Synthesis, Characterization, and Investigation of Novel Ionic Liquid-Based Tooth Bleaching Gels: A Step towards Safer and Cost-Effective Cosmetic Dentistry
Abstract
1. Introduction
2. Methodology
2.1. Materials
2.2. Synthesis of Choline Hydroxide-Based Gels
2.3. Synthesis of Choline Citrate Gel
2.4. Characterization of Synthesized Gels
2.4.1. pH Measurement
2.4.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.5. Tooth Sample Preparation
2.5.1. Collection of Tooth Samples
2.5.2. Cutting of Enamel Blocks
2.5.3. Mounting and Polishing of Enamel Blocks
2.6. Staining of Tooth Samples
2.6.1. Grouping for Bleaching Treatment of Tooth Samples
2.6.2. Tooth Color Analysis
2.7. Digital Colorimetry
- 1–0: Not perceptible by the human eye.
- 1–2: Perceptible through close observation.
- 2–10: Perceptible at a glance.
- 11–49: Colors are more similar than the opposite.
- 100: Colors are exactly opposite.
2.7.1. Microhardness
2.7.2. Scanning Electron Microscopy (SEM)
2.8. Optical Profilometry
Statistical Analysis
3. Results
3.1. pH Measurement
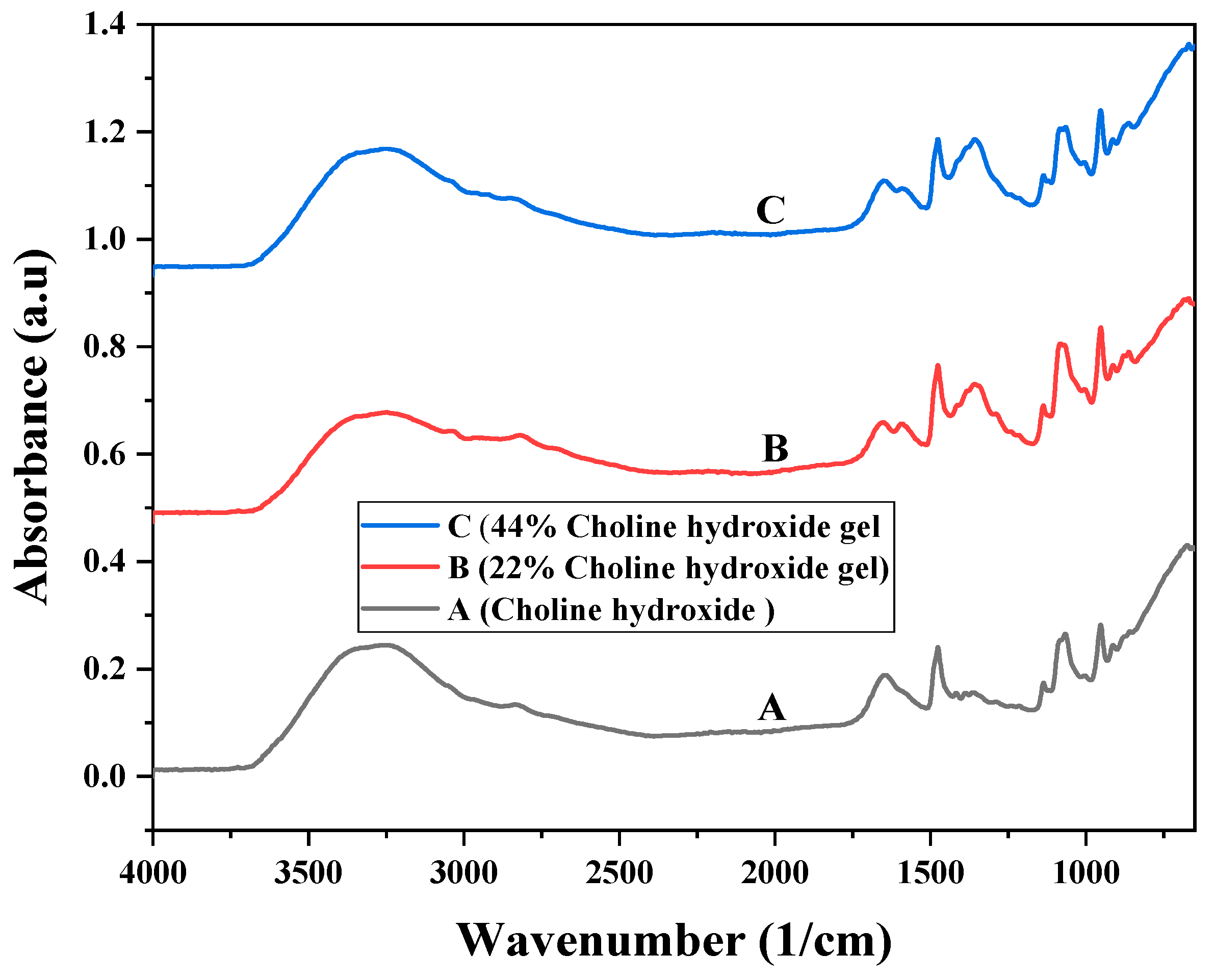
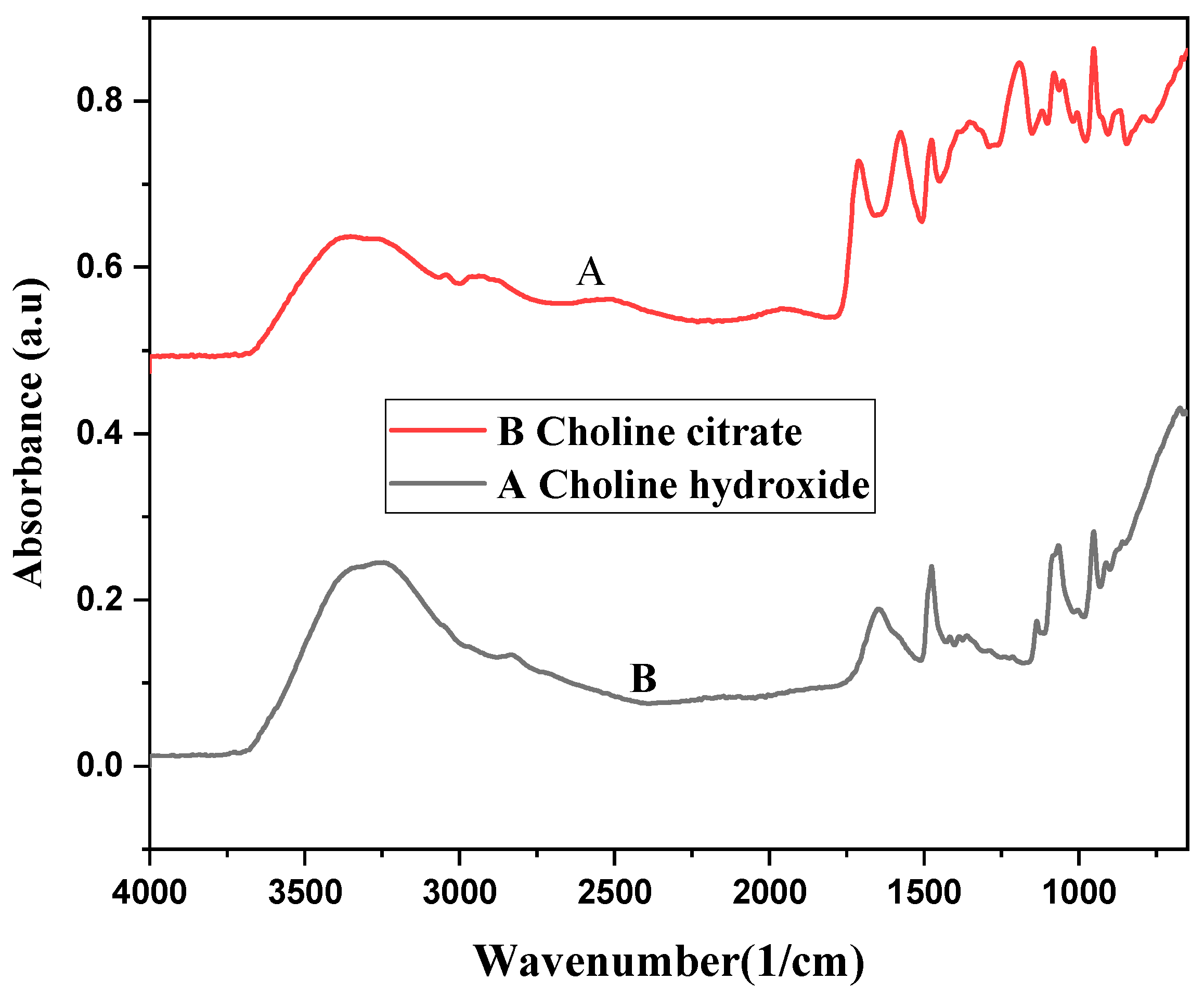
3.2. Fourier Transform Infrared Spectroscopy
3.3. Tooth Color Analysis
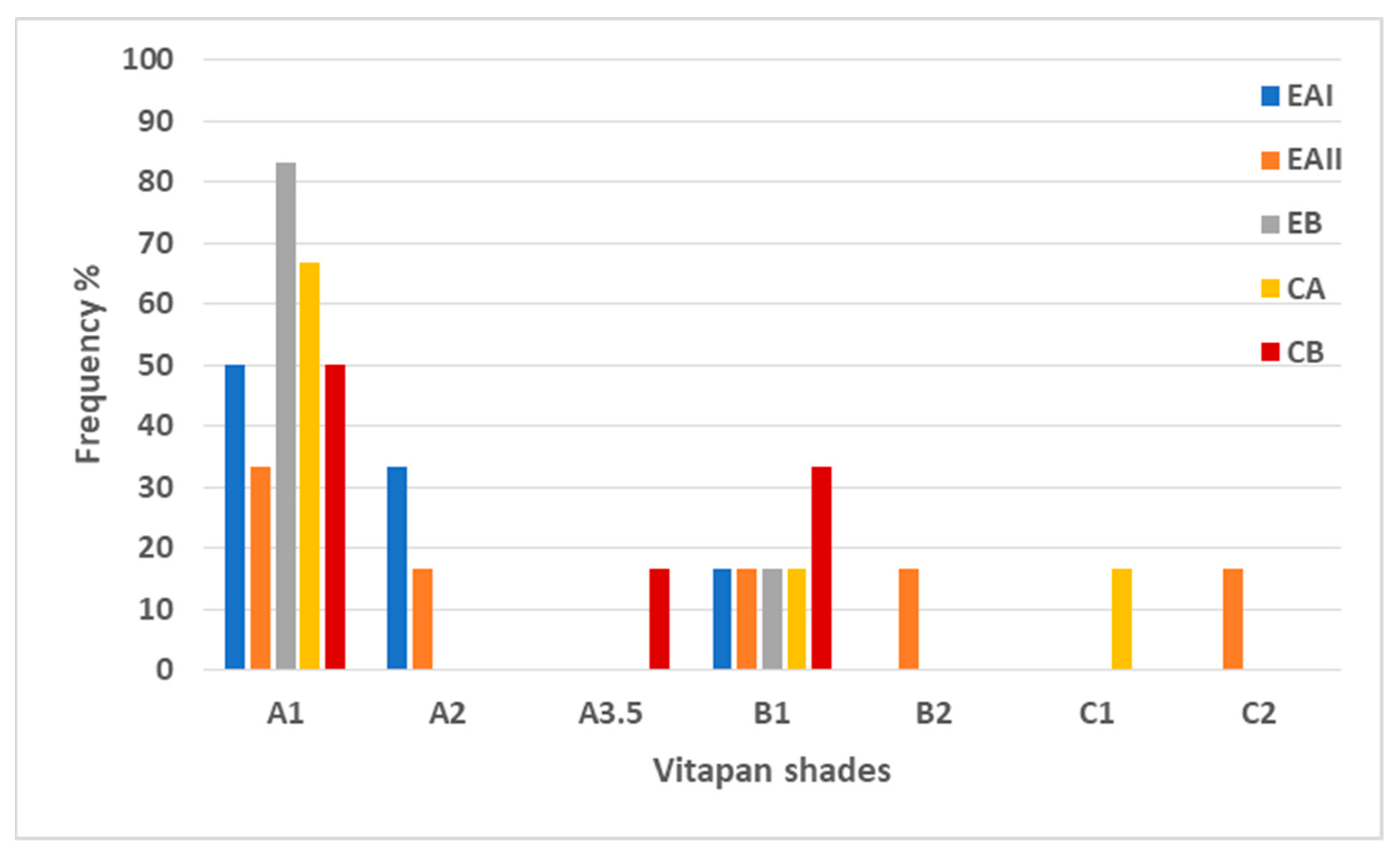
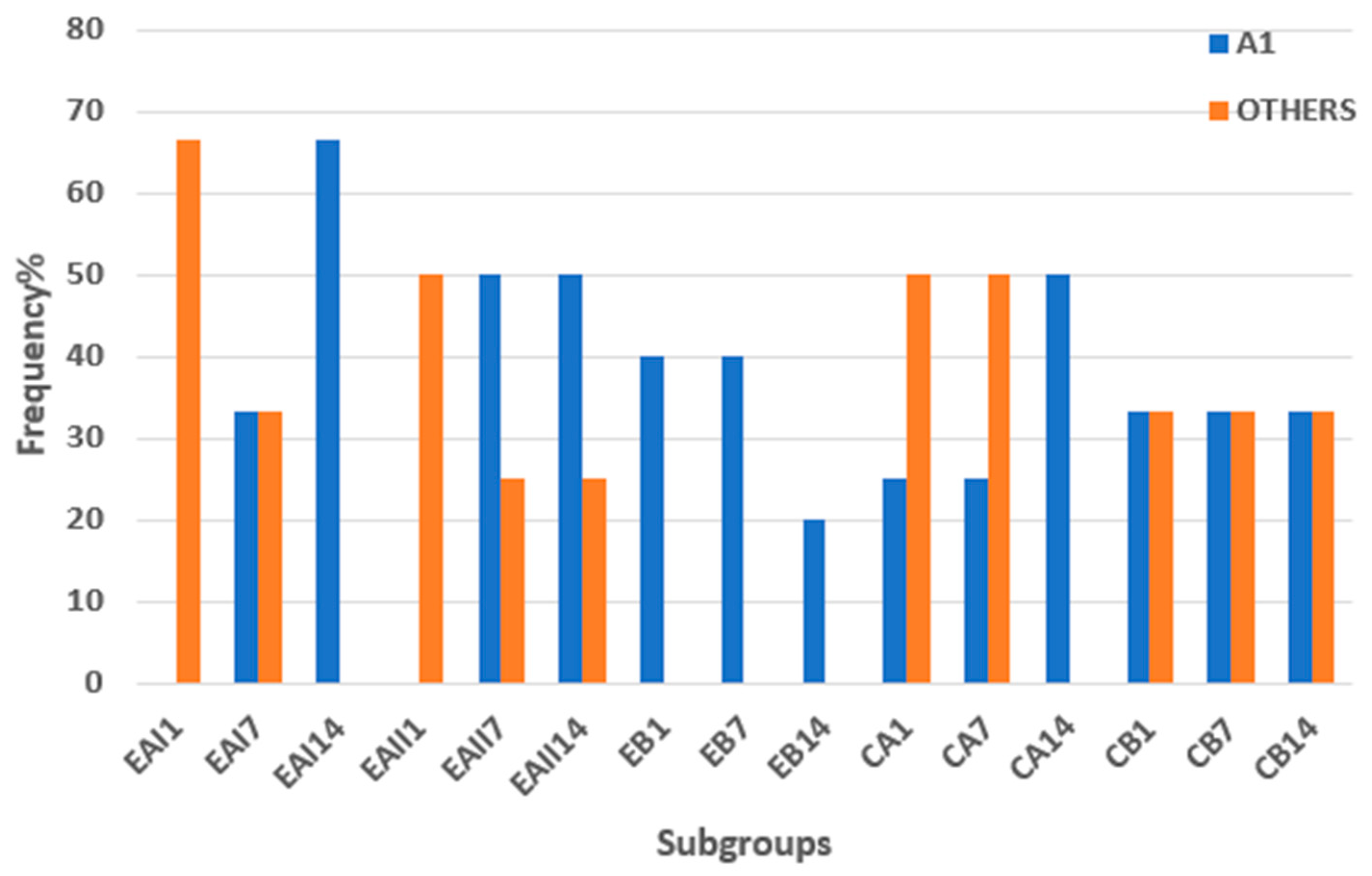
3.3.1. Shade Matching with Vitapan Shade Guide
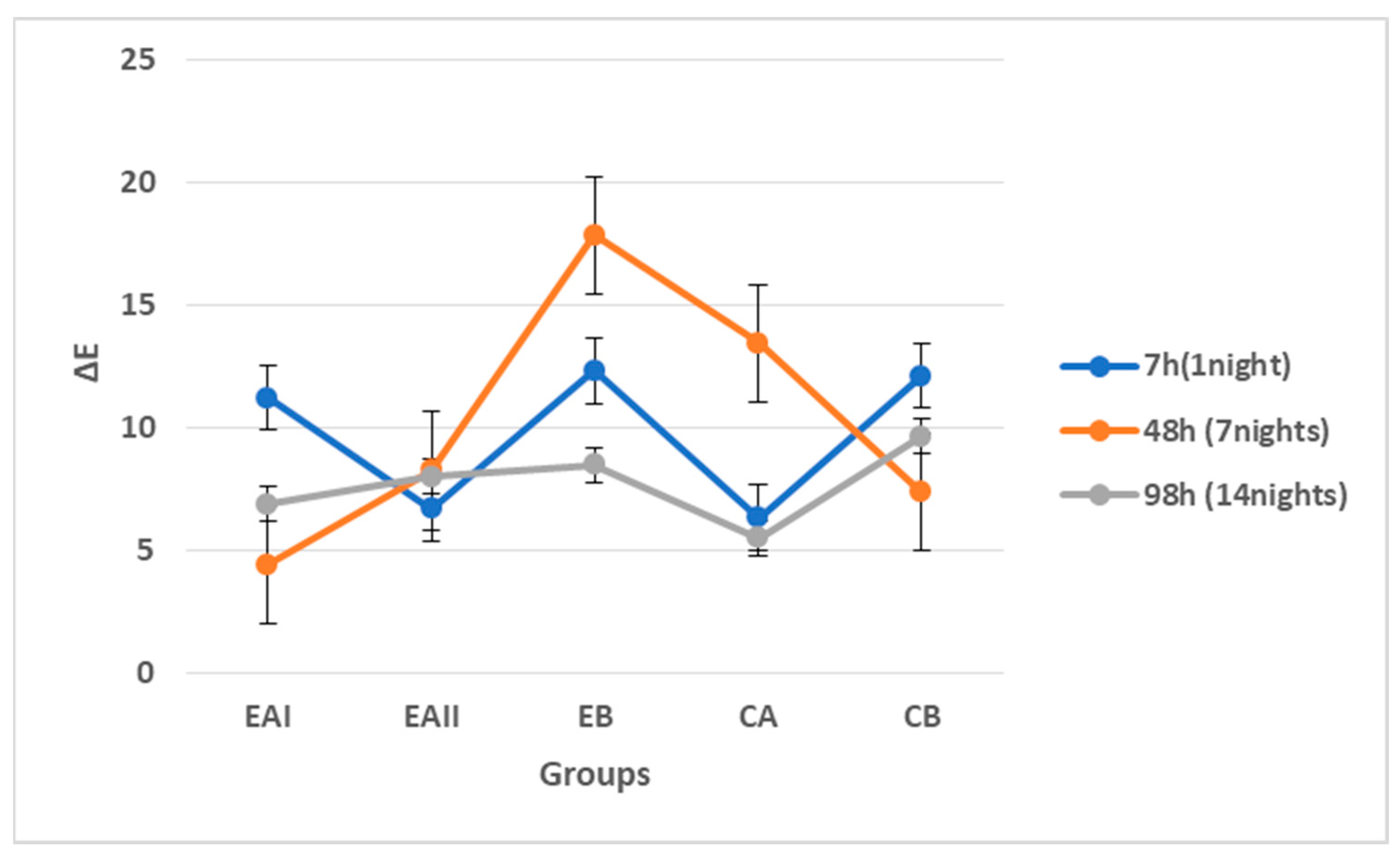
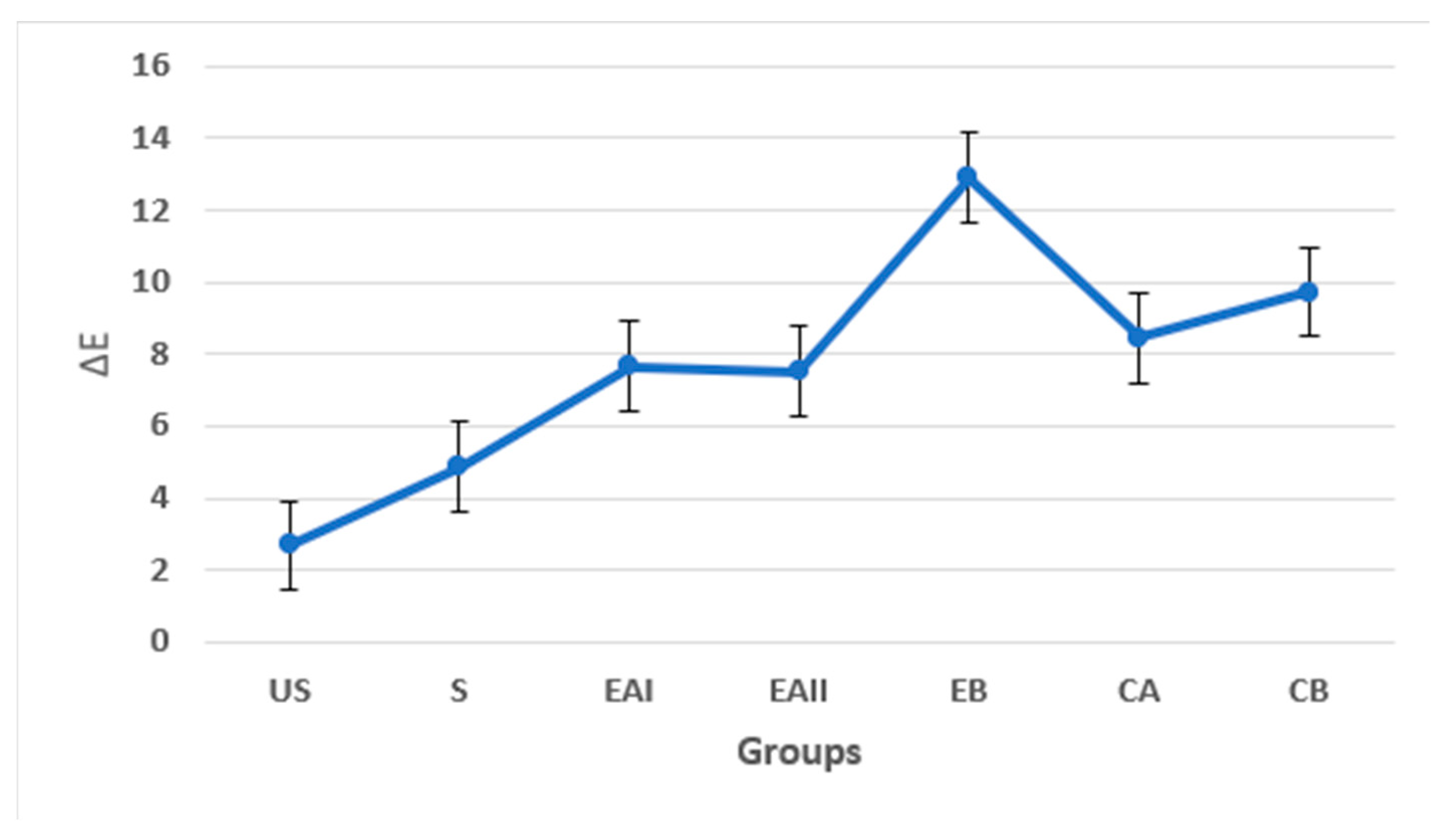
3.3.2. Digital Colorimetry
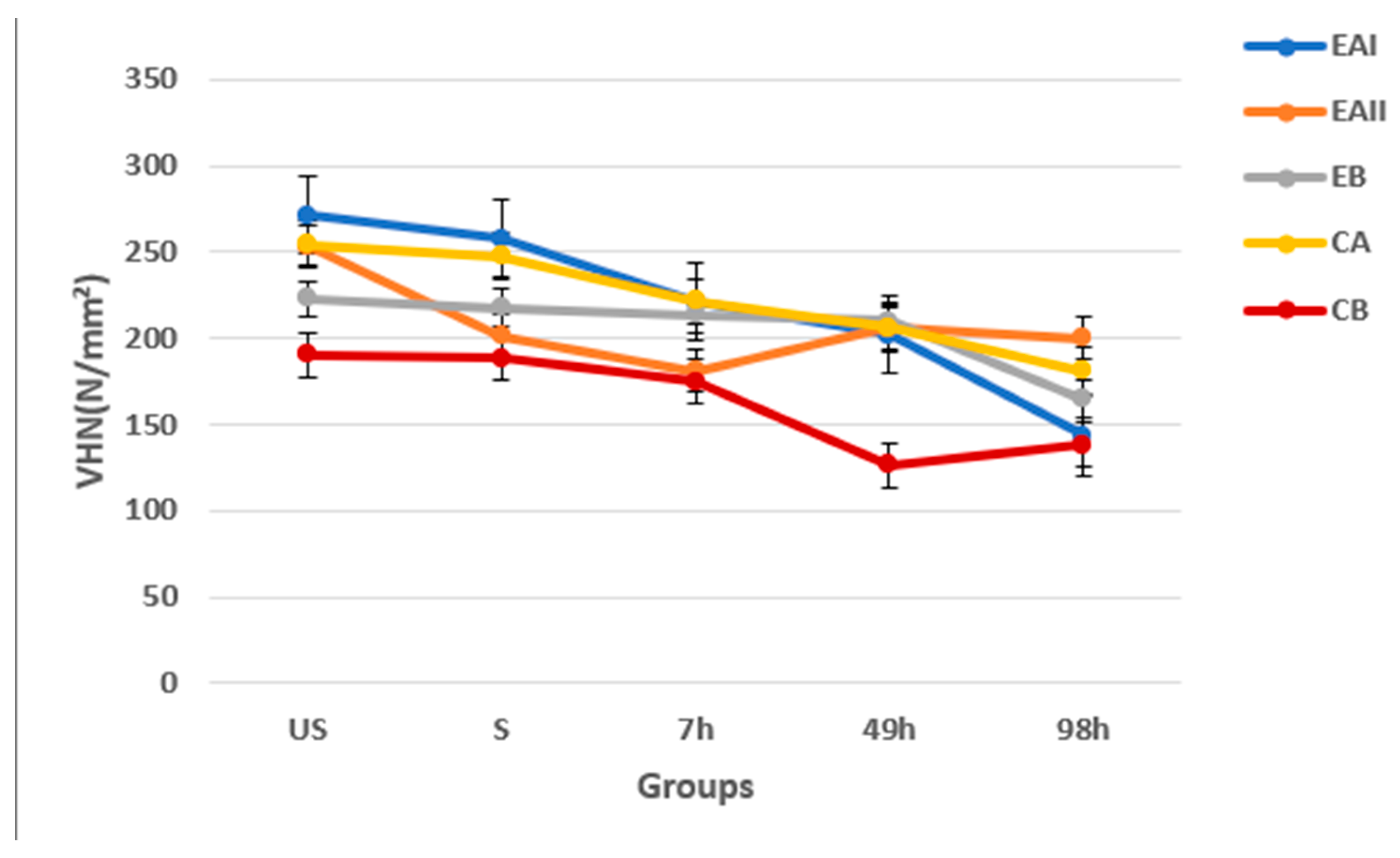
3.4. Microhardness
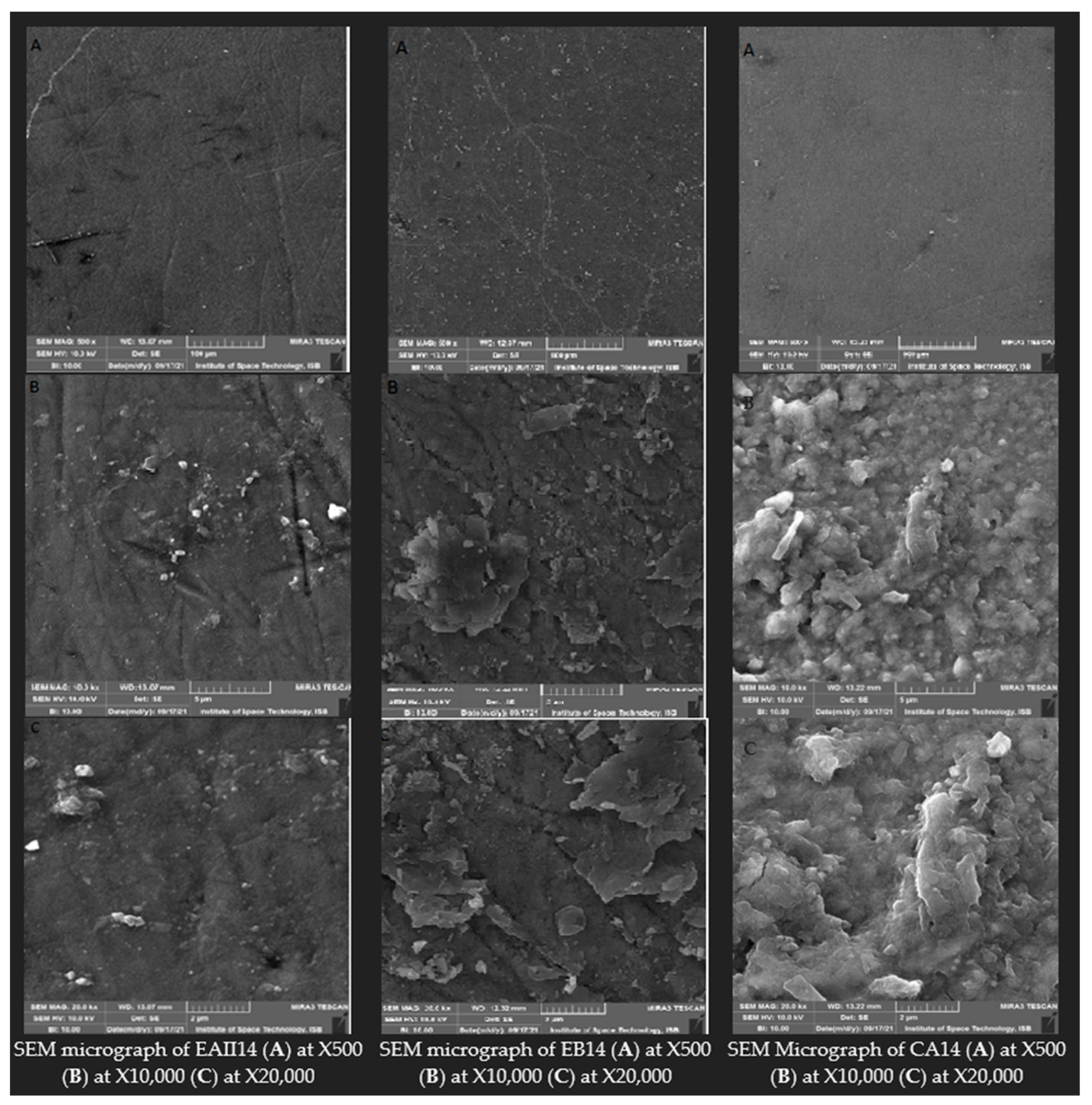
3.5. Scanning Electron Microscopy
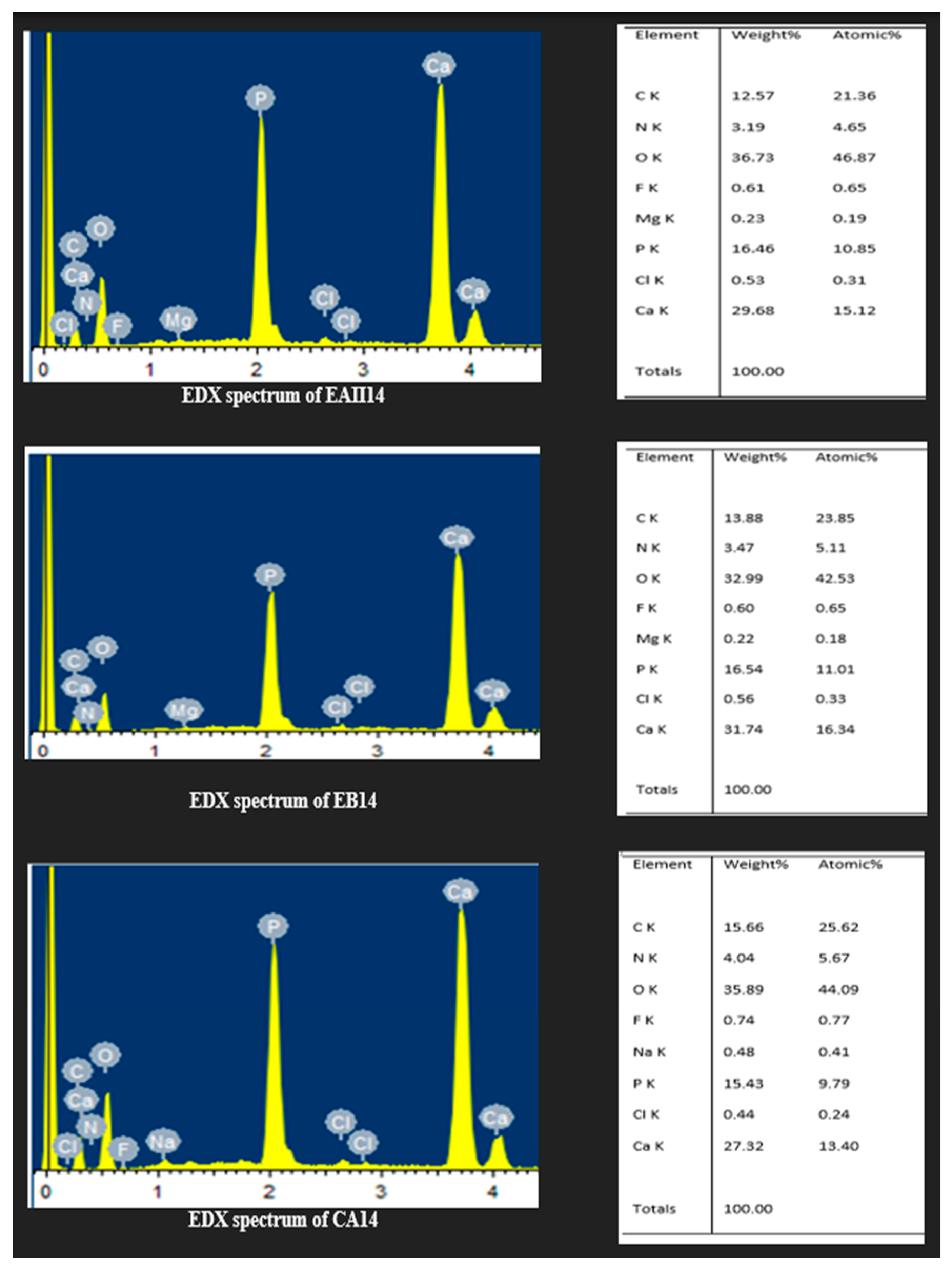
3.6. Energy Dispersive X-ray Spectroscopy
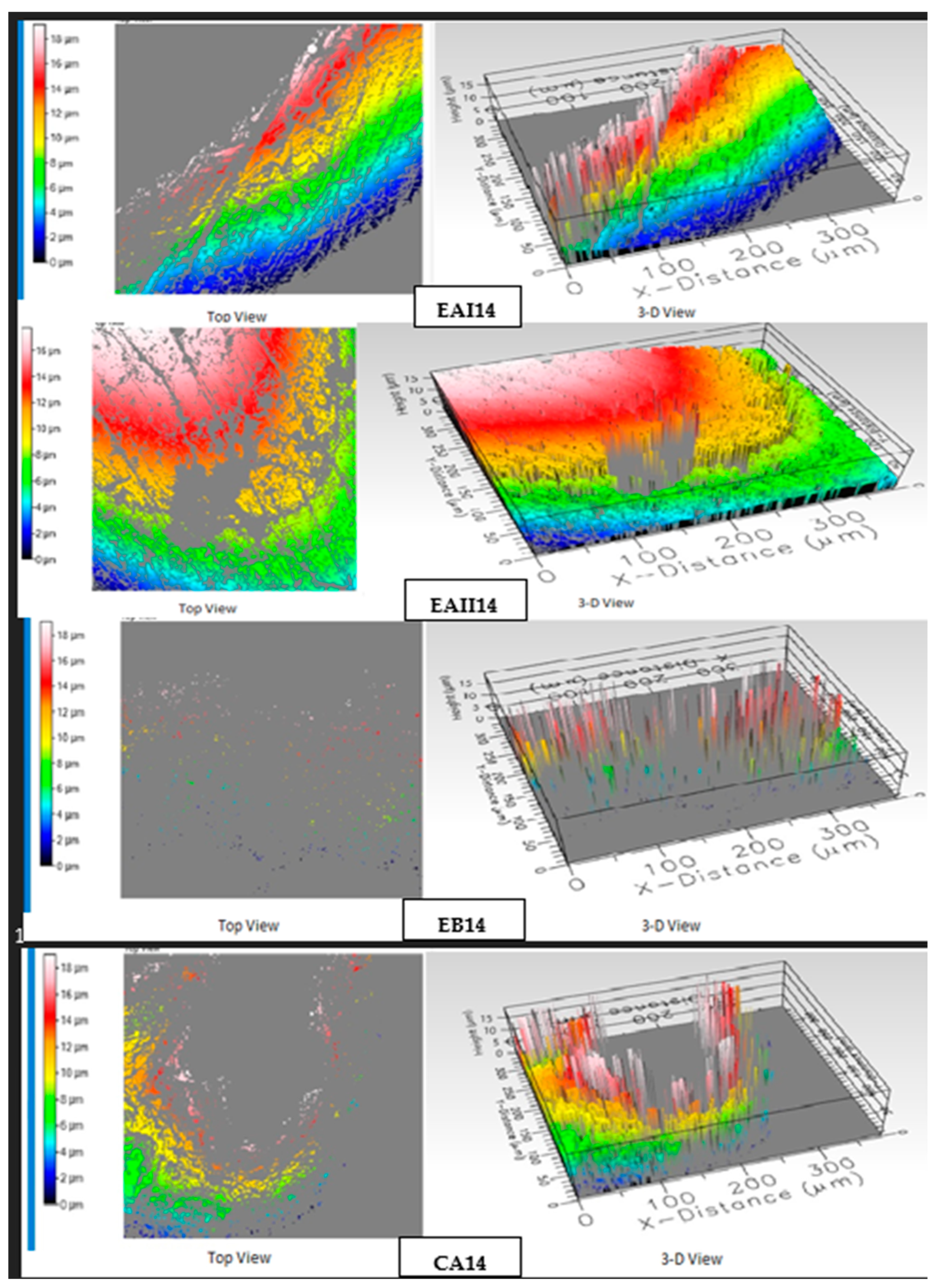
3.7. Optical Profilometry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pindborg, J.J. Pathology of the Dental Hard Tissues; Saunders: Philadelphia, PA, USA, 1970. [Google Scholar]
- de Paula, E.A.; Nava, J.A.; Rosso, C.; Benazzi, C.M.; Fernandes, K.T.; Kossatz, S.; Loguercio, A.D.; Reis, A. In-office bleaching with a two- and seven-day intervals between clinical sessions: A randomized clinical trial on tooth sensitivity. J. Dent. 2015, 43, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Addy, M.; Moran, J. Mechanisms of stain formation on teeth, in particular associated with metal ions and antiseptics. Adv. Dent. Res. 1995, 9, 450–456. [Google Scholar] [CrossRef]
