Preparation of Sulfonated Poly(arylene ether)/SiO2 Composite Membranes with Enhanced Proton Selectivity for Vanadium Redox Flow Batteries
Abstract
1. Introduction
2. Results and Discussion
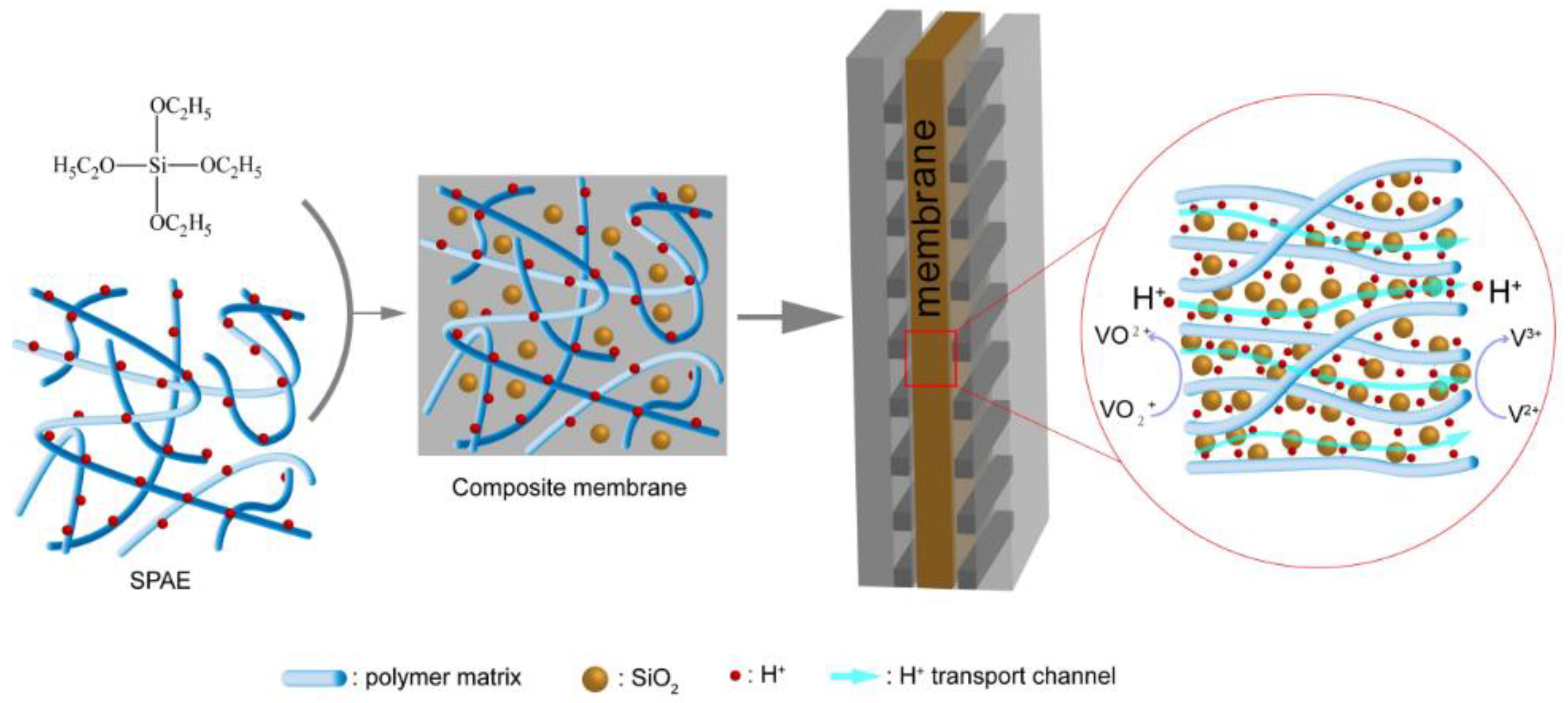
2.1. Preparation of the SPAE/SiO2 Composite PEMs
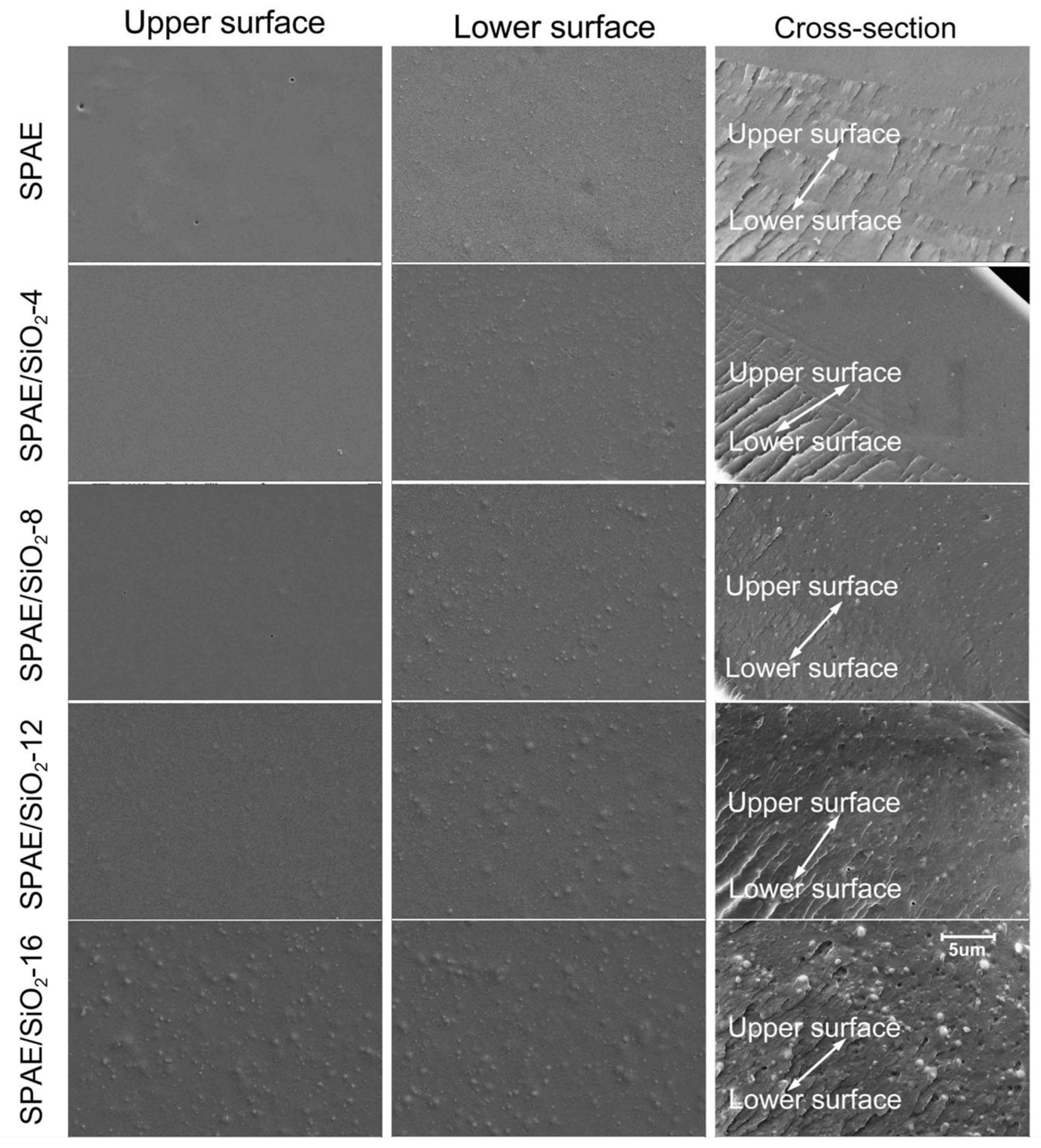
2.2. Microstructure of the SPAE/SiO2 Composite PEMs
2.3. IEC, Water Uptake, and Swelling Ratio
2.4. Proton Conductivity and Area Resistance
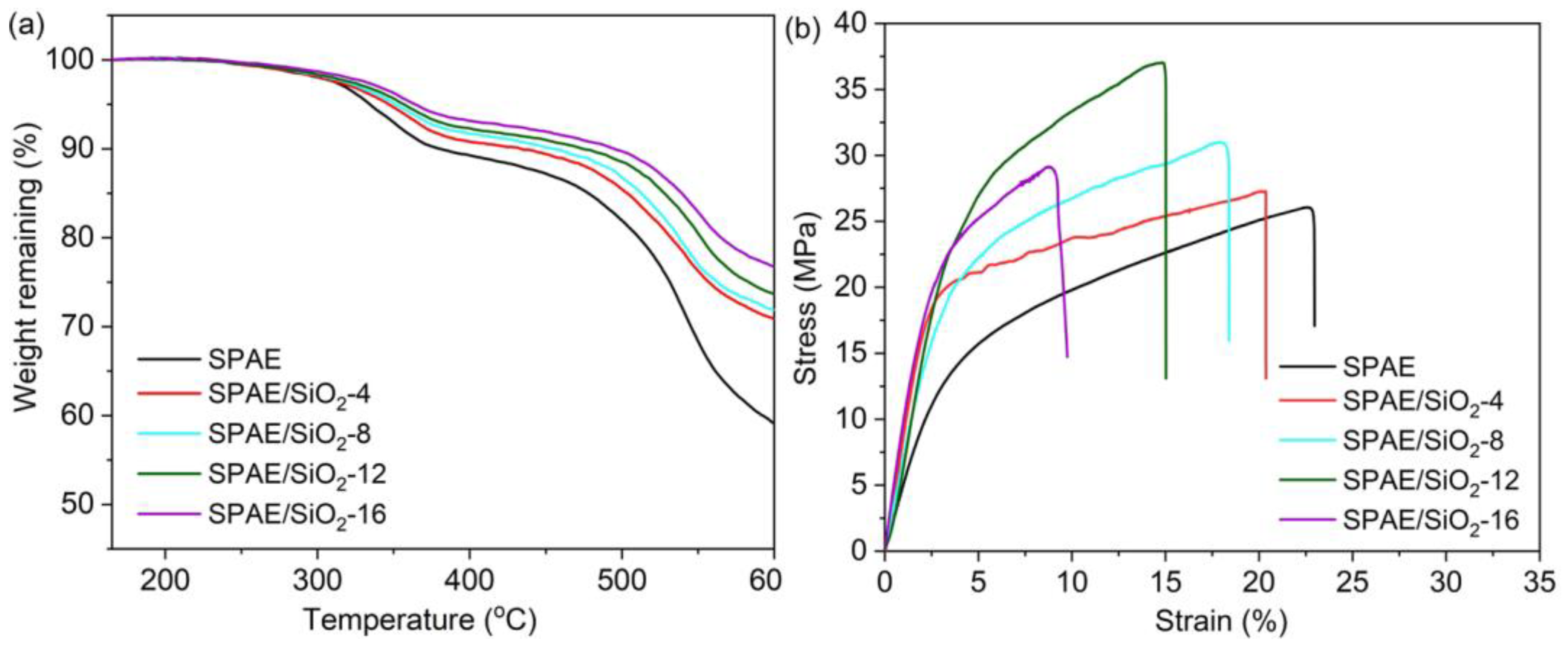
2.5. Thermal and Mechanical Properties
2.6. VO2+ Permeability and Ion Selectivity
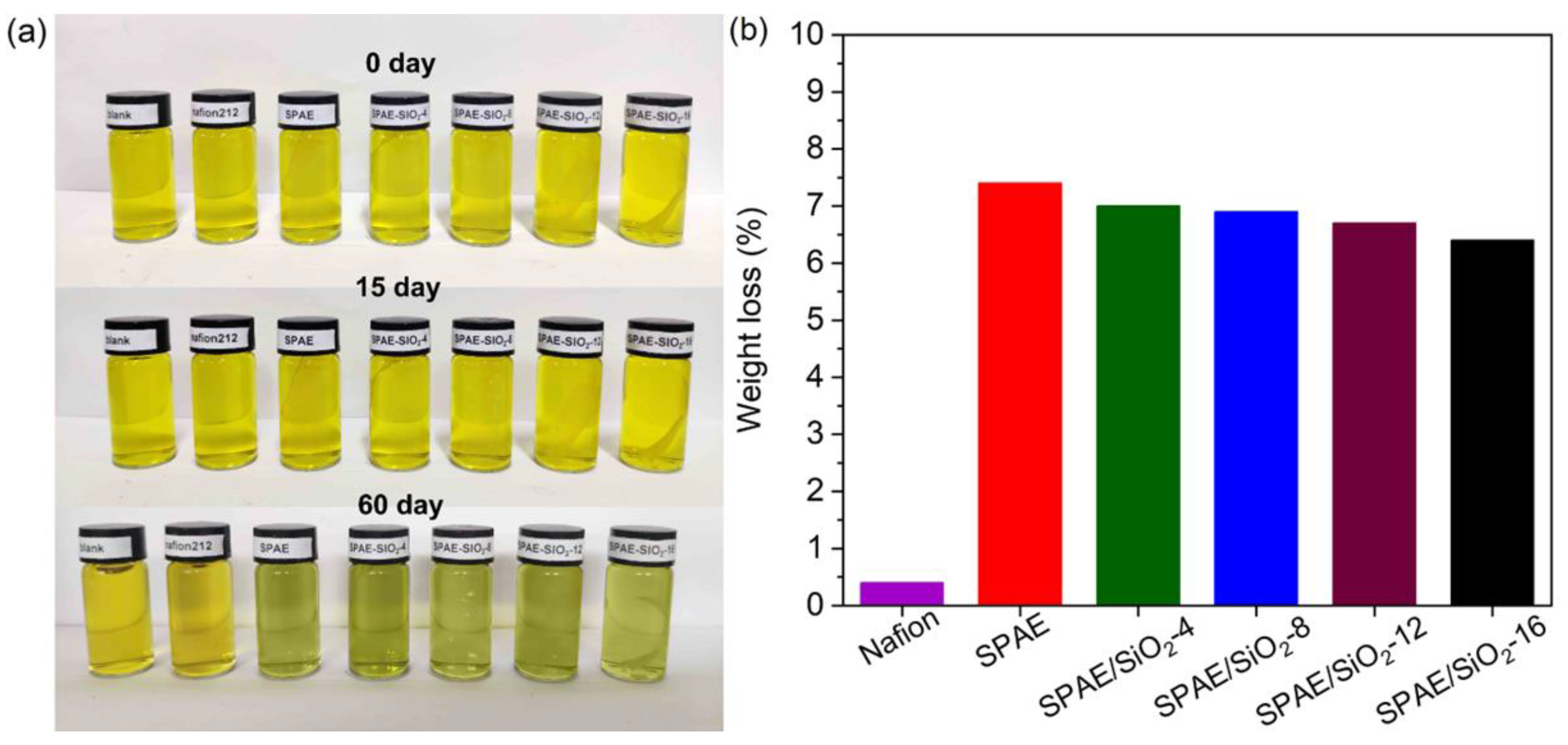
2.7. Oxidative Stability
3. Experimental Section
3.1. Materials
3.2. Preparation of SPAE/SiO2 Composite PEMs
3.3. Characterizations
3.3.1. Water Uptake and Swelling Ratio
3.3.2. Oxidative Stability
3.3.3. Mechanical Properties
3.3.4. Ion Exchange Capacity (IEC)
3.3.5. Proton Conductivity
3.3.6. Area Resistance
3.3.7. VO2+ Permeability and Ion Selectivity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Choi, C.; Kim, S.; Kim, R.; Choi, Y.; Jung, H.Y.; Yang, J.H.; Kim, H.T. A review of vanadium electrolytes for vanadium redox flow batteries. Renew. Sust. Energ. Rev. 2017, 69, 263–274. [Google Scholar] [CrossRef]
- Schwenzer, B.; Zhang, J.L.; Kim, S.; Li, L.Y.; Liu, J.; Yang, Z.G. Membrane development for vanadium redox flow batteries. ChemSusChem 2011, 4, 1388–1406. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Hickner, M.A.; Agar, E.; Kumbur, E.C. Selective anion exchange membranes for high coulombic efficiency vanadium redox flow batteries. Electrochem. Commun. 2013, 26, 37–40. [Google Scholar] [CrossRef]
- Zhang, S.H.; Yin, C.X.; Xing, D.B.; Yang, D.L.; Jian, X.G. Preparation of chloromethylated/quaternized poly(phthalazinone ether ketone) anion exchange membrane materials for vanadium redox flow battery applications. J. Membr. Sci. 2017, 363, 243–249. [Google Scholar] [CrossRef]
- Xi, J.Y.; Jiang, B.; Yu, L.H.; Liu, L. Membrane evaluation for vanadium flow batteries in a temperature range of −20–50 °C. J. Membr. Sci. 2017, 522, 45–55. [Google Scholar] [CrossRef]
- Wu, C.X.; Lu, S.F.; Wang, H.N.; Xu, X.; Peng, S.K.; Tan, Q.L.; Xiang, Y. A novel polysulfone-polyvinylpyrrolidone membrane with superior proton-to-vanadium ion selectivity for vanadium redox flow batteries. J. Mater. Chem. A 2016, 4, 1174–1179. [Google Scholar] [CrossRef]
