One-Pot Synthesis and Evaluation of Antioxidative Stress and Anticancer Properties of an Active Chromone Derivative
Abstract
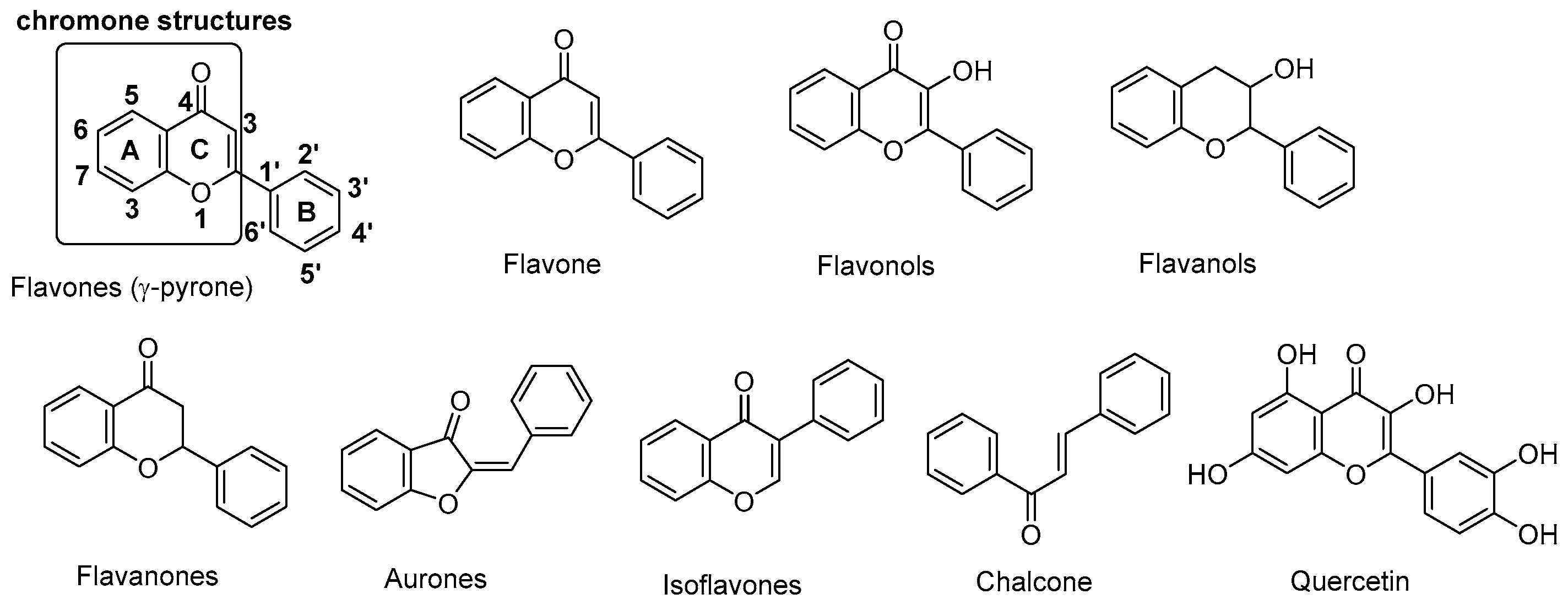
1. Introduction
2. Results and Discussion
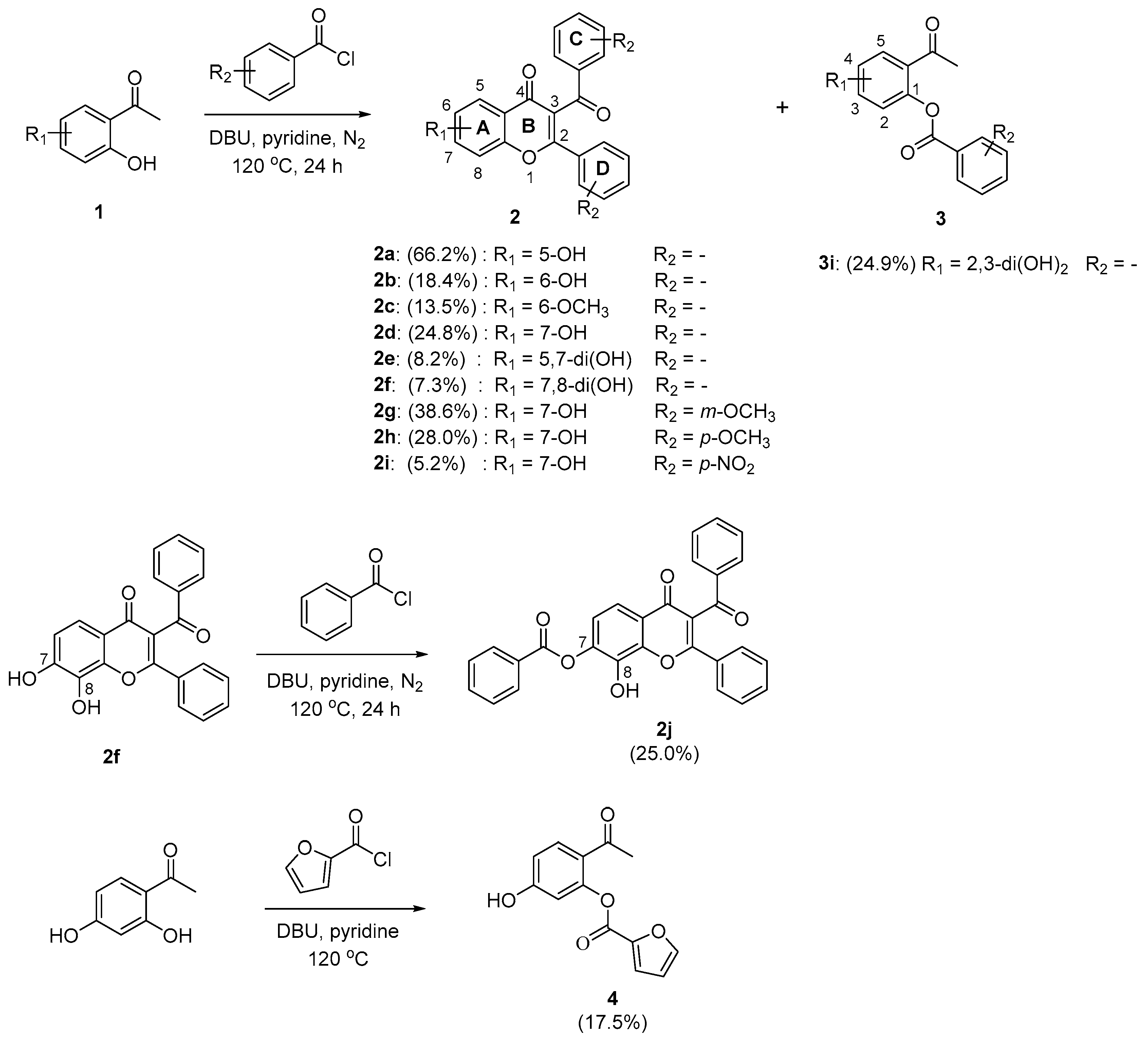
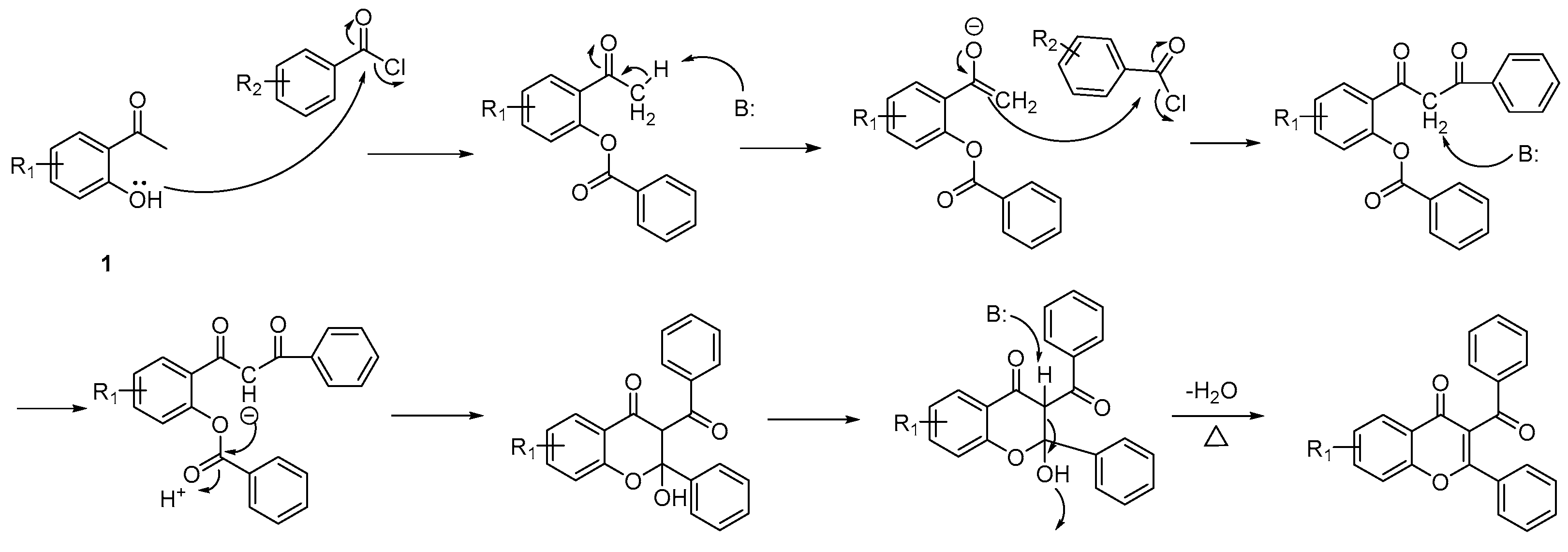
2.1. Synthesis
2.2. Antioxidant Activity
2.3. Cytotoxicity Assay
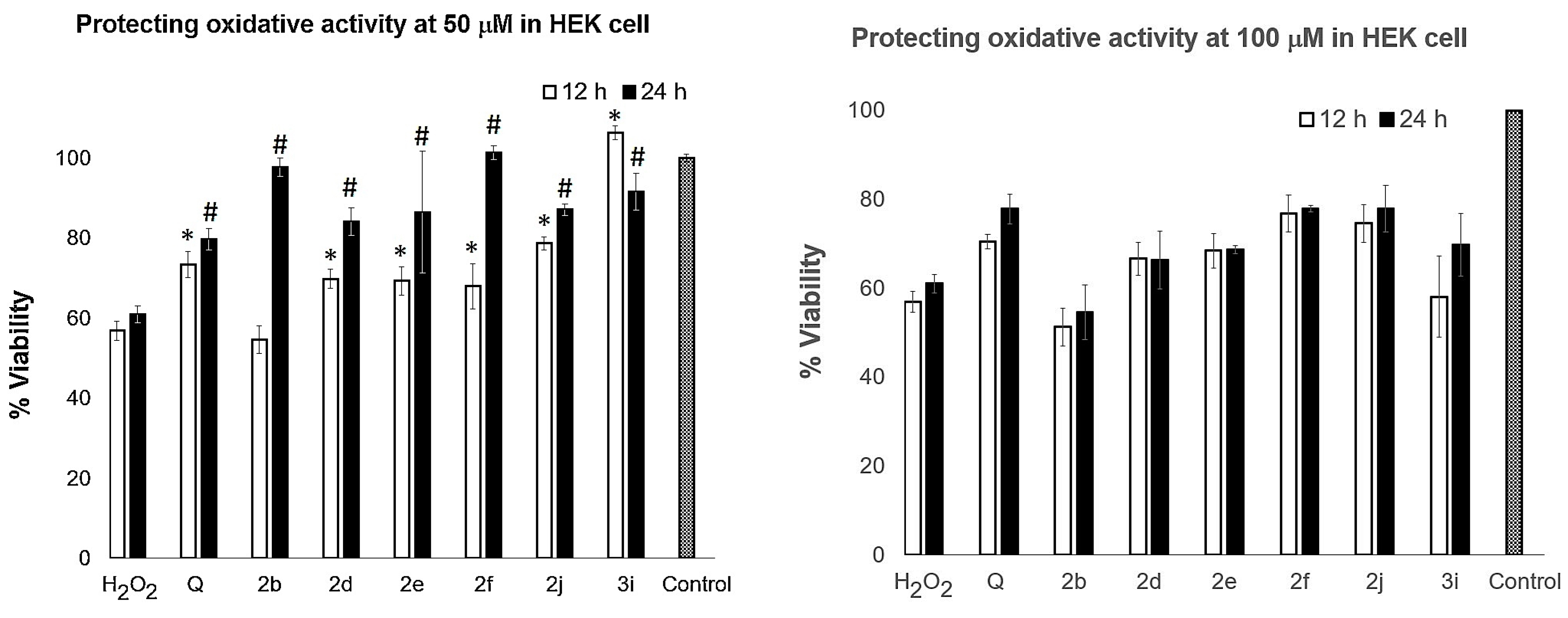
2.4. Antioxidant Activity against H2O2-Induced Cytotoxicity
2.5. Cellular ROS Assay
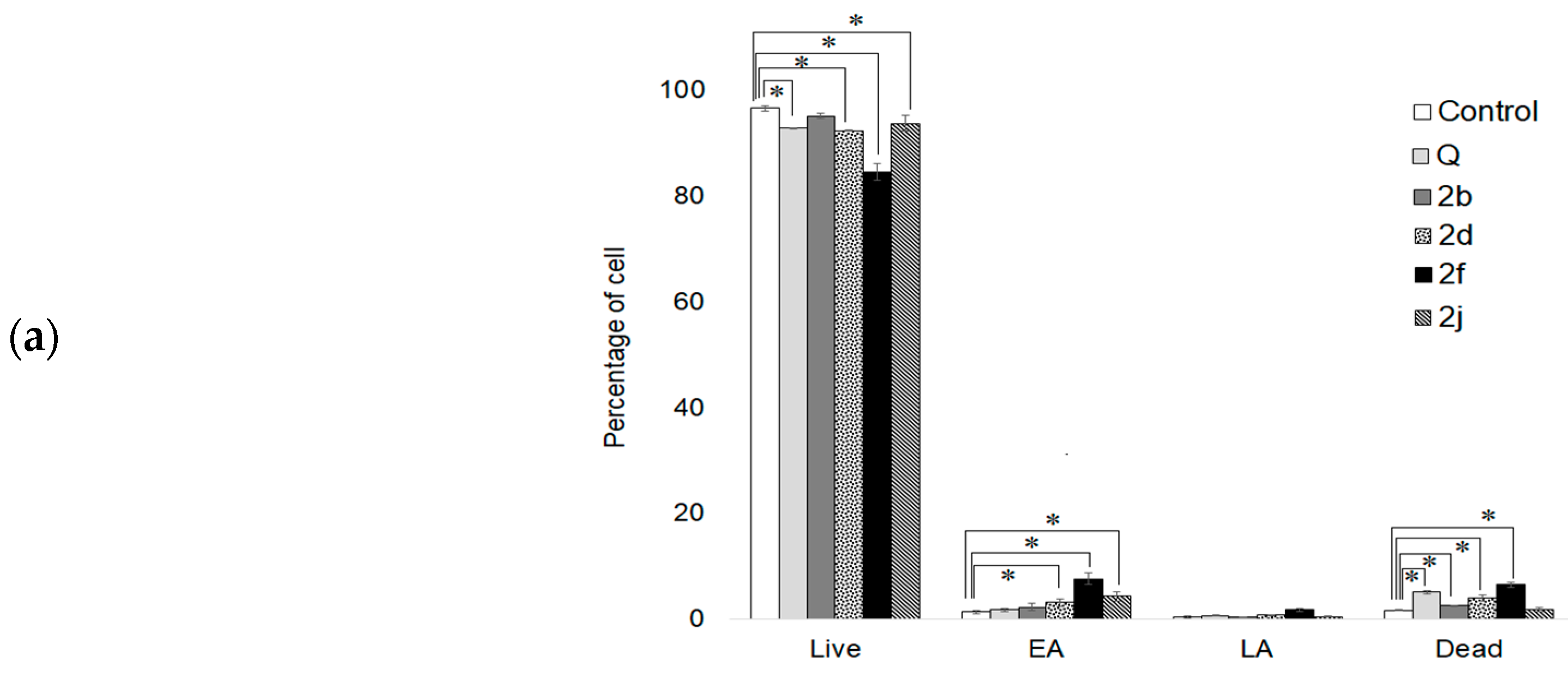
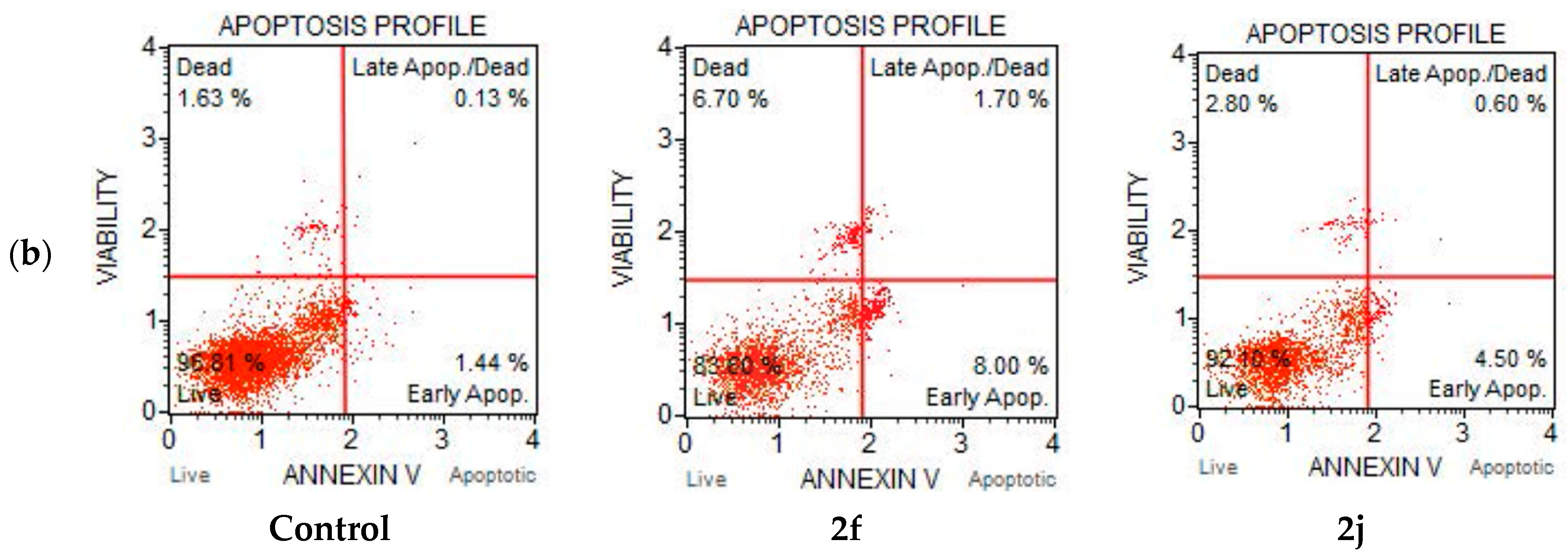
2.6. Apoptosis
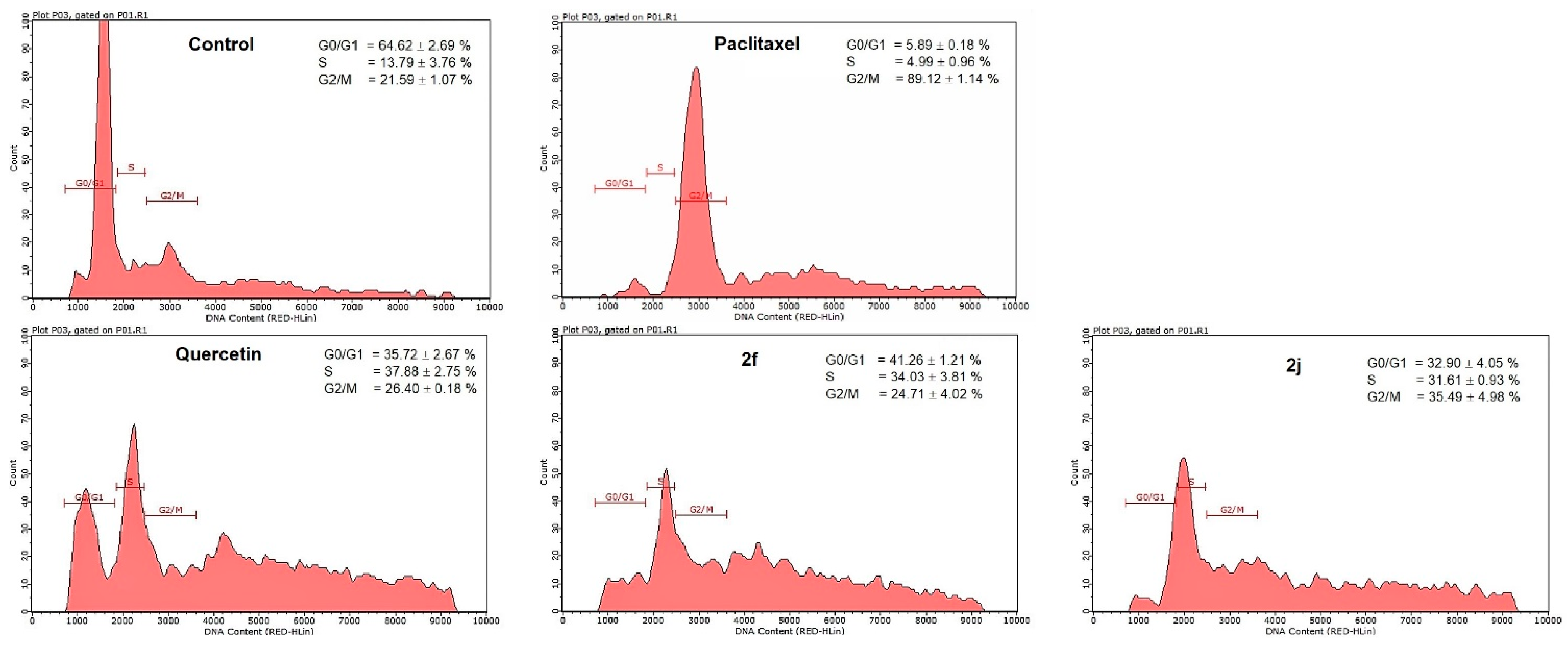
2.7. Cell Cycle Analysis
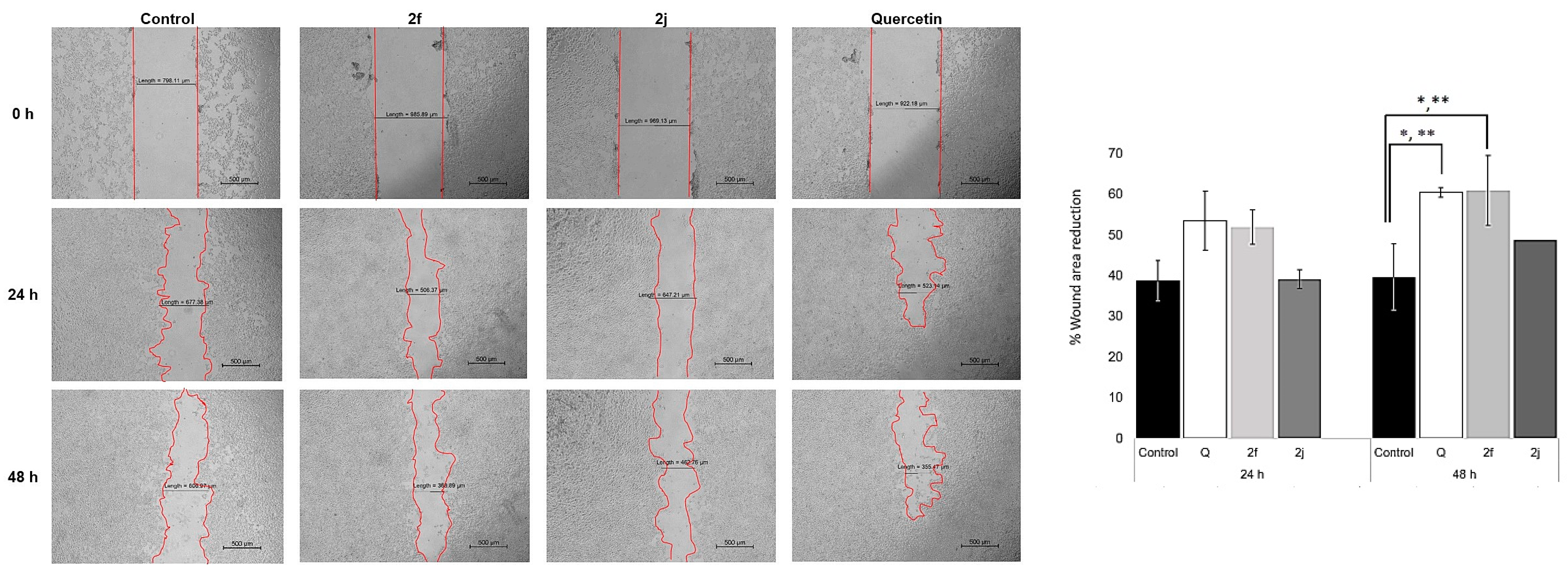
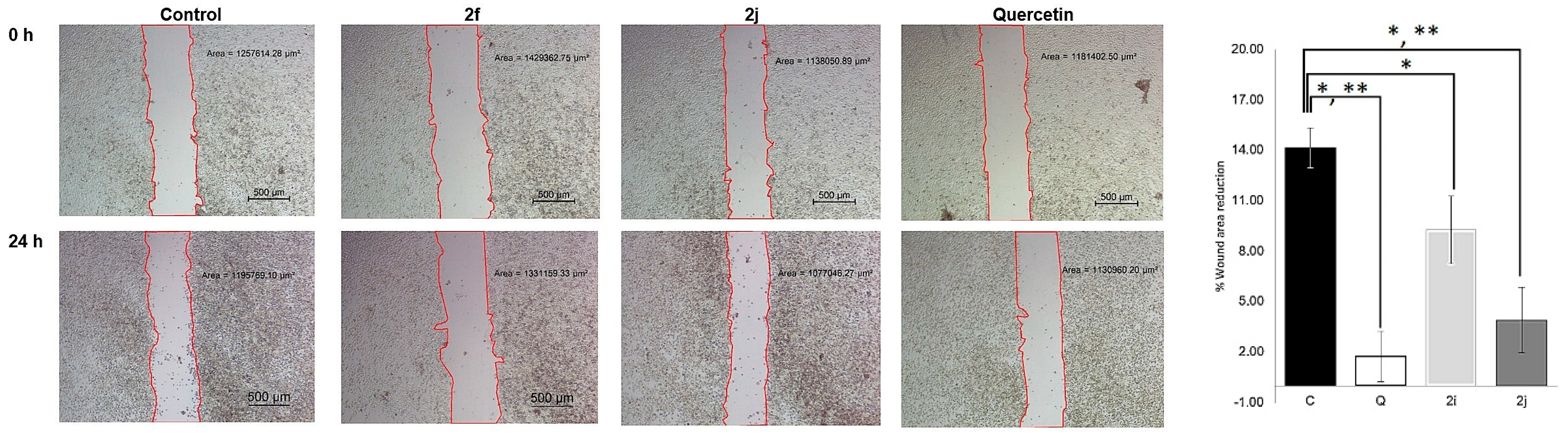
2.8. Cell Migration Assay
3. Materials and Methods
3.1. Apparatus, Chemical and Reagents
3.2. Synthesis
3.2.1. General Procedure for the Synthesis
3.2.2. Characterization of Synthesis Compounds
3.3. Antioxidant Activity
3.3.1. DPPH Assay
3.3.2. FRAP Assay
3.3.3. ABTS Assay
3.4. Cell Culture
3.5. Cytotoxicity Assay
3.6. Cellular ROS Assay
3.7. Apoptosis
3.8. Cell Cycle Analysis
3.9. Cell Migration Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Aminjan, H.H.; Abtahi, S.R.; Hazrati, E.; Chamanara, M.; Jalili, M.; Paknejad, B. Targeting of oxidative stress and inflammation through ROS/NF-kappaB pathway in phosphine-induced hepatotoxicity mitigation. Life Sci. 2019, 232, 116607. