Dual Anta-Inhibitors Targeting Protein Kinase CK1δ and A2A Adenosine Receptor Useful in Neurodegenerative Disorders
Abstract
1. Introduction
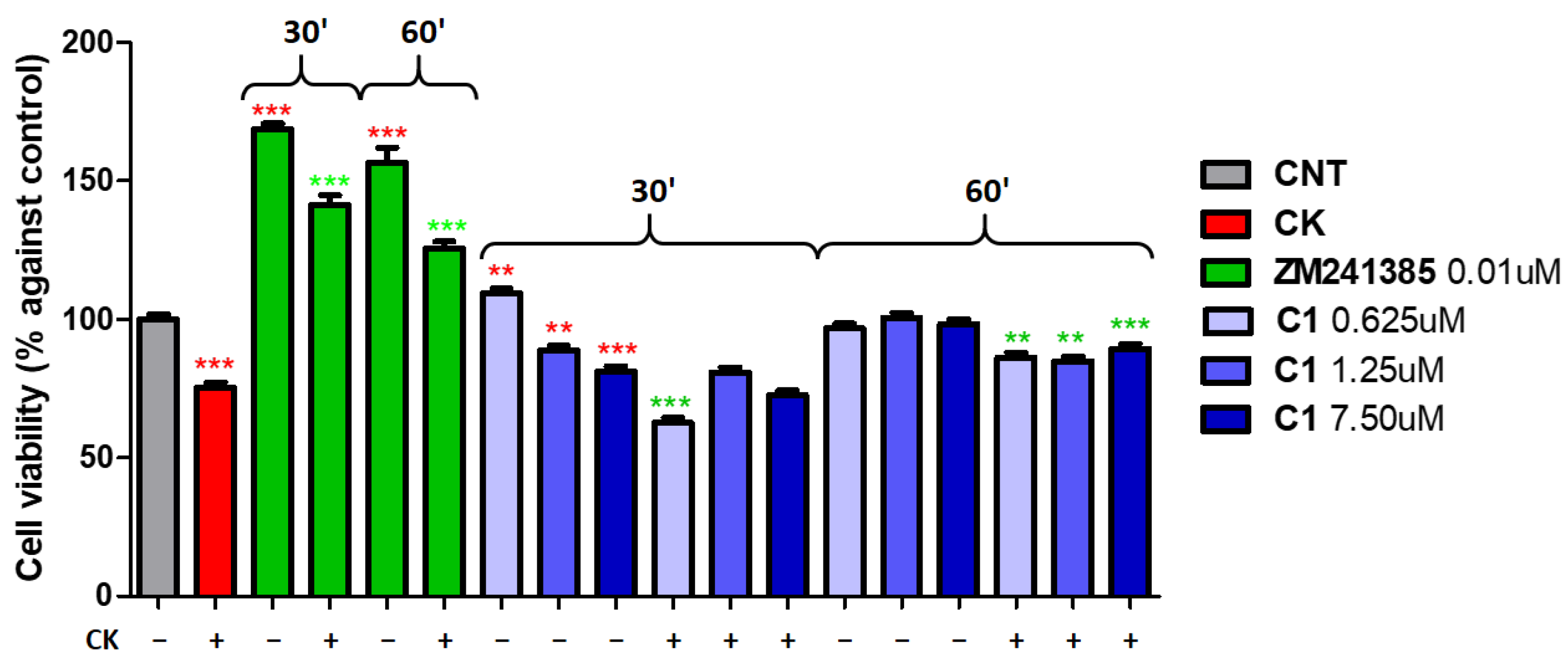
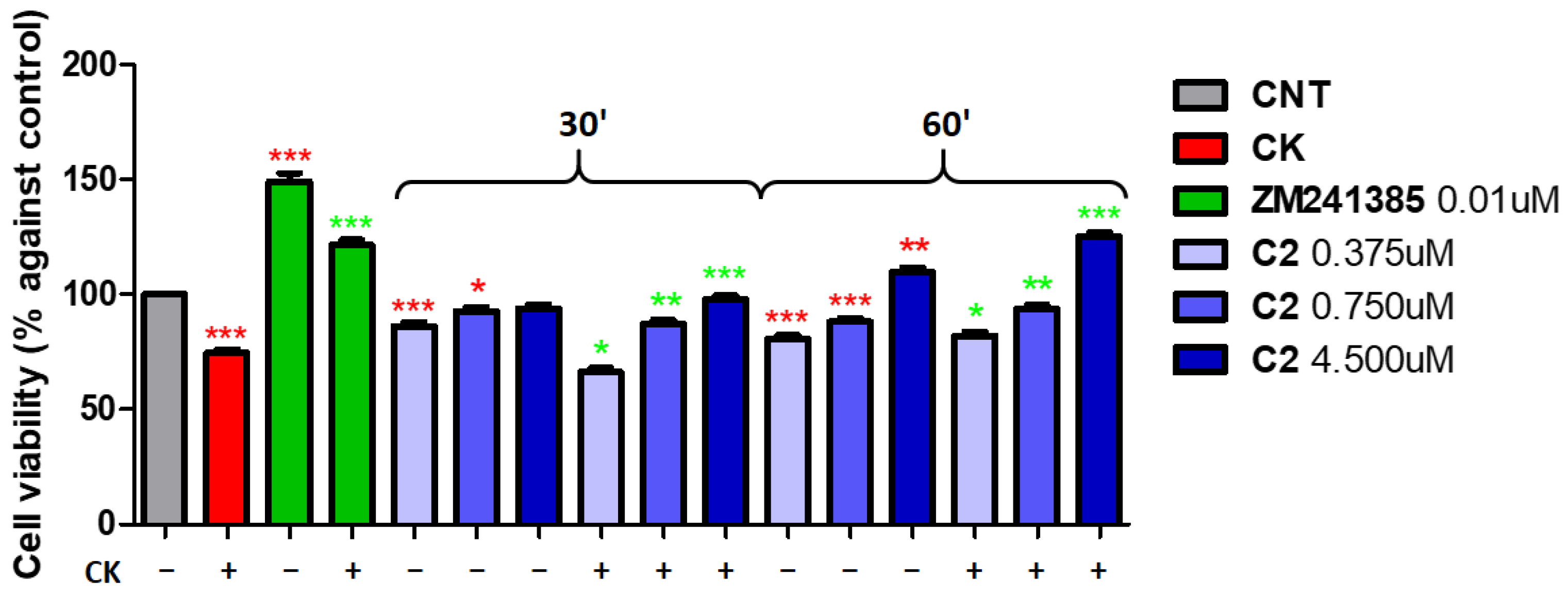
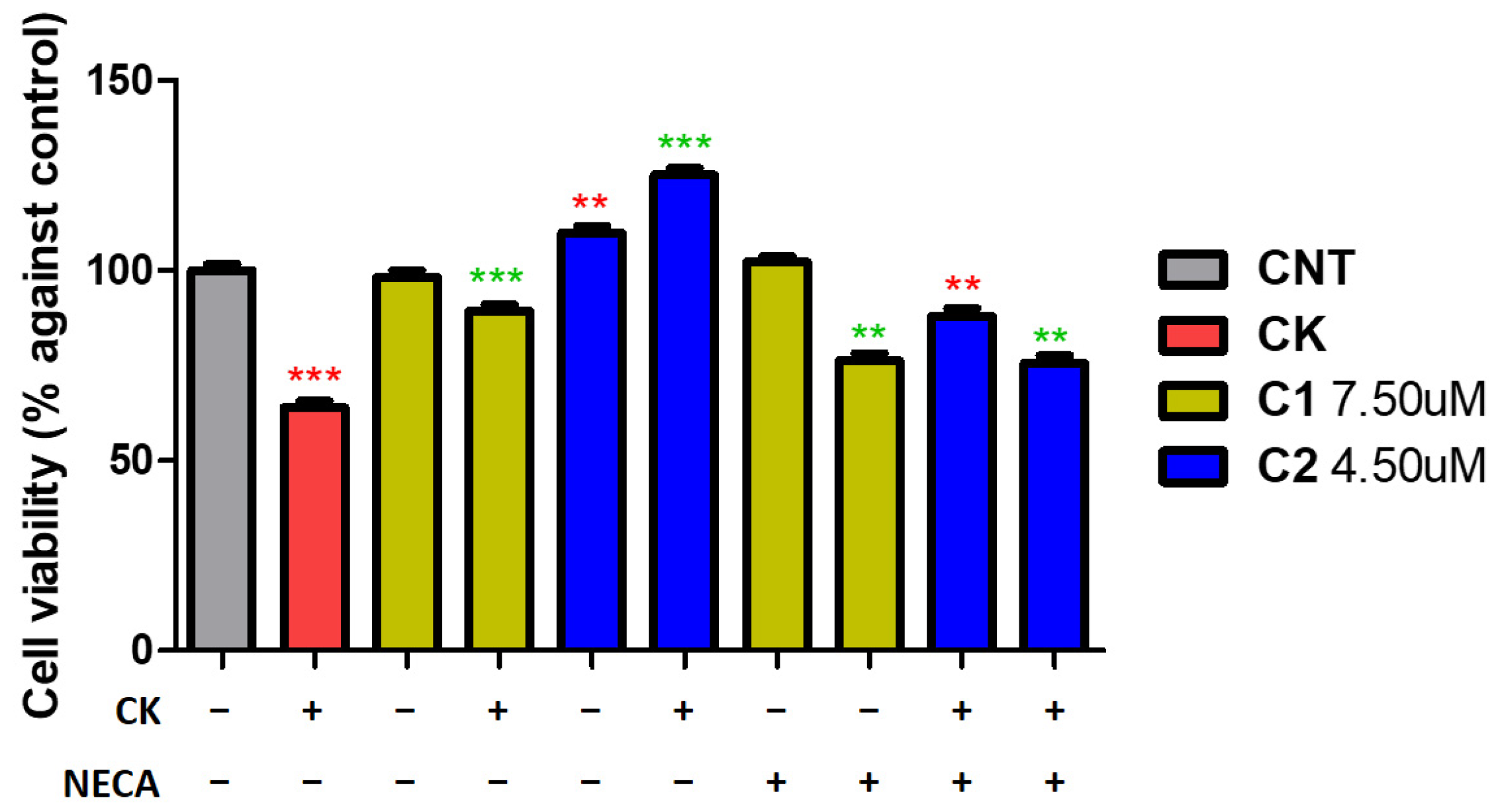
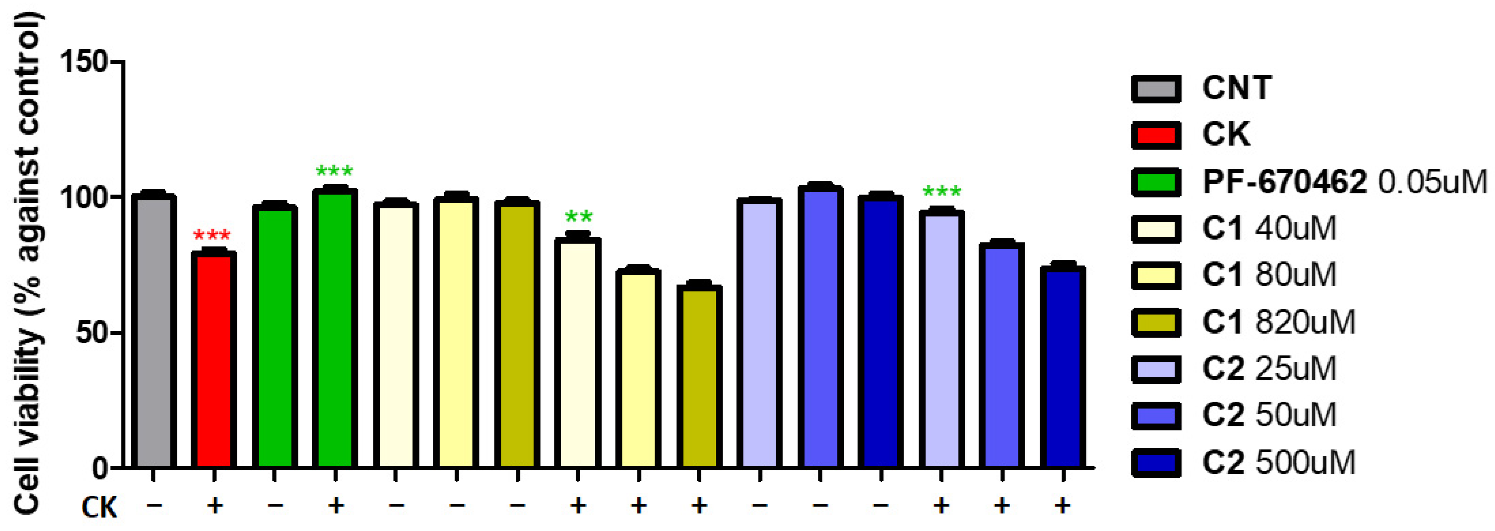
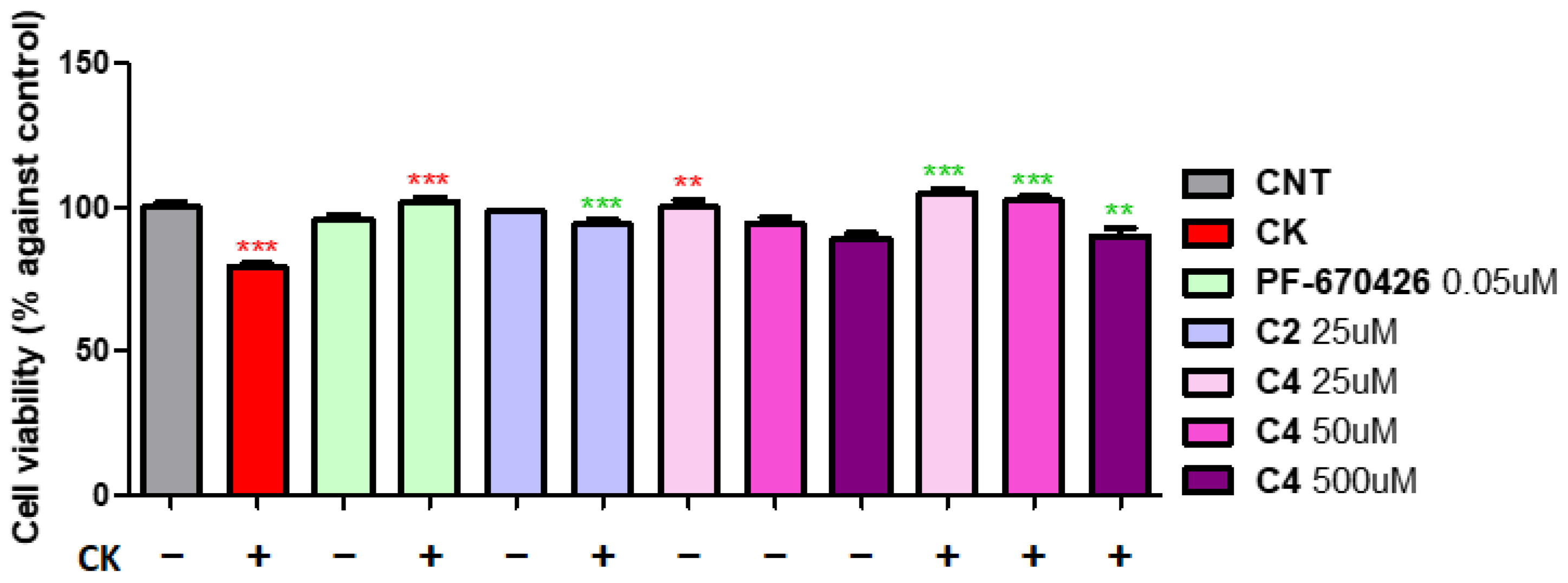
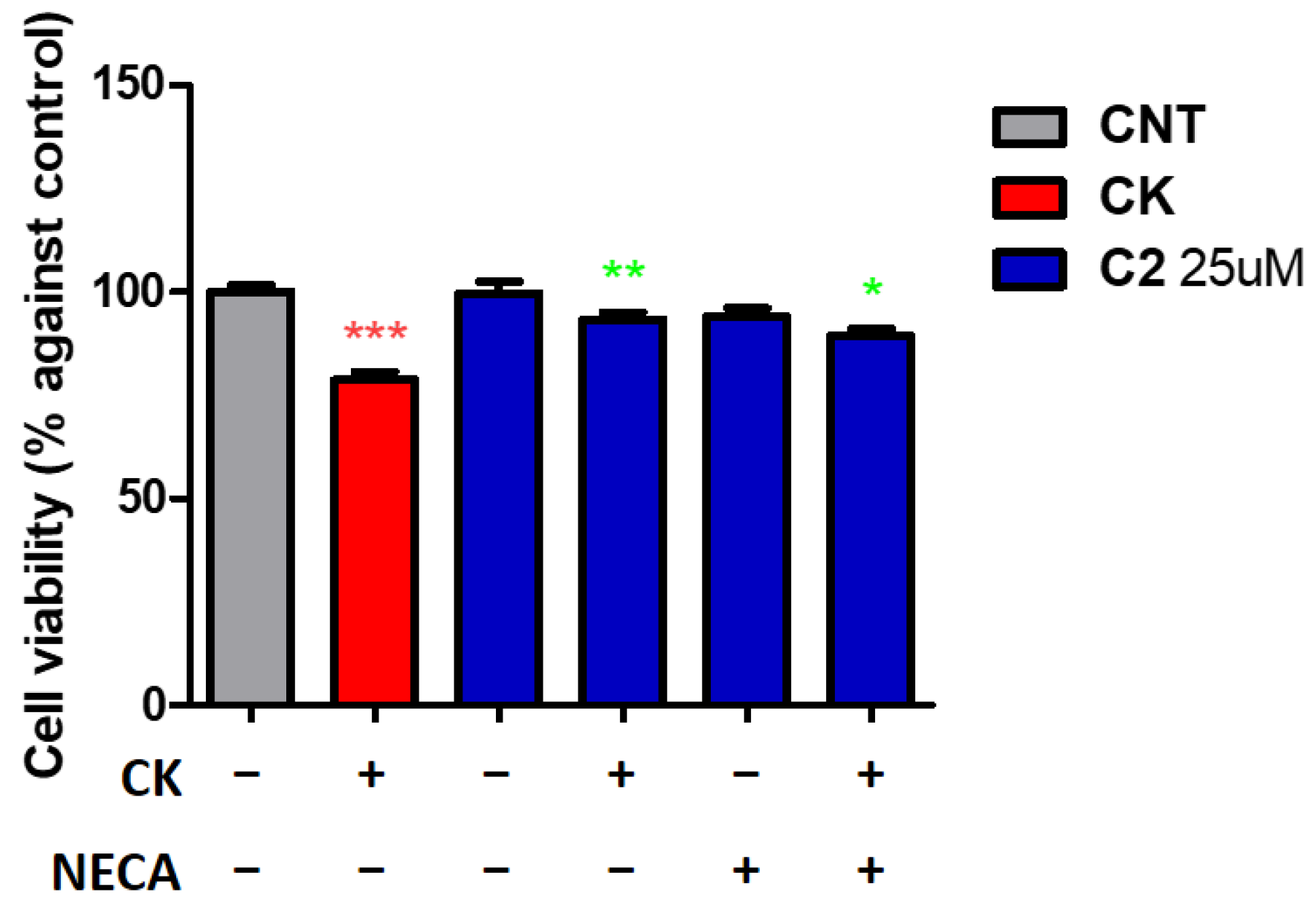
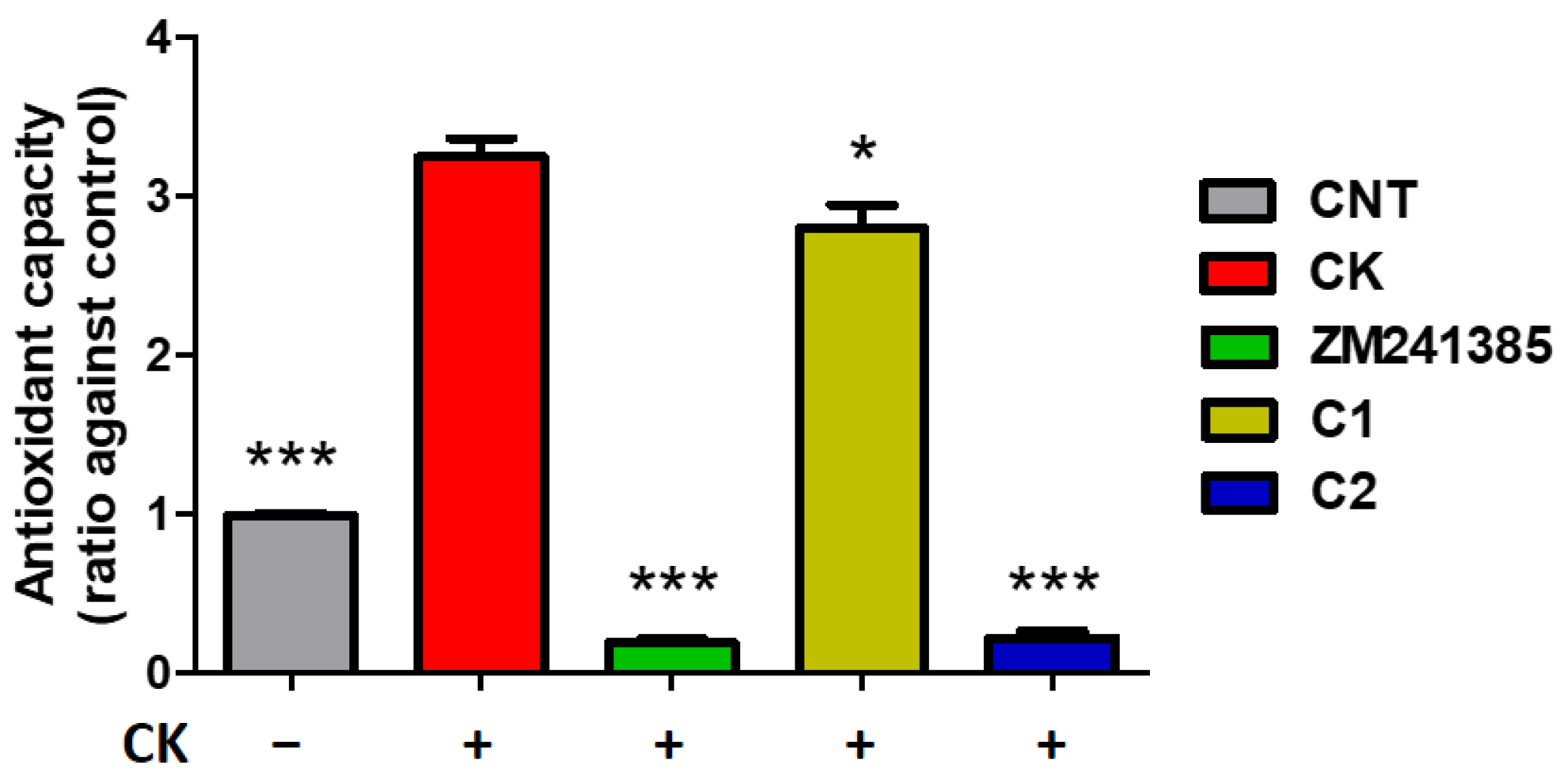
2. Results and Discussion

3. Materials and Methods
3.1. Test Materials
3.2. Animals
3.3. Cells Culture
3.4. Cell Titer 96® Aqueous One Solution Cell Proliferation Assay
3.5. CellTiter-Glo® Luminescent Cell Viability Assay
3.6. Griess Assay
3.7. Everted Gut Sac Studies
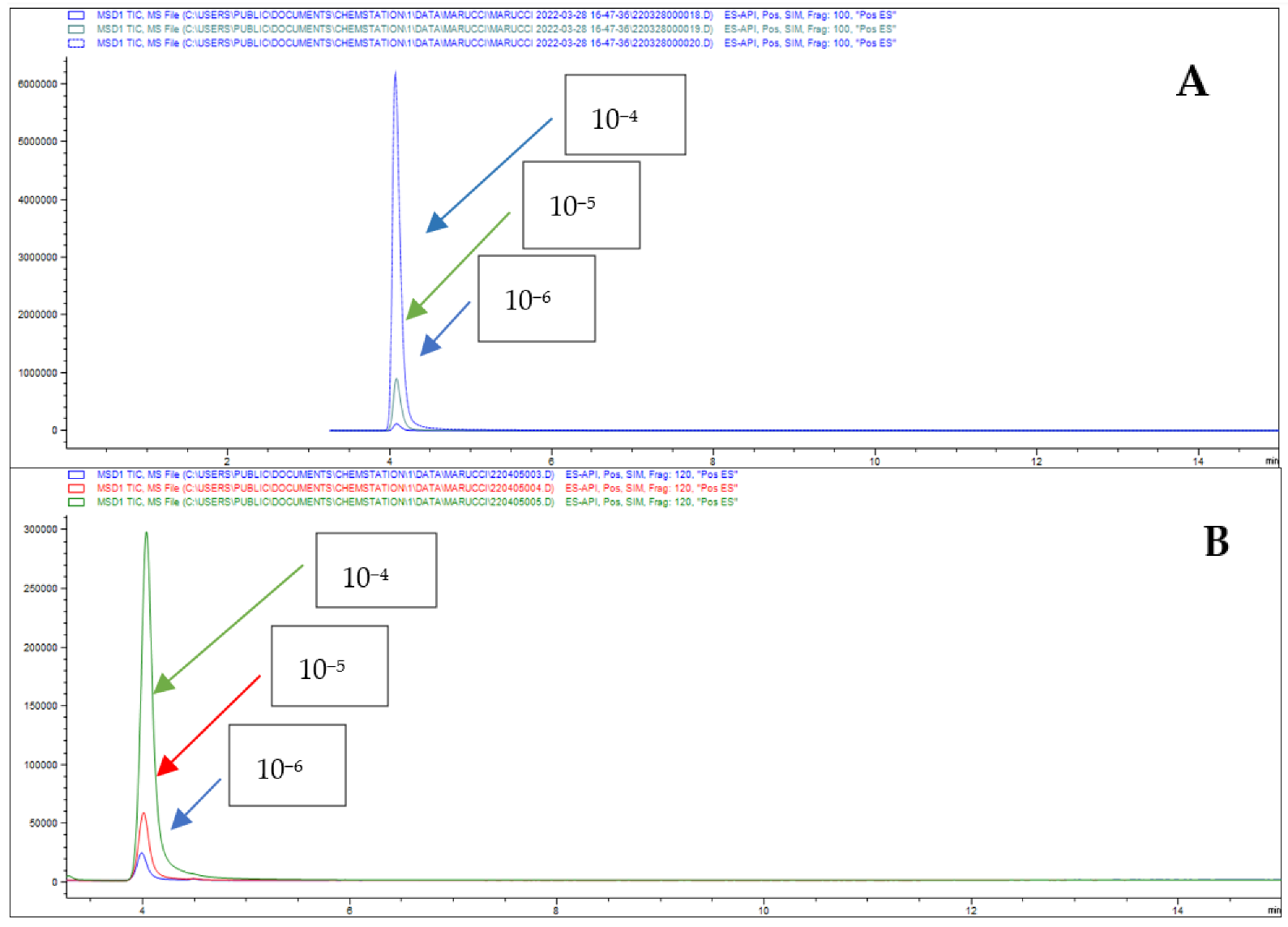
3.8. HPLC-MS Analysis
3.9. Calculation of the Apparent Permeability Coefficients
3.10. Percentage of Drug Absorption (A%) and Drug Retention (Ad%)
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Miller, R.L.; Dhavale, D.D.; O’Shea, J.Y.; Andruska, K.M.; Liu, J.; Franklin, E.E.; Buddhala, C.; Loftin, S.K.; Cirrito, J.R.; Perrin, R.J.; et al. Quantifying regional α-synuclein, amyloid β, and tau accumulation in lewy body dementia. Ann. Clin. Transl. Neurol. 2022, 9, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Catarzi, D.; Varano, F.; Vigiani, E.; Lambertucci, C.; Spinaci, A.; Volpini, R.; Colotta, V. Casein Kinase 1δ Inhibitors as Promising Therapeutic Agents for Neurodegenerative Disorders. Curr. Med. Chem. 2022, 29, 4698–4737. [Google Scholar] [CrossRef] [PubMed]
- Varano, F.; Catarzi, D.; Calenda, S.; Vigiani, E.; Colotta, V. CK1 delta inhibition: An emerging strategy to combat neurodegenerative diseases. Future Med. Chem. 2022, 14, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Malim, F.M.; Goswami, A.; Sharma, N.; Juvvalapalli, S.S.; Chatterjee, S.; Kate, A.S.; Khairnar, A. Neuroprotective Effect of Swertiamarin in a Rotenone Model of Parkinson’s Disease: Role of Neuroinflammation and Alpha-Synuclein Accumulation. ACS Pharmacol. Transl. Sci. 2022, 6, 40–51. [Google Scholar] [CrossRef]
