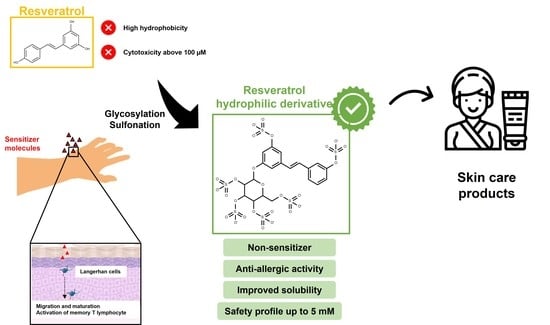
A Hydrophilic Sulfated Resveratrol Derivative for Topical Application: Sensitization and Anti-Allergic Potential
Abstract
1. Introduction
2. Results and Discussion
2.1. Comparison of Reactive Points Using in Silico Software ADMETLab 2.0
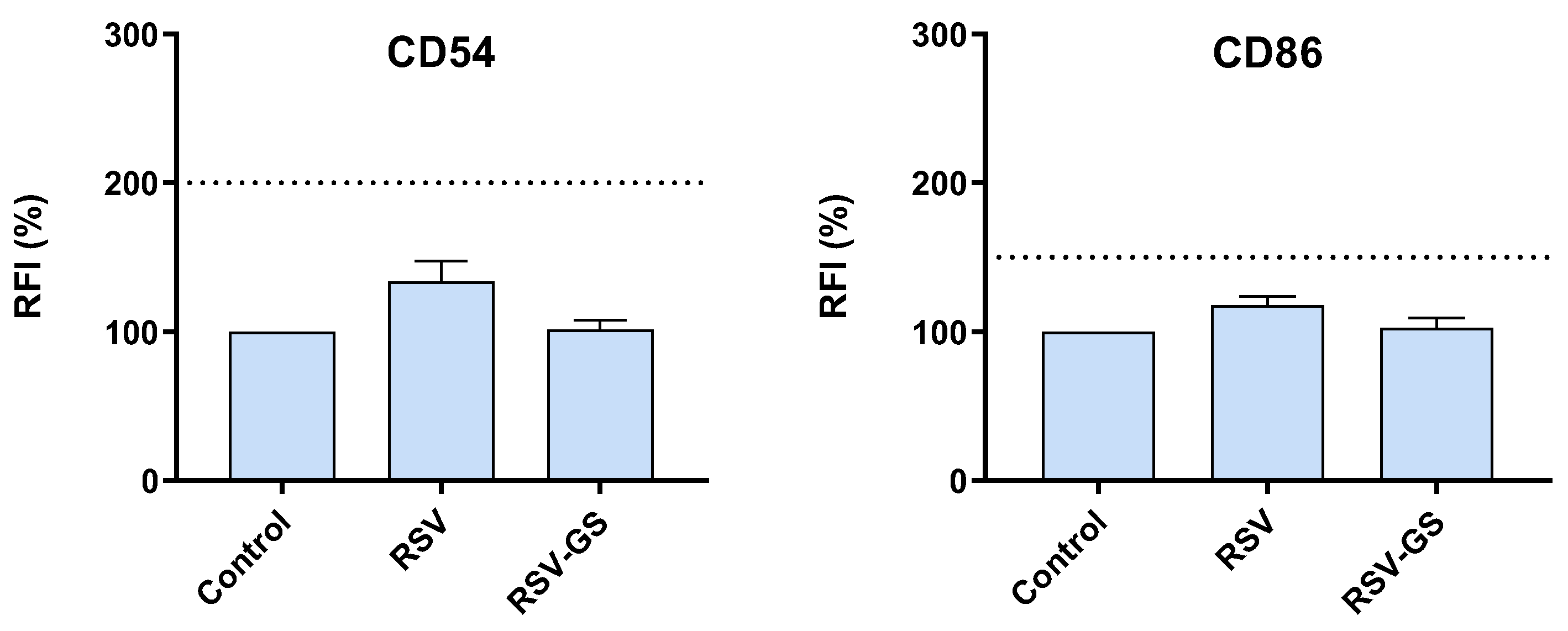
2.2. Skin Sensitization Potential of RSV and RSV-GS in THP-1 Cells
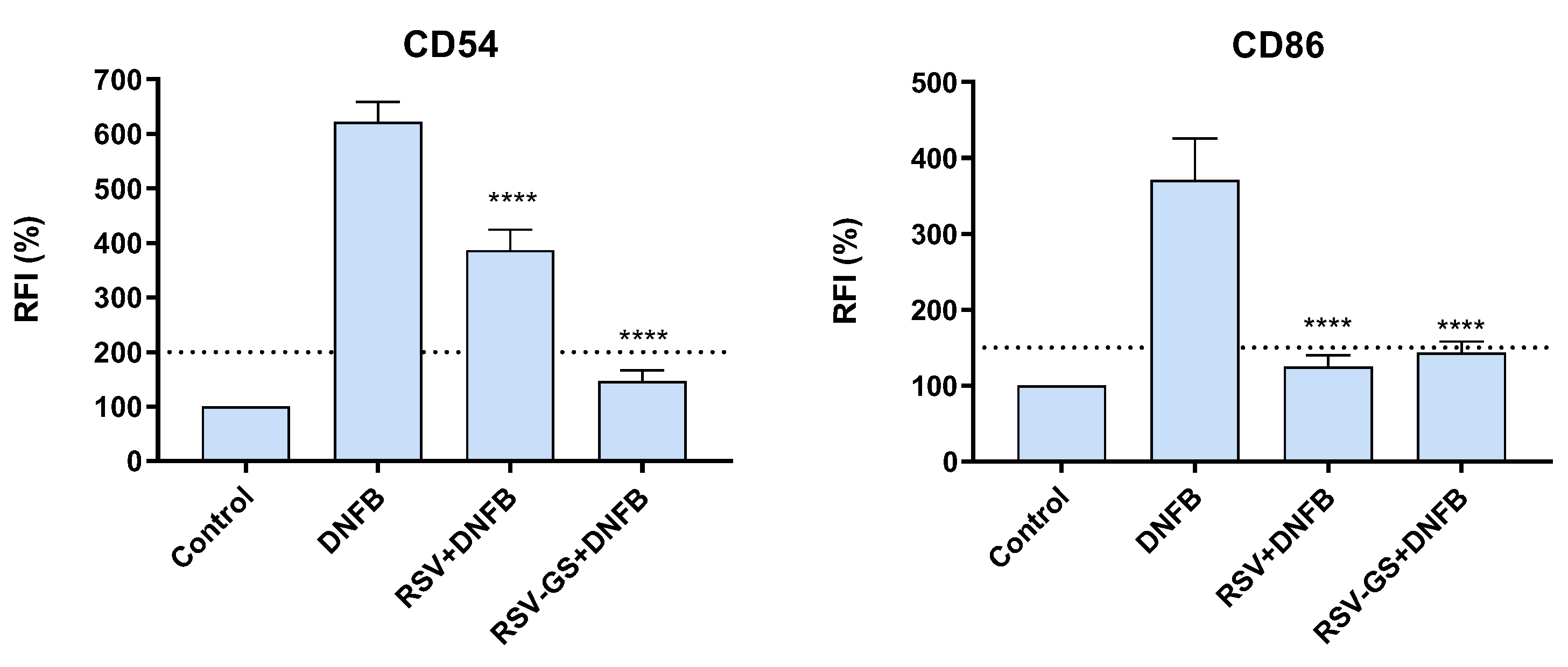
2.3. Anti-Allergic Potential of RSV and RSV-GS in THP-1 Cells
3. Materials and Methods
3.1. Materials
3.2. Software Used for the Prediction of Sensitization Potential and Reactive Points of Chemicals
3.3. Inhibition of THP-1 Maturation Profile Induced by the Strong Allergen DNFB
3.4. Flow Cytometry Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gilaberte, Y.; Prieto-Torres, L.; Pastushenko, I.; Juarranz, Á. Anatomy and Function of the Skin. In Nanoscience in Dermatology; Elsiever: Amsterdam, The Netherlands, 2016; pp. 1–14. [Google Scholar]
- Incorvaia, C.; Frati, F.; Verna, N.; D’Alo, S.; Motolese, A.; Pucci, S. Allergy and the skin. Clin. Exp. Immunol. 2008, 153 (Suppl. 1), 27–29. [Google Scholar] [CrossRef] [PubMed]
- Moreira, P.; Sousa, F.J.; Matos, P.; Brites, G.S.; Goncalves, M.J.; Cavaleiro, C.; Figueirinha, A.; Salgueiro, L.; Batista, M.T.; Branco, P.C.; et al. Chemical Composition and Effect against Skin Alterations of Bioactive Extracts Obtained by the Hydrodistillation of Eucalyptus globulus Leaves. Pharmaceutics 2022, 14, 561. [Google Scholar] [CrossRef] [PubMed]
- Luis, A.; Martins, J.D.; Silva, A.; Ferreira, I.; Cruz, M.T.; Neves, B.M. Oxidative stress-dependent activation of the eIF2alpha-ATF4 unfolded protein response branch by skin sensitizer 1-fluoro-2,4-dinitrobenzene modulates dendritic-like cell maturation and inflammatory status in a biphasic manner [corrected]. Free Radic. Biol. Med. 2014, 77, 217–229. [Google Scholar] [CrossRef]
- Silva, F.; Brites, G.; Ferreira, I.; Silva, A.; Miguel Neves, B.; Costa Pereira, J.; Cruz, M.T. Evaluating Skin Sensitization Via Soft and Hard Multivariate Modeling. Int. J. Toxicol. 2020, 39, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A.; Travers, P.; Walport, M.; Shlomchik, M.J. Effector mechanisms in allergic reactions. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001. [Google Scholar]
