Abstract
The purpose of this work was to prepare new isatin- and monothiomalondiamide-based indole derivatives, as well as to study the properties of the new compounds. The four-component reaction of 5-R-isatins (R = H, CH3), malononitrile, monothiomalonamide (3-amino-3-thioxo- propanamide) and triethylamine in hot EtOH yields a mixture of isomeric triethylammonium 6′-amino-3′-(aminocarbonyl)-5′-cyano-2-oxo-1,2-dihydro-1′H- and 6′-amino-3′-(aminocarbonyl)- 5′-cyano-2-oxo-1,2-dihydro-3′H-spiro[indole-3,4′-pyridine]-2′-thiolates. The reactivity and structure of the products was studied. We found that oxidation of spiro[indole-3,4′-pyridine]-2′-thiolates with DMSO-HCl system produced only acidification products, diastereomeric 6′-amino-5′-cyano-5-methyl-2-oxo-2′-thioxo-1,2,2′,3′-tetrahydro-1′H-spiro-[indole-3,4′-pyridine]- 3′-carboxamides, instead of the expected isothiazolopyridines. The alkylation of the prepared spiro[indole-3,4′-pyridine]-2′-thiolates upon treatment with N-aryl α-chloroacetamides and α-bromoacetophenones proceeds in a regioselective way at the sulfur atom. In the case of α-bromoacetophenones, ring-chain tautomerism was observed for the S-alkylation products. According to NMR data, the compounds consist of a mixture of stereoisomers of 2′-amino-6′-[(2-aryl-2-oxoethyl)thio]-3′-cyano-2-oxo-1′H-spiro[indoline-3,4′-pyridine]-5′-carboxamides and 5′-amino-3′-aryl-6′-cyano-3′-hydroxy-2-oxo-2′,3′-dihydrospiro[indoline-3,7′-thiazolo[3,2-a]pyridine]-8′-carboxamides in various ratios. The structure of the synthesized compounds was confirmed by IR spectroscopy, HRMS, 1H and 13C DEPTQ NMR studies and the results of 2D NMR experiments (1H-13C HSQC, 1H-13C HMBC). Molecular docking studies were performed to investigate suitable binding modes of some new compounds with respect to the transcriptional regulator protein PqsR of Pseudomonas aeruginosa. The docking studies revealed that the compounds have affinity for the bacterial regulator protein PqsR of Pseudomonas aeruginosa with a binding energy in the range of −5.8 to −8.2 kcal/mol. In addition, one of the new compounds, 2′-amino-3′-cyano-5-methyl-2-oxo-6′-{[2-oxo-2-(p-tolylamino)ethyl]thio}-1′H-spiro-[indoline-3,4′-pyridine]-5′-carboxamide, showed in vitro moderate antibacterial effect against Pseudomonas aeruginosa and good antioxidant properties in a test with 1,1-diphenyl-2-picrylhydrazyl radical. Finally, three of the new compounds were recognized as moderately active herbicide safeners with respect to herbicide 2,4-D in the laboratory experiments on sunflower seedlings.
1. Introduction
Nicotinonitriles, nicotinamides and their partially saturated analogs represent a promising class of heterocyclic compounds with an interesting profile of biological activity (for reviews, see [1,2,3,4,5,6,7,8,9,10,11]). However, while nicotinonitriles have been fairly well studied, the related synthetic nicotinamides and 1,4-dihydronicotinamides have been less studied and require further investigation. One of the most accessible and efficient approaches to the synthesis of functionalized nicotinamides is based on the reaction of active methylene malonamides and malonthioamides with 1,3-C3 dielectrophiles [12,13,14,15,16,17,18,19,20,21,22]. Monothiomalonamide 1 (3-amino-3-thioxopropanamide) is known as a very convenient reagent for the preparation of substituted nicotinamides. It can be easily synthesized by passing H2S through a hot saturated solution of cyanoacetamide in pyridine in the presence of Et3N [23,24]. Substituted nicotinamides can be prepared by the reaction of monothiomalonamide 1 with malononitrile and aldehydes (or with arylmethylene malononitriles originated from Knoevenagel-type condensation) (Scheme 1). At the same time, it was noted that the structure of the reaction products strongly depends on the conditions. Thus, the paper [25] describes the preparation of 1,4-dihydropyridines 2 by reaction of monothiomalonamide 1 with aldehydes and malononitrile in the presence of Et3N in hot EtOH solution. However, other authors demonstrated the formation of nicotinamides 3 under similar reaction conditions [26]. As shown in paper [27], the reaction of thioamide 1 with benzylidene malononitrile may result in the formation of three different products, depending on the temperature, base and solvent used: the Michael adducts 4, 1,4-dihydropyridin-2-thiolates similar to compounds 2, or their 3,4-dihydro isomers 5 (Scheme 1). The formation of 3,4-dihydronicotinamides was also reported in other papers [13,21,28].
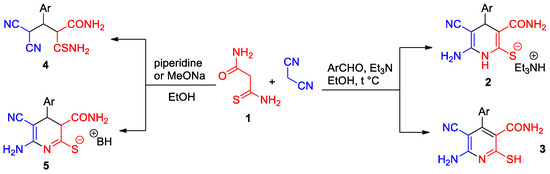
Scheme 1.
Possible pathways for reaction of monothiomalonamide 1 with malonononitrile and aldehydes.
As highly reactive indole derivatives, isatins are very widely used in fine organic synthesis (for a review of isatin chemistry, see [29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44]). Isatin is also probably one of the most important compounds among the biologically active indoles. In recent years, isatin and isatin-based molecular hybrids have found a variety of applications in pharmacy and drug design [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62].
Isatins have not previously been reacted with monothiomalonamide 1 and malononitrile. The expected products of spiro[indoline-3,4′-pyridine] structure are of interest as promising biologically active agents.
In particular, among the compounds with such structural motifs, acetyl and butyryl cholinesterase inhibitors 6 [63], antimicrobial agents 7 [64], insecticides 8 [65], antitumor agents 9 [66], on-off fluorescent chemosensors 10 for Cu2+ imaging in human hepatocellular liver carcinoma cells [67], fungicides 11 [68] and SARS-CoV-2 inhibitors 12 [69] were found (Scheme 2).
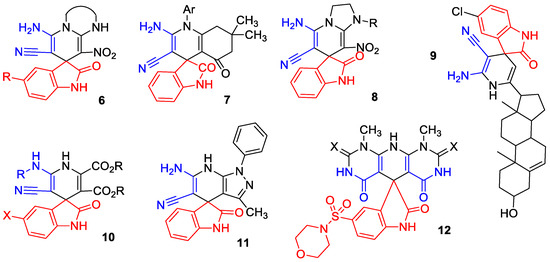
Scheme 2.
Biologically important spiro[indoline-3,4′-pyridines].
In the context of our interest in exploring the chemistry of biologically active nicotinonitriles [70,71,72,73,74,75,76,77,78,79] and indole–nicotinonitrile hybrids [80], it seemed reasonable to prepare new 6′-amino-5′-cyano-2-oxo-1,2-dihydro-1′H-spiro[indole-3,4′-pyridine]- 3′-carboxamides and to study their reactions and properties.
This work presents our research results concerning the reaction of monothiomalonamide 1 with isatins and malononitrile under basic conditions, as well as reactivity features, molecular docking studies and biological activities of the prepared 2′-amino-3′-cyano-2-oxospiro[indoline-3,4′-pyridine]-5′-carboxamides.
2. Results and Discussion
2.1. Synthesis
When a mixture of isatin and malononitrile was treated with excessive triethylamine and monothiomalonamide 1 (1.0 eq.), a white solid of a heterocyclization product was isolated in 88% yield. Detailed analysis of IR, 1H NMR, 13C DEPTQ NMR and 1H-13C HSQC and 1H-13C HMBC spectra revealed that the product was a mixture of triethylammonium 6′-amino-3′-(aminocarbonyl)-5′-cyano-2-oxo-1,2-dihydro-1′H-spiro-[indole-3,4′-pyridine]-2′-thiolate 13a and 3′H isomeric thiolate 13b in a molar ratio of ~7:1 (Scheme 3). A similar reaction with 5-methylisatin led to the formation of a mixture of 1′H-thiolate 14a and 3′H-thiolate 14b in a molar ratio of 7:3 and yield of 83%.
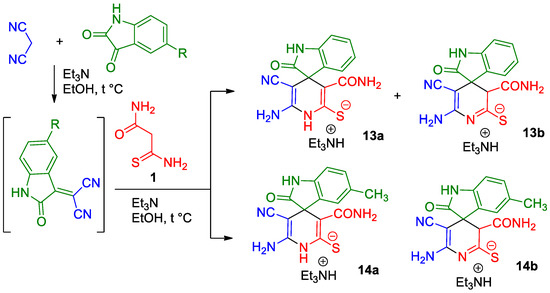
Scheme 3.
The reaction of isatins with malononitrile and monothiomalonamide 1 (3-amino-3-thioxopropanamide) in the presence of Et3N.
Full signal assignment for 13a + 13b and 14a + 14b mixtures was based on an analysis of the IR and NMR spectroscopy data, including 13C DEPTQ and 2D 1H-13C HSQC and 1H-13C HMBC experiments (Supplementary Data File). Thus, the 1H NMR spectra of 13a + 13b and 14a + 14b revealed a double set of aromatic proton signals and two peaks of NH2 protons (for major 1′H isomer at δ 5.36–5.37 ppm and for minor 3′H isomer at δ 6.23–6.25 ppm), as well as a common signal for NH isatin protons (δ 9.44–9.55 ppm) and one peak of pyridine NH proton of the major 1′H isomer at δ 7.67–7.69 ppm.
Overall, the NMR spectra of the compounds 13 and 14 show a complex picture. It is noteworthy that in some cases the signals of the minor 3′H-isomers 13b,14b exhibit signal doubling, apparently due to the splitting of the signals of two diastereomeric pairs (Scheme 4):
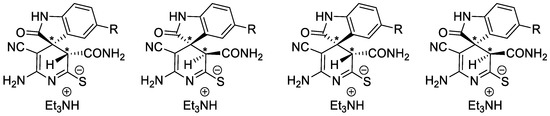
Scheme 4.
Diastereomeric pairs of minor isomers—2′-amino-5′-carbamoyl-3′-cyano- 2-oxo-3′H-spiro[indoline-3,4′-pyridine]-2′-thiolates 13b,14b. An asterisk (*) marks the chiral centers in the molecules.
Noteworthy is the fact that the signals of the CONH2 protons were observed as two peaks with a very significant difference in chemical shifts (∆δ ≥ 4 ppm). Thus, for the major 1′H isomer, CONH2 protons appeared at δ 5.75–5.77 ppm and δ 10.25–10.26 ppm, while the peaks of the minor 3′H isomer were observed at δ 6.32–6.37 ppm and δ 10.19–10.30 ppm. We assume that the S-C=C-C(O)NH2 fragment is planar because of conjugation. The observed difference in chemical shifts can be explained by the presence of an intramolecular hydrogen bond between one of the CONH2 protons and the negatively charged sulfur atom on the one hand, and the anisotropic shielding effect of the carbonyl group on the second proton on the other (Scheme 5).
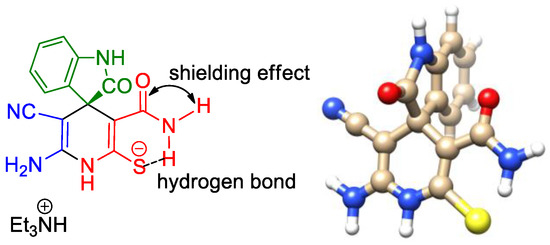
Scheme 5.
