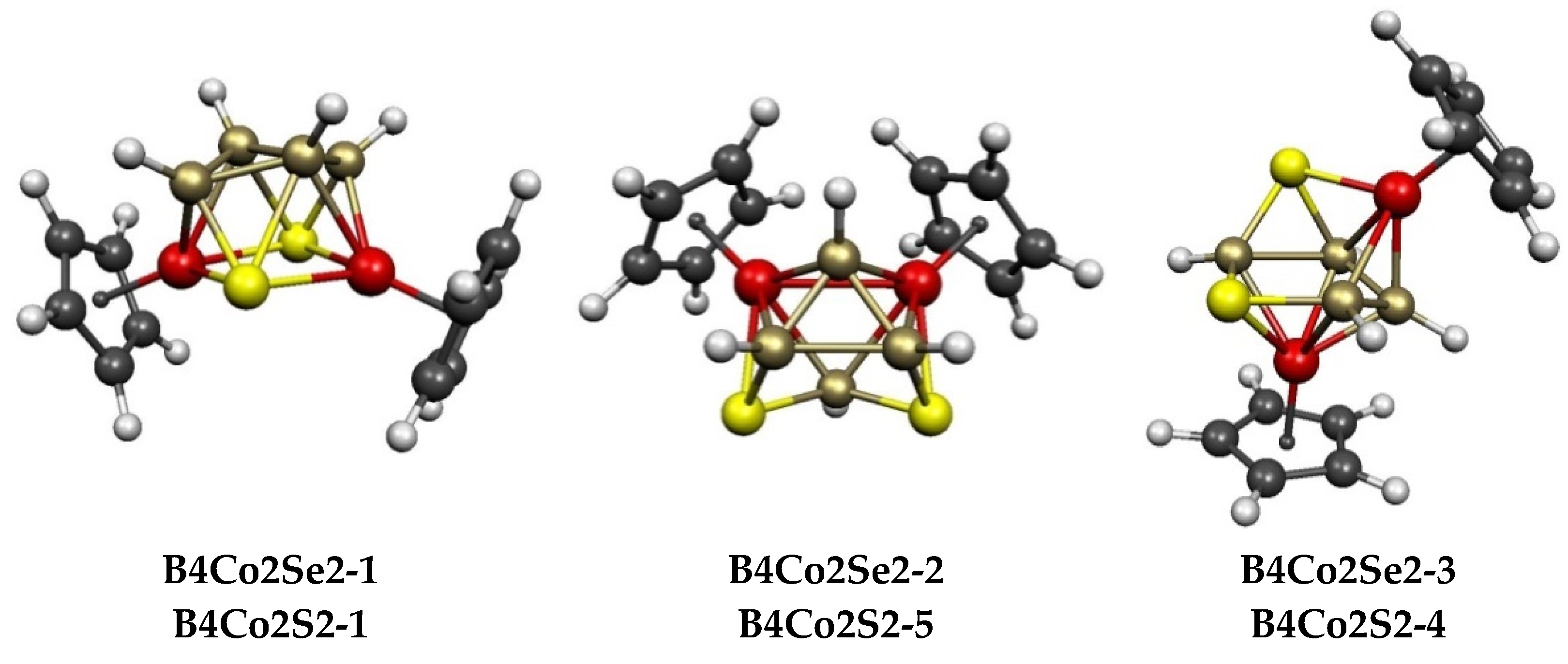
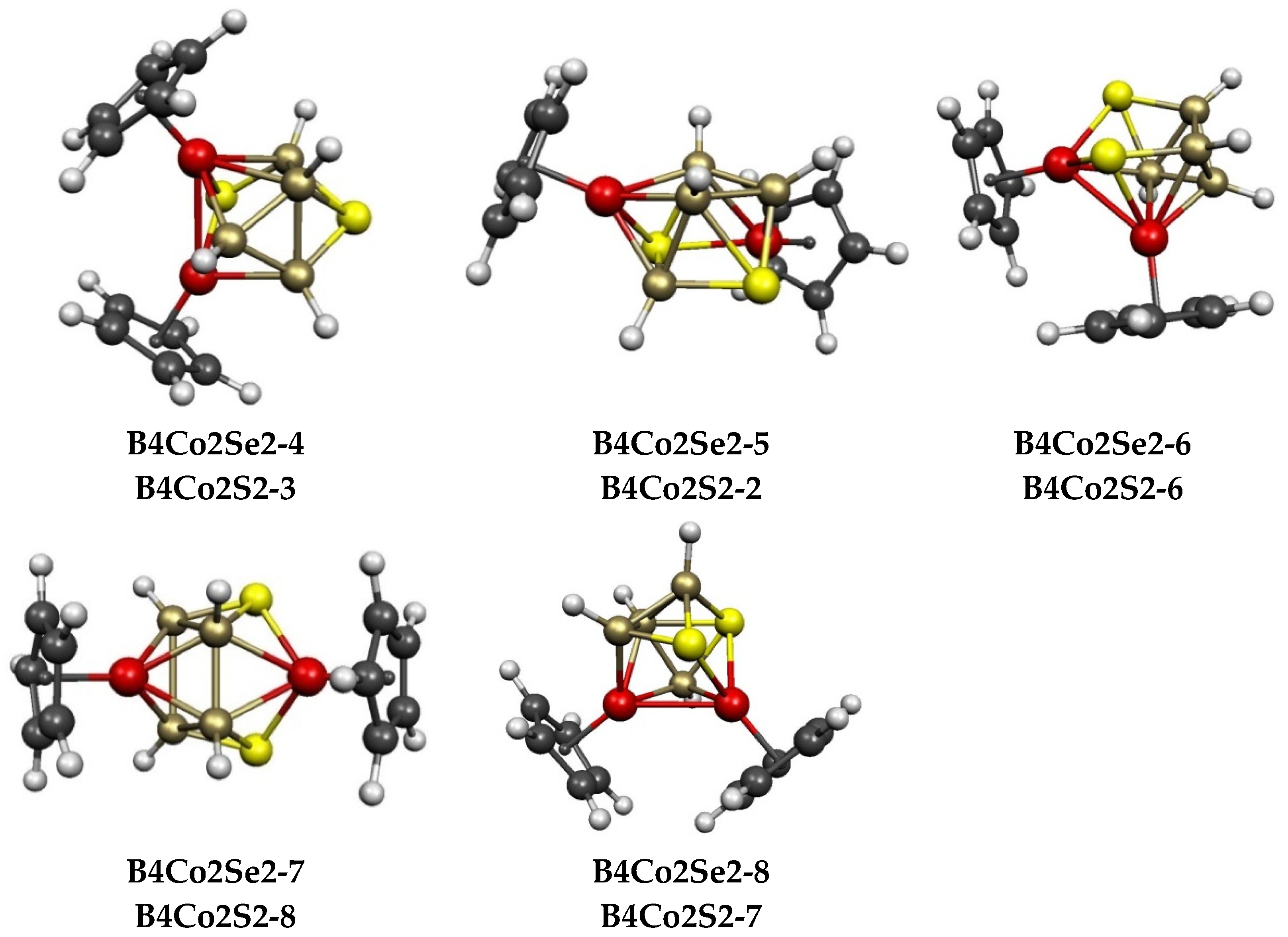
2.3. The Cp2Co2E2Bn−4Hn−4 (E = S, Se) Systems Having 8 to 10 Vertices
3. Theoretical Methods
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Muetterties, E.L. (Ed.) Boron Hydride Chemistry; Academic Press: New York, NY, USA, 1975. [Google Scholar]
- Williams, R.E. The polyborane, carborane, carbocation continuum—Architectural patterns. Chem. Rev. 1992, 92, 177–207. [Google Scholar] [CrossRef]
- Wade, K. The structural significance of the number of skeletal bonding electron-pairs in carboranes, the higher boranes and borane anions, and various transition-metal carbonyl cluster compounds. J. Chem. Soc. D Chem. Commun. 1971, 15, 792–793. [Google Scholar] [CrossRef]
- Mingos, D.M.P. A General theory for cluster and ring compounds of the main group and transition elements. Nat. Phys. Sci. 1972, 236, 99–102. [Google Scholar] [CrossRef]
- Mingos, D.M.P. Polyhedral skeletal electron pair approach. Accts. Chem. Res. 1984, 17, 311–319. [Google Scholar] [CrossRef]
- King, R.B.; Rouvray, D.H. A graph-theoretical interpretation of the bonding topology in polyhedral boranes, carboranes, and metal clusters. J. Am. Chem. Soc. 1977, 99, 7834–7840. [Google Scholar] [CrossRef]
- Aihara, J.-I. 3-Dimensional aromaticity of polyhedral boranes. J. Am. Chem. Soc. 1978, 100, 3339–3342. [Google Scholar]
- King, R.B. Three-dimensional aromaticity in polyhedral boranes and related molecules. Chem. Revs. 2001, 101, 1119–1152. [Google Scholar] [CrossRef]
- Callahan, K.P.; Hawthorne, M.F. Ten years of metallocarboranes. Adv. Organometal. Chem. 1976, 14, 145. [Google Scholar]
- Grimes, R.N. The role of metals in borane clusters. Accts. Chem. Res. 1983, 16, 22–26. [Google Scholar] [CrossRef]
- Kuan, H.; Yang, Z.; Zhang, L.; Xie, L.; Wang, L.; Xu, H.; Josephson, L.; Liang, S.H.; Zhang, M.-R. Boron agents for neutron capture therapy. Coord. Chem. Revs. 2020, 405, 213139. [Google Scholar]
- Avdeeva, V.V.; Garaev, T.M.; Malinina, E.A.; Zhizhin, K.Y.; Kuznetsov, N.T. Physiologically active compounds based on membranotropic cage carriers—Derivatives of adamantane and polyhedral boron clusters. Russ. J. Inorg. Chem. 2022, 67, 33–53. [Google Scholar] [CrossRef]
- Sivaev, I.B. Functional group directed B–H activation of polyhedral boron hydrides by transition metal complexes. Russ. J. Inorg. Chem. 2021, 66, 1192–1246. [Google Scholar] [CrossRef]
- Bould, J.; Kennedy, J.D.; Thornton-Pett, M. Ten-vertex metallaborane chemistry. Aspects of the iridadecaborane closo→isonido→isocloso structural continuum. J. Chem. Soc. Dalton 1992, 4, 563–576. [Google Scholar] [CrossRef]
- Kennedy, J.D.; Štibr, B. Current Topics in the Chemistry of Boron; Kabalka, G.W., Ed.; Royal Society of Chemistry: Cambridge, UK, 1994; pp. 285–292. [Google Scholar]
- Kennedy, J.D. The Borane-Carborane-Carbocation Continuum; Casanova, J., Ed.; Wiley: New York, NY, USA, 1998; Chapter 3; pp. 85–116. [Google Scholar]