- Brites, G.S.; Ferreira, I.; Sebastiao, A.I.; Silva, A.; Carrascal, M.; Neves, B.M.; Cruz, M.T. Allergic contact dermatitis: From pathophysiology to development of new preventive strategies. Pharmacol. Res. 2020, 162, 105282. [Google Scholar] [CrossRef]
- Martins, M.S.; Ferreira, M.S.; Almeida, I.F.; Sousa, E. Occurrence of Allergens in Cosmetics for Sensitive Skin. Cosmetics 2022, 9, 32. [Google Scholar] [CrossRef]
- Richard, M.A.; Paul, C.; Nijsten, T.; Gisondi, P.; Salavastru, C.; Taieb, C.; Trakatelli, M.; Puig, L.; Stratigos, A. Prevalence of most common skin diseases in Europe: A population-based study. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1088–1096. [Google Scholar] [CrossRef]
- Sebastiao, A.I.; Ferreira, I.; Brites, G.; Silva, A.; Neves, B.M.; Teresa Cruz, M. NLRP3 Inflammasome and Allergic Contact Dermatitis: A Connection to Demystify. Pharmaceutics 2020, 12, 867. [Google Scholar] [CrossRef]
- Johansen, J.D.; Bonefeld, C.M.; Schwensen, J.F.B.; Thyssen, J.P.; Uter, W. Novel insights into contact dermatitis. J. Allergy Clin. Immunol. 2022, 149, 1162–1171. [Google Scholar] [CrossRef]
- Kimber, I.; Poole, A.; Basketter, D.A. Skin and respiratory chemical allergy: Confluence and divergence in a hybrid adverse outcome pathway. Toxicol. Res. 2018, 7, 586–605. [Google Scholar] [CrossRef]
- OECD. Guidance Document on the Reporting of Defined Approaches and Individual Information Sources to Be Used within Integrated Approaches to Testing and Assessment (IATA) for the Skin Sensitization; ENV/JM/MONO(2016)29; OECD: Paris, France, 2016; Volume 256, pp. 1–317. [Google Scholar]
- OECD. Test Guideline No. 442E In Vitro Skin Sensitisation Assays Addressing the Key Event on Activation of Dendritic Cells on the Adverse Outcome Pathway for Skin Sensitisation; OECD: Paris, France, 2022; pp. 1–92. [Google Scholar]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Ferreira, M.S.; Magalhaes, M.C.; Oliveira, R.; Sousa-Lobo, J.M.; Almeida, I.F. Trends