Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
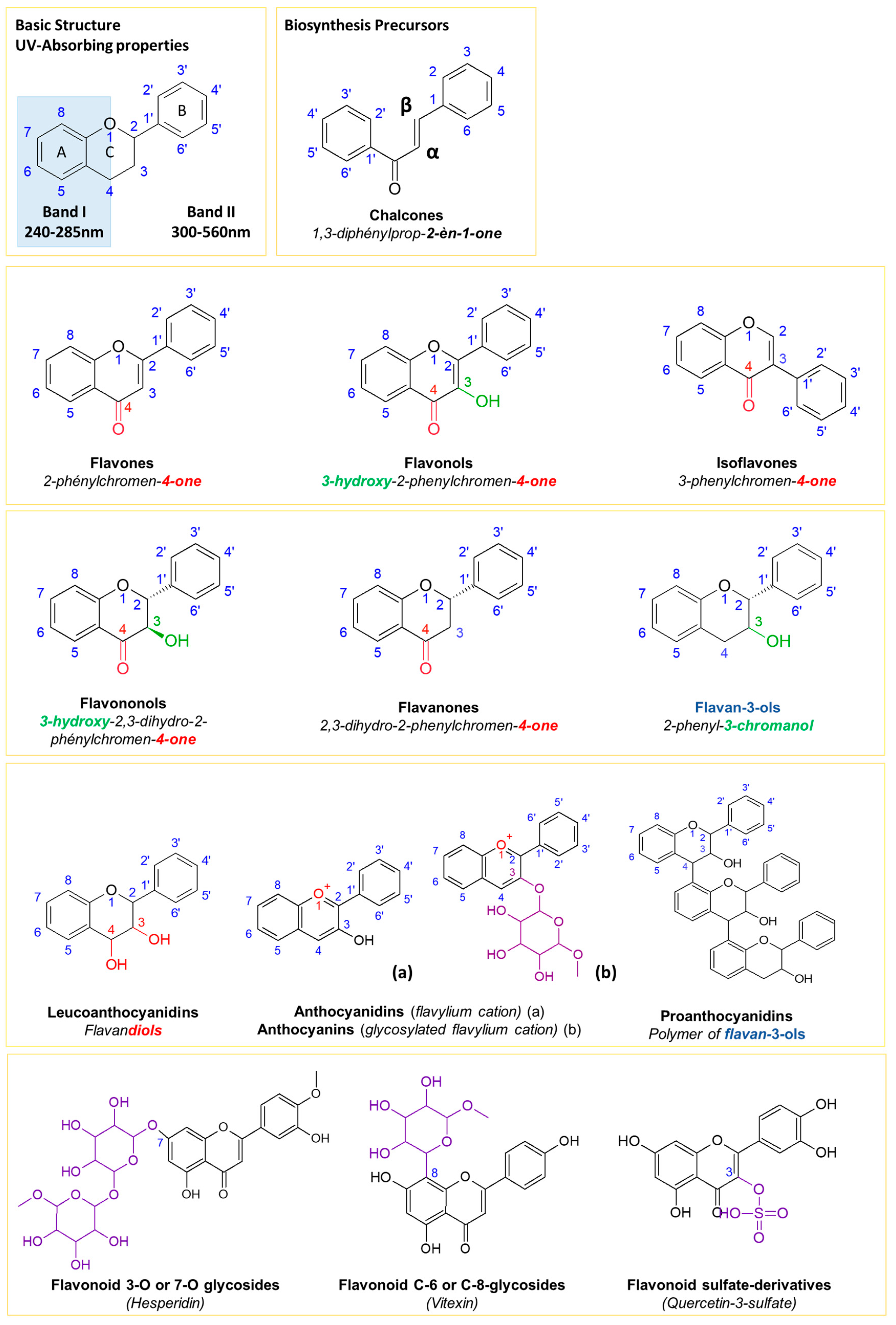
2.1.1. Fragmentation Pathways of Flavonoids
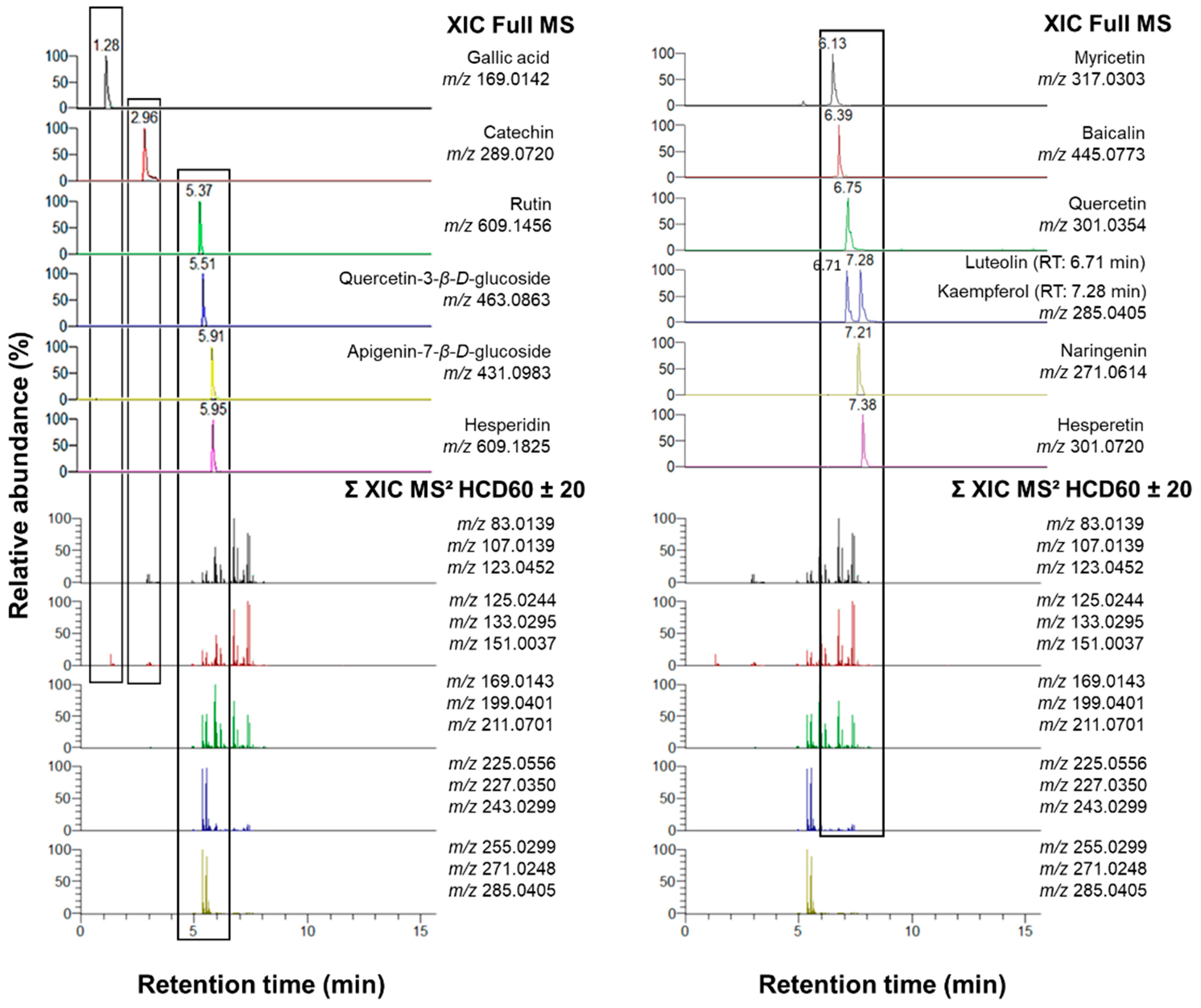
2.1.2. Validation of the Fragment Ion Set for the Flavonoid Annotation
2.1.3. Method Validation: Nontargeted Analysis of a Model Sample
2.2. Method Application
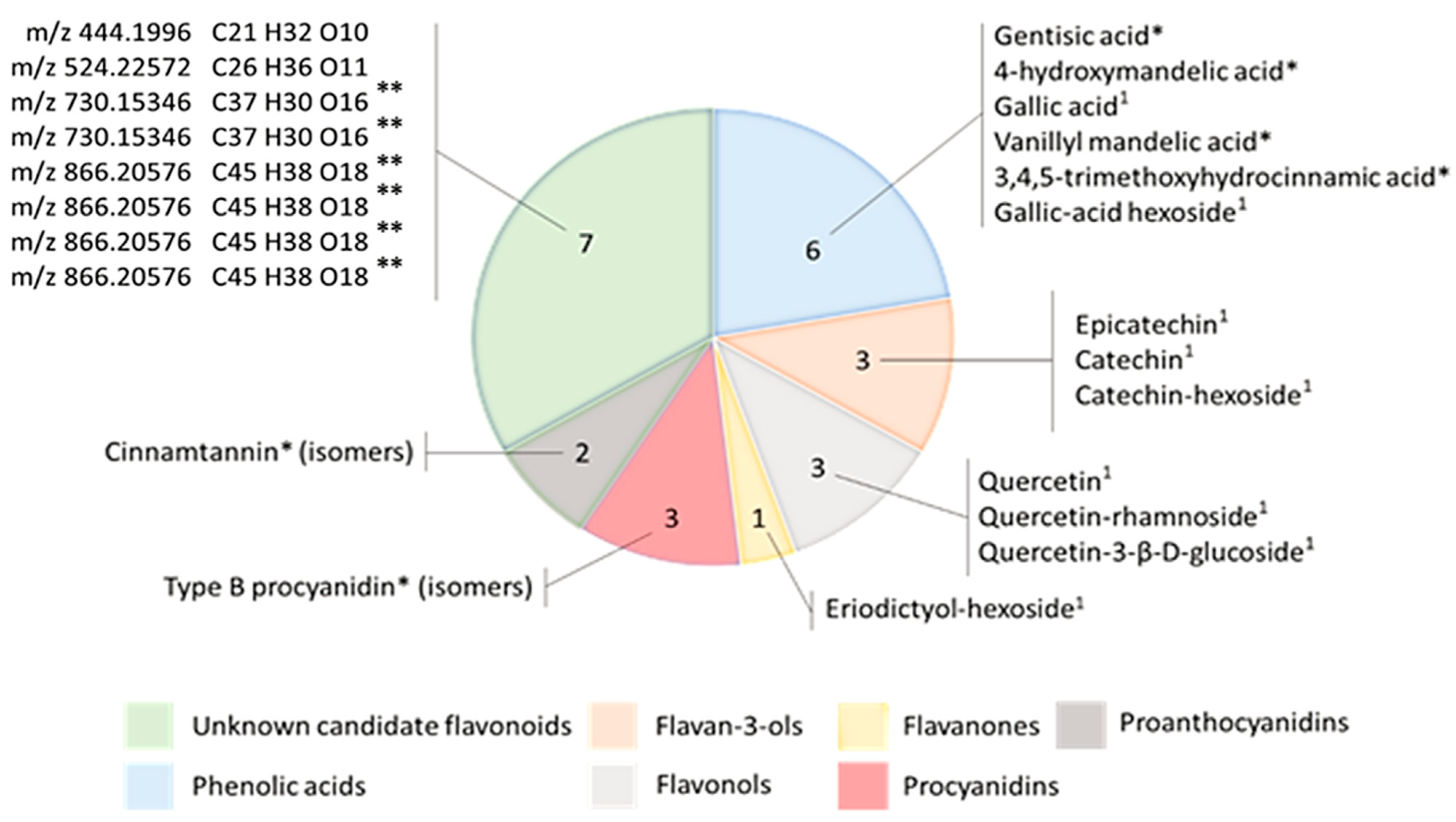
2.2.1. Method Application for the Nonscreening of Flavonoids in Salicornia europaea Extracts
2.2.2. Identification of Flavonoids in Salicornia europaea Extracts Based on In Silico Predictive Combination Flavonoid-Specific Chemical Substitutions in Known Parent Molecules
2.2.3. Evaluation of the Extraction Efficiency of Flavonoids from Salicornia Plant
3. Materials and Methods
3.1. Biological Materials, Chemical Reagents
3.2. Sample Preparation
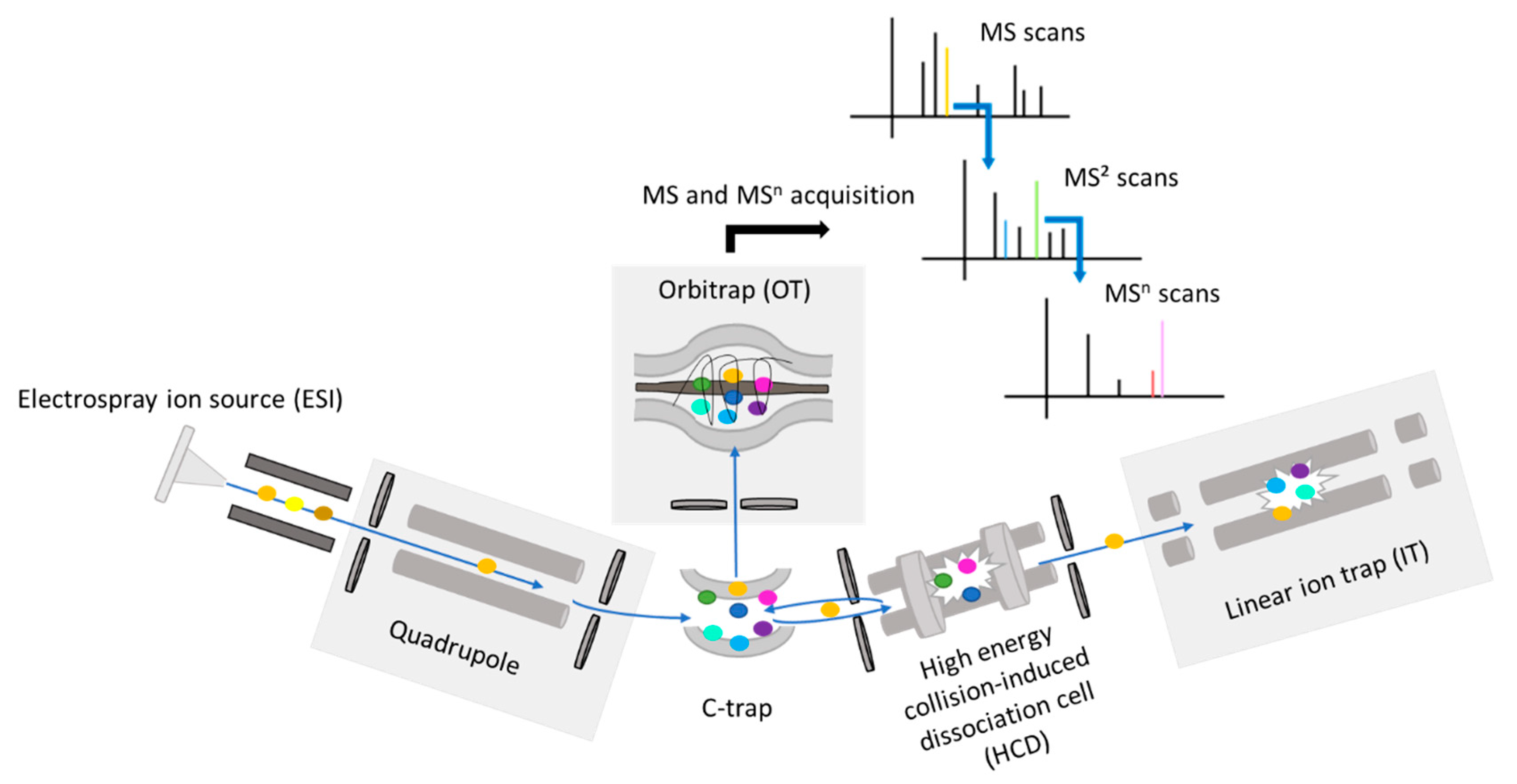
3.3. Instrumentation
3.4. Chromatographic Separation
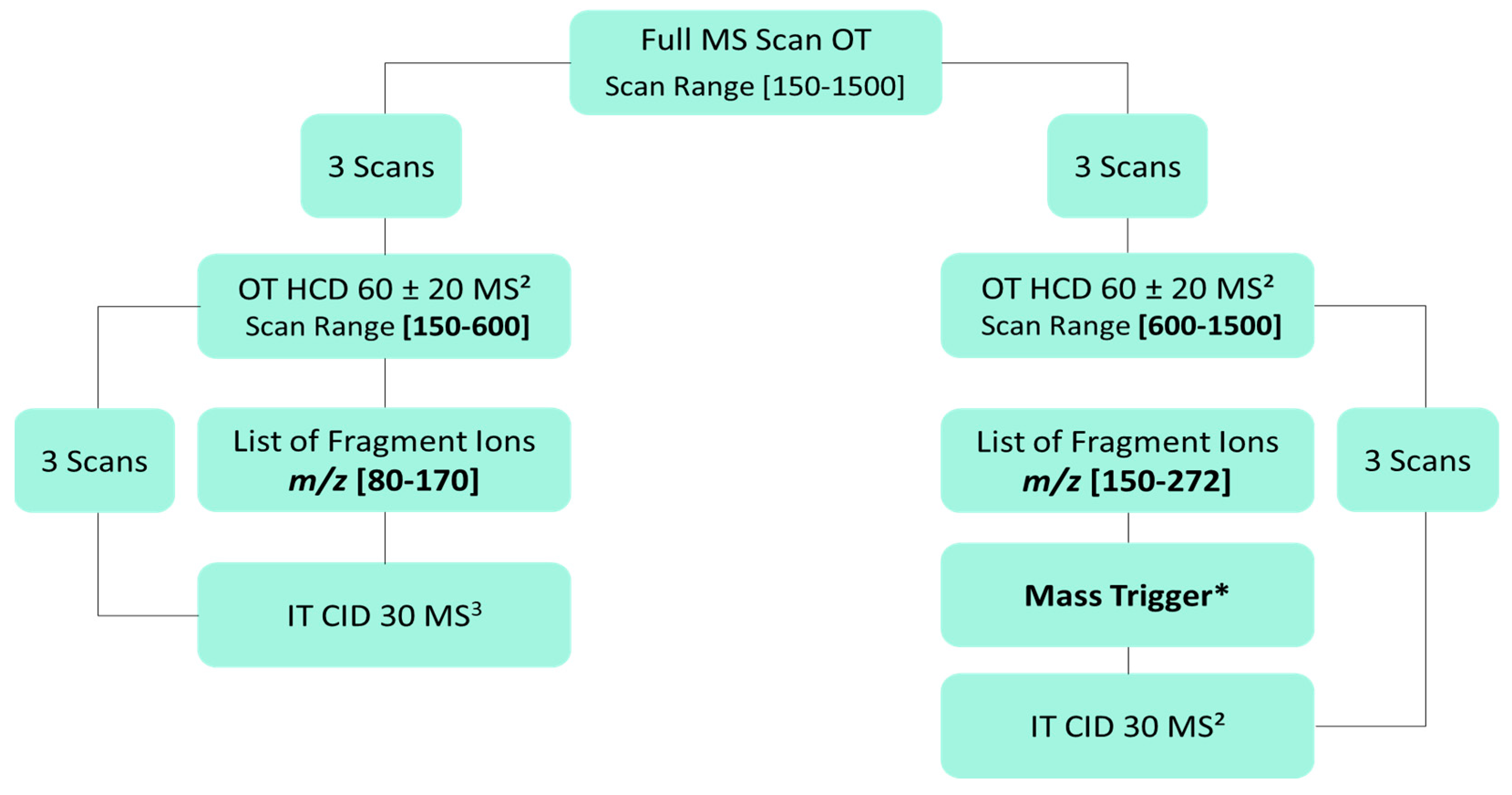
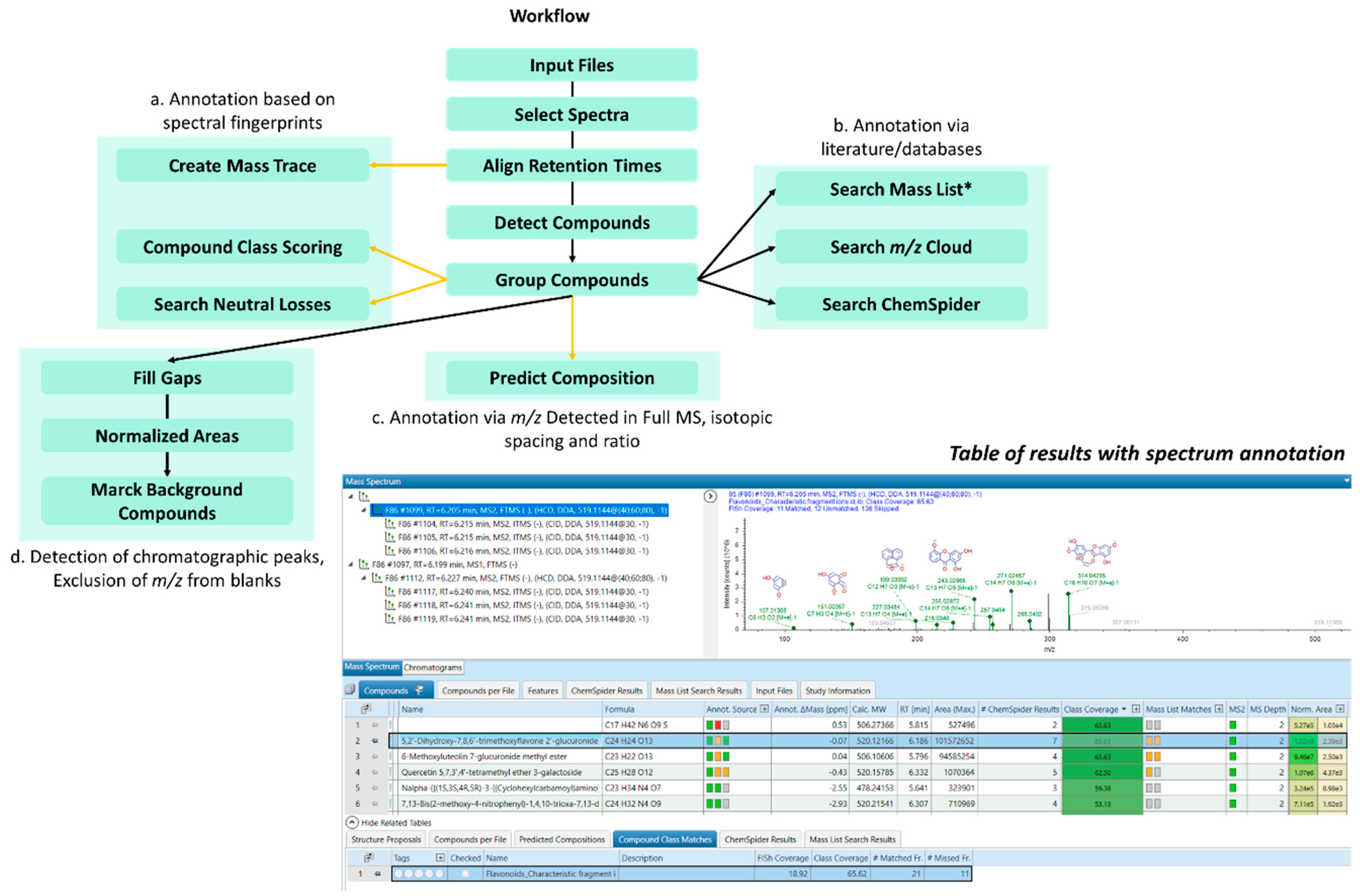
3.5. Untargeted Screening of Flavonoids Based on Fragment Ion Search (FISh) Strategy
3.6. Untargeted Screening of Flavonoids Based on Fragment Ion Search (FISh) Strategy
3.7. Evaluation of Extraction Performances of Flavonoids in Salicornia Europaea Extracts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Chapter Twelve—Bioactive Components from Seaweeds: Cosmetic Applications and Future Development. In Advances in Botanical Research; Bourgougnon, N., Ed.; Sea Plants; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 345–378. [Google Scholar]
- Suganya, T.; Varman, M.; Masjuki, H.H.; Renganathan, S. Macroalgae and Microalgae as a Potential Source for Commercial Applications along with Biofuels Production: A Biorefinery Approach. Renew. Sustain. Energy Rev. 2016, 55, 909–941. [Google Scholar] [CrossRef]
- Mishra, A.; Patel, M.K.; Jha, B. Non-Targeted Metabolomics and Scavenging Activity of Reactive Oxygen Species Reveal the Potential of Salicornia Brachiata as a Functional Food. J. Funct. Foods 2015, 13, 21–31. [Google Scholar] [CrossRef]
- Cárdenas-Pérez, S.; Piernik, A.; Chanona-Pérez, J.J.; Grigore, M.N.; Perea-Flores, M.J. An Overview of the Emerging Trends of the Salicornia L. Genus as a Sustainable Crop. Environ. Exp. Bot. 2021, 191, 104606. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoon, Y.S.; Cho, J.W. Quantitative Analysis of Flavonoids from Salicomia herbacea L. Extracst by LC-MS. Korean J. Med. Crop Sci. 2008, 16, 231–237. [Google Scholar]
- Santos, E.; Maia, B.; Ferriani, A.; Teixeira, S. Flavonoids: Classification, Biosynthesis and Chemical Ecology. Flavonoids-From Biosynth. Hum. Health 2017, 13, 78–94. [Google Scholar]
