Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione
Abstract
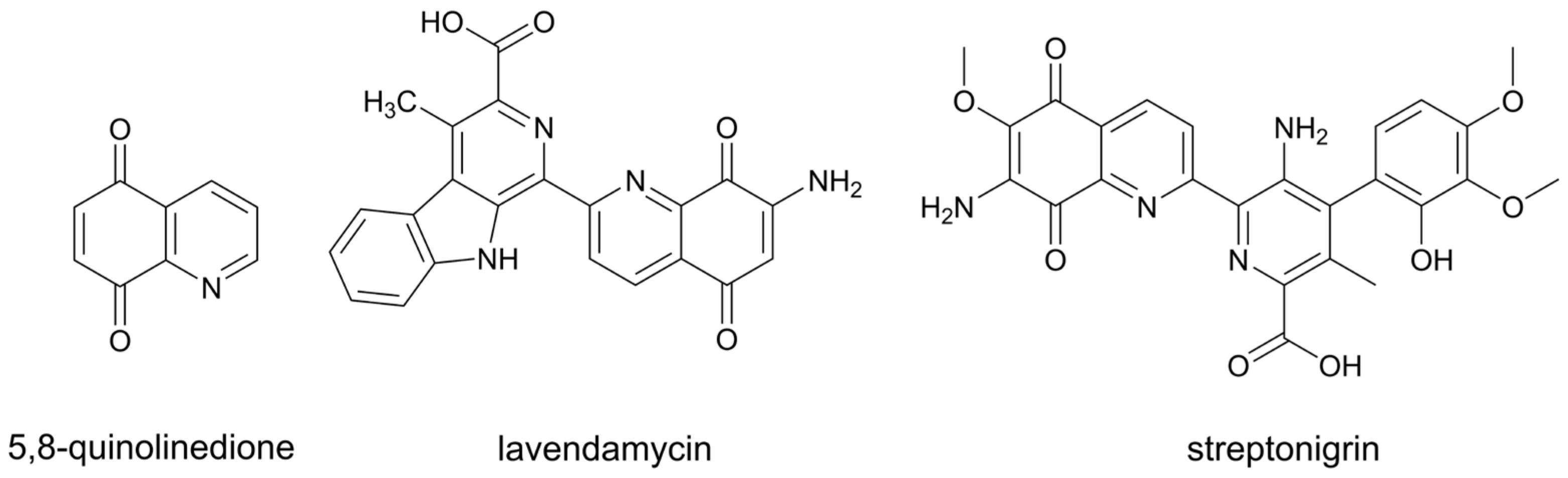
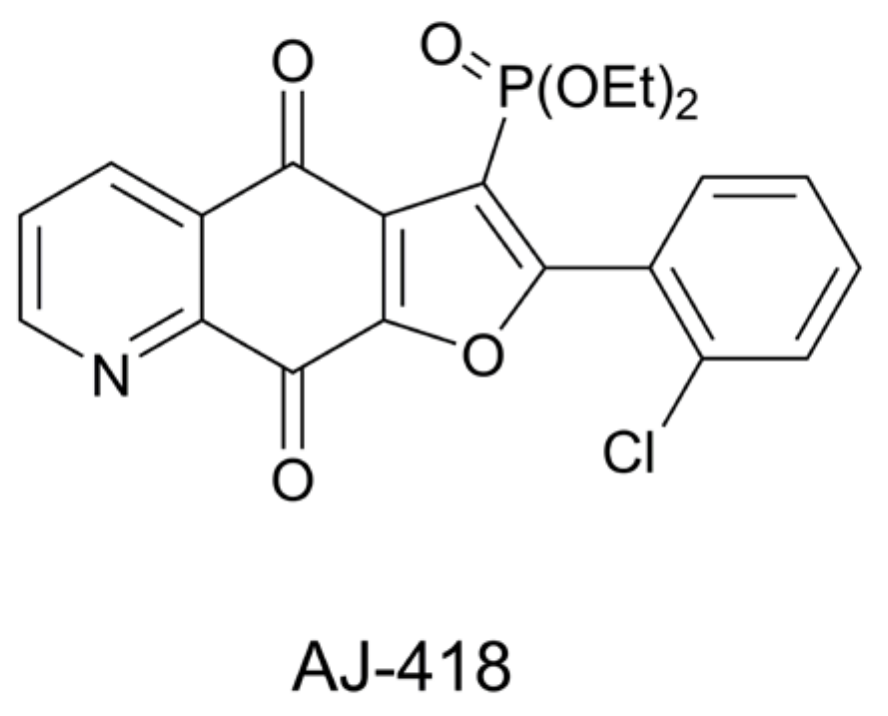
1. Introduction
2. Results
2.1. In Vitro Data
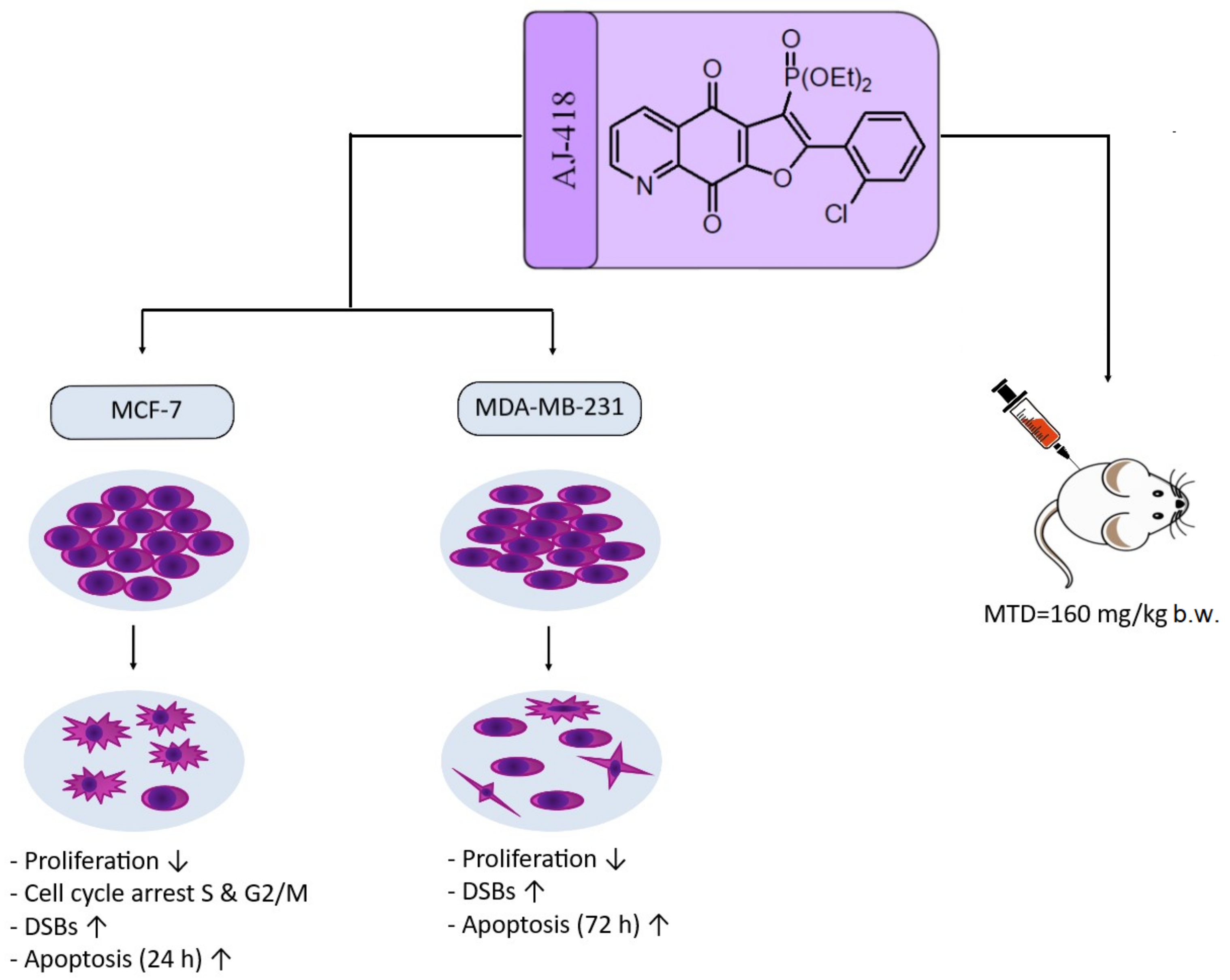
2.1.1. Cytotoxic Activity against Breast Cancer and Non-Tumorigenic Cell Lines (MTT Assay)
2.1.2. Morphology of MCF-7, MDA-MB-231 and MCF-10A Cells Incubated with AJ-418
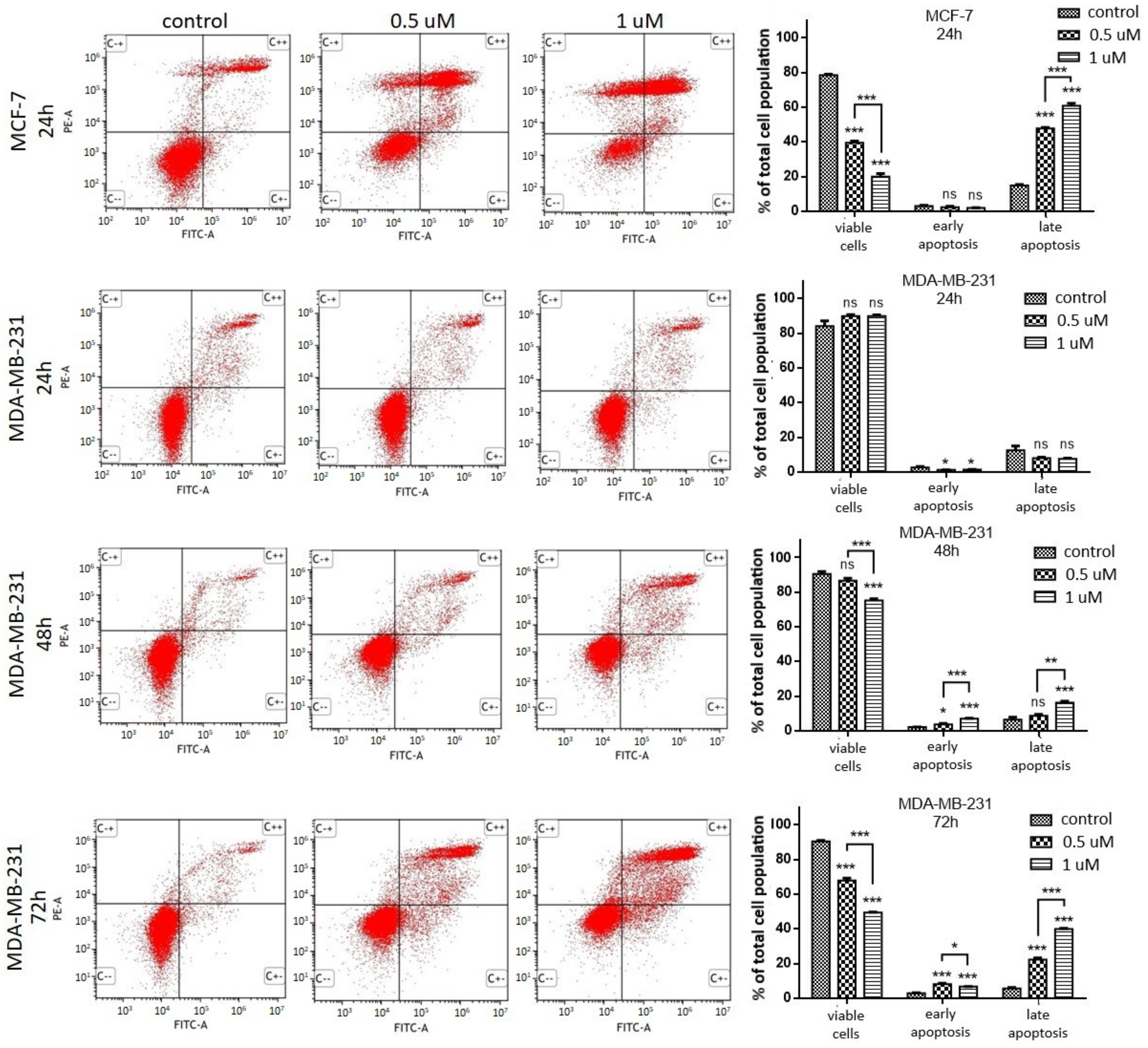
2.1.3. Apoptosis Induction
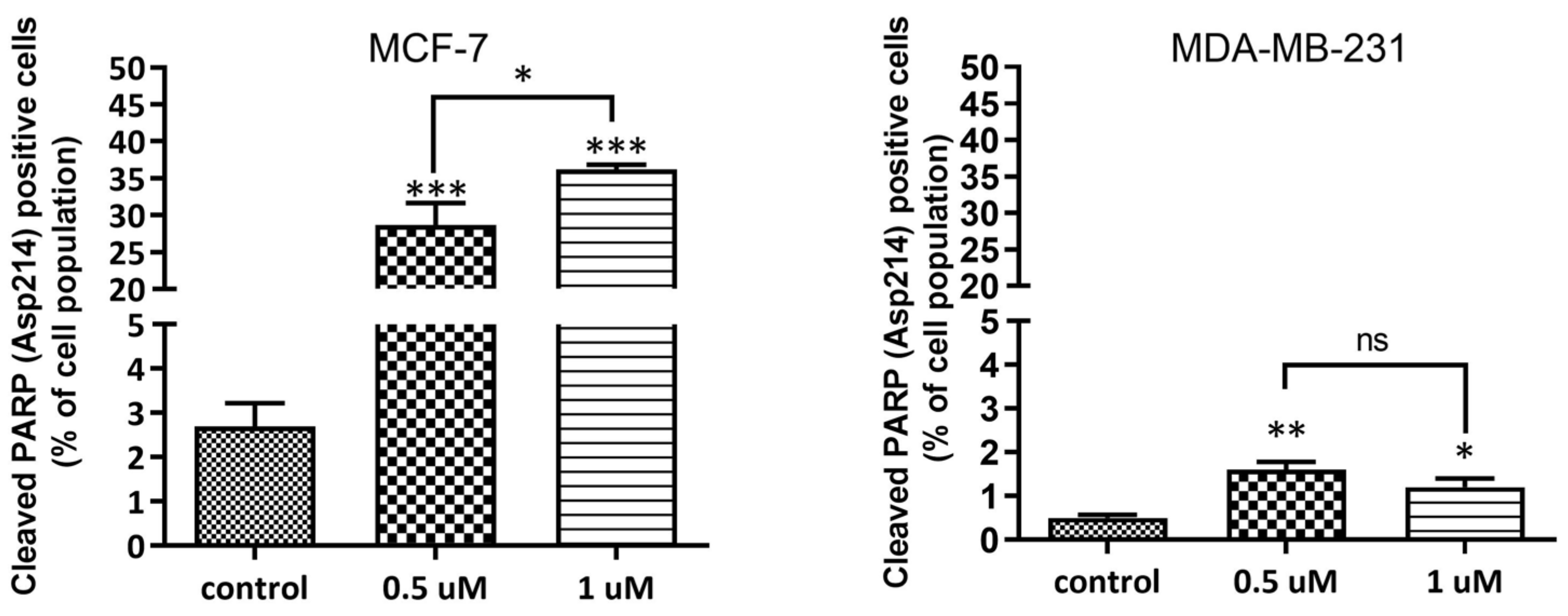
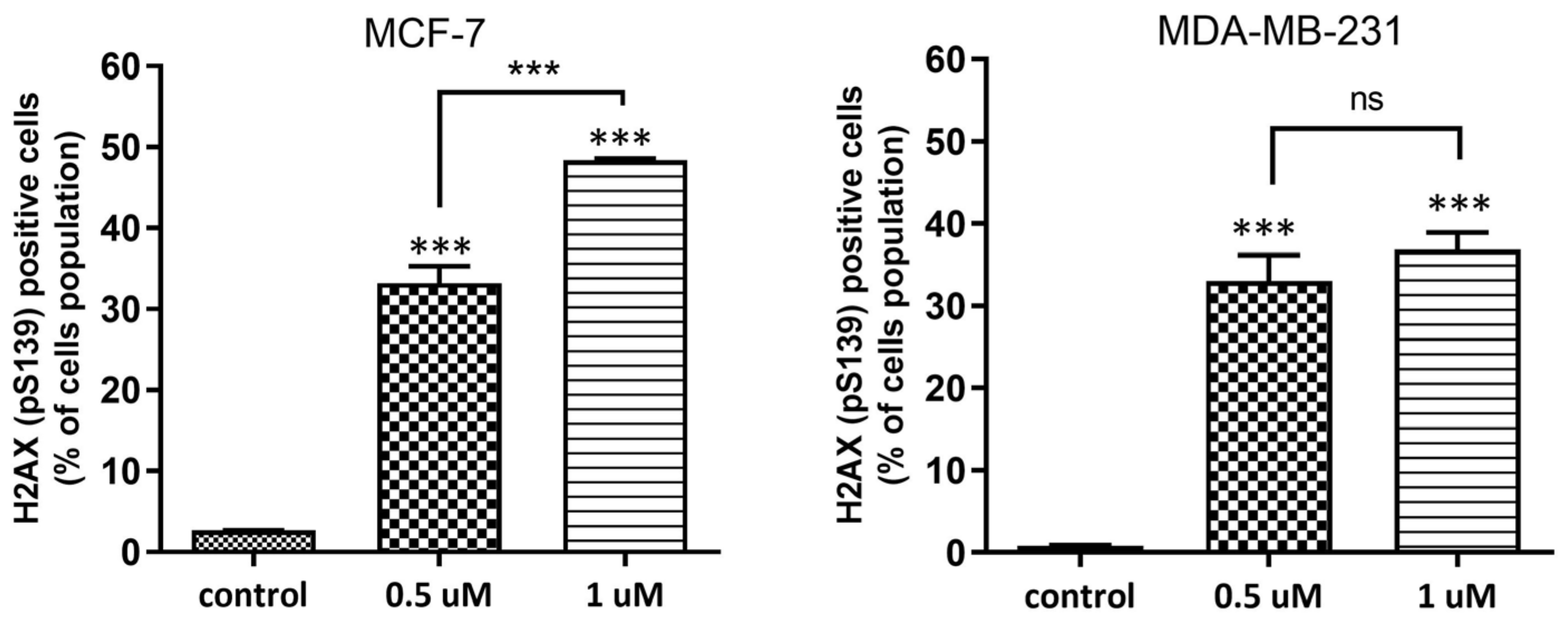
2.1.4. DNA Damage Assessment
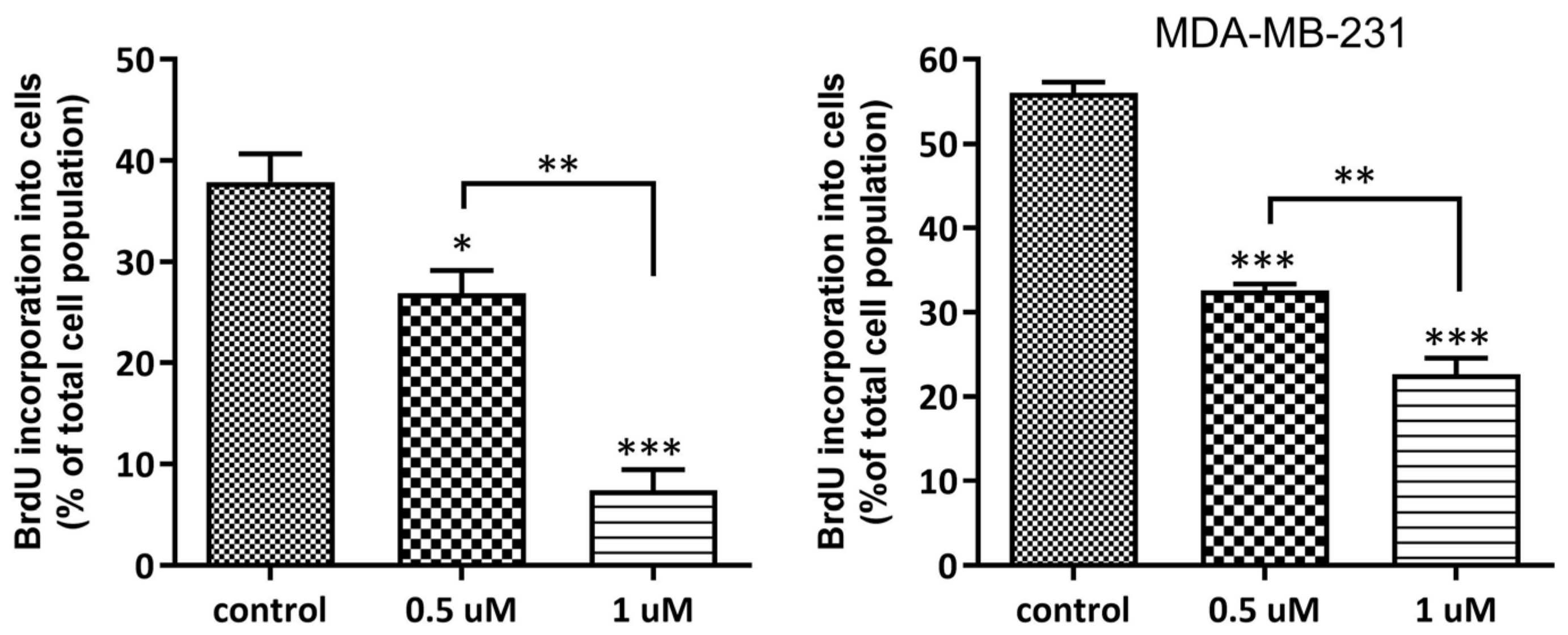
2.1.5. Antiproliferative Activity
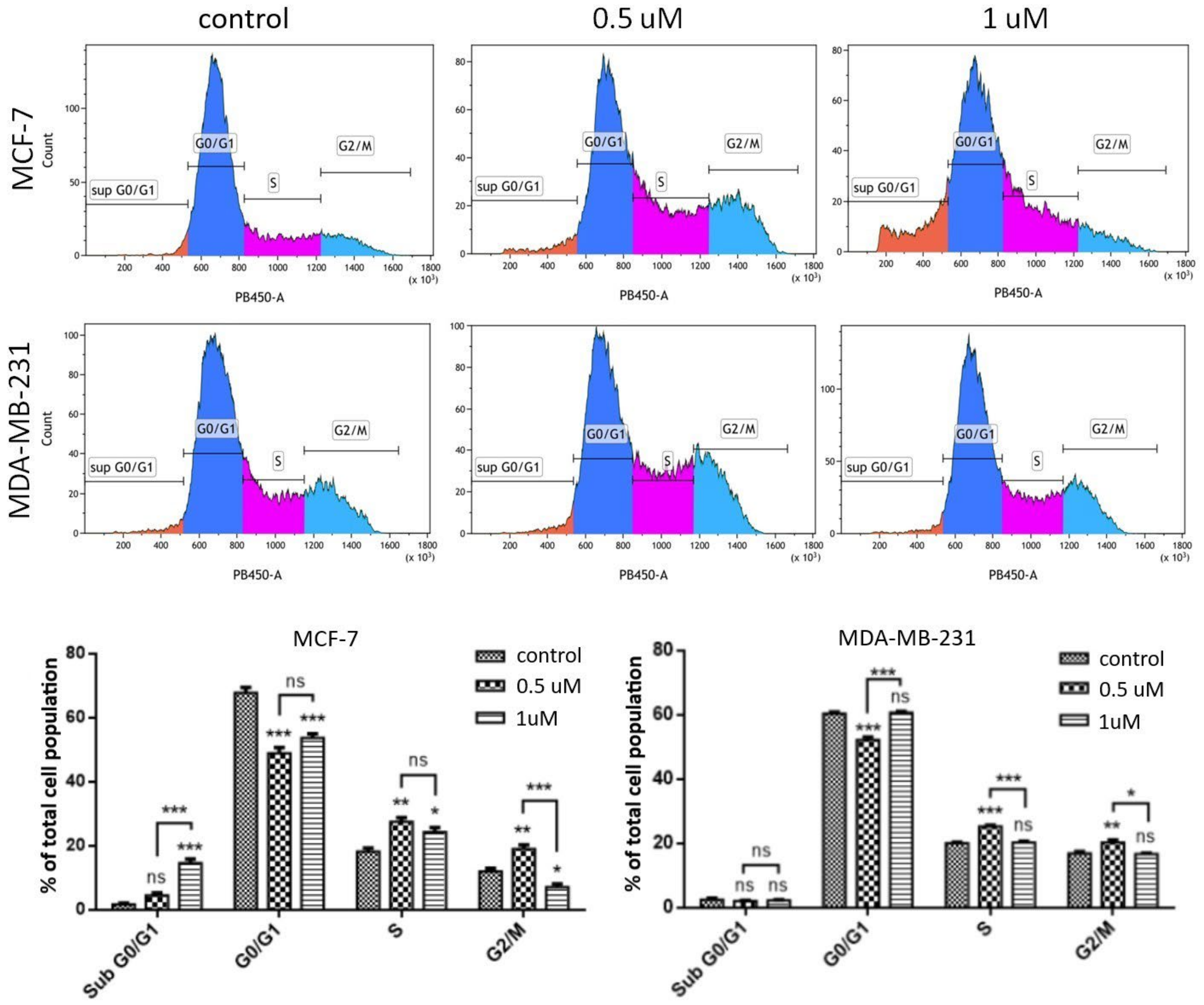
2.1.6. Cell Cycle Analysis
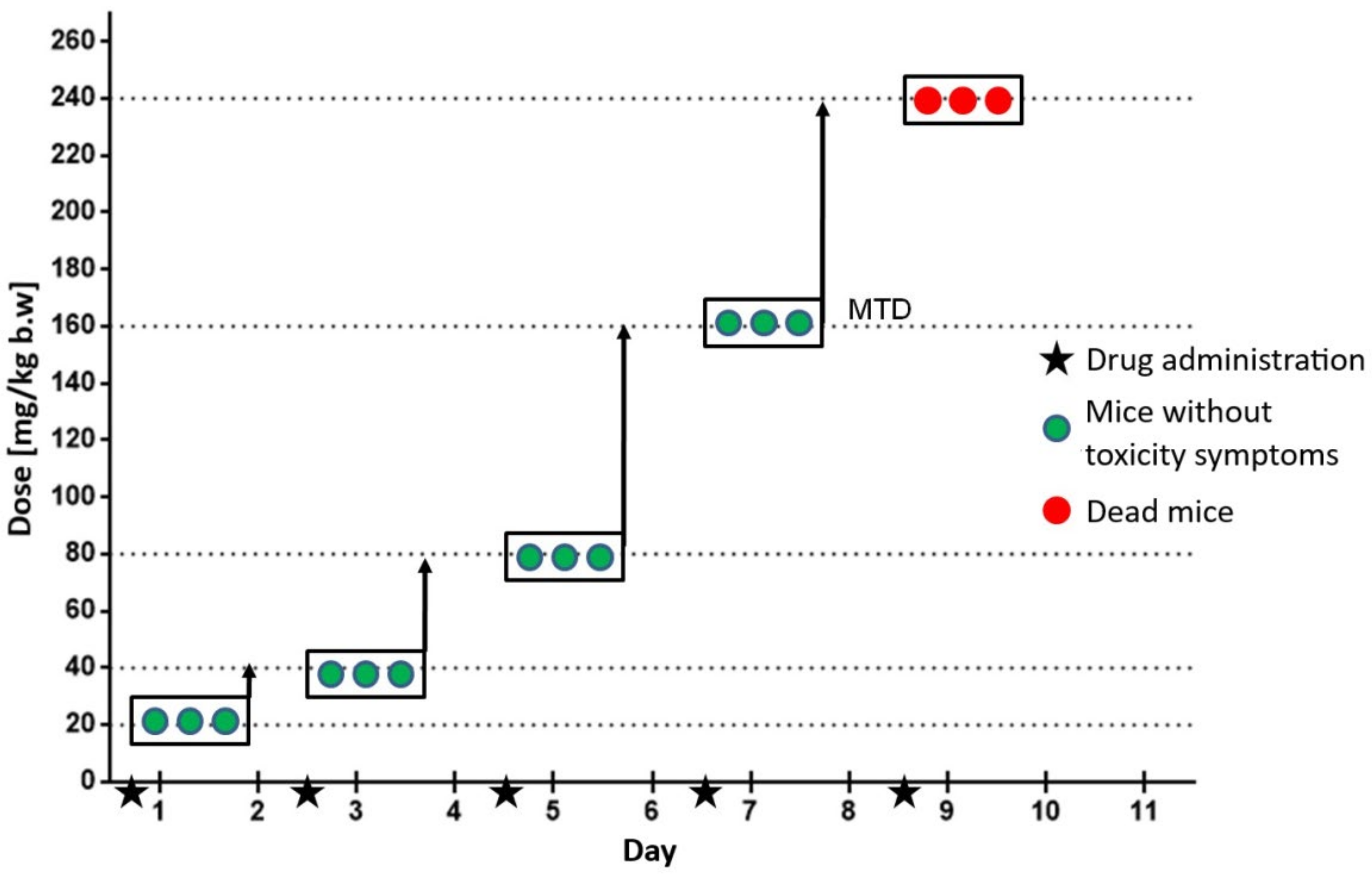
2.2. In Vivo Studies—Evaluation of Maximum Tolerated Dose in C3H Mice
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Sample Preparation
4.2. MTT Assay
4.3. Assessment of Morphological Changes
4.4. Apoptosis Detection by Annexin V and Propidium Iodide Staining
4.5. Evaluation of Cell Cycle Distribution, Proliferation, Apoptosis and DNA Damage Using the “Apoptosis, DNA Damage and Cell Proliferation Kit”
4.6. Animals
