Bioactive Compounds Produced by Endophytic Microorganisms Associated with Bryophytes—The “Bryendophytes”
Abstract
1. Introduction
2. Isolation of Endophytes
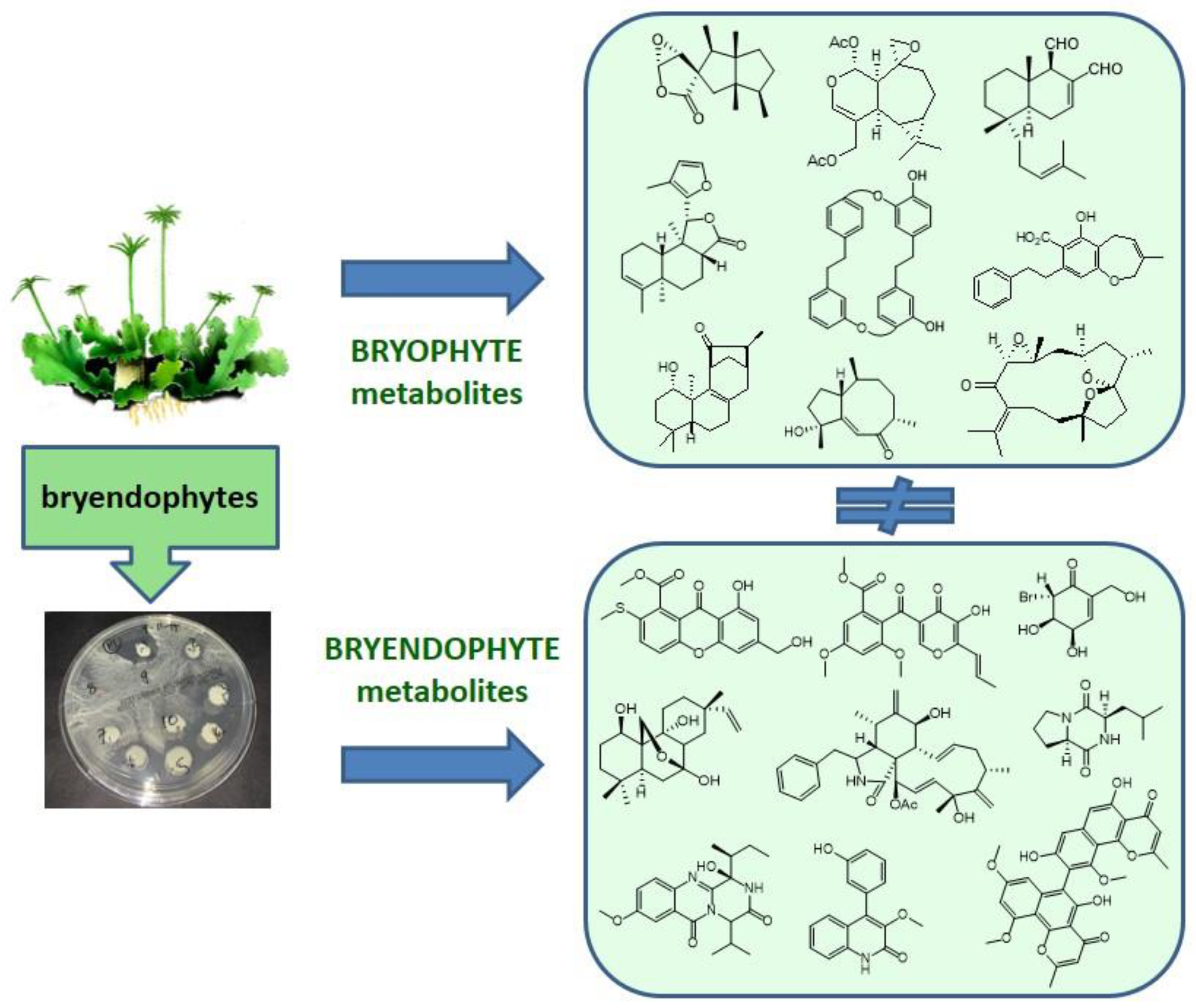
3. The Bryendophytes
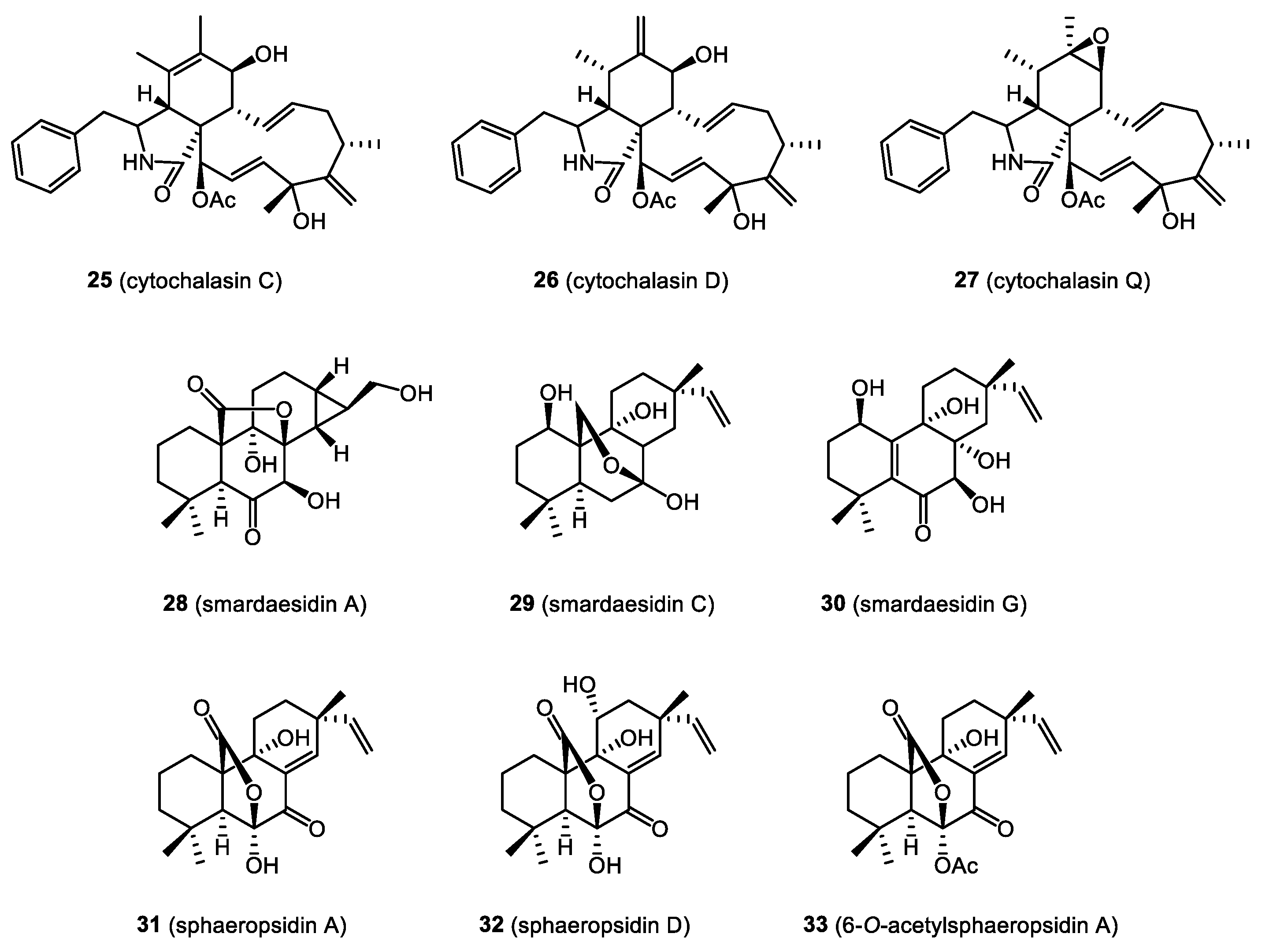
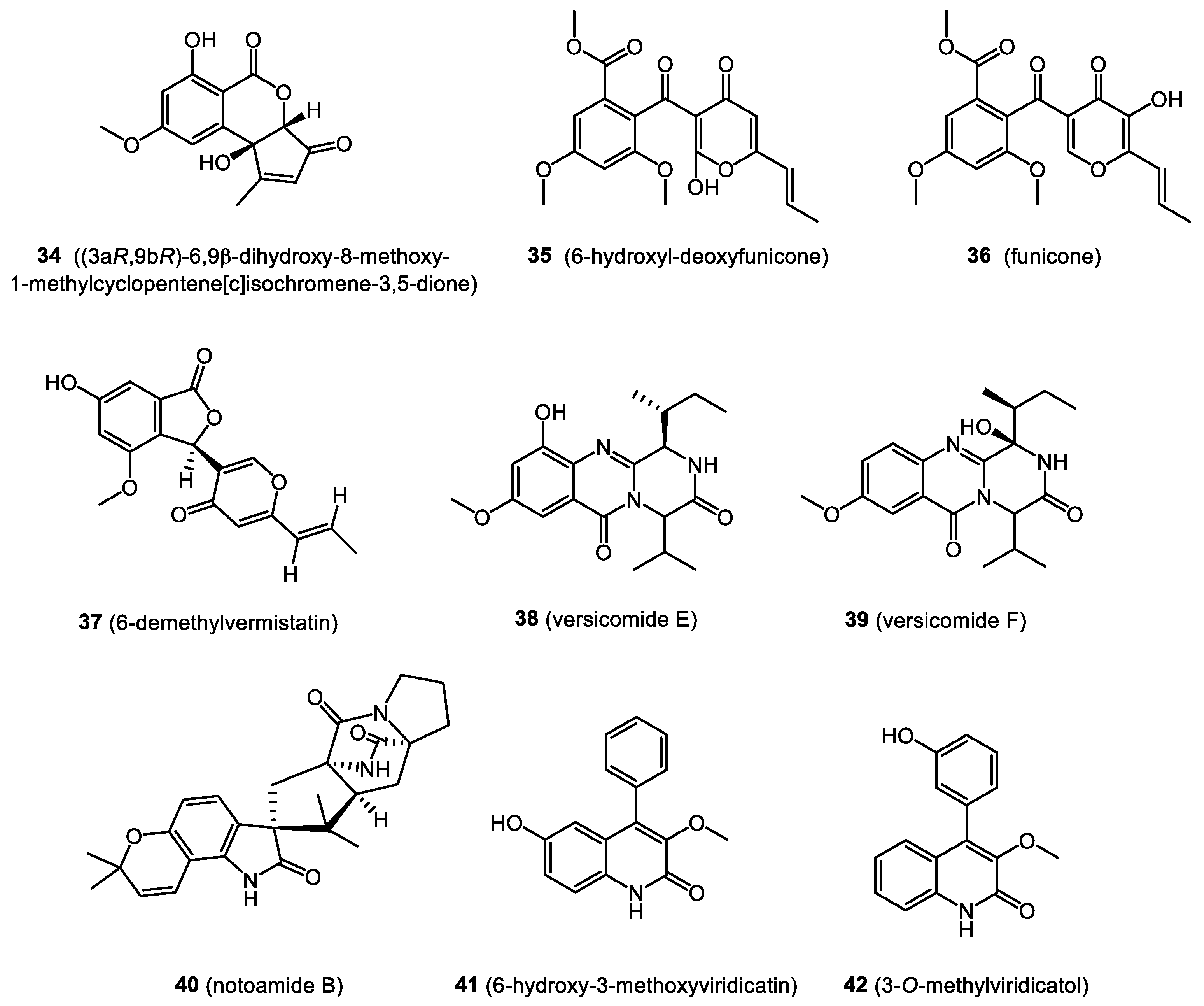
4. Bioactive Natural Products from Bryendophytes
4.1. Antibacterial and Antifungal Activity
4.2. Immunosuppressive Properties
4.3. Cytotoxicity and Anticancer Activity
4.4. Allelopathic Activity
4.5. Anti-Inflammatory Activity
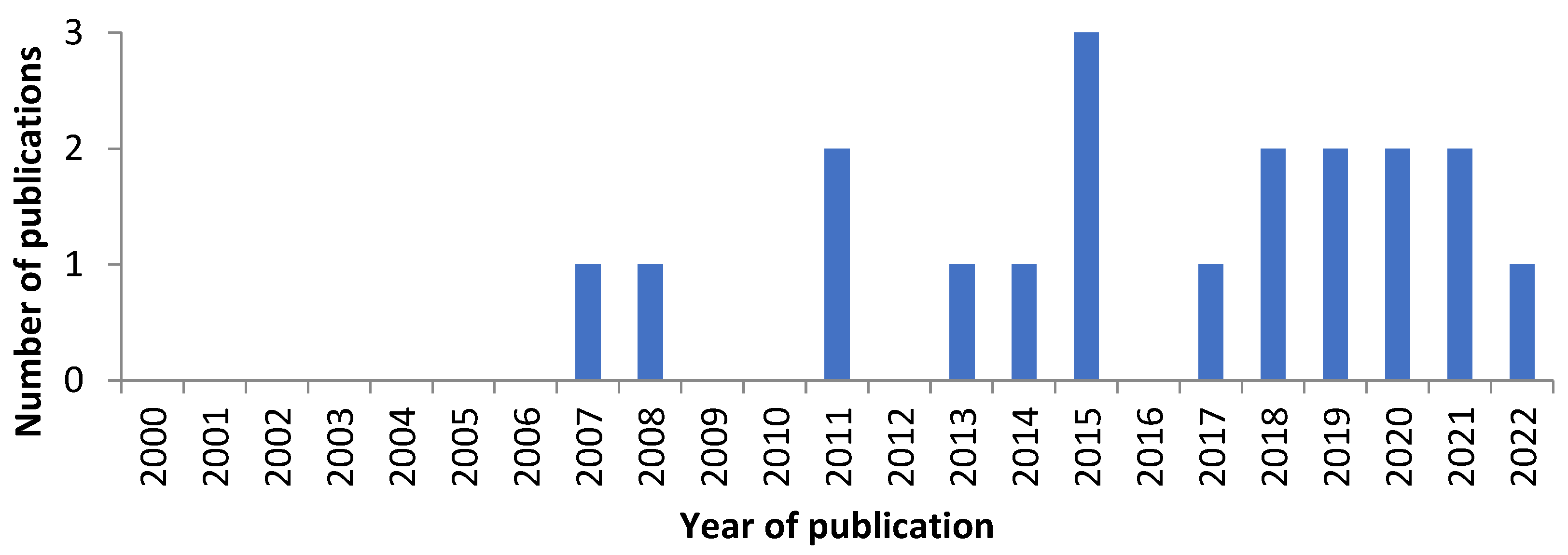
5. Chemical Diversity of Bryendophyte Metabolites
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clarke, J.T.; Warnock, R.C.M.; Donoghue, P.C.J. Establishing a time-scale for plant evolution. New Phytol. 2011, 192, 266–301. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.; Wittemann, M.; Cronberg, N. Allelopathy in bryophytes—A review. Lindbergia 2018, 41. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Phytochemical and biological studies of bryophytes. Phytochemistry 2013, 91, 52–80. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A. Chemical Constituents of Bryophytes: Structures and Biological Activity. J. Nat. Prod. 2018, 81, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ludwiczuk, A.; Wei, G.; Chen, X.; Crandall-Stotler, B.; Bowman, J.L. Terpenoid Secondary Metabolites in Bryophytes: Chemical Diversity, Biosynthesis and Biological Functions. CRC Crit. Rev. Plant Sci. 2018, 37, 210–231. [Google Scholar] [CrossRef]
- Opelt, K.; Berg, G. Diversity and antagonistic potential of bacteria associated with bryophytes from nutrient-poor habitats of the baltic sea coast. Appl. Environ. Microbiol. 2004, 70, 6569–6579. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Okyere, S.K.; Wang, S.; Wang, J.; Xie, L.; Ran, Y.; Hu, Y. Endophytic Fungi: An Effective Alternative Source of Plant-Derived Bioactive Compounds for Pharmacological Studies. J. Fungi 2022, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Pandey, S.; Alam, A. Isolation and characterization of endophytic bacteria associated with gametophytes of bryophytes in Mount Abu (Rajasthan). Rhizosphere 2022, 24, 100592. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.-S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Soares, D.A.; Rosa, L.H.; Da Silva, J.F.M.; Pimenta, R.S. A review of bioactive compounds produced by endophytic fungi associated with medicinal plants. Bol. Mus. Para. Emílio Goeldi-Ciências Nat. 2017, 12, 331–352. [Google Scholar] [CrossRef]
- Wicaksono, W.A.; Cernava, T.; Berg, C.; Berg, G. Bog ecosystems as a playground for plant–microbe coevolution: Bryophytes and vascular plants harbour functionally adapted bacteria. Microbiome 2021, 9, 170. [Google Scholar] [CrossRef]