in the Use of Botanicals in Anti-Aging Cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Khan, J.; Dukhyil, A.A.B.; Alarousy, R.; Attah, E.I.; Sharma, T.; Khairnar, S.J.; Bendale, A.R. Chalcone Scaffolds, Bioprecursors of Flavonoids: Chemistry, Bioactivities, and Pharmacokinetics. Molecules 2021, 26, 7177. [Google Scholar] [CrossRef]
- Hano, C.; Tungmunnithum, D. Plant Polyphenols, More than Just Simple Natural Antioxidants: Oxidative Stress, Aging and Age-Related Diseases. Medicines 2020, 7, 26. [Google Scholar] [CrossRef]
- Menaa, F.; Menaa, A.; Tréton, J. Polyphenols against Skin Aging. In Polyphenols in Human. Health and Disease, 1st ed.; Academic Press: Cambridge, MA, USA, 2014; pp. 819–830. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Neves, A.R.; Lúcio, M.; Lima, J.L.C.; Reis, S. Resveratrol in Medicinal Chemistry: A critical Review of its pharmacokinetics, drug-delivery, and membrane interactions. Curr. Med. Chem. 2012, 19, 1663–1681. [Google Scholar]
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664. [Google Scholar] [CrossRef]
- Regev-Shoshani, G.; Shoseyov, O.; Kerem, Z. Glycosylation of resveratrol protects it form enzymic oxidation. Biochem. J. 2003, 374, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Koudous, I.; Both, S.; Gudi, G.; Schulz, H.; Strube, J. Process design based on physicochemical properties for the example of obtaining valuable products from plant-based extracts. Comptes Rendus Chim. 2014, 17, 218–231. [Google Scholar] [CrossRef]
- Sousa, M.E.; Correia-da-Silva, M.; Pinto, M.M. Sulfated Flavonoids: Nature Playing with the Hydrophilic-Hydrophobic Balance. In Natural Products: Chemistry, Biochemistry and Pharmacology; Brahmachari, G., Ed.; Narosa Publishing House: New Delhi, India, 2008; pp. 392–416. [Google Scholar]
- Martins, B.T.; Correia da Silva, M.; Pinto, M.; Cidade, H.; Kijjoa, A. Marine natural flavonoids: Chemistry and biological activities. Nat. Prod. Res. 2019, 33, 3260–3272. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M. Emerging sulfated flavonoids and other polyphenols as drugs: Nature as an inspiration. Med. Res. Rev. 2014, 34, 223–279. [Google Scholar] [CrossRef] [PubMed]