The local shielding/deshielding effects on the CONH2 protons.
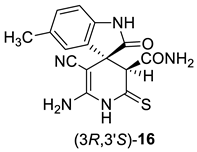
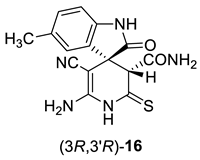
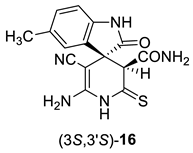
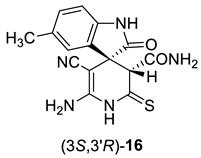
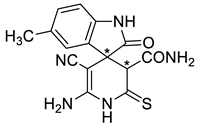
Due to the presence of a number of active functional groups, the resulting thiolates 13 and 14 seem to be attractive molecules for further transformations. Thus, in order to explore the reactivity of spiro[indole-3,4′-pyridine]-2′-thiolates, we attempted to oxidize thiolates 13 and 14 with dimethyl sulfoxide (DMSO)–HCl system. The oxidation of 2-mercaptonicotinamides and related compounds was reported as a general method for the synthesis of 3-oxo-2,3-dihydroisothiazolo[5,4-b]pyridines [24,81,82,83]. Such compounds are direct structural analogues of the practically important thieno[2,3-b]pyridines [84]. However, isothiazolo[5,4-b]pyridines are much less studied, although some compounds showed anti-tuberculosis [85] effects, were reported as COX-1 cyclooxygenase inhibitors [86], analgesics [87] and strong inhibitors of histone acetyltransferases with potential anticancer effects [88]. Previously, we have reported that the DMSO–HCl system can be successfully used as a mild oxidizing agent to convert 2-mercaptopyridin-3-carboxamides into isothiazolo[5,4-b]pyridines [72,89]. Nevertheless, upon treatment of thiolates 14a + 14b with excess HCl in DMSO solution, only corresponding 2-thioxopyridine 16 was isolated in 88% yield instead of the expected products 15. According to NMR spectroscopy data, compound 16 exists in the solution as a mixture of diastereomers in the ratio ~3:5 (Scheme 6).
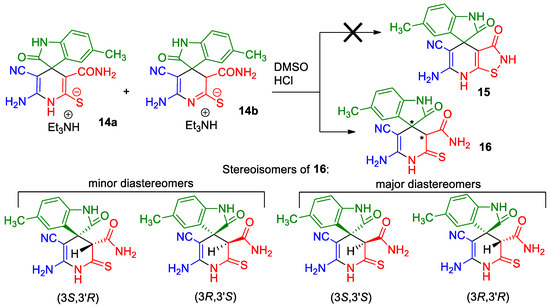
Scheme 6.
Synthesis and stereochemical features of 2-thioxopyridine 16. An asterisk (*) marks the chiral centers in the molecules.
In actuality, there are two chiral centers in the molecule 16; therefore, the compound can exist as a mixture of two diastereomeric pairs—(3S,3′R)/(3R,3′S) and (3S,3′S)(3R,3′R) (Scheme 6). The H-3′ proton signal of the minor isomers (δ 4.06 ppm) is noticeably shifted downfield with respect to the H-3′ signal of the major isomers (δ 3.78 ppm). Hence, we believe that the H-3′ proton of major isomer is affected by the anisotropic shielding effect of indoline C-2 carbonyl group, while the minor isomer H-3′ proton is not. Therefore, the (3S,3′S)/(3R,3′R) configuration should be assigned to the major diastereomers, and the (3S,3′R)(3R,3′S) configuration to the minor diastereomeric pair.
Alkylation of thiolates is a well-known approach to the diversity of heterocyclic thio ethers. Therefore, we decided to study the alkylation of thiolates 13 and 14 with some active α-halo carbonyls. It is well known that the alkylation of pyridine-2-thiolates and related compounds proceed in a regiospecific way at the sulfur atom. The S-alkylation products have been proven to be multifunctional reagents for fine organic synthesis and were recognized as biologically active compounds (for reviews, see [1,6,9,10,11,90,91,92]).
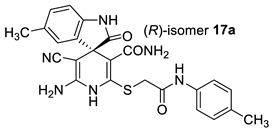
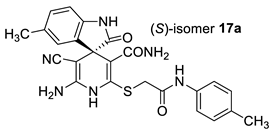
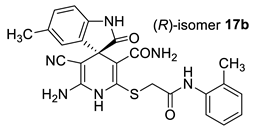
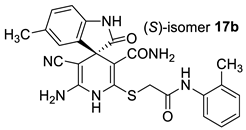
As expected, upon treatment with N-aryl α-chloroacetamides and α-bromoacetophenones, thiolates 13 and 14 underwent regiospecific alkylation to give exclusively S-alkylation products in high yields. The structure of the products was confirmed by means of IR, 1H NMR, 13C NMR DEPTQ and HRMS spectroscopy. Analysis of spectral data of compounds 17 (Scheme 7) showed that the relevant products had the structure of 1′H-isomers only, while 3′H-isomers were not detected. This can be explained by the ease of prototropic migration 3′H→1′H with the formation of thermodynamically more stable products; a similar phenomenon was previously observed in some other S-alkylation reactions [13,28].
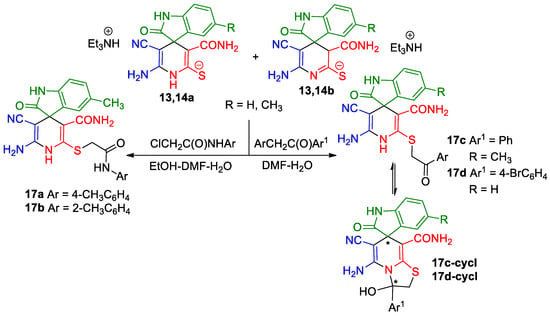
Scheme 7.
Alkylation of thiolates 13 and 14. An asterisk (*) marks the chiral centers in the molecules.
It should also be noted that the spectra of carboxamides 17a,b revealed the expected patterns, whereas the spectral picture of the alkylation products with α-bromacetophenones was very complex and contained signals of at least three different compounds. This pattern can be explained by the ring tautomerism of compounds 17c,d in a solution. In addition, cyclic tautomers—thiazolo[3,2-a]pyridines 17c-cycl and 17d-cycl each have two stereogenic centers and are mixtures of two diastereomeric pairs (Scheme 8). It is noteworthy that similar ring-chain tautomerism was also previously observed in a number of related compounds [28,93].
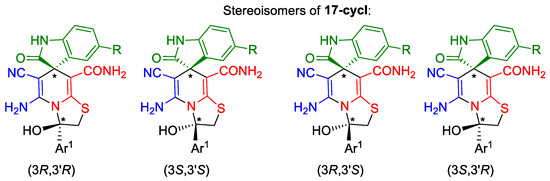
Scheme 8.
Diastereomeric pairs of thiazolo[3,2-a]pyridine tautomers of compounds 17c,d-cycl. An asterisk (*) marks the chiral centers in the molecules.
The signals in the 1H NMR spectra of ring and chain forms of 17c,d were assigned based on the integral intensities of SCH2 signals as well as on the presence of OH and pyridine NH proton signals. The major cyclic tautomers, thiazolo[3,2-a]pyridines 17c,d-cycl, have two sets of two doublets shifted upfield (δ 3.18–3.26 and 3.54–3.58 ppm) with respect to the characteristic AB-quartet of SCH2C(O)Ar protons of acyclic minor forms 17c,d (δ 4.71–4.76 ppm, 2J = 17.2 Hz) (Supplementary Data File).
Nevertheless, the unambiguous assignment of signals in the NMR spectra of all tautomers and stereoisomers in the 17c,d system seems difficult. Additionally, the question of which tautomeric form of 17c,d is preferred in the solid state remains unclear; the presence of a strong absorption band at ν 1697–1701 cm−1, which may be assigned to keto carbonyls, and the absence of band doubling indirectly indicate the preference of noncyclic forms. However, other authors [93] showed, using an X-ray method, that compounds with such type of ring-chain tautomerism in the solid state exist as thiazolo[3,2-a]pyridines. In general, this question requires further exploration.
2.2. Docking Studies
First, we used the Osiris property explorer on-line tool [94] for in silico prediction of the drug likeness of the synthesized compounds. Salts 13 and 14, as well as tautomeric mixtures 17c,d, were excluded from further consideration. All the molecules 16,17a,b were found to be non-toxic in terms of mutagenicity, carcinogenicity, skin irritancy and reproductive effects.
Many indoles/isatins, nicotinonitriles and hybrid compounds combining fragments of the above systems are of interest as antimicrobial agents. Recently, indole-containing compounds have been reported to have antibacterial activity as well as activity as resistance-modifying agents [95,96,97,98]. It is noteworthy that infections caused by Pseudomonas aeruginosa pose a real problem, especially in critically ill and immunodeficient patients. The main cause of high mortality is the emergence of drug-resistant strains [99].
For this reason, we decided to investigate the antibacterial potential of the new compounds 16,17a,b against Pseudomonas aeruginosa. The LysR-type transcriptional regulator protein PqsR (PDB ID 4JVC) was chosen as a target. This bacterial transcriptional regulator protein is a key component of alkyl-quinolone-dependent quorum sensing in Pseudomonas aeruginosa and could be considered as a potential target for new antibacterial agents that attenuate infection by blocking virulence [100].
The energy-minimized 3D structures for each individual stereoisomer of ligand molecules 16,17a,b were docked using Autodock Vina against the bacterial regulator protein PqsR of Pseudomonas aeruginosa (PDB ID 4JVC) at standard precision mode (SP). The docking score of all the ligand molecules 16,17a,b were found to be in the range of −5.8 to −8.2 kcal/mol.
The docking results are presented in Table 1. As follows from Table 1, both the (R)- and (S)-enantiomers of compound 17a had the minimum value of the scoring function and hence showed the best affinity to the protein target. The major interactions between the ligands with the target protein can be categorized as “hydrogen bonding, hydrophobic”, which was crucial to stabilize the inhibitors inside the binding pocket of the receptor. The most important interactions are shown in Figure 1 and Figure 2.

Table 1.
The results obtained from the rigid docking studies for stereoisomers of compounds 16,17a,b against bacterial transcriptional regulator protein PqsR of Pseudomonas aeruginosa (PDB ID 4JVC).
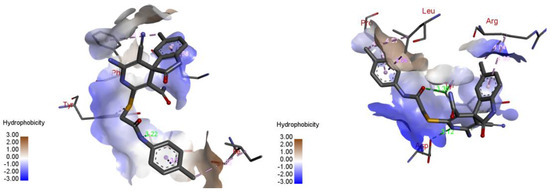
Figure 1.
Best-scored docking poses for (R)-17a (left) and (S)-17a (right) with bacterial protein PqsR (PDB ID 4JVC) (dotted lines show non-valent contacts of the ligand with amino acid residues of the target protein PqsR).
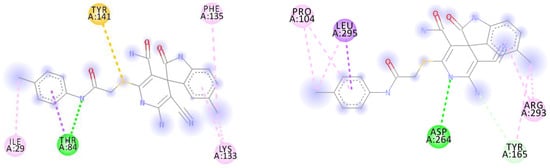
Figure 2.
