The Synthesis and Biological Applications of the 1,2,3-Dithiazole Scaffold
Abstract
1. Introduction
2. 1,2,3-Dithiazoles Synthesis Overview
2.1. Early Years before Appel Salt
2.2. Discovery of Appel Salt and Applications
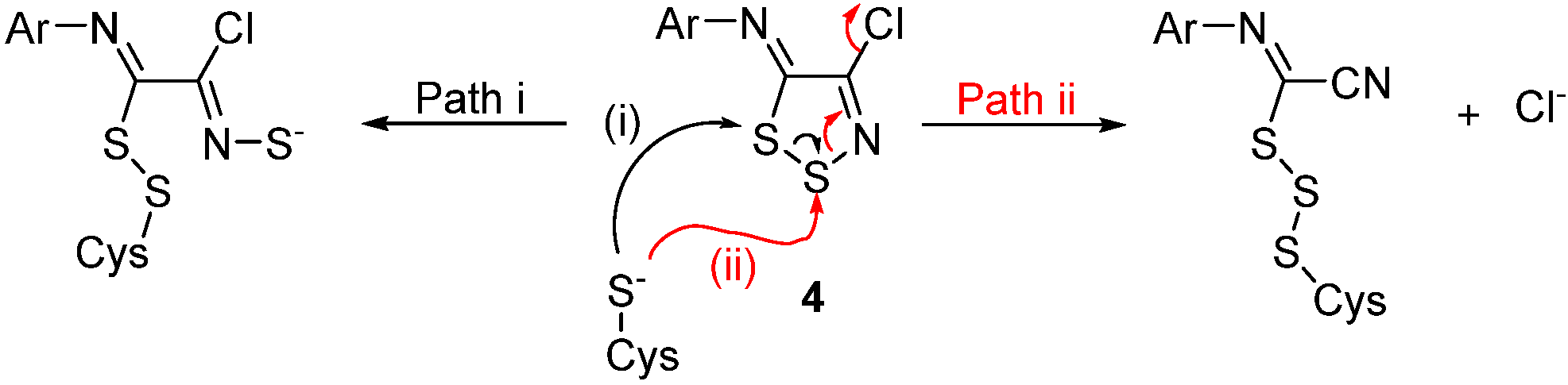
2.3. Reactivity of C-4 and the Displacement of the Chloride
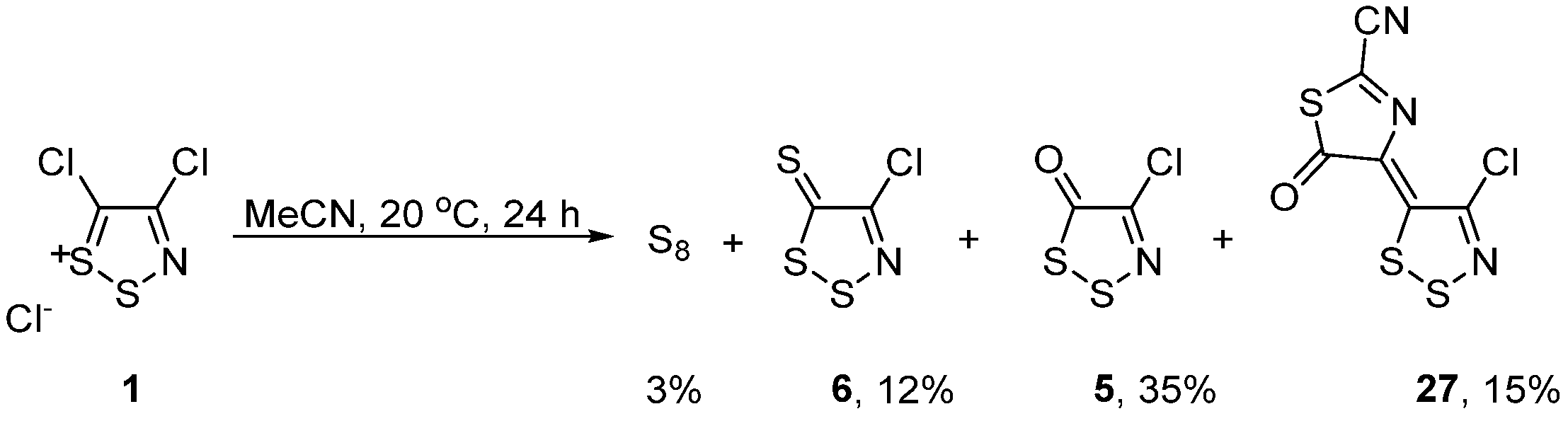
2.4. Alternatives beyond Appel Salt Chemistry
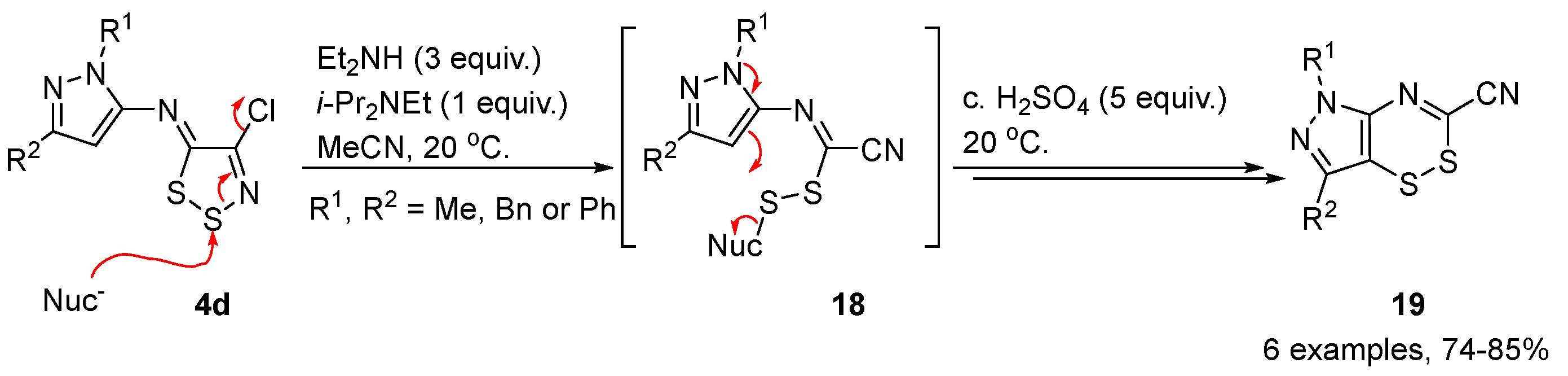
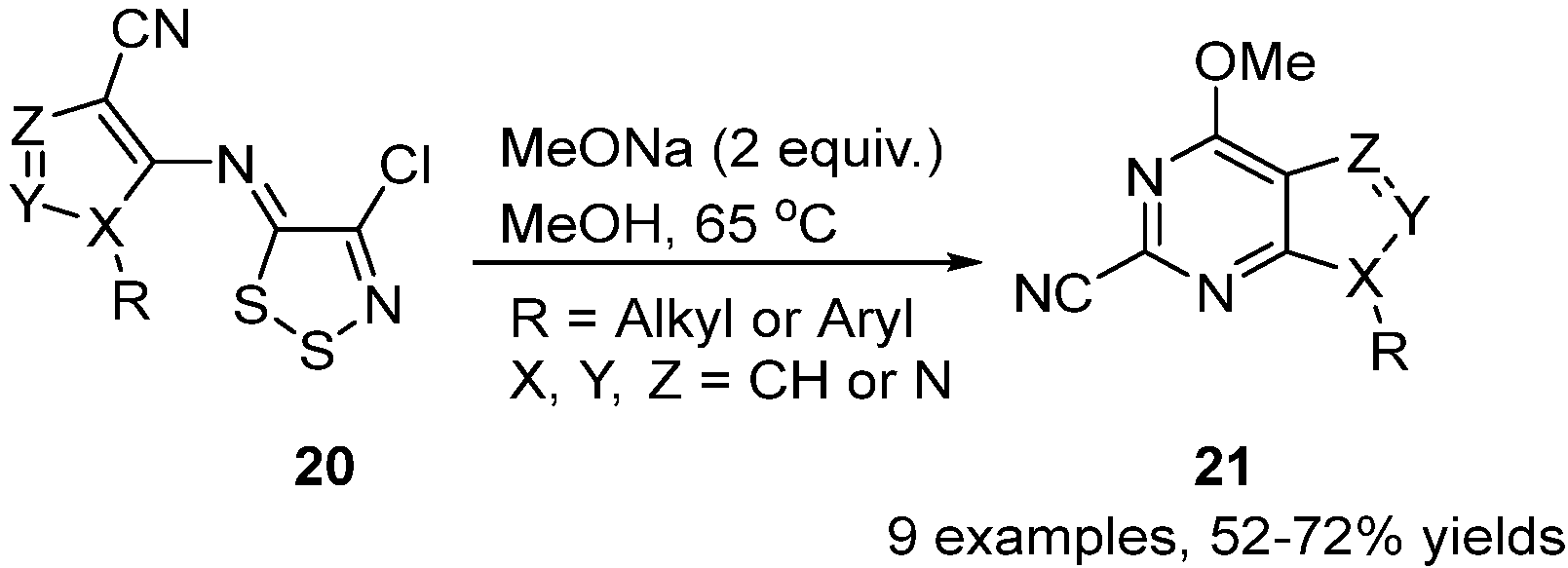
2.5. Reactivity of 1,2,3-Dithiazoles
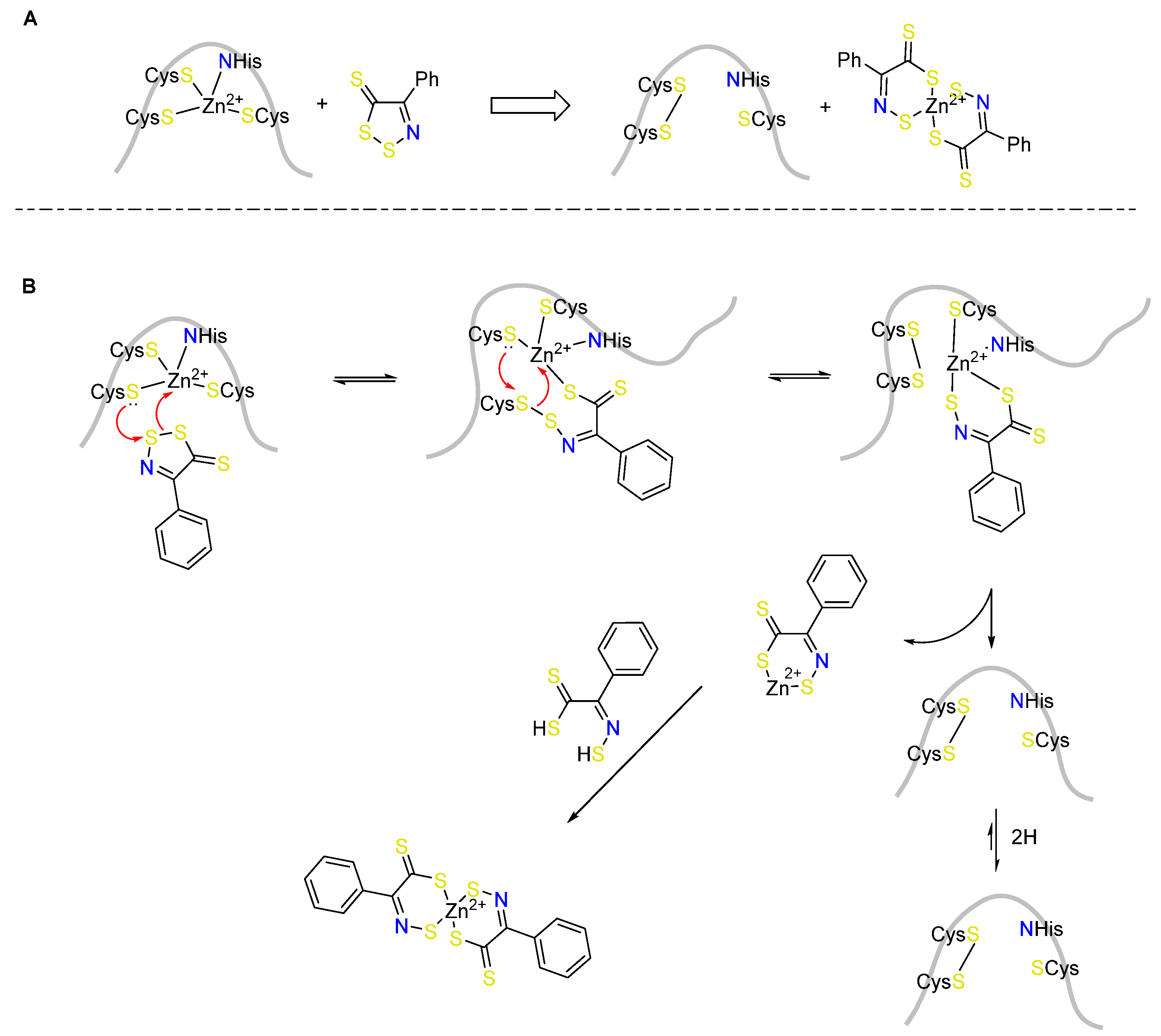
3. 1,2,3-Dithiazoles in Medicinal Chemistry
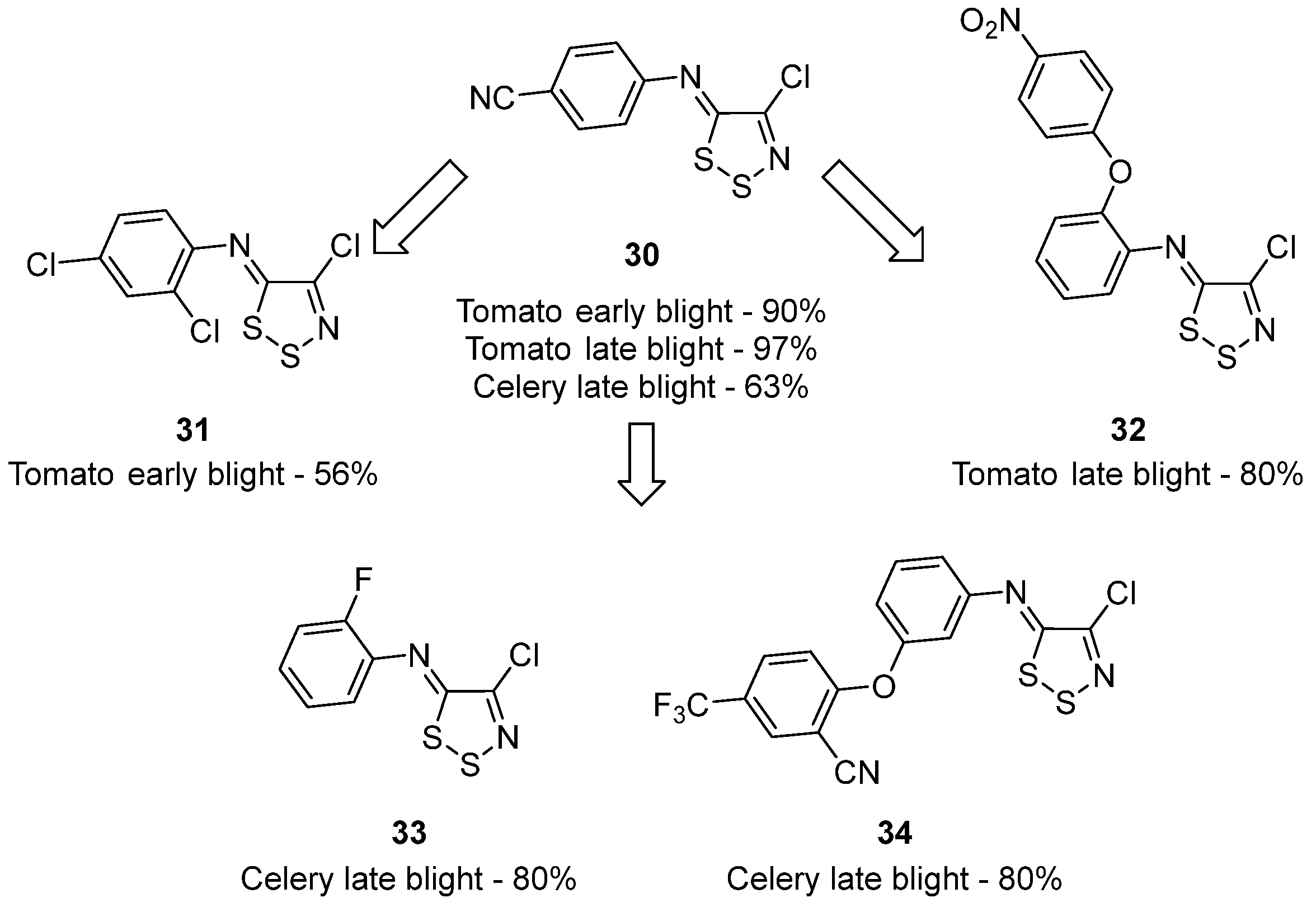
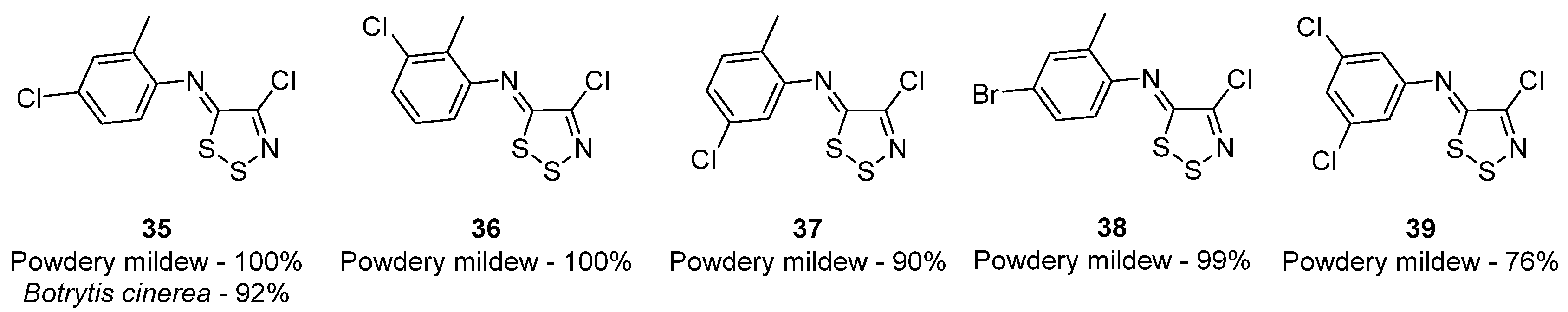
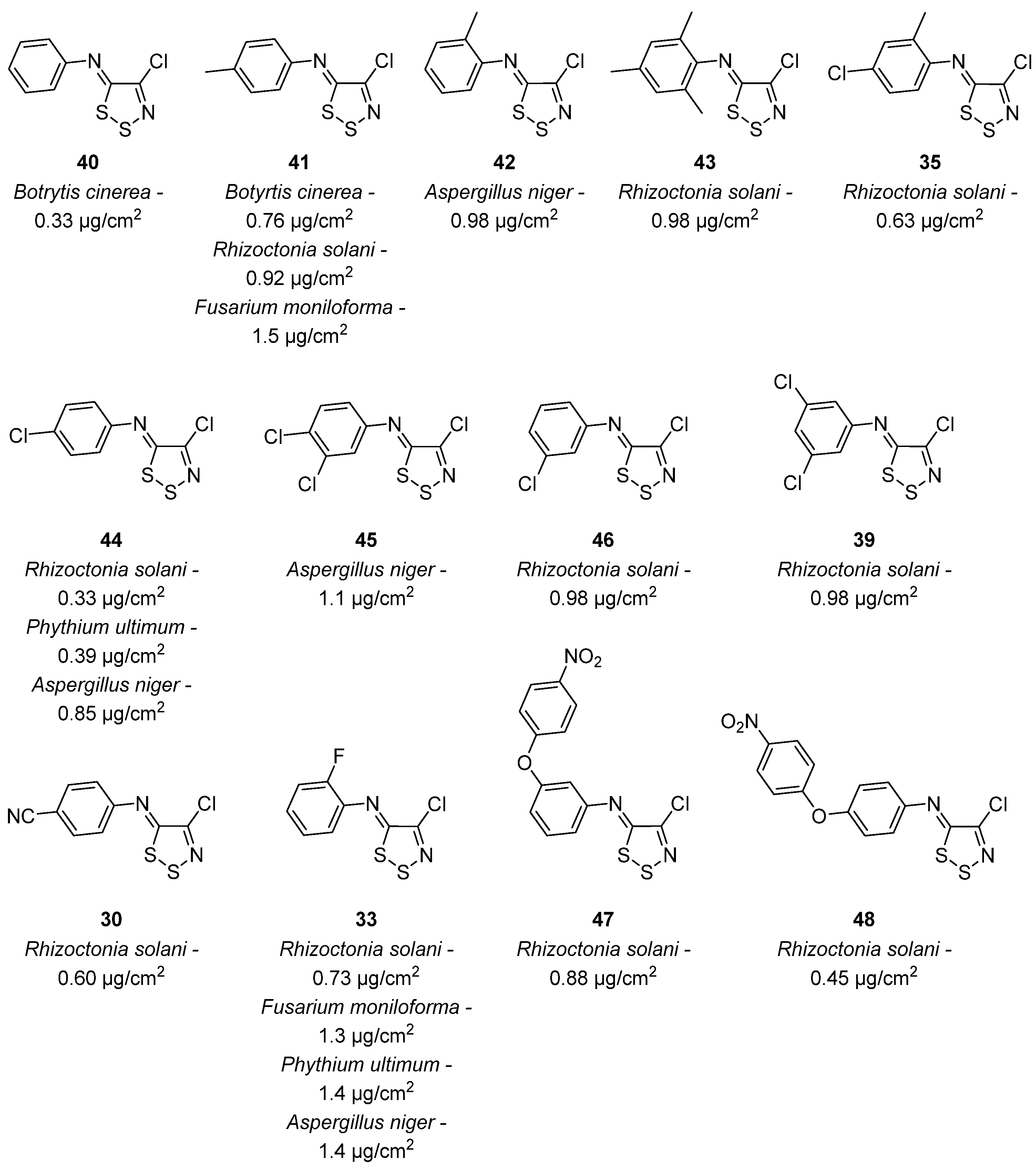
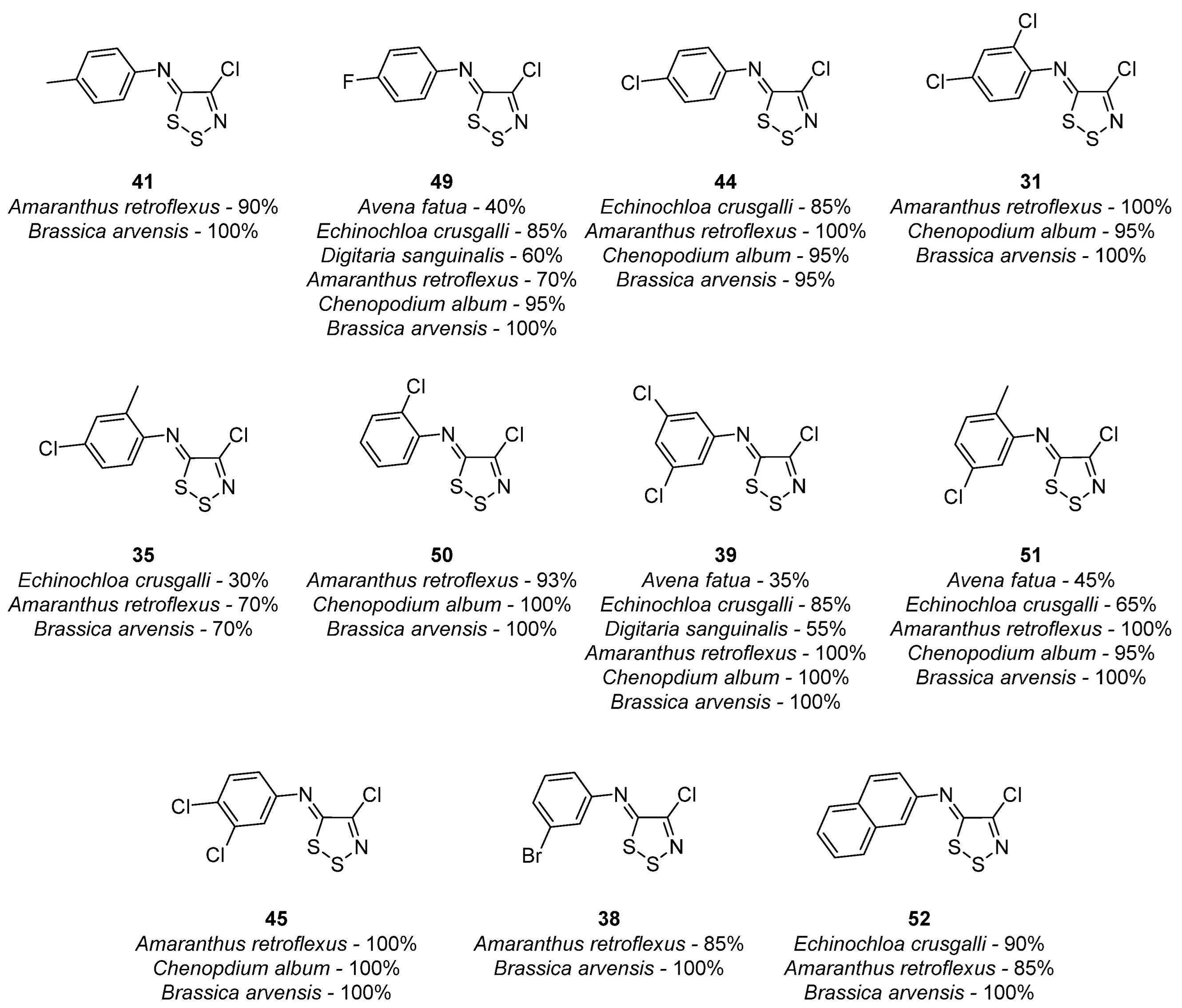
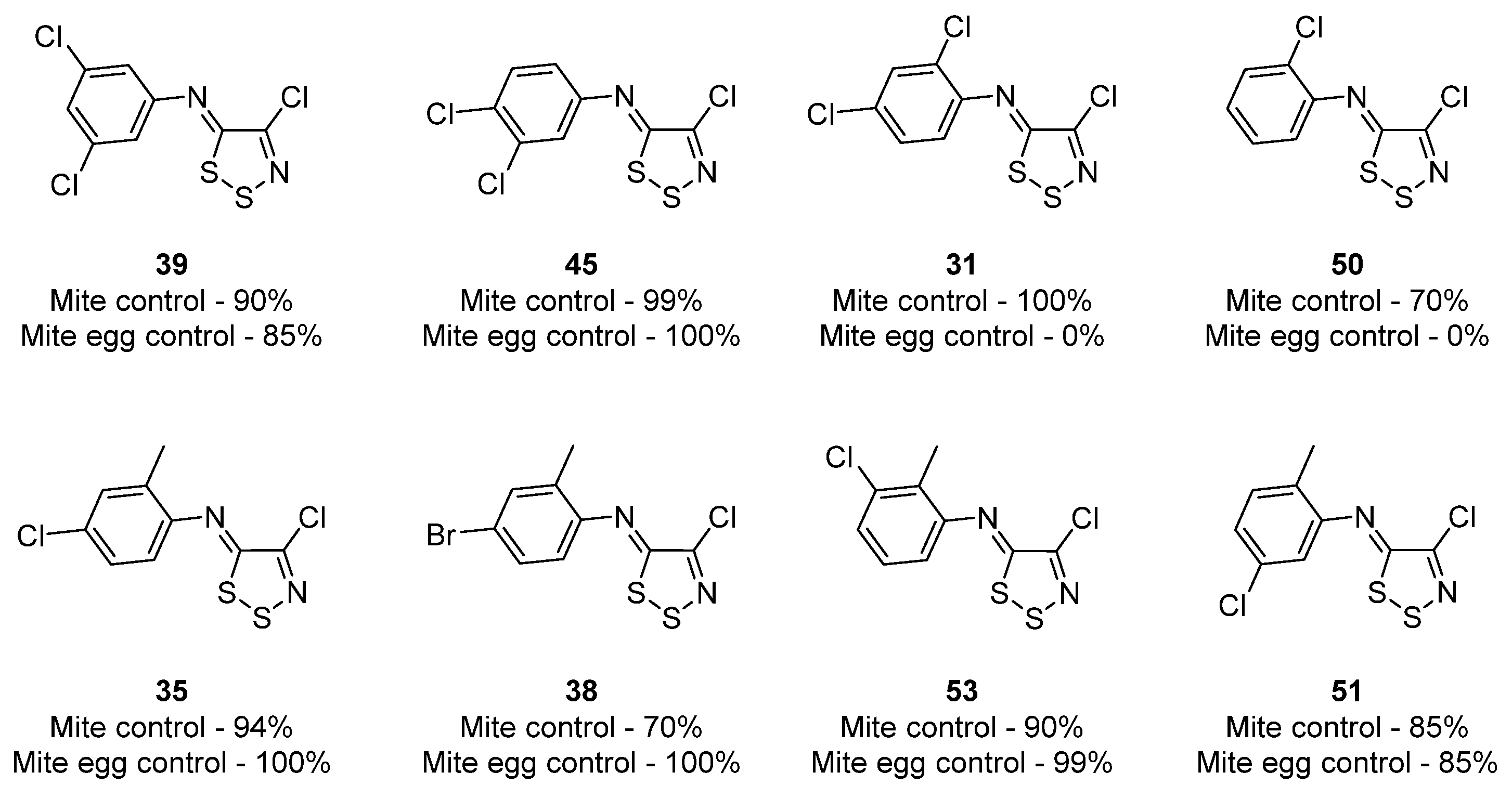
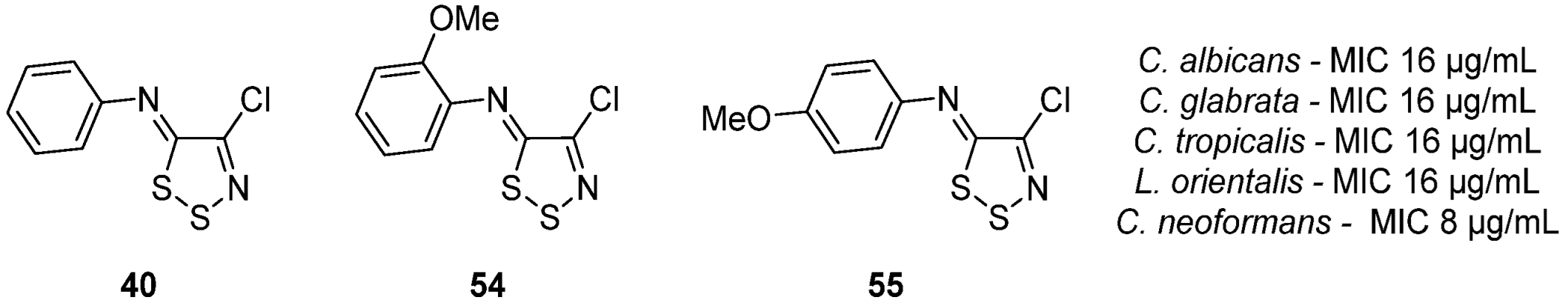
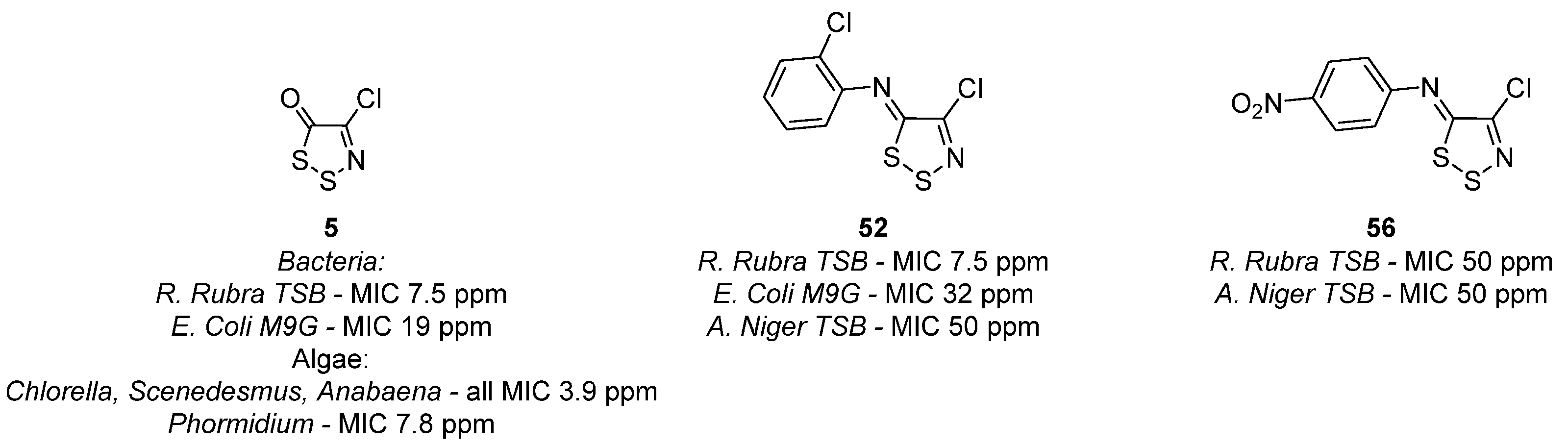
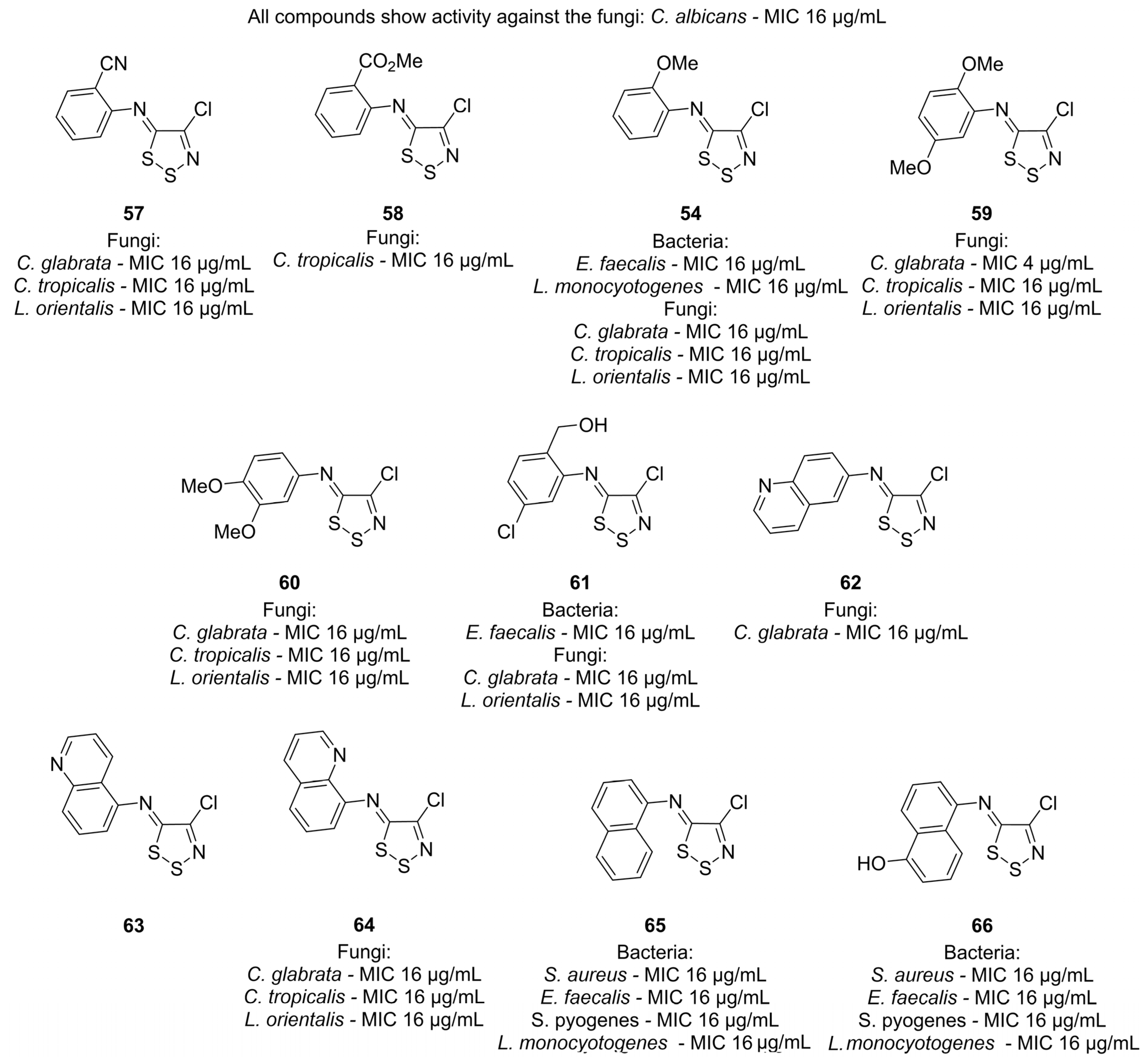
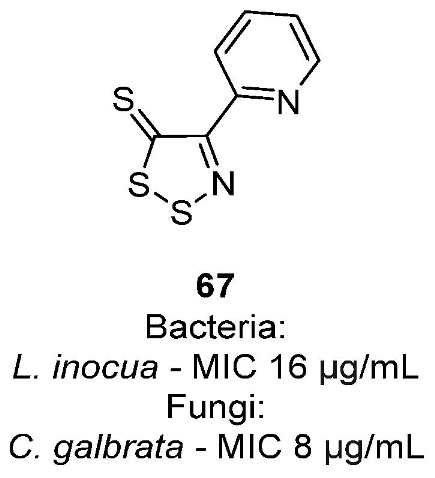
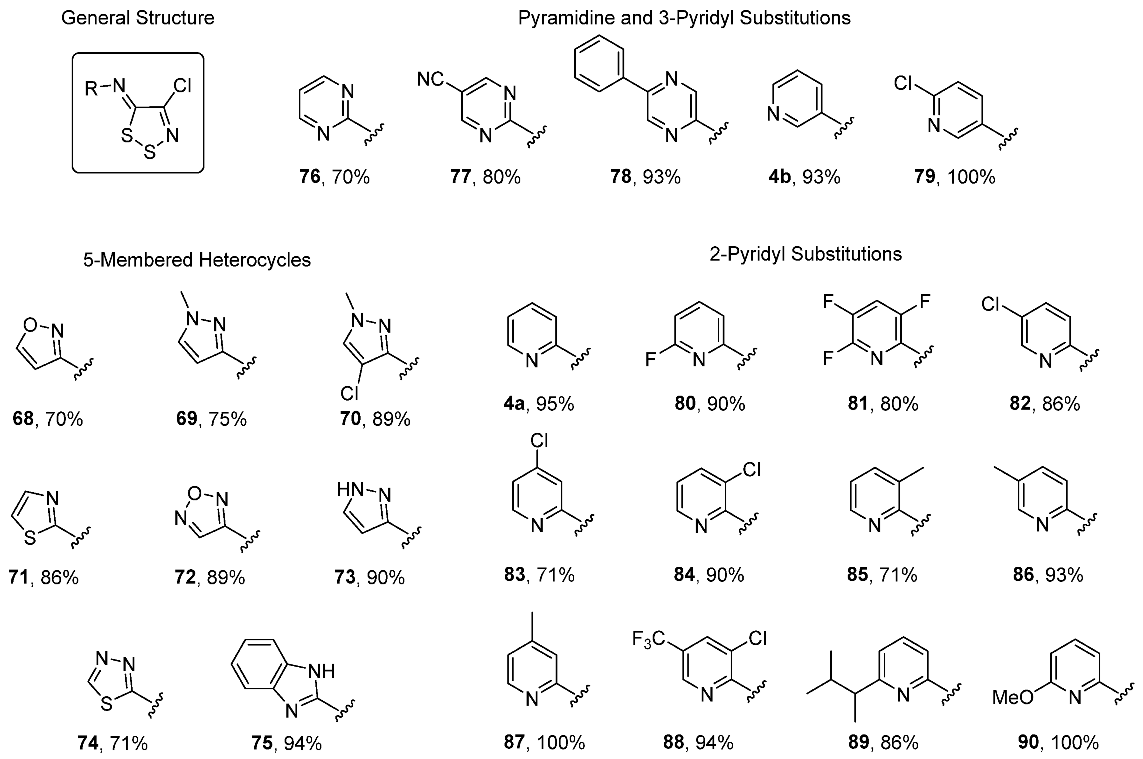
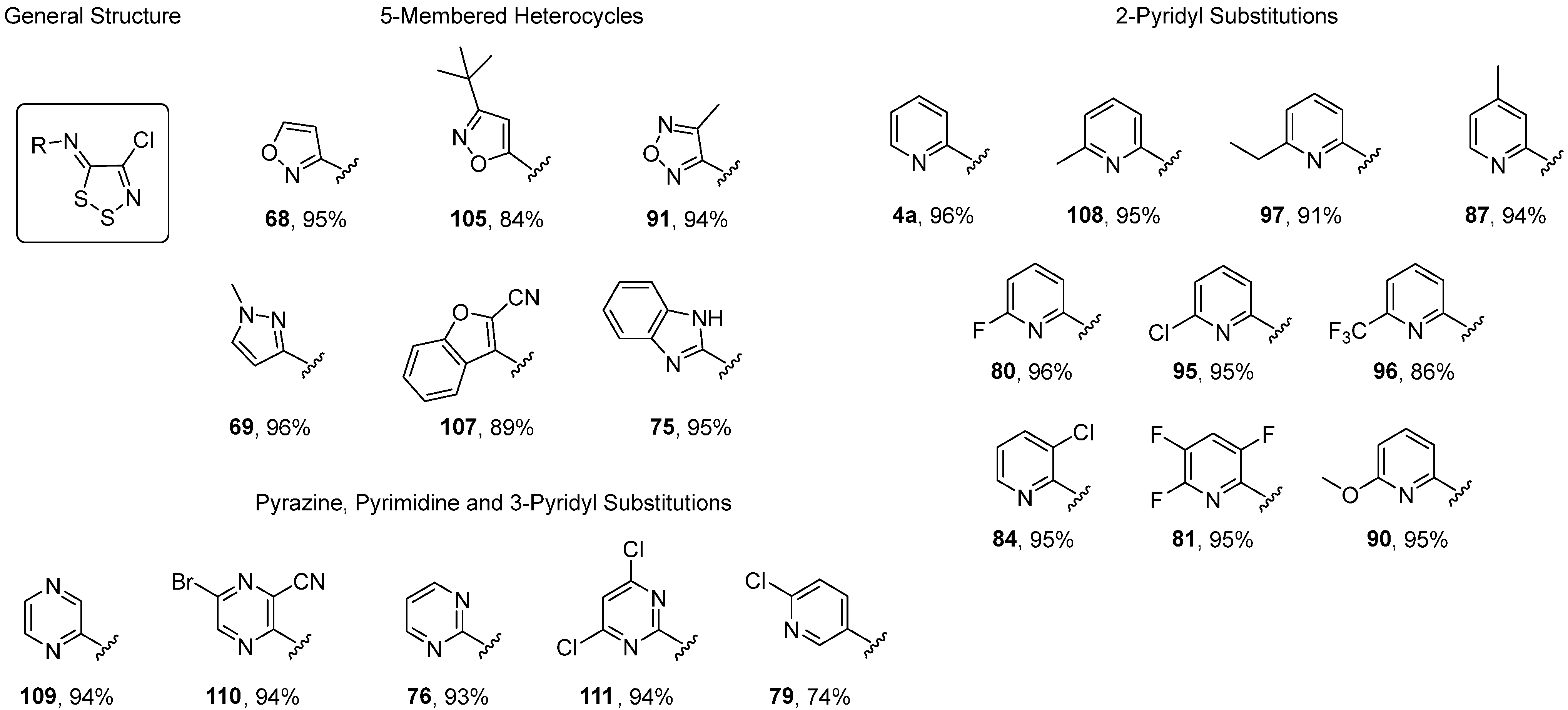
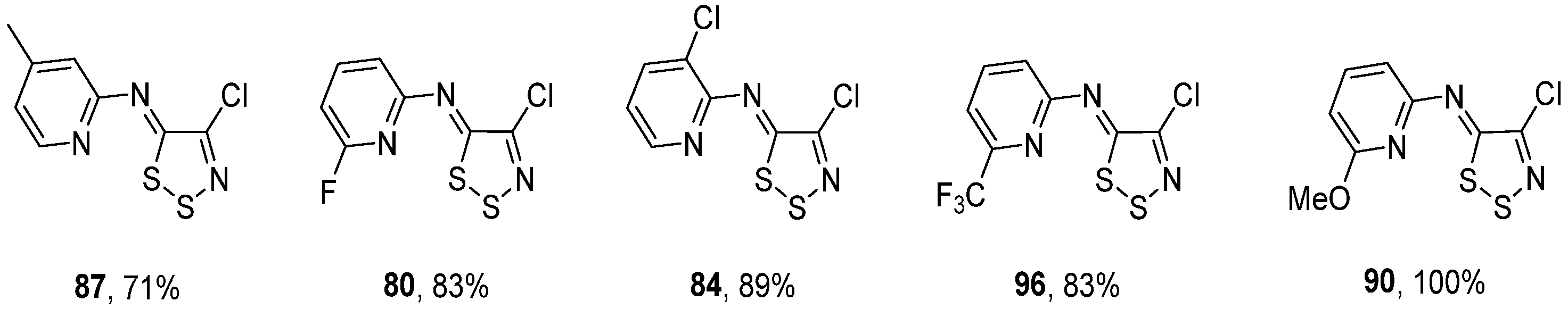
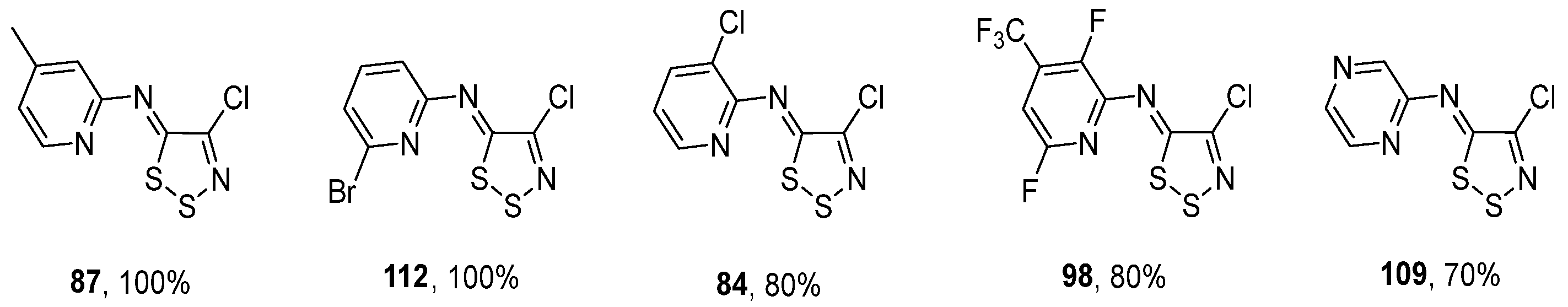
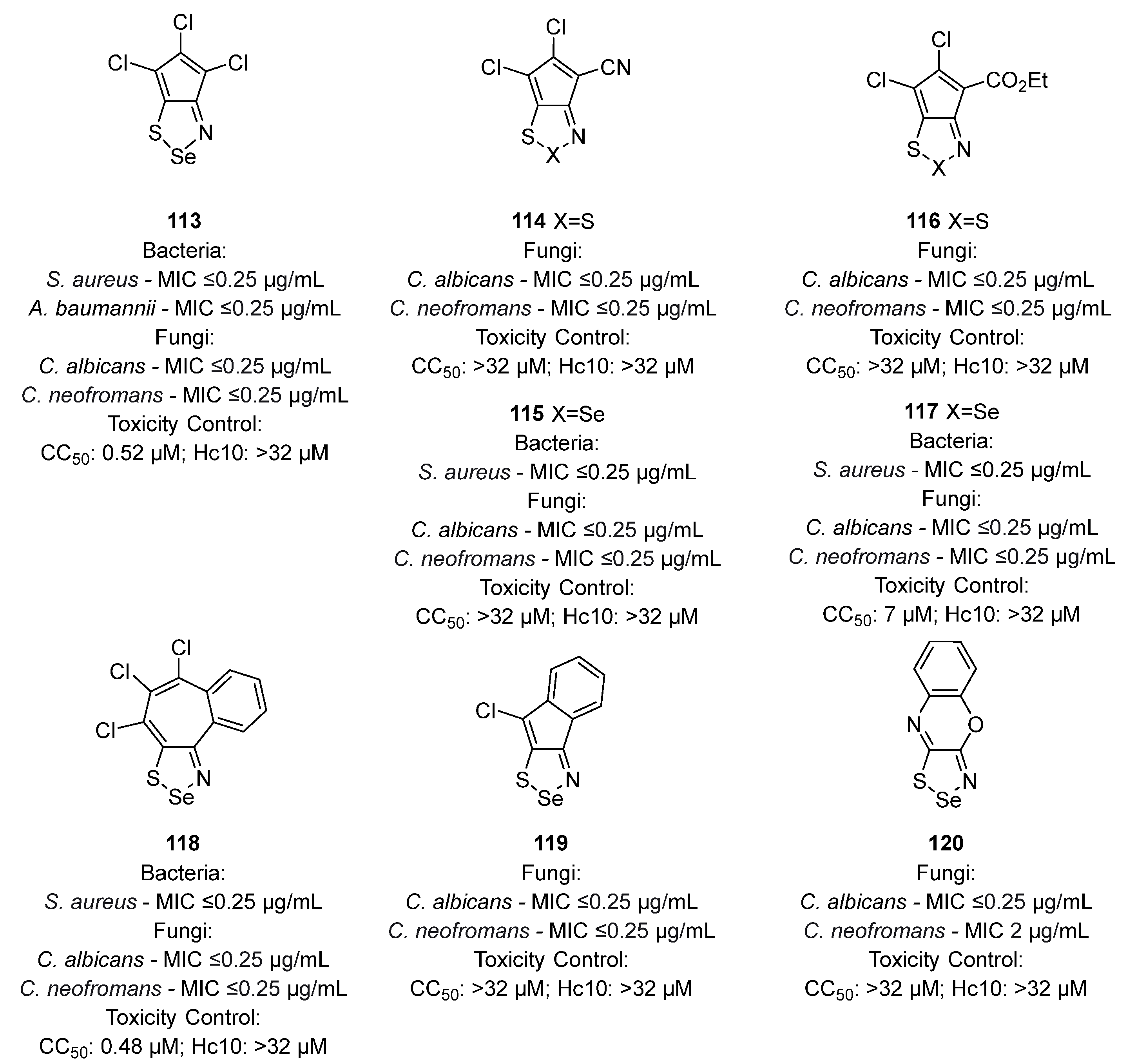
3.1. Antimicrobial Activities of 1,2,3-Dithiazoles, including Antifungal, Herbicidal, and Antibacterial
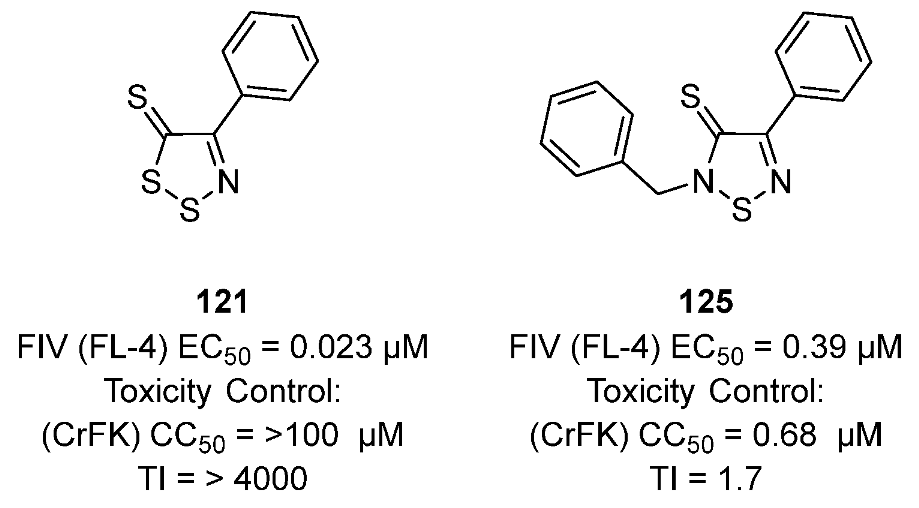
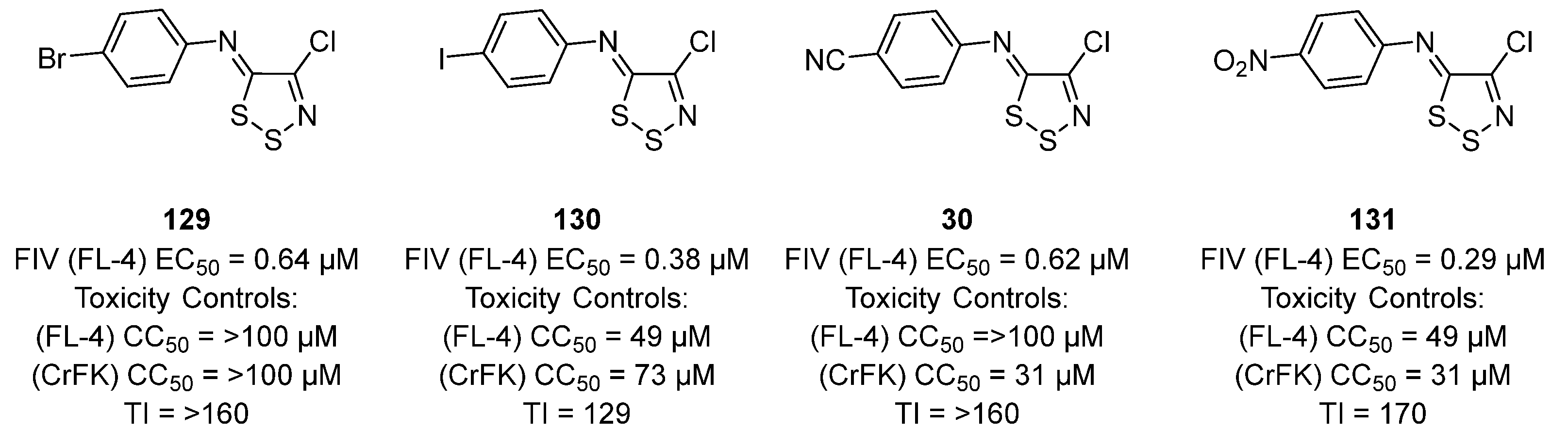
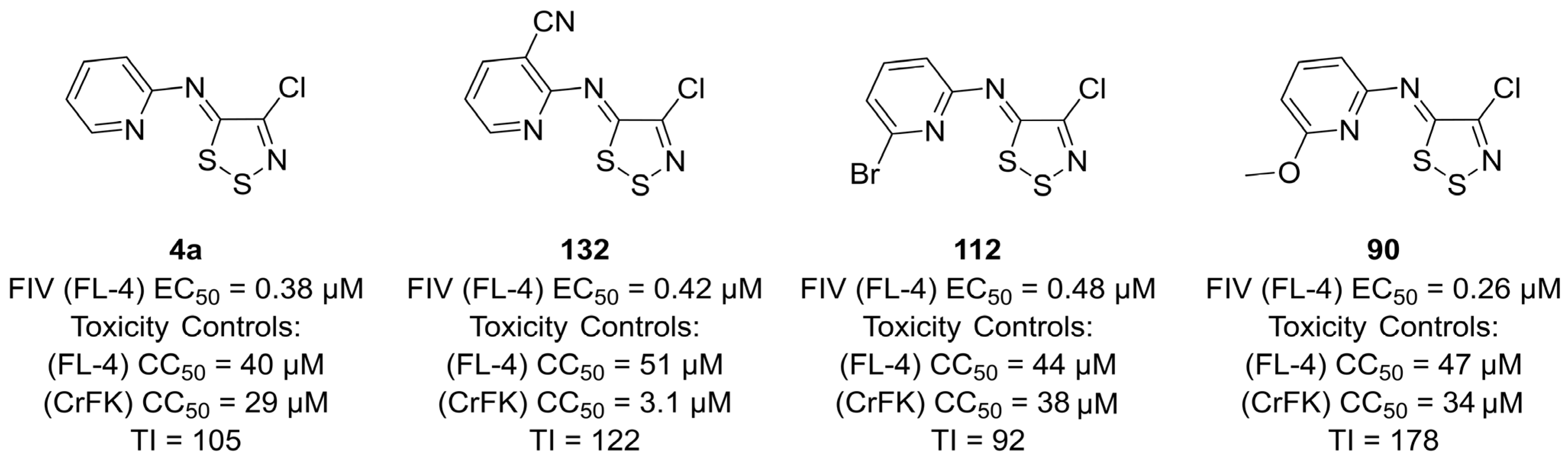
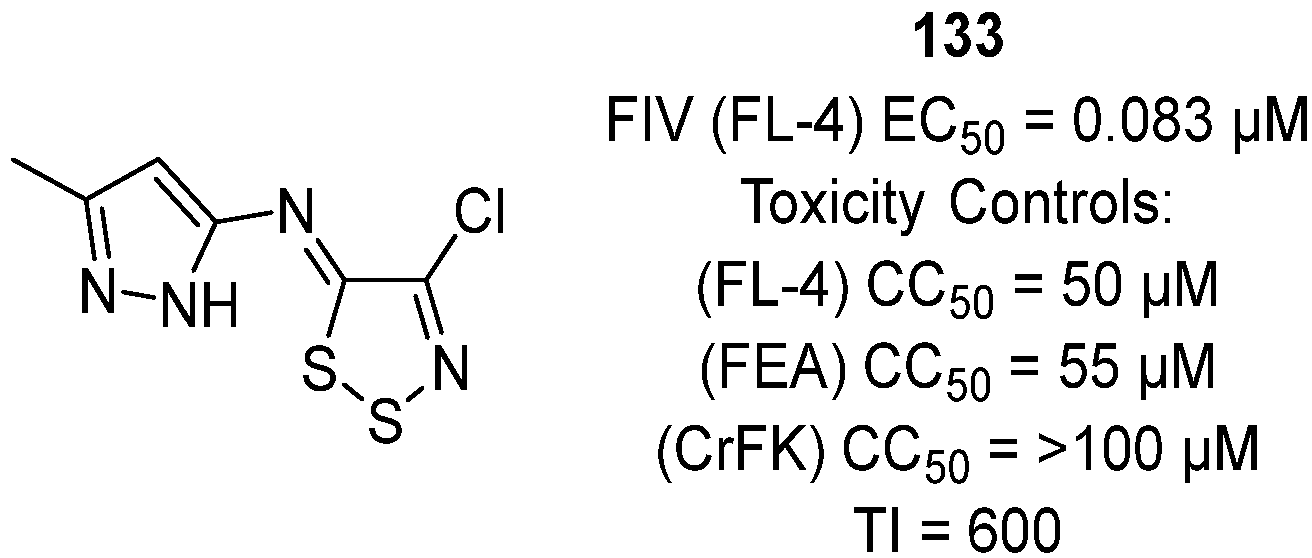
3.2. Antiviral Activities of 1,2,3-Dithiazoles
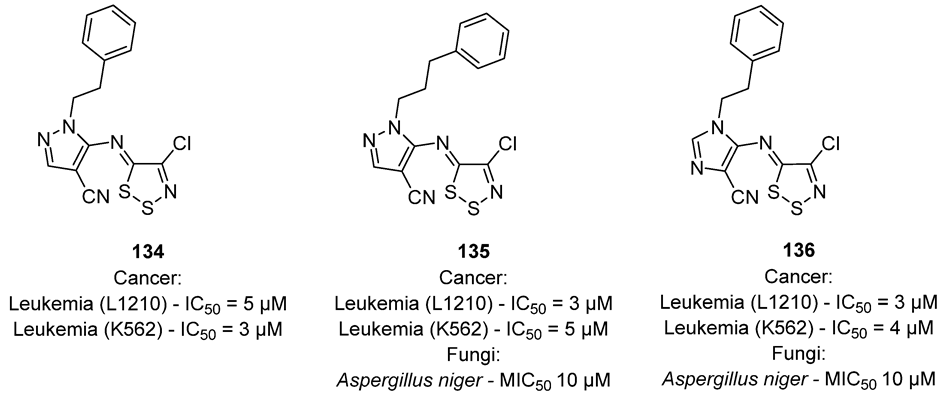
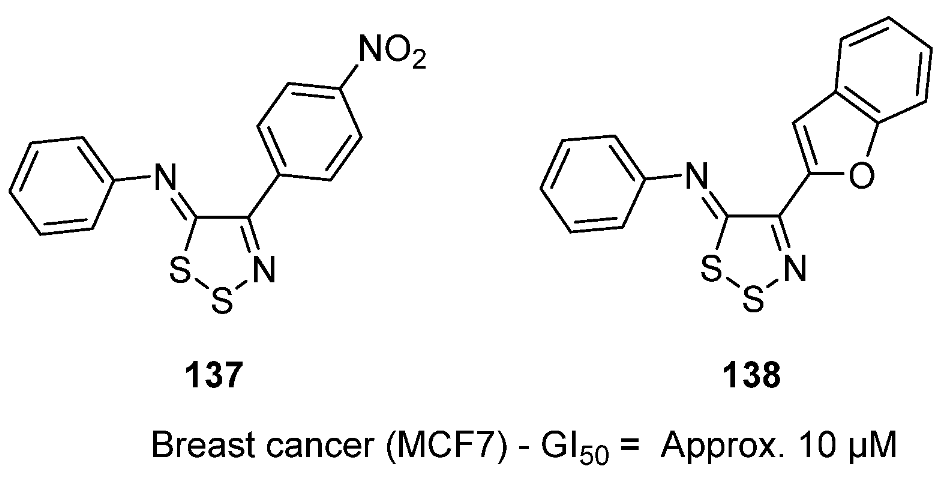
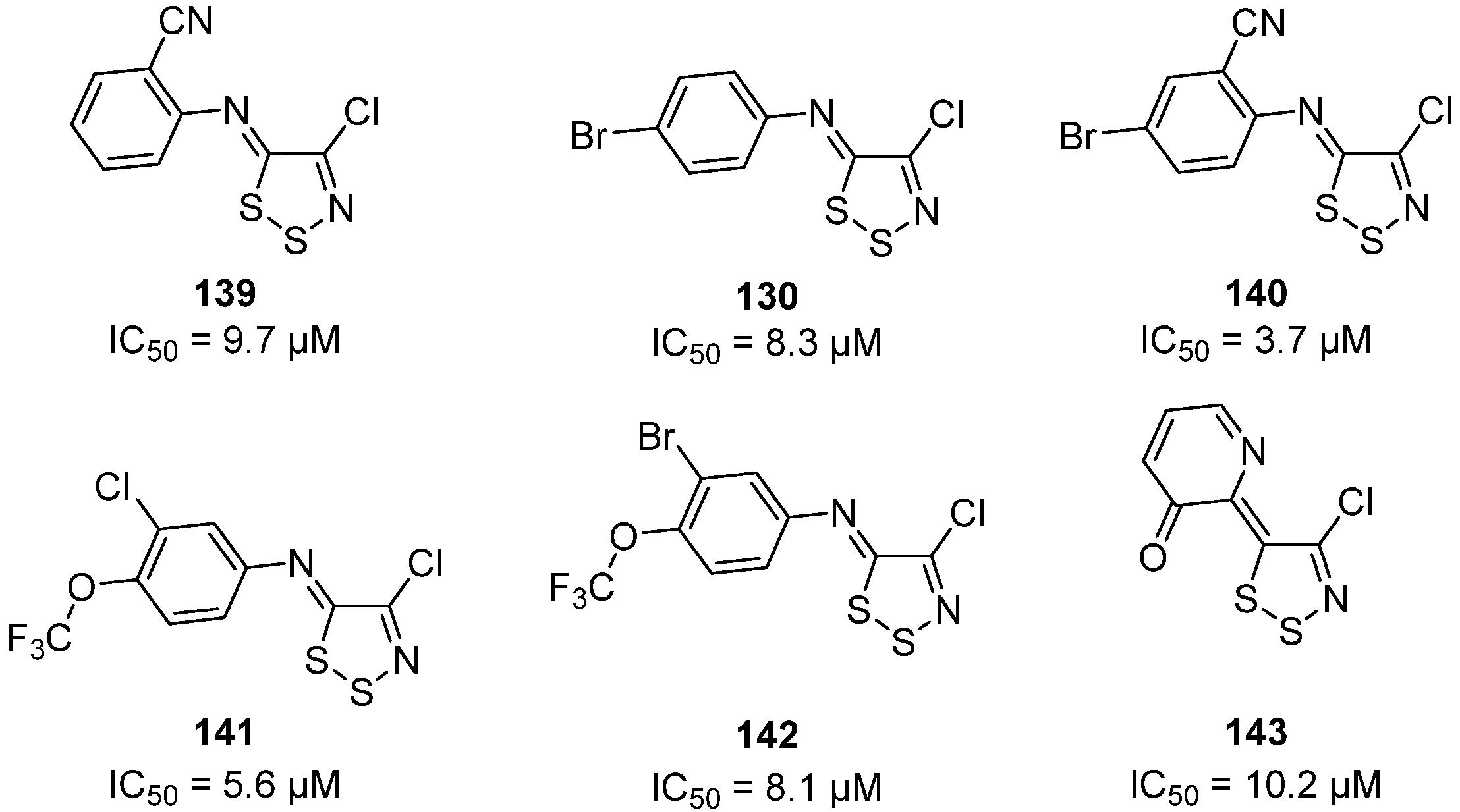
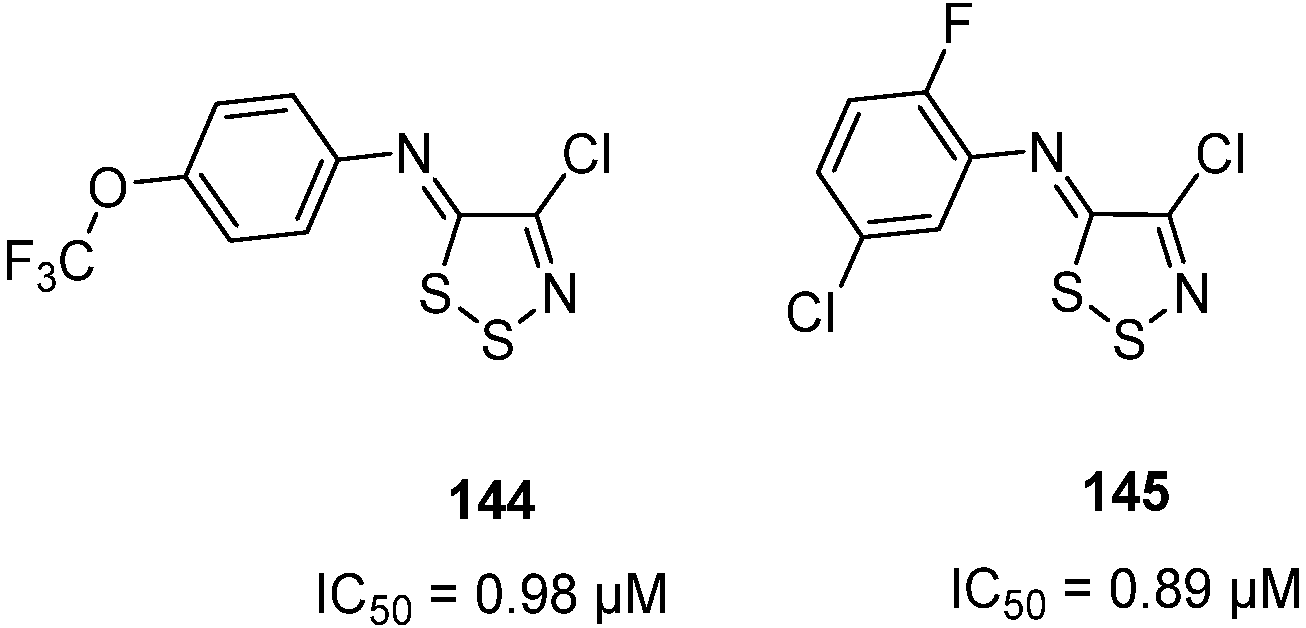
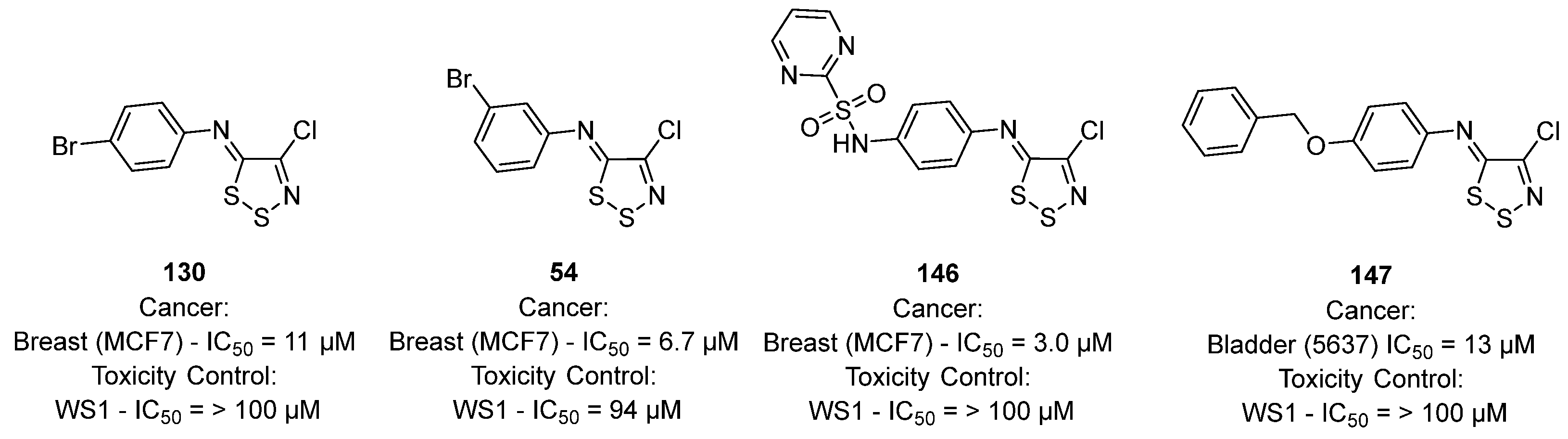
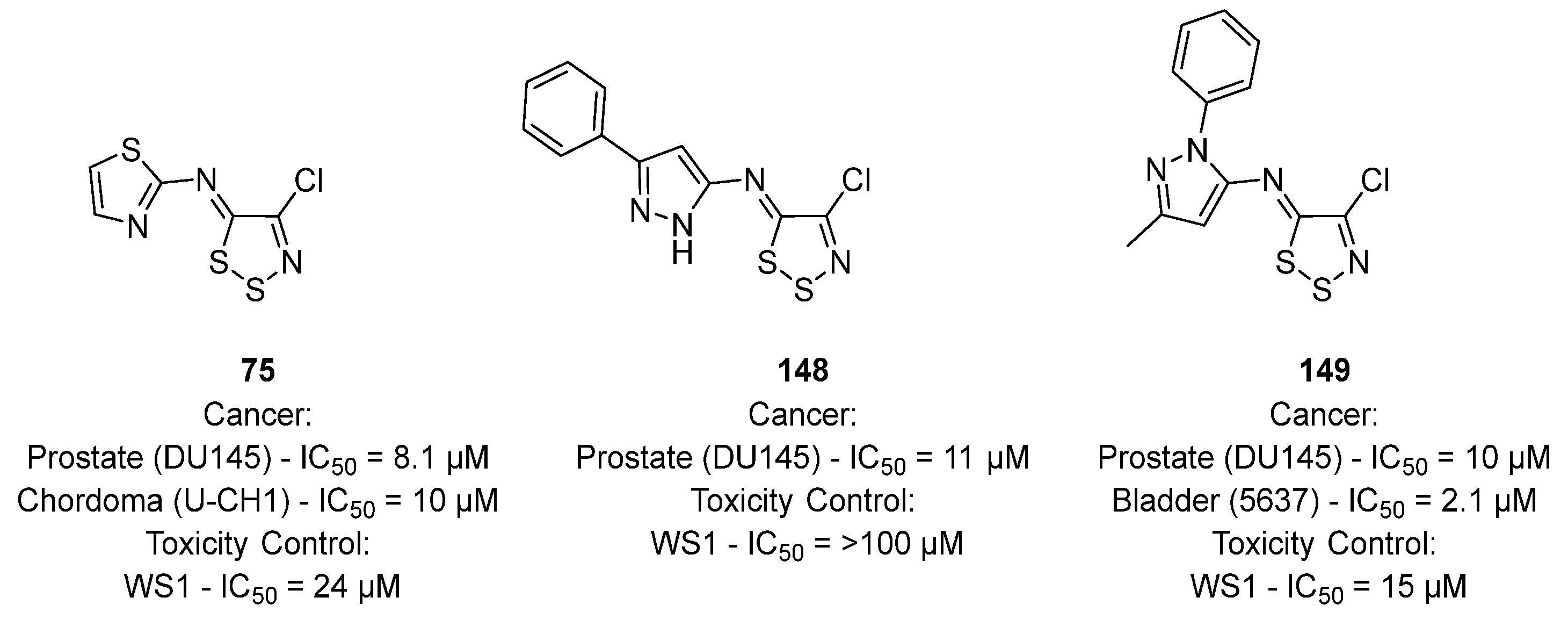
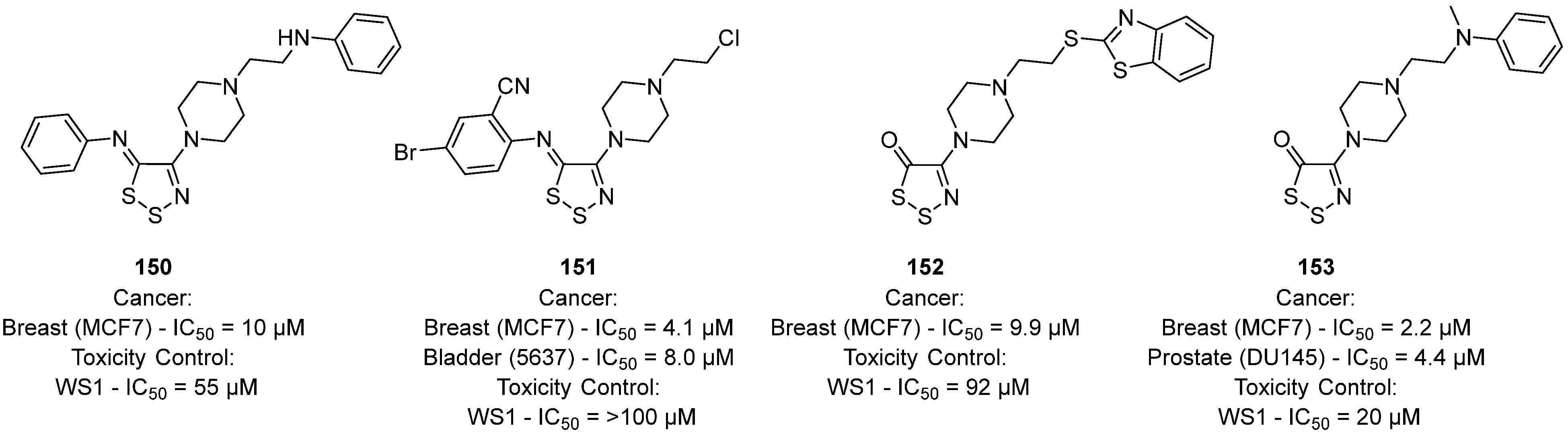
3.3. Anticancer Activities of 1,2,3-Dithiazoles
3.4. Other Biological Applications
3.4.1. Melanin Synthesis Inhibitors
3.4.2. Antifibrotic Collagen Specific Chaperone hsp47 Inhibitor
3.4.3. Arabidopsis Gibberellin 2-Oxidase Inhibitors
4. Summary and Overview
