The Hydrophobic Extract of Sorghum bicolor (L. Moench) Enriched in Apigenin-Protected Rats against Aflatoxin B1-Associated Hepatorenal Derangement
Abstract
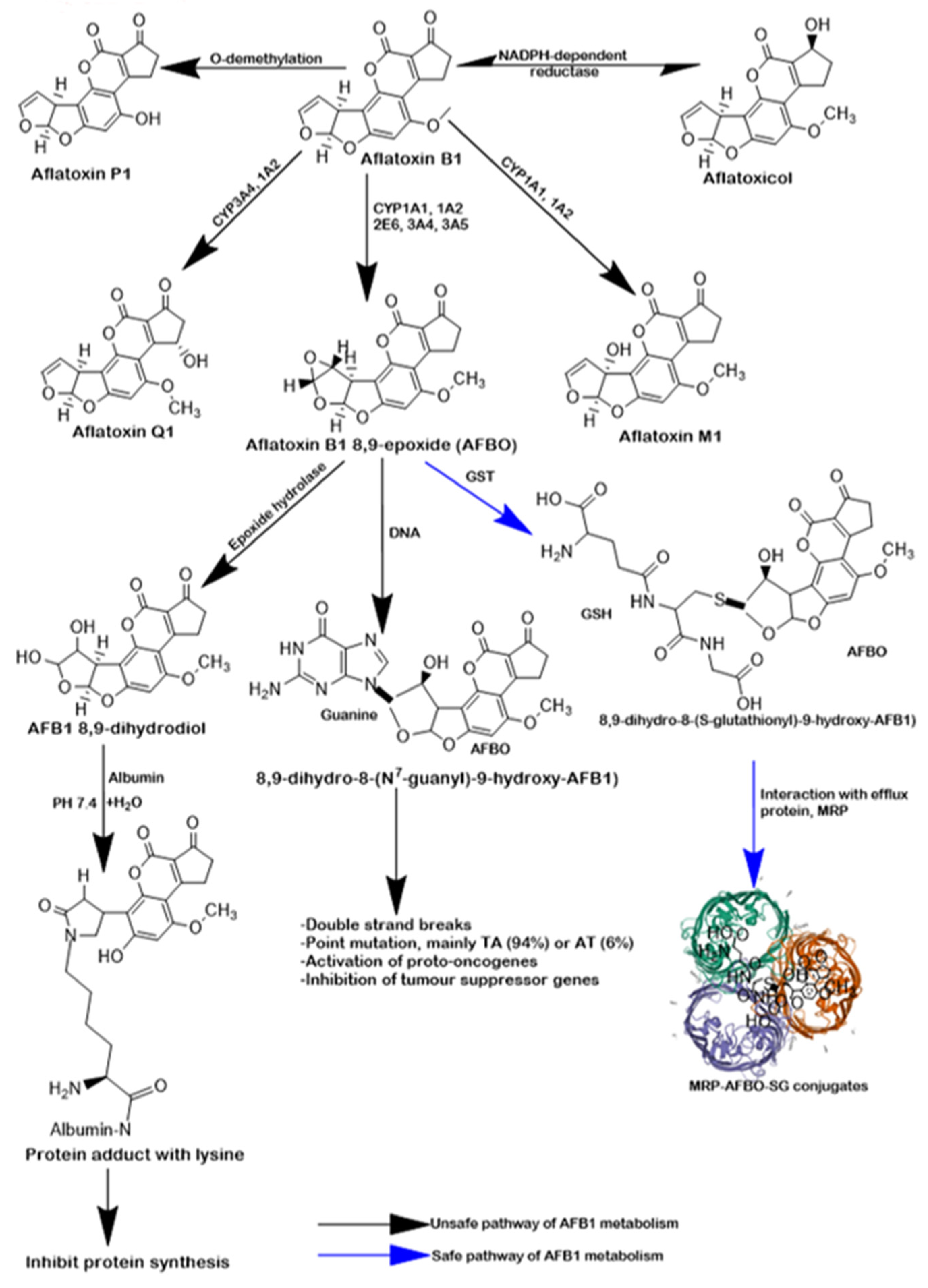
1. Introduction
2. Results
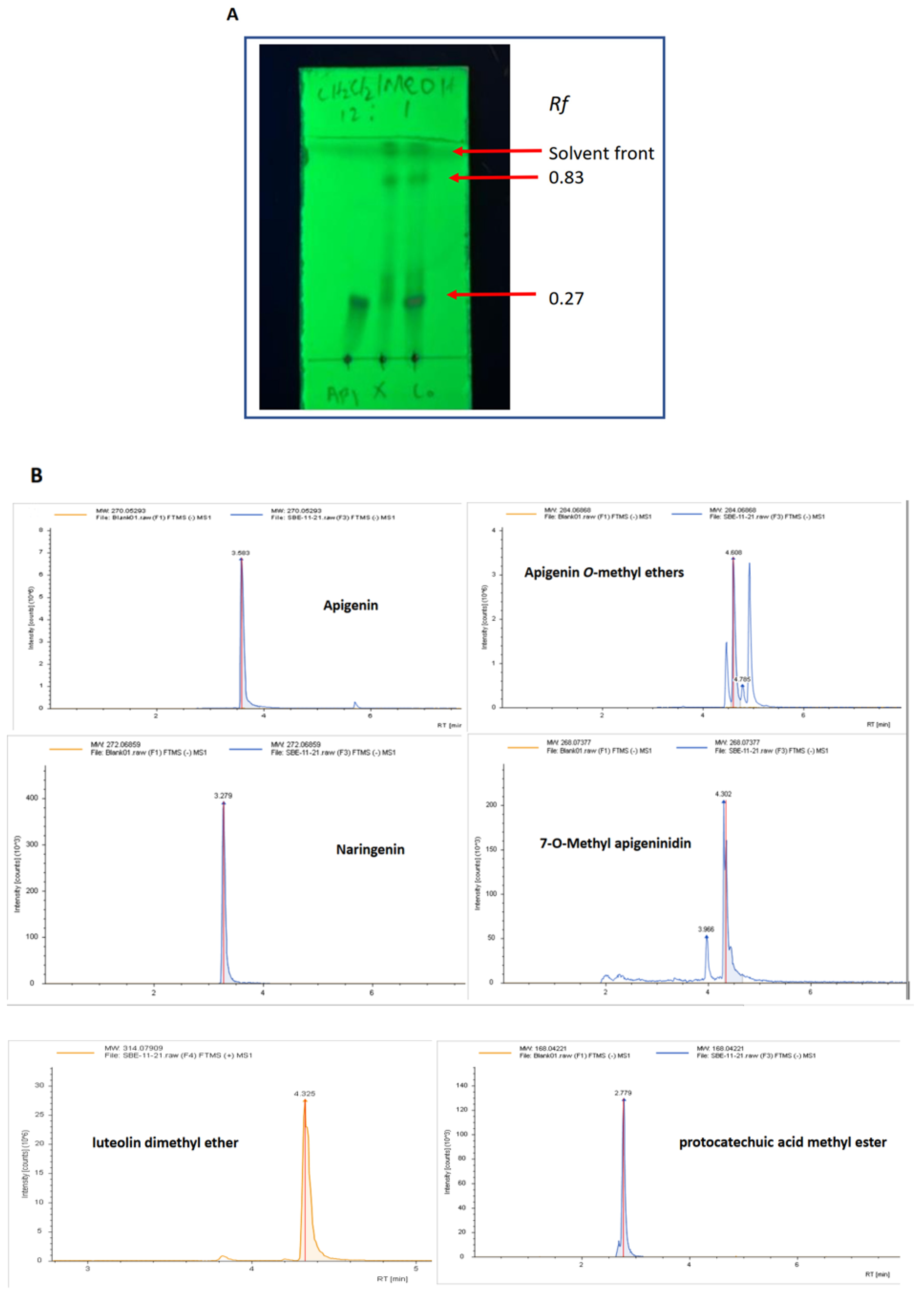
2.1. Characterisation of the Key Components in SBE-HP
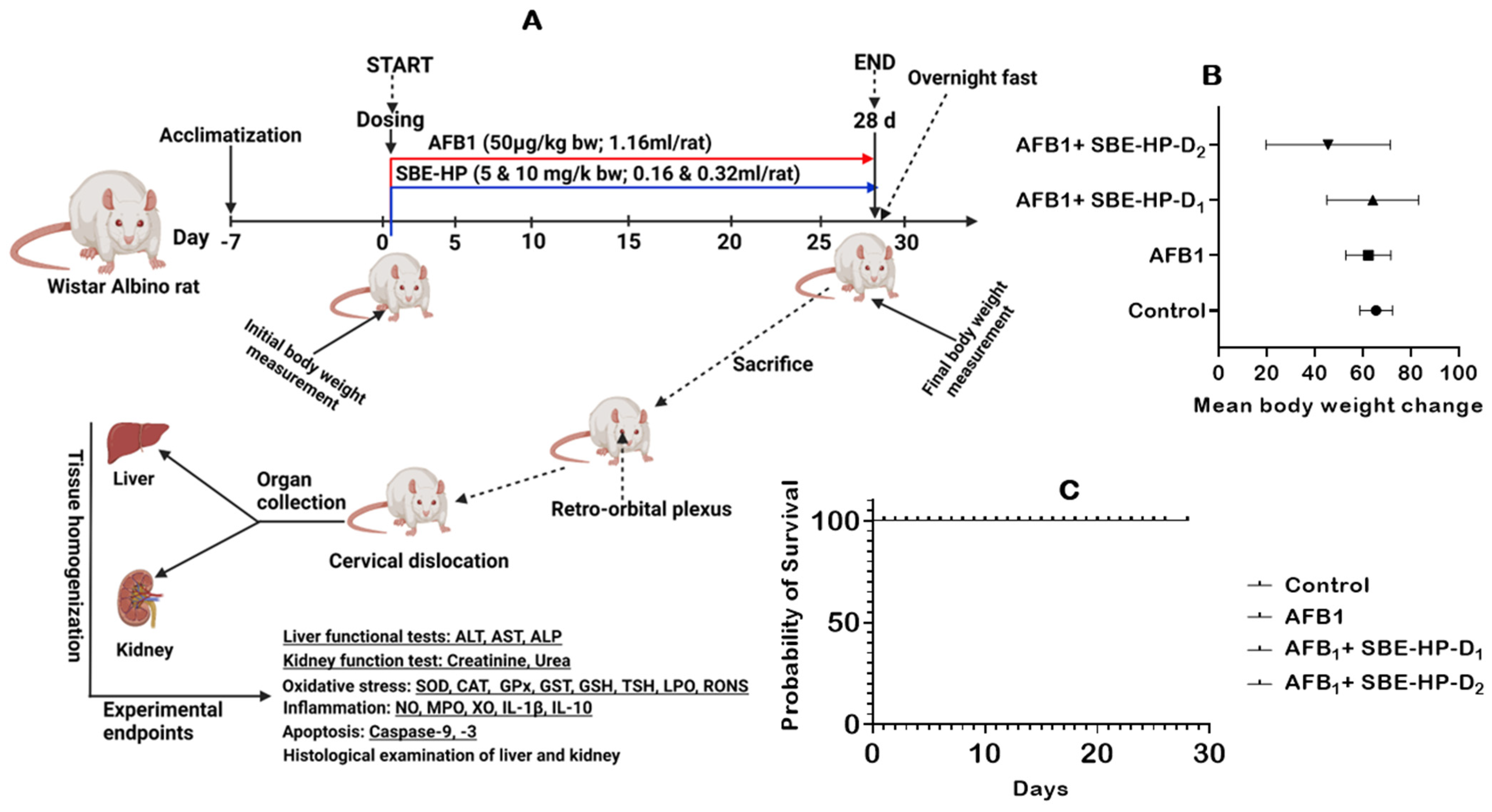
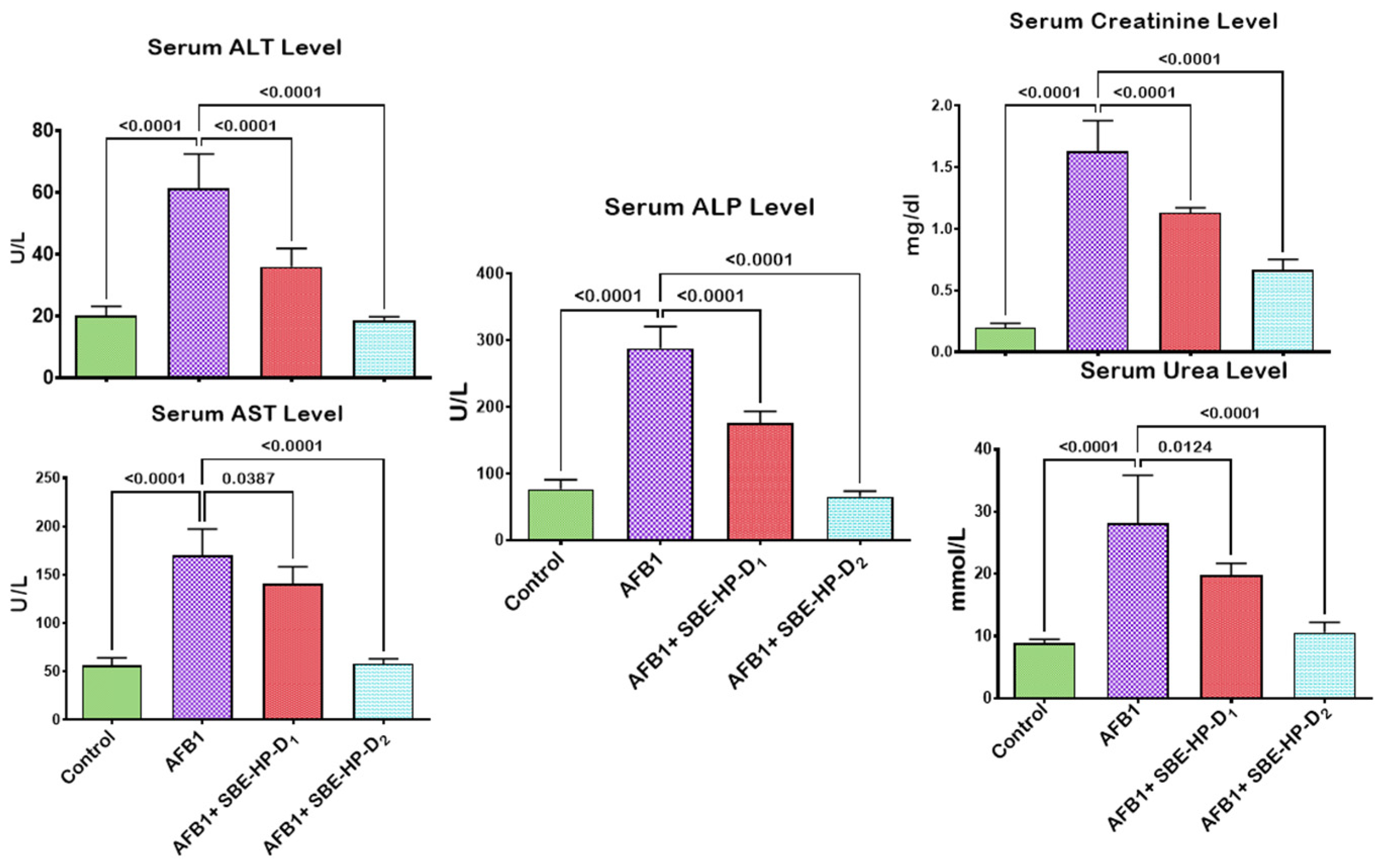
2.2. SBE-HP Prevented AFB1-Orchestrated Alterations in Body Weight and Organosomatic Indices, Rat Survivability, and Biomarkers of Hepatorenal Functions in Rats
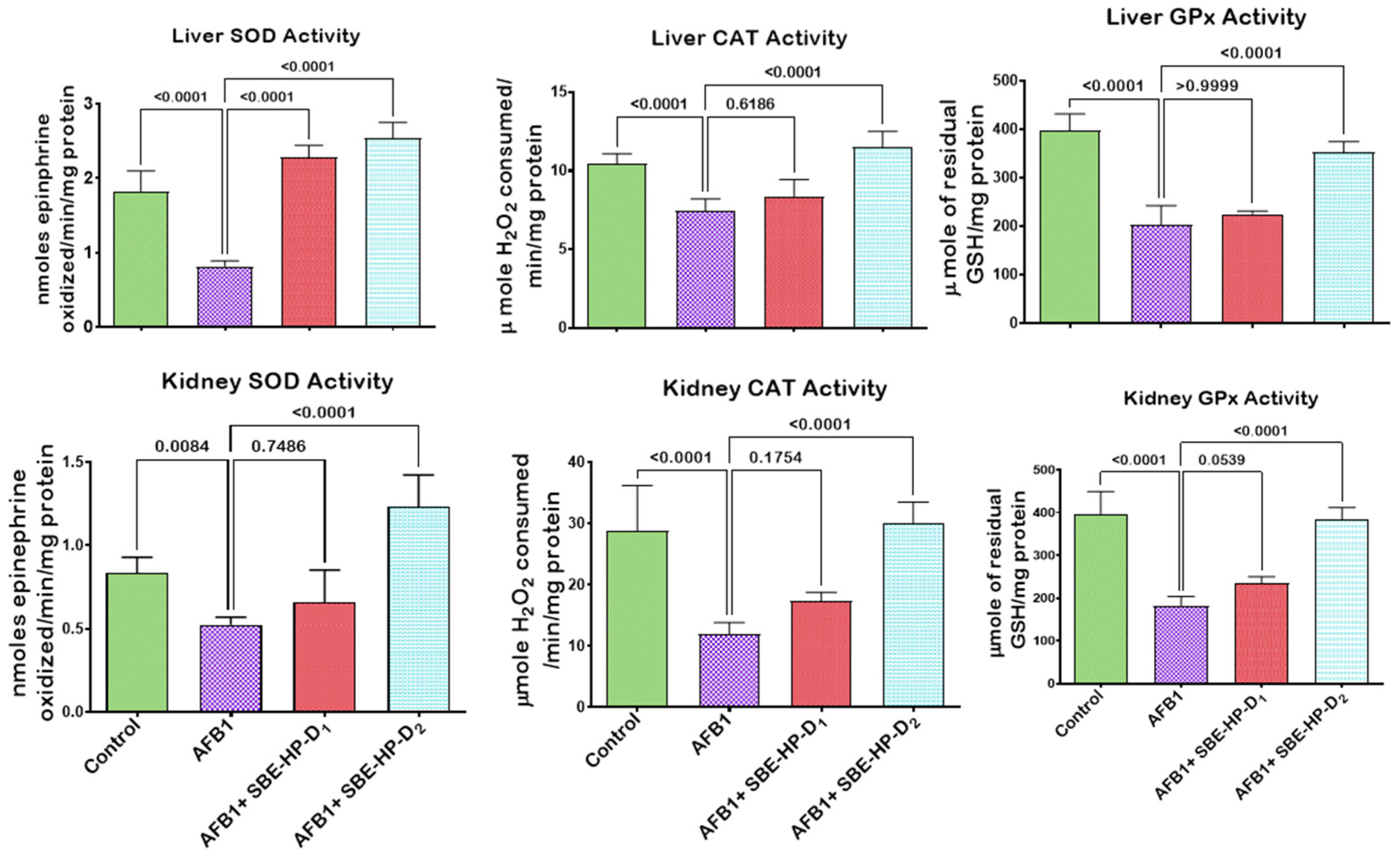
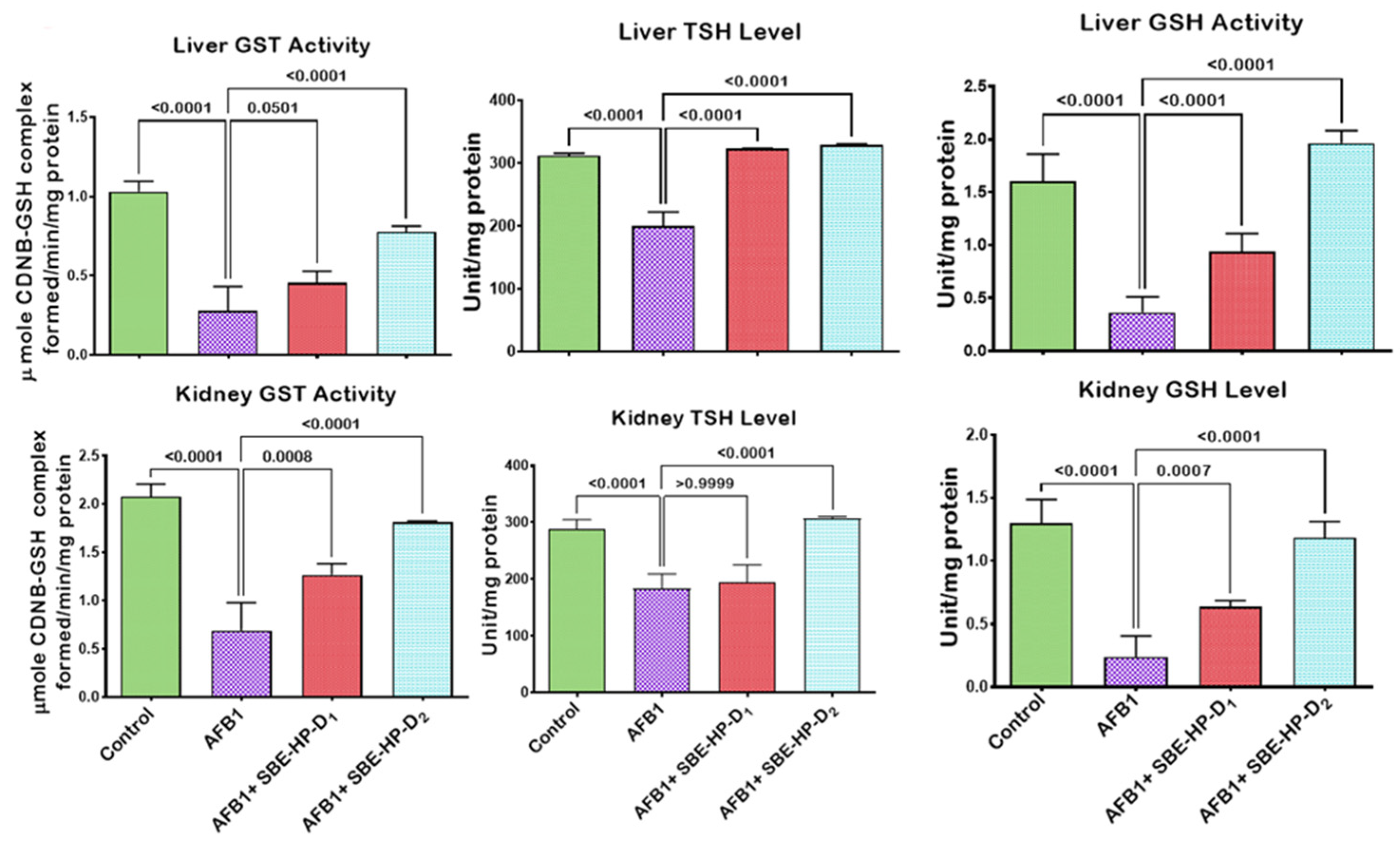
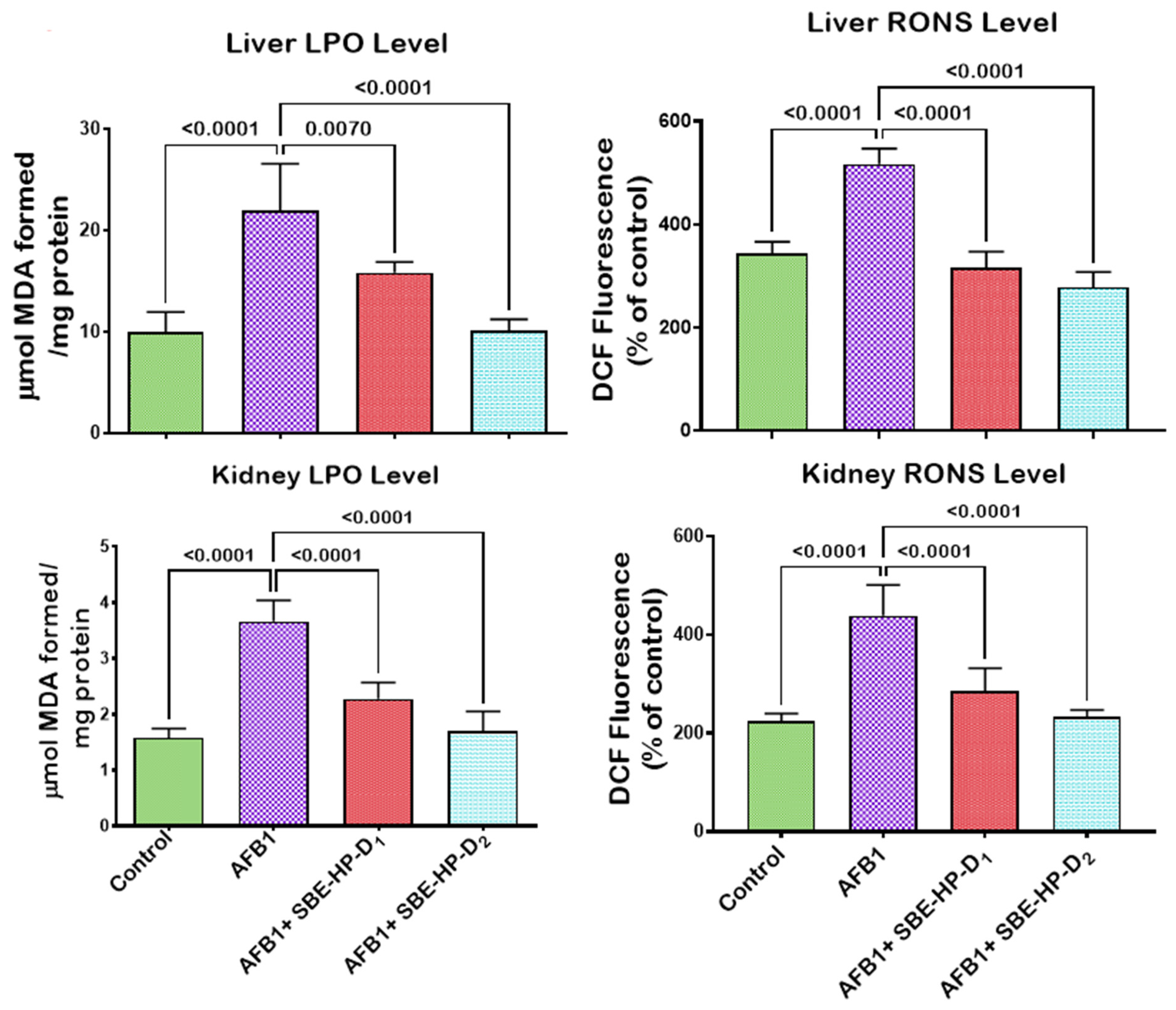
2.3. SBE-HP Mitigates Oxidative Stress and Restores Antioxidant Activities and Levels in the Liver and Kidney of AFB1-Treated Rats
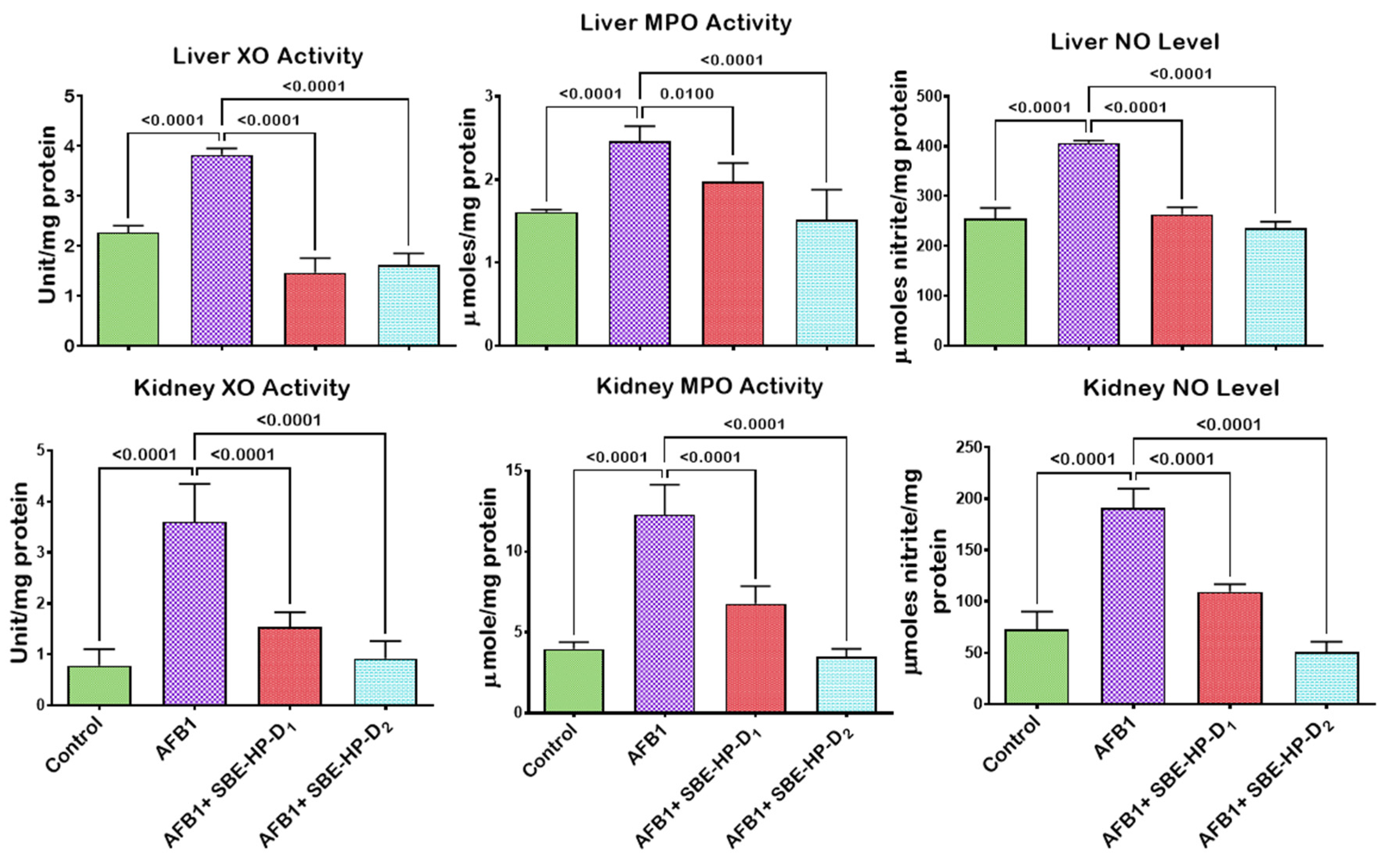