- Jiang, F.; Dusting, G.J. Natural Phenolic Compounds as Cardiovascular Therapeutics: Potential Role of Their Antiinflammatory Effects. Curr. Vasc. Pharmacol. 2003, 1, 135–156. [Google Scholar] [CrossRef]
- Nájar, A.M.; Romero-Bernal, M.; del Río, C.; Montaner, J. A Review on Polyphenols in Salicornia Ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia. Nutrients 2023, 15, 793. [Google Scholar] [CrossRef]
- Limongelli, F.; Crupi, P.; Clodoveo, M.L.; Corbo, F.; Muraglia, M. Overview of the Polyphenols in Salicornia: From Recovery to Health-Promoting Effect. Molecules 2022, 27, 7954. [Google Scholar] [CrossRef]
- Ameixa, O.M.C.C.; Rebelo, J.; Silva, H.; Pinto, D.C.G.A. Gall Midge Baldratia salicorniae Kieffer (Diptera: Cecidomyiidae) Infestation on Salicornia europaea L. Induces the Production of Specialized Metabolites with Biotechnological Potential. Phytochemistry 2022, 200, 113207. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Beji-Serairi, R.; Selmi, S.; Saidani-Tounsi, M.; Abdelly, C. Salicornia fruticosa L. and Portulaca oleracea L. Antioxidants as Affected by Domestic Cooking Processes. Int. J. Gastron. Food Sci. 2022, 27, 100462. [Google Scholar] [CrossRef]
- Falasca, S.L.; Ulberich, A.; Acevedo, A. Identification of Argentinian Saline Drylands Suitable for Growing Salicornia Bigelovii for Bioenergy. Int. J. Hydrogen Energy 2014, 39, 8682–8689. [Google Scholar] [CrossRef]
- Custódio, M.; Lillebø, A.I.; Calado, R.; Villasante, S. Halophytes as Novel Marine Products—A Consumers’ Perspective in Portugal and Policy Implications. Mar. Policy 2021, 133, 104731. [Google Scholar] [CrossRef]
- Lima, A.R.; Castañeda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of Cultivation Salinity in the Nutritional Composition, Antioxidant Capacity and Microbial Quality of Salicornia Ramosissima Commercially Produced in Soilless Systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Essaidi, I.; Brahmi, Z.; Snoussi, A.; Ben Haj Koubaier, H.; Casabianca, H.; Abe, N.; El Omri, A.; Chaabouni, M.M.; Bouzouita, N. Phytochemical Investigation of Tunisian Salicornia herbacea L., Antioxidant, Antimicrobial and Cytochrome P450 (CYPs) Inhibitory Activities of Its Methanol Extract. Food Control 2013, 32, 125–133. [Google Scholar] [CrossRef]
- Patel, S. Salicornia: Evaluating the Halophytic Extremophile as a Food and a Pharmaceutical Candidate. 3 Biotech 2016, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ventura, Y.; Wuddineh, W.A.; Myrzabayeva, M.; Alikulov, Z.; Khozin-Goldberg, I.; Shpigel, M.; Samocha, T.M.; Sagi, M. Effect of Seawater Concentration on the Productivity and Nutritional Value of Annual Salicornia and Perennial Sarcocornia Halophytes as Leafy Vegetable Crops. Sci. Hortic. 2011, 128, 189–196. [Google Scholar] [CrossRef]
