Characterization of Delonix regia Flowers’ Pigment and Polysaccharides: Evaluating Their Antibacterial, Anticancer, and Antioxidant Activities and Their Application as a Natural Colorant and Sweetener in Beverages
Abstract
1. Introduction
2. Results and Discussion
2.1. Proximate Composition of Delonix regia
2.2. Phenolic Compounds
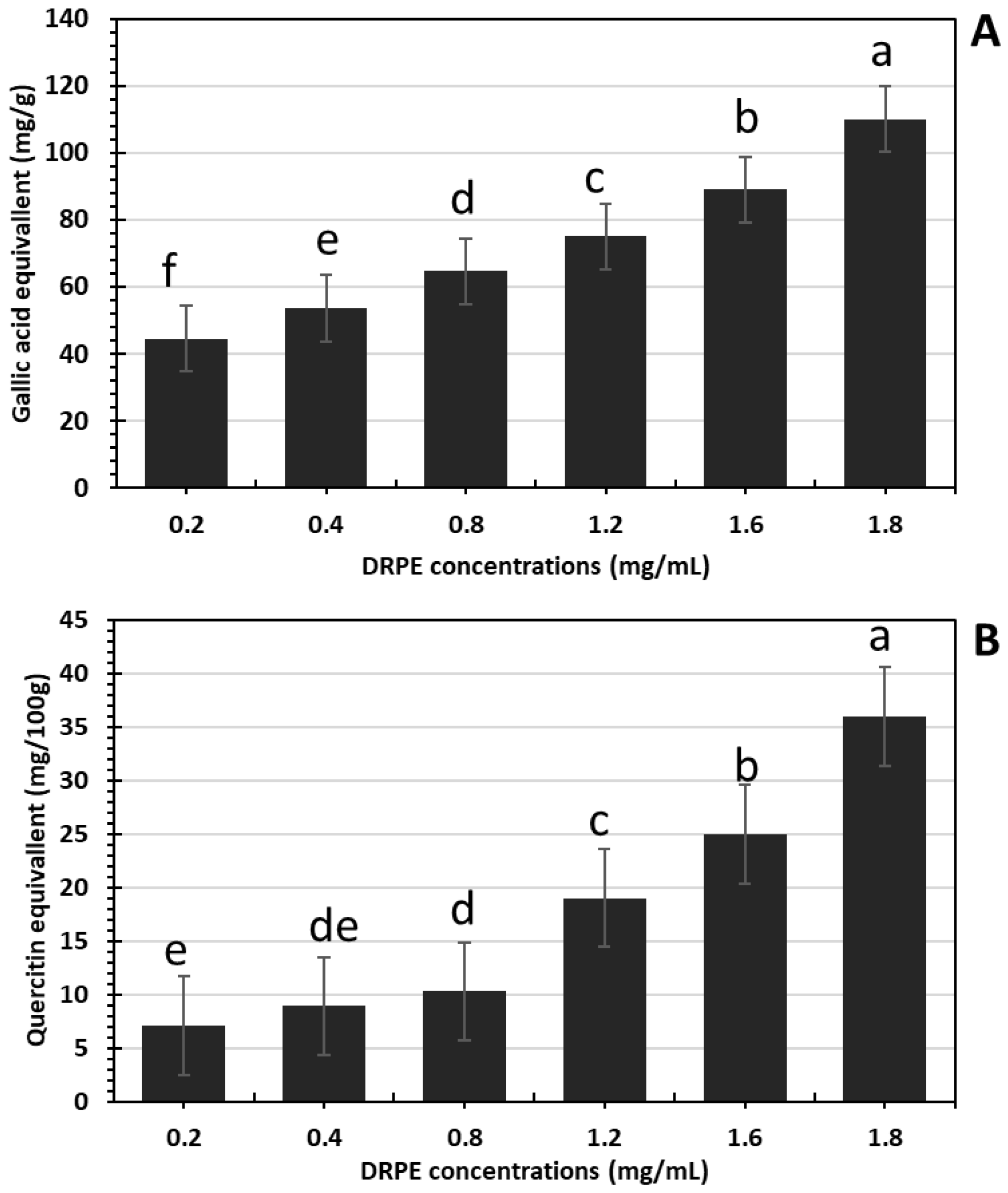
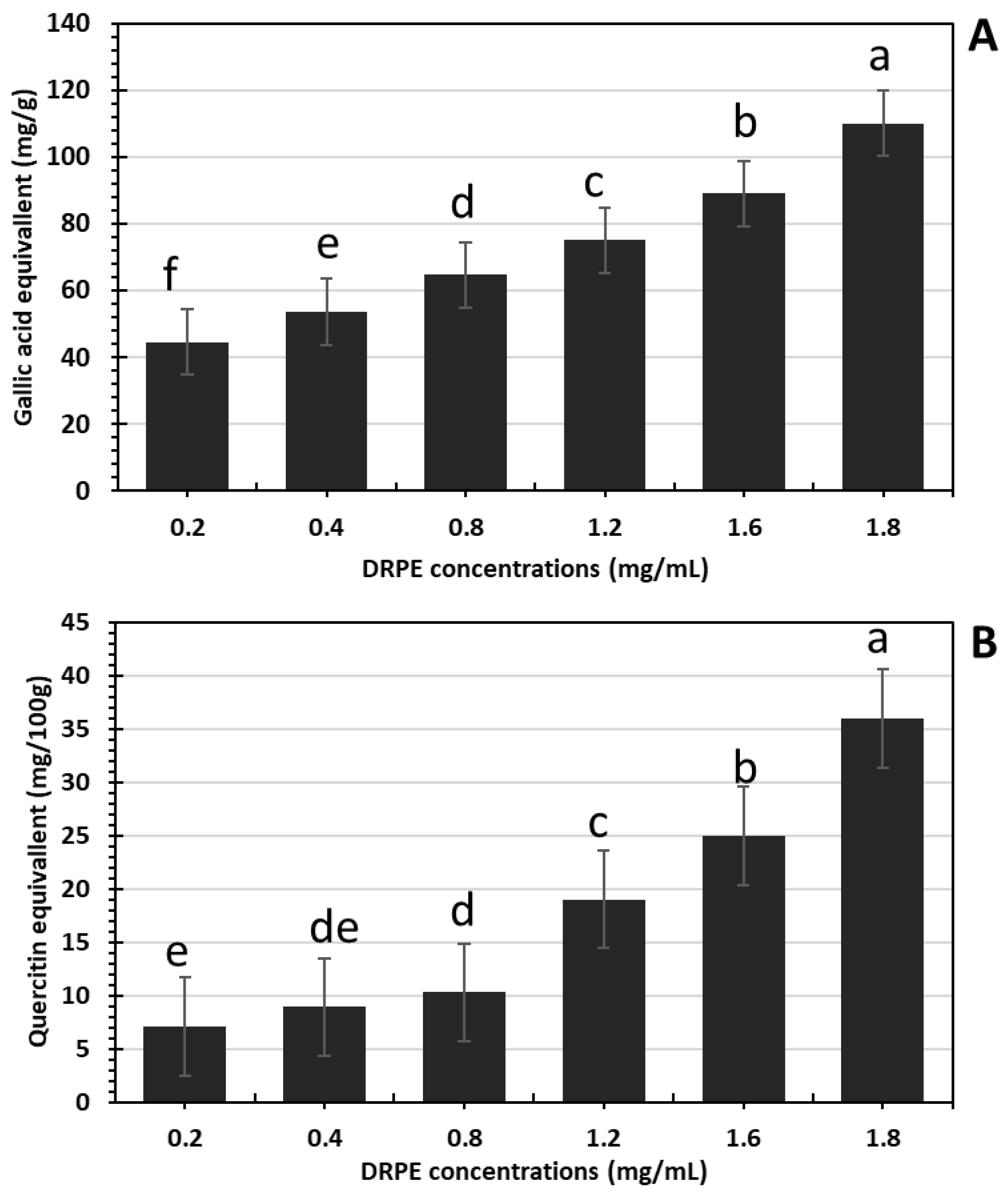
2.2.1. Total Phenols and Flavonoid Contents in DRPE
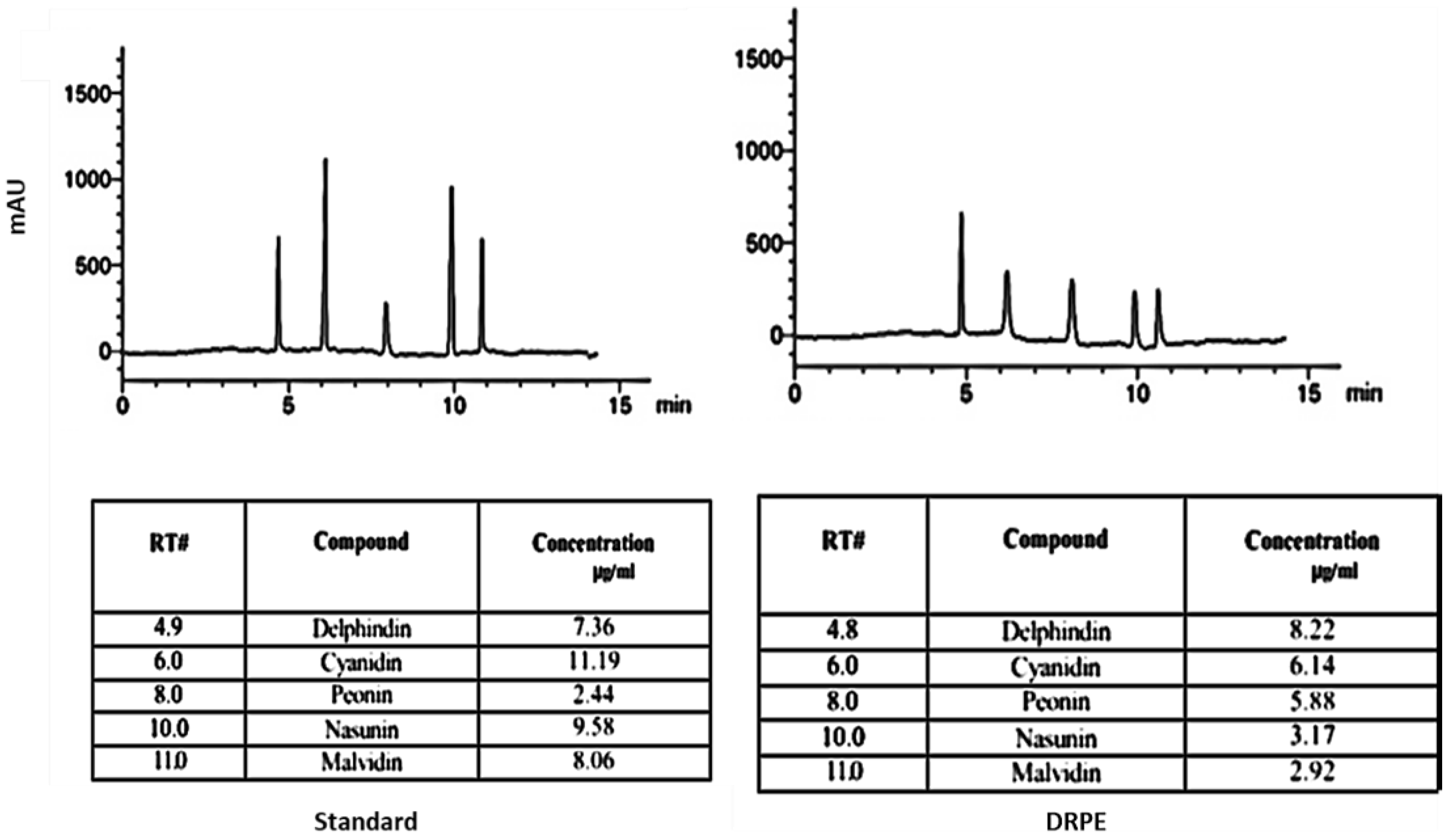
2.2.2. Anthocyanin Content
2.3. Isolation, Characterization, and Identification of Polysaccharide Contents in DRPE