- Amel, A.; Smedley, S.B.; Dekel, D.R.; Hickner, M.A.; Ein-Eli, Y. Characterization and chemical stability of anion exchange membranes cross-linked with polar electron-donating linkers. J. Electrochem. Soc. 2015, 162, 1047–1055. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis-effect of hydrophilicity of anion exchange membranes on perm selectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Monopoli, A.; Casiello, M.; Cotugno, P.; Milella, A.; Palumbo, F.; Fracassi, F.; Nacci, A. Synthesis of tailored perfluoro unsaturated monomers for potential applications in proton exchange membrane preparation. Molecules 2021, 26, 5592. [Google Scholar] [CrossRef]
- Yang, S.; Ahn, Y.; Kim, D. Poly(arylene ether ketone) proton exchange membranes grafted with long aliphatic pendant sulfonated groups for vanadium redox flow batteries. J. Mater. Chem. A 2017, 5, 2261–2270. [Google Scholar] [CrossRef]
- Cheng, H.L.; Xu, J.M.; Ma, L.; Xu, L.S.; Liu, B.J.; Wang, Z.; Zhang, H.X. Preparation and characterization of sulfonated poly(arylene ether ketone) copolymers with pendant sulfoalkyl groups as proton exchange membranes. J. Power Sources 2014, 260, 307–316. [Google Scholar] [CrossRef]
- Zhang, W.M.; Chen, S.Y.; Chen, D.Y.; Ye, Z.L. Sulfonated binaphthyl-containing poly(arylene ether ketone)s with rigid backbone and excellent film-forming capability for proton exchange membranes. Polymers 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Suryani; Chang, Y.N.; Lai, J.Y.; Liu, Y.L. Polybenzimidazole(PBI)-functionalized silica nanoparticles modified PBI nanocomposite membranes for proton exchange membranes fuel cells. J. Membr. Sci. 2012, 403, 1–7. [Google Scholar] [CrossRef]
- Lee, K.H.; Lee, S.Y.; Shin, D.W.; Wang, C.Y.; Ahn, S.H.; Lee, K.J.; Guiver, M.D.; Lee, Y.M. Structural influence of hydrophobic diamine in sulfonated poly(sulfide sulfone imide) copolymers on medium temperature PEM fuel cell. Polymer 2014, 55, 1317–1326. [Google Scholar] [CrossRef]
- Duburg, J.C.; Azizi, K.; Primdahl, S.; Hjuler, H.A.; Zanzola, E.; Schmidt, T.J.; Gubler, L. Composite polybenzimidazole membrane with high capacity retention for vanadium redox flow batteries. Molecules 2021, 26, 1679. [Google Scholar] [CrossRef]