- Rodrigues, J.L.; Rocha, P.S.; Pardim, S.L.d.S.; Machado, A.C.V.; Faria-e-Silva, A.L.; Seraidarian, P.I. Association between in-office and at-home tooth bleaching: A single blind randomized clinical trial. Braz. Dent. J. 2018, 29, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zeng, L.; Min, W.; Tan, L.; Lv, R.; Chen, Y. A bio-safety tooth-whitening composite gels with novel phthalimide peroxy caproic acid. Compos. Commun. 2019, 13, 107–111. [Google Scholar] [CrossRef]
- Kwon, S.R.; Kurti, S.R.; Oyoyo, U.; Li, Y. Effect of various tooth whitening modalities on microhardness, surface roughness and surface morphology of the enamel. Odontology 2015, 103, 274–279. [Google Scholar] [CrossRef]
- Lopes, G.C.; Bonissoni, L.; Baratieri, L.N.; Vieira, L.C.C.; Monteiro Jr, S. Effect of bleaching agents on the hardness and morphology of enamel. J. Esthet. Restor. Dent. 2002, 14, 24–30. [Google Scholar] [CrossRef]
- Akbari, M.; Nejat, A.; Farkhondeh, N.; Mehraban Moghadam, S.; Hashemy, S.; Mohammadipour, H. Does at-home bleaching induce systemic oxidative stress in healthy subjects? Aust. Dent. J. 2017, 62, 58–64. [Google Scholar] [CrossRef]
- Al-Tarakemah, Y.; Darvell, B.W. On the permanence of tooth bleaching. Dent. Mater. 2016, 32, 1281–1288. [Google Scholar] [CrossRef]
- Lin, K.-Y.; Chung, C.-H.; Ciou, J.-S.; Su, P.-F.; Wang, P.-W.; Shieh, D.-B.; Wang, T.-C. Molecular damage and responses of oral keratinocyte to hydrogen peroxide. BMC Oral Health 2019, 19, 10. [Google Scholar] [CrossRef]
- Li, Y. Stain removal and whitening by baking soda dentifrice: A review of literature. J. Am. Dent. Assoc. 2017, 148, S20–S26. [Google Scholar] [CrossRef]
- Shetty, K. Hydrogen peroxide burn of the oral mucosa. Ann. Pharmacother. 2006, 40, 351. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Pallesen, U. Medicine, Tooth bleaching—A critical review of the biological aspects. Crit. Rev. Oral Biol. Med. 2003, 14, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids; ACS Publications: Washington, DC, USA, 2017. [Google Scholar]
- Ahmad, H.; Zaharudin, N.S.; Majid, N.N.A.; Jumbri, K.; Rahman, M.B.A. Synthesis and characterization of new choline-based ionic liquids and their antimicrobial properties. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 54, 124–132. [Google Scholar]
- Herzog, T.; Lemmens, H.P.; Arlt, G.; Raakow, R.; Weimann, A.; Pascher, A.; Knoefel, W.T.; Hesse, U.; Scheithe, K.; Groll, S. Treatment of postoperative ileus with choline citrate—Results of a prospective, randomised, placebo-controlled, double-blind multicentre trial. Int. J. Color. Dis. 2011, 26, 645–652. [Google Scholar] [CrossRef] [PubMed]