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wannagat, U.; Schindler, G. Reaktionen des Schwefeldichlorids mit Pyridin und verwandten Verbindungen. Angew. Chem. 1957, 69, 784-784. [Google Scholar] [CrossRef]
- Moore, J.E. (Chevron Research Co.) Certain 4-Halo-5-aryl-1,2,3-dithiazole Compounds and their Preparation. U.S. Patent Application No. US4059590, 22 November 1977. [Google Scholar]
- Moore, J.E. (Chevron Research Co.) Method for control of fungi using 4-halo-5-aryl-1,2,3,-dithiazoles. U.S. Patent Application No. US4119722 A, 10 October 1978. [Google Scholar]
- Appel, R.; Janssen, H.; Siray, M.; Knoch, F. Synthese und Reaktionen des 4,5-Dichlor-1,2,3-dithiazolium-chlorids. Chem. Ber. 1985, 118, 1632–1643. [Google Scholar] [CrossRef]
- Cuadro, A.M.; Alvarez-Buila, J. 4,5-Dichloro-1,2,3-dithiazolium chloride (Appel’s Salt): Reactions with N-nucleophiles. Tetrahedron 1994, 50, 10037–10046. [Google Scholar] [CrossRef]
- Rees, C.W. Polysulfur-nitrogen Heterocyclic Chemistry. J. Heterocycl. Chem. 1992, 29, 639–651. [Google Scholar] [CrossRef]
- Rakitin, O.A. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., Taylor, R.J.K., Zhdankin, V.V., Eds.; Elsevier: Oxford, UK, 2008; Volume 6, Chapter 6.01; pp. 1–36. [Google Scholar]
- Navo, C.D.; Peccati, F.; Mazo, N.; Núñez-Franco, R.; Jiménez-Osés, G. 1,2-Oxa/thia-3-azoles. In Comprehensive Heterocyclic Chemistry IV; Black, D.S., Cossy, J., Stevens, C.V., Eds.; Elsevier: Oxford, UK, 2022; Volume 6, Chapter 6.01; pp. 1–55. [Google Scholar]
- Konstantinova, L.S.; Rakitin, O.A. Synthesis and properties of 1,2,3-dithiazoles. Russ. Chem. Rev. 2008, 77, 521–546. [Google Scholar] [CrossRef]
- Kim, K. Recent Advances in 1,2,3-Dithiazole Chemistry. Phosphorus Sulfur Silicon Relat. Elem. 1997, 120, 229–244. [Google Scholar] [CrossRef]
- Kim, K. Synthesis and Reactions of 1,2,3-Dithiazoles. Sulfur Rep. 1998, 21, 147–207. [Google Scholar] [CrossRef]