Two-dimensional docking pose interaction diagram with the key amino acids in bacterial protein PqsR binding site for (R)-17a (left) and (S)-17a (right) (conventional hydrogen bonds showed green, Pi-Sigma interactions showed purple, Pi-Sulfur interaction showed yellow and alkyl and Pi-alkyl interactions showed pink).
2.3. Antibacterial and Antioxidant Activity of Compound 17a
The leader compound 17a was also tested in vitro to determine its ability to inhibit pathogen growth by the agar well-diffusion method using Mueller–Hinton agar at 100 mg/mL concentration. Antibacterial activity was tested against two strains of Gram-negative bacteria: Escherichia coli ATCC 25922 and Pseudomonas aeruginosa ATCC 27853. The reference drug was Ciprofloxacin. The results of in vitro studies showed that 17a efficiently suppresses Gram-negative bacteria P. aeruginosa ATCC 27853, but has no effect against Gram-positive bacteria. Compound 17a also demonstrated a moderate inhibitory effect against E. coli. Then, the minimum inhibitory concentration (MIC) value for 17a was determined based on the results of the examination according to the protocol described in [101]. Ciprofloxacin was used as the reference drug. We found that compound 17a showed moderate antibacterial activity with MIC 12.5 μg/mL (Ciprofloxacin—1 μg/mL).
The ability of 17a to scavenge DPPH radical (1,1-diphenyl-2-picrylhydrazyl) was examined by the method proposed by Blois [102]. Antioxidant activity was calculated in % of inhibition according to Equation (1):
where I—inhibition effect, %; Acontrol—absorption in control experiment; and Atest—absorption in test with compound 17a.
The results are shown in Table 2. As follows from Table 2, compound 17a showed antioxidant effects comparable to those of ascorbic acid.

Table 2.
Percentage of free radical inhibition of 17a against DPPH compared with the control (ascorbic acid).
2.4. Agrochemical Studies
Some of the new compounds were tested as herbicide safeners with respect to 2,4-dichlorophenoxyacetic acid (2,4-D). 2,4-D is widely used and non-toxic for human herbicides [103]. However, 2,4-D is toxic for sunflowers. If the recommended dose for controlling weeds in resistant cereal crops is 0.5–0.8 kg/ha of 2,4-D by the active substance, the 15–18 g/ha dose for sunflower leads to a 40–60% decrease in yield.
One of the most effective approaches to increase plant resistance towards herbicides is the activation of metabolic processes affected by herbicide safeners (also called herbicide antidotes or detoxifiers) [104,105]. Herbicide safeners can be defined [106] as agrochemicals suitable for neutralization of phytotoxins in plants, thus protecting crop plants from herbicide injury. Safeners are harmless to crop plants (or even have a growth-stimulating effect), but do not affect the activity of herbicides against weeds.
It is known that some functionalized pyridines are efficient herbicide safeners and plant growth regulators [107,108,109,110,111]. Nicotinamides 16a and 17a,b were studied as 2,4-D antidotes with respect to sunflower seedlings using the reported procedure [107] (see the Materials and Methods). The antidote effect A was determined as a ratio of the hypocotyl (or root) length of sunflower seedlings in the “herbicide + antidote” experiments to the length in the reference group (where the seedlings were treated with 2,4-D only) (Equation (2)):
where Lexp is an organ length (mm) in the group of seedlings treated with 2,4-D and tested compound, and Lref is an organ length (mm) in the reference group of sunflower seedlings. We found that compounds 16a and 17a,b showed moderate 2,4-D antidote effect in the laboratory experiments (Table 3). As we can see, spiro[indole-3,4′-pyridines] 16a and 17a,b reduced the negative effect of 2,4-D on sunflower seedling hypocotyls by 16–33% and by 12–25% on sunflower seedling roots.
A = (Lexp/Lref) × 100%,

Table 3.
The antidote effects of the most active compounds 16a and 17a,b with respect to herbicide 2,4-D.
3. Materials and Methods
1H and 13C DEPTQ NMR spectra and 2D NMR experiments were recorded in solutions of DMSO-d6 on a Bruker AVANCE-III HD instrument (at 400.40 or 100.61 MHz, respectively). Residual solvent signals were used as internal standards in DMSO-d6—2.49 ppm for 1H, and 39.50 ppm for 13C nuclei. HRMS spectra were recorded using a Bruker MaXis Impact quadrupole time-of-flight mass spectrometer equipped with an electrospray ionization source in positive ion detection mode. The voltage at the ionization source was 3.5 kV, the drying gas flow rate was 8 L/min, the spray gas pressure was 2 bar, the temperature of the ionization source was 250 °C, the mass scanning range (m/z) was 50–1000 and the scanning speed was 3 Hz. The data were processed using Bruker Data Analysis 4.1 software. See Supplementary Materials File for NMR, FTIR and HRMS spectral charts.
FT-IR spectra were measured on a Bruker Vertex 70 instrument equipped with an ATR sampling module. Elemental analyses were carried out using a Carlo Erba 1106 Elemental Analyzer. Reaction progress and purity of isolated compounds were controlled by TLC on Sorbfil-A plates, eluent—acetone:hexane 1:1 or ethyl acetate:light petroleum 3:1, the spots were visualized in UV-light and iodine vapors. Monothiomalonamide 1 (3-amino-3-thioxopropanamide) was prepared from α-cyanoacetamide and hydrogen sulfide as described earlier [23,24]. Isatins, triethylamine, malononitrile and solvents were purchased from commercial vendors.
The reaction of isatins, malononitrile and monothiomalonamide. In a 50 mL beaker, 0.5 g (7.57 mmol) of malononitrile, 7.57 mmol of the corresponding isatin (1.11 g of isatin or 1.22 g of 5-methylisatin) and 20 mL of EtOH were added. Two drops of triethylamine were added to the suspension under heating (50 °C) and stirring. The reaction mixture became dark red due to the formation of the corresponding Knoevenagel condensation product. Stirring at 50 °C was continued for another 0.5 h, after which 0.89 g (7.57 mmol) of monothiomalonamide 1 and 1.6 mL (11.35 mmol, 1.5 eq.) of triethylamine were added. The mixture was stirred under vigorous heating (50 °C) for 2 h, then cooled and kept in a freezer overnight. The precipitate was filtered off and washed with acetone until colorless washings were obtained and then dried at 60 °C.
Triethylammonium 6′-amino-3′-(aminocarbonyl)-5′-cyano-2-oxo-1,2-dihydro-1′H-spiro-[indole-3,4′-pyridine]-2′-thiolate 13a and isomeric 6′-amino-3′-(aminocarbonyl)-5′-cyano-2-oxo- 1,2-dihydro-3′H-spiro-[indole-3,4′-pyridine]-2′-thiolate 13b. Off-white solid, yield was 2.74 g (88%). FTIR, νmax, cm−1: 3395, 3323, 2710 (N–H, +N–H); 2170 (C≡N); 1715, 1684 (C=O).
1H NMR (400 MHz, DMSO-d6): the signals of major isomer 13a—1.10 (t, 3J = 7.2 Hz, 9H, 3 CH3CH2N), 2.90–2.95 (m, 6H, 3 CH3CH2N), 5.37 (br s, 2H, NH2), 5.77 (br s, 1H, C(O)NH2), 6.55 (d, 3J = 7.5 Hz, 1H, H-7 indole), 6.70–6.74 (m, 1H, H-5 indole), 6.78 (d, 3J = 7.0 Hz, 1H, H-4 indole), 6.89–6.93 (m, 1H, H-6 indole), 7.69 (s, 1H, NH pyridine), 9.55 (s, 1H, NH indole), 10.25 (br s, 1H, C(O)NH2); the observed signals of minor isomer 13b—1.10 (t, 3J = 7.2 Hz, 9H, 3 CH3CH2N), 2.90–2.95 (m, 6H, 3 CH3CH2N), 3.80 (s, 1H, 3′H), 6.25 (br s, 2H, NH2), 6.34–6.37 (m, 1H, C(O)NH2), 6.75–6.76 (m, 1H, H-indole), 6.93–6.96 (m, 1H, H-indole), 7.10–7.14 (m, 1H, H-indole), 9.55 (s, 1H, NH indole), 10.30 (br s, 1H, C(O)NH2). NH+ signal was not observed, probably due to the fast H–D exchange. According to the integral signal intensities in the 1H NMR spectrum, the ratio of 1′H-isomer 13a to 3′H-isomer 13b is about 7:1.
13C DEPTQ NMR (101 MHz, DMSO-d6): the signals of major isomer 13a—9.3* (NCH2CH3), 45.7 (NCH2CH3), 52.2 (C spiro), 56.5 (C-5′), 100.4 (C-3′), 107.8* (CH-7 indole), 120.2* (CH-5 indole), 121.1 (C≡N), 122.3* (CH-4 indole), 125.7* (CH-6 indole), 141.0 (C-3a indole), 141.5 (C-7a indole), 150.3 (C-6′), 159.1 (C-2′), 170.0 (CONH2), 182.2 (C=O); the observed signals of minor isomer 13b—9.3* (NCH2CH3), 45.7 (NCH2CH3), 58.9* (CH-3′), 109.1* (CH-7 indole), 119.9 (C≡N), 121.8* (CH-5 indole), 124.2* (CH-4 indole), 128.1* (CH-6 indole), 135.3 (C-3a indole), 137.2 (C-7a indole), 151.8 (C-6′). *Negatively phased signals.
HRMS (ESI) m/z: calculated for C20H27N6O2S [M + H]+: 415.1916, found 415.1912 (Δ 1.01 ppm).
Triethylammonium 6′-amino-3′-(aminocarbonyl)-5′-cyano-5-methyl-2-oxo-1,2-dihydro- 1′H-spiro[indole-3,4′-pyridine]-2′-thiolate 14a and isomeric 6′-amino-3′-(aminocarbonyl)-5′- cyano-5-methyl-2-oxo-1,2-dihydro-3′H-spiro[indole-3,4′-pyridine]-2′-thiolate 14b. Off-white solid, yield was 2.68 g (83%). FTIR, νmax, cm−1: 3406, 3377, 2711 (N–H, +N–H); 2172 (C≡N); 1676, 1666 (C=O).
1H NMR (400 MHz, DMSO-d6): the signals of major isomer 14a—1.11 (t, 3J = 7.2 Hz, 9H, 3 CH3CH2N), 2.17 (s, 3H, ArCH3), 2.93–2.98 (m, 6H, 3 CH3CH2N), 5.36 (br s, 2H, NH2), 5.75 (br s, 1H, C(O)NH2), 6.44 (d, 3J = 7.5 Hz, 1H, H-7 indole), 6.61 (br s, 1H, H-4 indole), 6.72 (d, 3J = 7.5 Hz, 1H, H-6 indole), 7.67 (s, 1H, NH pyridine), 9.44 (s, 1H, NH indole), 10.26 (br s, 1H, C(O)NH2); the observed signals of minor isomer 14b—1.11 (t, 3J = 7.2 Hz, 9H, 3 CH3CH2N), 2.20 (s, 3H, ArCH3), 2.93–2.98 (m, 6H, 3 CH3CH2N), 4.35 (s, 1H, 3′H), 6.23 (br s, 2H, NH2), 6.32–6.35 (m, 1H, C(O)NH2), 6.64 (d, 3J = 7.7 Hz, 1H, H-7 indole), 6.76 (br s, 1H, H-4 indole), 6.93 (d, 3J = 7.7 Hz, 1H, H-6 indole), 9.44 (s, 1H, NH indole), 10.19 (br s, 1H, C(O)NH2); NH+ signal was not observed, probably due to the fast H–D exchange. According to the integral signal intensities in the 1H NMR spectrum, the ratio of 1′H-isomer 14a to 3′H-isomer 14b is about 7:3.