4.7. Determination of the Maximum Tolerated Dose (MTD)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jampilek, J. Heterocycles in medicinal chemistry. Molecules 2019, 24, 3839. [Google Scholar] [CrossRef] [PubMed]
- Mancini, I.; Vigna, J.; Sighel, D.; Defant, A. Hybrid molecules containing naphthoquinone and quinolinedione scaffolds as antineoplastic agents. Molecules 2022, 27, 4948. [Google Scholar] [CrossRef] [PubMed]
- Kadela-Tomanek, M.; Bębenek, E.; Chrobak, E.; Boryczka, S. 5, 8-Quinolinedione scaffold as a promising moiety of bioactive agents. Molecules 2019, 24, 4115. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Cullen, W.P. Streptonigrin, an antitumor substance. I. Isolation and characterization. Antibiot. Annu. 1959, 7, 950–953. [Google Scholar]
- Balitz, D.M.; Bush, J.A.; Bradner, W.T.; Doyle, T.W.; O’Herron, F.A.; Nettleton, D.E. Isolation of lavendamycin, a new antibiotic from Streptomyces lavendulae. J. Antibiot. 1982, 35, 259–265. [Google Scholar] [CrossRef]
- Bolzán, A.D.; Bianchi, M.S. Genotoxicity of streptonigrin: A review. Mutat. Res. 2001, 488, 25–37. [Google Scholar] [CrossRef]
- Hassani, M.; Cai, W.; Koelsch, K.H.; Holley, D.C.; Rose, A.S.; Olang, F.; Lineswala, J.P.; Holloway, W.G.; Gerdes, J.M.; Behforouz, M.; et al. Lavendamycin antitumor agents: Structure-based design, synthesis, and NAD(P)H:quinone oxidoreductase 1 (NQO1) model validation with molecular docking and biological studies. J. Med. Chem. 2008, 51, 3104–3115. [Google Scholar] [CrossRef]