- Barreira, L. Halophytes: Gourmet Food with Nutritional Health Benefits? J. Food Compos. Anal. 2017, 8, 35–42. [Google Scholar] [CrossRef]
- Stanković, M.S.; Petrović, M.; Godjevac, D.; Stevanović, Z.D. Screening Inland Halophytes from the Central Balkan for Their Antioxidant Activity in Relation to Total Phenolic Compounds and Flavonoids: Are There Any Prospective Medicinal Plants? J. Arid. Environ. 2015, 120, 26–32. [Google Scholar] [CrossRef]
- Kong, C.-S.; Kim, Y.A.; Kim, M.-M.; Park, J.-S.; Kim, J.-A.; Kim, S.-K.; Lee, B.-J.; Nam, T.J.; Seo, Y. Flavonoid Glycosides Isolated from Salicornia Herbacea Inhibit Matrix Metalloproteinase in HT1080 Cells. Toxicol. Vitr. 2008, 22, 1742–1748. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, J.-Y.; Ma, Y.-K.; Park, K.Y.; Lee, S.-H.; Ham, K.-S.; Lee, H.J.; Park, K.-H.; Moon, J.-H. Dicaffeoylquinic Acid Derivatives and Flavonoid Glucosides from Glasswort (Salicornia herbacea L.) and Their Antioxidative Activity. Food Chem. 2011, 125, 55–62. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, e162750. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Kim, S.; Lee, E.-Y.; Hillman, P.F.; Ko, J.; Yang, I.; Nam, S.-J. Chemical Structure and Biological Activities of Secondary Metabolites from Salicornia europaea L. Molecules 2021, 26, 2252. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, J.J.; Lucini, L.; Jothiramshekar, S.; Parida, A. Metabolomic Insights into the Mechanisms Underlying Tolerance to Salinity in Different Halophytes. Plant Physiol. Biochem. 2019, 135, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Alfheeaid, H.A.; Raheem, D.; Ahmed, F.; Alhodieb, F.S.; Alsharari, Z.D.; Alhaji, J.H.; BinMowyna, M.N.; Saraiva, A.; Raposo, A. Salicornia bigelovii, S. brachiata and S. herbacea: Their Nutritional Characteristics and an Evaluation of Their Potential as Salt Substitutes. Foods 2022, 11, 3402. [Google Scholar] [CrossRef] [PubMed]
- Bertin, R.L.; Gonzaga, L.V.; Borges, G.D.S.C.; Azevedo, M.S.; Maltez, H.F.; Heller, M.; Micke, G.A.; Tavares, L.B.B.; Fett, R. Nutrient Composition and, Identification/Quantification of Major Phenolic Compounds in Sarcocornia Ambigua (Amaranthaceae) Using HPLC–ESI-MS/MS. Food Res. Int. 2014, 55, 404–411. [Google Scholar] [CrossRef]