- Smithwick, G.; Vacik, J.; Schipper, I. Use of ferric choline citrate in the prevention of iron-deficiency anemia in baby pigs. Am. J. Vet. Res. 1967, 28, 469–474. [Google Scholar]
- Cuppini, M.; Garcia, I.M.; de Souza, V.S.; Zatta, K.C.; Visioli, F.; Leitune, V.C.B.; Guterres, S.S.; Scholten, J.D.; Collares, F.M. Ionic liquid-loaded microcapsules doped into dental resin infiltrants. Bioact. Mater. 2021, 6, 2667–2675. [Google Scholar] [CrossRef]
- Garcia, I.M.; Souza, V.S.; Souza, J.D.; Visioli, F.; Leitune, V.C.B.; Scholten, J.D.; Collares, F.M. Zinc-based particle with ionic liquid as a hybrid filler for dental adhesive resin. J. Dent. 2020, 102, 103477. [Google Scholar] [CrossRef]
- Sandhu, S.V.; Tiwari, R.; Bhullar, R.K.; Bansal, H.; Bhandari, R.; Kakkar, T.; Bhusri, R. Sterilization of extracted human teeth: A comparative analysis. J. Oral Biol. Craniofacial Res. 2012, 2, 170–175. [Google Scholar] [CrossRef]
- Vasques, C.T.; Domenech, S.C.; Severgnini, V.L.; Belmonte, L.A.; Soldi, M.S.; Barreto, P.L.; Soldi, V. Effect of thermal treatment on the stability and structure of maize starch cast films. Starch-Stärke 2007, 59, 161–170. [Google Scholar] [CrossRef]
- Vajekar, S.; Shankarling, G. Highly efficient green synthesis of the photochromic spironaphthoxazines using an eco-friendly choline hydroxide catalyst. Synth. Commun. 2019, 50, 1–10. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Shi, Z.; Gong, X.; Cao, J.; Wang, M.J.E. Facile hydrogen production from Al-water reaction promoted by choline hydroxide. Energy 2017, 131, 98–105. [Google Scholar] [CrossRef]
- Gupta, A.; Pal, A.K.; Woo, E.M.; Katiyar, V. Effects of Amphiphilic Chitosan on Stereocomplexation and Properties of Poly (lactic acid) Nano-biocomposite. Sci. Rep. 2018, 8, 4351. [Google Scholar] [CrossRef] [PubMed]
- Giri, A.; Makhal, A.; Ghosh, B.; Raychaudhuri, A.; Pal, S. Functionalization of manganite nanoparticles and their interaction with biologically relevant small ligands: Picosecond time-resolved FRET studies. Nanoscale 2010, 2, 2704–2709. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.Q. Tooth-bleaching procedures and their controversial effects: A literature review. Saudi Dent. J. 2014, 26, 33–46. [Google Scholar] [CrossRef]
- Beladi-Mousavi, S.S.; Hajibabaei, K.; Tamadon, M.-R.; Rafieian-Kopaei, M. Relationship between free radicals and risk of kidney diseases; the role of antioxidants and their reaction mechanisms. Ann. Res. Antioxid. 2016, 1, 161–170. [Google Scholar]