- Ling, Y.; Yang, Q.X.; Teng, Y.N.; Chen, S.; Gao, W.J.; Guo, J.; Hsu, P.L.; Liu, Y.; Morris-Natschke, S.L.; Hung, C.C.; et al. Development of novel amino-quinoline-5,8-dione derivatives as NAD(P)H:quinone oxidoreductase 1 (NQO1) inhibitors with potent antiproliferative activities. Eur. J. Med. Chem. 2018, 154, 199–209. [Google Scholar] [CrossRef]
- Kadela-Tomanek, M.; Jastrzębska, M.; Pawełczak, B.; Bębenek, E.; Chrobak, E.; Latocha, M.; Boryczka, S. Alkynyloxy derivatives of 5, 8-quinolinedione: Synthesis, in vitro cytotoxicity studies and computational molecular modeling with NAD (P) H:Quinone oxidoreductase 1. Eur. J. Med. Chem. 2017, 126, 969–982. [Google Scholar] [CrossRef]
- Zhou, B.-Q.; Huang, X.-L.; Qin, Q.-P.; Wang, Z.-F.; Wu, X.-Y.; Tan, M.-X.; Liang, H. Transition metal complexes with 6,7-dichloro-5,8-quinolinedione as mitochondria-targeted anticancer agents. Polyhedron 2020, 181, 114–482. [Google Scholar]
- Hsu, T.S.; Chen, C.; Lee, P.T.; Chiu, S.J.; Liu, H.F.; Tsai, C.C.; Chao, J.I. 7-Chloro-6-piperidin-1-yl-quinline-5, 8-dione (PT-262), a novel synthetic compound induces lung carcinoma cell death associated with inhibiting ERK and CDC2 phosphorylation via a p53-independent pathway. Cancer Chemother. Pharmacol. 2008, 62, 799–808. [Google Scholar] [CrossRef]