- Ancheeva, E.; Daletos, G.; Proksch, P. Bioactive Secondary Metabolites from Endophytic Fungi. Curr. Med. Chem. 2020, 27, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Liu, J.; Chen, C.; Mo, X.; Tan, Q.; He, Y.; Wang, Z.; Yin, J.; Zhou, G. The Multifunctions and Future Prospects of Endophytes and Their Metabolites in Plant Disease Management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- de Paula Nogueira Cruz, F.; Bogas, A.C.; Paiva de Sousa, C. Plant-Associated Microorganisms as a Potent Bio-Factory of Active Molecules against Multiresistant Pathogens. In Antimicrobial Resistance—A One Health Perspective; IntechOpen: London, UK, 2021. [Google Scholar]
- Costa, L.E.D.O.; de Queiroz, M.V.; Borges, A.C.; de Moraes, C.A.; de Araújo, E.F. Isolation and Characterization of Endophytic Bacteria Isolated from the Leaves of the Common Bean (Phaseolus vulgaris). Braz. J. Microbiol. 2012, 43, 1562–1575. [Google Scholar] [CrossRef]
- Wijekoon, C.; Quill, Z. Fungal endophyte diversity in table grapes. Can. J. Microbiol. 2021, 67, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Mei, Y.; Wang, L.; Wang, X.; Xu, Q.; Peng, S.; Zhou, Y.; Wei, C. Isolation, Diversity, and Growth-Promoting Activities of Endophytic Bacteria from Tea Cultivars of Zijuan and Yunkang-10. Front. Microbiol. 2018, 9, 1848. [Google Scholar] [CrossRef]
- Stelmasiewicz, M.; Światek, Ł.; Ludwiczuk, A. Phytochemical profile and anticancer potential of endophytic microorganisms from liverwort species, Marchantia polymorpha L. Molecules 2022, 27, 153. [Google Scholar] [CrossRef]
- Zinniel, D.K.; Lambrecht, P.; Harris, N.B.; Feng, Z.; Kuczmarski, D.; Higley, P.; Ishimaru, C.A.; Arunakumari, A.; Barletta, R.G.; Vidaver, A.K. Isolation and Characterization of Endophytic Colonizing Bacteria from Agronomic Crops and Prairie Plants. Appl. Environ. Microbiol. 2002, 68, 2198–2208. [Google Scholar] [CrossRef]
- Wang, W.; Zhai, Y.; Cao, L.; Tan, H.; Zhang, R. Endophytic bacterial and fungal microbiota in sprouts, roots and stems of rice (Oryza sativa L.). Microbiol. Res. 2016, 188–189, 1–8. [Google Scholar] [CrossRef]
- Ding, X.; Liu, K.; Deng, B.; Chen, W.; Li, W.; Liu, F. Isolation and characterization of endophytic fungi from Camptotheca acuminata. World J. Microbiol. Biotechnol. 2013, 29, 1831–1838. [Google Scholar] [CrossRef]
- Cappelletti, S.; Piacentino, D.; Fineschi, V.; Frati, P.; D’Errico, S.; Aromatario, M. Mercuric chloride poisoning: Symptoms, analysis, therapies, and autoptic findings. A review of the literature. Crit. Rev. Toxicol. 2019, 49, 329–341. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, J.B.A.; Lorenzi, A.S.; do Vale, H.M.M. Methods used for the study of endophytic fungi: A review on methodologies and challenges, and associated tips. Arch. Microbiol. 2022, 204, 675. [Google Scholar] [CrossRef]