- Wang, R.; Pang, S.C.; Li, J.Y.; Li, C.L.; Liu, J.M.; Wang, Y.M.; Chen, M.L.; Li, Y.B. A review of the current research on in vivo and in vitro detection for alpha-synuclein: A biomarker of Parkinson’s disease. Anal. Bioanal Chem. 2023, 415, 1589–1605. [Google Scholar] [CrossRef]
- Uchida, K.; Morikawa, K.; Muguruma, Y.; Hosokawa, M.; Tsutsumiuchi, K.; Kaneda, D.; Hashizume, Y.; Akatsu, H.; Inoue, K. LC- MS/MS assay for the investigation of acetylated Alpha-synuclein in serum from postmortem Alzheimer’s disease pathology. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2021, 1181, 122885. [Google Scholar] [CrossRef]
- Jin, Y.; Li, F.; Sonoustoun, B.; Kondru, N.C.; Martens, Y.A.; Qiao, W.; Heckman, M.G.; Ikezu, T.C.; Li, Z.; Burgess, J.D.; et al. APOE4 exacerbates α-synuclein seeding activity and contributes to neurotoxicity in Alzheimer’s disease with Lewy body pathology. Acta Neuropathol. 2022, 143, 641–662. [Google Scholar] [CrossRef]
- Shim, K.H.; Kang, M.J.; Youn, Y.C.; An, S.S.A.; Kim, S. Alpha-synuclein: A pathological factor with Aβ and tau and biomarker in Alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 201. [Google Scholar] [CrossRef]
- Beatino, M.F.; De Luca, C.; Campese, N.; Belli, E.; Piccarducci, R.; Giampietri, L.; Martini, C.; Perugi, G.; Siciliano, G.; Ceravolo, R.; et al. α-synuclein as an emerging pathophysiological biomarker of Alzheimer’s disease. Expert Rev. Mol. Diagn. 2022, 22, 411–425. [Google Scholar] [CrossRef]
- Rossi, M.; Baiardi, S.; Teunissen, C.E.; Quadalti, C.; van de Beek, M.; Mammana, A.; Stanzani-Maserati, M.; Van der Flier, W.M.; Sambati, L.; Zenesini, C.; et al. Diagnostic Value of the CSF α-Synuclein Real-Time Quaking-Induced Conversion Assay at the Prodromal MCI Stage of Dementia with Lewy Bodies. Neurology 2021, 97, 930–940. [Google Scholar] [CrossRef]