- Carvalhal, F.; Correia-da-Silva, M.; Sousa, E.; Pinto, M.; Kijoa, A. Sources and Biological activities of marine sulfated steroids. J. Mol. Endocrinol. 2018, 61, T211–T231. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, C.; Sousa, E.; Pinto, M.; Correia-da-Silva, M. An antifouling model from the sea: A review of 25 years of zosteric acid studies. Biofouling 2017, 33, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Correia-da-Silva, M.; Sousa, E.; Duarte, B.; Marques, F.; Carvalho, F.; Cunha-Ribeiro, L.M.; Pinto, M.M. Flavonoids with an oligopolysulfated moiety: A new class of anticoagulant agents. J. Med. Chem. 2011, 54, 95–106. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Sousa, E.; Duarte, B.; Marques, F.; Cunha-Ribeiro, L.M.; Pinto, M.M. Dual anticoagulant/antiplatelet persulfated small molecules. Eur. J. Med. Chem. 2011, 46, 2347–2358. [Google Scholar] [CrossRef]
- Correia-da-Silva, M.; Rocha, V.; Marques, C.; Deus, C.M.; Marques-Carvalho, A.; Oliveira, P.J.; Palmeira, A.; Pinto, M.; Sousa, E.; Sousa Lobo, J.M.; et al. SULFATION PATHWAYS: Potential benefits of a sulfated resveratrol derivative for topical application. J. Mol. Endocrinol. 2018, 61, M27–M39. [Google Scholar] [CrossRef]
- Poor, M.; Kaci, H.; Bodnarova, S.; Mohos, V.; Fliszar-Nyul, E.; Kunsagi-Mate, S.; Ozvegy-Laczka, C.; Lemli, B. Interactions of resveratrol and its metabolites (resveratrol-3-sulfate, resveratrol-3-glucuronide, and dihydroresveratrol) with serum albumin, cytochrome P450 enzymes, and OATP transporters. Biomed. Pharmacother. 2022, 151, 113136. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A.; et al. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef] [PubMed]
- Ashikaga, T.; Sakaguchi, H.; Sono, S.; Kosaka, N.; Ishikawa, M.; Nukada, Y.; Miyazawa, M.; Ito, Y.; Nishiyama, N.; Itagaki, H. A comparative evaluation of in vitro skin sensitisation testes: The human cell-line activation test (h-CLAT) versus the local lymh node assay (LLNA). ATLA 2010, 38, 275–284. [Google Scholar]
