Fluorination Improves the Electro-Optical Properties of Benzoxazole-Terminated Liquid Crystals in High Birefringence Liquid Crystal Mixtures: Experimental and Theoretical Investigations
Abstract
1. Introduction
2. Results and Discussion
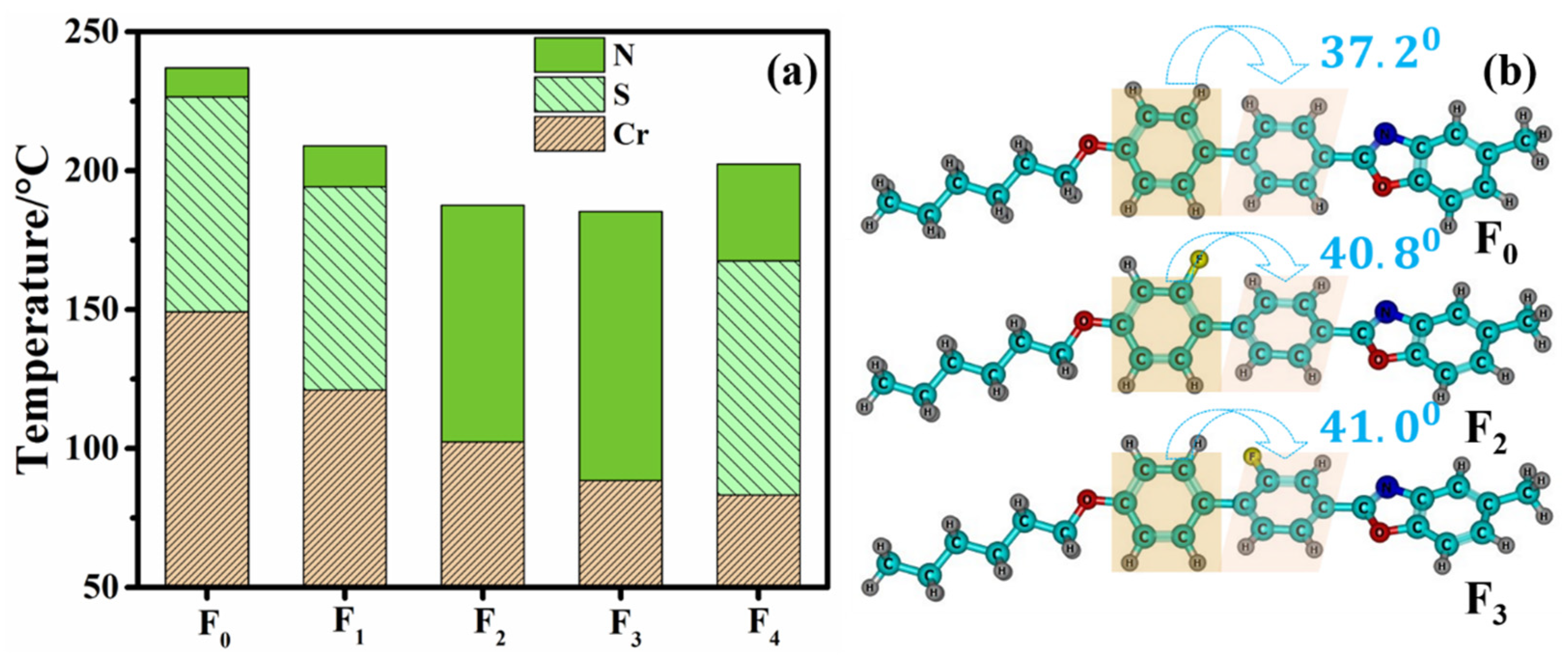
2.1. Effect of Fluorination on Solubility
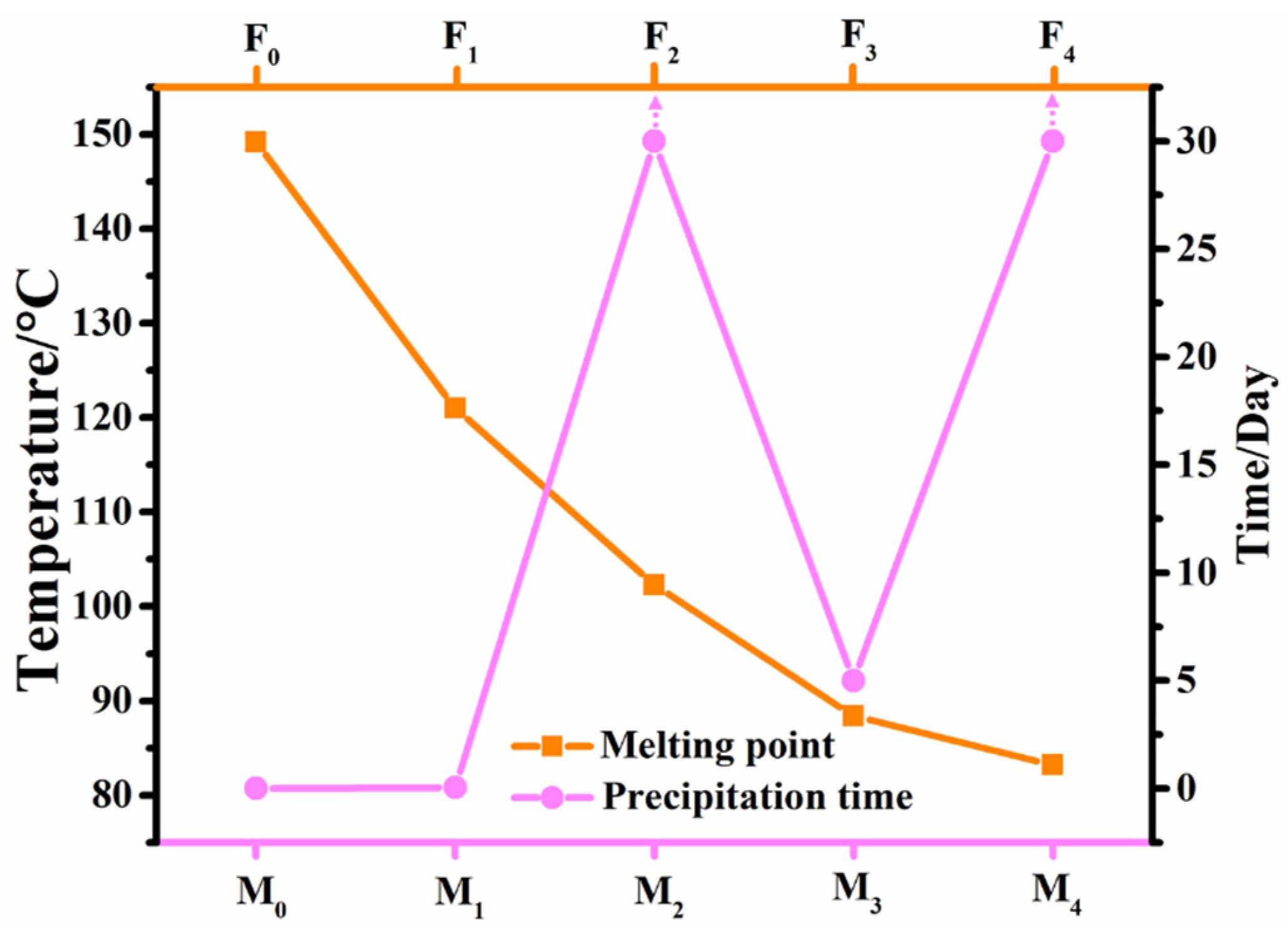
2.2. Effect of Fluorination on Mesomorphic Properties
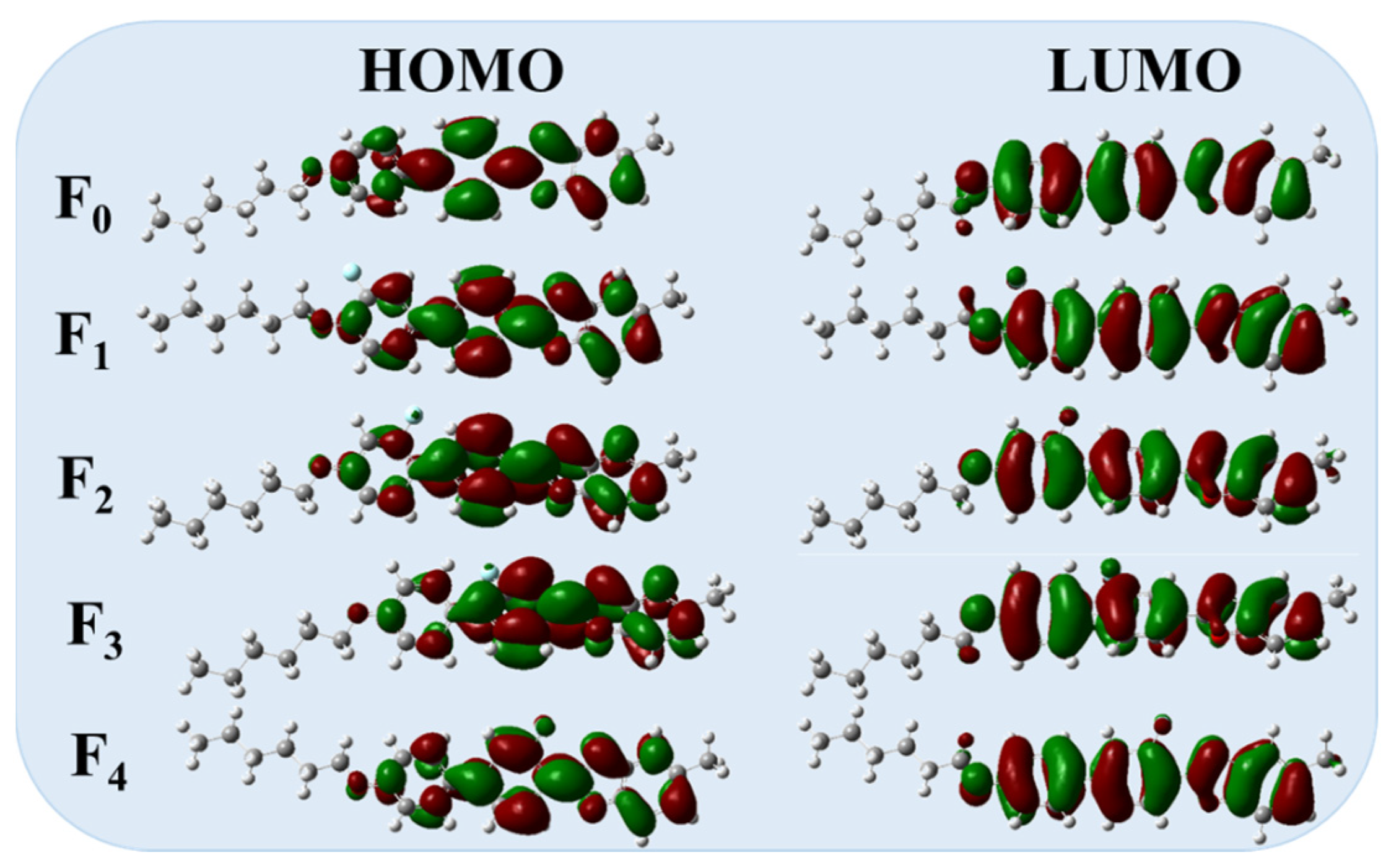
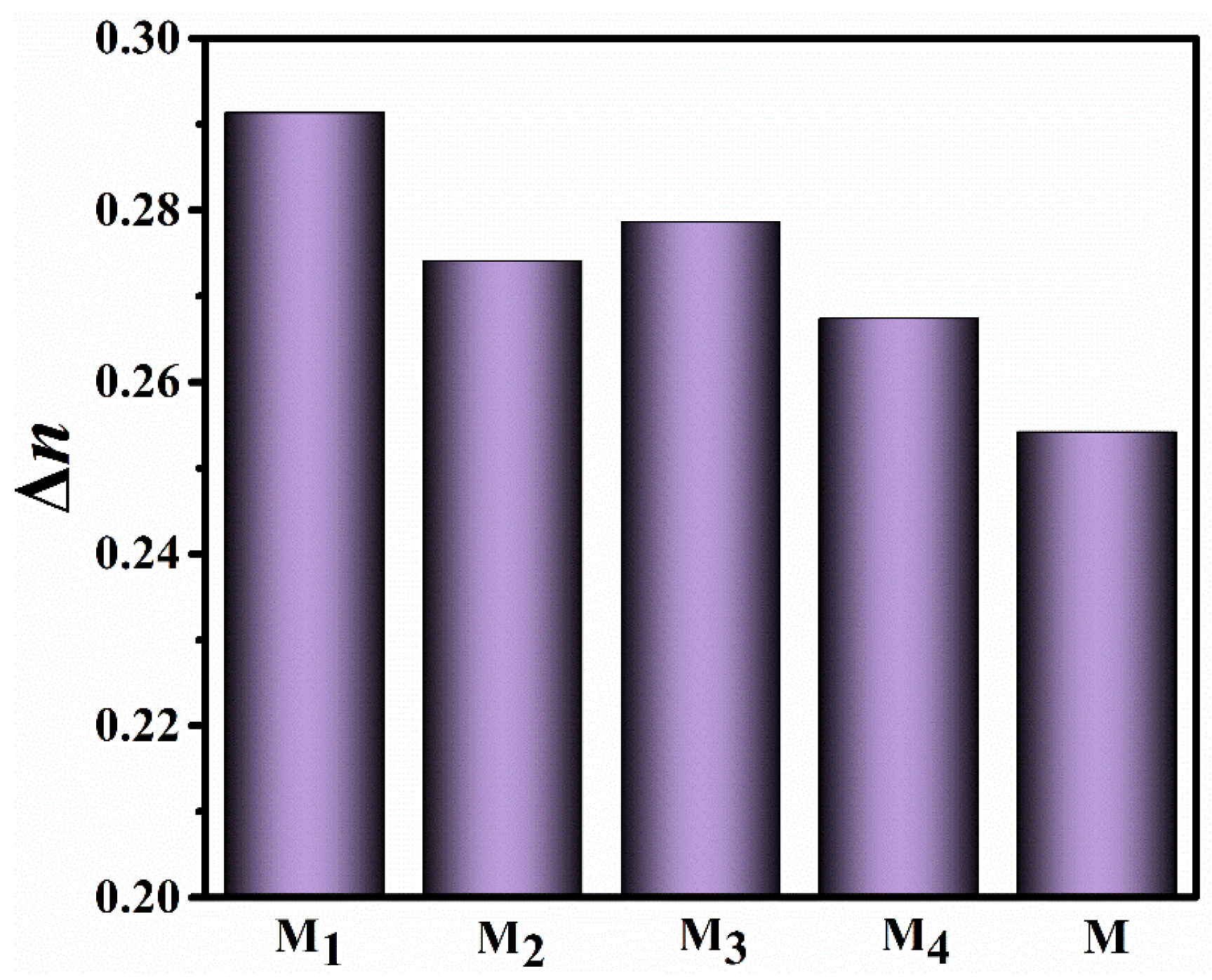
2.3. Effect of Fluorination on Birefringence
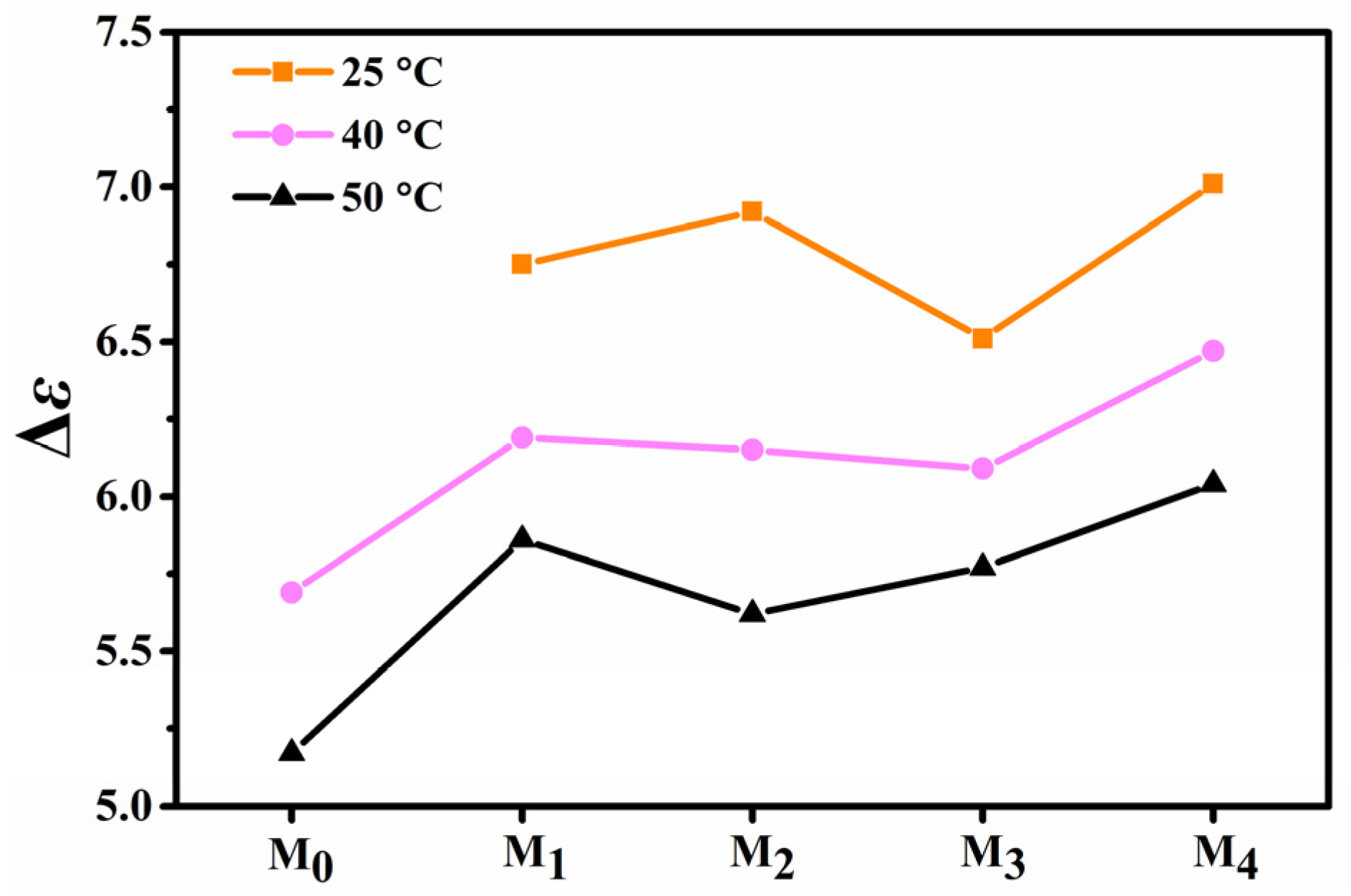
2.4. Effect of Fluorination on Dielectric Anisotropy
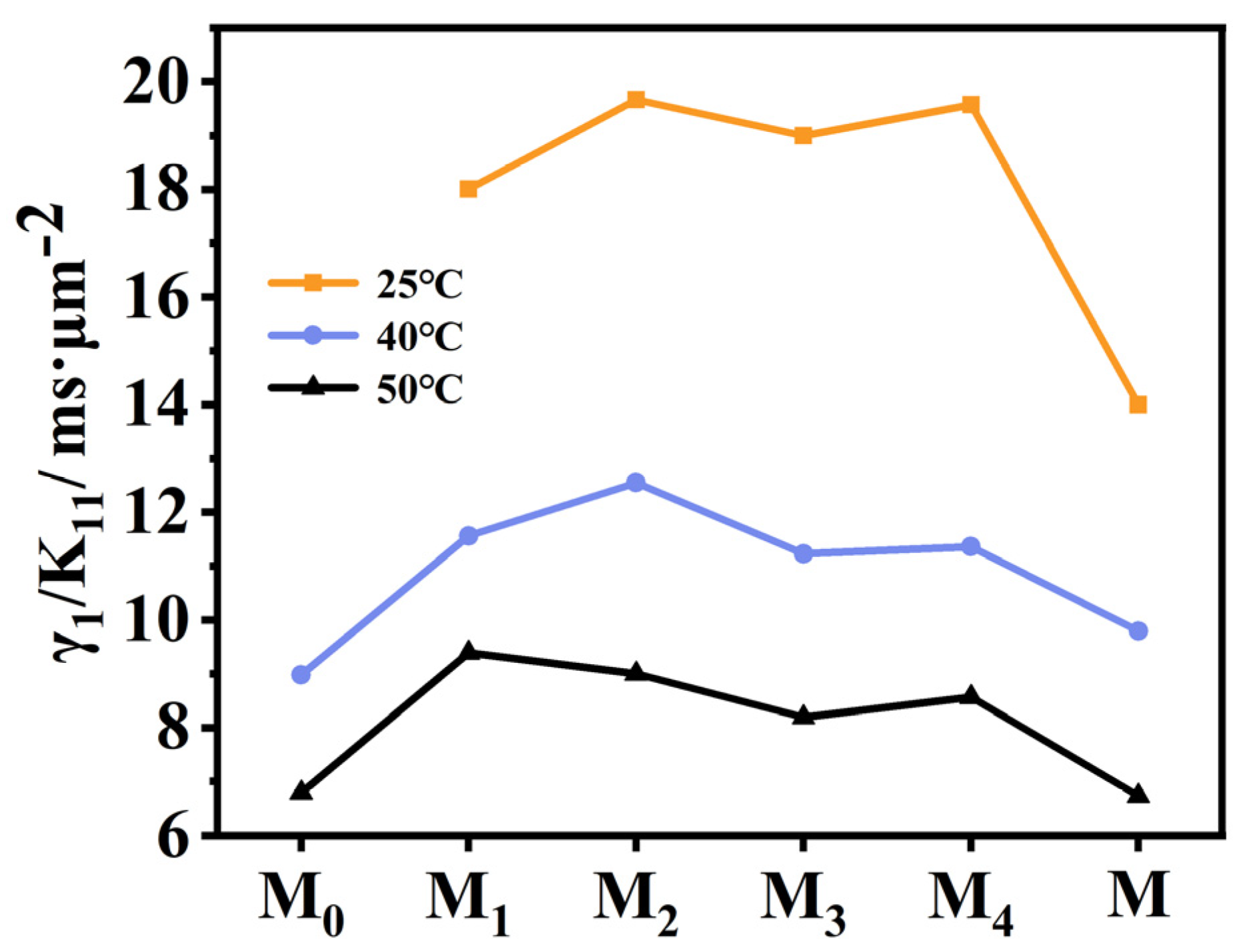
2.5. Effect of Fluorination on Viscoelastic Constant
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Target Compounds
3.3. The Preparation of LC Mixtures and Solubility Experiments
3.4. Characterization and Measurements
3.5. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhan, T.; Yin, K.; Xiong, J.; He, Z.; Wu, S.-T. Augmented Reality and Virtual Reality Displays: Perspectives and Challenges. iScience 2020, 23, 101397. [Google Scholar] [CrossRef]
- Yin, K.; Hsiang, E.-L.; Zou, J.; Li, Y.; Yang, Z.; Yang, Q.; Lai, P.-C.; Lin, C.-L.; Wu, S.-T. Advanced liquid crystal devices for augmented reality and virtual reality displays: Principles and applications. Light Sci. Appl. 2022, 11, 161. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Xiong, J.; Yin, K.; Wu, S.-T. 3D displays in augmented and virtual realities with holographic optical elements [Invited]. Opt. Express 2021, 29, 42696. [Google Scholar] [CrossRef]
