Core-Shell, Critical-Temperature-Suppressed V Alloy-Pd Alloy Hydrides for Hydrogen Storage—A Technical Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Description of Materials for Comparison
2.1.1. LaNi5 Powder
2.1.2. LaNi5-like Alloy Powder in ABS Copolymer
2.1.3. Core-Shell Vanadium–Palladium
2.1.4. Core-Shell Vanadium–Aluminium Alloy–Palladium–Silver Alloy
2.1.5. Summary of Material Densities
2.2. Comparison of Hydrogen Storage Capacities
2.3. Comparison of Hydrogen Storage Properties
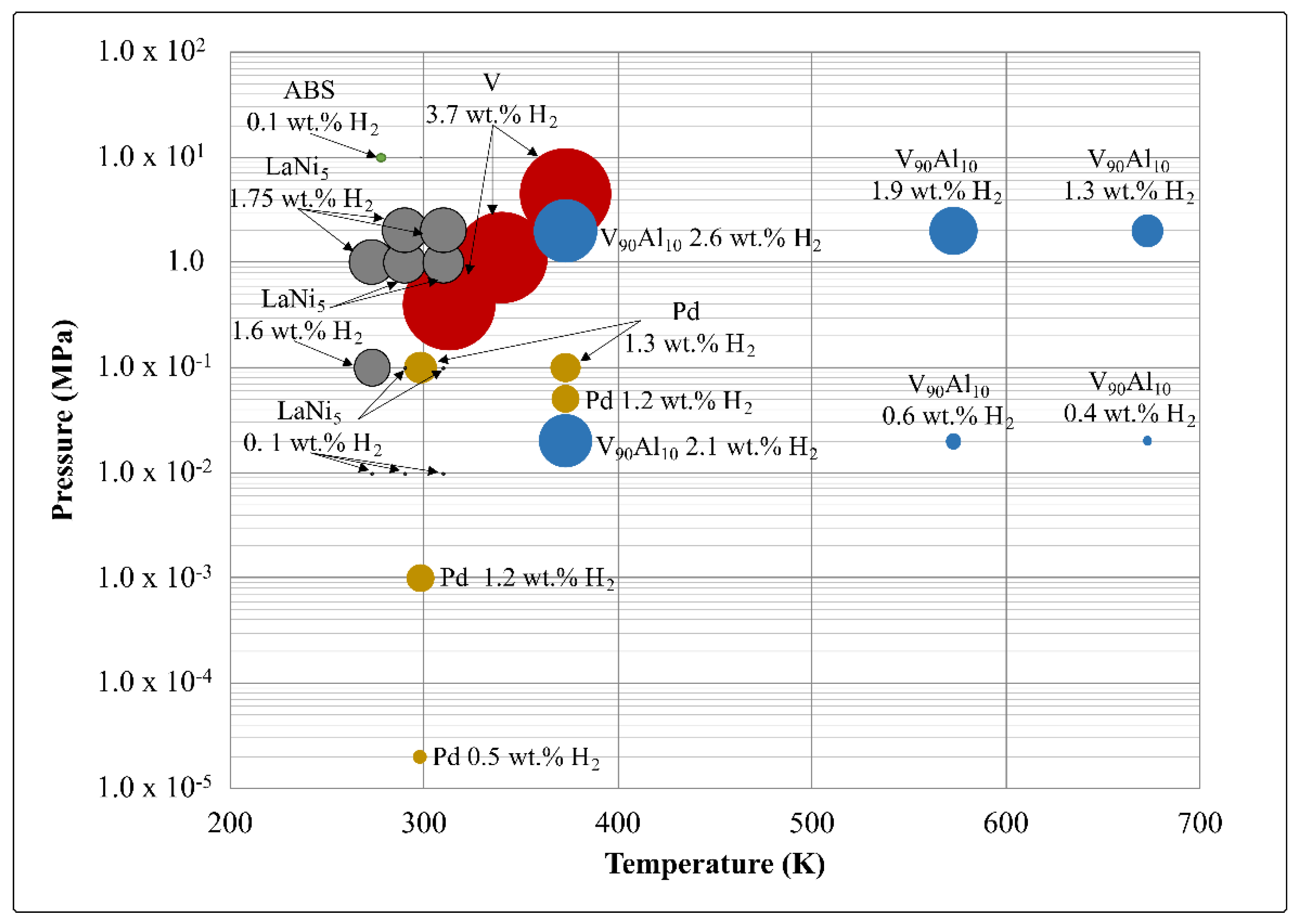
2.3.1. Reversible Hydrogen Storage Capacity
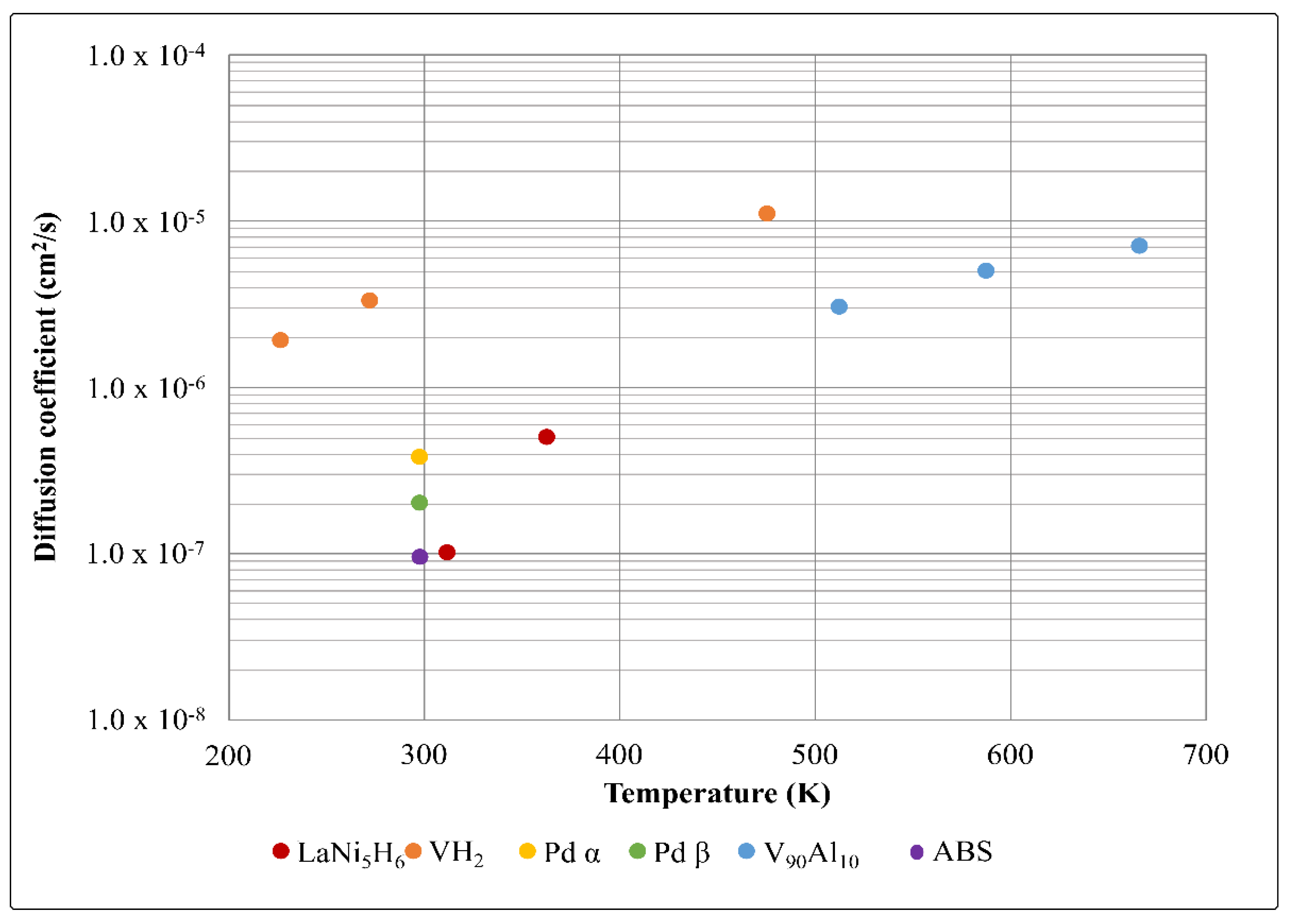
2.3.2. Hydrogen Diffusion Coefficient
2.3.3. Physical Expansion and Contraction
2.4. Comparison by Kinetics of Hydrogen Absorption and Desorption
| Material | Thermal Conductivity, w/m·K [ref] | Specific Heat, J/g·K [ref] | Hydrogen Absorption Energy, Average or (1st/2nd) kJ/mol H [ref] | Hydrogen Desorption Energy, Total or (1st/2nd) kJ/mol H [ref] | Phase Transition Losses, kJ/mol |
|---|---|---|---|---|---|
| LaNi5 | 0.72 [44] | 53.2 [44,45] | |||
| LaNi5H6 | 0.98 [44] | 0.35 [46] | |||
| AB5 | 1.2 [14] | 45.5 [47] | |||
| ABS | 0.25 [48] | 1.9 [38] | |||
| AB5/ABS | 48.6 [5] | ||||
| V | 40 [49] | 0.71 [50] | (34/40.6) [51] | (20/157) [20] | |
| Pd | 68.8 [52] | 0.24 | 19.1 [53] | 18.7 [53] | 0.95 [54] |
| V90Al10 | 12 (Estimate from) [55] | 29.6 [56] | |||
| Pd80Ag20 | 27.4 [52] | 7.81 (Pd90Ag10) * [57] |
3. Methods
4. Conclusions and Recommendations
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- James, N.; Braun, J.E.; Groll, E.A.; Horton, W.T. Compressor driven metal hydride heat pumps using an adsorptive slurry and isothermal compression. Sci. Technol. Built Environ. 2016, 22, 565–575. [Google Scholar] [CrossRef]
- Goto, K.; Hirata, T.; Yamamoto, I.; Nakao, W. Suitability Evaluation of LaNi5 as Hydrogen-Storage-Alloy Actuator by In-Situ Displacement Measurement during Hydrogen Pressure Change. Molecules 2019, 24, 2420. [Google Scholar] [CrossRef] [PubMed]