- Bae, B.; Miyatake, K.; Watanabe, M. Effect of the hydrophobic component on the properties of sulfonated poly(arylene ether sulfone)s. Macromolecules 2009, 42, 1873–1880. [Google Scholar] [CrossRef]
- Kim, Y.S.; Hickner, M.A.; Dong, L.M.; Pivovar, B.S.; McGrath, J.E. Sulfonated poly(arylene ether sulfone) copolymer proton exchange membranes: Composition and morphology effects on the methanol permeability. J. Membr. Sci. 2004, 243, 317–326. [Google Scholar] [CrossRef]
- Semiz, L.; Sankir, N.; Sankir, M. Directly copolymerized disulfonated poly(arylene ether sulfone) membranes for vanadium redox flow batteries. Int. J. Electrochem. Sci. 2014, 9, 3060–3067. [Google Scholar]
- Wang, C.; Shin, D.W.; Lee, S.Y.; Kang, N.R.; Lee, Y.M.; Guiver, M.D. Poly(arylene ether sulfone) proton exchange membranes with flexible acid side chains. J. Membr. Sci. 2012, 421, 375. [Google Scholar] [CrossRef]
- Park, C.H.; Lee, C.H.; Guiver, M.D.; Lee, Y.M. Sulfonated hydrocarbon membranes for medium-temperature and low-humidity proton exchange membrane fuel cells (PEMFCs). Prog. Polym. Sci. 2011, 36, 1443–1498. [Google Scholar] [CrossRef]
- Miyatake, K.; Chikashige, Y.; Higuchi, E.; Watanabe, M. Tuned polymer electrolyte membranes based on aromatic poly ethers for fuel cell applications. J. Am. Chem. Soc. 2007, 129, 3879–3887. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Fuel cells: Operating flexibly. Nat. Energy 2016, 1, 16136. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, L.; Goh, K.; Sui, X.L.; Meng, L.H.; Wang, Z.B. A highly proton-vanadium-selective perfluoro sulfonic acid membrane for vanadium redox flow batteries. New J. Chem. 2019, 43, 11374–11381. [Google Scholar] [CrossRef]
- Xi, J.Y.; Wu, Z.H.; Qiu, X.P.; Chen, L.Q. Nafion/SiO2 hybrid membrane for vanadium redox flow battery. J. Power Sources 2007, 166, 531–536. [Google Scholar] [CrossRef]