- Wang, X.; Fells, J.A.; Shi, Y.; Ali, T.; Welch, C.; Mehl, G.H.; Wilkinson, T.D.; Booth, M.J.; Morris, S.M.; Elston, S.J. A Compact Full 2π Flexoelectro-Optic Liquid Crystal Phase Modulator. Adv. Mater. Technol. 2020, 5, 2000589. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Wang, Y.-J.; Reshetnyak, V. Liquid crystal lenses with tunable focal length. Liq. Cryst. Rev. 2018, 5, 111–143. [Google Scholar] [CrossRef]
- Nyushkov, B.N.; Trashkeev, S.I.; Ivanenko, A.V.; Kolker, D.B.; Purtov, P.A. Fiber-to-fiber nonlinear coupling via a nematic liquid crystal. Laser Phys. Lett. 2017, 14, 15104. [Google Scholar] [CrossRef]
- Morris, S.M.; Gardiner, D.J.; Hands, P.J.W.; Qasim, M.M.; Wilkinson, T.D.; White, I.H.; Coles, H.J. Electrically switchable random to photonic band-edge laser emission in chiral nematic liquid crystals. Appl. Phys. Lett. 2012, 100, 071110. [Google Scholar] [CrossRef]
- Wang, L.; Ge, S.; Hu, W.; Nakajima, M.; Lu, Y. Tunable reflective liquid crystal terahertz waveplates. Opt. Mater. Express 2017, 7, 2023–2029. [Google Scholar] [CrossRef]
- Koh, T.-M.; Ha, S.-T.; Yeap, G.-Y.; Lin, H.-C. New mesomorphic benzothiazol derivatives: Synthesis and characterization. Chin. Chem. Lett. 2013, 24, 926–928. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, Y.; Du, S.; Yuan, D.; Chen, P.; Liu, G.; Dang, J.; Chen, X.; An, Z. Mesomorphic properties improved via lateral fluorine substituent on benzoxazole-terminated mesogenic compounds. Liq. Cryst. 2020, 47, 1555–1568. [Google Scholar] [CrossRef]