- Hafner, K.; Stowasser, B.; Sturm, V. Synthesis and properties of 4,6-Di-t-Butyl-Cyclopenta-1,2-Dithiole and its 3-aza-derivative. Tetrahedron Lett. 1985, 26, 189–192. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Laborie, H.; Tanga, A.; Sopéna, V.; Lanneluc, I.; Picot, L.; Sablé, S.; Thiéry, V.; et al. One-pot synthesis of 5-phenylimino, 5-thieno or 5-oxo-1,2,3-dithiazoles and evaluation of their antimicrobial and antitumor activity. Bioorg. Med. Chem. Lett. 2009, 19, 136–141. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. The Reaction of DABCO with 4-Chloro-5H-1,2,3-dithiazoles: Synthesis and Chemistry of 4-[N-(2-Chloroethyl)piperazin-1-yl]-5H-1,2,3-dithiazoles. J. Org. Chem. 2016, 81, 615–631. [Google Scholar] [CrossRef]
- Cottenceau, G.; Besson, T.; Gautier, V.; Rees, C.W.; Pons, A.-M. Antibacterial Evaluation of Novel N-Arylimino-1,2,3-dithiazoles and N-Arylcyanothioformamides. Bioorg. Med. Chem. Lett. 1996, 6, 529–532. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Pavani, M.G.; Nuñez, M.C.; Brigidi, P.; Vitali, B.; Gambari, R.; Romagnoli, R. Antimicrobial and antitumor activity of N-heteroimmine-1,2,3-dithiazoles and their transformation in triazolo-, imidazo-, and pyrazolopirimidines. Bioorg. Med. Chem. 2002, 10, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Asquith, C.R.M.; Konstantinova, L.S.; Meli, M.L.; Laitinen, T.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R.; Hilton, S.T. Evaluation of Substituted 1,2,3-Dithiazoles as Inhibitors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein via a Proposed Zinc Ejection Mechanism. ChemMedChem 2016, 11, 2119–2126. [Google Scholar] [CrossRef]
- Thomson, C.A.; Atkinson, H.M.; Ananthanarayanan, V.S. Identification of Small Molecule Chemical Inhibitors of the Collagen-Specific Chaperone Hsp47. J. Med. Chem. 2005, 48, 1680–1684. [Google Scholar] [CrossRef]
- Charalambous, A.; Koyioni, M.; Antoniades, I.; Pegeioti, D.; Eleftheriou, I.; Michaelidou, S.S.; Amelichev, S.A.; Konstantinova, L.S.; Rakitin, O.A.; Koutentis, P.A.; et al. 1,2,3-Dithiazoles—New reversible melanin synthesis inhibitors: A chemical genomics study. Med. Chem. Comm. 2015, 6, 935–946. [Google Scholar] [CrossRef]
- Otani, M.; Yoon, J.M.; Park, S.H.; Asami, T.; Nakajima, M. Screening and characterization of an inhibitory chemical specific to Arabidopsis gibberellin 2-oxidases. Bioorg. Med. Chem. Lett. 2010, 20, 4259–4262. [Google Scholar] [CrossRef]