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.G.; Mascio, P.D.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef] [PubMed]
- Finkel, T. Oxidant signals and oxidative stress. Curr. Opin. Cell Biol. 2003, 15, 247–254. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef]
- Kaminski, K.A.; Bonda, T.A.; Korecki, J.; Musial, W.J. Oxidative stress and neutrophil activation—The two keystones of ischemia/reperfusion injury. Int. J. Cardiol. 2002, 86, 41–59. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, S.; Wang, L.; Ceylan, A.F.; Ren, J.; Zhang, Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of drp1-mediated maladaptive mitochondrial fission. Pharmacol. Res. 2020, 157, 104846. [Google Scholar] [CrossRef]
- López-Otín, C.; Galluzzi, L.; Freije, J.M.; Madeo, F.; Kroemer, G. Metabolic control of longevity. Cell 2016, 166, 802–821. [Google Scholar] [CrossRef]
- Liguori, I.; Russo, G.; Aran, L.; Bulli, G.; Curcio, F.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Sarcopenia: Assessment of disease burden and strategies to improve outcomes. Clin. Interv. Aging 2018, 13, 913–927. [Google Scholar] [CrossRef]
- Sackesen, C.; Ercan, H.; Dizdar, E.; Soyer, O.; Gumus, P.; Tosun, B.N.; Büyüktuncer, Z.; Karabulut, E.; Besler, T.; Kalayci, O. A comprehensive evaluation of the enzymatic and nonenzymatic antioxidant systems in childhood asthma. J. Allergy Clin. Immunol. 2008, 122, 78–85. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Martorell, M.; Arbiser, J.L.; Sureda, A.; Martins, N.; Maurya, P.K.; Sharifi-Rad, M.; Kumar, P.; Sharifi-Rad, J. Antioxidants: Positive or negative actors? Biomolecules 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Blasiak, J. Role of mitochondria in carcinogenesis. Acta Biochim. Pol. 2014, 61, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Luo, X.; Murthy, M.R.V. Role of oxidative stress in neurodegeneration: Recent developments in assay methods for oxidative stress and nutraceutical antioxidants. Prog. Neuropsychopharmacol. Biol. Psych. 2004, 28, 771–799. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Wenying, R.; Zhenhua, Q.; Hongwei, W.; Lei, Z.; Li, Z. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar]
- Sharifi, R.J.; Hoseini-Alfatemi, S.M.; Sharifi-Rad, M.; Teixeira da Silva, J.A. Antibacterial, antioxidant, antifungal and anti-inflammatory activities of crude extract from Nitraria schoberi fruits. Biotech 2015, 5, 677–684. [Google Scholar] [CrossRef]