- Shanker, G.; Tschierske, C. Synthesis of non-symmetrically substituted 1,2,4-oxadiazole derived liquid crystals. Tetrahedron 2011, 67, 8635–8638. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Singh, G.; Fisch, M.R.; Agra-Kooijman, D.M.; Li, Q.; Kumar, S. Chiral and orientationally ordered fluid mesophases formed by oxadiazole bisaniline based achiral bent mesogens. Liq. Cryst. 2019, 46, 1373–1382. [Google Scholar] [CrossRef]
- Rahman, M.L.; Hegde, G.; Yusoff, M.M.; Malek, M.N.F.A.; Srinivasa, H.T.; Kumar, S. New pyrimidine-based photo-switchable bent-core liquid crystals. New J. Chem. 2013, 37, 2460–2467. [Google Scholar] [CrossRef]
- Chen, R.; Wang, L.; An, Z.; Chen, X.; Chen, P. Effect of π-conjugation units on the liquid crystal and photovoltaic performance of heterocyclic pyridine-based compounds. Liq. Cryst. 2021, 48, 2178–2187. [Google Scholar] [CrossRef]
- Chen, R.; Weng, Q.; An, Z.; Zhu, S.; Wang, Q.; Chen, X.; Chen, P. Investigation of 4-pyridyl liquid crystals on the photovoltaic performance and stability of dye sensitized solar cells by the co-sensitization. Dye. Pigment. 2018, 159, 527–532. [Google Scholar] [CrossRef]
- Hu, K.; Weng, Q.; Chen, R.; Li, J.; Shi, D.; Chen, P.; Gao, A.; Chen, X.; An, Z. Benzoxazole-terminated liquid crystals with high birefringence and large dielectric anisotropy. Liq. Cryst. 2020, 47, 1274–1280. [Google Scholar] [CrossRef]
- Li, J.; Mo, L.; Che, Z.; Li, J.; Hu, M.; Wan, D.; An, Z. New multi-fluorinated benzofuran liquid crystals with large dielectric anisotropy and improved solubility. Liq. Cryst. 2022, 49, 1753–1762. [Google Scholar] [CrossRef]