- Garrido, A.; Fairfoul, G.; Tolosa, E.; Marti, M.J.; Ezquerra, M.; Green, A.J.E. Brain and Cerebrospinal Fluid α-Synuclein Real-Time Quaking-Induced Conversion Identifies Lewy Body Pathology in LRRK2-PD. Mov. Disord. 2022, 38, 333–338. [Google Scholar] [CrossRef]
- Kim, W.S.; Kågedal, K.; Halliday, G.M. Alpha-synuclein biology in Lewy body diseases. Alzheimer’s Res. Ther. 2014, 6, 73. [Google Scholar] [CrossRef]
- Sevenich, M.; Honold, D.; Willuweit, A.; Kutzsche, J.; Mohrlüder, J.; Willbold, D. Development of an α-synuclein fibril and oligomer specific tracer for diagnosis of Parkinson’s disease, dementia with Lewy bodies and multiple system atrophy. Neurochem. Int. 2022, 161, 105422. [Google Scholar] [CrossRef]
- Roth, A.; Sander, A.; Oswald, M.S.; Gärtner, F.; Knippschild, U.; Bischof, J. Comprehensive Characterization of CK1δ-Mediated Tau Phosphorylation in Alzheimer’s Disease. Front. Mol. Biosci. 2022, 9, 872171. [Google Scholar] [CrossRef]
- Rubio de la Torre, E.; Luzón-Toro, B.; Forte-Lago, B.; Minguez-Castellanos, A.; Ferrer, I.; Hilfiker, S. Combined kinase inhibition modulates parkin inactivation. Hum. Mol. Genet. 2009, 18, 809–823. [Google Scholar] [CrossRef]
- Salado, I.G.; Redondo, M.; Bello, M.L.; Perez, C.; Liachko, N.F.; Kraemer, B.C.; Miguel, L.; Lecourtois, M.; Gil, C.; Martinez, A.; et al. Protein kinase CK-1 inhibitors as new potential drugs for amyotrophic lateral sclerosis. J. Med. Chem. 2014, 57, 2755–2772. [Google Scholar] [CrossRef]
- Joshi, K.; Goyal, S.; Grover, S.; Jamal, S.; Singh, A.; Dhar, P.; Grover, A. Novel group-based QSAR and combinatorial design of CK-1δ inhibitors as neuroprotective agents. BMC Bioinform. 2016, 17, 515. [Google Scholar] [CrossRef]