- Maurais, A.J.; Weerapana, E. Reactive-cysteine profiling for drug discovery. Curr. Opin. Chem. Biol. 2019, 50, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Bak, D.W.; Bechtel, T.J.; Falco, J.A.; Weerapana, E. Cysteine Reactivity Across the Sub-Cellular Universe. Curr. Opin. Chem. Biol. 2019, 48, 96–105. [Google Scholar] [CrossRef]
- Yang, F.; Chen, N.; Wang, F.; Jia, G.; Wang, C. Comparative reactivity profiling of cysteine-specific probes by chemoproteomics. Curr. Res. Chem. Biol. 2022, 2, 100024. [Google Scholar] [CrossRef]
- Hoch, D.G.; Abegg, D.; Adibekian, A. Cysteine-reactive probes and their use in chemical proteomics. Chem. Commun. 2018, 54, 4501–4512. [Google Scholar] [CrossRef]
- Chaikuad, A.; Koch, P.; Laufer, S.A.; Knapp, S. The Cysteinome of Protein Kinases as a Target in Drug Development. Angew. Chem. Int. Ed. 2018, 57, 4372–4385. [Google Scholar] [CrossRef]
- Besson, T.; Emayan, K.; Rees, C.W. 1,2,3-Dithiazoles and new routes to 3,l-benzoxazin-4-ones, 3,l -benzothiazin-4-ones and N-arylcyanothioformamides. J. Chem. Soc. Perkin Trans. 1995, 1, 2097–2102. [Google Scholar] [CrossRef]
- Besson, T.; Rees, C.W. Some chemistry of 4,5-dichloro-l,2,3-dithiazolium chloride and its derivatives. J. Chem. Soc. Perkin Trans. 1995, 1, 1659–1662. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K. A new procedure to N-Arylcyanothioformamides from 5-arylimino-4-chloro-5H-1,2,3-dithiazoles. Bull. Korean Chem. Soc. 1992, 13, 107–108. [Google Scholar] [CrossRef]
- Koutentis, P.A. The Preparation and Characterization of 5-Substituted-4-chloro-1,2,3-dithiazolium Salts and their Conversion into 4-Substituted-3-chloro-1,2,5-thiadiazoles. Molecules 2005, 10, 346–359. [Google Scholar] [CrossRef]
- Clarke, D.; Emayan, K.; Rees, C.W. New synthesis of isothiazoles from primary enamines. J. Chem. Soc. Perkin Trans. I 1998, 1, 77–82. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Christoforou, I.C.; Ioannidou, H.A.; Manos, M.J.; Koutentis, P.A. Ring transformation of (4-chloro-5H-1,2,3-dithiazol-5-ylidene)acetonitriles to 3-haloisothiazole-5-carbonitriles. RSC Adv. 2014, 4, 7735–7748. [Google Scholar] [CrossRef]