- Duan, L.; Shi, D.; Chen, P.; Zhang, L.; Ren, L.; Chen, X.; An, Z. Preparation and characterisation of laterally monofluorinated mesogenic benzimidazole-based compounds. Liq. Cryst. 2017, 44, 1678–1685. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Aboelnaga, A. Synthesis and mesomorphic study of new phenylthiophene liquid crystals. Liq. Cryst. 2021, 49, 804–811. [Google Scholar] [CrossRef]
- Xie, N.; Du, S.; Chen, R.; Liu, G.; Chen, P.; Weng, Q.; Wang, J.; Gao, A.; Chen, X.; An, Z. Synthesis and properties of benzoxazole-terminated mesogenic compounds containing tolane with high birefringence and large dielectric anisotropy. Liq. Cryst. 2021, 48, 1978–1991. [Google Scholar] [CrossRef]
- Hu, K.; Chen, P.; Chen, X.; An, Z. Synthesis and Characterization of Mesogenic Compounds Possessing Bithiophene and Benzoxazole Units. Mol. Cryst. Liq. Cryst. 2015, 608, 25–37. [Google Scholar] [CrossRef]
- Weng, Q.; Duan, L.; Chen, P.; Gao, A.; Chen, X.; An, Z. Synthesis and mesomorphic properties of the nematic mesophase benzoxazole derivatives with big twist angle of difluoro-biphenyl unit. Liq. Cryst. 2019, 46, 1013–1023. [Google Scholar] [CrossRef]
- Zhang, M.; Du, S.; Yuan, D.; Chen, P.; Liu, G.; Dang, J.; Chen, X.; An, Z. Benzoxazole-based nematic liquid crystals containing ethynyl and two lateral fluorine atoms with large birefringence. Liq. Cryst. 2021, 48, 157–167. [Google Scholar] [CrossRef]