2.3.1. Isolation
2.3.2. Characterization
FTIR Spectra
1 H-NMR Analysis of Delonix regia Polysaccharide
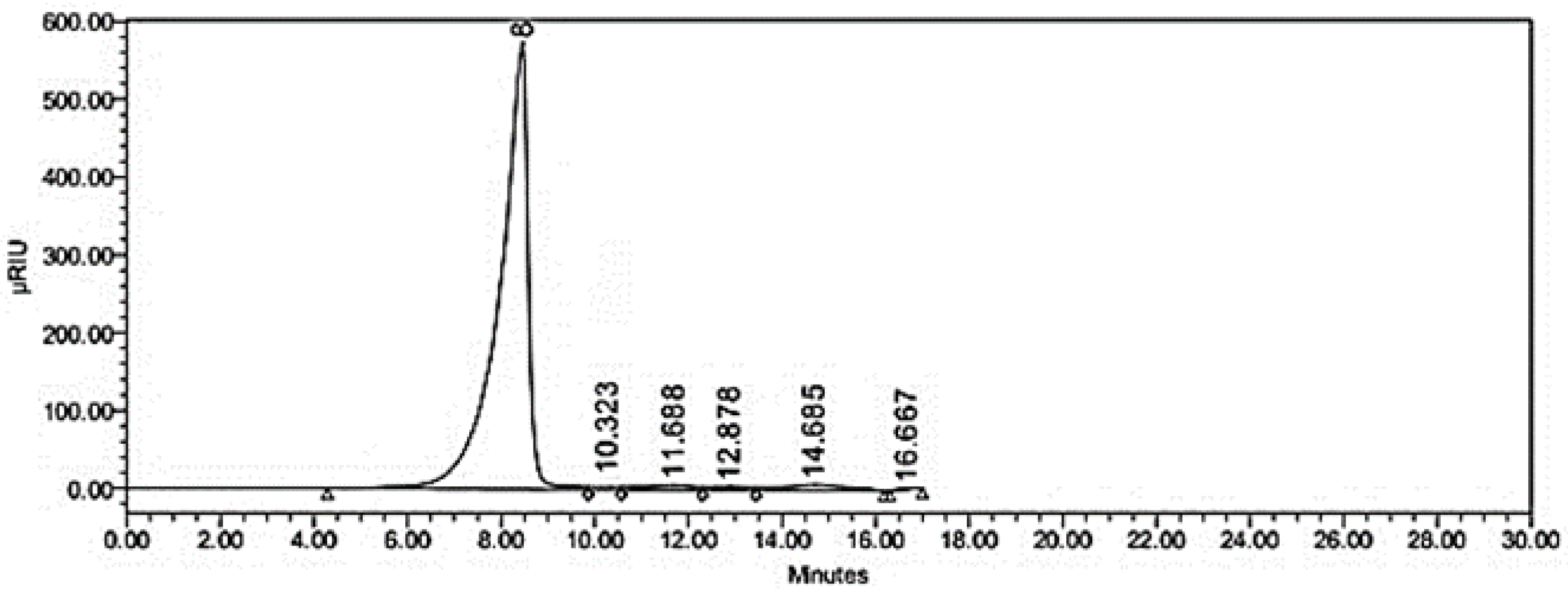
2.3.3. Identification of Sugar Profile of Isolated Galactomannans by HPLC
2.4. Biological Activities of DRPE
2.4.1. Antioxidant
DPPH Assay
Ferric-Reducing Antioxidant Power (FRAP)
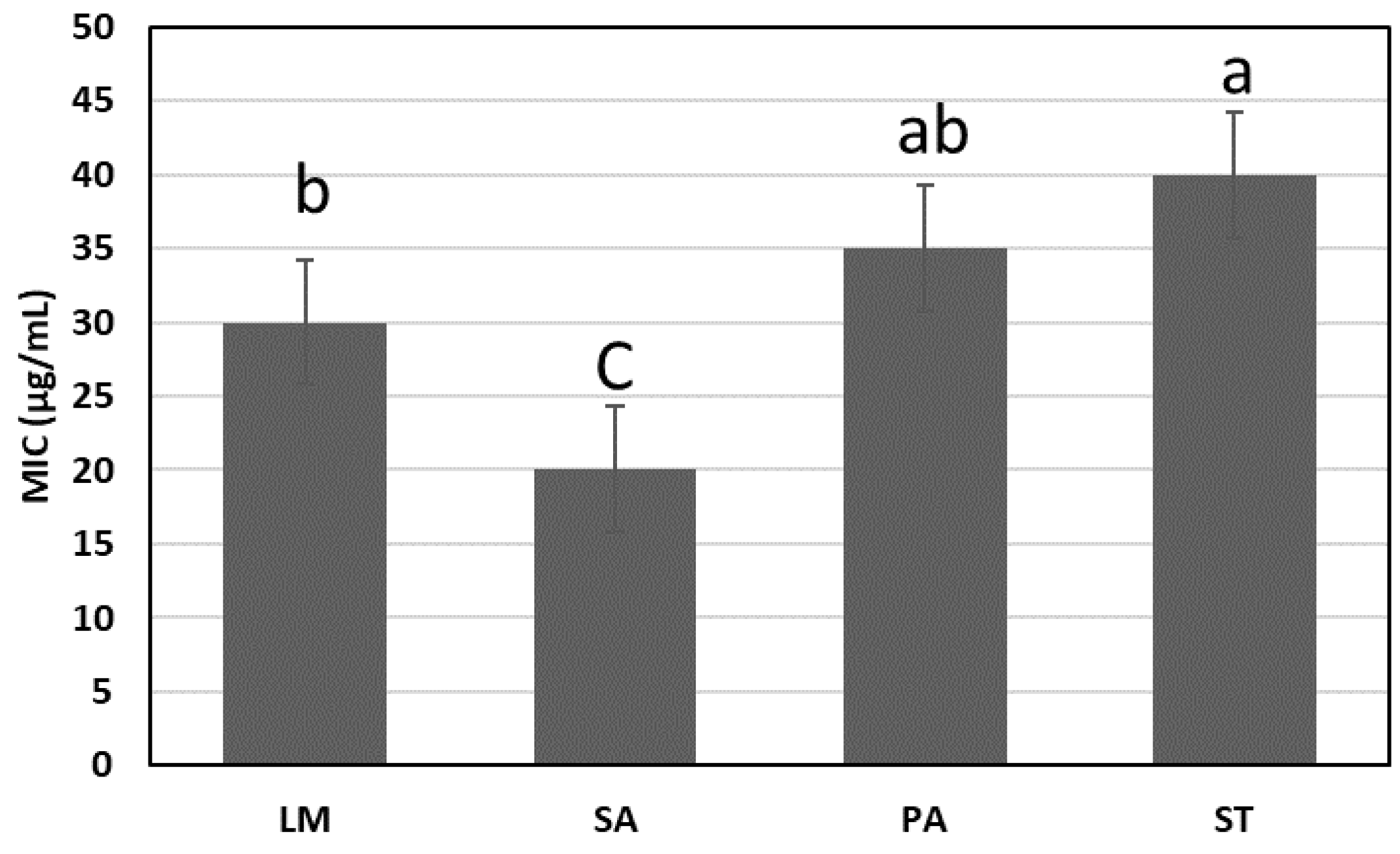
2.4.2. Antibacterial
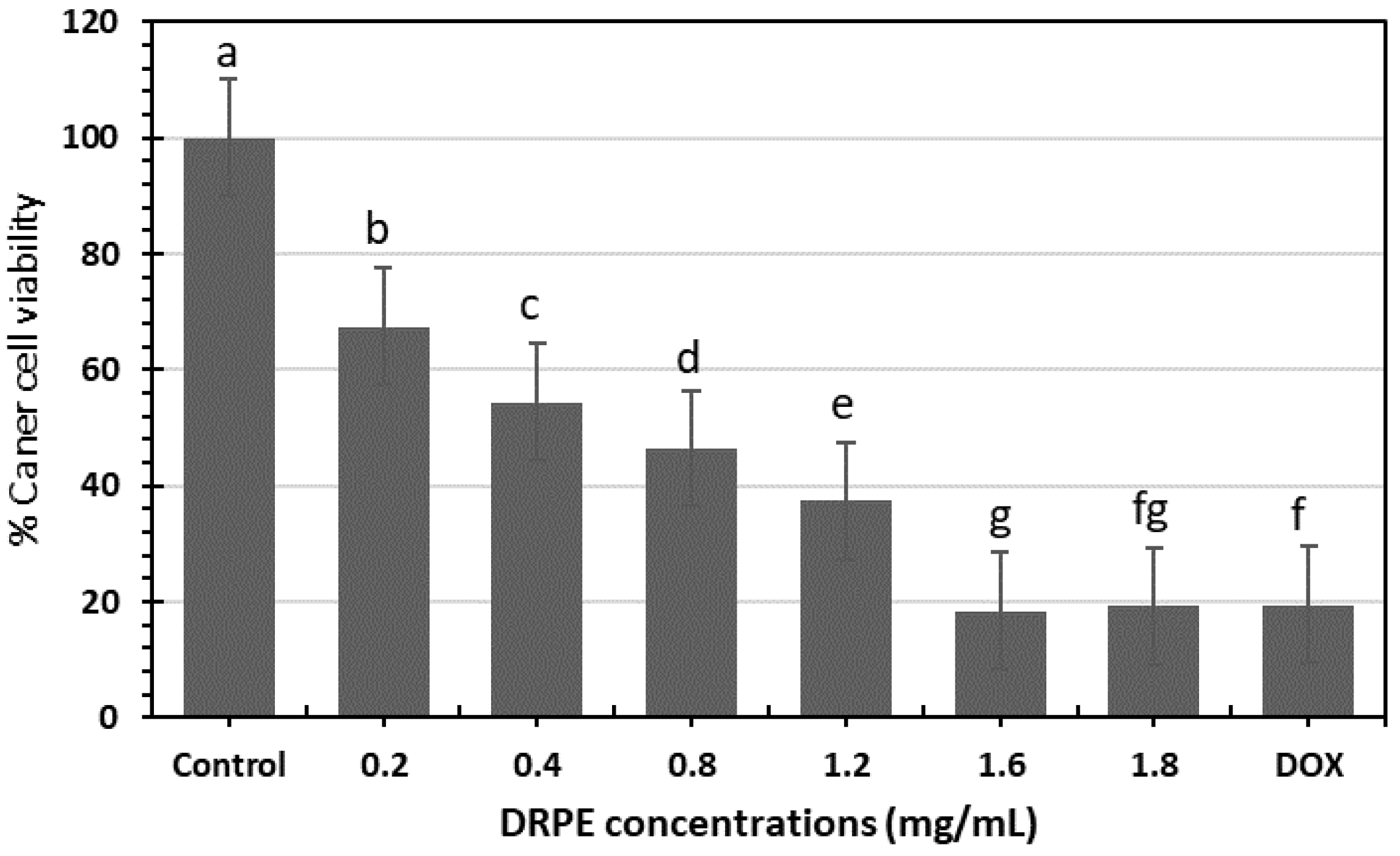
2.4.3. Anticancer
2.5. Safety Experiment
2.6. The Experiment of Strawberry Beverage Supplemented with DRPE at Different Concentrations (200, 800, and 1600 µg/g) as a Natural Colorant and Sweetener