- Štibr, B.; Kennedy, J.D.; Drdáková, E.; Thornton-Pett, M. Nine-vertex polyhedral iridamonocarbaborane chemistry. Products of thermolysis of [(CO)(PPh3)2IrCB7H8] and emerging alternative cluster-geometry patterns. J. Chem. Soc. Dalton 1994, 2, 229–236. [Google Scholar] [CrossRef]
- King, R.B. The oblate deltahedra in dimetallaboranes: Geometry and chemical bonding. Inorg. Chem. 2006, 45, 8211–8216. [Google Scholar] [CrossRef]
- Parry, R.W.; Walter, M.K.; Jolly, W.L. (Eds.) Preparative Inorganic Reactions; Interscience: New York, NY, USA, 1968; Volume 5, pp. 45–102. [Google Scholar]
- Kasper, J.S.; Lucht, C.M.; Harker, D. The structure of the decaborane molecule. J. Am. Chem. Soc. 1948, 70, 881–882. [Google Scholar] [CrossRef]
- Onak, T.P.; Wong, G.T.F. Preparation of the pentagonal pyramidal carborane, 2,3,4,5-tetracarba-nido-hexaborane(6). J. Am. Chem. Soc. 1970, 92, 5226. [Google Scholar] [CrossRef]
- Pasinski, J.P.; Beaudet, R.A. Microwave spectrum, structure, and dipole moment of 2,3,4,5-tetracarbahexaborane(6). J. Chem. Phys. 1974, 61, 683–691. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Schang, H.-J.; Hofmann, M.; Schleyer, P.v.R. A new carborane cage: Hexacarba-arachno-dodecaborane(12). Angew. Chem. Int. Ed. 1998, 37, 1245–1247. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Schanz, H.-J.; Hofmann, M.; Schleyer, P.v.R.; Boese, R. The structures of a tetracarba-nido-octaborane(8) and a novel spiro derivative of a 2,3,5-tricarba-nido-hexaborane(7). Eur. J. Inorg. Chem. 1999, 3, 533–537. [Google Scholar] [CrossRef]
- Köster, R.; Seidel, G.; Wrackmeyer, B.; Bläser, D.; Boese, R.; Bühl, M.; Schleyer, P.v.R. Decaethyl-2,6,8,10-tetracarba-nido-decaborane(10)—Preparation, structure in the solid state, and stability. Chem. Ber. 1991, 124, 2715–2724. [Google Scholar] [CrossRef]
- Rayment, T.; Shearer, H.M.M. Crystal structure of 2,4,6,8,9,10-hexamethyl-2,4,6,8,9,10-hexabora-adamantane. J. Chem. Soc. Dalton 1977, 2, 136–138. [Google Scholar] [CrossRef]
- Köster, R.; Seidel, G.; Wrackmeyer, B. Dimerization of a C2B3-closo-carbaborane(5) to the C4B6-adamantane and its 2Z/3Z valence isomerization to the C4B6-nido-carbaborane(10). Angew. Chem. Int. Ed. 1985, 24, 326–328. [Google Scholar] [CrossRef]
- Wrackmeyer, B.; Schanz, H.-J. Tetracarba-nido-hexa-, octa-, and –decaborane derivatives. NMR study and DFT calculations. J. Organomet. Chem. 2015, 798, 268–273. [Google Scholar] [CrossRef]
- Štíbr, B.; Jelínek, T.; Drdáková, E.; Heřmánek, S.; Plešek, J. A new family of stable parent nido-tetracarbaboranes 5,6,8,9-C4B6H10, 2,7,8,11-C4B7H11, and 7,8,9,10-C4B7H11. Polyhedron 1988, 7, 669–670. [Google Scholar] [CrossRef]
- Grimes, R.N. Carbon-rich carboranes and their metal derivatives. Adv. Inorg. Chem. Radiochem. 1983, 26, 55–117. [Google Scholar]
