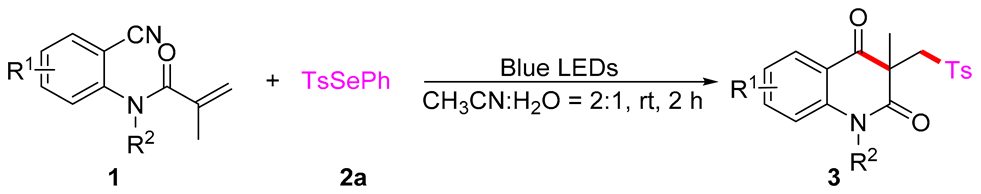
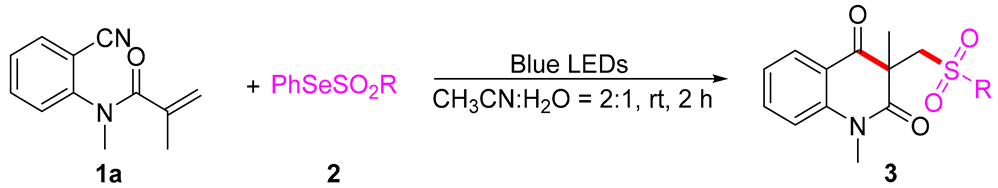3.2. The General Procedure for the Synthesis of 3
In a reaction tube, acrylamides 1 (0.5 mmol) and PhSeSO2R 2 (1.2 equiv, 0.6 mmol) were mixed in CH3CN/H2O (2:1, 3 mL) and irradiated for 2 h until complete consumption of starting material, as monitored by TLC analysis. After the completion of the reaction, the mixture was quenched by NaHCO3 (sat. aq. 10 mL) and extracted with CH2Cl2 (3 × 10 mL). Then the organic solvent was concentrated in vacuo. The residue was purified by flash column chromatography with ethyl acetate and petroleum ether as eluent to product 3.
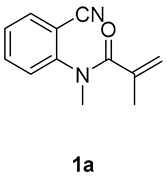
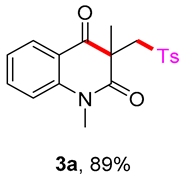
1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3a). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.25), white solid (95 mg, 89%): mp: 161–162 °C. 1H-NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 7.7, 1.5 Hz, 1H), 7.84–7.61 (m, 3H), 7.41–7.18 (m, 4H), 4.22 (s, 2H), 3.54 (s, 3H), 2.43 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.28, 171.69, 144.52, 143.15, 138.64, 136.50, 129.63, 128.66, 127.83, 123.29, 119.23, 115.16, 62.40, 55.25, 30.14, 25.91, 21.65. HRMS (ESI) calculated for C19H20NO4S [M+H]+: 358.1108, found: 358.1103.
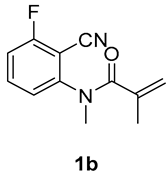
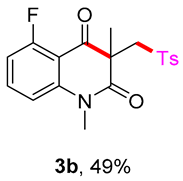
5-fluoro-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3b). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (52 mg, 49%): mp: 206–207 °C. 1H-NMR (500 MHz, CDCl3) δ 7.74 (d, J = 8.3 Hz, 2H), 7.61 (td, J = 8.4, 5.8 Hz, 1H), 7.33 (d, J = 8.1 Hz, 2H), 7.05 (d, J = 8.5 Hz, 1H), 6.91 (dd, J = 9.9, 8.7 Hz, 1H), 4.19 (d, J = 17.0 Hz, 2H), 3.55 (s, 3H), 2.44 (s, 3H), 1.43 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 191.50, 171.31, 162.74 (d, J = 267.2 Hz), 144.53, 144.29 (d, J = 2.8 Hz), 138.79, 136.69 (d, J = 11.9 Hz), 129.68, 127.83, 111.46 (d, J = 21.3 Hz), 111.02 (d, J = 3.5 Hz), 109.09 (d, J = 9.0 Hz), 61.93, 56.31, 30.97, 25.43, 21.65. 19F-NMR (471 MHz, CDCl3) δ -109.25. HRMS (ESI) calculated for C19H19FNO4S [M+H]+: 376.1013, found: 376.1009.
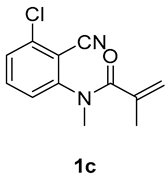
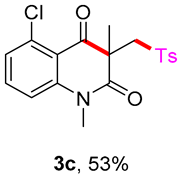
5-chloro-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3c). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.30), white solid (62 mg, 53%): mp: 182–183 °C. 1H-NMR (500 MHz, CDCl3) δ 7.79–7.67 (m, 2H), 7.46–7.41 (m, 1H), 7.32–7.25 (m, 2H), 7.20–7.17 (m, 1H), 7.12 (t, J = 9.1 Hz, 1H), 4.11 (d, J = 7.7 Hz, 2H), 3.49 (s, 3H), 2.37 (s, 3H), 1.34 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 190.97, 169.97, 143.88, 143.43, 138.00, 135.40, 133.79, 128.61, 126.86, 125.75, 116.13, 113.02, 60.77, 55.72, 30.06, 23.67, 20.62. HRMS (ESI) calculated for C19H19ClNO4S [M+H]+: 392.0718, found: 392.0710.