13C DEPTQ NMR (101 MHz, DMSO-d6): the signals of major isomer 14a—9.2* (NCH2CH3), 20.8* (ArCH3), 45.7 (NCH2CH3), 52.2 (C spiro), 56.7 (C-5′), 100.5 (C-3′), 107.6* (CH-7 indole), 121.2 (C≡N), 123.1* (CH-4 indole), 126.0* (CH-6 indole), 128.6 (C-5 indole), 139.0 (C-7a indole), 141.1 (C-3a indole), 150.3 (C-6′), 159.0 (C-2′), 170.0 (CONH2), 182.2 (C=O); the observed signals of minor isomer 14b—9.2* (NCH2CH3), 20.6* (ArCH3), 45.7 (NCH2CH3), 53.1* (CH-3′), 107.5 (C-5′), 108.9* (CH-7 indole), 120.0 (C≡N), 124.8* (CH-4 indole), 128.4* (CH-6 indole), 130.5 (C-5 indole), 135.4 (C-7a indole), 139.1 (C-3a indole), 151.7 (C-6′), 178.4 (C=O); *Negatively phased signals.
HRMS (ESI) m/z: calculated for C21H29N6O2S [M + H]+: 429.2073, found 429.2069 (Δ 0.86 ppm).
Synthesis of 6′-amino-5′-cyano-5-methyl-2-oxo-2′-thioxo-1,2,2′,3′-tetrahydro-1′H- spiro[indole-3,4′-pyridine]-3′-carboxamide 16. A 10 mL beaker was charged with 300 mg (0.7 mmol) of thiolate 14a + 14b and 2 mL of DMSO. To the solution formed, 0.2 mL of concentrated hydrochloric acid was added dropwise under vigorous stirring. Gas evolution (Caution! Me2S!) and formation of a precipitate were observed. The mixture was stirred for 10 min, diluted with EtOH up to a volume of 10 mL, filtered off, washed with EtOH and petroleum ether and dried at 60 °C. Compound 16 was obtained as a yellow finely crystalline powder, yield 201 mg (88%).
FTIR, νmax, cm−1: 3425, 3317, 3207 (N–H); 2193 (C≡N); 1705, 1653 (C=O).
1H NMR (400 MHz, DMSO-d6): the signals of major (3S,3′S)/(3R,3′R) diastereomers of 16—2.22 (s, 3H, ArCH3), 3.78 (s, 1H, H-3′), 6.32 (br s, 2H, NH2), 6.72 (d, 3J = 7.8 Hz, 1H, H-7 indole), 6.90–6.97 (m, 1H, H-4 indole, overlapped with the signals CONH2 and Ar–H of minor diastereomers), 7.03 (d, 3J = 7.8 Hz, 1H, H-6 indole), 7.15 (br s, 1H, CONH2), 7.33 (br s, 1H, CONH2), 10.40 (s, 1H, NH indole), 11.59 (br s, 1H, C(S)NH); the signals of minor (3S,3′R)(3R,3′S) diastereomers of 16—2.23 (s, 3H, ArCH3), 4.06 (s, 1H, H-3′), 6.35 (br s, 2H, NH2), 6.62 (d, 3J = 7.8 Hz, 1H, H-7 indole), 6.90–6.97 (m, 3H, 1H of CONH2 and indole H-4 and H-6, overlapped with H-4 signal of major diastereomers), 7.27 (br s, 1H, CONH2), 10.06 (s, 1H, NH indole), 11.55 (br s, 1H, C(S)NH).
13C DEPTQ NMR (101 MHz, DMSO-d6): the signals of major (3S,3′S)/(3R,3′R) diastereomers of 16—20.92* (ArCH3), 48.8 (C spiro), 56.59 (C-5′), 59.0* (CH-3′), 109.3* (CH-7 indole), 118.9 (C≡N), 126.6* (CH-4 indole), 127.7 (C-3a indole), 129.4* (CH-6 indole), 130.3 (C-5 indole), 139.7 (C-7a indole), 152.1 (C-6′), 166.6 (CONH2), 178.5 (C=O), 198.2 (C=S); the signals of minor (3S,3′R)(3R,3′S) diastereomers of 16—20.85* (ArCH3), 47.9 (C spiro), 56.64 (C-5′), 60.2* (CH-3′), 109.1* (CH-7 indole), 118.2 (C≡N), 124.1* (CH-4 indole), 129.1* (CH-6 indole), 129.6 (C-3a indole), 129.8 (C-5 indole), 140.5 (C-7a indole), 151.6 (C-6′), 166.9 (CONH2), 177.2 (C=O), 199.1 (C=S). *Negatively phased signals.
HRMS (ESI) m/z: calculated for C15H14N5O2S [M + H]+: 328.0868, found 328.0864 (Δ 1.22 ppm).
Preparation of compounds 17a–d. General procedure. A 25 mL beaker was charged with 300 mg of thiolates 13a + 13b (0.72 mmol) of 14a + 14b (0.70 mmol), DMF (5 mL) and water (3 mL). A hot (50 °C) solution of 0.75 mmol of the corresponding alkylating agent in 10 mL EtOH was added to the resulting solution of thiolates 13,14. A mixture was stirred for another 1 h at 40–50 °C until the reaction completion (TLC) and left for 24 h at room temperature. The precipitated solid was filtered off, washed with EtOH and dried at 60 °C.
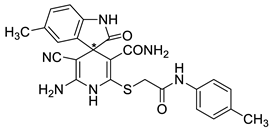
2′-Amino-3′-cyano-5-methyl-2-oxo-6′-{[2-oxo-2-(p-tolylamino)ethyl]thio}-1′H-spiro-[indoline-3,4′-pyridine]-5′-carboxamide 17a. Off-white solid, yield 92%.
FTIR, νmax, cm−1: 3476, 3406, 3358, 3298, 3175 (N–H); 2191 (C≡N); 1678, 1651 (C=O).
1H NMR (400 MHz, DMSO-d6): 2.15 (s, 3H, ArCH3), 2.26 (s, 3H, ArCH3), 3.86 (AB-q, 2J = 15.3 Hz, 2H, SCH2), 5.70 (br s, 2H, NH2), 6.54 (d, 3J = 7.7 Hz, 1H, H-7 indole), 6.75 (br s, 1H, H-4 indole), 6.87 (d, 3J = 7.7 Hz, 1H, H-6 indole), 7.00 (br s, 1H, CONH2), 7.14 (d, 3J = 8.1 Hz, 2H, H-2 H-6 4-MeC6H4NH), 7.45–7.49 (m, 3H, 1H CONH2 and H-3 H-5 4-MeC6H4NH overlapped), 8.74 (s, 1H, NH pyridine), 9.90 (s, 1H, NH indole), 10.25 (s, 1H, C(O)NHAr).
13C DEPTQ NMR (101 MHz, DMSO-d6): 20.3* (ArCH3), 20.8* (ArCH3), 37.3 (SCH2), 52.2 (C spiro), 56.4 (C-3′), 108.6* (CH-7 indole), 113.3 (C-5′), 119.7 (C≡N), 119.8* (2C, C-2 C-6 4-MeC6H4NH), 124.9* (CH-4 indole), 128.4* (CH-6 indole), 129.3* (2C, C-3 C-5 4-MeC6H4NH), 128.9 (C-Ar), 129.9 (C-Ar), 133.0 (C-Ar), 135.1 (C-Ar), 135.9 (C-Ar), 139.4 (C-2′), 151.9 (C-6′), 166.6 (CONH), 167.1 (CONH), 179.3 (C=O indoline); *Negatively phased signals.
HRMS (ESI) m/z: calculated for C24H23N6O3S [M + H]+: 475.1552, found 475.1551 (Δ 0.21 ppm).
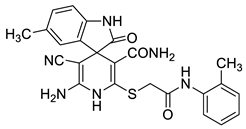
2′-Amino-3′-cyano-5-methyl-2-oxo-6′-{[2-oxo-2-(o-tolylamino)ethyl]thio}-1′H-spiro-[indoline-3,4′-pyridine]-5′-carboxamide 17b. Off-white solid, yield 82%. The compound is sparingly soluble in DMSO. FTIR, νmax, cm−1: 3437, 3358, 3302, 3283, 3236, 3165 (N–H); 2168 (C≡N); 1699, 1684 (C=O).
1H NMR (400 MHz, DMSO-d6): 2.13 (s, 3H, ArCH3), 2.22 (s, 3H, ArCH3), 3.94 (AB-q, 2J = 15.4 Hz, 2H, SCH2), 5.73 (br s, 2H, NH2), 6.54 (d, 3J = 7.7 Hz, 1H, H-7 indole), 6.77 (br s, 1H, H-4 indole), 6.87 (d, 3J = 7.7 Hz, 1H, H-6 indole), 6.97 (br s, 1H, CONH2), 7.12–7.20 (m, 2H, H-ArNH), 7.25 (d, 3J = 7.2 Hz, 1H, H-ArNH), 7.36 (d, 3J = 7.5 Hz, 1H, H-ArNH), 7.52 (br s, 1H, CONH2), 8.80 (s, 1H, NH pyridine), 9.80 (s, 1H, NH indole), 9.89 (s, 1H, C(O)NHAr).
13C DEPTQ NMR (101 MHz, DMSO-d6): 17.8* (ArCH3), 20.8* (ArCH3), 36.6 (SCH2), 49.9 (C spiro), 55.9 (C-3′), 108.7* (CH-7 indole), 112.2 (C-5′), 119.7 (C≡N), 124.8* (CH-4 indole), 125.6* (CH ArNH), 126.0* (CH ArNH), 126.1* (CH ArNH), 128.3* (CH-6 indole), 129.8 (C Ar), 129.9 (C Ar), 130.5* (CH ArNH), 133.4 (C-Ar), 135.1 (C-Ar), 135.9 (C-Ar), 138.3 (C-2′), 152.0 (C-6′), 166.6 (CONH), 167.5 (CONH), 179.3 (C=O indoline); *Negatively phased signals.
HRMS (ESI) m/z: calculated for C24H23N6O3S [M + H]+: 475.1552, found 475.1551 (Δ 0.21 ppm).
2′-Amino-3′-cyano-5-methyl-2-oxo-6′-[(2-oxo-2-phenylethyl)thio]-1′H-spiro[indoline-3,4′-pyridine]-5′-carboxamide 17c and 5′-amino-6′-cyano-3′-hydroxy-5-methyl-2-oxo-3′-phenyl- 2′,3′-dihydrospiro[indoline-3,7′-thiazolo[3,2-a]pyridine]-8′-carboxamide 17c-cycl. Light brown solid, yield 93%. According to 1H NMR, the compound exists as a mixture of linear and cyclic tautomers with a predominance of the 17c-cycl form in a ratio ~3:7. The compound is sparingly soluble in DMSO. FTIR, νmax, cm−1: 3441, 3368, 3308, 3234, 3200, 3161 (N–H); 2178 (C≡N); 1697, 1680, 1649 (C=O).