- Alquezar, C.; Salado, I.G.; de la Encarnación, A.; Pérez, D.I.; Moreno, F.; Gil, C.; de Munain, A.L.; Martínez, A.; Requero, A.M. Targeting TDP-43 phosphorylation by Casein Kinase-1δ inhibitors: A novel strategy for the treatment of frontotemporal dementia. Mol. Neurodegener. 2016, 11, 36. [Google Scholar] [CrossRef]
- Martínez-González, L.; Rodríguez-Cueto, C.; Cabezudo, D.; Bartolomé, F.; Andrés-Benito, P.; Ferrer, I.; Gil, C.; Martín-Requero, Á.; Fernández-Ruiz, J.; Martínez, A.; et al. Motor neuron preservation and decrease of in vivo TDP-43 phosphorylation by protein CK-1δ kinase inhibitor treatment. Sci. Rep. 2020, 10, 4449. [Google Scholar] [CrossRef]
- He, W.; Xie, X.; Li, C.; Ding, H.; Ye, J. Adenosine A2A Receptor Antagonist Improves Cognitive Impairment by Inhibiting Neuroinflammation and Excitatory Neurotoxicity in Chronic Periodontitis Mice. Molecules 2022, 27, 6267. [Google Scholar] [CrossRef]
- Rebola, N.; Simões, A.P.; Canas, P.M.; Tomé, A.R.; Andrade, G.M.; Barry, C.E.; Agostinho, P.M.; Lynch, M.A.; Cunha, R.A. Adenosine A2A receptors control neuroinflammation and consequent hippocampal neuronal dysfunction. J. Neurochem. 2011, 117, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Poloni, T.E.; Pelloni, L.; Pasquini, S.; Varani, K.; Vincenzi, F.; Borea, P.A.; Gessi, S. An Open Question: Is the A2A Adenosine Receptor a Novel Target for Alzheimer’s Disease Treatment? Front. Pharmacol. 2021, 12, 652455. [Google Scholar] [CrossRef] [PubMed]
- Lambertucci, C.; Marucci, G.; Catarzi, D.; Colotta, V.; Francucci, B.; Spinaci, A.; Varano, F.; Volpini, R. A2A Adenosine Receptor Antagonists and their Potential in Neurological Disorders. Curr. Med. Chem. 2022, 29, 4780–4795. [Google Scholar] [CrossRef] [PubMed]