- Liu, G.; Ren, L.; Zhang, M.; Du, S.; Chen, P.; Gao, A.; Chen, X.; An, Z. Synthesis and properties of benzoxazole-based liquid crystals containing ethynyl group. Liq. Cryst. 2020, 47, 1719–1728. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2019, 47, 404–413. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino)phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Alhaddad, O.A.; Ahmed, H.A.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef]
- Praveen, L. Nematic and smectic bithiophenes for UV sensing mechanism: Comparative calculations on different homologues. J. Mol. Liq. 2021, 341, 117424. [Google Scholar] [CrossRef]
- Alamro, F.S.; Al-Kadhi, N.S.; Gomha, S.M.; Popoola, S.A.; Khushaim, M.S.; Alhaddad, O.A.; Ahmed, H.A. Experimental and Theoretical Investigations of Three-Ring Ester/Azomethine Materials. Materials 2022, 15, 2312. [Google Scholar] [CrossRef]
- Al-Hamdani, U.J.; Abdulwahhab, H.A.; Hussein, K.A. Effects of terminal substituents on msomorphic properties of Schiff base—Ester mesogens and DFT calculations. Liq. Cryst. 2022, 49, 1998–2007. [Google Scholar] [CrossRef]
- Nada, S.; Hagar, M.; Farahat, O.; Hasanein, A.A.; Emwas, A.-H.; Sharfalddin, A.A.; Jaremko, M.; Zakaria, M.A. Three Rings Schiff Base Ester Liquid Crystals: Experimental and Computational Approaches of Mesogenic Core Orientation Effect, Heterocycle Impact. Molecules 2022, 27, 2304. [Google Scholar] [CrossRef]
- Supreet; Singh, G. Recent advances on cadmium free quantum dots-liquid crystal nanocomposites. Appl. Mater. Today 2020, 21, 100840. [Google Scholar] [CrossRef]