- Losada-Barreiro, S.; Bravo-Diaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Kothari, S.; Jum’ah, A.A.; Gray, A.R.; Lyons, K.M.; Yap, M.; Brunton, P.A. A randomized clinical trial investigating three vital tooth bleaching protocols and associated efficacy, effectiveness and participants’ satisfaction. J. Dent. 2020, 95, 103322. [Google Scholar] [CrossRef]
- Stephanie, S.; Hayati, A.T.; Sukartini, E. Differences in the tooth whitening effect between strawberry juice and apple juice in-vitro. Padjadjaran J. Dent. 2012, 24, 145–150. [Google Scholar] [CrossRef]
- Aframian, D.; Davidowitz, T.; Benoliel, R. The distribution of oral mucosal pH values in healthy saliva secretors. Oral Dis. 2006, 12, 420–423. [Google Scholar] [CrossRef]
- McGuckin, R.S.; Babin, J.; Meyer, B. Alterations in human enamel surface morphology following vital bleaching. J. Prosthet. Dent. 1992, 68, 754–760. [Google Scholar] [CrossRef]
- Weiger, R.; Kuhn, A.; Löst, C.J. Effect of various types of sodium perborate on the pH of bleaching agents. J. Endod. 1993, 19, 239–241. [Google Scholar] [CrossRef] [PubMed]
- Driessens, F.; Theuns, H.; Borggreven, J.; Van Dijk, J. Solubility behaviour of whole human enamel. Caries Res. 1986, 20, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Titley, K.; Torneck, C.; Ruse, N.J. The effect of carbamide-peroxide gel on the shear bond strength of a microfil resin to bovine enamel. J. Dent. Res. 1992, 71, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Price, R.B.; Sedarous, M.; Hiltz, G.S. The pH of tooth-whitening products. J. Can. Dent. Assoc. 2000, 66, 421–426. [Google Scholar] [PubMed]
- Majeed, A.; Grobler, S.R.; Moola, M.H. The pH of various tooth-whitening products on the South African market: Scientific. S. Afr. Dent. J. 2011, 66, 278–281. [Google Scholar]
- Yu, H.-F.; Huang, K.-C. Effects of pH and citric acid contents on characteristics of ester-derived BaFe12O19 powder. J. Magn. Magn. Mater. 2003, 260, 455–461. [Google Scholar] [CrossRef]
- Abelló, S.; Medina, F.; Rodríguez, X.; Cesteros, Y.; Salagre, P.; Sueiras, J.E.; Tichit, D.; Coq, B. Supported choline hydroxide (ionic liquid) as heterogeneous catalyst for aldol condensation reactions. Chem. Commun. 2004, 9, 1096–1097. [Google Scholar] [CrossRef]
- Bravo-Núñez, Á.; Garzón, R.; Rosell, C.M.; Gómez, M.J.F. Evaluation of starch–protein interactions as a function of pH. Foods 2019, 8, 155. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.V.; Gómez, X.; González-Andrés, F.; Morán, A.J.B. Characterization of different compost extracts using Fourier-transform infrared spectroscopy (FTIR) and thermal analysis. Biodegradation 2008, 19, 815–830. [Google Scholar] [CrossRef]