- Paje, L.A.; Choi, J.; Lee, H.-D.; Kim, J.; Yu, A.R.; Bae, M.-J.; Geraldino, P.J.L.; Lee, S. Phenolic Acids and Flavonoids from Salvia Plebeia and HPLC-UV Profiling of Four Salvia Species. Heliyon 2022, 8, e09046. [Google Scholar] [CrossRef]
- das Neves Costa, F.; Jerz, G.; de Souza Figueiredo, F.; Winterhalter, P.; Leitão, G.G. Solvent System Selectivities in Countercurrent Chromatography Using Salicornia Gaudichaudiana Metabolites as Practical Example with Off-Line Electrospray Mass-Spectrometry Injection Profiling. J. Chromatogr. A 2015, 1385, 20–27. [Google Scholar] [CrossRef]
- Sammani, M.S.; Clavijo, S.; Cerdà, V. Recent, Advanced Sample Pretreatments and Analytical Methods for Flavonoids Determination in Different Samples. TrAC Trends Anal. Chem. 2021, 138, 116220. [Google Scholar] [CrossRef]
- Kiyonami, R. Novel Structure-Based Profiling and Annotation Workflow—High-Throughput Analysis of Flavonoids Using the Thermo Scientific Orbitrap ID-X Tribrid MS; Thermo Fisher Scientific Inc.: Waltham, MA, USA, 2018; Volume 15, Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/an-65363-ms-msn-flavonoids-an65363-en.pdf (accessed on 15 February 2023).
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, A.; Cannazza, G.; Capriotti, A.L.; Citti, C.; La Barbera, G.; Laganà, A.; Montone, C.M.; Piovesana, S.; Cavaliere, C. A New Software-Assisted Analytical Workflow Based on High-Resolution Mass Spectrometry for the Systematic Study of Phenolic Compounds in Complex Matrices. Talanta 2020, 209, 120573. [Google Scholar] [CrossRef]
- Costa, F.D.N.; Borges, R.M.; Leitão, G.G.; Jerz, G. Preparative Mass-Spectrometry Profiling of Minor Concentrated Metabolites in Salicornia gaudichaudiana Moq by High-Speed Countercurrent Chromatography and off-Line Electrospray Mass-Spectrometry Injection. J. Sep. Sci. 2019, 42, 1528–1541. [Google Scholar] [CrossRef]
- Gates, P.J.; Lopes, N.P. Characterisation of Flavonoid Aglycones by Negative Ion Chip-Based Nanospray Tandem Mass Spectrometry. Int. J. Anal. Chem. 2012, 2012, e259217. [Google Scholar] [CrossRef]
- Barnaba, C.; Dellacassa, E.; Nicolini, G.; Nardin, T.; Serra, M.; Larcher, R. Non-Targeted Glycosidic Profiling of International Wines Using Neutral Loss-High Resolution Mass Spectrometry. J. Chromatogr. A 2018, 1557, 75–89. [Google Scholar] [CrossRef]