- Sahu, P.K.; Tilgam, J.; Mishra, S.; Hamid, S.; Gupta, A.; Verma, S.K.; Kharwar, R.N. Surface sterilization for isolation of endophytes: Ensuring what (not) to grow. J. Basic Microbiol. 2022, 62, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Alvin, A.; Kalaitzis, J.A.; Sasia, B.; Neilan, B.A. Combined genetic and bioactivity-based prioritization leads to the isolation of an endophyte-derived antimycobacterial compound. J. Appl. Microbiol. 2016, 120, 1229–1239. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.; Chang, C.-F.; Chi, W.-C. Isolation of endophytic fungi with antimicrobial activity from medicinal plant Zanthoxylum simulans Hance. Folia Microbiol. 2021, 66, 385–397. [Google Scholar] [CrossRef]
- Cun, H.; Munir, S.; He, P.; Wu, Y.; He, P.; Ahmed, A.; Che, H.; Li, J.; He, Y. Diversity of root endophytic bacteria from maize seedling involved in biocontrol and plant growth promotion. Egypt. J. Biol. Pest Control 2022, 32, 129. [Google Scholar] [CrossRef]
- Costa, D.; Fernandes, T.; Martins, F.; Pereira, J.A.; Tavares, R.M.; Santos, P.M.; Baptista, P.; Lino-Neto, T. Illuminating Olea europaea L. endophyte fungal community. Microbiol. Res. 2021, 245, 126693. [Google Scholar] [CrossRef]
- Ameen, F.; Stephenson, S.L.; AlNadhari, S.; Yassin, M.A. Isolation, identification and bioactivity analysis of an endophytic fungus isolated from Aloe vera collected from Asir desert, Saudi Arabia. Bioprocess Biosyst. Eng. 2021, 44, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Dwibedi, V.; Saxena, S. Arcopilus aureus, a Resveratrol-Producing Endophyte from Vitis vinifera. Appl. Biochem. Biotechnol. 2018, 186, 476–495. [Google Scholar] [CrossRef]
- Koua, F.H.M.; Kimbara, K.; Tani, A. Bacterial-biota dynamics of eight bryophyte species from different ecosystems. Saudi J. Biol. Sci. 2015, 22, 204–210. [Google Scholar] [CrossRef]
- Guo, L.; Wu, J.Z.; Han, T.; Cao, T.; Rahman, K.; Qin, L.P. Chemical composition, antifungal and antitumor properties of ether extracts of Scapania verrucosa Heeg. and its endophytic fungus Chaetomium fusiforme. Molecules 2008, 13, 2114–2125. [Google Scholar] [CrossRef]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Schüßler, A. Glomus claroideum forms an arbuscular mycorrhiza-like symbiosis with the hornwort Anthoceros punctatus. Mycorrhiza 2000, 10, 15–21. [Google Scholar] [CrossRef]
- Turnau, K.; Ronikier, M.; Unrug, J. Role of mycorhizal links between plants in establishment of liverworts thalli in natural habitats. Acta Soc. Bot. Pol. 2014, 68, 63–68. [Google Scholar] [CrossRef]
- Deshmukh, S.K.; Dufossé, L.; Chhipa, H.; Saxena, S.; Mahajan, G.B.; Gupta, M.K. Fungal Endophytes: A Potential Source of Antibacterial Compounds. J. Fungi 2022, 8, 164. [Google Scholar] [CrossRef] [PubMed]