- Mead, J.R.; McNair, N. Antiparasitic activity of flavonoids and isoflavones against Cryptosporidium parvum and Encephalitozoon intestinalis. FEMS Microbiol. Lett. 2006, 259, 153–157. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.F.M.; Batista, V.F.; Pinto, D.; Silva, A.M.S. Challenges with chromone as a privileged scaffold in drug discovery. Expert Opin. Drug Discov. 2018, 13, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a Natural Flavonoid Interacts with DNA, Arrests Cell Cycle and Causes Tumor Regression by Activating Mitochondrial Pathway of Apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.K.; Kaur, S.; Mahata, P.K.; Biswas, D.; Pramanik, B.N.; Chan, T.M. Synthesis and properties of 3-acyl-γ-pyrones, a novel class of flavones and chromones. Tetrahedron. Lett. 2005, 46, 4119–4121. [Google Scholar] [CrossRef]
- Maicheen, C.; Jittikoon, J.; Vajragupta, O.; Ungwitayatorn, J. Synthesis, topoisomerase I inhibitory and cytotoxic activities of chromone derivatives. Med. Chem. 2013, 9, 329–339. [Google Scholar] [CrossRef]
- Riva, C.D.; Toma, C.; Donadd, L.; Boi, C.; Pennini, R.; Motta, G.; Leonardi, A. New DBU (1,8-diazabicyclo [5.4.0]undec-7-ene) assisted one-pot synthesis of 2,8-disubstituted 4H-1-benzopyran-4-ones. Synthesis 1997, 2, 195–201. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 4th ed.; Clarendon Press: Oxford, UK, 2006. [Google Scholar]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochim. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Nenadis, N.; Wang, L.F.; Tsimidou, M.; Zhang, H.Y. Estimation of Scavenging Activity of Phenolic Compounds Using the ABTS•+ Assay. J. Agric. Food Chem. 2004, 52, 4669–4674. [Google Scholar] [CrossRef]
- Vidya Priyadarsini, R.; Senthil Murugan, R.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Moore, J.; Yu, L. High-Throughput Relative DPPH Radical Scavenging Capacity Assay. J. Agric. Food Chem. 2006, 54, 7429–7436. [Google Scholar] [CrossRef] [PubMed]
- Re, P.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]

| DPPH517nm | FRAP593nm Value ± SD (TE, µM Trolox/1 µM of Synthesized Compounds) | ABTS734nm | |||
|---|---|---|---|---|---|
| Entry | % SA ± SD at 10 mg/mL | EC50 ± SD (μM) | % Scavenging at 500 µM | EC50 ± SD (μM) | |
| 2a | 2.23 ± 1.99 | - | 0.02 ± 0.004 | 10.93 ± 1.44 | - |
| 2b | 5.32 ± 2.43 | - | 0.02 ± 0.003 | 35.99 ± 1.32 | - |
| 2c | 5.88 ± 2.65 | - | 0.07 ± 0.028 | 13.16 ± 0.78 | - |
| 2d | 15.85 ± 4.61 | >750 | 0.02 ± 0.003 | 27.59 ± 2.46 | - |
| 2e | 46.30 ± 3.61 | >750 | 0.02 ± 0.002 | 80.67 ± 0.30 | 158.27 ± 10.87 |
| 2f | 93.55 ± 3.20 | 34.06 ± 3.28 | 1.70 ± 0.138 | 81.63 ± 1.80 | 10.15 ± 0.70 |
| 2g | 18.32 ± 0.40 | - | 0.02 ± 0.008 | 11.52 ± 3.56 | - |
| 2h | 12.57 ± 6.18 | - | 0.06 ± 0.024 | 6.55 ± 4.19 | - |