- Hossain, S.I.; Aziz, M.A.; Han, D.B.; Selvam, P.; Shanmugam, S. Fabrication of SPAEK-cerium zirconium oxide nanotube composite membrane with outstanding performance and durability for vanadium redox flow batteries. J. Mater. Chem. A. 2018, 6, 20205–20213. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Yan, X.M.; Gao, L.; Hu, L.; Ruan, X.H.; Zheng, W.J.; He, G.H. Novel triple tertiary amine polymer-based hydrogen bond network inducing highly efficient proton conducting channels of amphoteric membrane for high-performance vanadium redox flow battery. ACS Appl. Mater. Interfaces 2019, 11, 5003–5014. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, H.X.; Qian, P.H.; Zhou, Y.; Shi, J.Y.; Shi, H.F. Sulfonated poly(ether ether ketone)/amine-functionalized graphene oxide hybrid membrane with various chain lengths for vanadium redox flow battery: A comparative study. J. Membr. Sci. 2020, 610, 118232. [Google Scholar] [CrossRef]
- Liu, B.; Jiang, Y.H.; Wang, H.X.; Ge, J.; Shi, H.F. Sulfonated poly(ether ether ketone) hybrid membranes with amphoteric graphene oxide nanosheets as interfacial reinforcement for vanadium redox flow battery. Energy Fuels 2020, 34, 2452–2461. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, H.X.; Liu, B.; Shi, J.L.; Zhang, J.; Shi, H.F. An ultra-high ion selective hybrid proton exchange membrane incorporated with zwitterion-decorated graphene oxide for vanadium redox flow batteries. J. Mater. Chem. A 2019, 7, 12669–12680. [Google Scholar] [CrossRef]
- Zheng, L.Y.; Wang, H.X.; Niu, R.T.; Zhang, Y.X.; Shi, H.F. Sulfonated poly(ether ether ketone)/sulfonated graphene oxide hybrid membrane for vanadium redox flow battery. Electrochim. Acta 2018, 282, 437–447. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, H.X.; Yu, W.K.; Shi, J.L.; Shi, H.F. Sulfonated poly(ether ether ketone)-based hybrid membranes containing polydopamine-decorated multiwalled carbon nanotubes with acid-base pairs for all vanadium redox flow battery. J. Membr. Sci. 2018, 564, 916–925. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, H.X.; Yu, W.K.; Shi, H.F. Structure and properties of sulfonated poly(ether ether ketone) hybrid membrane with polyaniline-chains-modified graphene oxide and its application for vanadium redox flow battery. ChemistrySelect 2018, 3, 9249–9258. [Google Scholar] [CrossRef]
- Niu, R.T.; Kong, L.Q.; Zheng, L.Y.; Wang, H.X.; Shi, H.F. Novel graphitic carbon nitride nanosheets/sulfonated poly(ether ether ketone) acid-base hybrid membrane for vanadium redox flow battery. J. Membr. Sci. 2017, 525, 220–228. [Google Scholar] [CrossRef]