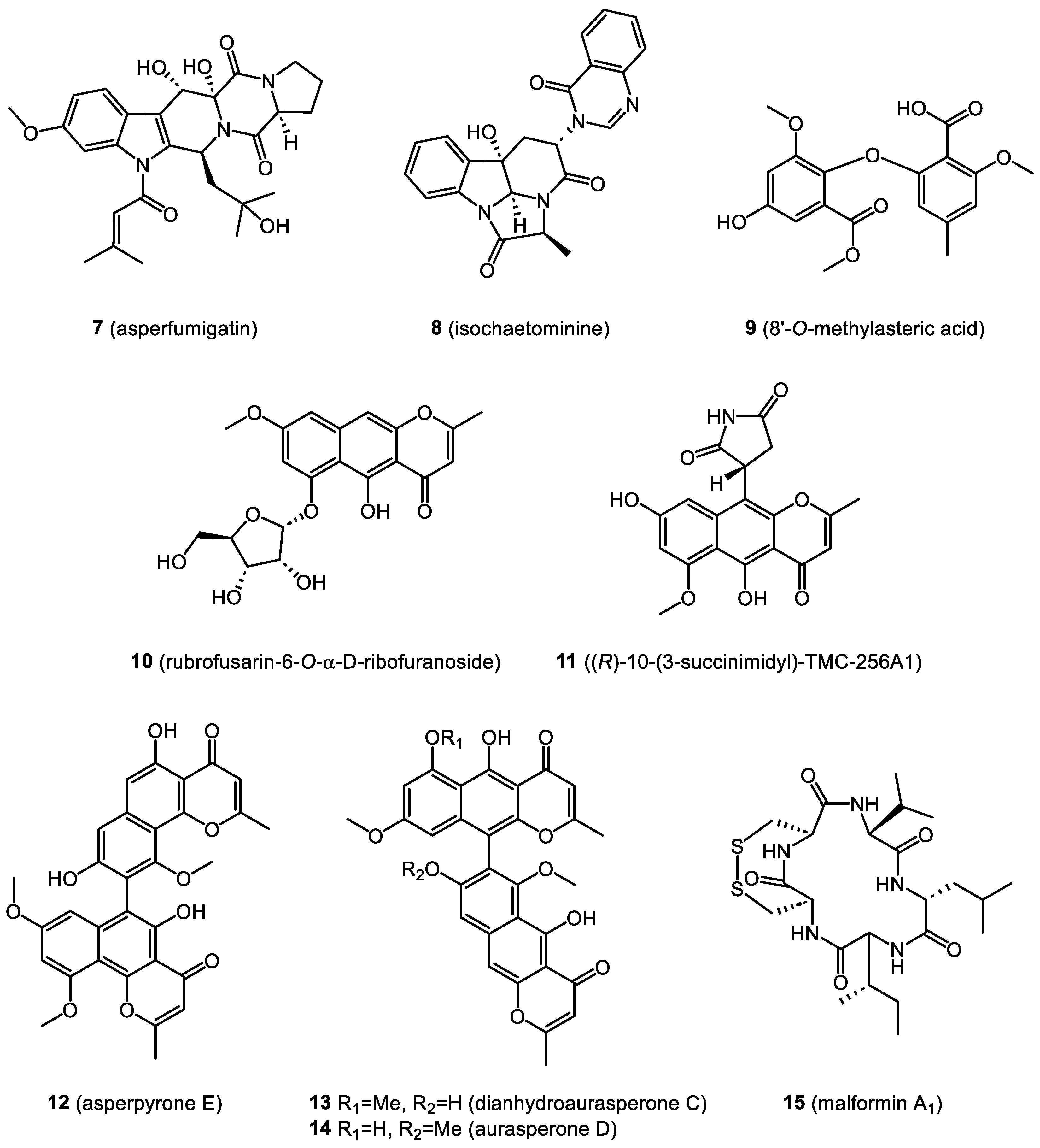
- Xie, F.; Li, X.-B.; Zhou, J.-C.; Xu, Q.-Q.; Wang, X.-N.; Yuan, H.-Q.; Lou, H.-X. Secondary metabolites from Aspergillus fumigatus, an endophytic fungus from the liverwort Heteroscyphus tener (Steph.) Schiffn. Chem. Biodivers. 2015, 12, 1313–1321. [Google Scholar] [CrossRef]
- Yu, N.H.; Park, S.-Y.; Kim, J.A.; Park, C.-H.; Jeong, M.-H.; Oh, S.-O.; Hong, S.G.; Talavera, M.; Divakar, P.K.; Hur, J.-S. Endophytic and endolichenic fungal diversity in maritime Antarctica based on cultured material and their evolutionary position among Dikarya. Fungal Syst. Evol. 2018, 2, 263–272. [Google Scholar] [CrossRef]
- Arnold, A.E.; Harrington, A.H.; Huang, Y.-L.; U’Ren, J.M.; Massimo, N.C.; Knight-Connoni, V.; Inderbitzin, P. Coniochaeta elegans sp. nov., Coniochaeta montana sp. nov. and Coniochaeta nivea sp. nov., three new species of endophytes with distinctive morphology and functional traits. Int. J. Syst. Evol. Microbiol. 2021, 71, 005003. [Google Scholar] [CrossRef]
- Davey, M.L.; Currah, R.S. A new species of Cladophialophora (hyphomycetes) from boreal and montane bryophytes. Mycol. Res. 2007, 111, 106–116. [Google Scholar] [CrossRef]
- Melo, I.S.; Santos, S.N.; Rosa, L.H.; Parma, M.M.; Silva, L.J.; Queiroz, S.C.N.; Pellizari, V.H. Isolation and biological activities of an endophytic Mortierella alpina strain from the Antarctic moss Schistidium antarctici. Extremophiles 2014, 18, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-N.; Bashyal, B.P.; Wijeratne, E.M.K.; U’Ren, J.M.; Liu, M.X.; Gunatilaka, M.K.; Arnold, A.E.; Gunatilaka, A.A.L. Smardaesidins A–G, Isopimarane and 20- nor -Isopimarane Diterpenoids from Smardaea sp., a Fungal Endophyte of the Moss Ceratodon purpureus. J. Nat. Prod. 2011, 74, 2052–2061. [Google Scholar] [CrossRef]
- Wei, H.; Xu, Y.; Espinosa-Artiles, P.; Liu, M.X.; Luo, J.-G.; U’Ren, J.M.; Arnold, A.E.; Gunatilaka, A.A.L. Sesquiterpenes and other constituents of Xylaria sp. NC1214, a fungal endophyte of the moss Hypnum sp. Phytochemistry 2015, 118, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.M.; Hauser, D.A.; Hinson, R.; Shaw, A.J. A novel experimental system using the liverwort Marchantia polymorpha and its fungal endophytes reveals diverse and context-dependent effects. New Phytol. 2018, 218, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.; Shaw, A.J. Exploring the natural microbiome of the model liverwort: Fungal endophyte diversity in Marchantia polymorpha L. Symbiosis 2019, 78, 45–59. [Google Scholar] [CrossRef]
- Bradbury, S.M. Response of the post-fire bryophyte community to salvage logging in boreal mixedwood forests of northeastern Alberta, Canada. For. Ecol. Manag. 2006, 234, 313–322. [Google Scholar] [CrossRef]