- Jia, W.; Shi, L.; Zhang, F.; Chang, J.; Chu, X. High-Throughput Mass Spectrometry Scheme for Screening and Quantification of Flavonoids in Antioxidant Nutraceuticals. J. Chromatogr. A 2019, 1608, 460408. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Jiang, Y.; Liu, R.; Liu, M.; Yi, L.; Liao, N.; Liu, S. Structural Features Guided “Fishing” Strategy to Identification of Flavonoids from Lotus Plumule in a Self-Built Data “Pool” by Ultra-High Performance Liquid Chromatography Coupled with Hybrid Quadrupole-Orbitrap High Resolution Mass Spectrometry. J. Chromatogr. B 2019, 1124, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Demarque, D.P.; Crotti, A.E.; Vessecchi, R.; Lopes, J.L.; Lopes, N.P. Fragmentation Reactions Using Electrospray Ionization Mass Spectrometry: An Important Tool for the Structural Elucidation and Characterization of Synthetic and Natural Products. Nat. Prod. Rep. 2016, 33, 432–455. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural Characterization of Flavonoid Glycoconjugates and Their Derivatives with Mass Spectrometric Techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef]
- Georgiev, V.; Ananga, A.; Tsolova, V. Recent Advances and Uses of Grape Flavonoids as Nutraceuticals. Nutrients 2014, 6, 391–415. [Google Scholar] [CrossRef]
- Zhong, J.; Ren, D.; Shang, Y.; Huang, S.; Li, Y.; Hu, Y.; Yi, L. Targeted identification of glycosylated flavones and isomers in green tea through integrated ion-filtering strategy and mass-fragmentation characteristics based on the UPLC–Q–Orbitrap–MS/MS platform. Food Chem. 2022, 377, 131901. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Caçador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima J. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Sánchez-Gavilán, I.; Ramírez, E.; de la Fuente, V. Bioactive Compounds in Salicornia Patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Jurinjak Tušek, A.; Šamec, D.; Šalić, A. Modern Techniques for Flavonoid Extraction—To Optimize or Not to Optimize? Appl. Sci. 2022, 12, 11865. [Google Scholar] [CrossRef]
- Chaves, J.O.; De Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.-S.; Lee, J.I.; Kim, Y.A.; Kim, J.-A.; Bak, S.S.; Hong, J.W.; Park, H.Y.; Yea, S.S.; Seo, Y. Evaluation on Anti-Adipogenic Activity of Flavonoid Glucopyranosides from Salicornia Herbacea. Process Biochem. 2012, 47, 1073–1078. [Google Scholar] [CrossRef]



| C4H3O2 | C6H3O2 | C7H7O2 | C6H5O3 | C8H5O3 | C8H5O2 | C7H3O4 | C7H5O5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Standards | m/z 83.0139 | m/z 107.0139 | m/z 123.0452 | m/z 125.0244 | m/z 133.0295 | m/z 151.0037 | m/z 169.0143 | m/z 199.0401 | ||||||
| Phenolic acid | Gallic acid | [(M-H)-C3H2O3]− | - | - | [(M-H)-CO2]− | - | - | [M-H]− | - | ||||||
| Flavonol | Quercetin | 1,3A−C3O2 | 1,3A−CO2 | - | 1,4A− | 1,3A−H2O | 1,3A− | [(M-H)-C8H4O2]− | - | ||||||
| Flavonol glycoside | Quercetin-β-d-glucoside | 1,3A−C3O2 | 1,3A−CO2 | - | - | - | 1,3A− | - | [301.0354A-C2H2O2−CO2]− | ||||||