- Kong, L.Q.; Zheng, L.Y.; Niu, R.T.; Wang, H.X.; Shi, H.F. A sulfonated poly(ether ether ketone)/amine-functionalized graphene oxide hybrid membrane for vanadium redox flow batteries. RSC Adv. 2016, 6, 100262–100270. [Google Scholar] [CrossRef]
- Xiong, K.N.; Wu, W.; Wang, S.F.; Zhang, L. Modeling, design, materials and fabrication of bipolar plates for proton exchange membrane fuel cell: A review. Appl. Energy 2021, 301, 117443. [Google Scholar] [CrossRef]
- Elwan, H.A.; Thimmappa, R.; Mamlouk, M.; Scott, K. Applications of poly ionic liquids in proton exchange membrane fuel cells: A review. J. Power Sources 2021, 510, 230371. [Google Scholar] [CrossRef]
- Liu, L.; Wang, C.; He, Z.F.; Das, R.A.J.; Dong, B.B.; Xie, X.F.; Guo, Z.H. An overview of amphoteric ion exchange membranes for vanadium redox flow batteries. J. Mater. Sci. Technol. 2021, 69, 212–227. [Google Scholar] [CrossRef]
- Jing, X.; Li, X.; Han, W. Research progress of proton exchange membranes and electrode materials for all-vanadium flow batteries. Polym. Bull. 2021, 5, 1–13. [Google Scholar]
- Gubler, L. Membranes and separators for redox flow batteries. Curr. Opin. Electrochem. 2019, 18, 31–36. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.W.; Chen, X.L.; Chen, D.Y.; Zheng, Y.Y. Side chain engineering of sulfonated poly(arylene ether)s for proton exchange membranes. Chin. J. Polym. Sci. 2020, 38, 644–652. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.Y.; Xu, J.Q.; Chen, S.Y.; Chen, D.Y. Densely quaternized fluorinated poly(fluorenyl ether)s with excellent conductivity and stability for vanadium redox flow batteries. ACS Appl. Mater. Interfaces 2021, 13, 18923–18933. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Hickner, M. Anion exchange membranes by bromination of benzyl methyl-containing poly(sulfone)s. Macromolecules 2010, 43, 2349–2356. [Google Scholar] [CrossRef]
- Lee, K.H.; Chu, J.Y.; Kim, A.R.; Kim, H.G.; Yoo, D.J. Functionalized TiO2 mediated organic-inorganic composite membranes based on quaternized poly(arylene ether ketone) with enhanced ionic conductivity and alkaline stability for alkaline fuel cells. J. Membr. Sci. 2021, 634, 119435. [Google Scholar] [CrossRef]