1H NMR (400 MHz, DMSO-d6): the signals of major cyclic tautomeric form 17c-cycl; signals of 17c-cycl are doubled due to existence of diastereomeric forms in 1:1 ratio—2.28 (s, 3H, ArCH3), 2.31 (s, 3H, ArCH3), 3.18–3.22 (two d overlapped, each 1H, SCH2 thiazole), 3.54–3.58 (two d overlapped, each 1H, SCH2 thiazole), 6.10 (br s, 2H, NH2), 6.17 (br s, 2H, NH2), 6.72–6.75 (two d overlapped, each 1H, H-7 indole), 6.88–6.89 (m, two s overlapped, each 1H, CONH2), 7.03–7.05 (m, 2H CONH2 and 2H H-4 indole of both diastereomers 17c-cycl overlapped), 7.49–7.45 (m, 4H, H-Ar), 7.51–7.52 (m, 4H, H-Ar), 7.56–7.60 (m, 4H, H-Ar), 8.57 (s, 1H, OH), 8.67 (s, 1H, OH), 10.34 (s, 1H, NH indole), 10.35 (s, 1H, NH indole); The observed signals of minor non-cyclic tautomer 17c—2.23 (s, 3H, ArCH3), 4.76 (AB-q, 2J = 17.2 Hz, 2H, SCH2C(O)), 5.68 (br s, 2H, NH2), 6.56 (d, 3J = 8.1 Hz, 1H, H-7 indole), 6.99 (br s, 1H, H-4 indole), 7.25 (br s, 1H, CONH2), 7.69–7.73 (m, 1H, H-4 C(O)Ph), 8.02 (d, 3J = 8.3 Hz, 2H, H-2 H-6 C(O)Ph), 8.53 (s, 1H, NH pyridine), 9.91 (s, 1H, NH indole).
13C DEPTQ NMR (101 MHz, DMSO-d6) (all observed signals): 21.22* (CH3), 21.24* (CH3), 21.3* (CH3), 41.4 (SCH2), 42.46 (SCH2), 43.1 (SCH2), 56.4, 110.2*(CH-7 indole), 110.3* (CH-7 indole), 119.5 (CN), 124.0 (C Ar), 125.1* (CH Ar), 125.34* (CH Ar), 125.5* (CH Ar), 128.9* (CH Ar), 129.0* (CH Ar), 129.1* (CH Ar), 129.2* (CH Ar), 129.5* (CH Ar), 129.9* (CH Ar), 130.1 (C Ar), 130.3 (C Ar), 131.8 (C Ar), 131.9 (C Ar), 135.66 (C Ar), 135.68 (C Ar), 142.1 (C Ar), 151.8 (C-6′), 159.9 (C), 167.1 (CONH), 167.8 (CONH), 179.4 (C=O indoline), 179.6 (C=O indoline), 180.5 (C=O indoline), 185.6 (C(O)Ph); *Negatively phased signals.
HRMS (ESI) m/z: calculated for C23H20N5O3S [M + H]+: 446.1289, found 446.1283 (Δ 1.35 ppm); calculated for C23H19N5NaO3S [M + Na]+: 468.1106, found 468.1101 (Δ 1.07 ppm).
2′-Amino-6′-[(2-(4-bromophenyl)-2-oxoethyl)thio]-3′-cyano-2-oxo-1′H-spiro[indoline-3,4′-pyridine]-5′-carboxamide 17d and 5′-amino-3′-(4-bromophenyl)-6′-cyano-3′-hydroxy-2-oxo- 2′,3′-dihydrospiro[indoline-3,7′-thiazolo[3,2-a]pyridine]-8′-carboxamide 17d-cycl. Beige solid, yield 92%. According to 1H NMR, the compound exists as a mixture of linear and cyclic tautomers with a predominance of the 17d-cycl form in a ratio ~1:4. The compound is sparingly soluble in DMSO. FTIR, νmax, cm−1: 3450, 3337, 3312, 3202, 3163 (N–H); 2181 (C≡N); 1701, 1680 (C=O).
1H NMR (400 MHz, DMSO-d6): the signals of major cyclic tautomeric form 17d-cycl; signals of 17d-cycl are doubled due to existence of diastereomeric forms in 1:1 ratio—3.19–3.26 (two d overlapped, each 1H, SCH2 thiazole), 3.55–3.58 (two d overlapped, each 1H, SCH2 thiazole), 6.08 (br s, 2H, NH2), 6.14 (br s, 2H, NH2), 6.81–6.83 (two d overlapped, each 1H, H-7 indole), 6.99–7.28 (m, 10H, H-Ar, CONH2 overlapped), 7.44 (d, 3J = 8.3 Hz, 2H, H-3 H-5 C(O)Ar), 7.55 (d, 3J = 8.4 Hz, 2H, H-3 H-5 C(O)Ar), 7.63 (d, 3J = 8.4 Hz, 2H, H-2 H-6 C(O)Ar), 7.71 (d, 3J = 8.3 Hz, 2H, H-2 H-6 C(O)Ar), 8.69 (s, 1H, OH), 8.79 (s, 1H, OH), 10.38 (s, 1H, NH indole), 10.41 (s, 1H, NH indole);
The observed signals of minor non-cyclic tautomer 17d—4.71 (AB-q, 2J = 17.2 Hz, 2H, SCH2C(O)), 5.70 (br s, 2H, NH2), 6.67 (d, 3J = 7.6 Hz, 1H, H-7 indole), 6.88–6.92 (m, 1H, H-indole), 7.80 (d, 3J = 8.3 Hz, 2H, H-3 H-5 C(O)Ar), 7.94 (d, 3J = 8.3 Hz, 2H, H-2 H-6 C(O)Ar), 8.53 (s, 1H, NH pyridine), 10.02 (s, 1H, NH indole).
13C DEPTQ NMR (101 MHz, DMSO-d6) (all observed signals): 41.8 (SCH2), 41.6 (SCH2), 42.5 (SCH2), 58.8, 61.6, 96.3, 100.0, 108.9* (CH-7 indole), 109.8* (CH-7 indole), 109.9* (CH-7 indole), 118.8 (CN), 118.9 (CN), 121.3* (CH Ar), 121.9 (C Ar), 122.0 (C Ar), 122.1 (C Ar), 122.3* (CH Ar), 122.5* (CH Ar), 124.3* (CH Ar), 124.4* (CH Ar), 127.0* (CH Ar), 127.3* (CH Ar), 128.1* (CH Ar), 128.9* (CH Ar), 129.5 (C Ar), 130.5* (CH Ar), 131.5* (CH Ar), 131.7* (CH Ar), 132.1* (CH Ar), 134.2 (C Ar), 134.5 (C Ar), 134.7 (C Ar), 134.8 (C Ar), 1401.0 (C Ar), 141.1 (C Ar), 141.6 (C Ar), 141.9 (C Ar), 146.6 (C Ar), 151.2 (C), 151.6 (C), 152.0 (C), 167.1 (CONH), 167.3 (CONH), 178.49 (C=O indoline), 179.1 (C=O indoline), 188.7 (C(O)Ar); *Negatively phased signals.
HRMS (ESI) m/z: calculated for C22H17BrN5O3S [M + H]+: 510.0236, found 510.0235 (Δ 0.1 ppm); calculated for C22H15BrN5O2S [M + H–H2O]+: 492.0130, found 492.0130 (Δ 0 ppm).
3.1. Antibacterial Studies
A suspension of pathogens isolated from a swollen ear canal was prepared by transferring a loop full of isolated colony to 5 mL of normal saline in tubes. The turbidity of the bacterial growth in tubes was then compared with the turbidity of 0.5 McFarland suspension to give an approximate number of live cells equal to 0.5 × 108 CFU/mL. Then, 0.1 mL of the bacterial suspension was transferred and spread homogeneously over the Mueller–Hinton agar with a sterile swab and the plate was allowed to stand for 10 min at room temperature to dry. Using a sterile cork borer, a 6 mm diameter hole was made in each prepared plate. A solution of compound 17a at 100 mg/mL concentration was prepared by dissolving 17a in dimethyl sulfoxide (DMSO). Then, 100 μL of the solution was added using a micropipette in each hole under sterile conditions; DMSO was also added as control. The plate was kept for 24 h at 37 °C in an incubator. The diameter of the inhibitory zone around each hole was measured in millimeters.
The minimum inhibitory concentration (MIC) against P. aeruginosa ATCC 27,853 was determined using the classical broth dilution method [101] by preparing differently concentrated solutions of 17a using nutrient broth along with positive and negative controls for each pathogenic bacteria. The turbidity of P. aeruginosa suspension was brought to 0.5 McFarland and 100 μL of bacterial suspension was added to the solutions of 17a. The positive control consisted of 1 mL of nutrient broth and 100 μL of bacterial suspension, and 1 mL of nutrient broth was used only as a negative control. All tubes were incubated at 37 °C for 24 h. After incubation, 100 μL of suspension was dropped onto sterile Mueller–Hinton agar and the plates were incubated at 37 °C for 24 h.
3.2. Antioxidant Activity of Compound 17a
The solutions of different concentrations (100,000, 10,000, 1000 and 100 µg/mL) were prepared from compound 17a. Methanolic solution of DPPH was prepared by dissolving 4 mg of DPPH in 100mL of MeOH. Then, 1 mL of DPPH methanolic solution was added to 1 mL of each solution of 17a. The reaction mixture was incubated for 30 min in the dark at room temperature. The absorption of the resulting solutions was recorded at 517 nm. The DPPH color changes from deep violet (free radical form) to pale yellow (1,1-diphenyl-2-picryl hydrazine, reduced form). Ascorbic acid was used as a positive control and methanolic solution was used as a negative control.
3.3. Herbicide-Safening Effect Studies
The germinated sunflower seeds (cv. Master) with 2–4 mm long embryo roots were placed in a solution of 2,4-D (10−3 % by weight) for 1 h to achieve 40–60% inhibition of hypocotyl growth. After treatment, the seedlings were washed with pure water and placed into a solution of the corresponding compound 16a and 17a,b (concentrations 10−2, 10−3, 10−4 or 10−5% by weight, “herbicide + antidote” experiments). After 1 h, the seedlings were washed with pure water and placed on paper strips (10 × 75 cm, 20 seeds per strip). The strips were rolled and placed into beakers with water (50 cm3). The reference group of seedlings (“herbicide” experiments) was kept in 2,4-D solution (10−3%) for 1 h and then in water for 1 h. The “control” seedlings were kept in water for 2 h. The temperature of all solutions was maintained at 28 °C. The seedlings were then thermostated for 3 days at 28 °C. Each experiment was performed in triplicate; 20 seeds were used in each experiment. The results are given in Table 3.