- Yu, L.-M.; Hu, Z.; Chen, Y.; Ravji, A.; Lopez, S.; Plescia, C.B.; Yu, Q.; Yang, H.; Abdelmalak, M.; Saha, S.; et al. Synthesis and structure-activity relationship of furoquinolinediones as inhibitors of tyrosyl-DNA phosphodiesterase 2 (TDP2). Eur. J. Med. Chem. 2018, 151, 777–802. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.Y.; Zou, Y.X. Recent advances in the construction of phosphorus-substituted heterocycles, 2009–2019. Adv. Synth. Catal. 2020, 362, 1724–1818. [Google Scholar] [CrossRef]
- Yu, H.; Yang, H.; Shi, E.; Tang, W. Development and clinical application of phosphorus-containing drugs. Med. Drug Discov. 2020, 8, 100063. [Google Scholar] [CrossRef]
- Rodriguez, G. Fludarabine phosphate. Investig. New Drugs 1994, 12, 75–92. [Google Scholar] [CrossRef]
- Wiemer, A.J.; Wiemer, D.F. Prodrugs of phosphonates and phosphates: Crossing the membrane barrier. Top. Curr. Chem. 2015, 360, 115–160. [Google Scholar]
- Bagchi, S.; Rathee, P.; Jayaprakash, V.; Banerjee, S. Farnesyl transferase inhibitors as potential anticancer agents. Mini Rev. Med. Chem. 2018, 18, 1611–1623. [Google Scholar] [CrossRef]