Sensory Properties and Color Changes
3. Materials and Methods
3.1. Materials
3.2. Proximate Analysis of Delonix regia Flowers
3.3. Preparation of Delonix regia Pigment Extract
3.4. Polyphenolic Content in DRPE
3.4.1. Total Phenolics (TFs) and Flavonoids (TFs)
3.4.2. Anthocyanins
Total Anthocyanins (TAs)
Anthocyanins Profile by HPLC
3.5. Polysaccharides Content in Delonix regia Flowers
3.5.1. Extraction of the Crude Polysaccharide
3.5.2. Monosaccharide Profile
3.5.3. Molecular Weight Determination
3.5.4. Characterization of DR Polysaccharides
FT-IR Analysis
1H-NMR Spectroscopy Analysis
3.6. Biological Activities of DRPE
3.6.1. Antioxidant
DPPH Assay
Ferric-Reducing Antioxidant Power (FRAP)
3.6.2. Cytotoxicity Effects
3.6.3. Antibacterial Activity
3.7. Safety Experiment
- The rats were fed a basal diet without additions as a negative control;
- As a positive control, the rats were fed a basal diet supplemented with a synthetic dye (1.6 mg/mL);
- As a positive control, the rats were fed a basal diet supplemented with aspartame (1.6 mg/mL);
- The rats’ group was fed a diet supplemented with DRPE (0.2 mg/g);
- The rats’ group was fed a diet supplemented with DRPE (1.6 mg/g).