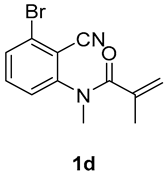
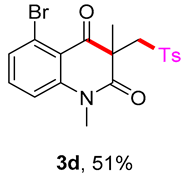
5-bromo-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3d). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.30), white solid (67 mg, 51%): mp: 140–141 °C. 1H-NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.2 Hz, 2H), 7.48–7.40 (m, 2H), 7.34 (d, J = 8.1 Hz, 2H), 7.25–7.22 (m, 1H), 4.19 (d, J = 4.7 Hz, 2H), 3.55 (s, 3H), 2.44 (s, 3H), 1.40 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 192.19, 170.87, 145.01, 144.47, 139.04, 135.07, 130.43, 129.84, 129.65, 127.88, 123.89, 114.84, 61.80, 56.52, 31.03, 24.57, 21.66. HRMS (ESI) calculated for C19H19BrNO4S [M+H]+: 436.0213, found: 436.0200.
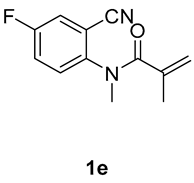
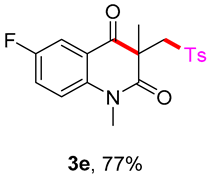
5-fluoro-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3e). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (87 mg, 77%): mp: 135–136 °C. 1H-NMR (500 MHz, CDCl3) δ 7.69 (dd, J = 8.0, 3.1 Hz, 1H), 7.62 (d, J = 8.2 Hz, 2H), 7.31 (ddd, J = 9.2, 7.5, 3.1 Hz, 1H), 7.25 (d, J = 8.1 Hz, 2H), 7.15 (dd, J = 9.1, 4.0 Hz, 1H), 4.14 (d, J = 5.7 Hz, 2H), 3.46 (s, 3H), 2.36 (s, 3H), 1.34 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.60 (d, J = 1.6 Hz), 171.27, 158.58 (d, J = 245.4 Hz), 144.65, 139.61 (d, J = 1.9 Hz), 138.50, 129.69, 127.80, 123.49 (d, J = 23.3 Hz), 120.40 (d, J = 6.4 Hz), 117.06 (d, J = 7.2 Hz), 114.28 (d, J = 23.4 Hz), 62.54, 55.05, 30.41, 25.78, 21.65. 19F-NMR (471 MHz, CDCl3) δ −119.37. HRMS (ESI) calculated for C19H19FNO4S [M+H]+: 376.1013, found: 376.1009.
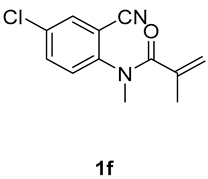
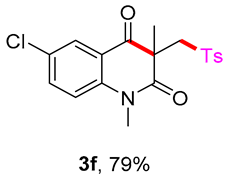
6-chloro-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3f). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (93 mg, 79%): mp: 139–140 °C. 1H-NMR (500 MHz, CDCl3) δ 7.76 (d, J = 8.2 Hz, 2H), 7.49 (d, J = 7.8 Hz, 1H), 7.43 (d, J = 8.2 Hz, 1H), 7.34 (d, J = 8.1 Hz, 2H), 7.23 (d, J = 8.3 Hz, 1H), 4.19 (d, J = 4.8 Hz, 2H), 3.56 (s, 3H), 2.44 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.25, 171.76, 144.67, 142.85, 138.42, 130.05, 129.70, 127.77, 126.41, 123.59, 117.60, 115.53, 62.42, 55.21, 30.29, 25.82, 21.66. HRMS (ESI) calculated for C19H19ClNO4S [M+H]+: 392.0718, found: 392.0710.
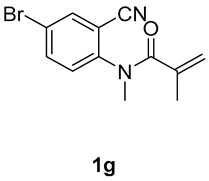
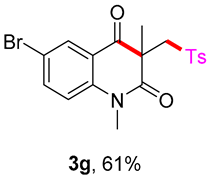
5-bromo-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3g). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.21), white solid (80 mg, 61%): mp: 148–149 °C. 1H-NMR (500 MHz, CDCl3) δ 8.00 (dd, J = 43.4, 8.3 Hz, 1H), 7.70 (d, J = 8.2 Hz, 2H), 7.44–7.20 (m, 4H), 4.20 (s, 2H), 3.53 (s, 3H), 2.44 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.22, 171.76, 144.63, 142.85, 138.49, 130.09, 129.69, 127.80, 126.55, 123.59, 118.42, 115.48, 62.51, 55.23, 30.28, 25.84, 21.66. HRMS (ESI) calculated for C19H19BrNO4S [M+H]+: 436.0213, found: 436.0200.