- Clark, E.B. An analysis of tooth color. J. Am. Dent. Assoc. 1931, 18, 2093–2103. [Google Scholar] [CrossRef]
- Browning, W.D.; Dentistry, R. Use of shade guides for color measurement in tooth-bleaching studies. J. Esthet. Restor. Dent. 2003, 15, S13–S20. [Google Scholar] [CrossRef] [PubMed]
- Eachempati, P.; Nagraj, S.K.; Krishanappa, S.K.K.; Gupta, P.; Yaylali, I.E. Home-based chemically-induced whitening (bleaching) of teeth in adults. Cochrane Database Syst. Rev. 2018, 18, 2093–2103. [Google Scholar] [CrossRef] [PubMed]
- Delgado, E.; Hernández-Cott, P.L.; Stewart, B.; Collins, M.; De Vizio, W. Tooth-whitening efficacy of custom tray-delivered 9% hydrogen peroxide and 20% carbamide peroxide during daytime use: A 14-day clinical trial. Puerto Rico Health Sci. J. 2007, 26, 367–372. [Google Scholar]
- Haeghen, Y.V.; Naeyaert, J.M.; Lemahieu, I.; Philips, W. An imaging system with calibrated color image acquisition for use in dermatology. IEEE Trans. Med. Imaging 2000, 19, 722–730. [Google Scholar] [CrossRef]
- Torres, C.R.G.; Wiegand, A.; Sener, B.; Attin, T.J. Influence of chemical activation of a 35% hydrogen peroxide bleaching gel on its penetration and efficacy—In vitro study. J. Dent. 2010, 38, 838–846. [Google Scholar] [CrossRef]
- Barnes, D.; Kihn, P.; Romberg, E.; George, D.; De Paola, L.; Medina, E. Clinical evaluation of a new 10% carbamide peroxide tooth-whitening agent. Compend. Contin. Educ. Dent. 1998, 19, 968–980. [Google Scholar]
- Batista, G.R.; Barcellos, D.C.; Torres, C.R.; Goto, E.H.; Pucci, C.R.; Borges, A.B. The influence of chemical activation on tooth bleaching using 10% carbamide peroxide. Oper. Dent. 2011, 36, 162–168. [Google Scholar] [CrossRef]
- Ghanbarzadeh, M.; Ahrari, F.; Akbari, M.; Hamzei, H.; Dentistry, E. Microhardness of demineralized enamel following home bleaching and laser-assisted in office bleaching. J. Clin. Exp. Dent. 2015, 7, e405. [Google Scholar] [CrossRef]
- Cavalli, V.; Giannini, M.; Carvalho, R.M. Effect of carbamide peroxide bleaching agents on tensile strength of human enamel. Dent. Mater. 2004, 20, 733–739. [Google Scholar] [CrossRef]
- Fatima, N. In-vitro comparative study of in-office and home bleaching agents on surface micro-morphology of enamel. J. Coll. Physicians Surg. Pak. 2016, 26, 9–12. [Google Scholar]