- Al-Kali, A.; Gandhi, V.; Ayoubi, M.; Keating, M.; Ravandi, F. Forodesine: Review of preclinical and clinical data. Future Oncol. 2010, 6, 1211–1217. [Google Scholar] [CrossRef]
- Modranka, J.; Drogosz-Stachowicz, J.; Pietrzak, A.; Janecka, A.; Janecki, T. Synthesis and structure–activity relationship study of novel 3-diethoxyphosphorylfuroquinoline-4, 9-diones with potent antitumor efficacy. Eur. J. Med. Chem. 2021, 219, 113–429. [Google Scholar] [CrossRef]
- Mashimo, M.; Onishi, A.; Uno, A.; Tanimichi, A.; Nobeyama, M.; Mori, T.; Yamada, S.; Negi, S.; Bu, X.; Kato, J. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021, 296, 100046–100058. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Fernandez-Capetillo, O.; Lee, A.; Nussenzweig, M.; Nussenzweig, A. H2AX: The histone guardian of the genome. DNA Repair 2004, 3, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Marusyk, A.; Almendro, V.; Polyak, K. Intra-tumour heterogeneity: A looking glass for cancer? Nat. Rev. Cancer 2012, 12, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.H.; Lu, D.I.; Wang, N.; Liu, L.Y.; Wang, Y.; Li, Y.Q.; Yan, T.B.; Sun, X.G.; Hu, P.; Zhang, T.C. Estrogen receptor alpha mediate proliferation of breast cancer MCF-7 cells via a p21/PCNA/E2F1-dependent pathway. FEBS J. 2014, 17, 581–590. [Google Scholar]