- Moon-Kook, J.; Kim, K. Synthesis of new 5-alkylidene-4-chloro-5H-1,2,3-dithiazoles and their stereochemistry. Tetrahedron 1999, 55, 9651–9667. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. The reaction of 4,5-dichloro-1,2,3-dithiazolium chloride with DMSO: An improved synthesis of 4-chloro-1,2,3-dithiazol-5H-one. Tetrahedron 2009, 65, 6855–6858. [Google Scholar] [CrossRef]
- Kalogirou, A.S.; Koutentis, P.A. A qualitative comparison of the reactivities of 3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine and 4,5-dichloro-1,2,3-dithiazolium chloride. Molecules 2015, 20, 14576–14594. [Google Scholar] [CrossRef]
- Folmer, J.J.; Weinreb, S.M. Generation of esters from carboxylic acids using Appel’s salt (4,5-dichloro-1,2,3-dithiazolium chloride). Tetrahedron Lett. 1993, 34, 2737–2740. [Google Scholar] [CrossRef]
- Laitinen, T.; Meili, T.; Koyioni, M.; Koutentis, P.A.; Poso, A.; Hofmann-Lehmann, R.; Asquith, C.R.M. Synthesis and evaluation of 1,2,3-dithiazole inhibitors of the nucleocapsid protein of feline immunodeficiency virus (FIV) as a model for HIV infection. Bioorg. Med. Chem. 2022, 68, 116834. [Google Scholar] [CrossRef] [PubMed]
- Koutentis, P.A.; Koyioni, M.; Michaelidou, S.S. Synthesis of [(4-Chloro-5H-1,2,3-dithiazol-5-ylidene)amino]azines. Molecules 2011, 16, 8992–9002. [Google Scholar] [CrossRef] [PubMed]
- Van der Plas, H.C. Chapter II SN(ANRORC) Reactions in Azines, Containing an “Outside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 9–86. [Google Scholar] [CrossRef]
- Van der Plas, H.C. Chapter III SN(ANRORC) Reactions in Azaheterocycles Containing an “Inside” Leaving Group. Adv. Heterocycl. Chem. 1999, 74, 87–151. [Google Scholar] [CrossRef]
- Lee, H.; Kim, K.; Whang, D.; Kim, K. Novel Synthesis of 5-(arylimino)-4-(dialkylamino)-5H-l,2,3-dithiazoles and the mechanism of their formation. J. Org. Chem. 1994, 59, 6179–6183. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Baranovsky, I.V.; Irtegova, I.G.; Bagryanskaya, I.Y.; Shundrin, L.A.; Zibarev, A.V.; Rakitin, O.A. Fused 1,2,3-dithiazoles: Convenient synthesis, structural characterization, and electrochemical properties. Molecules 2016, 21, 596. [Google Scholar] [CrossRef]