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Tolidis, K.; Gerasimou, P. Effect of fluoride treatments on bleached enamel microhardness and surface morphology. Oral Health Prev. Dent. 2017, 15, 169–175. [Google Scholar] [PubMed]
- Gutiérrez-Salazar, M.d.P.; Reyes-Gasga, J. Microhardness and chemical composition of human tooth. Mater. Res. 2003, 6, 367–373. [Google Scholar] [CrossRef]
- Zanolla, J.; Marques, A.; da Costa, D.; de Souza, A.; Coutinho, M. Influence of tooth bleaching on dental enamel microhardness: A systematic review and meta-analysis. Aust. Dent. J. 2017, 62, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, O.; Hellwig, E.; Hahn, P. The efficacy of three different in-office bleaching systems and their effect on enamel microhardness. Oper. Dent. 2008, 33, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Attin, T.; Schmidlin, P.R.; Wegehaupt, F.; Wiegand, A. Influence of study design on the impact of bleaching agents on dental enamel microhardness: A review. Dent. Mater. 2009, 25, 143–157. [Google Scholar] [CrossRef]
- Junior, E.P.; Fidel, R.; da Cruz Filho, A.M.; Silva, R.G.; Pécora, J. In vitro action of various carbamide peroxide gel bleaching agents on the microhardness of human enamel. Braz. Dent. J. 1996, 7, 75–79. [Google Scholar]
- Markowitz, K. Pretty painful: Why does tooth bleaching hurt? Med. Hypotheses 2010, 74, 835–840. [Google Scholar] [CrossRef]
- Minoux, M.; Serfaty, R. Vital tooth bleaching: Biologic adverse effects—A review. Quintessence Int. 2008, 39, 645–659. [Google Scholar]
- Sasaki, R.T.; Arcanjo, A.J.; Flório, F.M.; Basting, R.T. Micromorphology and microhardness of enamel after treatment with home-use bleaching agents containing 10% carbamide peroxide and 7.5% hydrogen peroxide. J. Appl. Oral Sci. 2009, 17, 611–616. [Google Scholar] [CrossRef]
- Abouassi, T.; Wolkewitz, M.; Hahn, P. Effect of carbamide peroxide and hydrogen peroxide on enamel surface: An in vitro study. Clin. Oral Investig. 2011, 15, 673–680. [Google Scholar] [CrossRef]
- Cvikl, B.; Lussi, A.; Moritz, A.; Flury, S. Enamel surface changes after exposure to bleaching gels containing carbamide peroxide or hydrogen peroxide. Oper. Dent. 2016, 41, E39–E47. [Google Scholar] [CrossRef] [PubMed]
- Tezel, H.; Ertaş, Ö.S.; Özata, F.; Dalgar, H.; Korkut, Z.O. Effect of bleaching agents on calcium loss from the enamel surface. Quintessence Int. 2007, 38, 339–347. [Google Scholar] [PubMed]