- Maynard, R.B.; Grimes, R.N. Oxidative fusion of carborane ligands in iron and cobalt complexes—A systematic study. J. Am. Chem. Soc. 1982, 104, 5983–5986. [Google Scholar] [CrossRef]
- Maxwell, W.M.; Miller, V.R.; Grimes, R.N. 4-Carbon carboranes—Synthesis of tetra-C-methyltetracarba-dodecaborane(12) and its metallocarborane derivatives. J. Am. Chem. Soc. 1974, 99, 7116–7117. [Google Scholar] [CrossRef]
- Maxwell, W.M.; Miller, V.R.; Grimes, R.N. Iron-hydrogen and iron-cobalt metallocarboranes—Synthesis and chemistry of [(CH3)2C2B4H4]2FeIIH2 and a novel tetracarbon carborane system (CH3)4C4B8H8. Inorg. Chem. 1976, 15, 1343–1348. [Google Scholar] [CrossRef]
- Freyberg, D.P.; Weiss, R.; Sinn, E.; Grimes, R.N. Crystal and molecular structure of a tetracarbon carborane, (CH3)4C4B8H8, a new type of nido cage system. Inorg. Chem. 1977, 16, 1847–1851. [Google Scholar] [CrossRef]
- Venable, T.L.; Maynard, R.B.; Grimes, R.N. Crystal and molecular structure of a tetracarbon carborane, (CH3)4B8H8, a new type of nido cage system. J. Am. Chem. Soc. 1984, 106, 6187–6193. [Google Scholar] [CrossRef]
- Spencer, J.T.; Pourlan, M.R.; Butcher, R.J.; Sinn, E.; Grimes, R.N. Pi-complexation of nido-(PhCH2)2C2B4H6 at the C2B3 and C6 rings—Synthesis and crystal structures of nido-2,3-(CO)3Cr[(.eta.6-C6H5)CH2]2-2,3,-C2B4H6 and (PhCH2)4C4B8H8, a nonfluxional C4B8 cluster. Organometallics 1987, 6, 335–343. [Google Scholar] [CrossRef]
- Attia, A.A.A.; Lupan, A.; King, R.B. Tetracarbaboranes: nido structures without bridging hydrogens. Dalton Trans. 2016, 45, 18541–18551. [Google Scholar] [CrossRef] [PubMed]
- Friesen, G.D.; Barriola, A.; Daluga, P.; Ragatz, P.; Huffman, J.; Todd, L. Chemistry of dithiaboranes, selenathiaboranes, and diselenaboranes. Inorg. Chem. 1980, 19, 458–462. [Google Scholar] [CrossRef]
- Attia, A.A.A.; Lupan, A.; King, R.B. Polyhedral cobaltadiselenaboranes: Nido strucures without bridging hydrogens. RSC Adv. 2016, 6, 53635–53642. [Google Scholar] [CrossRef]
- Kang, S.O.; Sneddon, L.G. Synthesis of new dithiacobaltaborane clusters derived from arachno-6,8-S2B7H9. Inorg. Chem. 1988, 27, 3769–3772. [Google Scholar] [CrossRef]
- Robertson, A.P.M.; Beattie, N.A.; Scott, C.; Man, W.Y.; Jones, J.J.; Macgregor, S.A.; Rosair, G.M.; Welch, A.J. 14-Vertex heteroboranes with 14 skeletal electron pairs: An experimental and computational study. Angew. Chem. Int. Ed. 2016, 55, 8706–8710. [Google Scholar] [CrossRef]
- Szabolcs, J.; Lupan, A.; Kun, A.-Z.; King, R.B. Isocloso versus closo deltahedra in slightly hypoelectronic supraicosahedral 14-vertex dimetallaboranes with 28 skeletal electrons; Relationship to icosahedral dimetallaboranes. New J. Chem. 2020, 44, 16977–16984. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6169. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef]
- Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA. 2016. Available online: https://gaussian.com/g09citation/ (accessed on 10 February 2023).