- Zeng, L.; Li, W.; Chen, C.S. Breast cancer animal models and applications. Zool. Res. 2020, 41, 477–494. [Google Scholar] [CrossRef]
- Lamb, C.A.; Vanzulli, S.I.; Lanari, C. Hormone receptors in breast cancer: More than estrogen receptors. Medicina 2019, 79, 540–545. [Google Scholar]
- Zheng, A.; Kallio, A.; Harkonen, P. Tamoxifen-induced rapid death of MCF-7 breast cancer cells is mediated via extracellularly signal-regulated kinase signaling and can be abrogated by oestrogen. Endocrinology 2007, 148, 2764–2777. [Google Scholar] [CrossRef]
- Akekawatchai, C.; Roytrakul, S.; Kittisenachai, S.; Isarankura-Na-Ayudhya, P.; Jitrapakdee, S. Protein profiles associated with anoikis resistance of metastatic MDA-MB-231 breast cancer cells. Asian Pac. J. Cancer Prev. 2016, 17, 1007–1012. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, D.H.; Chun, S.Y.; Nam, K.S. Metastatic potential in MDA-MB-231 human breast cancer cells is inhibited by proton beam irradiation via the Akt/nuclear factor-kappaB signaling pathway. Mol. Med. Rep. 2014, 10, 1007–1012. [Google Scholar] [CrossRef]
- Lyons, T.G. Targeted therapies for triple-negative breast cancer. Curr. Treat. Options Oncol. 2019, 20, 82–94. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020, 22, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Tait, L.; Soule, H.D.; Russo, J. Ultrastructural and immunocytochemical characterization of an immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6087–6094. [Google Scholar] [PubMed]