- Gessi, S.; Poloni, T.E.; Negro, G.; Varani, K.; Pasquini, S.; Vincenzi, F.; Borea, P.A.; Merighi, S. A2A Adenosine Receptor as a Potential Biomarker and a Possible Therapeutic Target in Alzheimer’s Disease. Cells 2021, 10, 2344. [Google Scholar] [CrossRef] [PubMed]
- Colella, M.; Zinni, M.; Pansiot, J.; Cassanello, M.; Mairesse, J.; Ramenghi, L.; Baud, O. Modulation of Microglial Activation by Adenosine A2a Receptor in Animal Models of Perinatal Brain Injury. Front. Neurol. 2018, 9, 605. [Google Scholar] [CrossRef]
- Aires, I.D.; Madeira, M.H.; Boia, R.; Rodrigues-Neves, A.C.; Martins, J.M.; Ambrósio, A.F.; Santiago, A.R. Intravitreal injection of adenosine A2A receptor antagonist reduces neuroinflammation, vascular leakage and cell death in the retina of diabetic mice. Sci. Rep. 2019, 9, 17207. [Google Scholar] [CrossRef]
- Martí Navia, A.; Dal Ben, D.; Lambertucci, C.; Spinaci, A.; Volpini, R.; Coelho, J.E.; Lopes, L.V.; Marques-Morgado, I.; Marucci, G.; Buccioni, M. Adenosine receptors as neuroinflammation modulators: Role of A1 agonists and A2A antagonists. Cells 2020, 9, 1739. [Google Scholar] [CrossRef]
- Marucci, G.; Dal Ben, D.; Lambertucci, C.; Martì Navia, A.; Spinaci, A.; Volpini, R.; Buccioni, M. Combined Therapy of A1AR Agonists and A2AAR Antagonists in Neuroinflammation. Molecules 2021, 26, 1188. [Google Scholar] [CrossRef]
- Merighi, S.; Borea, P.A.; Varani, K.; Vincenzi, F.V.; Jacobson, K.A.; Gessi, S. A2A Adenosine Receptor Antagonists in Neurodegenerative Diseases. Curr. Med. Chem. 2022, 29, 4138–4151. [Google Scholar] [CrossRef]
- Cai, Q.; Xu, N.; He, Y.; Zhu, J.; Ye, F.; Luo, Z.; Lu, R.; Huang, L.; Zhang, F.; Chen, J.F.; et al. α-Synuclein Aggregates in the Nigro-Striatal Dopaminergic Pathway Impair Fine Movement: Partial Reversal by the Adenosine A2A Receptor Antagonist. Int. J. Mol. Sci. 2023, 24, 1365. [Google Scholar] [CrossRef]