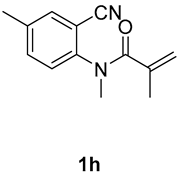
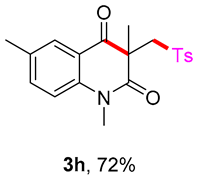
1,3,6-trimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3h). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (80 mg, 72%): mp: 174–175 °C. 1H-NMR (500 MHz, CDCl3) δ 7.89 (d, J = 1.7 Hz, 1H), 7.71 (d, J = 8.3 Hz, 2H), 7.47 (dd, J = 8.4, 2.1 Hz, 1H), 7.31 (d, J = 8.2 Hz, 2H), 7.13 (d, J = 8.5 Hz, 1H), 4.21 (s, 2H), 3.51 (s, 3H), 2.43 (s, 3H), 2.38 (s, 3H), 1.40 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.48, 171.49, 144.45, 141.00, 138.68, 137.31, 133.01, 129.60, 128.53, 127.83, 119.02, 115.17, 62.42, 55.10, 30.09, 25.96, 21.65, 20.35. HRMS (ESI) calculated for C20H22NO4S [M+H]+: 372.1264, found: 372.1261.
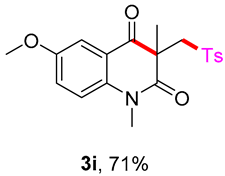
6-methoxy-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3i). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.24), yellow solid (82 mg, 71%): mp: 87–88 °C. 1H-NMR (500 MHz, CDCl3) δ 7.72 (d, J = 8.3 Hz, 2H), 7.57 (d, J = 3.1 Hz, 1H), 7.32 (d, J = 8.0 Hz, 2H), 7.28–7.24 (m, 1H), 7.17 (d, J = 9.0 Hz, 1H), 4.21 (d, J = 2.5 Hz, 2H), 3.87 (s, 3H), 3.52 (s, 3H), 2.44 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.39, 171.18, 155.59, 144.48, 138.63, 137.34, 129.62, 127.85, 124.52, 119.86, 116.70, 110.19, 62.50, 55.83, 54.94, 30.21, 26.05, 21.65. HRMS (ESI) calculated for C20H22NO5S [M+H]+: 383.1213, found: 383.1234.
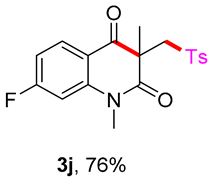
7-fluoro-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3j). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.21), yellow solid (86 mg, 76%): mp: 114–115 °C. 1H-NMR (500 MHz, CDCl3) δ 8.24–8.08 (m, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.2 Hz, 2H), 6.92 (d, J = 9.2 Hz, 2H), 4.20 (s, 2H), 3.52 (s, 3H), 2.44 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 192.79, 171.87, 167.81 (d, J = 256.5 Hz), 145.48 (d, J = 11.8 Hz), 144.62, 138.50, 131.67 (d, J = 11.4 Hz), 129.68, 127.79, 115.88 (d, J = 2.5 Hz), 110.75 (d, J = 22.4 Hz), 102.79 (d, J = 27.6 Hz), 62.44, 55.07, 30.32, 25.91, 21.66. 19F-NMR (471 MHz, CDCl3) δ −98.47. HRMS (ESI) calculated for C19H19FNO4S [M+H]+: 376.1013, found: 376.1008.
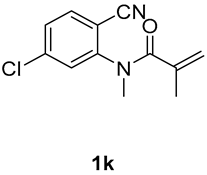
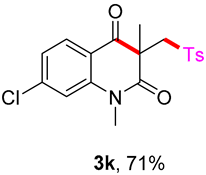
7-chloro-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3k). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), yellow solid (84 mg, 71%): mp: 132–133 °C. 1H-NMR (500 MHz, CDCl3) δ 8.03 (d, J = 2.5 Hz, 1H), 7.69 (d, J = 8.2 Hz, 2H), 7.60 (dd, J = 8.8, 2.6 Hz, 1H), 7.33 (d, J = 8.3 Hz, 2H), 7.20 (d, J = 8.9 Hz, 1H), 4.21 (d, J = 2.3 Hz, 2H), 3.52 (s, 3H), 2.44 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.36, 171.35, 144.68, 141.69, 138.45, 136.11, 129.70, 129.06, 127.96, 127.77, 120.19, 116.96, 62.51, 55.21, 30.32, 25.73, 21.66. HRMS (ESI) calculated for C19H19ClNO4S [M+H]+: 392.0718, found: 392.0712.