- Soule, H.D.; Maloney, T.M.; Wolman, S.R.; Peterson, W.D.; Brenz, R.; McGrath, C.M.; Russo, J.; Pauley, R.J.; Jones, R.F.; Brooks, S.C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6075–6086. [Google Scholar] [PubMed]
- Lee, K.J.; Mann, E.; Wright, G.; Piett, C.G.; Nagel, Z.D.; Gassman, N.R. Exploiting DNA repair defects in triple negative breast cancer to improve cell killing. Ther. Adv. Med. Oncol. 2020, 12, 1758835920958354–1758835920958370. [Google Scholar] [CrossRef]
- McLaren, A.N.N.E.; Taita, A. Cytoplasmic isocitrate dehydrogenase variation within the C3H inbred strain. Gen. Res. 1969, 14, 93–94. [Google Scholar] [CrossRef]
- Shao, R.; Dao, M.L.; Day, N.K.; Good, R.A. Dietary manipulation of mammary tumor development in adult C3H/Bi Mice. Exp. Biol. Med. 1990, 193, 313–317. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. NC3Rs Reporting Guidelines Working Group. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Aston, W.J.; Hope, D.E.; Nowak, A.K.; Robinson, B.W.; Lake, R.A.; Lesterhuis, W.J. A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice. BMC Cancer 2017, 17, 684–693. [Google Scholar] [CrossRef]