- Tsutsui, S.; Schnermann, J.; Noorbakhsh, F.; Henry, S.; Yong, V.W.; Winston, B.W.; Warren, K.; Power, C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004, 11, 1521–1529. [Google Scholar] [CrossRef]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A(2A) receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef]
- Spinaci, A.; Buccioni, M.; Catarzi, D.; Cui, C.; Colotta, V.; Dal Ben, D.; Cescon, E.; Francucci, B.; Grieco, I.; Lambertucci, C.; et al. “Dual Anta-Inhibitors” of the A2A Adenosine Receptor and Casein Kinase CK1delta: Synthesis, Biological Evaluation, and Molecular Modeling Studies. Pharmaceuticals 2023, 16, 167. [Google Scholar] [CrossRef]
- Hunter, T. Protein kinases and phosphatases: The yin and yang of protein phosphorylation and signaling. Cell 1995, 80, 225–236. [Google Scholar] [CrossRef]
- Cohen, P. The origins of protein phosphorylation. Nat. Cell Biol. 2002, 4, E127–E130. [Google Scholar] [CrossRef]
- Tarrant, M.K.; Cole, P.A. The chemical biology of protein phosphorylation. Annu. Rev. Biochem. 2009, 78, 797–825. [Google Scholar] [CrossRef]
- Karve, T.M.; Cheema, A.K. Small changes huge impact: The role of protein posttranslational modifications in cellular homeostasis and disease. J. Amino Acids 2011, 2011, 207691. [Google Scholar] [CrossRef]
- Jin, J.; Pawson, T. Modular evolution of phosphorylation-based signalling systems. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2012, 367, 2540–2555. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Muzio, L.L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy. Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef]
- Taylor, J.M.; Main, B.S.; Crack, P.J. Neuroinflammation and oxidative stress: Co-conspirators in the pathology of Parkinson’s disease. Neurochem. Int. 2013, 62, 803–819. [Google Scholar] [CrossRef]
- He, J.; Zhu, G.; Wang, G.; Zhang, F. Oxidative Stress and Neuroinflammation Potentiate Each Other to Promote Progression of Dopamine Neurodegeneration. Oxid. Med. Cell. Longev. 2020, 2020, 6137521. [Google Scholar] [CrossRef] [PubMed]