- Schneider, W.B.; Bistoni, G.; Sparta, M.; Saitow, M.; Riplinger, C.; Auer, A.A.; Neese, F. Decomposition of intermolecular interaction energies within the local pair natural orbital coupled cluster framework. J. Chem. Theory Comput. 2016, 12, 4778–4792. [Google Scholar] [CrossRef] [PubMed]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse maps—A systematic infrastructure for reduced-scaling electronic structure methods. II. Linear scaling domain based pair natural orbital coupled cluster theory. J. Chem. Phys. 2016, 144, 024109. [Google Scholar] [CrossRef]
- Pavošević, F.; Pinski, P.; Riplinger, C.; Neese, F.; Valeev, E.F. SparseMaps—A systematic infrastructure for reduced-scaling electronic structure methods. IV. Linear-scaling second-order explicitly correlated energy with pair natural orbitals. J. Chem. Phys. 2016, 144, 144109. [Google Scholar] [CrossRef] [PubMed]
- Kubas, A.; Berger, D.; Oberhofer, H.; Maganas, D.; Reuter, K.; Neese, F. Surface adsorption energetics studied with “gold standard” wave-function-based ab initio methods: Small-molecule binding to TiO2 (110). J. Phys. Chem. Lett. 2016, 7, 4207–4212. [Google Scholar] [CrossRef] [PubMed]
- Isegawa, M.; Neese, F.; Pantazis, D.A. Ionization energies and aqueous redox potentials of organic molecules: Comparison of DFT, correlated ab initio theory and pair natural orbital approaches. J. Chem. Theory Comput. 2016, 12, 2272–2284. [Google Scholar] [CrossRef]
- Guo, Y.; Sivalingam, K.; Valeev, E.F.; Neese, F. SparseMaps—A systematic infrastructure for reduced-scaling electronic structure methods. III. Linear-scaling multireference domain-based pair natural orbital N-electron valence perturbation theory. J. Chem. Phys. 2016, 144, 094111. [Google Scholar] [CrossRef]
- Dutta, A.K.; Neese, F.; Izsák, R. Towards a pair natural orbital coupled cluster method for excited states. J. Chem. Phys. 2016, 145, 034102. [Google Scholar] [CrossRef]
- Datta, D.; Kossmann, S.; Neese, F. Analytic energy derivatives for the calculation of the first-order molecular properties using the domain-based local pair-natural orbital coupled-cluster theory. J. Chem. Phys. 2016, 145, 114101. [Google Scholar] [CrossRef]