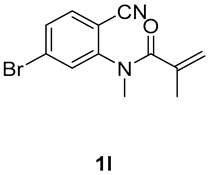
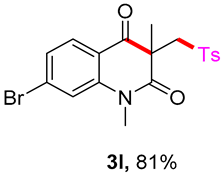
7-bromo-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3l). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (95 mg, 81%): mp: 174–175 °C. 1H-NMR (500 MHz, CDCl3) δ 7.95 (d, J = 8.3 Hz, 1H), 7.69 (d, J = 8.2 Hz, 2H), 7.41 (d, J = 0.8 Hz, 1H), 7.32 (d, J = 8.1 Hz, 3H), 4.20 (s, 2H), 3.53 (s, 3H), 2.43 (s, 3H), 1.40 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.42, 171.69, 144.63, 143.97, 138.48, 131.60, 129.97, 129.68, 127.79, 126.53, 118.43, 117.97, 62.49, 55.23, 30.28, 25.80, 21.66. HRMS (ESI) calculated for C19H19BrNO4S [M+H]+: 392.0718, found: 392.0710.
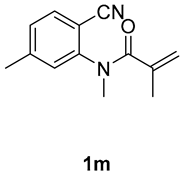
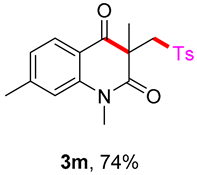
1,3,7-trimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3m). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.22), white solid (83 mg, 74%): mp: 137–138 °C. 1H-NMR (500 MHz, CDCl3) δ 8.03–7.95 (m, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 8.2 Hz, 2H), 7.03 (s, 2H), 4.20 (s, 2H), 3.53 (s, 3H), 2.47 (s, 3H), 2.43 (s, 3H), 1.40 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.80, 171.94, 147.98, 144.44, 143.21, 138.68, 129.60, 128.71, 127.84, 124.34, 117.11, 115.60, 62.43, 54.98, 30.07, 26.08, 22.49, 21.65. HRMS (ESI) calculated for C20H22NO4S [M+H]+: 372.1264, found: 372.1261.
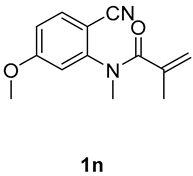
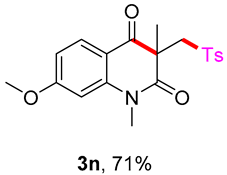
7-methoxy-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3n). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), yellow solid (83 mg, 71%): mp: 192–193 °C. 1H-NMR (500 MHz, CDCl3) δ 7.72 (d, J = 8.3 Hz, 2H), 7.57 (d, J = 3.1 Hz, 1H), 7.32 (d, J = 8.0 Hz, 2H), 7.29–7.24 (m, 1H), 7.17 (d, J = 9.0 Hz, 1H), 4.21 (d, J = 2.4 Hz, 2H), 3.87 (s, 3H), 3.52 (s, 3H), 2.43 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.39, 171.17, 155.58, 144.48, 138.62, 137.33, 129.62, 127.85, 124.51, 119.85, 116.72, 110.20, 62.49, 55.83, 54.94, 30.20, 26.04, 21.65. HRMS (ESI) calculated for C20H22NO4S [M+H]+: 388.1213, found: 383.1235.
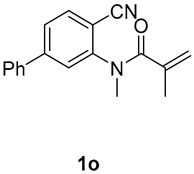
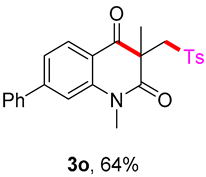
1,3-dimethyl-7-phenyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3o). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (83 mg, 64%): mp: 100–101 °C. 1H-NMR (500 MHz, CDCl3) δ 8.16 (d, J = 8.0 Hz, 1H), 7.72 (d, J = 8.2 Hz, 2H), 7.64 (d, J = 7.3 Hz, 2H), 7.54–7.38 (m, 5H), 7.32 (d, J = 8.1 Hz, 2H), 4.23 (s, 2H), 3.62 (s, 3H), 2.44 (s, 3H), 1.45 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 193.94, 171.94, 149.61, 144.53, 143.54, 139.81, 138.63, 129.66, 129.28, 129.13, 128.92, 127.85, 127.44, 126.46, 122.34, 118.00, 113.87, 62.50, 55.16, 30.23, 26.04, 21.67. HRMS (ESI) calculated for C25H24NO4S [M+H]+: 434.1421, found: 434.1410.
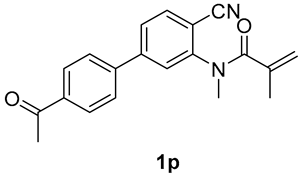
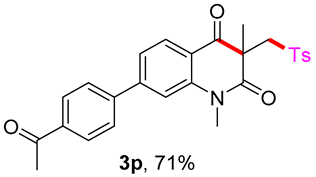
7-(4-acetylphenyl)-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3p). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), yellow solid (101 mg, 88%): mp: 192–193 °C. 1H-NMR (500 MHz, CDCl3) δ 8.19 (d, J = 8.0 Hz, 1H), 8.09 (d, J = 8.3 Hz, 2H), 7.73 (t, J = 8.0 Hz, 4H), 7.48–7.41 (m, 2H), 7.33 (d, J = 8.1 Hz, 2H), 4.24 (s, 2H), 3.63 (s, 3H), 2.66 (s, 3H), 2.44 (s, 3H), 1.45 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 197.54, 193.88, 171.85, 148.08, 144.59, 144.15, 143.62, 138.60, 137.06, 129.68, 129.42, 129.11, 127.81, 127.68, 122.35, 118.56, 114.03, 62.55, 55.22, 30.27, 26.77, 25.94, 21.67. HRMS (ESI) calculated for C27H26NO5S [M+H]+: 476.1526, found: 476.1520.