- Huang, G.; Lin, W.; Li, H.; Tang, Q.; Hu, Z.; Huang, H.; Deng, X.; Xu, Q. Pentacyclic Cytochalasins and Their Derivatives from the Endophytic Fungus Phomopsis sp. xz-18. Molecules 2021, 26, 6505. [Google Scholar] [CrossRef]
- Costa, J.-L.; Paulsrud, P.; Rikkinen, J.; Lindblad, P. Genetic Diversity of Nostoc Symbionts Endophytically Associated with Two Bryophyte Species. Appl. Environ. Microbiol. 2001, 67, 4393–4396. [Google Scholar] [CrossRef]
- Warshan, D.; Liaimer, A.; Pederson, E.; Kim, S.-Y.; Shapiro, N.; Woyke, T.; Altermark, B.; Pawlowski, K.; Weyman, P.D.; Dupont, C.L.; et al. Genomic Changes Associated with the Evolutionary Transitions of Nostoc to a Plant Symbiont. Mol. Biol. Evol. 2018, 35, 1160–1175. [Google Scholar] [CrossRef]
- Zhuang, X.; Peng, C.; Wang, Z.; Zhao, J.; Shen, Y.; Liu, C.; Xiang, W. Actinomadura physcomitrii sp. nov., a novel actinomycete isolated from moss [Physcomitrium sphaericum (Ludw) Fuernr]. Antonie Van Leeuwenhoek 2020, 113, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, Y.; Liu, C.; Wang, H.; Zhao, J.; Li, L.; Zhang, Z.; Wang, X.; Xiang, W. Microbispora bryophytorum sp. nov., an actinomycete isolated from moss (Bryophyta). Int. J. Syst. Evol. Microbiol. 2015, 65, 1274–1279. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Jin, P.; Zheng, W.; Chu, L.; Liu, C.; Li, J.; Xiang, W.; Wang, X. Actinoallomurus bryophytorum sp. nov., an endophytic actinomycete isolated from moss (Bryophyta). Antonie Van Leeuwenhoek 2015, 108, 453–459. [Google Scholar] [CrossRef]
- Li, C.; Jin, P.; Liu, C.; Ma, Z.; Zhao, J.; Li, J.; Wang, X.; Xiang, W. Streptomyces bryophytorum sp. nov., an endophytic actinomycete isolated from moss (Bryophyta). Antonie Van Leeuwenhoek 2016, 109, 1209–1215. [Google Scholar] [CrossRef]
- Opelt, K.; Chobot, V.; Hadacek, F.; Schönmann, S.; Eberl, L.; Berg, G. Investigations of the structure and function of bacterial communities associated with Sphagnum mosses. Environ. Microbiol. 2007, 9, 2795–2809. [Google Scholar] [CrossRef]
- Liu, X.L.; Liu, S.L.; Liu, M.; Kong, B.H.; Liu, L.; Li, Y.H. A primary assessment of the endophytic bacterial community in a xerophilous moss (Grimmia montana) using molecular method and cultivated isolates. Braz. J. Microbiol. 2014, 45, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Xiang, H.-B.; Zhang, Y.-Q.; Liu, H.-Y.; Wei, Y.-Z.; Zhao, L.-X.; Yu, L.-Y. Molecular analysis of fungal diversity associated with three bryophyte species in the Fildes Region, King George Island, maritime Antarctica. Extremophiles 2013, 17, 757–765. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Rumschlag-Booms, E.; Guan, Y.-F.; Liu, K.-L.; Wang, D.-Y.; Li, W.