- Shi, D.; Hu, K.; Chen, P.; Du, W.; Gao, A.; Chen, R.; Chen, X.; An, Z. Improved nematic mesophase stability of benzoxazole-liquid crystals via modification of inter-ring twist angle of biphenyl unit. Liq. Cryst. 2016, 43, 1397–1407. [Google Scholar] [CrossRef]
- Shi, D.; Hu, K.; Chen, P.; Gao, A.; Du, W.; Chen, R.; Chen, X.; An, Z. Nematic mesophase enhanced via lateral monofluorine substitution on benzoxazole-liquid crystals. Liq. Cryst. 2016, 43, 1341–1350. [Google Scholar] [CrossRef]
- Gray, G.W. IV, molecular arrangement and order in the nematic phase-the swarm theory and distortion hypothesis. In Molecular Structure and the Properties of Liquid Crystals. Structure and the Properties of Liquid Crystals; Reinitzers, C., Ed.; Academic Press: Cambridge, UK, 1962; pp. 66–80. [Google Scholar]
- Chen, R.; Huang, Y.; Li, J.; Hu, M.; Li, J.; Chen, X.; Chen, P.; Wu, S.-T.; An, Z. High-frame-rate liquid crystal phase modulator for augmented reality displays. Liq. Cryst. 2019, 46, 309–315. [Google Scholar] [CrossRef]
- Chen, R.; Jiang, Y.; Li, J.; An, Z.; Chen, X.; Chen, P. Dielectric and optical anisotropy enhanced by 1,3-dioxolane terminal substitution on tolane-liquid crystals. J. Mater. Chem. C 2015, 3, 8706–8711. [Google Scholar] [CrossRef]
- Wu, S.-T.; Wu, C.-S. Rotational viscosity of nematic liquid crystals A critical examination of existing models. Liq. Cryst. 1990, 8, 171–182. [Google Scholar] [CrossRef]
- Vuks, M.F. Determination of the optical anisotropy of aromatic molecules from the double refraction of crystals. Opt. Spectrosc. 1966, 20, 361–368. [Google Scholar]