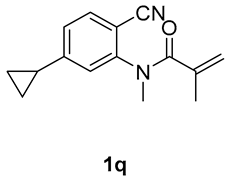
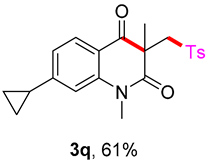
7-cyclopropyl-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3q). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.22), white solid (73 mg, 61%): mp: 151–152 °C. 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.1 Hz, 1H), 7.70 (d, J = 8.3 Hz, 2H), 7.29 (t, J = 10.3 Hz, 2H), 6.93 (d, J = 0.7 Hz, 1H), 6.82 (dd, J = 8.1, 1.1 Hz, 1H), 4.19 (s, 2H), 3.54 (s, 3H), 2.43 (s, 3H), 2.04–1.95 (m, 1H), 1.40 (s, 3H), 1.19–1.10 (m, 2H), 0.91–0.82 (m, 2H). 13C-NMR (126 MHz, CDCl3) δ 193.58, 172.02, 154.61, 144.43, 143.24, 138.67, 129.59, 128.82, 127.84, 119.83, 116.96, 112.53, 62.42, 54.90, 30.05, 26.12, 21.65, 16.62, 10.77, 10.75. HRMS (ESI) calculated for C22H24NO4S [M+H]+: 398.1421, found: 398.1420.
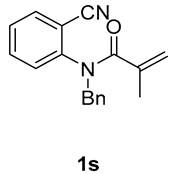
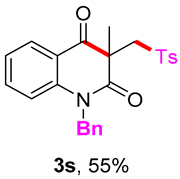
1-benzyl-3-methyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3r). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.21), white solid (72 mg, 55%): mp: 166–167 °C. 1H-NMR (500 MHz, CDCl3) δ 8.11 (dd, J = 7.8, 1.6 Hz, 1H), 7.74 (d, J = 8.3 Hz, 2H), 7.49 (s, 1H), 7.33 (ddd, J = 18.7, 10.8, 7.7 Hz, 6H), 7.25 (d, J = 4.6 Hz, 1H), 7.16 (d, J = 7.5 Hz, 1H), 7.08 (d, J = 8.4 Hz, 1H), 5.53–5.23 (m, 2H), 4.30 (s, 2H), 2.42 (s, 3H), 1.50 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.15, 172.30, 144.47, 142.28, 138.94, 136.33, 135.88, 129.65, 128.99, 128.81, 127.84, 127.41, 126.38, 123.37, 119.43, 116.15, 62.28, 55.78, 46.44, 25.92, 21.66. HRMS (ESI) calculated for C25H24NO4S [M+H]+: 434.1421, found: 434.1414.
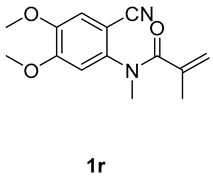
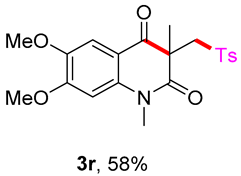
6,7-dimethoxy-1,3-dimethyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (3s). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), yellow liquid (73 mg, 58%): mp: 180–181 °C. 1H-NMR (500 MHz, CDCl3) δ 7.71 (d, J = 8.3 Hz, 2H), 7.53 (s, 1H), 7.31 (d, J = 8.1 Hz, 2H), 6.67 (s, 1H), 4.19 (d, J = 6.5 Hz, 2H), 4.02 (s, 3H), 3.94 (s, 3H), 3.55 (s, 3H), 2.43 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 192.79, 172.08, 156.01, 145.42, 144.45, 139.45, 138.57, 129.61, 127.82, 111.92, 109.19, 98.39, 62.52, 56.41, 56.25, 54.40, 30.17, 26.49, 21.64. HRMS (ESI) calculated for C21H24NO6S [M+H]+: 418.1319, found: 418.1315.