| 2i | 13.75 ± 3.31 | - | 0.08 ± 0.006 | 8.52 ± 3.89 | - |
| 2j | 92.88 ± 0.46 | 49.91 ± 10.99 | 0.34 ± 0.078 | >95 | 22.18 ± 0.39 |
| 3i | 90.55 ± 0.36 | 58.98 ± 11.20 | 3.16 ± 0.350 | 78.80 ± 1.14 | 13.89 ± 0.74 |
| 4 | 0.99 ± 0.71 | - | 0.00 ± 0.001 | 14.51 ± 1.94 | - |
| Quercetin | 93.89 ± 2.04 | 18.29 ± 5.62 | 3.35 ± 0.231 | 88.51 ± 0.80 | 11.41 ± 0.17 |
| % Viability ± SD (at 100 μM) | ||||
|---|---|---|---|---|
| HEK293 | HACAT | |||
| Entry | 24 h | 48 h | 24 h | 48 h |
| 2a | 110.88 ± 6.37 | 102.57 ± 6.05 | 63.80 ± 3.36 | 88.54 ± 6.86 |
| 2b | 105.16 ± 2.70 | 72.41 ± 8.55 | 75.71 ± 3.38 | 74.93 ± 1.55 |
| 2c | 72.48 ± 6.46 | 74.03 ± 1.32 | 62.26 ± 3.04 | 60.42 ± 0.73 |
| 2d | 88.65 ± 1.33 | 87.84 ± 1.80 | 77.76 ± 6.93 | 72.51 ± 3.41 |
| 2e | 99.73 ± 2.41 | 93.93 ± 5.34 | 79.70 ± 3.26 | 69.11 ± 3.07 |
| 2f | 105.39 ± 1.19 | 74.68 ± 0.83 | 81.60 ± 8.46 | 77.07 ± 5.03 |
| 2g | 79.07 ± 0.90 | 76.74 ± 1.16 | 81.55 ± 5.17 | 78.39 ± 5.44 |
| 2h | 77.24 ± 1.93 | 73.10 ± 0.79 | 75.16 ± 7.39 | 71.76 ± 3.27 |
| 2i | 65.14 ± 1.21 | 41.16 ± 2.11 | 59.76 ± 5.77 | 27.51 ± 1.24 |
| 2j | 96.02 ± 2.62 | 71.60 ± 5.19 | 72.58 ± 5.53 | 58.03 ± 2.63 |
| 3i | 110.06 ± 1.20 | 107.23 ± 4.18 | 104.81 ± 0.88 | 84.87 ± 3.05 |
| 4 | 97.99 ± 0.40 | 108.38 ± 2.84 | 85.70 ± 5.60 | 72.17 ± 1.81 |