- Wiedemann, E.; Heintz, A.; Lichtenthaler, R. Transport properties of vanadium ions in cation exchange membranes: Determination of diffusion coefficients using a dialysis cell. J. Membr. Sci. 1998, 141, 215–221. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, L.; Goh, K.; Sui, X.L.; Meng, L.H.; Wang, Z.B. A phosphotungstic acid coupled silica-Nafion composite membrane with significantly enhanced ion selectivity for vanadium redox flow battery. J. Energy Chem. 2020, 41, 177–184. [Google Scholar] [CrossRef]
- Xi, X.L.; Ding, C.; Zhang, H.Z.; Li, X.F.; Cheng, Y.H.; Zhang, H.M. Solvent responsive silica composite nanofiltration membrane with controlled pores and improved ion selectivity for vanadium flow battery application. J. Power Sources 2015, 274, 1126–1134. [Google Scholar] [CrossRef]
- Chen, D.Y.; Wang, S.J.; Xiao, M.; Han, D.M.; Meng, Y.Z. Sulfonated poly (fluorenyl ether ketone) membrane with embedded silica rich layer and enhanced proton selectivity for vanadium redox flow battery. J. Power Sources 2010, 195, 7701–7708. [Google Scholar] [CrossRef]
- Kondratenko, M.S.; Karpushkin, E.A.; Gvozdik, N.A.; Gallyamov, M.O.; Stevenson, K.J.; Sergeyev, V.G. Influence of aminosilane precursor concentration on physicochemical properties of composite Nafion membranes for vanadium redox flow battery applications. J. Power Sources 2017, 340, 32–39. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, X.J.; Xue, R.; Yu, Q.C.; Jiang, F.J.; Zhong, Y.G. Proton exchange membranes with ultra-low vanadium ions permeability improved by sulfated zirconia for all vanadium redox flow battery. Int. J. Hydrog. Energy 2019, 44, 5997–6006. [Google Scholar] [CrossRef]
- Rajput, A.; Sharma, P.P.; Yadav, V.; Kulshrestha, V. Highly stable graphene oxide composite proton exchange membrane for electro-chemical energy application. Int. J. Hydrog. Energy 2020, 45, 16976–16983. [Google Scholar] [CrossRef]
- Yang, X.B.; Zhao, L.; Sui, X.L.; Meng, L.H.; Wang, Z.B. Phosphotungstic acid immobilized nanofibers-Nafion composite membrane with low vanadium permeability and high selectivity for vanadium redox flow battery. J. Colloid Interface Sci. 2019, 542, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Hickner, M.A. V5+ degradation of sulfonated Radel membrane for vanadium redox flow batteries. Phys. Chem. Chem. Phys. 2013, 15, 11299–11305. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.J.; Song, X.Q.; Ye, G.D.; Xu, J.J. Preparation of PVA/paraffin thermal regulating fiber by in situ microencapsulation. Compos. Sci. Technol. 2008, 68, 2231–2237. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.Y.; Wang, B.S.; Lin, M.J.; Xie, Z.L.; Chen, D.Y. Fluorinated poly(fluorenyl ether)s with linear multicationic side chains for vanadium redox flow batteries. Sci. China Mater. 2021, 64, 349–361. [Google Scholar] [CrossRef]
- Fujimoto, C.H.; Hickner, M.A.; Cornelius, C.J.; Loy, D.A. Ionomeric poly(phenylene) prepared by Diels-Alder polymerization: Synthesis and physical properties of a novel polyelectrolyte. Macromolecules 2005, 38, 5010–5016. [Google Scholar] [CrossRef]
- Largier, T.; Cornelius, C. Random quaternary ammonium Diels-Alder poly(phenylene) copolymers for improved vanadium redox flow batteries. J. Power Sources 2017, 352, 149–155. [Google Scholar] [CrossRef]
- Ling, X.; Jia, C.K.; Liu, J.G.; Yan, C.W. Preparation and characterization of sulfonated poly (ether sulfone)/sulfonated poly(ether ether ketone) blend membrane for vanadium redox flow battery. J. Membr. Sci. 2012, 415, 306–312. [Google Scholar] [CrossRef]
- Hwang, C.W.; Park, H.M.; Oh, C.M.; Hwang, T.S.; Shim, J.; Jin, C.S. Synthesis and characterization of vinylimidazole-co-trifluoroethylmethacrylate-co-divinylbenzene anion-exchange membrane for all-vanadium redox flow battery. J. Membr. Sci. 2014, 468, 98–106. [Google Scholar] [CrossRef]
- Pang, B.; Zhang, Q.; Xiao, X. Superior acidic sulfate ester group based high conductive membrane for vanadium redox flow battery. J. Power Sources 2021, 506, 230203. [Google Scholar] [CrossRef]
- Shang, X.Y.; Shu, D.; Wang, S.J.; Xiao, M.; Meng, Y.Z. Fluorene-containing sulfonated poly(arylene ether 1,3,4-oxadiazole) as proton-exchange membrane for PEM fuel cell application. J. Membr. Sci. 2007, 291, 140–147. [Google Scholar] [CrossRef]