- Pinski, P.; Riplinger, C.; Valeev, E.F.; Neese, F. Sparse maps—A systematic infrastructure for reduced-scaling electronic structure methods. I. An efficient and simple linear scaling local MP2 method that uses an intermediate basis of pair natural orbitals. J. Chem. Phys. 2015, 143, 034108. [Google Scholar] [CrossRef]
- Mondal, B.; Neese, F.; Ye, S. Control in the rate-determining step provides a promising strategy to develop new catalysts for CO2 hydrogenation: A local pair natural orbital coupled cluster theory study. Inorg. Chem. 2015, 54, 7192–7198. [Google Scholar] [CrossRef]
- Liakos, D.G.; Sparta, M.; Kesharwani, M.K.; Martin, J.M.L.; Neese, F. Exploring the accuracy limits of local pair natural orbital coupled-cluster theory. J. Chem. Theory Comput. 2015, 11, 1525–1539. [Google Scholar] [CrossRef] [PubMed]
- Liakos, D.G.; Neese, F. Domain based pair natural orbital coupled cluster studies on linear and folded alkane chains. J. Chem. Theory Comput. 2015, 11, 2137–2143. [Google Scholar] [CrossRef] [PubMed]
- Liakos, D.G.; Neese, F. Is it possible to obtain coupled cluster quality energies at near density functional theory cost? domain-based local pair natural orbital coupled cluster vs. modern density functional theory. J. Chem. Theory Comput. 2015, 11, 4054–4063. [Google Scholar] [CrossRef] [PubMed]
- Demel, O.; Pittner, J.; Neese, F. A local pair natural orbital-based multireference Mukherjee’s coupled cluster method. J. Chem. Theory Comput. 2015, 11, 3104–3114. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. Speeding up spin-component-scaled third-order pertubation theory with the chain of spheres approximation: The COSX-SCS-MP3 method. Mol. Phys. 2013, 111, 1190–1195. [Google Scholar] [CrossRef]
- Izsák, R.; Neese, F. An overlap fitted chain of spheres exchange method. J. Chem. Phys. 2011, 135, 144105. [Google Scholar] [CrossRef]
- Kossmann, S.; Neese, F. Efficient structure optimization with second-order many-body perturbation theory: The RIJCOSX-MP2 Method. J. Chem. Theory Comput. 2010, 6, 2325–2338. [Google Scholar] [CrossRef]