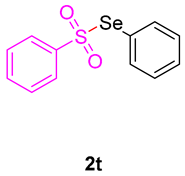
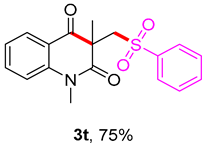
1,3-dimethyl-3-((phenylsulfonyl)methyl)quinoline-2,4(1H,3H)-dione (3t). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (77 mg, 75%): mp: 118–119 °C. 1H-NMR (500 MHz, CDCl3) δ 8.10 (dd, J = 7.7, 1.4 Hz, 1H), 7.85 (d, J = 7.4 Hz, 2H), 7.71–7.51 (m, 4H), 7.24 (dt, J = 9.8, 8.9 Hz, 2H), 4.24 (s, 2H), 3.55 (s, 3H), 1.43 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.26, 171.67, 143.15, 141.55, 136.53, 133.58, 129.03, 128.70, 127.81, 123.34, 119.23, 115.17, 62.21, 55.32, 30.16, 25.91. HRMS (ESI) calculated for C18H18NO4S [M+H]+: 344.0951, found: 344.0947.
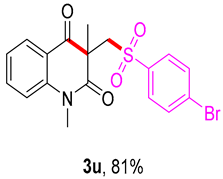
3-(((4-bromophenyl)sulfonyl)methyl)-1,3-dimethylquinoline-2,4(1H,3H)-dione (3u). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (102 mg, 81%): mp: 174–175 °C. 1H-NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 7.7, 1.5 Hz, 1H), 8.00 (d, J = 8.2 Hz, 2H), 7.82 (d, J = 8.3 Hz, 2H), 7.70 (s, 1H), 7.26 (dd, J = 8.0, 2.6 Hz, 2H), 4.27 (s, 2H), 3.56 (s, 3H), 1.43 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.26, 171.63, 145.11, 143.05, 136.68, 128.69, 128.54, 126.18, 126.15, 123.49, 119.13, 115.24, 61.82, 55.78, 30.20, 25.75. HRMS (ESI) calculated for C18H17BrNO4S [M+H]+: 422.0056, found: 422.0051.
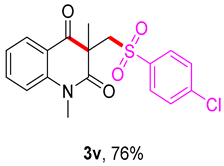
3-(((4-chlorophenyl)sulfonyl)methyl)-1,3-dimethylquinoline-2,4(1H,3H)-dione (3v). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (86 mg, 76%): mp: 180–181 °C. 1H-NMR (500 MHz, CDCl3) δ 8.08 (d, J = 7.6 Hz, 1H), 7.78 (d, J = 8.5 Hz, 2H), 7.68 (s, 1H), 7.50 (d, J = 8.5 Hz, 2H), 7.32–7.18 (m, 2H), 4.24 (s, 2H), 3.54 (s, 3H), 1.42 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.26, 171.63, 143.07, 140.25, 140.13, 136.64, 129.40, 129.31, 128.64, 123.41, 119.13, 115.24, 62.10, 55.60, 30.18, 25.82. HRMS (ESI) calculated for C18H16ClNNaO4S [M+H]+: 400.0381, found: 400.0411.
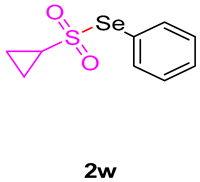
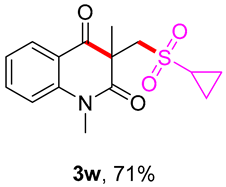
3-((cyclopropylsulfonyl)methyl)-1,3-dimethylquinoline-2,4(1H,3H)-dione (3w). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.25), yellow liquid (66 mg, 71%): mp: 153–155 °C. 1H-NMR (500 MHz, CDCl3) δ 8.08 (dd, J = 8.1, 1.7 Hz, 1H), 7.72–7.62 (m, 1H), 7.28–7.17 (m, 2H), 4.21 (d, J = 1.3 Hz, 2H), 3.53 (s, 3H), 2.70–2.58 (m, 1H), 1.46 (s, 3H), 1.24–1.15 (m, 2H), 1.00 (dd, J = 8.0, 2.0 Hz, 2H). 13C-NMR (126 MHz, CDCl3) δ 194.82, 172.13, 143.04, 136.48, 128.63, 123.33, 119.14, 115.14, 59.87, 55.72, 33.32, 30.14, 25.64, 5.13, 5.02. HRMS (ESI) calculated for C15H18NO4S [M+H]+: 308.0951, found: 308.0947.
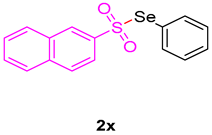
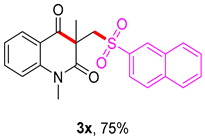
1,3-dimethyl-3-((naphthalen-2-ylsulfonyl)methyl)quinoline-2,4(1H,3H)-dione (3x). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.24), white solid (89 mg, 75%): mp: 135–136 °C. 1H-NMR (500 MHz, CDCl3) δ 8.35 (s, 1H), 8.08 (d, J = 7.3 Hz, 1H), 7.98 (d, J = 8.7 Hz, 1H), 7.91 (t, J = 7.3 Hz, 2H), 7.85 (dd, J = 8.6, 1.4 Hz, 1H), 7.72–7.53 (m, 3H), 7.21 (dd, J = 12.2, 5.4 Hz, 2H), 4.30 (s, 2H), 3.51 (s, 3H), 1.43 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.19, 171.61, 143.14, 138.20, 136.58, 135.30, 131.99, 129.66, 129.53, 129.42, 129.21, 128.67, 127.98, 127.53, 123.34, 122.63, 119.25, 115.20, 62.30, 55.17, 30.14, 26.09. HRMS (ESI) calculated for C22H20NO4S [M+H]+: 394.1180, found: 394.1094.