| Quercetin | 102.45 ± 1.58 | 86.62 ± 4.66 | 85.57 ± 125 | 76.82 ± 2.32 |
| Entry | % Viability ± SD (at 100 μM) | ||
|---|---|---|---|
| 24 h | 48 h | IC50 (μM, at 24 h) | |
| 2a | 91.89 ± 2.06 | 58.75 ± 2.02 | ND |
| 2b | 40.90± 0.41 | 24.82 ± 1.05 | 95.69 ± 1.16 |
| 2c | 60.67 ± 0.57 | 82.12 ± 0.11 | ND |
| 2d | 85.17 ± 0.79 | 42.36 ± 0.09 | 166.94 ± 0.49 |
| 2e | 75.95 ± 1.52 | 26.46 ± 1.13 | 107.60 ± 4.04 |
| 2f | 109.27 ± 0.92 | 29.16 ± 0.40 | 190.90 ± 6.01 |
| 2g | 47.98. ± 3.10 | 46.28 ± 1.14 | 172.73 ± 1.66 |
| 2h | 84.86± 2.55 | 64.25 ± 0.76 | ND |
| 2i | 38.71 ± 5.22 | 12.51 ± 1.45 | 34.93 ± 2.41 |
| 2j | 65.15 ± 1.42 | 30.01 ± 1.23 | 101.00 ± 3.18 |
| 3i | 99.88 ± 4.19 | 37.13 ± 1.96 | ND |
| 4 | 86.14 ± 3.24 | 91.07 ± 0.65 | ND |
| Quercetin | 69.13 ± 1.93 | 19.21 ± 0.25 | 192.43 ± 1.27 |
| Compounds | G0/G1 (%) | S (%) | G2/M (%) |
|---|---|---|---|
| Paclitaxel | 5.89 ± 0.18 | 4.99 ± 0.96 | 89.12 ± 1.14 * |
| Quercetin | 35.72 ± 2.67 | 37.88 ± 2.75 * | 26.40 ± 0.18 |
| 2f | 41.26 ± 1.21 | 34.03 ± 3.81 * | 24.71 ± 4.02 |
| 2j | 32.90 ± 4.05 | 31.61 ± 0.93 * | 35.49 ± 4.98 |
| Untreated control | 64.62 ± 2.69 | 13.79 ± 3.76 | 21.59 ± 1.