- Kossmann, S.; Neese, F. Comparison of two efficient approximate Hartee–Fock approaches. Chem. Phys. Lett. 2009, 481, 240–243. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Hansen, A.; Becker, U. Efficient, approximate and parallel Hartree–Fock and hybrid DFT calculations. A “chain-of-spheres” algorithm for the Hartree–Fock exchange. Chem. Phys. 2009, 356, 98–109. [Google Scholar] [CrossRef]
- Neese, F. An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix. J. Comput. Chem. 2003, 24, 1740–1747. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.K.; Neese, F.; Izsák, R. Speeding up equation of motion coupled cluster theory with the chain of spheres approximation. J. Chem. Phys. 2016, 144, 034102. [Google Scholar] [CrossRef] [PubMed]
- Christian, G.J.; Neese, F.; Ye, S. Unravelling the molecular origin of the regiospecificity in extradiol catechol dioxygenases. Inorg. Chem. 2016, 55, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
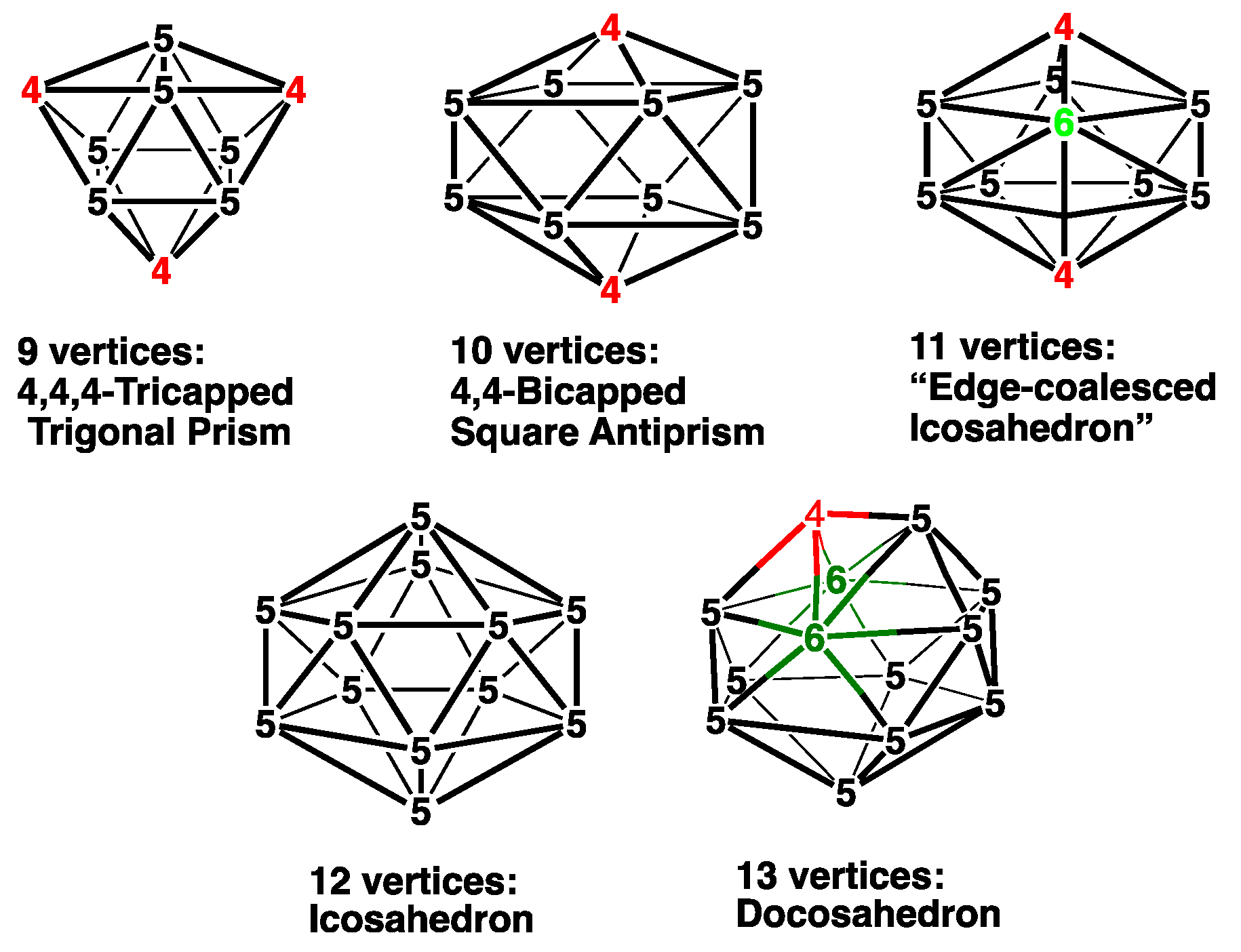
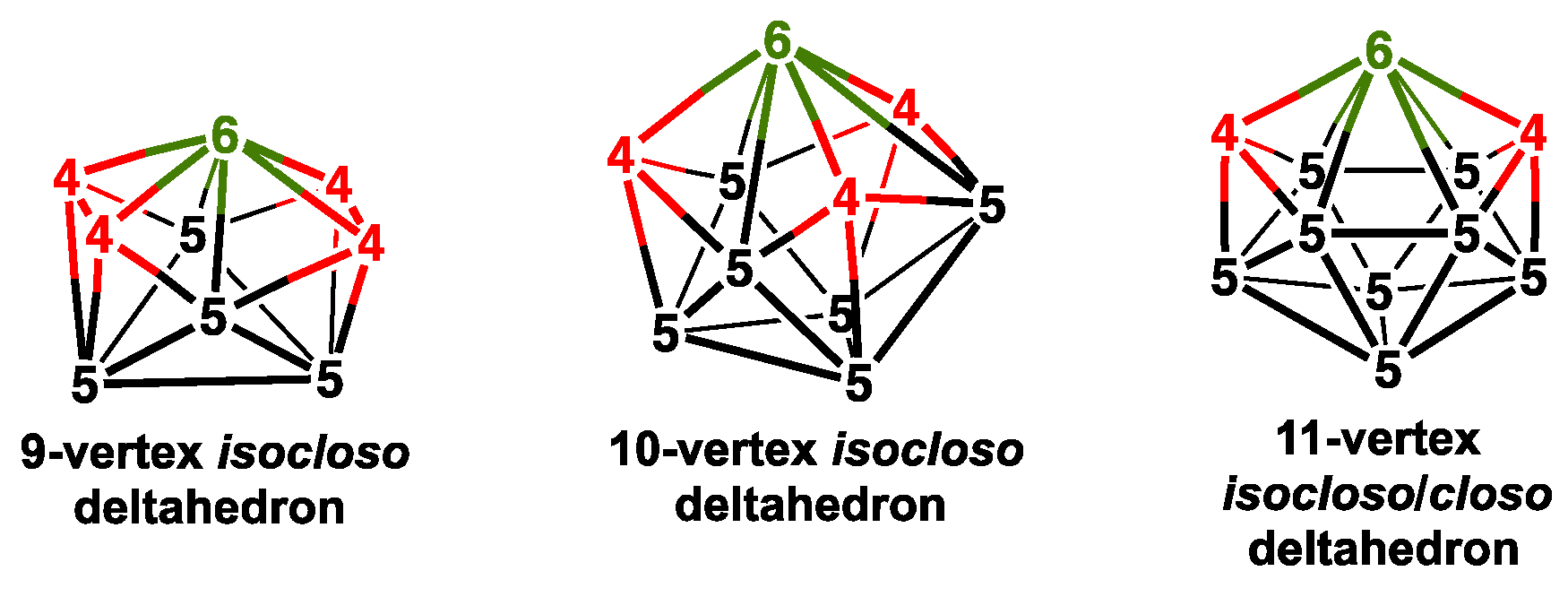

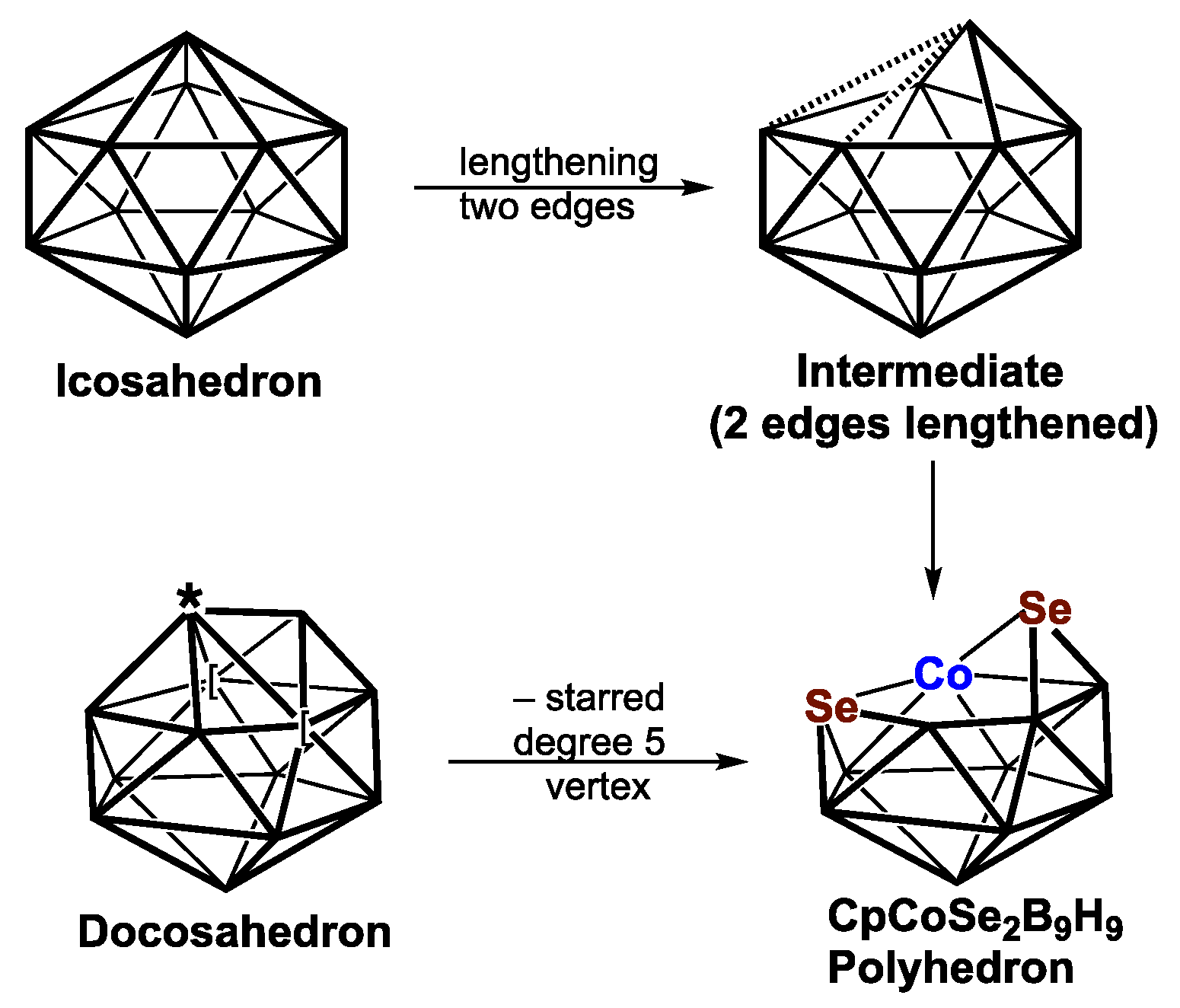
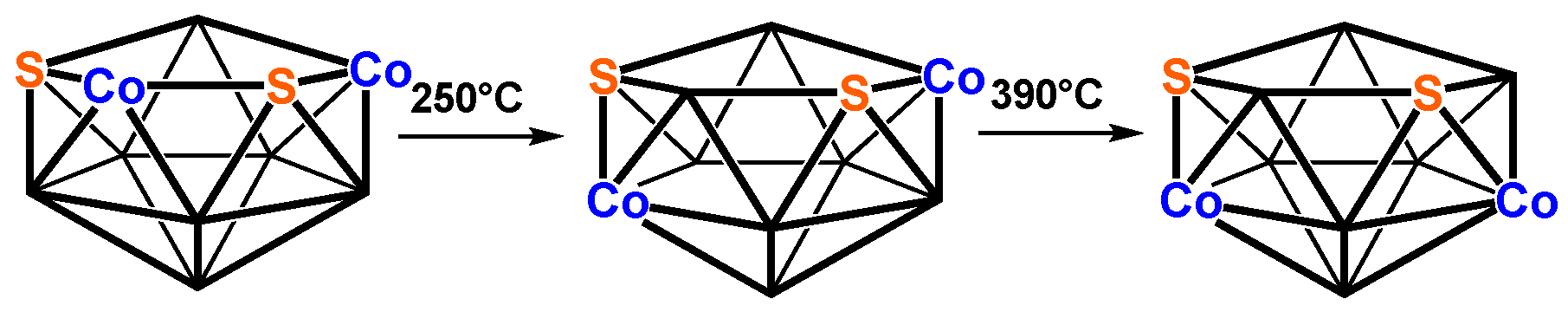

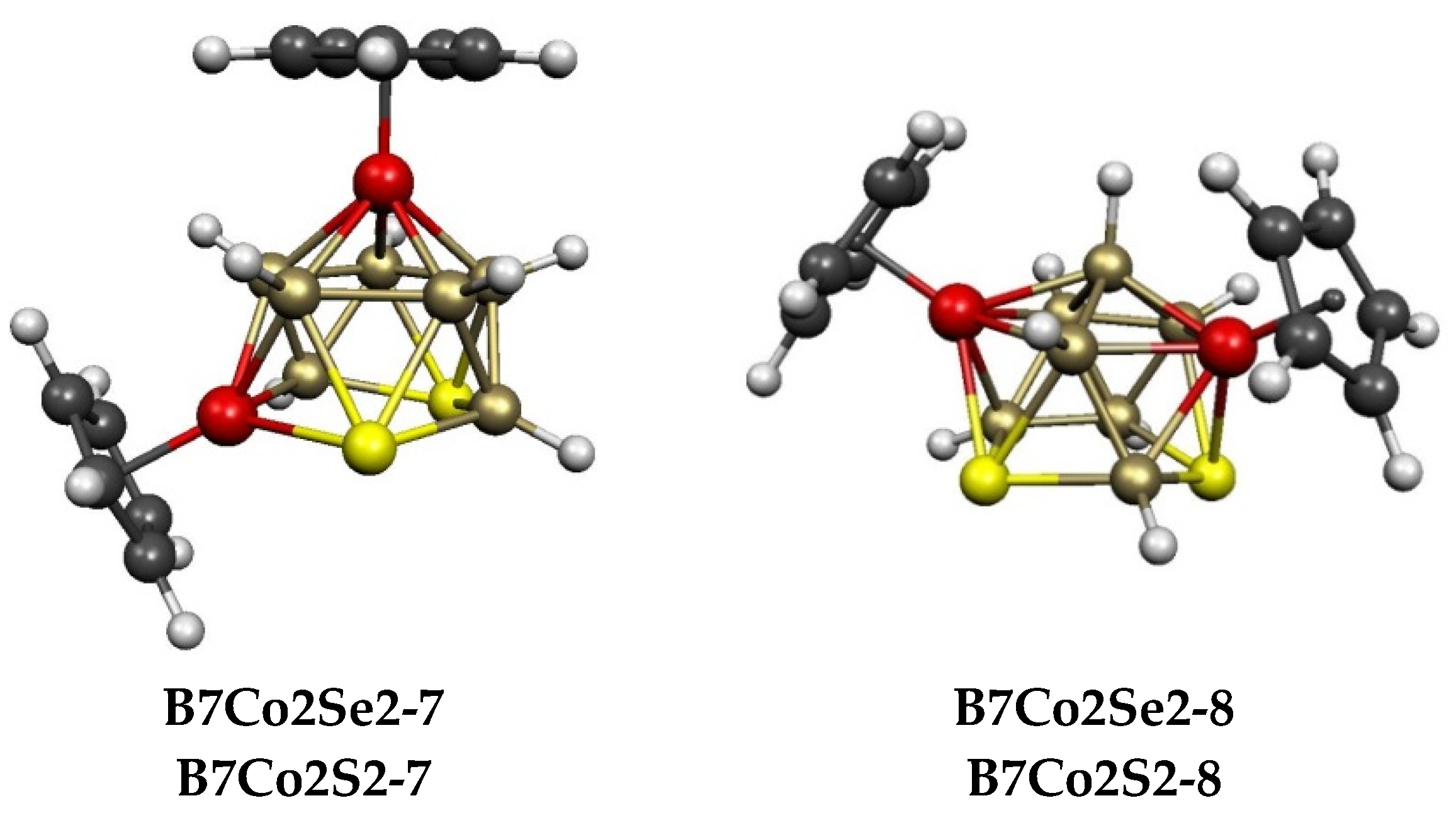


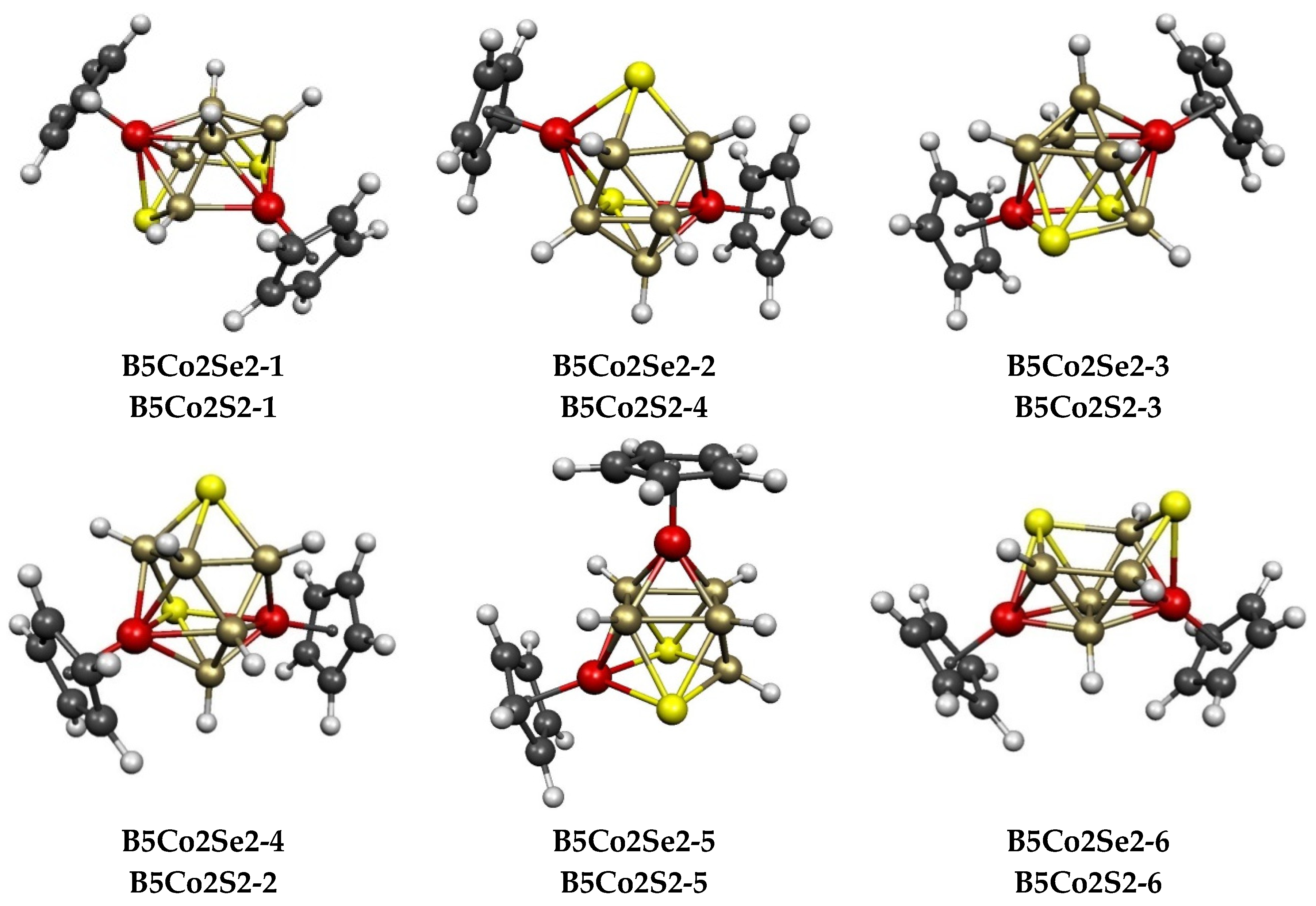

| Cp2Co2Se2B7H7 | Cp2Co2S2B7H7 | Vertex Degrees | Co…S | Co…Co (E = Se) | Pentagonal | ||||
|---|---|---|---|---|---|---|---|---|---|
| Structure (sym) | ∆E | Structure | ∆E | S | Co | Edges | Dist (Å) | WBI | Face Atoms |
| B7Co2Se2-1 (C1) | 0.0 | B7Co2S2-1 | 0.0 | 4, 4 | 4, 4 | 3 | 3.91 | 0.10 | SCoSBCo |
| B7Co2Se2-2 (C1) | 2.5 | B7Co2S2-2 | 1.8 | 4, 4 | 4, 5 | 3 | 3.81 | 0.10 | SCoSBB |
| B7Co2Se2-3 (C1) | 8.1 | B7Co2S2-3 | 6.2 | 4, 4 | 4, 5 | 2 | 3.79 | 0.09 | SCoBSB |
| B7Co2Se2-4 (C1) | 9.4 | B7Co2S2-4 | 7.7 | 4, 4 | 4, 5 | 2 | 3.71 | 0.10 | SCoBSB |
| B7Co2Se2-5 (Cs) | 10.1 | B7Co2S2-5 | 18.2 | 4, 4 | 4, 5 | 2 | 3.68 | 0.09 | SCoSBB |
| B7Co2Se2-6 (Cs) | 11.8 | B7Co2S2-6 | 19.9 | 4, 4 | 5, 5 | 2 | 3.73 | 0.09 | SBSBB |
| B7Co2Se2-7 (C1) | 13.5 | B7Co2S2-7 | 10.7 | 4, 4 | 4, 5 | 1 | 3.67 | 0.08 | SCoBSB |
| B4Co2Se2-8 (C1) | 14.7 | B7Co2S2-8 | 12.6 | 4, 4 | 5, 5 | 2 | 3.73 | 0.08 | SBSBB |
| Cp2Co2Se2B8H8 | Cp2Co2S2B8H8 | Vertex Degrees | Co…S | Co…Co (E = Se) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Structure (sym) | ∆E | Structure | ∆E | S | Co | Edges | Dist (Å) | WBI | Polyhedron |