- Joubert, J.M.; Paul-Boncour, V.; Cuevas, F.; Zhang, J.; Latroche, M. LaNi5 related AB5 compounds: Structure, properties and applications. J. Alloys Compd. 2021, 862, 158163. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Clewley, J.D. Hysteresis in metal hydrides. J. Less Common Met. 1982, 83, 127–141. [Google Scholar] [CrossRef]
- Pentimalli, M.; Padella, F.; Pilloni, L.; Imperi, E.; Matricardi, P. AB5/ABS composite material for hydrogen storage. J. Hydrogen Energy 2009, 34, 4592–4596. [Google Scholar] [CrossRef]
- Dolan, M.D.; McLennan, K.G.; Chandra, D.; Kochanek, M.A.; Song, G. Suppression of the critical temperature in binary vanadium hydrides. J. Alloys Compd. 2014, 586, 385–391. [Google Scholar] [CrossRef]
- Buxbaum, R.E.; Marker, T.L. Hydrogen transport through non-porous membranes of palladium-coated niobium, tantalum and vanadium. J. Membr. Sci. 1993, 85, 29–38. [Google Scholar] [CrossRef]
- Palladium Spot Price Live Chart. BullionVault n.d. Available online: https://www.bullionvault.com/palladium-price-chart.do (accessed on 23 September 2022).
- Fuerst, T.F.; Petsalis, E.P.; Lundin, S.-T.B.; Wilcox, J.; Way, J.D.; Wolden, C.A. Experimental and Theoretical Insights into the Potential of V2O3 Surface Coatings for Hydrogen Permeable Vanadium Membranes. J. Phys. Chem. C 2018, 122, 3488–3496. [Google Scholar] [CrossRef]
- Dolan, M.D.; Lamb, K.E.; Evtimova, J.B.; Viano, D.M. Deuterium enrichment using vanadium membranes. J. Hydrogen Energy 2017, 42, 24183–24188. [Google Scholar] [CrossRef]
- Blinov, D.V.; Dunikov, D.O.; Kazakov, A.N.; Romanov, I.A. Influence of geometrical non-uniformities of LaNi5 metal hydride bed on its structure and heat and mass transfer at hydrogen absorption. J. Phys. Conf. Ser. 2017, 891, 012119. [Google Scholar] [CrossRef]
- Mostofinejad, D.; Reisi, M. A new DEM-based method to predict packing density of coarse aggregates considering their grading and shapes. Constr. Build. Mater. 2012, 35, 414–420. [Google Scholar] [CrossRef]
- Jia, X.; Gan, M.; Williams, R.A.; Rhodes, D. Validation of a digital packing algorithm in predicting powder packing densities. Powder Technol. 2007, 174, 10–13. [Google Scholar] [CrossRef]