- Amenta, F.; Buccioni, M.; Dal Ben, D.; Lambertucci, C.; Navia, A.M.; Ngouadjeu Ngnintedem, M.A.; Ricciutelli, M.; Spinaci, A.; Volpini, R.; Marucci, G. Ex-vivo absorption study of lysine R-lipoate salt, a new pharmaceutical form of R-ALA. Eur. J. Pharm. Sci. 2018, 118, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, M.; Kandhavelu, M.; Angeli, P.; Cristalli, G.; Dal Ben, D.; Giardinà, D.; Lambertucci, C.; Lammi, C.; Volpini, R.; Marucci, G. Identification of α1-adrenoceptor subtypes involved in contraction of young CD rat epididymal vas deferens. Eur. J. Pharmacol. 2009, 602, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Lassoued, M.A.; Khemissb, F.; Sfar, S. Comparative study of two in vitro methods for assessing drug absorption: Sartorius SM 16750 apparatus versus everted gut sac. J. Pharm. Pharmaceut. Sci. 2011, 14, 117–127. [Google Scholar] [CrossRef]


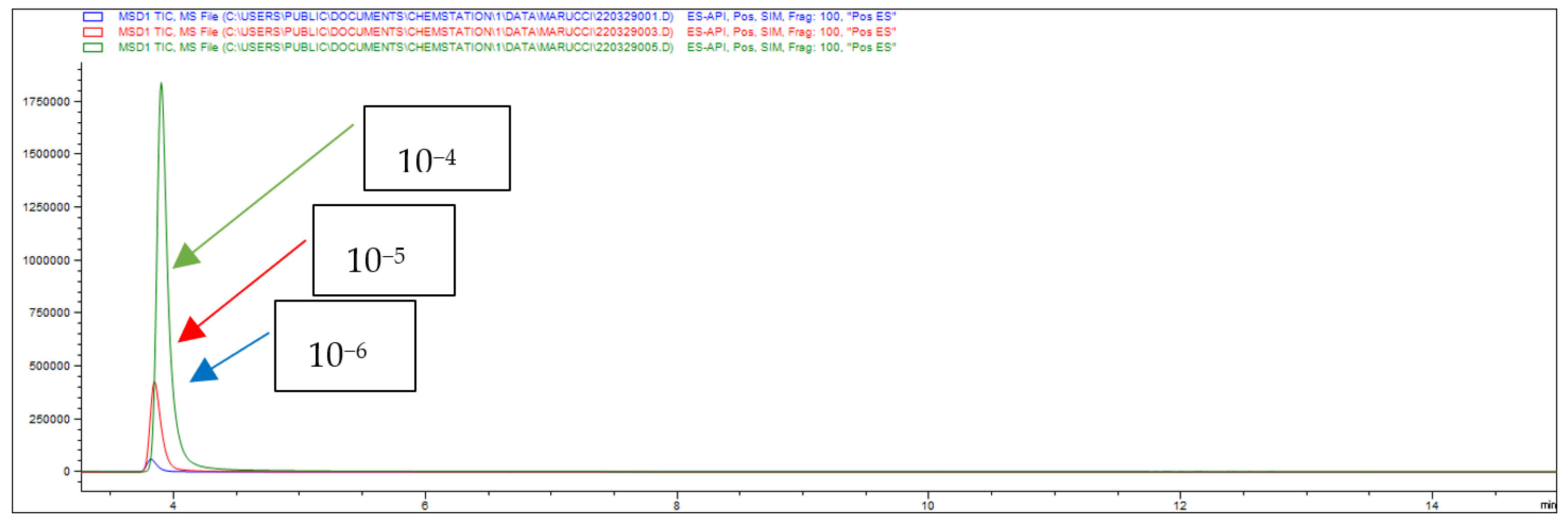
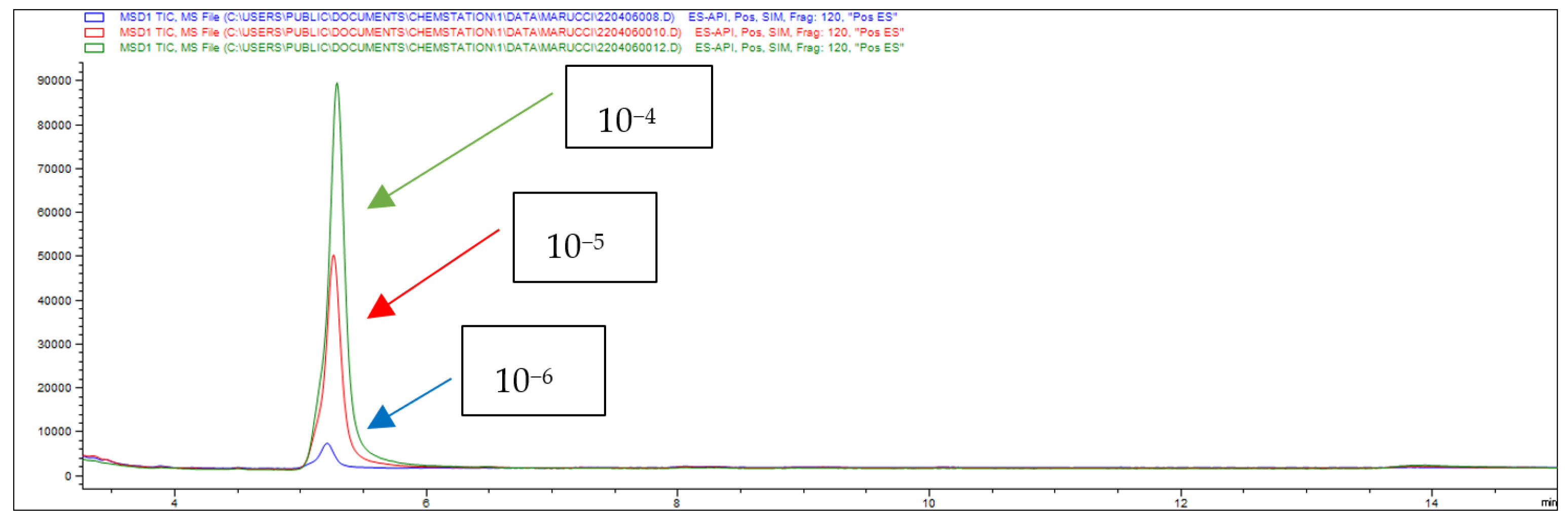

| A | B | |||||
|---|---|---|---|---|---|---|
| Cmp | KiA1 (μM) a | KiA2A (μM) b | KiA3 (μM) c | % Residual Activity at 40 μM ± sd | % Residual Activity at 10 μM ± sd | IC50 (μM) |
| 1 | 3.339 ± 0.658 | 0.123 ± 0.002 | 2.878 ± 0.695 | 19 ± 9.4 | 27 ± 4.0 | 1.75 ± 0.2 |
| 2 | 2.903 ± 0.648 | 0.0762 ± 0.173 | 1.146 ± 0.107 | 22 ± 1.0 | 34 ± 5.0 | 0.59 ± 0.2 |
| 3 | 0.118 ± 0.010 | 0.007 ± 0.00001 | 0.140 ± 0.035 | 86 ± 1.6 | n.d. | >40 |
| 4 | 8.657 ± 1.524 | 3.478 ± 0.312 | 5.957 ± 1.021 | 25 ± 9.6 | 13 ± 24 | 0.36 ± 0.1 |
| ZM 241385 | 348 ± 11 | 1.250 ± 0.21 | 1072 ± 207 | - | - | - |
| PF-670462 | - | - | - | - | - | 0.014 [33] |
| Initial Concentration | Absorbed Concentration |
|---|---|
| 24.6 | 11.5 |
| 2.46 | 1.03 |
| 0.246 | 0.059 |
| Initial Concentration | Absorbed Concentration |
|---|---|
| 37.6 | 8.66 |