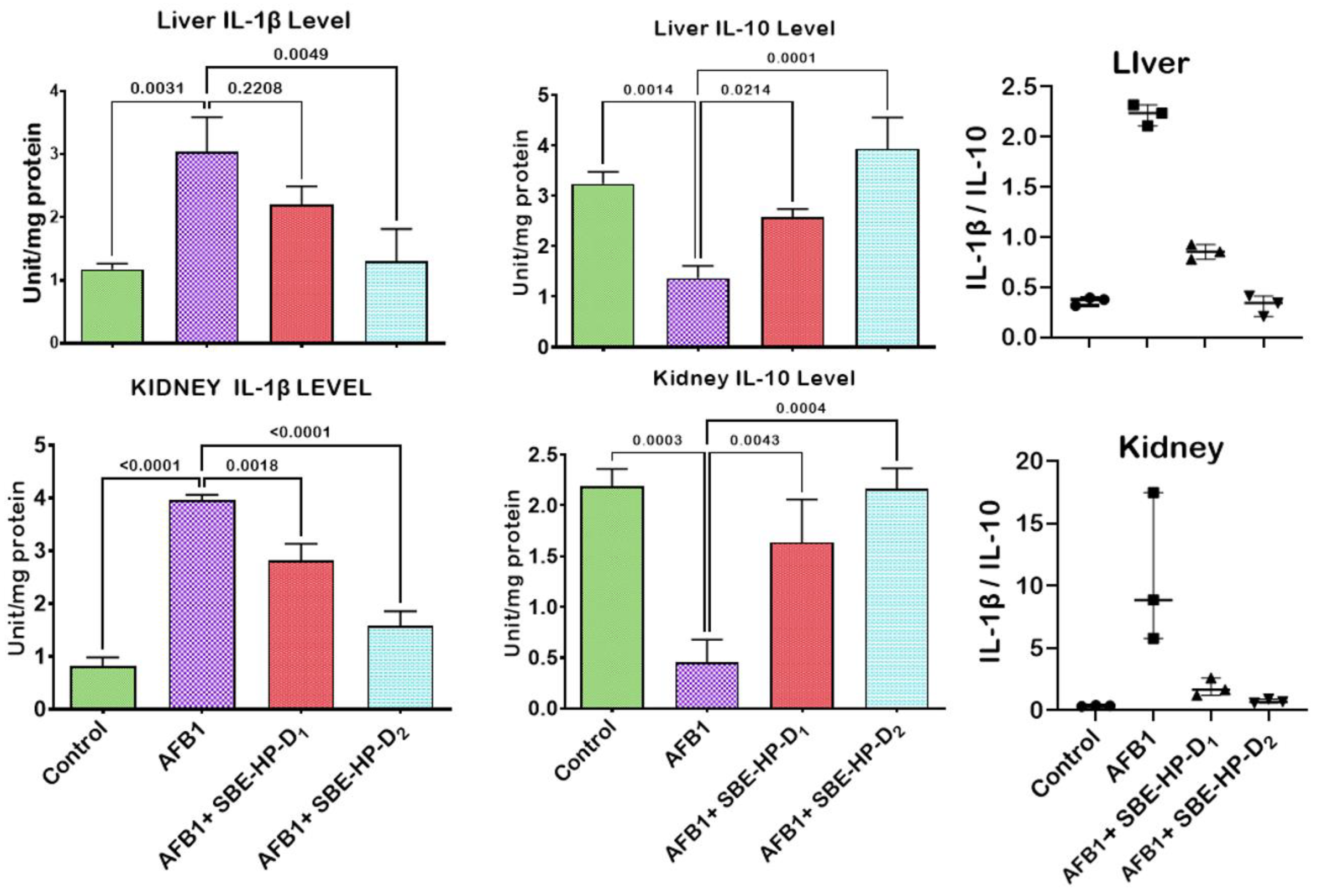
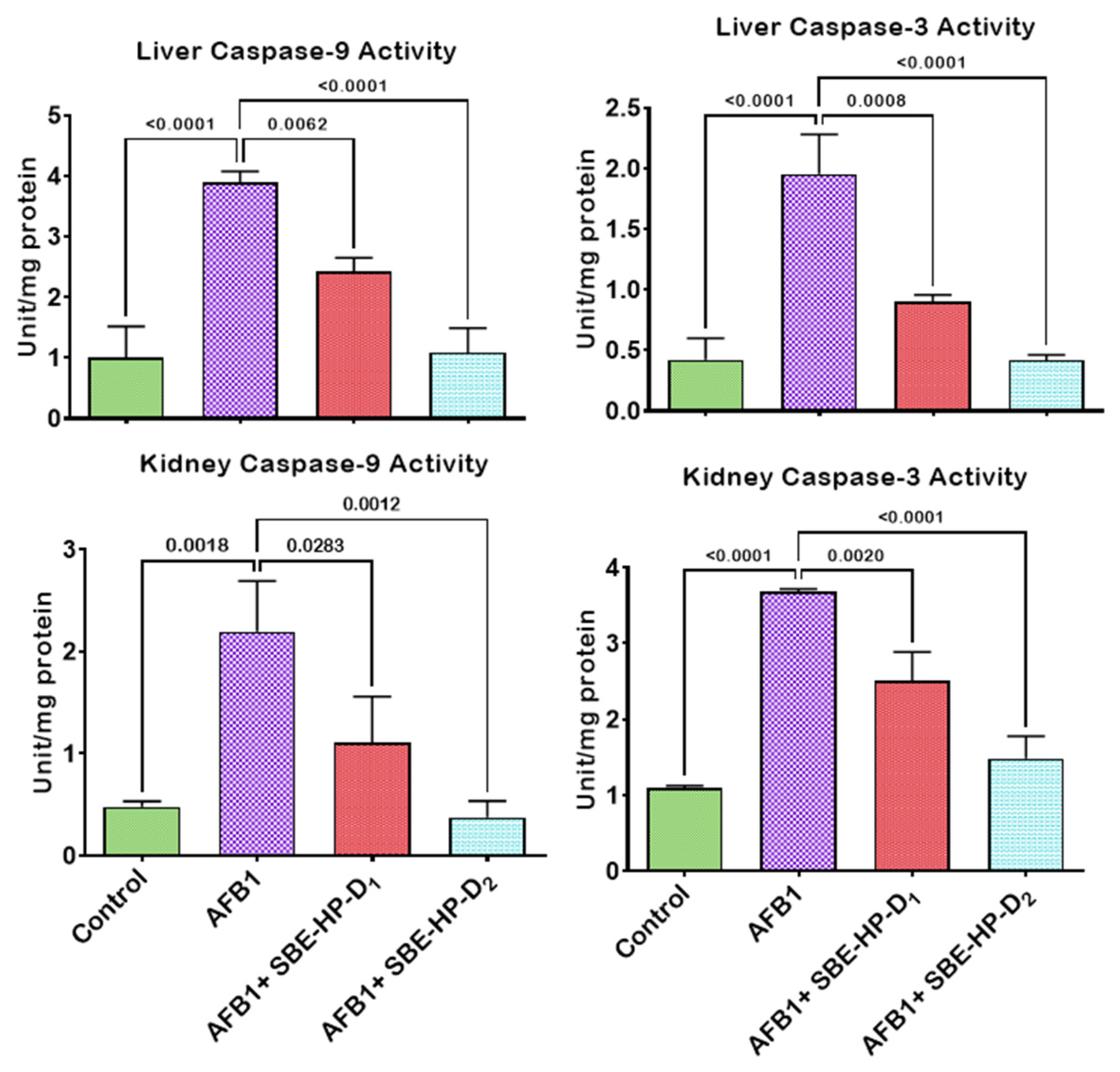
2.4. SBE-HP Resolves Inflammation and Abrogates Apoptosis in the Liver and Kidney of AFB1-Treated Rats
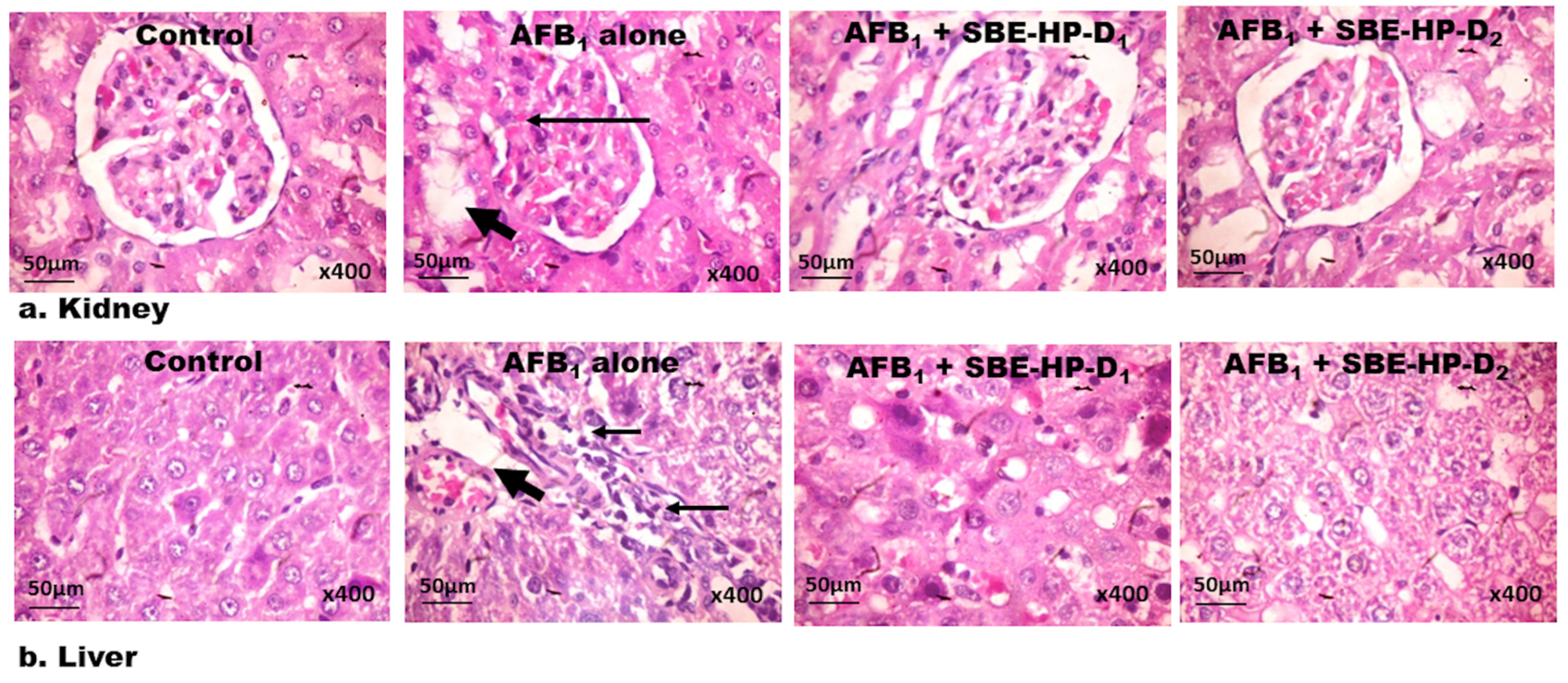
2.5. SBE-HP Abrogates Histological Lesions in the Liver and Kidney of AFB1-Exposed Rats
3. Discussion
4. Materials and Methods
4.1. Chemicals, Reagents, and Kits
4.2. Collection, Identification, and Processing of Plant Sample
4.3. Extraction and Phytochemical Characterisation of S. bicolor
4.4. Animal Welfare, Sample Size Estimation, and Experimental Design
4.5. Termination of the Experiment, Organ Harvest, and Tissue Processing
4.6. Estimation of Function and Integrity of the Hepatorenal System
4.7. Estimation of the Biomarkers of Oxidative Stress, Inflammation, and Apoptosis