- Rotstein, I.; Dankner, E.; Goldman, A.; Heling, I.; Stabholz, A.; Zalkind, M. Histochemical analysis of dental hard tissues following bleaching. J. Endod. 1996, 22, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Zekonis, R.; Matis, B.A.; Cochran, M.A.; Shetri, S.A.; Eckert, G.J.; Carlson, T. Clinical evaluation of in-office and at-home bleaching treatments. Oper. Dent. 2003, 28, 114–121. [Google Scholar]
- Schaad, P.; Paris, E.; Cuisinier, F.; Voegel, J.-C. Atomic force microscopy study of human tooth enamel surfaces. Scanning Microsc. 1993, 7, 3. [Google Scholar]
- Nogueira, R.D.; Silva, C.B.; Lepri, C.P.; Palma-Dibb, R.G.; Geraldo-Martins, V.R. Evaluation of surface roughness and bacterial adhesion on tooth enamel irradiated with high intensity lasers. Braz. Dent. J. 2017, 28, 24–29. [Google Scholar] [CrossRef]
- Hafez, R.; Ahmed, D.; Yousry, M.; El-Badrawy, W.; El-Mowafy, O.J. Effect of in-office bleaching on color and surface roughness of composite restoratives. Eur. J. Dent. 2010, 4, 118–127. [Google Scholar] [CrossRef]
- Azrak, B.; Callaway, A.; Kurth, P.; Willershausen, B.J.; Dentistry, R. Influence of bleaching agents on surface roughness of sound or eroded dental enamel specimens. J. Esthet. Restor. Dent. 2010, 22, 391–399. [Google Scholar] [CrossRef]
- Moraes, R.; Marimon, J.; Schneider, L.; Sobrinho, L.C.; Camacho, G.; Bueno, M. Carbamide peroxide bleaching agents: Effects on surface roughness of enamel, composite and porcelain. Clin. Oral Investig. 2006, 10, 23–28. [Google Scholar] [CrossRef]
| Groups | Description | Subgroups (n = 3) | Treatment Duration |
|---|---|---|---|
| Experimental groups (E) | |||
| EAI | 22% Choline Hydroxide gel | EAI1 | 7 h (1 night) |
| EAI7 | 49 h (7 nights) | ||
| EAI14 | 98 h (14 nights) | ||
| EAII | 44% Choline Hydroxide gel | EAII1 | 7 h (1 night) |
| EAII7 | 49 h (7 nights) | ||
| EAII14 | 98 h (14 nights) | ||
| EB | Choline Citrate gel | EB1 | 7 h (1 night) |
| EB7 | 49 h (7 nights) | ||
| EB14 | 98 h (14 nights) | ||
| Control groups (C) | |||
| CA | Commercial at-home carbamide peroxide gel (positive control) | CA1 | 7 h (1 night) |
| CA7 | 49 h (7 nights) | ||
| CA14 | 98 h (14 nights) | ||
| CB | Deionized water (negative control) | CB1 | 7 h (1 night) |
| CB7 | 49 h (7 nights) | ||
| CB14 | 98 h (14 nights) | ||
| Samples | Mean pH & SD |
|---|---|
| Parent ionic liquid | |
| 44% choline hydroxide Liquid | 12.4 ± 0.15 |
| Gels | |
| 22% choline hydroxide gel | 8.43 ± 0.51 |
| 44% choline hydroxide gel | 11.2 ± 0.1 |
| Choline citrate gel | 5.86 ± 0. 05 |
| 16% at-home carbamide peroxide gel | 5.53 ± 0.20 |
| Mean pH | 8.70 ± 2.63 |
| Groups | Description | Mean ΔE and SD |
|---|---|---|
| Experimental groups (E) | ||