- Plater, M.J.; Rees, C.W.; Roe, D.G.; Torroba, T. Cyclopenta-1,2,3-dithiazoles and related compounds. J. Chem. Soc. Chem. Commun. 1993, 7, 293–294. [Google Scholar] [CrossRef]
- Koyioni, M.; Manoli, M.; Manos, M.J.; Koutentis, P.A. Reinvestigating the Reaction of 1H-Pyrazol-5-amines with 4,5-Dichloro-1,2,3-dithiazolium Chloride: A Route to Pyrazolo [3,4-c]isothiazoles and Pyrazolo[3,4-d]thiazoles. J. Org. Chem. 2014, 79, 4025–4037. [Google Scholar] [CrossRef]
- Besson, T.; Guillaumet, G.; Lamazzi, C.; Rees, C.W. Synthesis of 3,1-Benzoxazines, 3,1-Benzothiazines and 3,1-Benzoxazepines via N-Arylimino-1,2,3-dithiazoles. Synlett 1997, 6, 704–706. [Google Scholar] [CrossRef]
- Letribot, B.; Delatouche, R.; Rouillard, H.; Bonnet, A.; Chérouvrier, J.-R.; Domon, L.; Besson, T.; Thiéry, V. Synthesis of 2-Mercapto-(2-Oxoindolin-3-Ylidene)Acetonitriles from 3-(4-Chloro-5H-1,2,3-Dithiazol-5-Ylidene)Indolin-2-ones. Molecules 2018, 23, 1390. [Google Scholar] [CrossRef] [PubMed]
- Kalogirou, A.S.; Koutentis, P.A. The degradation of 4,5-dichloro-1,2,3-dithiazolium chloride in wet solvents. Tetrahedron 2009, 65, 6859–6862. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Bol’shakov, O.I.; Obruchnikova, N.V.; Golova, S.P.; Nelyubina, Y.V.; Lyssenko, K.A.; Rakitin, O.A. Reactions of 4-substituted 5H-1,2,3-dithiazoles with primary and secondary amines: Fast and convenient synthesis of 1,2,5-thiadiazoles, 2-iminothioacetamides and 2-oxoacetamides. Tetrahedron 2010, 66, 4330–4338. [Google Scholar] [CrossRef]
- Appel, R.; Janssen, H.; Haller, I.; Plempel, M. (Bayer AG) 1,2,3-Dithiazolderivate, Verfahren zu ihrer Herstellung Sowie ihre Verwendung als Arzneimittel. Ger. Patent Application No. DE2848221 A1, 7 November 1980. [Google Scholar]
- Mayer, R.; Fçrster, E.; Matauschek, B.D. Verfahren zur Herstellung von Aromatisch oder Heteroaromatisch Substituierten Cyanthioformamiden. Ger. Patent Application No. DD212387, 8 August 1984. [Google Scholar]