- Gallo, R.; Viglizzo, G.; Vecchio, F.; Parodi, A. Allergic contact dermatitis from pentylene glycol in an emollient cream, with possible co-sensitization to resveratrol. Contact Derm. 2003, 48, 176–177. [Google Scholar] [CrossRef]
- Degraeuwe, A.; Jacobs, M.-C.; Herman, A. Alergic contact dermatitis caused by resveratrol in a cosmetic cream. Contact Derm. 2020, 82, 412–413. [Google Scholar] [CrossRef]
- Khaimov, V.; Reske, T.; Matschegewski, C.; Grabow, N.; Eickner, T. Safety evaluation of resveratrol as an active compound for drug-eluting cardiovascular implants. Curr. Dir. Biomed. Eng. 2019, 5, 331–333. [Google Scholar] [CrossRef]
| Compound | Skin Sensitization Rule | Reactive Points (Highlighted with Red) |
|---|---|---|
| RSV | Four alerts (reactive points in the structure) |  |
| RSV-GS | Three alerts (reactive points in the structure) |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jesus, A.; Sebastião, A.I.; Brites, G.; Correia-da-Silva, M.; Cidade, H.; Cruz, M.T.; Sousa, E.; Almeida, I.F. A Hydrophilic Sulfated Resveratrol Derivative for Topical Application: Sensitization and Anti-Allergic Potential. Molecules 2023, 28, 3158. https://doi.org/10.3390/molecules28073158
Jesus A, Sebastião AI, Brites G, Correia-da-Silva M, Cidade H, Cruz MT, Sousa E, Almeida IF. A Hydrophilic Sulfated Resveratrol Derivative for Topical Application: Sensitization and Anti-Allergic Potential. Molecules. 2023; 28(7):3158. https://doi.org/10.3390/molecules28073158
Chicago/Turabian StyleJesus, Ana, Ana I. Sebastião, Gonçalo Brites, Marta Correia-da-Silva, Honorina Cidade, Maria T. Cruz, Emília Sousa, and Isabel F. Almeida. 2023. "A Hydrophilic Sulfated Resveratrol Derivative for Topical Application: Sensitization and Anti-Allergic Potential" Molecules 28, no. 7: 3158. https://doi.org/10.3390/molecules28073158
APA StyleJesus, A., Sebastião, A. I., Brites, G., Correia-da-Silva, M., Cidade, H., Cruz, M. T., Sousa, E., & Almeida, I. F. (2023). A Hydrophilic Sulfated Resveratrol Derivative for Topical Application: Sensitization and Anti-Allergic Potential. Molecules, 28(7), 3158. https://doi.org/10.3390/molecules28073158