Biochemical Examination
3.8. Preparation of Strawberry Drink Supplemented with DRPE
3.9. Color Analysis and Sensory Properties
3.10. Statistical Evaluation
4. Conclusions
Author Contributions
Funding
Dedication
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Bhardwaj, S.; Verma, R.; Gupta, J. Challenges and future prospects of herbal medicine. Int. Res. Med. Health Sci. 2018, 1, 12–15. [Google Scholar] [CrossRef]
- Alamgir, A.; Alamgir, A. Medicinal, non-medicinal, biopesticides, color-and dye-yielding plants; secondary metabolites and drug principles; significance of medicinal plants; use of medicinal plants in the systems of traditional and complementary and alternative medicines (CAMs). Ther. Use Med. Plants Extr. Vol. 1 Pharmacogn. 2017, 1, 61–104. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Abeyrathne, E.D.N.S.; Nam, K.; Huang, X.; Ahn, D.U. Plant-and animal-based antioxidants’ structure, efficacy, mechanisms, and applications: A review. Antioxidants 2022, 11, 1025. [Google Scholar] [CrossRef] [PubMed]
- Zinczenko, D. Eat This, Not That (Revised): The Best (& Worst) Foods in America; Ballantine Books: New York, NY, USA, 2019. [Google Scholar]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2021, 427, 127891. [Google Scholar] [CrossRef] [PubMed]
- Rahman, F.B.; Ahmed, S.; Noor, P.; Rahman, M.M.M.; Huq, S.A.; Akib, M.T.E.; Shohael, A.M. A comprehensive multi-directional exploration of phytochemicals and bioactivities of flower extracts from Delonix regia (Bojer ex Hook.) Raf., Cassia fistula L. and Lagerstroemia speciosa L. Biochem. Biophys. Rep. 2020, 24, 100805. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Gul, I.; Basharat, A.; Qamar, S.A. Polysaccharides-based bio-nanostructures and their potential food applications. Int. J. Biol. Macromol. 2021, 176, 540–557. [Google Scholar] [CrossRef]
- Zdunek, A.; Pieczywek, P.M.; Cybulska, J. The primary, secondary, and structures of higher levels of pectin polysaccharides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1101–1117. [Google Scholar] [CrossRef]
- Arora, S.; Singh, D.; Rajput, A.; Bhatia, A.; Kumar, A.; Kaur, H.; Sharma, P.; Kaur, P.; Singh, S.; Attri, S. Plant-based polysaccharides and their health functions. Funct. Foods Health Dis. 2021, 11, 179–200. [Google Scholar] [CrossRef]
- Yang, S.; Mi, L.; Wu, J.; Liao, X.; Xu, Z. Strategy for anthocyanins production: From efficient green extraction to novel microbial biosynthesis. Crit. Rev. Food Sci. Nutr. 2022, 29, 1–16. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; El-Saadony, M.T.; Saghir, S.A.M.; Abd El-Hack, M.E.; Al-Shargi, O.Y.A.; Al-Gabri, N.; Salama, A. Mitigating negative impacts of heat stress in growing rabbits via dietary prodigiosin supplementation. Livestock Sci. 2020, 240, 104220. [Google Scholar] [CrossRef]
- Murugan, P. Antioxidants effect on herbs. Int. J. Rec. Res. Life Sci. 2021, 10, 3399–3402. [Google Scholar]
- Mishra, D. Food colors and associated oxidative stress in chemical carcinogenesis. In Handbook of Oxidative Stress in Cancer: Mechanistic Aspects; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–14. [Google Scholar]
- Adeonipekun, P.A.; Adeniyi, T.A.; Chidinma, O.Q.; Omolayo, R.O. Proximate, phytochemical, and antimicrobial evaluation of flowers of Mangifera indica L., stamens of Terminalia catappa L., and anther of Delonix regia (Bojer ex Hook.) Raf. South Afr. J. Bot. 2023, 155, 223–229. [Google Scholar] [CrossRef]
- El-Sayed, A.M.; Ezzat, S.M.; Salama, M.M.; Sleem, A.A. Hepatoprotective and cytotoxic activities of Delonix regia flower extracts. Pharmacogn. J. 2011, 3, 49–56. [Google Scholar] [CrossRef]
- Chitra, V.; Ilango, K.; Rajanandh, M.G.; Soni, D. Evaluation of Delonix regia Linn. flowers for antiarthritic and antioxidant activity in female wistar rats. Ann. Biol. Res. 2010, 1, 142–147. [Google Scholar]
- Morsi, M.K.S.; Morsy, N.F.S.; Golshany, H.S. Efficiency of ultrasound assisted extract of Delonix regia petals as natural antioxidant on the oxidative stability of sunflower oil. Grasas Y Aceites 2019, 70, 332. [Google Scholar] [CrossRef]
- Oyedeji, O.; Azeez, L.; Osifade, B. Chemical and nutritional compositions of flame of forest (Delonix regia) seeds and seed oil. S. Afr. J. Chem. 2017, 70, 16–20. [Google Scholar] [CrossRef]
- Kouassi, E.; Coulibaly, I.; Rodica, P.; Pintea, A.; Ouattara, S.; Odagiu, A. HPLC Phenolic Compounds Analysis and Antifungal Activity of extracts from Cymbopogon citratus (DC) Stapf. against Fusarium graminearum and Fusarium oxysporum sp. tulipae. J. Sci. Res. Rep. 2017, 14, 1–11. [Google Scholar]
- Chhabra, D.; Gupta, R.K. Fortification of curd using Delonix regia flower petal extract and estimation of its phytochemical, antibacterial & antioxidant activity. J. pharmacogn. phytochem. 2015, 4, 299–307. [Google Scholar]