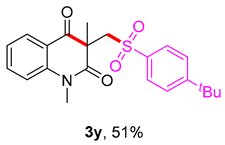
3-(((4-(tert-butyl)phenyl)sulfonyl)methyl)-1,3-dimethylquinoline-2,4(1H,3H)-dione (3y). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.25), white liquid (61 mg, 51%): mp: 95–96 °C. 1H-NMR (500 MHz, CDCl3) δ 8.11 (dd, J = 7.7, 1.6 Hz, 1H), 7.80–7.75 (m, 2H), 7.76–7.68 (m, 1H), 7.56–7.50 (m, 2H), 7.27–7.21 (m, 2H), 4.23 (s, 2H), 3.55 (s, 3H), 1.42 (s, 3H), 1.35 (s, 9H). 13C-NMR (126 MHz, CDCl3) δ 194.31, 171.73, 157.40, 143.17, 138.50, 136.47, 128.70, 127.69, 126.05, 123.29, 119.26, 115.15, 62.36, 55.26, 35.24, 31.09, 30.16, 25.89. HRMS (ESI) calculated for C22H26NO4S [M+H]+: 400.1577, found: 400.1572.
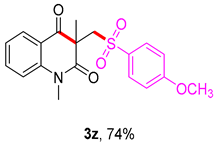
3-(((4-methoxyphenyl)sulfonyl)methyl)-1,3-dimethylquinoline-2,4(1H,3H)-dione (3z). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.25), white solid (83 mg, 74%): mp: 145–146 °C. 1H-NMR (500 MHz, CDCl3) δ 7.99 (dd, J = 7.7, 1.4 Hz, 1H), 7.66 (d, J = 8.9 Hz, 2H), 7.58 (s, 1H), 7.18–7.09 (m, 2H), 6.89 (d, J = 8.9 Hz, 2H), 4.13 (s, 2H), 3.77 (s, 3H), 3.44 (s, 3H), 1.33 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.33, 171.73, 163.63, 143.16, 136.49, 133.15, 130.05, 128.66, 123.28, 119.25, 115.15, 114.16, 62.63, 55.67, 55.26, 30.14, 25.92. HRMS (ESI) calculated for C19H19NO5S [M+H]+: 374.1057, found: 374.1052.
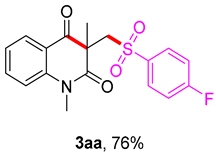
3-(((4-fluorophenyl)sulfonyl)methyl)-1,3-dimethylquinoline-2,4(1H,3H)-dione (3aa). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.21), white solid (83 mg, 76%): mp: 147-148 °C. 1H-NMR (500 MHz, CDCl3) δ 8.09 (dd, J = 7.7, 1.5 Hz, 1H), 7.92–7.79 (m, 2H), 7.72–7.64 (m, 1H), 7.29–7.16 (m, 4H), 4.25 (s, 2H), 3.55 (s, 3H), 1.42 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.30, 171.67, 165.73 (d, J = 255.8 Hz), 143.09, 137.69, 136.61, 130.78, 128.65, 123.40, 119.16, 116.27, 115.22, 62.21, 55.56, 30.17, 25.82. 19F-NMR (471 MHz, CDCl3) δ -103.92. HRMS (ESI) calculated for C18H17FNO5S [M+H]+: 362.0857, found: 362.0852.