4. Conclusions
In summary, we studied for the first time the reaction between isatin, malononitrile and monothiomalonamide in the presence of triethylamine. It was found that the reaction proceeds with the formation of two isomeric 1′H and 3′H products—triethylammonium 6′-amino-3′-(aminocarbonyl)-5′-cyano-2-oxo-1,2-dihydro-1′H-spiro-[indole-3,4′-pyridine]-2′-thiolate and 6′-amino-3′-(aminocarbonyl)-5′-cyano-2-oxo-1,2-dihydro-3′H-spiro-[indole-3,4′-pyridine]-2′-thiolate. These results are in good agreement with the results of other works on the preparation of related nicotinamides, starting from monothiomalonamide. We failed to oxidize the above thiolates to isothiazolo[5,4-b]pyridines upon treatment with DMSO-HCl system. Instead, diastereomers of 6′-amino-5′-cyano-5-methyl-2-oxo-2′-thioxo-1,2,2′,3′-tetrahydro-1′H- spiro[indole-3,4′-pyridine]-3′-carboxamide were isolated. The alkylation of a mixture of the above 1′H/3′H thiolates proceeded in a regionspecific way on the sulfur atom and led to the formation of only 1′H derivatives in high yields. In the case of the reaction involving α-bromoketones, the isolated alkylation products were found to exist as a mixture of linear and chained (thiazolo[3,2-a]pyridine) tautomers. The structure of the new compounds was thoroughly investigated using IR, HRMS and NMR spectroscopy, including DEPTQ and 2D 1H-13C HSQC and 1H-13C HMBC experiments. Molecular docking studies for some of the new compounds against the regulatory protein PqsR of Pseudomonas aeruginosa showed promising results. Among the most active compounds, both enantiomers of 2′-amino-3′-cyano-5-methyl-2-oxo-6′-{[2-oxo-2-(p-tolylamino)ethyl]thio}- 1′H-spiro[indoline-3,4′-pyridine]-5′-carboxamide 17a were recognized. The antibacterial effect of 17a was confirmed in experiments in vitro. Additionally, compound 17a revealed a pronounced antioxidant effect comparable with that for ascorbic acid in the experiments with 1,1-diphenyl-2-picrylhydrazyl radical. Finally, spiro[indole-3,4′-pyridines] 16a and 17a,b were recognized as moderately active 2,4-D herbicide safeners and reduced the negative effect of 2,4-D on sunflower seedling hypocotyls by 16–33% and by 12–25% on sunflower seedling roots.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28073161/s1. 1H and 13C DEPTQ NMR, 2D NMR 1H-13C HSQC and 1H-13C HMBC, FTIR, HRMS spectral charts for new compounds (Figures S1–S39, Tables S1 and S2).
Author Contributions
V.V.D.—conceptualization, supervision, investigation (synthesis), data analysis, agrochemical studies, funding acquisition, writing (original draft, review and editing); N.T.J.—investigation (synthesis and docking studies); A.Z.T.—data analysis; Z.R.A.-H.—investigation (biological studies); N.A.A.—data analysis; I.V.A.—supervision, data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of the Russian Ministry of Education and Science (FSRN-2023-0005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
File Electronic Supplementary Material.pdf containing 1H and 13C DEPTQ NMR, 2D NMR 1H-13C HSQC and 1H-13C HMBC, FTIR, HRMS spectral charts for new compounds (Figures S1–S39, Tables S1 and S2).
Acknowledgments
The studies were performed using the equipment of the scientific and educational center “Diagnostics of the Structure and Properties of Nanomaterials” and the equipment of the Center for Collective Use “Ecological Analytical Center” of Kuban State University, Krasnodar.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Hassan, H.; Hisham, M.; Osman, M.; Hayallah, A. Nicotinonitrile as an essential scaffold in medicinal chemistry: An updated review. J. Adv. Biomed. Pharm. Sci. 2023, 6, 1–11. [Google Scholar] [CrossRef]
- Shamroukh, A.H.; Kotb, E.R.; Anwar, M.M.; Sharaf, M. A Review on the Chemistry of Nicotinonitriles and Their applications. Egypt. J. Chem. 2021, 64, 4509–4529. [Google Scholar] [CrossRef]
- Makarov, M.V.; Migaud, M.E. Syntheses and chemical properties of β-nicotinamide riboside and its analogues and derivatives. Beilstein J. Org. Chem. 2019, 15, 401–430. [Google Scholar] [CrossRef]
- Velena, A.; Zarkovic, N.; Gall Troselj, K.; Bisenieks, E.; Krauze, A.; Poikans, J.; Duburs, G. 1, 4-dihydropyridine derivatives: Dihydronicotinamide analogues—Model compounds targeting oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 1892412. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.A.; Hussein, B.H.; Helal, M.H.; Salem, M.A. A Review: Synthesis and Medicinal Importance of Nicotinonitriles and Their Analogous. J. Heterocycl. Chem. 2018, 55, 1524–1553. [Google Scholar] [CrossRef]
- Gouda, M.A.; Attia, E.; Helal, M.H.; Salem, M.A. Recent Progress on Nicotinonitrile Scaffold-based Anticancer, Antitumor, and Antimicrobial Agents: A Literature Review. J. Heterocycl. Chem. 2018, 55, 2224–2250. [Google Scholar] [CrossRef]
- Salem, M.A.; Helel, M.H.; Gouda, M.A.; Ammar, Y.A.; El-Gaby, M.S.A. Overview on the synthetic routes to nicotine nitriles. Synth. Commun. 2018, 48, 345–374. [Google Scholar] [CrossRef]
- Gouda, M.A.; Berghot, M.A.; Abd El Ghani, G.E.; Khalil, A.E.G.M. Chemistry of 2-Amino-3-cyanopyridines. Synth. Commun. 2014, 44, 297–330. [Google Scholar] [CrossRef]
- Litvinov, V.P. The chemistry of 3-cyanopyridine-2(1H)-chalcogenones. Russ. Chem. Rev. 2006, 75, 577–599. [Google Scholar] [CrossRef]
- Litvinov, V.P. Partially hydrogenated pyridinechalcogenones. Russ. Chem. Bull. 1998, 47, 2053–2073. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Krivokolysko, S.G.; Dyachenko, V.D. Synthesis and properties of 3-cyanopyridine-2(1H)-chalcogenones. Review. Chem. Heterocycl. Compd. 1999, 35, 509–540. [Google Scholar] [CrossRef]
- Dyachenko, I.V.; Dyachenko, V.D.; Dorovatovskii, P.V.; Khrustalev, V.N.; Nenaidenko, V.G. New Options of Multicomponent Condensations Leading to Functional Derivatives of 2-Pyridons. Russ. J. Org. Chem. 2021, 57, 1809–1823. [Google Scholar] [CrossRef]
- Dyachenko, I.V.; Dyachenko, V.D.; Dorovatovskii, P.V.; Khrustalev, V.N.; Nenajdenko, V.G. New method for the synthesis of 4-spirocyclopentane-and 4-spirocyclohexanenicotinic acid nitriles and amides. Russ. Chem. Bull. 2021, 70, 949–959. [Google Scholar] [CrossRef]
- Frolov, K.A.; Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P. Three-component condensation in the synthesis of substituted tetrahydropyridinethiolates. Russ. Chem. Bull. 2005, 54, 1335–1336. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Karpov, E.N. 4-Alkyl-6-amino-N3,N5-diaryl-2-thioxo-1,2,3,4-tetrahydropyridine-3,5-dicarboxamides: I. Tandem synthesis and alkylation. Molecular and crystal structure of 6-allylsulfanyl-2-amino-4-isobutyl-N3,N5-di-m-tolyl-3,4-dihydropyridine-3, 5-dicarboxamide. Russ. J. Gen. Chem. 2013, 83, 1394–1401. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Karpov, E.N.; Feskov, I.A. 4-Alkyl-6-amino-N3,N5-diaryl-2-thioxo-1,2,3,4-tetrahydropyridine-3,5-dicarboxamides: II. Synthesis and selected reactions. Russ. J. Gen. Chem. 2013, 83, 1716–1723. [Google Scholar] [CrossRef]
- Faty, R.M.; Youssef, M.M.; Youssef, A.M. Microwave assisted synthesis and unusual coupling of some novel pyrido[3,2-f][1,4] thiazepines. Molecules 2011, 16, 4549–4559. [Google Scholar] [CrossRef]
- Dyachenko, I.V.; Vovk, M.V. Synthesis and transformations of new 3-oxo(thioxo)-1-phenyl-2,3,5,6,7,8-hexahydroisoquinoline-4-carboxylic acid derivatives. Russ. J. Gen. Chem. 2012, 82, 697–702. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Litvinov, V.P. The Mannich reaction in the synthesis of N,S-containing heterocycles. 12. First example of aminomethylation involving 2-thioxonicotinamide derivative: Synthesis of 3,5,7,11-tetraazatricyclo[7.3.1.02,7] tridec-2-ene-9-carboxamides. Russ. Chem. Bull. 2012, 61, 136–140. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Krasnikov, D.A. Unusual Michael Reaction of 3-(4-Chlorophenyl)-1-phenylprop-2-en-1-one with 3-Amino-N-phenyl-3-thioxopropanamide. Russ. J. Org. Chem. 2012, 48, 953–956. [Google Scholar] [CrossRef]
- Dyachenko, A.D.; Desenko, S.M.; Dyachenko, V.D. Regioselective synthesis and properties of 6-amino-3-carbamoyl-5-cyano-3,4-dihydrospirocyclohexane-4-pyridine-2-thiol and 5-cyano-3-thiocarbamoyl-4-spirocyclohexanepiperidine-2,6-dione. Chem. Heterocycl. Compd. 2004, 40, 1017–1023. [Google Scholar] [CrossRef]
- Dyachenko, V.D.; Tkachev, R.P. 3-Amino-3-thioxopropanamide in the Synthesis of Functionally Substituted Nicotinamides. Russ. J. Org. Chem. 2003, 39, 1174–1179. [Google Scholar] [CrossRef]
- Sasse, K. 1-Substituierte 1,6-Dihydro-4-mercapto-6-pyrimidinone und 1,2,3,6-Tetrahydro-4-(methylthio)-2,6-pyrimidindione. Liebigs Ann. Chem. 1976, 1976, 768–780. [Google Scholar] [CrossRef]
- Schaper, W. Heterocyclen-Synthesen mit Monothiomalonsäure-Amiden: Synthese von 3-Oxo-2,3-dihydroisothiazolo[5,4-b] pyridinen und 3-Oxo-2, 3-dihydroisothiazolo[5,4-d]pyrimidinen. Synthesis 1985, 1985, 861–867. [Google Scholar] [CrossRef]
- Rodinovskaya, L.A.; Shestopalov, A.M.; Nesterov, V.N. Stereoselective synthesis and structure of 3,4-trans-6-amino-4-aryl-3-carbamoyl-5-cyano-1,2,3,4-tetrahydropyridin-2(1H)-thiones. Chem. Heterocycl. Compd. 1996, 32, 1182–1188. [Google Scholar] [CrossRef]
- Hussain, S.M.; Sherif, S.M.; Youssef, M.M. New synthesis of polyfunctionally substituted 2-mercaptopyridines and fused pyridines. Gazz. Chim. Ital. 1994, 124, 97–101. [Google Scholar]
- Krauze, A.; Popelis, J.; Duburs, G. Synthesis of 4,7(2H)-dihydrothiazolo[3,2-a]pyridines from 3-carbamoyl-1,4-dihydropyridine-2(3H)-thiones. Heterocycl. Commun. 1996, 3, 515–520. [Google Scholar] [CrossRef]