| EAI | 22% choline hydroxide gel | 7.527 ± 5.66 |
| EAII | 44% choline hydroxide gel | 7.663 ± 1.84 |
| EB | Choline citrate gel | 12.908 ± 6.67 |
| Control groups (C) | ||
| CA | Commercial at-home carbamide peroxide gel (Positive control) | 8.445 ± 5.20 |
| CB | Deionized water (Negative control) | 9.722 ± 3.19 |
| Groups | Description | Mean VHN (N/mm²) and SD |
|---|---|---|
| Experimental groups (E) | ||
| EAI | 22% choline hydroxide gel | 188.92 ± 42.59 |
| EAII | 44% choline hydroxide gel | 195.77 ± 52.58 |
| EB | Choline citrate gel | 196.29 ± 54.51 |
| Control groups (C) | ||
| CA | Commercial at-home carbamide peroxide gel (positive control) | 202.92 ± 44.23 |
| CB | Deionized water (negative control) | 161.29 ± 58.45 |
| Subgroups | Description | Roughness Average (µ-in) |
|---|---|---|
| EAI14 | 22% choline hydroxide | 7.84 |
| EAII14 | 44% choline hydroxide | 10.9 |
| EB14 | Choline citrate | 5.91 |
| CA14 | At-home carbamide peroxide | 10.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satti, M.K.; Nayyer, M.; Alshamrani, M.; Kaleem, M.; Salawi, A.; Safhi, A.Y.; Alsalhi, A.; Sabei, F.Y.; Khan, A.S.; Muhammad, N. Synthesis, Characterization, and Investigation of Novel Ionic Liquid-Based Tooth Bleaching Gels: A Step towards Safer and Cost-Effective Cosmetic Dentistry. Molecules 2023, 28, 3131. https://doi.org/10.3390/molecules28073131
Satti MK, Nayyer M, Alshamrani M, Kaleem M, Salawi A, Safhi AY, Alsalhi A, Sabei FY, Khan AS, Muhammad N. Synthesis, Characterization, and Investigation of Novel Ionic Liquid-Based Tooth Bleaching Gels: A Step towards Safer and Cost-Effective Cosmetic Dentistry. Molecules. 2023; 28(7):3131. https://doi.org/10.3390/molecules28073131
Chicago/Turabian StyleSatti, Memuna Kausar, Maleeha Nayyer, Meshal Alshamrani, Muhammad Kaleem, Ahmad Salawi, Awaji Y. Safhi, Abdullah Alsalhi, Fahad Y. Sabei, Abdul Samad Khan, and Nawshad Muhammad. 2023. "Synthesis, Characterization, and Investigation of Novel Ionic Liquid-Based Tooth Bleaching Gels: A Step towards Safer and Cost-Effective Cosmetic Dentistry" Molecules 28, no. 7: 3131. https://doi.org/10.3390/molecules28073131
APA StyleSatti, M. K., Nayyer, M., Alshamrani, M., Kaleem, M., Salawi, A., Safhi, A. Y., Alsalhi, A., Sabei, F. Y., Khan, A. S., & Muhammad, N. (2023). Synthesis, Characterization, and Investigation of Novel Ionic Liquid-Based Tooth Bleaching Gels: A Step towards Safer and Cost-Effective Cosmetic Dentistry. Molecules, 28(7), 3131. https://doi.org/10.3390/molecules28073131