4.8. Examination of the Histological Sections of the Liver and Kidney
4.9. Statistical Analysis of Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000, 56, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Palacios, H.A.; Stefanello, A.; Gavilánez, M.S.G.; Demera, D.A.C.; Garcia, M.V.; Castillo, W.A.V.; Marcano, M.A.A.; Maigua, I.R.S.; Copetti, M.V. Relationship between the Fungal Incidence, Water Activity, Humidity, and Aflatoxin Content in Maize Samples from the Highlands and Coast of Ecuador. Toxins 2022, 14, 196. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J.; Mellon, J.E. Ecology of aflatoxin producing fungi and biocontrol of aflatoxin contamination. Mycotoxin Res. 2006, 22, 110–117. [Google Scholar] [CrossRef]
- Schroeder, H.W. Factors influencing the development of aflatoxins in some field crops. J. Stored Prod. Res. 1969, 5, 187–192. [Google Scholar] [CrossRef]
- Benkerroum, N. Aflatoxins: Producing-Molds, Structure, Health Issues and Incidence in Southeast Asian and Sub-Saharan African Countries. Int. J. Environ. Res. Public Health 2020, 17, 1215. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Huang, Z.; Liu, X.; Bi, J.; Shu, Z.; Xiao, A.; Wang, J. Colorimetric ELISA based on urease catalysis curcumin as a ratiometric indicator for the sensitive determination of aflatoxin B1 in grain products. Talanta 2022, 246, 123495. [Google Scholar] [CrossRef]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential economic losses to the US corn industry from aflatoxin contamination. Food Addit. Contam. Part A 2016, 33, 540–550. [Google Scholar] [CrossRef]
- Sudakin, D.L. Dietary Aflatoxin Exposure and Chemoprevention of Cancer: A Clinical Review. J. Toxicol. Clin. Toxicol. 2003, 41, 195–204. [Google Scholar] [CrossRef]