- Bhorga, P.H.; Kamle, S. Comparative phytochemical investigation and determination of total phenols and flavonoid concentration in leaves and flowers extract of Delonix regia (Boj. Ex. Hook). J. Drug Deliv. Ther. 2019, 9, 1034–1037. [Google Scholar]
- Ranjah, M.A. Lemongrass: A useful ingredient for functional foods. Int. J. Food Allied Sci. 2019, 4, 11–19. [Google Scholar]
- Hosny, M. Phytoconstituents from Delonix regia raf roots and their biological activities. Al-Azhar J. Pharm. Sci. 2012, 46, 149–160. [Google Scholar] [CrossRef]
- Veigas, J.M.; Divya, P.; Neelwarne, B. Identification of previously unreported pigments among carotenoids and anthocyanins in floral petals of Delonix regia (Hook.) Raf. Food Res. Int. 2012, 47, 116–123. [Google Scholar] [CrossRef]
- Rodriguez-Canto, W.; Chel-Guerrero, L.; Fernandez, V.; Aguilar-Vega, M. Delonix regia galactomannan hydrolysates: Rheological behavior and physicochemical characterization. Carbohydr. Polym. 2019, 206, 573–582. [Google Scholar] [CrossRef]
- Chouana, T.; Pierre, G.; Vial, C.; Gardarin, C.; Wadouachi, A.; Cailleu, D.; Le Cerf, D.; Boual, Z.; El Hadj, M.O.; Michaud, P. Structural characterization and rheological properties of a galactomannan from Astragalus gombo Bunge seeds harvested in Algerian Sahara. Carbohydr. Polym. 2017, 175, 387–394. [Google Scholar] [CrossRef]
- Mittal, N.; Mattu, P.; Kaur, G. Extraction and derivatization of Leucaena leucocephala (Lam.) galactomannan: Optimization and characterization. Int. J. Biol. Macromol. 2016, 92, 831–841. [Google Scholar] [CrossRef]
- Chakou, F.Z.; Boual, Z.; Hadj, M.D.O.E.; Belkhalfa, H.; Bachari, K.; Alaoui-Talibi, E.; El Modafar, C.; Hadjkacem, F.; Fendri, I.; Abdelkafi, S. Pharmacological investigations in traditional utilization of Alhagi maurorum Medik. in Saharan Algeria: In Vitro study of anti-inflammatory and antihyperglycemic activities of water-soluble polysaccharides extracted from the seeds. Plants 2021, 10, 2658. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.; Sanjeewa, K.K.A.; Samarakoon, W.; Lee, W.W.; Kim, H.-S.; Kim, E.-A.; Gunasekara, U.; Abeytunga, D.; Nanayakkara, C.; De Silva, E. FTIR characterization and antioxidant activity of water soluble crude polysaccharides of Sri Lankan marine algae. Algae 2017, 32, 75–86. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhang, W.; Sun, R. Characterization of galactomannan gum from fenugreek (Trigonella foenum-graecum) seeds and its rheological properties. Int. J. Polym. Mater. 2007, 56, 1145–1154. [Google Scholar] [CrossRef]
- Nwokocha, L.M.; Williams, P.A.; Yadav, M.P. Physicochemical characterisation of the galactomannan from Delonix regia seed. Food Hydrocoll. 2018, 78, 132–139. [Google Scholar] [CrossRef]
- da Silva, L.M.; Araújo, L.F.S.; Alvez, R.C.; Ono, L.; Sá, D.A.T.; da Cunha, P.L.; de Paula, R.C.M.; Maciel, J.S. Promising alternative gum: Extraction, characterization, and oxidation of the galactomannan of Cassia fistula. Int. J. Biol. Macromol. 2020, 165, 436–444. [Google Scholar] [CrossRef]
- Ai, Y.; Yu, Z.; Chen, Y.; Zhu, X.; Ai, Z.; Liu, S.; Ni, D. Rapid determination of the monosaccharide composition and contents in tea polysaccharides from Yingshuang green tea by pre-column derivatization HPLC. J. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Abuljadayel, D.A.; Shafi, M.E.; Albaqami, N.M.; Desoky, E.-S.M.; El-Tahan, A.M.; Mesiha, P.K.; Elnahal, A.S.; Almakas, A.; Taha, A.E. Control of foliar phytoparasitic nematodes through sustainable natural materials: Current progress and challenges. Saudi J. Biol. Sci. 2021, 28, 7314–7326. [Google Scholar] [CrossRef]
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia. J. Pharm. Anal. 2013, 3, 298–302. [Google Scholar] [CrossRef]
- Orumwensodia, K.O.; Uadia, P.O.; Choudhary, M.I. Phytotoxicity, cytotoxicity and chemical composition of Spondias mombin Linn. Stem bark. Clin. Phytosci. 2021, 7, 1–9. [Google Scholar] [CrossRef]
- Shameli Rajiri, M.; Aminsalehi, M.; Shahbandeh, M.; Maleki, A.; Jonoubi, P.; Rad, A.C. Anticancer and therapeutic potential of Delonix regia extract and silver nanoparticles (AgNPs) against pancreatic (Panc-1) and breast (MCF-7) cancer cell. Toxicol Environ Health Sci. 2021, 13, 45–56. [Google Scholar] [CrossRef]
- Lotfy, L.; Bakr, E.-S.; Mansour, R. Potential nutraceutical and therapeutic effects of Delonix regia leaves and pods on kidneys vitality in rats inflicted with renal failure. J. Sci. Educ. Technol. 2021, 21, 614–630. [Google Scholar]