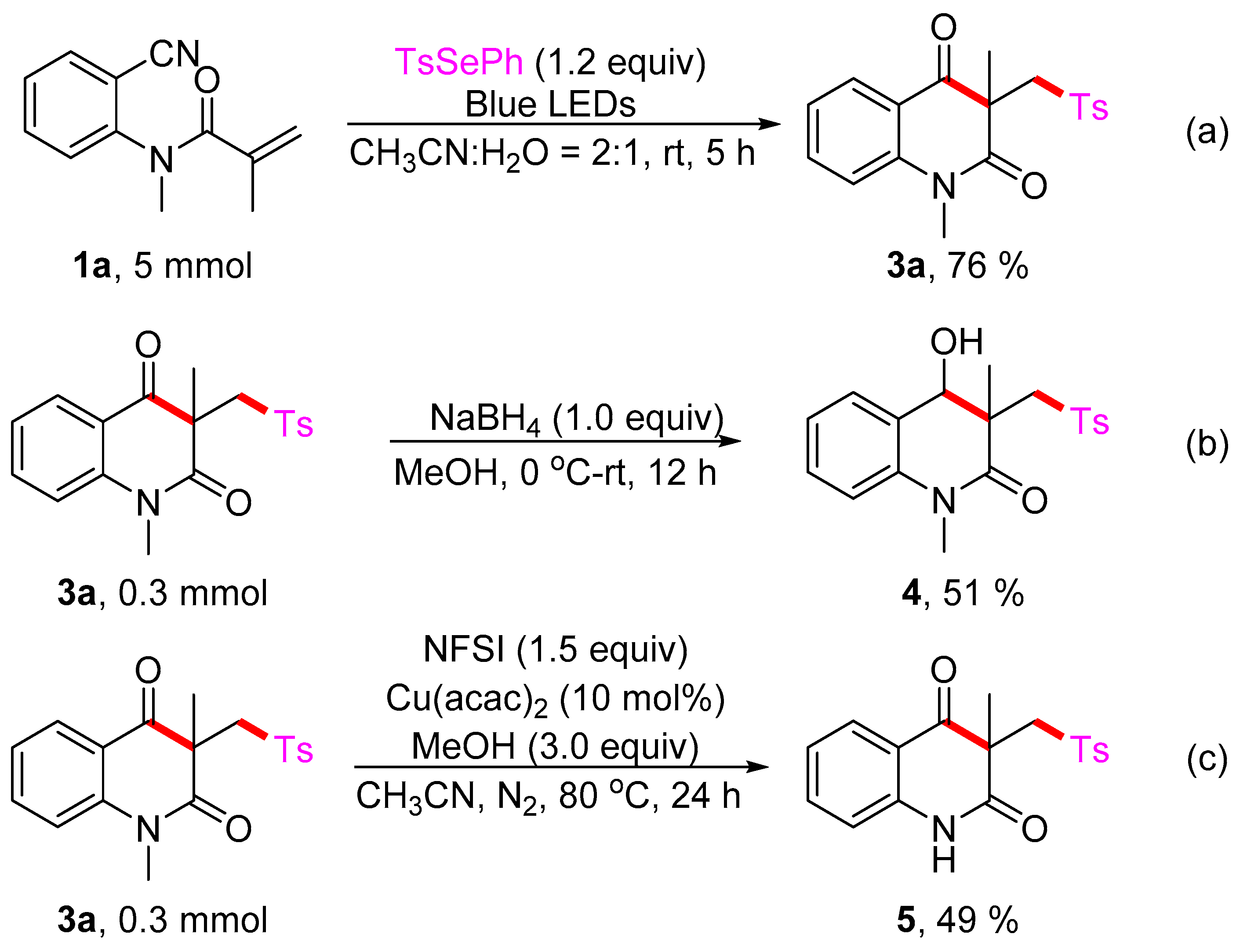
4-hydroxy-1,3-dimethyl-3-(tosylmethyl)-3,4-dihydroquinolin-2(1H)-one (4). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.23), white solid (55 mg, 51%): mp: 96–97 °C. 1H-NMR (500 MHz, CDCl3) δ 7.85 (d, J = 8.3 Hz, 2H), 7.40 (ddd, J = 14.9, 7.3, 1.4 Hz, 4H), 7.14 (td, J = 7.5, 0.7 Hz, 1H), 7.02 (d, J = 8.1 Hz, 1H), 5.20 (d, J = 3.0 Hz, 1H), 3.79 (dd, J = 14.4, 9.6 Hz, 1H), 3.66 (d, J = 14.5 Hz, 2H), 3.35 (s, 3H), 2.47 (s, 3H), 1.41 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 171.34, 145.14, 138.50, 137.84, 130.04, 130.00, 129.82, 127.70, 124.55, 123.75, 114.70, 72.01, 58.52, 47.95, 30.14, 21.69, 20.59. HRMS (ESI) calculated for C19H22NO4S [M+H]+: 360.1264, found: 360.1259.
3-methyl-3-(tosylmethyl)quinoline-2,4(1H,3H)-dione (5). The product was purified by column chromatography on silica gel (petroleum ether/ethyl acetate = 5:1, Rf = 0.24), white solid (51 mg, 49%): mp: 141–142 °C. 1H-NMR (500 MHz, CDCl3) δ 9.70 (s, 1H), 8.04–7.99 (m, 1H), 7.76 (d, J = 8.3 Hz, 2H), 7.56–7.52 (m, 1H), 7.29 (d, J = 8.1 Hz, 2H), 7.18 (t, J = 7.5 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 4.26 (dd, J = 29.7, 14.1 Hz, 2H), 2.36 (s, 3H), 1.48 (s, 3H). 13C-NMR (126 MHz, CDCl3) δ 194.31, 173.37, 144.65, 140.74, 138.52, 136.36, 129.72, 128.23, 127.84, 123.70, 117.99, 116.82, 61.91, 55.07, 25.58, 21.59. HRMS (ESI) calculated for C18H18NO4S [M+H]+: 344.0951, found: 344.0947.