- Holcomb, M.; Wilson, D.; Trucksess, M.; Thompson, H. Determination of aflatoxins in food products by chromatography. J. Chromatogr. A 1992, 624, 341–352. [Google Scholar] [CrossRef]
- Zhang, K.; Banerjee, K. A Review: Sample Preparation and Chromatographic Technologies for Detection of Aflatoxins in Foods. Toxins 2020, 12, 539. [Google Scholar] [CrossRef]
- Salisu, B.; Anua, S.M.; Ishak, W.R.W.; Mazlan, N. Development and validation of quantitative thin layer chromatographic technique for determination of total aflatoxins in poultry feed and food grains without sample clean-up. J. Adv. Veter. Anim. Res. 2021, 8, 656–670. [Google Scholar] [CrossRef]
- IARC. IARC Monographs on the Identification of Carcinogenic Hazards to Humans. In Book IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 131, pp. 1–40. [Google Scholar]
- Hanigan, H.M.; Laishes, B.A. Toxicity of aflatoxin B1 in rat and mouse hepatocytes in vivo and in vitro. Toxicology 1984, 30, 185–193. [Google Scholar] [CrossRef]
- Jiang, Y.; Ogunade, I.; Vyas, D.; Adesogan, A. Aflatoxin in Dairy Cows: Toxicity, Occurrence in Feedstuffs and Milk and Dietary Mitigation Strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef]
- Shephard, G.S. Risk assessment of aflatoxins in food in Africa. Food Addit. Contam. Part A 2008, 25, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Kortei, N.K.; Annan, T.; Kyei-Baffour, V.; Essuman, E.K.; Okyere, H.; Tettey, C.O. Exposure and risk characterizations of ochratoxins A and aflatoxins through maize (Zea mays) consumed in different agro-ecological zones of Ghana. Sci. Rep. 2021, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bammler, T.K.; Guo, Y.; Kelly, E.J.; Eaton, D.L. Mu-class