- Semiz, L.; Demirci, N.; Sankir, M. Influence of the basic membrane properties of the disulfonated poly(arylene ether sulfone) copolymer membranes on the vanadium redox flow battery performance. J. Membr. Sci. 2014, 468, 209–215. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.H.; Li, D.; Liu, B.Q.; Wang, J.J.; Song, Y.L. Novel amphoteric ion exchange membranes by blending sulfonated poly(ether ether ketone)/quaternized poly(ether imide) for vanadium redox flow battery applications. J. Mater. Chem. A. 2015, 3, 17590–17597. [Google Scholar] [CrossRef]



| Polymer | IEC (mmol g−1) | Water Uptake (%) | Swelling Ratio (%) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|
| SPAE | 1.97 | 25.6 | 13.3 | 26.33 | 24.8 |
| SPAE/SiO2-4 | 1.90 | 23.5 | 11.6 | 27.28 | 21.5 |
| SPAE/SiO2-8 | 1.82 | 21.6 | 10.5 | 31.01 | 18.3 |
| SPAE/SiO2-12 | 1.76 | 18.3 | 9.6 | 37.03 | 15.0 |
| SPAE/SiO2-16 | 1.70 | 15.2 | 8.7 | 29.13 | 9.7 |
| Polymer | Thickness (μm) | σ (mS cm−1) | Ea (KJ mol−1) | Area Resistance (Ω cm2) | VO2+ Permeability (10−12 m2 s−1) | Ion Selectivity (103 S min cm−3) |
|---|---|---|---|---|---|---|
| SPAE | 58 | 93.4 | 16.31 | 0.71 | 7.62 | 20.42 |
| SPAE/SiO2-4 | 53 | 88.2 | 15.58 | 0.87 | 6.11 | 24.06 |
| SPAE/SiO2-8 | 55 | 83.9 | 15.44 | 0.93 | 4.31 | 32.44 |
| SPAE/SiO2-12 | 51 | 79.9 | 15.00 | 1.17 | 2.57 | 51.82 |
| SPAE/SiO2-16 | 50 | 76.9 | 14.03 | 1.59 | 3.64 | 35.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, Z.; Chen, N.; Zheng, Z.; Xiong, L.; Chen, D. Preparation of Sulfonated Poly(arylene ether)/SiO2 Composite Membranes with Enhanced Proton Selectivity for Vanadium Redox Flow Batteries. Molecules 2023, 28, 3130. https://doi.org/10.3390/molecules28073130
Ye Z, Chen N, Zheng Z, Xiong L, Chen D. Preparation of Sulfonated Poly(arylene ether)/SiO2 Composite Membranes with Enhanced Proton Selectivity for Vanadium Redox Flow Batteries. Molecules. 2023; 28(7):3130. https://doi.org/10.3390/molecules28073130
Chicago/Turabian StyleYe, Zhoulin, Nanjie Chen, Zigui Zheng, Lei Xiong, and Dongyang Chen. 2023. "Preparation of Sulfonated Poly(arylene ether)/SiO2 Composite Membranes with Enhanced Proton Selectivity for Vanadium Redox Flow Batteries" Molecules 28, no. 7: 3130. https://doi.org/10.3390/molecules28073130
APA StyleYe, Z., Chen, N., Zheng, Z., Xiong, L., & Chen, D. (2023). Preparation of Sulfonated Poly(arylene ether)/SiO2 Composite Membranes with Enhanced Proton Selectivity for Vanadium Redox Flow Batteries. Molecules, 28(7), 3130. https://doi.org/10.3390/molecules28073130