- Horwitz, W.; Latimer, G.W. Association of Official Analytical Chemists International. In Official Methods of Analysis of AOAC International, 19th ed.; Oxford University Press: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Falah, S.; Ayunda, R.; Faridah, D. Potential of lemongrass leaves extract (Cymbopogon citratus) as prevention for oil oxidation. J. Chem. Pharm. Res. 2015, 7, 55–60. [Google Scholar]
- Wolfe, K.; Wu, X.; Liu, R.H. antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.M.; Mohamed, A.S.; El-Saadony, M.T.; Sitohy, M.Z. Palatable functional cucumber juices supplemented with polyphenols-rich herbal extracts. LWT—Food Sci. Technol. 2021, 148, 111668. [Google Scholar] [CrossRef]
- Singh, A.; Kitts, D.D. In vitro bioaccessibility of tart cherry anthocyanins in a health supplement mix containing mineral clay. J. Food Sci. 2019, 84, 475–480. [Google Scholar] [CrossRef]
- Udchumpisai, W.; Bangyeekhun, E. Purification, structural characterization, and biological activity of polysaccharides from Lentinus velutinus. Mycobiology 2020, 48, 51–57. [Google Scholar] [CrossRef]
- Montesano, D.; Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.; Blasi, F. A simple HPLC-ELSD method for sugar analysis in goji berry. J. Chem. 2016, 2016, 6271808. [Google Scholar] [CrossRef]
- Ji, X.; Guo, J.; Ding, D.; Gao, J.; Hao, L.; Guo, X.; Liu, Y. Structural characterization and antioxidant activity of a novel high-molecular-weight polysaccharide from Ziziphus jujuba cv. Muzao. J. Food Meas. Charact. 2022, 16, 2191–2200. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Mu, D.; Yang, H.; Feng, Y.; Ji, R.; Wu, R.; Wu, J. Preparation, structural characterization and bioactivities of polysaccharides from mulberry (Mori Fructus). Food Biosci. 2022, 46, 101604. [Google Scholar] [CrossRef]
- Moein, M.; Moein, S.; Farmani, F.; Rozbehan, S.; Sabahi, Z. Examination the antioxidant potentials and antidiabetic properties of phenolic extracts of some Iranian honeys. J. Nephropharmacol. 2021, 11, e6. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Ashour, E.A.; El-Hack, M.E.A.; Shafi, M.E.; Alghamdi, W.Y.; Taha, A.E.; Swelum, A.A.; Tufarelli, V.; Mulla, Z.S.; El-Ghareeb, W.R.; El-Saadony, M.T. Impacts of green coffee powder supplementation on growth performance, carcass characteristics, blood indices, meat quality and gut microbial load in broilers. Agriculture 2020, 10, 457. [Google Scholar] [CrossRef]
- Schumann, G.; Klauke, R. New IFCC reference procedures for the determination of catalytic activity concentrations of five enzymes in serum: Preliminary upper reference limits obtained in hospitalized subjects. Clin. Chim. Acta 2003, 327, 69–79. [Google Scholar] [CrossRef]
- Beutler, E. G6PD deficiency. Blood 1994, 84, 3613–3636. [Google Scholar] [CrossRef] [PubMed]
- Bulut, M.; Selek, S.; Bez, Y.; Kaya, M.C.; Gunes, M.; Karababa, F.; Celik, H.; Savas, H.A. Lipid peroxidation markers in adult attention deficit hyperactivity disorder: New findings for oxidative stress. Psychiatry Res. 2013, 209, 638–642. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Lambert, P.A. Direct assay of LDL Cholesterol: Comparing measurement and calculation. Lab. Med. 1996, 27, 613–617. [Google Scholar] [CrossRef]
- Devi, R.; Sharma, D. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J. Ethnopharmacol. 2004, 90, 63–68. [Google Scholar] [CrossRef]
- Namir, M.; Iskander, A.; Alyamani, A.; Sayed-Ahmed, E.T.A.; Saad, A.M.; Elsahy, K.; El-Tarabily, K.A.; Conte-Junior, C.A. Upgrading common wheat pasta by fiber-rich fraction of potato peel byproduct at different particle sizes: Effects on physicochemical, thermal, and sensory properties. Molecules 2022, 27, 2868. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Sitohy, M.Z.; Ramadan, M.F.; Saad, A.M. Green nanotechnology for preserving and enriching yogurt with biologically available iron (II). Innov. Food Sci. Emerg. Technol. 2021, 69, 102645. [Google Scholar] [CrossRef]
- Saville, D.J. Multiple comparison procedures—Cutting the gordian knot. Agron. J. 2015, 107, 730–735. [Google Scholar] [CrossRef]





| Proximate Composition | Values (%) |
|---|---|
| Moisture | 10.14 ± 0.1 c |
| Ash | 2.05 ± 0.2 d |
| Protein | 10.01 ± 0.1 c |
| Fiber | 14.04 ± 0.9 b |
| Fat | 13.68 ± 0.5 b |
| Carbohydrates | 50.24 ± 0.2 a |
| Elemental content | Values (ppm) |
| Mn | 13.81 ± 0.1 e |
| Mg | 2220.36 ± 1.8 c |
| Zn | 671.95 ± 2.1 d |
| Co | 0.0125 ± 0.0002 g |
| K | 16,358.12 ± 3.3 a |
| P | 2.087 ± 0.1 f |
| Ca | 4617.15 ± 4.3 b |
| RT | Monosaccharide | Concentration (µM) |
|---|---|---|
| 8.00 | Mannose | 3.60 a |
| 10.32 | Glucose | 1.00 c |
| 12.78 | Galactose | 2.70 b |
| 14.68 | Xylose | 0.78 d |
| 16.66 | Arabinose | 1.10 c |
| Blood Parameters | Control | Synthetic Dye(µg/g) | Aspartame (µg/g) | DRPE (µg/g) | |
|---|---|---|---|---|---|
| Liver parameters | 1600 | 1600 | 200 | 1600 | |