| Flavonol | Myricetin | 1,3A−C3O2 | 1,3A−CO2 | - | 1,4A− | 1,3A−H2O | 1,3A− | [(M-H)-C8H4O3]− | - | ||||||
| Flavonol | Rutin | 1,3A−C3O2 | 1,3A−CO2 | - | - | 1,3A−H2O | 1,3A− | [301.0354A-C8H4O3]− | [301.0354A-C2H2O2−CO2]− | ||||||
| Flavonol | Kaempferol | 1,3A−C3O2 | 1,3A−CO2 | 1,2B− | - | - | - | - | - | ||||||
| Flavanol | Catechin | 1,4A−C2H2O | - | 1,3B−CO | 1,4A− | - | − | - | - | ||||||
| Flavone | Apigenin | 1,3A−C3O2 | 1,3A−CO2 | - | - | - | 1,3A− | [(M-H)-C8H4]− | - | ||||||
| Flavone glycoside | Apigenin-7-β-d-glucoside | 1,3A−C3O2 | 1,3A−CO2 | - | - | - | 1,3A− | [(M-H)-C8H4]− | [269.0455C-C2H2O-CO]− | ||||||
| Flavone | Baicalin | - | - | - | - | - | - | - | [269.0455D-C2H2]− | ||||||
| Flavone | Luteolin | 1,3A−C3O2 | 1,3A−CO2 | - | - | 1,3B− | 1,3A− | - | [(M-H)-C2H2O-CO2]− | ||||||
| Flavanone | Hesperetin | 1,3A−C3O2 | 1,3A−CO2 | - | - | 1,3A−H2O | 1,3A− | - | [(M-H)-C3H6O3]− | ||||||
| Flavanone | Hesperidin | 1,3A−C3O2 | 1,3A−CO2 | - | 1,4A− | - | 1,3A− | - | [301.0720D-C4H6O3]− | ||||||
| Isoflavone | Naringenin | 1,3A−C3O2 | 1,3A−CO2 | - | [(M-H)-CO2]− | 1,3A−H2O | 1,3A− | - | [(M-H)-C2H4-CO2]− | ||||||
| O-methylated isoflavone | Genistein | 1,3A−C3O2 | 1,3A−CO2 | - | - | [(M-H)-C7H4O3]− | 1,3A− | [(M-H)-C8H4]− | - | ||||||
| C13H7O3 | C14H9O3 | C13H7O4 | C13H7O5 | C14H7O5 | C14H7O6 | C15H9O6 | |||||||||
| Class | Standards | m/z 211.0401 | m/z 225.0556 | m/z 227.0350 | m/z 243.0299 | m/z 255.0299 | m/z 271.0248 | m/z 285.0405 | |||||||
| Phenolic acid | Gallic acid | - | - | - | - | - | - | - | |||||||
| Flavonol | Quercetin | [(M-H)-CH2O2-CO2]− | - | [(M-H)-C2H2O3]− | [(M-H)-C2H2O2]− | [(M-H)-CH2O2]− | [(M-H)-CH2O]− | - | |||||||
| Flavonol glycoside | Quercetin-β-d-glucoside | [301.0354A-CH2O2-CO2]− | [301.0354A-C2H2O2-H2O]− | [301.0354A-C2H2O3]− | [301.0354A-C2H2O2]− | [301.0354A-CH2O2]− | [M-C6H10O5-CH2O]− or [301.0354A-CH2O]− | - | |||||||
| Flavonol | Myricetin | - | - | [(M-H)-C2H2O4] | - | - | [(M-H)-CH2O2]− | - | |||||||
| Flavonol | Rutin | [301.0354A-CH2O2-CO2]− | - | [301.0354A-C2H2O3]− | [301.0354A-C2H2O2]− | [301.0354A-CH2O2]− | [M-C12H20O9-CH2O]− or [301.0354A-CH2O]− | - | |||||||
| Flavonol | Kaempferol | [(M-H)-CH2O-CO2]− | - | - | [(M-H)-C2H2O]− | [(M-H)-CH2O]− | - | [M-H]− | |||||||
| Flavanol | Catechin | - | - | - | - | - | - | - | |||||||
| Flavone | Apigenin | - | [(M-H)-CO2]− | [(M-H)-C2H2O]− | - | - | - | - | |||||||
| Flavone glycoside | Apigenin-7-β-d-glucoside | [M-C6H10O5-C2H2O2]− | - | [M-C6H10O5- C2H2O]− [269.0455B- C2H2O]− | - | - | - | - | |||||||