| B8Co2Se2-1 (C2v) | 0.0 | B8Co2S2-9 | 9.0 | 5, 5 | 4, 4 | 4 | 3.18 | 0.17 | ni-12〈IV〉 |
| B8Co2Se2-2 (C1) | 4.0 | B8Co2S2-1 | 0.0 | 3, 3 | 5, 5 | 2 | 3.85 | 0.12 | meta Co2 open icosahedron |
| B8Co2Se2-3 (C1) | 4.5 | B8Co2S2-5 | 3.8 | 3, 4 | 4, 5 | 2 | 3.83 | 0.09 | meta Co2 open icosahedron |
| B8Co2Se2-4 (C2v) | 5.3 | B8Co2S2-8 | 7.9 | 4, 4 | 5, 5 | 4 | 3.48 | 0.15 | ni-12〈IV〉 |
| B8Co2Se2-5 (C1) | 8.2 | B8Co2S2-3 | 3.1 | 3, 4 | 5, 5 | 1 | 3.61 | 0.09 | ar-12〈IV,V〉 |
| B8Co2Se2-6 (C2) | 8.2 | B8Co2S2-4 | 3.5 | 3, 3 | 5, 5 | 2 | 4.22 | 0.05 | para Co2 open icosahedron |
| B8Co2Se2-7 (C2) | 9.3 | B8Co2S2-2 | 3.0 | 3, 3 | 5, 5 | 2 | 4.82 | 0.08 | para Co2 open icosahedron |
| B8Co2Se2-8 (C1) | 11.8 | B8Co2S2-6 | 5.6 | 3, 4 | 5, 5 | 1 | 3.73 | 0.11 | meta Co2 open icosahedron |
| B8Co2Se2-9 (C1) | 12.3 | B8Co2S2-7 | 7.1 | 3, 4 | 5, 5 | 1 | 3.72 | 0.10 | ar-12〈IV,V〉 |
| Cp2Co2Se2B4H4 | Cp2Co2S2B4H4 | Vertex Degrees | Co…S | Co…Co (E = Se) | Non-Triang Face | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structure (sym) | ∆E | Structure | ∆E | S | Co | Edges | Dist (Å) | WBI | Atoms | Polyhedron |
| B4Co2Se2-1 (C2) | 0.0 | B4Co2S2-1 | 0.0 | 4, 4 | 4, 4 | 4 | 3.39 | 0.12 | SCoSCo | bicap trig prism |
| B4Co2Se2-2 (Cs) | 7.1 | B4Co2S2-5 | 5.2 | 4, 4 | 4, 4 | 3 | 3.75 | 0.14 | SCoSB | bicap trig prism |
| B4Co2Se2-3 (C1) | 11.1 | B4Co2S2-4 | 1.9 | 3, 3 | 4, 5 | 2 | 2.55 | 0.38 | SCoBBB | ni-8〈V〉 |
| B4Co2Se2-4 (C1) | 11.4 | B4Co2S2-3 | 0.7 | 3, 3 | 4, 5 | 2 | 3.44 | 0.10 | SCoBSB | ni-8〈V〉 |
| B4Co2Se2-5 (Cs) | 12.5 | B4Co2S2-2 | 0.1 | 3, 3 | 5, 5 | 2 | 2.64 | 0.24 | SCoBSB | ni-8〈V〉 |
| B4Co2Se2-6 (C1) | 13.5 | B4Co2S2-6 | 6.5 | 3, 3 | 4, 5 | 3 | 2.59 | 0.40 | SCoSBB | ni-8〈V〉 |
| B4Co2Se2-7 (C1) | 14.9 | B4Co2S2-8 | 10.3 | 3, 4 | 4, 4 | 2 | 2.65 | 0.44 | 2 × SCoSB | ar-8〈IV,IV〉 |
| B4Co2Se2-8 (Cs) | 15.3 | B4Co2S2-7 | 8.3 | 3, 3 | 4, 4 | 2 | 2.62 | 0.09 | SCoSBB | ni-8〈V〉 |
| Cp2Co2Se2B5H5 | Cp2Co2S2B5H5 | Vertex Degrees | Co…S | Co…Co (E = Se) | Non-Triang Face | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structure (sym) | ∆E | Structure | ∆E | S | Co | Edges | Dist(Å) | WBI | Atoms | Polyhedron |