| 3.76 | 0.576 |
| 0.376 | - |
| Concentration | Percentage of Drug Absorption (A%) | Percentage of Drug Retention (Ad%) | Papp (×10−6 cm/s) |
|---|---|---|---|
| 10−4 M | 47 | 2 | 33 |
| 10−5 M | 42 | 10 | 29.6 |
| 10−6 M | 24 | 35 | 16.9 |
| Concentration | Percentage of Drug Absorption (A%) | Percentage of Drug Retention (Ad%) | Papp (×10−6 cm/s) |
|---|---|---|---|
| 10−4 M | 23 | 55 | 16.1 |
| 10−5 M | 15 | 66 | 10.8 |
| 10−6 M | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francucci, B.; Angeloni, S.; Dal Ben, D.; Lambertucci, C.; Ricciutelli, M.; Spinaci, A.; Smirnov, A.; Volpini, R.; Buccioni, M.; Marucci, G. Dual Anta-Inhibitors Targeting Protein Kinase CK1δ and A2A Adenosine Receptor Useful in Neurodegenerative Disorders. Molecules 2023, 28, 4762. https://doi.org/10.3390/molecules28124762
Francucci B, Angeloni S, Dal Ben D, Lambertucci C, Ricciutelli M, Spinaci A, Smirnov A, Volpini R, Buccioni M, Marucci G. Dual Anta-Inhibitors Targeting Protein Kinase CK1δ and A2A Adenosine Receptor Useful in Neurodegenerative Disorders. Molecules. 2023; 28(12):4762. https://doi.org/10.3390/molecules28124762
Chicago/Turabian StyleFrancucci, Beatrice, Simone Angeloni, Diego Dal Ben, Catia Lambertucci, Massimo Ricciutelli, Andrea Spinaci, Aleksei Smirnov, Rosaria Volpini, Michela Buccioni, and Gabriella Marucci. 2023. "Dual Anta-Inhibitors Targeting Protein Kinase CK1δ and A2A Adenosine Receptor Useful in Neurodegenerative Disorders" Molecules 28, no. 12: 4762. https://doi.org/10.3390/molecules28124762
APA StyleFrancucci, B., Angeloni, S., Dal Ben, D., Lambertucci, C., Ricciutelli, M., Spinaci, A., Smirnov, A., Volpini, R., Buccioni, M., & Marucci, G. (2023). Dual Anta-Inhibitors Targeting Protein Kinase CK1δ and A2A Adenosine Receptor Useful in Neurodegenerative Disorders. Molecules, 28(12), 4762. https://doi.org/10.3390/molecules28124762