- Kumar, E.A.; Maiya, M.P.; Murthy, S.S. Measurement and Analysis of Effective Thermal Conductivity of MmNi4.5Al0.5 Hydride Bed. Ind. Eng. Chem. Res. 2011, 50, 12990–12999. [Google Scholar] [CrossRef]
- Axelrod, S.D.; Makrides, A.C. X-ray Studies of Hydrogen—Silver—Palladium Electrodes. J. Phys. Chem. 1964, 68, 2154–2159. [Google Scholar] [CrossRef]
- Lodders, K. Solar system abundances and condensation temperatures of the elements. Astrophys. J. 2003, 591, 1220. [Google Scholar] [CrossRef]
- Briki, C.; Rango, P.; Belkhiria, S.; Dhaou, H.; Jemni, A. Measurements of expansion of LaNi5 compacted powder during hydrogen absorption/desorption cycles and their influences on the reactor wall. Int. J. Hydrogen Energy 2019, 44, 13647–13654. [Google Scholar] [CrossRef]
- Knapton, A.G. Palladium alloys for hydrogen diffusion membranes. Platin. Met. Rev. 1977, 21, 44–50. [Google Scholar]
- Kumar, S.; Jain, A.; Ichikawa, T.; Kojima, Y.; Dey, G.K. Development of vanadium based hydrogen storage material: A review. Renew. Sustain. Energy Rev. 2017, 72, 791–800. [Google Scholar] [CrossRef]
- Kumar, S.; Jain, A.; Kojima, Y. Thermodynamics and kinetics of hydrogen absorption–desorption of vanadium synthesized by aluminothermy. J. Therm. Anal. Calorim. 2017, 130, 721–726. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Higher hydrides of vanadium and niobium. Inorg. Chem. 1970, 9, 1678–1682. [Google Scholar] [CrossRef]
- Kuji, T.; Matsumura, Y.; Uchida, H.; Aizawa, T. Hydrogen absorption of nanocrystalline palladium. J. Alloys Compd. 2002, 330–332, 718–722. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. (Ed.) Chapter 18—Properties Determining Mass Transfer in Polymeric Systems. In Properties of Polymers, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1997; pp. 535–583. [Google Scholar] [CrossRef]
- Cohen, R.L.; West, K.W.; Wernick, J.H. Degradation of Lani5 hydrogen-absorbing material by cycling. J. Less Common Met. 1980, 70, 229–241. [Google Scholar] [CrossRef]