| ALT | 45.9 ± 0.2 b | 89.6 ± 0.9 a | 85.4 ± 0.2 ab | 47.2 ± 0.2 b | 46.5 ± 0.3 b |
| AST | 32.6 ± 0.3 c | 81.2 ± 0.3 a | 77.6 ± 0.1 b | 33.9 ± 0.3 c | 33.6 ± 0.7 c |
| MDA | 46.3 ± 0.7 c | 71.3 ± 0.5 a | 69.2 ± 0.3 ab | 47.2 ± 0.1 c | 46.9 ± 0.9 c |
| GSH | 55.6 ± 0.9 ab | 41.6 ± 0.8 b | 39.6 ± 0.9 b | 55.9 ± 0.6 ab | 56.2 ± 0.8 a |
| Kidney parameters | |||||
| Urea | |||||
| Creatinine | 0.41 ± 0.01 b | 0.89 ± 0.02 a | 0.88 ± 0.03 a | 0.46 ± 0.01 b | 0.45 ± 0.09 b |
| Lipid profile | |||||
| LDL | 16.8 ± 0.2 c | 87.3 ± 0.6 a | 85.6 ± 0.2 ab | 18.6 ± 0.9 b | 17.1 ± 0.2 c |
| HDL | 35.2 ± 0.9 b | 21.7 ± 0.1 c | 22.6 ± 0.1 c | 36.2 ± 0.1 b | 41.3 ± 0.3 a |
| TG | 72.3 ± 0.8 c | 140.3 ± 0.2 a | 133.5 ± 0.6 b | 73.9 ± 0.2 c | 71.2 ± 0.8 c |
| TC | 66.9 ± 0.2 c | 126.4 ± 0.9 a | 121.9 ± 0.5 b | 68.2 ± 0.7 c | 66.3 ± 0.4 c |
| Yogurt Samples | Storage (Days) | Color | Flavor | Texture | Taste | Overall Acceptability | |
|---|---|---|---|---|---|---|---|
| DR concentration (µg/g) | Control (0) | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.2 ± 0.0 cd | 8.4 ± 0.2 a | 8.7 ± 0.0 bc |
| 7 | 8.7 ± 0.2 bc | 8.4 ± 0.1 c | 8.2 ± 0.1 cd | 8.1 ± 0.1 bc | 8.4 ± 0.1 cd | ||
| 14 | 8.5 ± 0.3 cd | 8.1 ± 0.0 d | 8.0 ± 0.2 d | 7.9 ± 0.0 c | 8.2 ± 0.2 d | ||
| 21 | 8.0 ± 0.2 e | 7.6 ± 0.2 e | 7.5 ± 0.1 e | 7.4 ± 0.1 e | 7.7 ± 0.3 e | ||
| 30 | 7.5 ± 0.7 f | 7.5 ± 0.3 e | 7.0 ± 0.0 f | 6.9 ± 0.2 f | 7.3 ± 0.4 f | ||
| 200 | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.5 ± 0.2 c | 8.4 ± 0.4 a | 8.8 ± 0.1 b | |
| 7 | 8.7 ± 0.1 bc | 8.5 ± 0.2 c | 8.4 ± 0.1 c | 8.1 ± 0.2 bc | 8.5 ± 0.2 cd | ||
| 14 | 8.6 ± 0.2 c | 8.3 ± 0.3 cd | 8.2 ± 0.3 cd | 8.0 ± 0.1 c | 8.4 ± 0.1 d | ||
| 21 | 8.2 ± 0.3 d | 8.1 ± 0.2 d | 8.0 ± 0.2 d | 7.6 ± 0.1 d | 8.1 ± 0.0 de | ||
| 30 | 8.1 ± 0.2 | 8.0 ± 0.1 d | 7.6 ± 0.1 f | 7.5 ± 0.2 de | 8.0 ± 0.2 e | ||
| 800 | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.9 ± 0.0 a | 8.4 ± 0.0 a | 9.0 ± 0.0 a | |
| 7 | 8.8 ± 0.0 b | 8.8 ± 0.1 b | 8.7 ± 0.1 b | 8.2 ± 0.2 b | 8.8 ± 0.1 b | ||
| 14 | 8.6 ± 0.1 c | 8.7 ± 0.2 bc | 8.5 ± 0.2 c | 8.1 ± 0.1 bc | 8.6 ± 0.2 c | ||
| 21 | 8.6 ± 0.1 c | 8.5 ± 0.1 c | 8.3 ± 0.1 cd | 8.0 ± 0.1 c | 8.5 ± 0.1 cd | ||
| 30 | 8.5 ± 0.2 cd | 8.3 ± 0.2 cd | 8.0 ± 0.0 d | 7.9 ± 0.1 cd | 8.3 ± 0.2 d | ||
| 1600 | 0 | 9.0 ± 0.0 a | 9.0 ± 0.0 a | 8.6 ± 0.0 bc | 8.4 ± 0.1 a | 8.9 ± 0.0 ab | |
| 7 | 8.8 ± 0.1 b | 8.7 ± 0.2 bc | 8.5 ± 0.1 c | 8.2 ± 0.2 b | 8.7 ± 0.1 bc | ||
| 14 | 8.7 ± 0.2 bc | 8.5 ± 0.1 c | 8.3 ± 0.2 cd | 8.1 ± 0.1 bc | 8.5 ± 0.0 cd | ||
| 21 | 8.5 ± 0.1 cd | 8.4 ± 0.2 c | 8.0 ± 0.2 d | 7.9 ± 0.2 c | 8.3 ± 0.2 d | ||
| 30 | 8.3 ± 0.1 d | 8.2 ± 0.1 cd | 7.7 ± 0.1 f | 7.7 ± 0.1 d | 8.1 ± 0.1 de | ||
| Ingredients | SB | DRSB1 | DRSB2 | DRSB3 |
|---|---|---|---|---|
| Juice (mL) | 250 | 250 | 250 | 250 |
| yogurt (g) | 50 | 50 | 50 | 50 |
| Sugar (g) | 10 | - | - | - |
| DRPE (mL) | - | 90 | 90 | 90 |
| Water (mL) | 90 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebada, D.; Hefnawy, H.T.; Gomaa, A.; Alghamdi, A.M.; Alharbi, A.A.; Almuhayawi, M.S.; Alharbi, M.T.; Awad, A.; Al Jaouni, S.K.; Selim, S.; et al. Characterization of Delonix regia Flowers’ Pigment and Polysaccharides: Evaluating Their Antibacterial, Anticancer, and Antioxidant Activities and Their Application as a Natural Colorant and Sweetener in Beverages. Molecules 2023, 28, 3243. https://doi.org/10.3390/molecules28073243
Ebada D, Hefnawy HT, Gomaa A, Alghamdi AM, Alharbi AA, Almuhayawi MS, Alharbi MT, Awad A, Al Jaouni SK, Selim S, et al. Characterization of Delonix regia Flowers’ Pigment and Polysaccharides: Evaluating Their Antibacterial, Anticancer, and Antioxidant Activities and Their Application as a Natural Colorant and Sweetener in Beverages. Molecules. 2023; 28(7):3243. https://doi.org/10.3390/molecules28073243
Chicago/Turabian StyleEbada, Doaa, Hefnawy T. Hefnawy, Ayman Gomaa, Amira M. Alghamdi, Asmaa Ali Alharbi, Mohammed S. Almuhayawi, Mohanned Talal Alharbi, Ahmed Awad, Soad K. Al Jaouni, Samy Selim, and et al. 2023. "Characterization of Delonix regia Flowers’ Pigment and Polysaccharides: Evaluating Their Antibacterial, Anticancer, and Antioxidant Activities and Their Application as a Natural Colorant and Sweetener in Beverages" Molecules 28, no. 7: 3243. https://doi.org/10.3390/molecules28073243
APA StyleEbada, D., Hefnawy, H. T., Gomaa, A., Alghamdi, A. M., Alharbi, A. A., Almuhayawi, M. S., Alharbi, M. T., Awad, A., Al Jaouni, S. K., Selim, S., Eldeeb, G. S., & Namir, M. (2023). Characterization of Delonix regia Flowers’ Pigment and Polysaccharides: Evaluating Their Antibacterial, Anticancer, and Antioxidant Activities and Their Application as a Natural Colorant and Sweetener in Beverages. Molecules, 28(7), 3243. https://doi.org/10.3390/molecules28073243