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maicheen, C.; Churnthammakarn, C.; Pongsroypech, N.; Khamkhenshorngphanuch, T.; Ungwitayatorn, J.; Rungsardthong, K.; Asasutjarit, R.; Theeramunkong, S. One-Pot Synthesis and Evaluation of Antioxidative Stress and Anticancer Properties of an Active Chromone Derivative. Molecules 2023, 28, 3129. https://doi.org/10.3390/molecules28073129
Maicheen C, Churnthammakarn C, Pongsroypech N, Khamkhenshorngphanuch T, Ungwitayatorn J, Rungsardthong K, Asasutjarit R, Theeramunkong S. One-Pot Synthesis and Evaluation of Antioxidative Stress and Anticancer Properties of an Active Chromone Derivative. Molecules. 2023; 28(7):3129. https://doi.org/10.3390/molecules28073129
Chicago/Turabian StyleMaicheen, Chirattikan, Chokchaloemwat Churnthammakarn, Nichapat Pongsroypech, Thitiphong Khamkhenshorngphanuch, Jiraporn Ungwitayatorn, Kanin Rungsardthong, Rathapon Asasutjarit, and Sewan Theeramunkong. 2023. "One-Pot Synthesis and Evaluation of Antioxidative Stress and Anticancer Properties of an Active Chromone Derivative" Molecules 28, no. 7: 3129. https://doi.org/10.3390/molecules28073129
APA StyleMaicheen, C., Churnthammakarn, C., Pongsroypech, N., Khamkhenshorngphanuch, T., Ungwitayatorn, J., Rungsardthong, K., Asasutjarit, R., & Theeramunkong, S. (2023). One-Pot Synthesis and Evaluation of Antioxidative Stress and Anticancer Properties of an Active Chromone Derivative. Molecules, 28(7), 3129. https://doi.org/10.3390/molecules28073129