- Park, J.-M.; Lee, J.-Y. The intrinsic degradation phenomena of LaNi5 and LaNi4.7Al0.3 by temperature induced hydrogen absorption-desorption cycling. Mater. Res. Bull. 1987, 22, 455–465. [Google Scholar] [CrossRef]
- Wicke, E.; Brodowsky, H.; Züchner, H. Hydrogen in palladium and palladium alloys. In Hydrogen in Metals II: Application-Oriented Properties; Alefeld, G., Völkl, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; pp. 73–155. [Google Scholar] [CrossRef]
- Wiswall, R. Hydrogen storage in metals. In Hydrogen in Metals II; Alefeld, G., Völkl, J., Eds.; Springer: Berlin/Heidelberg, Germany, 1978; Volume 29, pp. 201–242. [Google Scholar] [CrossRef]
- Leung, W.B.; March, N.H.; Motz, H. Primitive phase diagram for hydrogen. Phys. Lett. A 1976, 56, 425–426. [Google Scholar] [CrossRef]
- Li, S.; Zhao, J.; Lu, P.; Xie, Y. Maximum packing densities of basic 3D objects. Chin. Sci. Bull. 2010, 55, 114–119. [Google Scholar] [CrossRef]
- Yao, X.; Zhu, Z.H.; Cheng, H.M.; Lu, G.Q. Hydrogen diffusion and effect of grain size on hydrogenation kinetics in magnesium hydrides. J. Mater. Res. 2008, 23, 336–340. [Google Scholar] [CrossRef]
- Bowman, R.C.; Fultz, B. Metallic Hydrides I: Hydrogen Storage and Other Gas-Phase Applications. MRS Bull. 2002, 27, 688–693. [Google Scholar] [CrossRef]
- Nakajima, H.; Yoshioka, M.; Koiwa, M. Electromigration of hydrogen in vanadium and its alloys. Acta Metall. 1987, 35, 2731–2736. [Google Scholar] [CrossRef]
- Jung, J.K.; Kim, I.G.; Kim, K.T.; Ryu, K.S.; Chung, K.S. Evaluation techniques of hydrogen permeation in sealing rubber materials. Polym. Test. 2021, 93, 107016. [Google Scholar] [CrossRef]
- Catalano, J.; Giacinti Baschetti, M.; Sarti, G.C. Hydrogen permeation in palladium-based membranes in the presence of carbon monoxide. J. Membr. Sci. 2010, 362, 221–233. [Google Scholar] [CrossRef]
- Zhang, Y.; Ozaki, T.; Komaki, M.; Nishimura, C. Hydrogen permeation characteristics of vanadium–aluminium alloys. Scr. Mater. 2002, 47, 601–606. [Google Scholar] [CrossRef]