-F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S.; et al. Anti-HIV diphyllin glycosides from Justicia gendarussa. Phytochemistry 2017, 136, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.M.; Hauser, D.A.; Li, F. The diversity and community structure of symbiotic cyanobacteria in hornworts inferred from long-read amplicon sequencing. Am. J. Bot. 2021, 108, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Raghoebarsing, A.A.; Smolders, A.J.P.; Schmid, M.C.; Rijpstra, W.I.C.; Wolters-Arts, M.; Derksen, J.; Jetten, M.S.M.; Schouten, S.; Sinninghe Damsté, J.S.; Lamers, L.P.M.; et al. Methanotrophic symbionts provide carbon for photosynthesis in peat bogs. Nature 2005, 436, 1153–1156. [Google Scholar] [CrossRef]
- Rai, N.; Kumari Keshri, P.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Kumar Singh, S.; Gautam, V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycology 2021, 12, 139–159. [Google Scholar] [CrossRef]
- Caruso, D.J.; Palombo, E.A.; Moulton, S.E.; Zaferanloo, B. Exploring the Promise of Endophytic Fungi: A Review of Novel Antimicrobial Compounds. Microorganisms 2022, 10, 1990. [Google Scholar] [CrossRef]
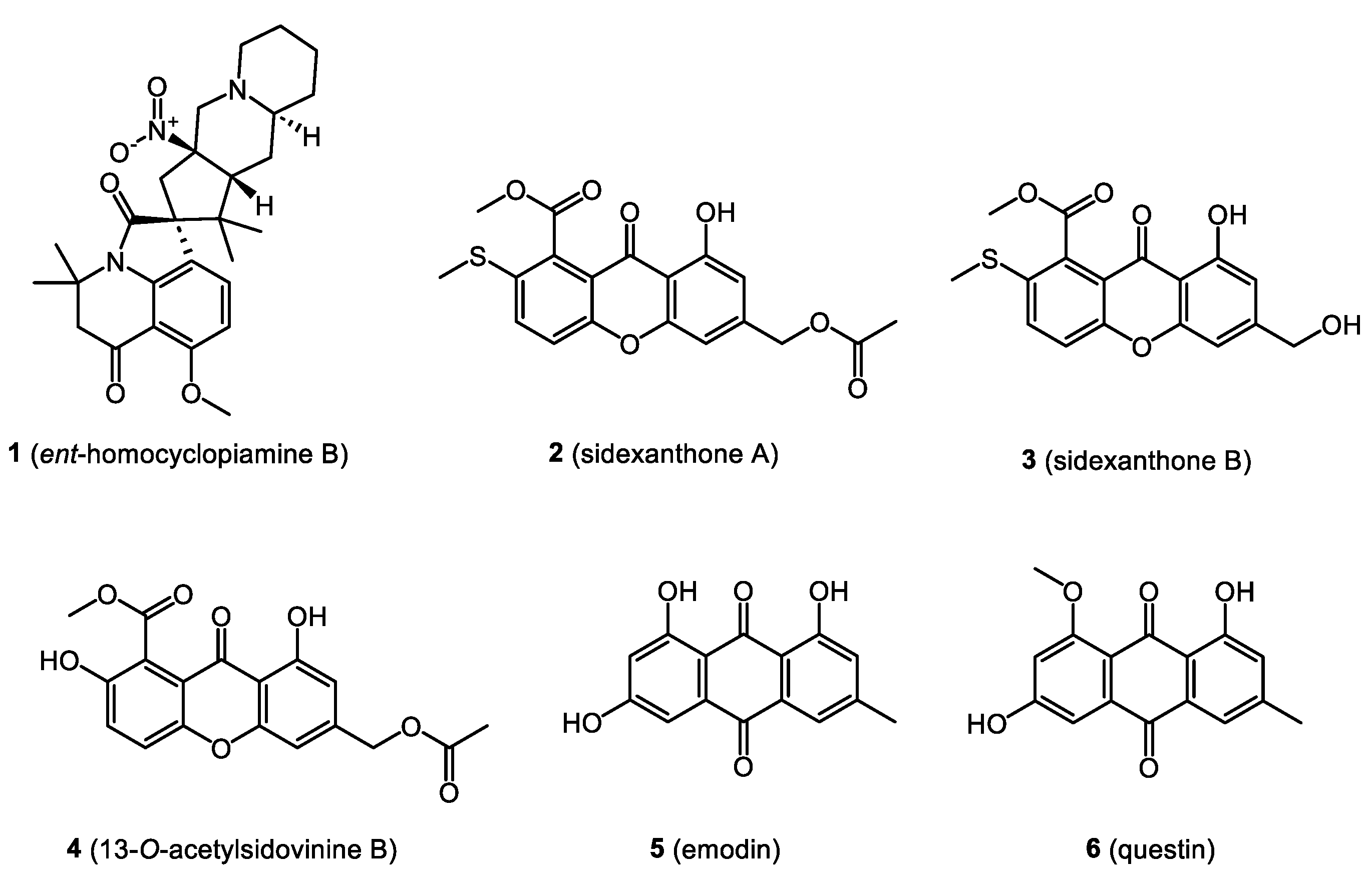
- Ali, T.; Pham, T.M.; Ju, K.-S.; Rakotondraibe, H.L. Ent-homocyclopiamine B, a Prenylated Indole Alkaloid of Biogenetic Interest from the Endophytic Fungus Penicillium concentricum. Molecules 2019, 24, 218. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-Q.; Zhang, X.; Han, Q.-J.; Li, X.-B.; Li, G.; Li, R.-J.; Jiao, Y.; Zhou, J.-C.; Lou, H.-X. Xanthone derivatives from Aspergillus sydowii, an endophytic fungus from the liverwort Scapania ciliata S. Lac and their immunosuppressive activities. Phytochem. Lett. 2013, 6, 318–321. [Google Scholar] [CrossRef]
- Li, X.B.; Xie, F.; Liu, S.S.; Li, Y.; Zhou, J.C.; Liu, Y.Q.; Yuan, H.Q.; Lou, H.X. Naphtho-γ-pyrones from endophyte Aspergillus niger occurring in the liverwort Heteroscyphus tener (Steph.) Schiffn. Chem. Biodivers. 2013, 10, 1193–1201. [Google Scholar] [CrossRef]
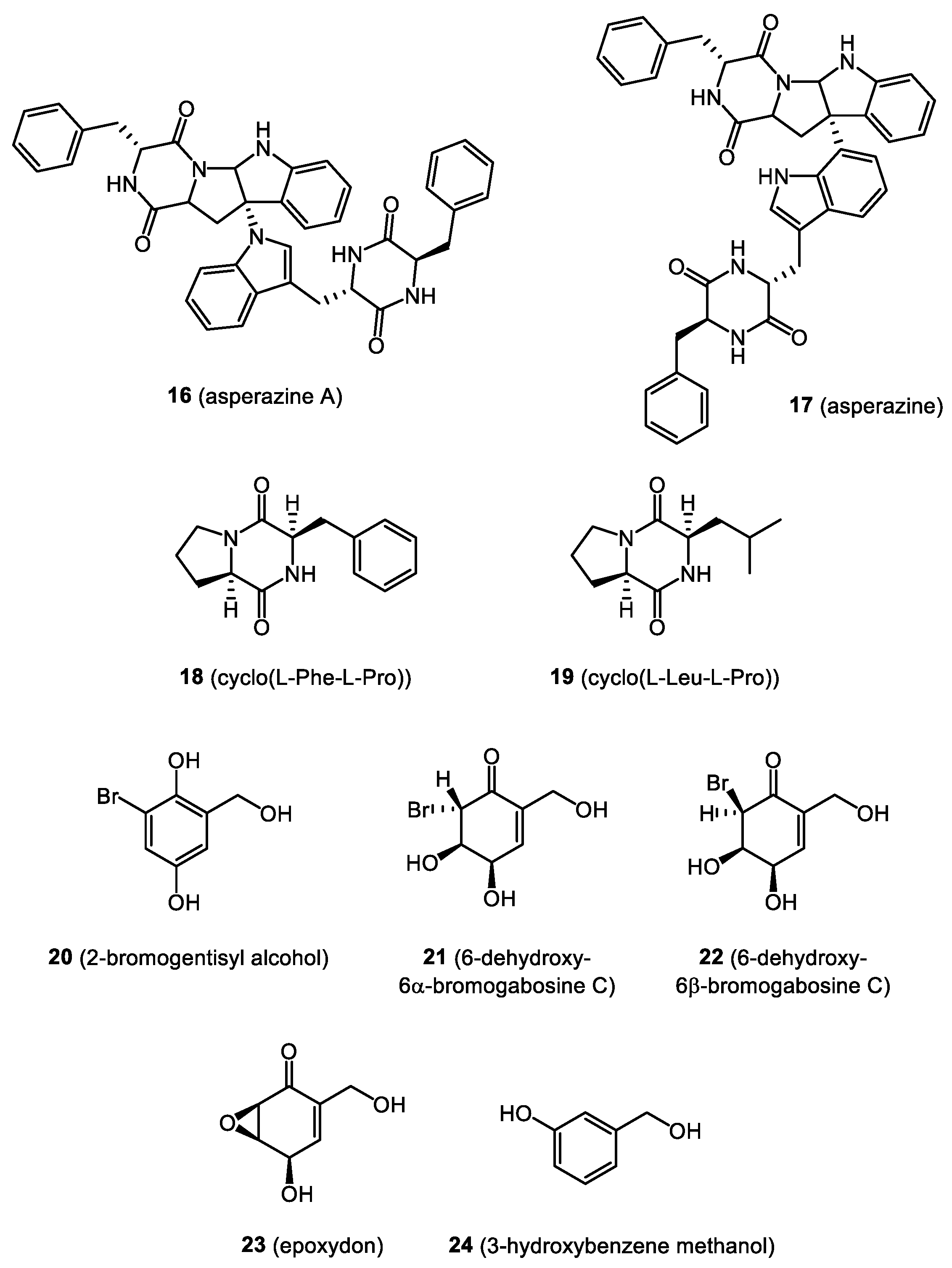
- Li, X.B.; Li, Y.L.; Zhou, J.C.; Yuan, H.Q.; Wang, X.N.; Lou, H.X. A new diketopiperazine heterodimer from an endophytic fungus Aspergillus niger. J. Asian Nat. Prod. Res. 2015, 17, 182–187. [Google Scholar] [CrossRef]
- Stelmasiewicz, M.; Światek, Ł.; Ludwiczuk, A. Chemical and Biological Studies of Endophytes Isolated from Marchantia polymorpha. Molecules 2023, 28, 2202. [Google Scholar] [CrossRef]
- Ali, T.; Inagaki, M.; Chai, H.B.; Wieboldt, T.; Rapplye, C.; Harinantenaina Rakotondraibe, L. Halogenated Compounds from Directed Fermentation of Penicillium concentricum, an Endophytic Fungus of the Liverwort Trichocolea tomentella. J. Nat. Prod. 2017, 80, 1397–1403. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Eugenio, G.D.; Ali, T.; Rakotondraibe, L.H.; Carcache De Blanco, E. Cytotoxic constituents from Penicillium concentricum, an endophytic fungus from Trichocolea tomentella. Anticancer. Drugs 2019, 30, 323–329. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, X.; Wang, L.; Li, G.; Zhou, J.C.; Lou, H.X. Metabolites from Penicillium sp.; An endophytic fungus from the liverwort Riccardia multifida (L.) S. Gray. Phytochem. Lett. 2013, 6, 14–17. [Google Scholar] [CrossRef]