| Flavone | Baicalin | - | [M-C6H8O6- CO2]− or [269.0455C-CO2]− | - | - | - | - | - | |||||||
| Flavone | Luteolin | - | - | - | [(M-H)-CH2O2]− | - | - | [M-H]− | |||||||
| Flavanone | Hesperetin | - | - | [(M-H)-C3H6O2]− | - | - | - | [(M-H)-CH4]− | |||||||
| Flavanone | Hesperidin | - | - | [M-C12H20O9-C3H6O2]− or [301.0720D-C3H6O2]− | - | - | - | - | |||||||
| Isoflavone | Naringenin | - | [(M-H)-CH2O2]− | [(M-H)-CO2]− | [(M-H)-C2H4]− | ||||||||||
| O-methylated isoflavone | Genistein | - | [(M-H)-CO2]− | [(M-H)-C2H2O]− | - | - | - | - | |||||||
| Flavonoids | Formula | Monoisotopic Mass (Da) | Mass Error (ppm) | [M-H]− m/z | Area Peak (106) | RT (min) | Nb of CFI (/15) | |
|---|---|---|---|---|---|---|---|---|
| 30 mM Tris-HCl pH 7 | 10 mM NH4Ac pH 5.4 | |||||||
| Multiple matches * | C16H12O5 | 284.0685 | 0.08 | 283.0612 | 12.6 ± 0.7 | 10.5 ± 1.2 | 6.48 | 10 |
| Luteolin | C15H10O6 | 286.0478 | 0.33 | 285.0406 | 9.18 ± 0.8 | 19.4 ± 3.3 | 6.73 | 13 |
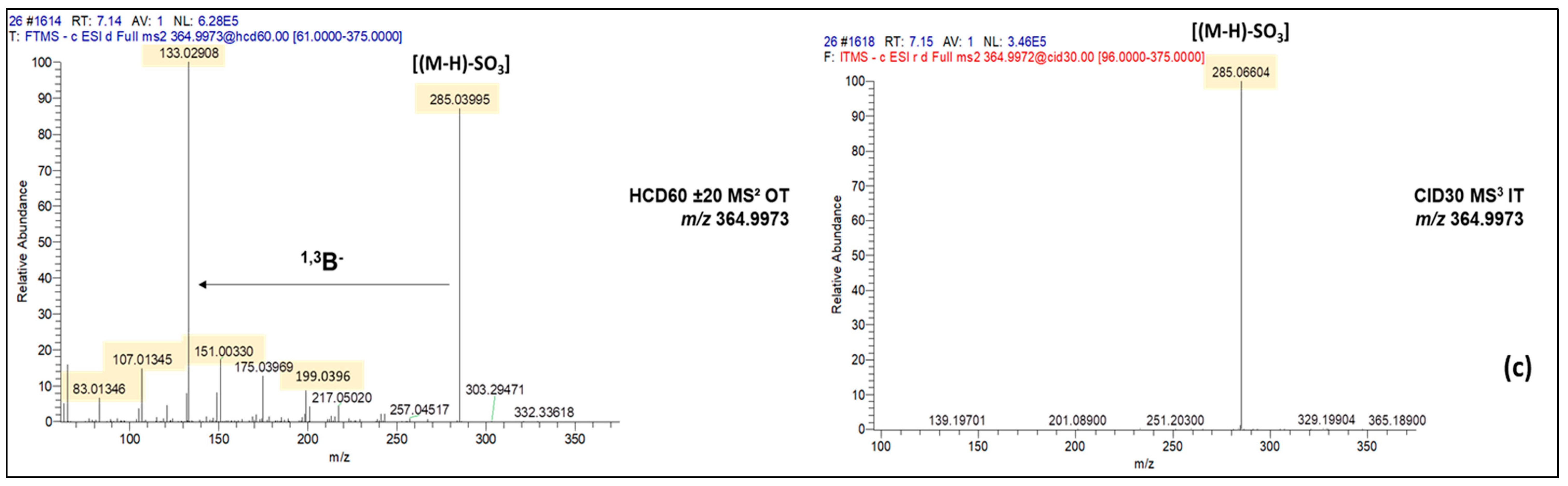
| Luteolin-O-sulfate | C15H10O9S | 366.0045 | −0.06 | 364.9973 | 31.1 ±7 | 42.7 ± 13.3 | 7.36 | 6 |
| Multiple matches * | C20H20O8 | 388.1158 | −0.05 | 387.1085 | 10.4 ±0.9 | 10.5 ± 2 | 5.80 | 10 |
| Quercetin-3-β-d-glucoside | C21H20O12 | 464.0956 | 0.19 | 463.0863 | 16.3 ± 2 | 13.2 ± 1.7 | 5.54 | 5 |
| Isorhamnetin-hexoside | C22H22O12 | 478.1111 | −0.12 | 477.1038 | 32.2 ±3 | 19.6 ± 2.7 | 5.88 | 9 |
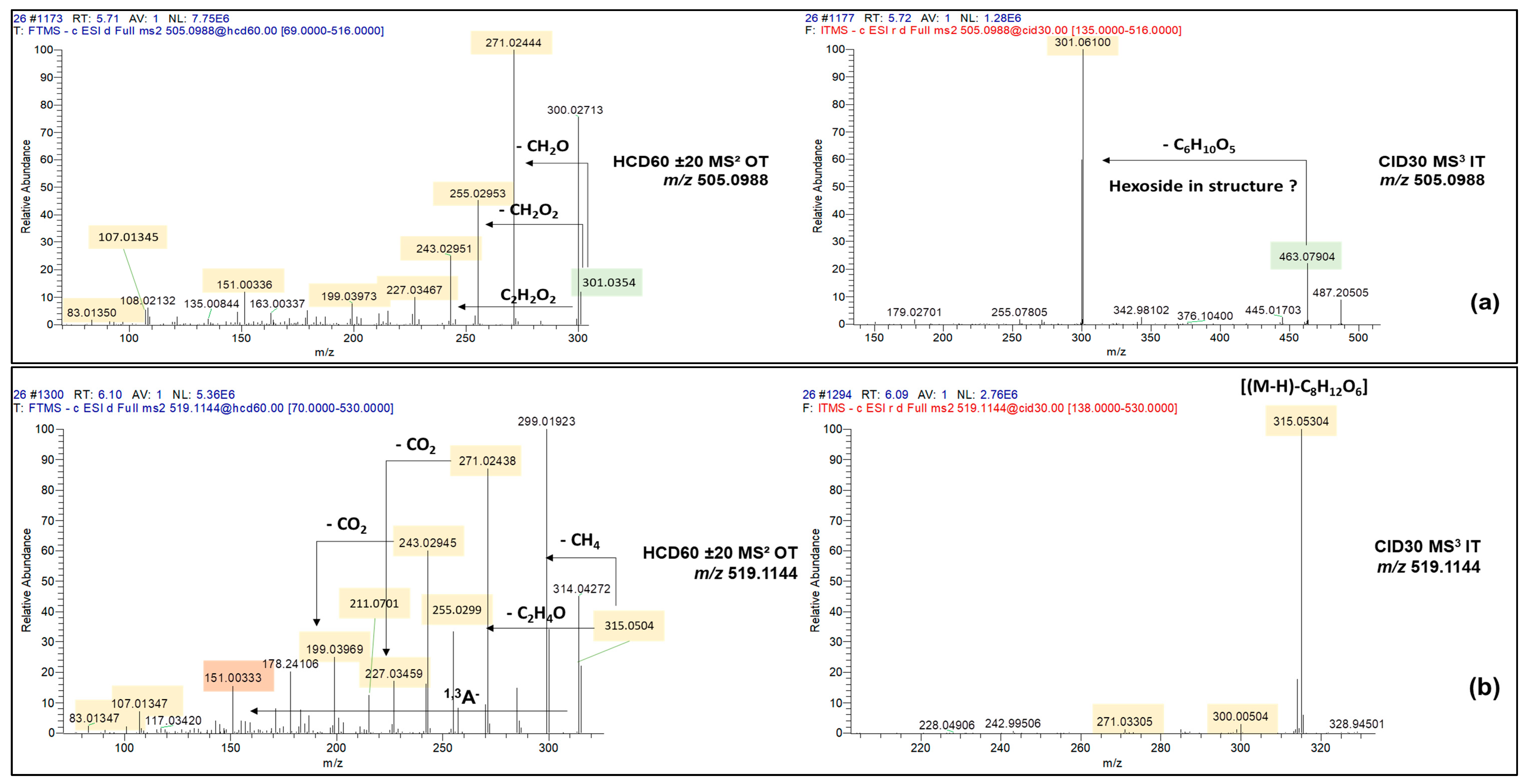
| Unknown | C23H22O13 | 506.1062 | 0.28 | 505.0989 | 47.8 ± 0.8 | 177 ± 2.7 | 5.71 | 8 |
| Multiple matches * | C25H24O12 | 516.1301 | −0.13 | 515.1229 | 7.51 ± 0.8 | 7.95 ± 1.7 | 5.38 | 5 |
| Unknown | C24H24O13 | 520.1217 | −0.07 | 519.1144 | 82.7 ± 12 | 194 ± 42 | 6.09 | 9 |
| Quercetin-3-(6″-malonyl) -glucoside | C24H22O15 | 550.0960 | 0.17 | 549.0887 | Nd | 10.1 ± 2.1 | 5.69 | 9 |
| Multiple matches * | C30H38O14 | 622.2262 | 0.09 | 621.2189 | 5.83 ± 0.9 | 8.64 ± 1.7 | 6.07 | 7 |
| Luteolin di-hexoside | C27H26O18 | 638.1120 | 0.16 | 637.1047 | Nd | 1.37 ± 0.3 | 5.25 | 6 |
| 4′-OH-5,7,2′-trimethoxyflavanone 4′-rhamnoside | C30H38O15 | 638.2210 | −0.08 | 637.2138 | 7.16 ± 0.7 | 11.7 ± 1.9 | 5.78 | 9 |
| Unknown | C22H18O23 | 650.0211 | 3.37 | 649.0135 | 2.22 ± 2 | 7.81 ± 1.8 | 5.71 | 7 |
| Unknown | C28H28O18 | 652.1276 | 5.45 | 651.1205 | Nd | 1.06 ± 0.4 | 5.45 | 9 |
| Unknown | C27H20O18S | 664.0368 | −0.33 | 663.0294 | Nd | 14.8 ± 1.2 | 6.09 | 10 |
| Unknown | C27H26O21S | 718.0689 | 0.21 | 717.0615 | 26.7 ± 5 | 26.6 ± 5.7 | 5.27 | 13 |
| Unknown | C31H42O26 | 830.1964 | −0.01 | 829.1892 | 1.89 ± 0.2 | Nd | 4.8 | 4 |