- Chen, D.; Chen, J.-D.; Zhao, L.-H.; Wang, C.-L.; Yu, B.-H.; Shi, D.-H. First-principles calculations of elasticity and thermodynamic properties of LaNi5 crystal under pressure. Chin. Phys. B 2009, 18, 738. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Tolj, I.; Davids, M.W.; Klochko, Y.; Parsons, A.; Swanepoel, D.; Smith, F.; Pollet, B.G.; Sita, C.; Linkov, V. Metal Hydride Hydrogen Storage and Supply Systems for Electric Forklift with LT PEMFC Power Module. In Proceedings of the 10th Conference on Sustainable Development of Energy, Water and Environment Systems (SWEDES2015), Dubrovnik, Croatia, 27 September–3 October 2015. [Google Scholar]
- Modern Plastics Handbook. McGraw-Hill Education—Access Engineering n.d. Available online: https://www.accessengineeringlibrary.com/content/book/9780070267145 (accessed on 23 December 2022).
- Westlake, D.G.; Ockers, S.T. Thermal expansion of vanadium and vanadium hydride at low temperature. J. Less Common Met. 1970, 22, 225–230. [Google Scholar] [CrossRef]
- Maeland, A.J. Investigation of the Vanadium—Hydrogen System by X-ray Diffraction Techniques. J. Phys. Chem. 1964, 68, 2197–2200. [Google Scholar] [CrossRef]
- White, G.K.; Pawlowicz, A.T. Thermal expansion of rhodium, iridium, and palladium at low temperatures. J. Low Temp. Phys. 1970, 2, 631–639. [Google Scholar] [CrossRef]
- Suleiman, M.; Jisrawi, N.M.; Dankert, O.; Reetz, M.T.; Bähtz, C.; Kirchheim, R.; Pundt, A. Phase transition and lattice expansion during hydrogen loading of nanometer sized palladium clusters. J. Alloys Compd. 2003, 356–357, 644–648. [Google Scholar] [CrossRef]
- Rusman, N.N.A.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 48, 12108–12126. [Google Scholar] [CrossRef]
- Yang, Y.; Mou, X.; Zhu, Z.; Bao, Z. Measurement and analysis of effective thermal conductivity of LaNi5 and its hydride under different gas atmospheres. Int. J. Hydrogen Energy 2021, 46, 19467–19477. [Google Scholar] [CrossRef]
- Boser, O. Hydrogen sorption in LaNi5. J. Less Common Met. 1976, 46, 91–99. [Google Scholar] [CrossRef]
- Gorbachuk, N.P.; Muratov, V.B. Heat Capacity and Enthalpy of LaNi5 in the Temperature Range 57–1542 K. Powder Met. Met Ceram 2005, 44, 467–471. [Google Scholar] [CrossRef]