- Klasen, M.; Bremer, M.; Götz, A.; Manabe, A.; Naemura, S.; Tarumi, K. Calculation of Optical and Dielectric Anisotropy of Nematic Liquid Crystals. Jpn. J. Appl. Phys. 1998, 37, L945. [Google Scholar] [CrossRef]


| Compound | Transition Temperature/°C (Enthalpy Change/kJ mol−1) | |
|---|---|---|
| Heating Process a | Cooling Process a | |
| F0 | Cr 149.2 (7.6) SmC 226.6 (3.90) N 237.0 (0.90) I | I 234.7 (−1.00) N 224.2 (−3.70) SmC 144.6 (−7.20) Cr |
| F1 | Cr 121.0 (23.25) SmC 194.2 (1.75) N 208.9 (0.76) I | I 207.3 (−0.89) N 192.5 (−1.47) SmC 85.4 (−19.90) Cr |
| F2 | Cr 102.3 (24.40) N 187.5 (0.60) I | I 186.4 (−0.54) N 79.5 (−20.50) Cr |
| F3 | Cr 88.4 (25.94) N 185.3 (0.55) I | I 185.5 (−0.67) N 75.8 (−22.93) Cr |
| F4 | Cr 83.2 (24.11) SmC 167.5 (1.38) N 202.3 (0.73) I | I 201.0 (−0.84) N 166.2 (−1.00) SmC 48.1 (−9.22) Cr |
| LC Mixture | M1 | M2 | M3 | M4 |
|---|---|---|---|---|
| ε‖ | 9.77 | 10.08 | 9.62 | 9.93 |
| ε⊥ | 3.02 | 3.16 | 3.11 | 2.92 |
| Δε | 6.75 | 6.92 | 6.51 | 7.01 |
| Δε′ | 4.28 a | 5.43 a | 2.68 a | 6.03 a |
| Δn′ | 0.50 a | 0.39 a | 0.42 a | 0.34 a |
| Compound | F0 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| αXX | 649.96 | 645.16 | 641.99 | 648.08 | 653.64 |
| αYY | 265.65 | 262.84 | 264.39 | 264.65 | 266.15 |
| αZZ | 164.51 | 168.71 | 166.47 | 166.33 | 164.33 |
| 360.04 | 358.90 | 357.62 | 359.69 | 361.38 | |
| Δα | 434.88 | 429.39 | 426.56 | 432.59 | 438.40 |
| Δn1 | 0.4958 | 0.4566 | 0.4446 | 0.4499 | 0.4634 |
| Compound | F0 | F1 | F2 | F3 | F4 |
|---|---|---|---|---|---|