| Unknown | C33H34O29 | 894.1162 | −2.49 | 893.1086 | 4.9 ± 0.7 | 4.91 ± 2.5 | 6.11 | 9 |
| Unknown | C40H38O24 | 902.1757 | 0.48 | 901.1681 | 4.32 ± 0.9 | 10.7 ± 2.5 | 4.84 | 5 |
| Unknown | C41H40O24 | 916.1911 | 0.11 | 915.1837 | 4.79 ± 0.6 | 7.66 ± 2.1 | 5.15 | 4 |
| Unknown | C45H40O27 | 1012.175 | −0.32 | 1011.1693 | Nd | 4.61 ± 1.3 | 5.07 | 10 |
| Unknown | C46H42O27 | 1026.1914 | 0.13 | 1025.1842 | 2.72 ± 0.5 | Nd | 5.29 | 5 |
| Unknown | C48H42O30 | 1098.1757 | −0.32 | 1097.1688 | 18.6 ± 2.6 | 39.4 ± 10.2 | 5.16 | 8 |
| Unknown | C49H44O30 | 1112.1916 | −0.12 | 1111.1846 | 10 ± 1.8 | 12.6 ± 2.4 | 5.38 | 6 |
| Formula | Monoisotopic Mass (Da) | Parent Compound | Transformations | Structural Modifications | Mass Error (ppm) | FISh Coverage (%) |
|---|---|---|---|---|---|---|
| C16H12O5 | 284.0685 | Genistein | CH2OH addition | +(CH2) | 0.08 | 43.82 |
| C16H12O6 | 300.0634 | Naringenin | Desaturation, O-methylation | + (CO) | 0.03 | 30.32 |
| C20H20O8 | 388.1158 | Hesperetin | Acylation, CH2OH addition, O-methylation | +(C4H6O2) | −0.05 | 42.81 |
| C23H22O13 | 506.1063 | Quercetin-3-β-d-glucoside | Acylation | +(C2H2O) | 0.4 | 42.11 |
| C24H24O13 | 520.1217 | Luteolin-O-glucoside | Acylation, CH2OH addition | +(C3H4O) | 0.17 | 67.88 |
| C28H28O18 | 652.1278 | Glycosylated gallic acid | CH2OH addition, gallic acid addition | +(C15H12O8) | 0.3 | 51.85 |
| C27H26O21S | 718.0689 | 6-methoxyluteolin 7-glucuronide methyl ester | Hydroxylation, methyloxalate addition, sulfation | +(C4H4O8S) | 0.27 | 57.41 |
| Name | Isorhamnetin-7-O-(6″acetyl glucoside) | 6-Methoxy-luteolin-sulfate-O-(dimethyloxalate-glucoside) |
|---|---|---|
| Structure |  | 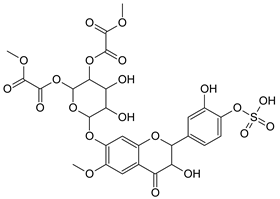 |
| Monoisotopic mass (Da) | 520.1217 | 718.0689 |
| FISh score (%) | 67.88 | 57.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parailloux, M.; Godin, S.; Lobinski, R. Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3. Molecules 2023, 28, 3022. https://doi.org/10.3390/molecules28073022
Parailloux M, Godin S, Lobinski R. Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3. Molecules. 2023; 28(7):3022. https://doi.org/10.3390/molecules28073022
Chicago/Turabian StyleParailloux, Maroussia, Simon Godin, and Ryszard Lobinski. 2023. "Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3" Molecules 28, no. 7: 3022. https://doi.org/10.3390/molecules28073022
APA StyleParailloux, M., Godin, S., & Lobinski, R. (2023). Nontargeted Screening for Flavonoids in Salicornia Plant by Reversed-Phase Liquid Chromatography–Electrospray Orbitrap Data-Dependent MS2/MS3. Molecules, 28(7), 3022. https://doi.org/10.3390/molecules28073022