- Wang, X.-L.; Suda, S. Reaction kinetics of the mmni4.5al0.5-h system. J. Alloys Compd. 1992, 184, 109–120. [Google Scholar] [CrossRef]
- Sonsalla, T.; Moore, A.L.; Meng, W.J.; Radadia, A.D.; Weiss, L. 3-D printer settings effects on the thermal conductivity of acrylonitrile butadiene styrene (ABS). Polym. Test. 2018, 70, 389–395. [Google Scholar] [CrossRef]
- Jung, W.D.; Schmidt, F.A.; Danielson, G.C. Thermal conductivity of high-purity vanadium. Phys. Rev. B 1977, 15, 659–665. [Google Scholar] [CrossRef]
- Watanabe, K.; Fukai, Y. Calorimetric Studies of the Behavior of Hydrogen in Vanadium and Vanadium Alloys. J. Phys. Soc. Jpn. 1985, 54, 3415–3424. [Google Scholar] [CrossRef]
- Bowman, R.C.; Freeman, B.D.; Phillips, J.R. Application of Vanadium Hydride Compressors for Joule-Thomson Cryocoolers. In Advances in Cryogenic Engineering; Fast, R.W., Ed.; Springer: Boston, MA, USA, 1991; pp. 973–980. [Google Scholar] [CrossRef]
- Ho, C.Y.; Ackerman, M.W.; Wu, K.Y.; Oh, S.G.; Havill, T.N. Thermal conductivity of ten selected binary alloy systems. J. Phys. Chem. Ref. Data 1978, 7, 959–1178. [Google Scholar] [CrossRef]
- Lässer, R.; Klatt, K.-H. Solubility of hydrogen isotopes in palladium. Phys. Rev. B 1983, 29, 748–758. [Google Scholar] [CrossRef]
- Flanagan, T.B.; Oates, W.A. The Palladium-Hydrogen System. Annu. Rev. Mater. Sci. 1991, 21, 269–304. [Google Scholar] [CrossRef]
- Klemens, P.G.; Williams, R.K. Thermal conductivity of metals and alloys. Int. Met. Rev. 1986, 31, 197–215. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnamurthy, N. Variation of activation energy of hydrogen absorption of vanadium as a function of aluminum. Int. J. Hydrogen Energy 2012, 37, 13429–13436. [Google Scholar] [CrossRef]
- Arratibel, A.; Pacheco Tanaka, A.; Laso, I.; van Sint Annaland, M.; Gallucci, F. Development of Pd-based double-skinned membranes for hydrogen production in fluidized bed membrane reactors. J. Membr. Sci. 2018, 550, 536–544. [Google Scholar] [CrossRef]
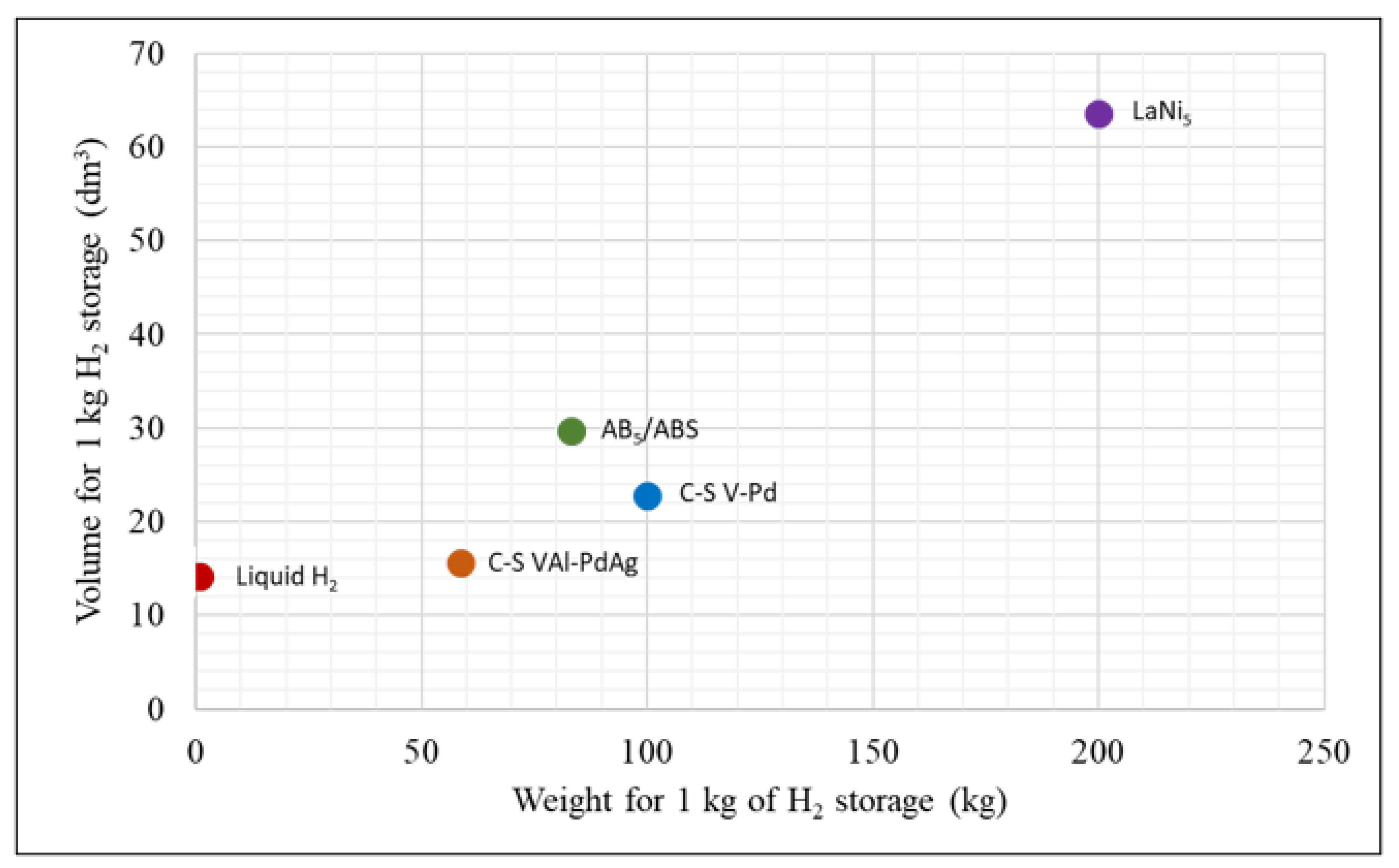