- Wang, N.N.; Liu, C.Y.; Wang, T.; Li, Y.L.; Xu, K.; Lou, H.X. Two New Quinazoline Derivatives from the Moss Endophytic Fungus Aspergillus sp. and Their Anti-inflammatory Activity. Nat. Prod. Bioprospect. 2021, 11, 105–110. [Google Scholar] [CrossRef]
- Xu, K.; Li, X.-Q.; Zhao, D.-L.; Zhang, P. Antifungal Secondary Metabolites Produced by the Fungal Endophytes: Chemical Diversity and Potential Use in the Development of Biopesticides. Front. Microbiol. 2021, 12, 689527. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Ionta, M.; Horvath, R.O.; Soares, M.G.; Silva, D.O.; Kawafune, E.S.; Ferreira, M.J.P.; Sartorelli, P. Dereplication of Cytochalasans and Octaketides in Cytotoxic Extracts of Endophytic Fungi from Casearia arborea (Salicaceae). Metabolites 2022, 12, 903. [Google Scholar] [CrossRef] [PubMed]
- Qader, M.; Zaman, K.A.U.; Hu, Z.; Wang, C.; Wu, X.; Cao, S. Aspochalasin H1: A New Cyclic Aspochalasin from Hawaiian Plant-Associated Endophytic Fungus Aspergillus sp. FT1307. Molecules 2021, 26, 4239. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, R.; Shanmuganathan, R.; Pugazhendhi, A. Vinblastine production by the endophytic fungus Curvularia verruculosa from the leaves of Catharanthus roseus and its in vitro cytotoxicity against HeLa cell line. Anal. Biochem. 2020, 593, 113530. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.P.; Abraham, W.-R. Antimicrobial and Biofilm Inhibiting Diketopiperazines. Curr. Med. Chem. 2012, 19, 3564–3577. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, Y.; Ludwiczuk, A.; Nagashima, F. Chemical constituents of bryophytes: Bio- and chemical diversity, biological activity, and chemosystematics. In Progress in the Chemistry of Organic Natural Products; Kinghorn, A.D., Falk, H., Kobayashi, J., Eds.; Springer-Verlag: Vienna, Austria, 2013; Volume 95, pp. 1–796. [Google Scholar]

| Source | Endophyte | Type | Identification | References |
|---|---|---|---|---|
| Hornwort Anthoceros fusiformis | Nostoc sp. | Cyanobacteria | tRNALeu gene | [49] |
| Liverwort Blasia pusilla | Nostoc sp. | Cyanobacteria | tRNALeu gene | |
| Feathermosses Pleurozium schreberi and Hylocomium splendens Liverwort Blasia pusilla | Nostoc sp. | Cyanobacteria | DNA sequencing; PacBio technology | [50] |
| Moss Physcomitrium sphaericum | Actinomadura physcomitrii sp. nov. | Bacteria (actinomycete) | 16S rRNA | [51] |
| Moss ** | Microbispora bryophytorum sp. nov. | Bacteria (actinomycete) | 16S rRNA | [52] |