- Besson, T.; Rees, C.W.; Cottenceau, G.; Pons, A.-M. Antimicrobial evaluation of 3,1-benzoxazin-4-ones, 3,1-benzothiazin-4-ones, 4-alkoxyquinazolin-2-carbonitriles and N-arylimino-1,2,3-dithiazoles. Bioorg. Med. Chem. Lett. 1996, 6, 2343–2348. [Google Scholar] [CrossRef]
- Joseph, R.W.; Antes, D.L.; Osei-Gyimah, P. (Rohm & Haas Co.) Antimicrobial Compounds with Quick Speed of Kill. U.S. Patent Application No. US5688744 A, 18 November 1997. [Google Scholar]
- Thiéry, V.; Rees, C.W.; Besson, T.; Cottenceau, G.; Pons, A.-M. Antimicrobial activity of novel N-quinolinyl and N-naphthylimino-1,2,3-dithiazoles. Eur. J. Med. Chem. 1998, 33, 149–153. [Google Scholar] [CrossRef]
- Thiéry, V.; Bébéteau, V.; Guillard, J.; Lamazzi, C.; Besson, T.; Cottenceau, G.; Pons, A.M. Antimicrobial activity of novel N-arylimino-1,2,3-dithiazoles. Pharm. Pharmacol. Commun. 1998, 4, 39–42. Available online: https://onlinelibrary.wiley.com/doi/epdf/10.1111/j.2042-7158.1998.tb00315.x (accessed on 21 March 2023).
- Benting, J.; Dahmen, P.; Wachendorff-Neumann, U.; Hadano, H.; Vors, J.-P. (Bayer Cropscience AG) Int. 5-heteroarylimino-1,2,3-dithiazoles PCT Pub. No. WO2012045726 A2, 12 April 2012. [Google Scholar]
- Laitinen, T.; Baranovsky, I.V.; Konstantinova, L.S.; Poso, A.; Rakitin, O.A.; Asquith, C.R.M. Antimicrobial and antifungal activity of rare substituted 1,2,3-Thiaselenazoles and Corresponding Match Pair 1,2,3-Dithiazoles. Antibiotics 2020, 9, 369. [Google Scholar] [CrossRef]
- Konstantinova, L.S.; Baranovsky, I.V.; Pritchina, E.A.; Mikhailov, M.S.; Bagryanskaya, I.Y.; Semenov, N.A.; Irtegova, I.G.; Salnikov, G.E.; Lyssenko, K.A.; Gritsan, N.P.; et al. Fused 1,2,3-Thiaselenazoles Synthesized from 1,2,3-Dithiazoles through Selective Chalcogen Exchange. Chem. Eur. J. 2017, 23, 17037–17047. [Google Scholar] [CrossRef]
- Blaskovich, M.A.; Zuegg, J.; Elliott, A.G.; Cooper, M.A. Helping chemists discover new antibiotics. ACS Infect. Dis. 2015, 1, 285–287. [Google Scholar] [CrossRef]
- WHO. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. 2017. Available online: http://www.who.int/mediacentre/news/releases/2017/bacteria-antibiotics-needed/en/ (accessed on 28 February 2023).