| Materials | Densities—First Material/Second Material (Effective Density) g/cm3 | First Material Weight per Pellet (g) | Second Material Weight per Pellet (g) | Reference |
|---|---|---|---|---|
| LaNi5 | 7.95 (4.37) | 1.24 | N/A | [11] |
| AB5/ABS | 8.91/1.03 (3.91 *) | 2.52 | 0.11 | [5,14] |
| C-S V-Pd | 6.1/12 (6.10 *) | 1.72 | 0.0015 | [16] |
| C-S VAl-PdAg | 5.43/5.97 (5.24 *) | 1.53 | 0.006 | [15] |
| Material | Reversible Storage Capacity in wt.% | Reference |
|---|---|---|
| LaNi5 | 0.55 | [24] |
| AB5/ABS | 1.2 | [5] |
| V | 1 | [19] |
| Pd | 1.8 | [27] |
| V90Al10 | 1.65 | Calculated from information in [6] |
| Pd80Ag20 | 1.35 | Calculated from information in [15] |
| Material | Thermal Expansion, K−1 | Expansion over 200 K, % | Thermal Expansion Reference | Lattice Expansion with Full Hydrogenation, % | Lattice Expansion Reference |
|---|---|---|---|---|---|
| LaNi5 | 4.5 × 10−5 | 0.90 | [36] | 7.4 | [17] |
| AB5 | 16 | [37] | |||
| ABS | 8.2 × 10−5 | 1.64 | [38] | None | |
| V | 8.4 × 10−6 | 0.17 | [39] | 37.7 (phase change) | [40] |
| Pd | 1.18 × 10−5 | 0.24 | [41] | 2.55 | [42] |
| V90Al10 | Not found | 3.278 | [6] | ||
| Pd80Ag20 | Not found | 2.651 | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamb, K.E.; Webb, C.J. Core-Shell, Critical-Temperature-Suppressed V Alloy-Pd Alloy Hydrides for Hydrogen Storage—A Technical Evaluation. Molecules 2023, 28, 3024. https://doi.org/10.3390/molecules28073024
Lamb KE, Webb CJ. Core-Shell, Critical-Temperature-Suppressed V Alloy-Pd Alloy Hydrides for Hydrogen Storage—A Technical Evaluation. Molecules. 2023; 28(7):3024. https://doi.org/10.3390/molecules28073024
Chicago/Turabian StyleLamb, Krystina E., and Colin J. Webb. 2023. "Core-Shell, Critical-Temperature-Suppressed V Alloy-Pd Alloy Hydrides for Hydrogen Storage—A Technical Evaluation" Molecules 28, no. 7: 3024. https://doi.org/10.3390/molecules28073024
APA StyleLamb, K. E., & Webb, C. J. (2023). Core-Shell, Critical-Temperature-Suppressed V Alloy-Pd Alloy Hydrides for Hydrogen Storage—A Technical Evaluation. Molecules, 28(7), 3024. https://doi.org/10.3390/molecules28073024