- Krauze, A.; Popelis, J.; Duburs, G. Efficient regioselective one-pot synthesis of partially hydrogenated thiazolo[3,2-a]pyridines. Tetrahedron 1998, 54, 9161–9168. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Bayat, M. Green synthesis of isatin-based compounds. Res. Chem. Intermed. 2022, 48, 3987–4016. [Google Scholar] [CrossRef]
- Gataullin, R.R. Advances in the Synthesis of Benzo-Fused Spiro Nitrogen Heterocycles: New Approaches and Modification of Old Strategies. Helv. Chim. Acta 2020, 103, e2000137. [Google Scholar] [CrossRef]
- Izmest’ev, A.N.; Gazieva, G.A.; Kravchenko, A.N. Regioselectivity of (3+2) cycloaddition of azomethine ylides to activated olefins in the synthesis of spiro [oxindole-3, 2′-pyrrolidine] derivatives. Chem. Heterocycl. Compd. 2020, 56, 255–264. [Google Scholar] [CrossRef]
- Bogdanov, A.V.; Mironov, V.F. Advances in the synthesis of isatins: A survey of the last decade. Synthesis 2018, 50, 1601–1609. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N. Asymmetric synthesis of chiral oxindoles using isatin as starting material. Tetrahedron 2018, 74, 1323–1353. [Google Scholar] [CrossRef]
- Moradi, R.; Ziarani, G.M.; Lashgari, N. Recent applications of isatin in the synthesis of organic compounds. Arkivoc 2017, 1, 148–201. [Google Scholar] [CrossRef]
- Yan, L.J.; Wang, Y.C. Recent Advances in Green Synthesis of 3, 3′-Spirooxindoles via Isatin–based One–pot Multicomponent Cascade Reactions in Aqueous Medium. ChemistrySelect 2016, 1, 6948–6960. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Moradi, R.; Lashgari, N. Synthesis of spiro-fused heterocyclic scaffolds through multicomponent reactions involving isatin. Arkivoc 2016, 1, 1–81. [Google Scholar] [CrossRef]
- Gazieva, G.A.; Izmest’ev, A.N. Oxoindolinylidene derivatives of thiazolidin-4-ones: Methods of synthesis and biological activity. Chem. Heterocycl. Compd. 2015, 50, 1515–1527. [Google Scholar] [CrossRef]
- Musin, L.I.; Bogdanov, A.V.; Mironov, V.F. Isatin derivatives in reactions with phosphorus (III–V) compounds. Chem. Heterocycl. Compd. 2015, 51, 421–439. [Google Scholar] [CrossRef]
- Borad, M.A.; Bhoi, M.N.; Prajapati, N.P.; Patel, H.D. Review of synthesis of multispiro heterocyclic compounds from isatin. Synth. Commun. 2014, 44, 1043–1057. [Google Scholar] [CrossRef]
- Xia, M.; Ma, R.Z. Recent progress on routes to spirooxindole systems derived from isatin. J. Heterocycl. Chem. 2014, 51, 539–554. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Wan, J. Recent advances in diversity oriented synthesis through isatin-based multicomponent reactions. Asian J. Org. Chem. 2013, 2, 374–386. [Google Scholar] [CrossRef]
- Lashgari, N.; Ziarani, G.M. Synthesis of heterocyclic compounds based on isatin through 1,3-dipolar cycloaddition reactions. Arkivoc 2012, 1, 277–320. [Google Scholar] [CrossRef]
- Da Silva, J.F.; Garden, S.J.; Pinto, A.C. The chemistry of isatins: A review from 1975 to 1999. J. Braz. Chem. Soc. 2001, 12, 273–324. [Google Scholar] [CrossRef]
- Shvekhgeimer, M.-G.A. Synthesis of heterocyclic compounds by the cyclization of isatin and its derivatives. Chem. Heterocycl. Compd. 1996, 32, 249–276. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, S.; Gao, C.; Fan, J.; Zhao, F.; Lv, Z.S.; Feng, L.S. Isatin hybrids and their anti-tuberculosis activity. Chin. Chem. Lett. 2017, 28, 159–167. [Google Scholar] [CrossRef]
- Cheke, R.S.; Firke, S.D.; Patil, R.R.; Bari, S.B. Isatin: New hope against convulsion. CNS Agents Med. Chem. 2018, 18, 76–101. [Google Scholar] [CrossRef] [PubMed]
- Guo, H. Isatin derivatives and their anti-bacterial activities. Eur. J. Med. Chem. 2019, 164, 678–688. [Google Scholar] [CrossRef]
- Vine, K.L.; Matesic, L.; Locke, J.M.; Ranson, M.; Skropeta, D. Cytotoxic and anticancer activities of isatin and its derivatives: A comprehensive review from 2000–2008. Anti-Cancer Agents Med. Chem. 2009, 9, 397–414. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, G.Q.; Liu, X.; Zhang, Z.; Feng, L.S.; Liu, M.L. Isatin derivatives with potential antitubercular activities. J. Heterocycl. Chem. 2018, 55, 1263–1279. [Google Scholar] [CrossRef]
- De Moraes Gomes, P.A.T.; Pena, L.J.; Leite, A.C.L. Isatin derivatives and their antiviral properties against arboviruses: A review. Mini Rev. Med. Chem. 2019, 19, 56–62. [Google Scholar] [CrossRef]
- Ding, Z.; Zhou, M.; Zeng, C. Recent advances in isatin hybrids as potential anticancer agents. Arch. Pharm. 2020, 353, 1900367. [Google Scholar] [CrossRef]
- Chauhan, G.; Pathak, D.P.; Ali, F.; Bhutani, R.; Kapoor, G.; Khasimbi, S. Advances in Synthesis, Derivatization and Bioactivity of Isatin: A Review. Curr. Org. Synth. 2021, 18, 37–74. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Pathania, S.; Grover, G.; Akhtar, M.J. Isatin containing heterocycles for different biological activities: Analysis of structure activity relationship. J. Mol. Struct. 2020, 1222, 128900. [Google Scholar] [CrossRef]
- Song, F.; Li, Z.; Bian, Y.; Huo, X.; Fang, J.; Shao, L.; Zhou, M. Indole/isatin-containing hybrids as potential antibacterial agents. Arch. Pharm. 2020, 353, 2000143. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Shang, C.; Wang, H.; Yun, J. Isatin–azole hybrids and their anticancer activities. Arch. Pharm. 2020, 353, 1900272. [Google Scholar] [CrossRef]
- Brandão, P.; Marques, C.; Burke, A.J.; Pineiro, M. The application of isatin-based multicomponent-reactions in the quest for new bioactive and druglike molecules. Eur. J. Med. Chem. 2021, 211, 113102. [Google Scholar] [CrossRef]
- Ferraz de Paiva, R.E.; Vieira, E.G.; Rodrigues da Silva, D.; Wegermann, C.A.; Costa Ferreira, A.M. Anticancer compounds based on isatin-derivatives: Strategies to ameliorate selectivity and efficiency. Front. Mol. Biosci. 2021, 7, 627272. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patil, V.M.; Firke, S.D.; Ambhore, J.P.; Ansari, I.A.; Patel, H.M.; Shinde, S.D.; Pasupuleti, V.R.; Hassan, M.I.; Adnan, M.; et al. Therapeutic Outcomes of Isatin and Its Derivatives against Multiple Diseases: Recent Developments in Drug Discovery. Pharmaceuticals 2022, 15, 272. [Google Scholar] [CrossRef]
- Chowdhary, S.; Shalini; Arora, A.; Kumar, V. A Mini Review on Isatin, an Anticancer Scaffold with Potential Activities against Neglected Tropical Diseases (NTDs). Pharmaceuticals 2022, 15, 536. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, D.; Hu, G. The antibacterial activity of isatin hybrids. Curr. Top. Med. Chem. 2022, 22, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Varun; Sonam; Kakkar, R. Isatin and its derivatives: A survey of recent syntheses, reactions, and applications. Med. Chem. Commun. 2019, 10, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Elsaman, T.; Mohamed, M.S.; Eltayib, E.M.; Abdel-Aziz, H.A.; Abdalla, A.E.; Munir, M.U.; Mohamed, M.A. Isatin derivatives as broad-spectrum antiviral agents: The current landscape. Med. Chem. Res. 2022, 31, 244–273. [Google Scholar] [CrossRef] [PubMed]
- Maryamabadi, A.; Hasaninejad, A.; Nowrouzi, N.; Mohebbi, G.; Asghari, B. Application of PEG-400 as a green biodegradable polymeric medium for the catalyst-free synthesis of spiro-dihydropyridines and their use as acetyl and butyrylcholinesterase inhibitors. Bioorg. Med. Chem. 2016, 24, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Ghozlan, S.A.; Mohamed, M.F.; Ahmed, A.G.; Shouman, S.A.; Attia, Y.M.; Abdelhamid, I.A. Cytotoxic and antimicrobial evaluations of novel apoptotic and anti-angiogenic spiro cyclic 2-oxindole derivatives of 2-amino-tetrahydroquinolin-5-one. Arch. Pharm. 2015, 348, 113–124. [Google Scholar] [CrossRef]
- Zou, M.; Tian, X.; Chen, N.; Shao, X. Nematicidal activity of sprio and bridged heterocyclic neonicotinoid analogues against Meloidogyne incognita. Lett. Drug Des. Discov. 2015, 12, 439–445. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Li, Y.F.; Wang, J.W.; Yu, B.; Shi, Y.K.; Liu, H.M. Multicomponent assembly of novel antiproliferative steroidal dihydropyridinyl spirooxindoles. Steroids 2016, 109, 22–28. [Google Scholar] [CrossRef]
- Mondal, A.; Naskar, B.; Goswami, S.; Prodhan, C.; Chaudhuri, K.; Mukhopadhyay, C. I2 catalyzed access of spiro[indoline-3,4′-pyridine] appended amine dyad: New ON–OFF chemosensors for Cu2+ and imaging in living cells. Org. Biomol. Chem. 2018, 16, 302–315. [Google Scholar] [CrossRef]
- Yagnam, S.; Akondi, A.M.; Trivedi, R.; Rathod, B.; Prakasham, R.S.; Sridhar, B. Spirooxindole-fused pyrazolo pyridine derivatives: NiO–SiO2 catalyzed one-pot synthesis and antimicrobial activities. Synth. Commun. 2018, 48, 255–266. [Google Scholar] [CrossRef]
- El-Kalyoubi, S.A.; Ragab, A.; Abu Ali, O.A.; Ammar, Y.A.; Seadawy, M.G.; Ahmed, A.; Fayed, E.A. One-pot synthesis and molecular modeling studies of new bioactive spiro-oxindoles based on uracil derivatives as SARS-CoV-2 inhibitors targeting rna polymerase and spike glycoprotein. Pharmaceuticals 2022, 15, 376. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Sinotsko, A.E.; Strelkov, V.D.; Varzieva, E.A.; Russkikh, A.A.; Levchenko, A.G.; Temerdashev, A.Z.; Aksenov, N.A.; Aksenova, I.V. Alkyl 4-aryl-6-amino-7-phenyl-3-(phenylimino)-4,7-dihydro-3H-[1,2]dithiolo[3,4-b]pyridine- 5-carboxylates: Synthesis and Agrochemical Studies. Molecules 2023, 28, 609. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, B.S.; Bibik, E.Y.; Frolov, K.A.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G. Synthesis and in vivo evaluation of hepatoprotective effects of novel sulfur-containing 1,4-dihydropyridines and 1,2,3,4-tetrahydropyridines. Curr. Bioact. Compds. 2023, 19, e171022210054. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Jassim, N.T.; Temerdashev, A.Z.; Aksenov, N.A.; Aksenova, I.V. Synthesis and structure of 4-aryl-3,6-dioxo-2,3,4,5,6,7-hexahydroisothiazolo[5,4-b]pyridine-5-carbonitriles. Russ. J. Gen. Chem. 2022, 92, 2861–2869. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Samokish, A.A.; Venidiktova, Y.S.; Frolov, K.A.; Krivokolysko, S.G.; Pankov, A.A.; Aksenov, N.A.; Aksenova, I.V. New hybrid molecules based on sulfur-containing nicotinonitriles: Synthesis, analgesic activity in acetic acid-induced writhing test, and molecular docking studies. Russ. J. Bioorg. Chem. 2022, 48, 628–635. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Khrustaleva, A.N.; Frolov, K.A.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G. 1,6-Diamino-2-oxopyridine-3, 5-dicarbonitrile derivatives in the Mannich reaction. Russ. J. Gen. Chem. 2021, 91, 44–56. [Google Scholar] [CrossRef]