| Cell Line | IC50 (µM) a | ||
|---|---|---|---|
| AJ-418 | Doxorubicin | ||
| 48 h | 24 h | 48 h | |
| MCF-7 | 0.10 ± 0.01 | 0.46 ± 0.01 | 0.89 ± 0.12 |
| MDA-MB-231 | 0.12 ± 0.01 | 0.49 ± 0.01 | 0.68 ± 0.08 |
| MCF-10A | 0.50 ± 0.01 | 1.82 ± 0.04 | 1.2 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drogosz-Stachowicz, J.; Gach-Janczak, K.; Mirowski, M.; Pietrzak, J.; Janecki, T.; Janecka, A. Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione. Molecules 2023, 28, 3128. https://doi.org/10.3390/molecules28073128
Drogosz-Stachowicz J, Gach-Janczak K, Mirowski M, Pietrzak J, Janecki T, Janecka A. Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione. Molecules. 2023; 28(7):3128. https://doi.org/10.3390/molecules28073128
Chicago/Turabian StyleDrogosz-Stachowicz, Joanna, Katarzyna Gach-Janczak, Marek Mirowski, Jacek Pietrzak, Tomasz Janecki, and Anna Janecka. 2023. "Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione" Molecules 28, no. 7: 3128. https://doi.org/10.3390/molecules28073128
APA StyleDrogosz-Stachowicz, J., Gach-Janczak, K., Mirowski, M., Pietrzak, J., Janecki, T., & Janecka, A. (2023). Anticancer Properties of 3-Dietoxyphosphorylfuroquinoline-4,9-dione. Molecules, 28(7), 3128. https://doi.org/10.3390/molecules28073128