| Moss ** | Actinoallomurus bryophytorum sp. nov. | Bacteria (actinomycete) | 16S rRNA | [53] |
| Moss ** | Streptomyces bryophytorum sp. nov. | Bacteria (actinomycete) | 16S rRNA | [54] |
| Mosses Sphagnum magellanicum and Sphagnum fallax | Burkholderia sp., Rahnella aquatilis, Paenibacillus polymyxa, Pseudomonas tolaasii, Hafnia sp., Microbacterium phyllosphaerae, and Streptomyces purpurascens | Bacteria | 16S rRNA | [55] |
| Moss Grimmia montana | Gammaproteobacteria: Acinetobacter sp., Leclercia sp., Aeromonas sp., Aeromonas sp., et al. | Bacteria | 16S rRNA | [56] |
| Alphaproteobacteria: Rhizobium sp., Brevundimonas sp., Methylobacterium sp., et al. | ||||
| Betaproteobacteria: Bordetella sp., Comamonas sp., Methylophilus sp. et al. | ||||
| Firmicutes: Planococcus sp., Planomicrobium sp., Bacillus sp., et al. | ||||
| Moss Hylocomiaceae ** | Coniochaeta nivea sp. nov. | Fungi | ITS rDNA | [40] |
| Moss Schistidium antarctici | Mortierella alpina | Fungi | 18S rRNA | [42] |
| Moss Ceratodon purpureus | Smardaea sp. | Fungi | ITS rDNA LSU rDNA | [43] |
| Mosses Polytrichum juniperinum, Aulacomnium palustre, and Sphagnum fuscum | Cladophialophora minutissima | Fungi | ITS 18S SSU (18S rRNA) | [41] |
| Moss, Hypnum sp. (Hypnaceae) | Xylaria sp. * | Fungi | ITS rDNA, LSU rDNA | [44] |
| Liverwort, Marchantia polymorpha | Biscogniauxia mediterranea | Fungi | ITS1, ITS2, LSU rDNA | [45] |
| Colletotrichum truncatum | ||||
| Daldinia loculata | ||||
| Hypoxylon submonticulosum | ||||
| Nemania serpens | ||||
| Phoma herbarum | ||||
| Toxicocladosporium irritans | ||||
| Xylaria cubensis, Xylaria arbuscula | ||||
| Candida sp., Coniochaetaceae sp., Helotiaceae sp., Pezizales sp., Plectosphaerella sp., Pleosporales sp., Schizothecium sp. * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stelmasiewicz, M.; Świątek, Ł.; Gibbons, S.; Ludwiczuk, A. Bioactive Compounds Produced by Endophytic Microorganisms Associated with Bryophytes—The “Bryendophytes”. Molecules 2023, 28, 3246. https://doi.org/10.3390/molecules28073246
Stelmasiewicz M, Świątek Ł, Gibbons S, Ludwiczuk A. Bioactive Compounds Produced by Endophytic Microorganisms Associated with Bryophytes—The “Bryendophytes”. Molecules. 2023; 28(7):3246. https://doi.org/10.3390/molecules28073246
Chicago/Turabian StyleStelmasiewicz, Mateusz, Łukasz Świątek, Simon Gibbons, and Agnieszka Ludwiczuk. 2023. "Bioactive Compounds Produced by Endophytic Microorganisms Associated with Bryophytes—The “Bryendophytes”" Molecules 28, no. 7: 3246. https://doi.org/10.3390/molecules28073246
APA StyleStelmasiewicz, M., Świątek, Ł., Gibbons, S., & Ludwiczuk, A. (2023). Bioactive Compounds Produced by Endophytic Microorganisms Associated with Bryophytes—The “Bryendophytes”. Molecules, 28(7), 3246. https://doi.org/10.3390/molecules28073246