- Bibik, I.V.; Bibik, E.Y.; Dotsenko, V.V.; Frolov, K.A.; Krivokolysko, S.G.; Aksenov, N.A.; Aksenova, I.V.; Shcherbakov, S.V.; Ovcharov, S.N. Synthesis and analgesic activity of new heterocyclic cyanothioacetamide derivatives. Russ. J. Gen. Chem. 2021, 91, 154–166. [Google Scholar] [CrossRef]
- Kurskova, A.O.; Dotsenko, V.V.; Frolov, K.A.; Aksenov, N.A.; Aksenova, I.V.; Shcherbakov, S.V.; Ovcharov, S.N.; Krivokolysko, D.S.; Krivokolysko, S.G. New methods of synthesis, structure and aminomethylation of 4-imino-2-(dicyanomethylene)-3-azaspiro[5.5]undecane-1, 5-dicarbonitrile. Russ. J. Gen. Chem. 2021, 91, 971–984. [Google Scholar] [CrossRef]
- Kurskova, A.O.; Dotsenko, V.V.; Frolov, K.A.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, B.S.; Krivokolysko, S.G. Synthesis and Aminomethylation of 6-Amino-2-(dicyanomethylene)-4-phenyl-1,2-dihydropyridine-3,5-dicarbonitrile morpholinium salt. Russ. J. Gen. Chem. 2021, 91, 1471–1483. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Samokish, A.A.; Venidiktova, Y.S.; Frolov, K.A.; Krivokolysko, S.G.; Vasilin, V.K.; Pankov, A.A.; Aksenov, N.A.; et al. New 4-(2-furyl)-1,4-dihydronicotinonitriles and 1,4,5,6-tetrahydro-nicotinonitriles: Synthesis, structure, and analgesic activity. Russ. J. Gen. Chem. 2021, 91, 1646–1660. [Google Scholar] [CrossRef]
- Krivokolysko, D.S.; Dotsenko, V.V.; Bibik, E.Y.; Myazina, A.V.; Krivokolysko, S.G.; Vasilin, V.K.; Pankov, A.A.; Aksenov, N.A.; Aksenova, I.V. Synthesis, structure, and analgesic activity of 4-(5-cyano-{4-(fur-2-yl)-1,4-dihydropyridin-3-yl} carboxamido)benzoic acids ethyl esters. Russ. J. Gen. Chem. 2021, 91, 2588–2605. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G.; Krivokolysko, B.S.; Frolov, K.A. A New approach to the synthesis of functional derivatives of 3-(4-pyridinyl)-1H-indole and 4-(1H-indol-3-yl)thieno[2,3-b]pyridine. Russ. J. Gen. Chem. 2018, 88, 682–688. [Google Scholar] [CrossRef]
- Terauchi, H.; Tanitame, A.; Tada, K.; Nishikawa, Y. A Convenient Synthesis of N-Substituted 2,3-Dihydro-3-oxoisothiazolo[5, 4-b]pyridines in Acidic Conditions. Heterocycles 1996, 43, 1719–1734. [Google Scholar] [CrossRef]
- Martinez-Merino, V.; Gil, M.J.; Gonzalez, A.; Zabalza, J.M.; Navarro, J.; Mañu, M.A. New 5-substituted derivatives of ethyl 2,3-dihydro-3-oxoisothiazolo[5, 4-b]pyridine-2-acetate. Heterocycles 1994, 38, 333–344. [Google Scholar] [CrossRef]
- Monge, A.; Martinez-Merino, V.; Fernandez-Alvarez, E. Synthesis of 2-substituted 3-oxoisothiazolo[5,4-b]pyridines. J. Heterocycl. Chem. 1985, 22, 1353–1356. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Krivokolysko, S.G. Recent advances in the chemistry of thieno[2,3-b]pyridines 1. Methods of synthesis of thieno[2,3-b]pyridines. Russ. Chem. Bull. Int. Ed. 2020, 69, 1829–1858. [Google Scholar] [CrossRef]
- Malinka, W.; Świątek, P.; Śliwińska, M.; Szponar, B.; Gamian, A.; Karczmarzyk, Z.; Fruziński, A. Synthesis of novel isothiazolopyridines and their in vitro evaluation against Mycobacterium and Propionibacterium acnes. Bioorg. Med. Chem. 2013, 21, 5282–5291. [Google Scholar] [CrossRef] [PubMed]
- Świątek, P.; Strzelecka, M.; Urniaz, R.; Gębczak, K.; Gębarowski, T.; Gąsiorowski, K.; Malinka, W. Synthesis, COX-1/2 inhibition activities and molecular docking study of isothiazolopyridine derivatives. Bioorg. Med. Chem. 2017, 25, 316–326. [Google Scholar] [CrossRef]
- Malinka, W.; Świątek, P.; Filipek, B.; Sapa, J.; Jezierska, A.; Koll, A. Synthesis, analgesic activity and computational study of new isothiazolopyridines of Mannich base type. Farmaco 2005, 60, 961–968. [Google Scholar] [CrossRef]
- Gorsuch, S.; Bavetsias, V.; Rowlands, M.G.; Aherne, G.W.; Workman, P.; Jarman, M.; McDonald, E. Synthesis of isothiazol-3-one derivatives as inhibitors of histone acetyltransferases (HATs). Bioorg. Med. Chem. 2009, 17, 467–474. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Krivokolysko, S.G. Oxidation of thioamides with the DMSO–HCl system: A convenient and efficient method for the synthesis of 1,2,4-thiadiazoles, isothiazolo[5,4-b]pyridines, and heterocyclic disulfides. Chem. Heterocycl. Compd. 2013, 49, 636–644. [Google Scholar] [CrossRef]
- Litvinov, V.P.; Rodinovskaya, L.A.; Sharanin, Y.A.; Shestopalov, A.M.; Senning, A. Advances in the chemistry of 3-cyanopyridin-2 (1H)-ones, -thiones, and -selenones. Sulfur Rep. 1992, 13, 1–142. [Google Scholar] [CrossRef]
- Becker, J.; Stidsen, C.E. Recent Developments in the Synthesis and Chemistry of 2(1H)-Pyridinethiones and Related Compounds. Sulfur Rep. 1988, 8, 105–146. [Google Scholar] [CrossRef]
- Litvinov, V.P. Advances in the Chemistry of Hydrogenated 3-Cyanopyridine-2(1H)-Thiones and -Selenones. Phosphorus Sulfur Silicon Relat. Elem. 1993, 74, 139–156. [Google Scholar] [CrossRef]
- Krivokolysko, S.G.; Rusanov, E.B.; Litvinov, V.P. Reaction of N-methylmorpholinium 5-cyano-2-oxo-4-(2-thienyl)-1,2,3,4-tetrahydropyridine-6-thiolate with α-bromo ketones. Chem. Heterocycl. Compd. 2002, 38, 1397–1405. [Google Scholar] [CrossRef]
- Sander, T. OSIRIS Property Explorer. Idorsia Pharmaceuticals Ltd., Switzerland. Available online: http://www.organic-chemistry.org/prog/peo/ (accessed on 27 February 2023).
- Parrino, B.; Carbone, D.; Cascioferro, S.; Pecoraro, C.; Giovannetti, E.; Deng, D.; Di Sarno, V.; Musella, S.; Auriemma, G.; Cusimano, M.G.; et al. 1,2,4-Oxadiazole topsentin analogs as staphylococcal biofilm inhibitors targeting the bacterial transpeptidase sortase A. Eur. J. Med. Chem. 2021, 209, 112892. [Google Scholar] [CrossRef]
- Kaur, J.; Utreja, D.; Jain, N.; Sharma, S. Recent developments in the synthesis and antimicrobial activity of indole and its derivatives. Curr. Org. Synth. 2019, 16, 17–37. [Google Scholar] [CrossRef]
- Nieto, M.J.; Lupton, H.K. Indole and indoline scaffolds in antimicrobials: Overview, synthesis and recent advances in antimicrobial research. Curr. Med. Chem. 2021, 28, 4828–4844. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Singh, R.K. Medicinal chemistry of indole derivatives: Current to future therapeutic prospectives. Bioorg. Chem. 2019, 89, 103021. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Vena, A.; Croxatto, A.; Righi, E.; Guery, B. How to manage Pseudomonas aeruginosa infections. Drugs Context 2018, 7, 212527. [Google Scholar] [CrossRef]
- Ilangovan, A.; Fletcher, M.; Rampioni, G.; Pustelny, C.; Rumbaugh, K.; Heeb, S.; Cámara, M.; Truman, A.; Chhabra, S.R.; Emsley, J.; et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR). PLoS Pathog. 2013, 9, e1003508. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D past, present, and future: A review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Chkanikov, N.D.; Spiridonov, Y.Y.; Khalikov, S.S.; Muzafarova, A.M. Antidotes for reduction of phytotoxicity of the residues of sulfonylurea herbicides. INEOS Open 2019, 2, 145–152. [Google Scholar] [CrossRef]
- Deng, X. Current advances in the action mechanisms of safeners. Agronomy 2022, 12, 2824. [Google Scholar] [CrossRef]
- Jia, L.; Jin, X.Y.; Zhao, L.X.; Fu, Y.; Ye, F. Research progress in the design and synthesis of herbicide safeners: A review. J. Agric. Food Chem. 2022, 70, 5499–5515. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Buryi, D.S.; Lukina, D.Y.; Stolyarova, A.N.; Aksenov, N.A.; Aksenova, I.V.; Strelkov, V.D.; Dyadyuchenko, L.V. Substituted N-(thieno[2,3-b]pyridine-3-yl)acetamides: Synthesis, reactions, and biological activity. Monatsh. Chem. 2019, 150, 1973–1985. [Google Scholar] [CrossRef]
- Dotsenko, V.V.; Muraviev, V.S.; Lukina, D.Y.; Strelkov, V.D.; Aksenov, N.A.; Aksenova, I.V.; Krapivin, G.D.; Dyadyuchenko, L.V. Reaction of 3-Amino-4,6-diarylthieno[2,3-b]pyridine-2-carboxamides with ninhydrin. Russ. J. Gen. Chem. 2020, 90, 948–960. [Google Scholar] [CrossRef]
- Buryi, D.S.; Dotsenko, V.V.; Aksenov, N.A.; Aksenova, I.V.; Krivokolysko, S.G.; Dyadyuchenko, L.V. Synthesis and properties of 4,6-dimethyl-5-pentyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile and 3-amino-4,6-dimethyl-5-pentylthieno[2,3-b]pyridines. Russ. J. Gen. Chem. 2019, 89, 1575–1585. [Google Scholar] [CrossRef]
- Stroganova, T.A.; Vasilin, V.K.; Krapivin, G.D.; Strelkov, V.D.; Dyadyuchenko, L.V. Synthesis of N-alkylated benzo- and pyridothienopyrrolo[1,2 a][1,4]diazepin-6-ones acting as antidotes against the herbicide 2,4-D. Chem. Heterocycl. Compd. 2016, 52, 45–51. [Google Scholar] [CrossRef]
- Dyadyuchenko, L.V.; Dmitrieva, I.G.; Aksenov, N.A.; Dotsenko, V.V. Synthesis, structure, and biological activity of 2, 6-diazido-4-methylnicotinonitrile derivatives. Chem. Heterocycl. Compd. 2018, 54, 964–970. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).