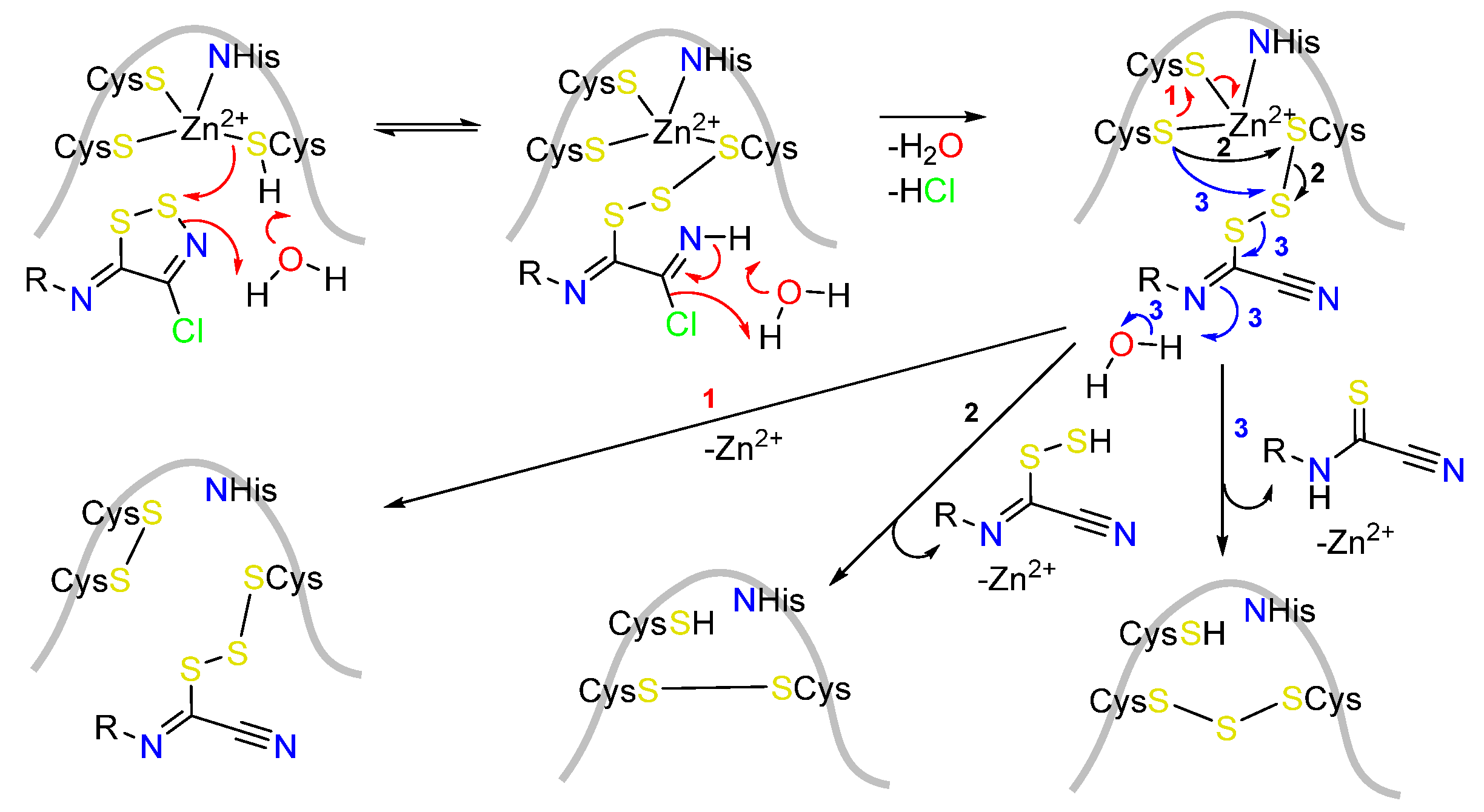
- Cook, K.M.; Hilton, S.T.; Mecinović, J.; Motherwell, W.B.; Figg, W.D.; Schofield, C.J. Epidithiodiketopiperazines block the interaction between hypoxia-inducible factor-1α (HIF-1α) and p300 by a zinc ejection mechanism. J. Biol. Chem. 2009, 284, 26831–26838. [Google Scholar] [CrossRef]
- Sekirnik, R.; Rose, N.R.; Thalhammer, A.; Seden, P.T.; Mecinović, J.; Schofield, C.J. Inhibition of the histone lysine demethylase JMJD2A by ejection of structural Zn(II). Chem. Commun. 2009, 42, 6376–6378. [Google Scholar] [CrossRef]
- Woodcock, J.C.; Henderson, W.; Miles, C.O. Metal complexes of the mycotoxins sporidesmin A and gliotoxin, investigated by electrospray ionisation mass spectrometry. J. Inorg. Biochem. 2001, 85, 187–199. [Google Scholar] [CrossRef]
- Woodcock, J.C.; Henderson, W.; Miles, C.O.; Nicholson, B.K. Metal complexes of sporidesmin D and dimethylgliotoxin, investigated by electrospray ionisation mass spectrometry. J. Inorg. Biochem. 2001, 84, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Asquith, C.R.M.; Meili, T.; Laitinen, T.; Baranovsky, I.V.; Konstantinova, L.S.; Poso, A.; Rakitin, O.A.; Hofmann-Lehmann, R. Synthesis and comparison of substituted 1,2,3-dithiazole and 1,2,3-thiaselenazole as inhibitors of the feline immunodeficiency virus (FIV) nucleocapsid protein as a model for HIV infection. Bioorg. Med. Chem. Lett. 2019, 29, 1765–1768. [Google Scholar] [CrossRef]
- Sancineto, L.; Mariotti, A.; Bagnoli, L.; Marini, F.; Desantis, J.; Iraci, N.; Santi, C.; Pannecouque, C.; Tabarrini, O. Design and Synthesis of DiselenoBisBenzamides (DISeBAs) as Nucleocapsid Protein 7 (NCp7) Inhibitors with anti-HIV Activity. J. Med. Chem. 2015, 58, 9601–9614. [Google Scholar] [CrossRef] [PubMed]
- Oppedisano, F.; Catto, M.; Koutentis, P.A.; Nicolotti, O.; Pochini, L.; Koyioni, M.; Introcaso, A.; Michaelidou, S.S.; Carotti, A.; Indiveri, C. Inactivation of the Glutamine/Amino Acid Transporter ASCT2 by 1,2,3-dithiazoles: Proteoliposomes as a Tool to Gain Insights in the Molecular Mechanism of Action and of Antitumor Activity. Toxicol. Appl. Pharmacol. 2012, 265, 93–102. [Google Scholar] [CrossRef]
- Scalise, M.; Pochini, L.; Console, L.; Losso, M.A.; Indiveri, C. The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology. Front. Cell Dev. Biol. 2018, 6, 96. [Google Scholar] [CrossRef]
- Bröer, S. Amino Acid Transporters as Disease Modifiers and Drug Targets. SLAS Discov. 2018, 23, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, T.; Li, Z.; Wang, L.; Yuan, S.; Sun, L. The role of ASCT2 in cancer: A review. Eur. J. Pharmacol. 2018, 837, 81–87. [Google Scholar] [CrossRef]
- Tailor, C.S.; Marin, M.; Nouri, A.; Kavanaugh, M.P.; Kabat, D. Truncated forms of the dual function human ASCT2 neutral amino acid transporter/retroviral receptor are translationally initiated at multiple alternative CUG and GUG codons. J. Biol. Chem. 2001, 276, 27221–27230. [Google Scholar] [CrossRef]
- Napolitano, L.; Scalise, M.; Koyioni, M.; Koutentis, P.; Catto, M.; Eberini, I.; Parravicini, C.; Palazzolo, L.; Pisani, L.; Galluccio, M.; et al. Potent Inhibitors of Human LAT1 (SLC7A5) Transporter Based on Dithiazole and Dithiazine Compounds for Development of Anticancer Drugs. Biochem. Pharmacol. 2017, 143, 39–52. [Google Scholar] [CrossRef]
- Lopes, C.; Pereira, C.; Medeiros, R. ASCT2 and LAT1 Contribution to the Hallmarks of Cancer: From a Molecular Perspective to Clinical Translation. Cancers 2021, 13, 203. [Google Scholar] [CrossRef]
- Okano, N.; Naruge, D.; Kawai, K.; Kobayashi, T.; Nagashima, F.; Endou, H.; Furuse, J. First-in-human phase I study of JPH203, an L-type amino acid transporter 1 inhibitor, in patients with advanced solid tumors. Investig. New Drugs 2020, 38, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Prejanò, M.; Romeo, I.; La Serra, M.A.; Russo, N.; Marino, T. Computational Study Reveals the Role of Water Molecules in the Inhibition Mechanism of LAT1 by 1,2,3-Dithiazoles. J. Chem. Inf. Model. 2021, 61, 5883–5892. [Google Scholar] [CrossRef]
- Maffuid, K.A.; Koyioni, M.; Torrice, C.D.; Murphy, W.A.; Mewada, H.K.; Koutentis, P.A.; Crona, D.J.; Asquith, C.R.M. Design and evaluation of 1,2,3-dithiazoles and fused 1,2,4-dithiazines as anti-cancer agents. Bioorg. Med. Chem. Lett. 2021, 43, 128078. [Google Scholar] [CrossRef]
- Wentrup, G.-J.; Koepke, M.; Boberg, F. Über 1,2-Dithiacyclopentene; XXIX. 3-Thioxo-3H-1,2-Dithiole aus 3-Chloro-1,2-dithio-lium-chloriden. Synthesis 1975, 525–526. [Google Scholar] [CrossRef]
- Lowe, P.A. The Chemistry of the Sulphonium Group; Stirling, C.J.M., Ed.; Wiley: Chichester, UK, 1981; Volume 1, Chapter 11; pp. 267–312. [Google Scholar] [CrossRef]
- Ogurtsov, V.A.; Rakitin, O.A.; Rees, C.W.; Smolentsev, A.A.; Lyssenko, K.A. New routes to 1,2-dithiole-3-thiones and 3-imines. Mendeleev Commun. 2005, 15, 20–21. [Google Scholar] [CrossRef]
- Masuda, H.; Fukumoto, M.; Hirayoshi, K.; Nagata, K. Coexpression of the collagen-binding stress protein Hsp47 gene and the alpha 1(I) and alpha 1(III) collagen genes in carbon tetrachloride induced rat liver fibrosis. J. Clin. Investig. 1994, 94, 2481–2488. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Taguchi, T. Collagen-binding heat shock protein (Hsp) 47 expression in anti-thymocyte serum (ATS)-induced glomerulonephritis. J. Pathol. 1997, 183, 24–29. [Google Scholar] [CrossRef]
- Razzaque, M.S.; Nazneen, A.; Taguchi, T. Immunolocalization of collagen and collagen-binding heat shock protein 47 in fibrotic lung diseases. Mod. Pathol. 1998, 11, 1183–1188. [Google Scholar]
- Razzaque, M.S.; Ahmed, A.R. Collagens, collagen-binding heat shock protein 47 and transforming growth factor-beta 1 are induced in cicatricial pemphigoid: Possible role(s) in dermal fibrosis. Cytokine 2002, 17, 311–316. [Google Scholar] [CrossRef]
- Rocnik, E.; Chow, L.H.; Pickering, J.G. Heat shock protein 47 is expressed in fibrous regions of human atheroma and is regulated by growth factors and oxidized low-density lipoprotein. Circulation 2000, 101, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Nagata, K.; Yamada, K.M. Phosphorylation and transformation sensitivity of a major collagen-binding protein of fibroblasts. J. Biol. Chem. 1986, 261, 7531–7536. [Google Scholar] [CrossRef]
- Nakayama, I.; Miyazawa, T.; Kobayashi, M.; Kamiya, Y.; Abe, H.; Sakurai, A. Effects of a New Plant Growth Regulator Prohexadione Calcium (BX-112) on Shoot Elongation Caused by Exogenously Applied Gibberellins in Rice (Oryza sativa L.) Seedlings. Plant Cell Physiol. 1990, 31, 195–200. [Google Scholar] [CrossRef]
- Rademacher, W. GROWTH RETARDANTS: Effects on Gibberellin Biosynthesis and Other Metabolic Pathways. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2000, 51, 501–531. [Google Scholar] [CrossRef]
- Mazum, T.K.; Bricker, B.A.; Flores-Rozas, H.; Ablordeppey, S.Y. The Mechanistic Targets of Antifungal Agents: An Overview. Mini. Rev. Med. Chem. 2016, 16, 555–578. [Google Scholar] [CrossRef] [PubMed]

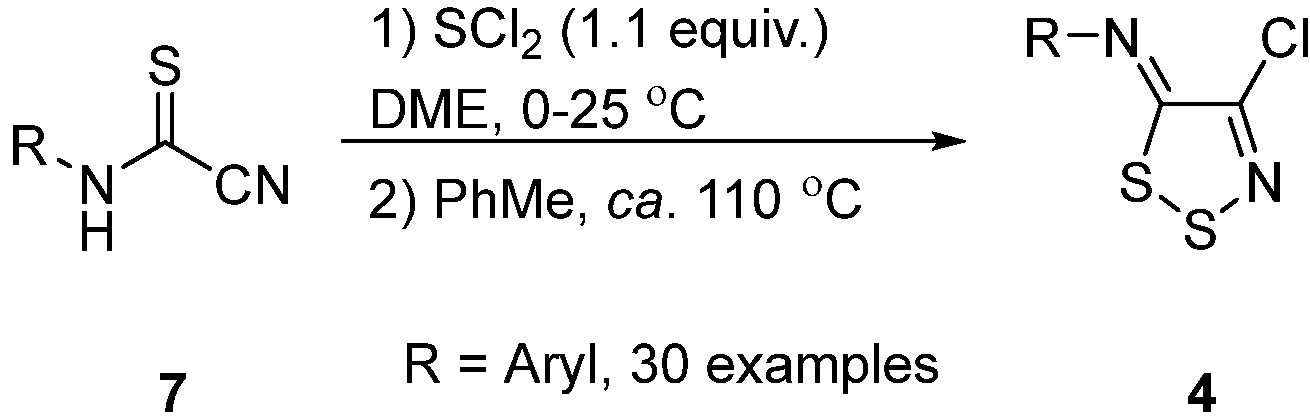



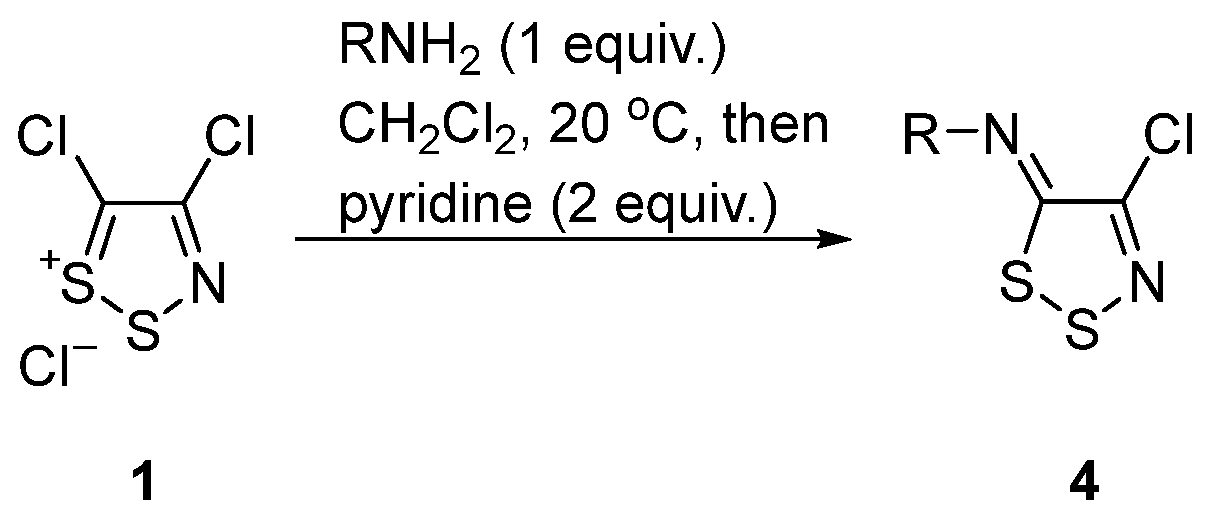



















Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalogirou, A.S.; Oh, H.J.; Asquith, C.R.M. The Synthesis and Biological Applications of the 1,2,3-Dithiazole Scaffold. Molecules 2023, 28, 3193. https://doi.org/10.3390/molecules28073193
Kalogirou AS, Oh HJ, Asquith CRM. The Synthesis and Biological Applications of the 1,2,3-Dithiazole Scaffold. Molecules. 2023; 28(7):3193. https://doi.org/10.3390/molecules28073193
Chicago/Turabian StyleKalogirou, Andreas S., Hans J. Oh, and Christopher R. M. Asquith. 2023. "The Synthesis and Biological Applications of the 1,2,3-Dithiazole Scaffold" Molecules 28, no. 7: 3193. https://doi.org/10.3390/molecules28073193
APA StyleKalogirou, A. S., Oh, H. J., & Asquith, C. R. M. (2023). The Synthesis and Biological Applications of the 1,2,3-Dithiazole Scaffold. Molecules, 28(7), 3193. https://doi.org/10.3390/molecules28073193