| B5Co2Se2-1 (C1) | 0.0 | B5Co2S2-1 | 0.0 | 3, 4 | 4, 5 | 2 | 3.67 | 0.11 | SCoBSB | ni-9〈V〉 |
| B5Co2Se2-2 (C1) | 0.7 | B5Co2S2-4 | 3.9 | 3, 4 | 4, 5 | 3 | 3.82 | 0.07 | SCoBSCo | ni-9〈V〉 |
| B5Co2Se2-3 (C1) | 0.9 | B5Co2S2-3 | 3.6 | 4, 4 | 4, 5 | 3 | 3.77 | 0.12 | SCoSB | capped square antiprism |
| B5Co2Se2-4 (C1) | 2.7 | B5Co2S2-2 | 2.8 | 3, 4 | 4, 5 | 3 | 3.40 | 0.07 | SCoBSB | ni-9〈V〉 |
| B5Co2Se2-5 (Cs) | 3.7 | B5Co2S2-5 | 6.0 | 4, 4 | 4, 4 | 2 | 3.65 | 0.11 | SCoSB | capped square antiprism |
| B5Co2Se2-6 (C1) | 6.9 | B5Co2S2-6 | 6.2 | 3, 4 | 4, 5 | 2 | 3.72 | 0.12 | SBBSB | ni-9〈V〉 |
| Cp2Co2Se2B6H6 | Cp2Co2S2B6H6 | Vertex Degrees | Co…S | Co…Co (E = Se) | Hexagonal Face | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Structure (sym) | ∆E | Structure | ∆E | S | Co | Edges | Dist (Å) | WBI | Atoms | Polyhedron |
| B6Co2Se2-1 (C2v) | 0.0 | B6Co2S2-1 | 0.0 | 3, 3 | 5, 5 | 2 | 3.77 | 0.10 | SBBBBS | B10H14 framework |
| B6Co2Se2-2 (C1) | 10.6 | B6Co2S2-2 | 12.4 | 3, 3 | 4, 5 | 2 | 3.78 | 0.09 | SBBSCoBS | B10H14 framework |
| B6Co2Se2-3 (C1) | 15.7 | B6Co2S2-3 | 18.1 | 3, 3 | 4, 5 | 2 | 2.49 | 0.41 | SBBSCoBS | B10H14 framework |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attia, A.A.A.; Lupan, A.; King, R.B. Polyhedral Dicobaltadithiaboranes and Dicobaltdiselenaboranes as Examples of Bimetallic Nido Structures without Bridging Hydrogens. Molecules 2023, 28, 2988. https://doi.org/10.3390/molecules28072988
Attia AAA, Lupan A, King RB. Polyhedral Dicobaltadithiaboranes and Dicobaltdiselenaboranes as Examples of Bimetallic Nido Structures without Bridging Hydrogens. Molecules. 2023; 28(7):2988. https://doi.org/10.3390/molecules28072988
Chicago/Turabian StyleAttia, Amr A. A., Alexandru Lupan, and Robert Bruce King. 2023. "Polyhedral Dicobaltadithiaboranes and Dicobaltdiselenaboranes as Examples of Bimetallic Nido Structures without Bridging Hydrogens" Molecules 28, no. 7: 2988. https://doi.org/10.3390/molecules28072988
APA StyleAttia, A. A. A., Lupan, A., & King, R. B. (2023). Polyhedral Dicobaltadithiaboranes and Dicobaltdiselenaboranes as Examples of Bimetallic Nido Structures without Bridging Hydrogens. Molecules, 28(7), 2988. https://doi.org/10.3390/molecules28072988