| µ (Debye) | 2.8938 | 1.8148 | 4.0915 | 3.3497 | 3.1741 |
| µx (Debye) | −2.2189 | −0.7043 | 2.8062 | 1.4570 | −2.8422 |
| Cos2θ a | 0.59 | 0.15 | 0.47 | 0.19 | 0.80 |
| Δα | 434.88 | 429.39 | 426.56 | 432.59 | 438.40 |
| µ′ (Debye) b | 6.45 | −1.81 | 6.86 | −4.82 | 14.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Mao, Z.; An, Z.; Chen, X.; Chen, P. Fluorination Improves the Electro-Optical Properties of Benzoxazole-Terminated Liquid Crystals in High Birefringence Liquid Crystal Mixtures: Experimental and Theoretical Investigations. Molecules 2023, 28, 3019. https://doi.org/10.3390/molecules28073019
Chen R, Mao Z, An Z, Chen X, Chen P. Fluorination Improves the Electro-Optical Properties of Benzoxazole-Terminated Liquid Crystals in High Birefringence Liquid Crystal Mixtures: Experimental and Theoretical Investigations. Molecules. 2023; 28(7):3019. https://doi.org/10.3390/molecules28073019
Chicago/Turabian StyleChen, Ran, Zihao Mao, Zhongwei An, Xinbing Chen, and Pei Chen. 2023. "Fluorination Improves the Electro-Optical Properties of Benzoxazole-Terminated Liquid Crystals in High Birefringence Liquid Crystal Mixtures: Experimental and Theoretical Investigations" Molecules 28, no. 7: 3019. https://doi.org/10.3390/molecules28073019
APA StyleChen, R., Mao, Z., An, Z., Chen, X., & Chen, P. (2023). Fluorination Improves the Electro-Optical Properties of Benzoxazole-Terminated Liquid Crystals in High Birefringence Liquid Crystal Mixtures: Experimental and Theoretical Investigations. Molecules, 28(7), 3019. https://doi.org/10.3390/molecules28073019