GSTs are responsible for aflatoxin B(1)-8, 9-epoxide-conjugating activity in the nonhuman primate macaca fascicularis liver. Toxicol. Sci. 2000, 56, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tulayakul, P.; Sakuda, S.; Dong, K.; Kumagai, S. Comparative activities of glutathione-S-transferase and dialdehyde reductase toward aflatoxin B1 in livers of experimental and farm animals. Toxicon 2005, 46, 204–209. [Google Scholar] [CrossRef]
- Min, L.; Fink-Gremmels, J.; Li, D.; Tong, X.; Tang, J.; Nan, X.; Yu, Z.; Chen, W.; Wang, G. An overview of aflatoxin B1 biotransformation and aflatoxin M1 secretion in lactating dairy cows. Anim. Nutr. 2021, 7, 42–48. [Google Scholar] [CrossRef]
- Shen, H.-M.; Ong, C.-N. Mutations of the p53 tumor suppressor gene and ras oncogenes in aflatoxin hepatocarcinogenesis. Mutat. Res. Genet. Toxicol. 1996, 366, 23–44. [Google Scholar] [CrossRef]
- Owumi, S.E.; Irozuru, C.E.; Arunsi, U.O.; Faleke, H.O.; Oyelere, A.K. Caffeic acid mitigates aflatoxin B1-mediated toxicity in the male rat reproductive system by modulating inflammatory and apoptotic responses, testicular function, and the redox-regulatory systems. J. Food Biochem. 2022, 46, e14090. [Google Scholar] [CrossRef]
- Owumi, S.E.; Irozuru, C.E.; Arunsi, U.O.; Oyelere, A.K. Caffeic acid protects against DNA damage, oxidative and inflammatory mediated toxicities, and upregulated caspases activation in the hepatorenal system of rats treated with aflatoxin B(1). Toxicon 2022, 207, 1–12. [Google Scholar] [CrossRef]
- Loe, D.W.; Stewart, R.K.; Massey, T.E.; Deeley, R.G.; Cole, S.P.C. ATP-Dependent Transport of Aflatoxin B1and Its Glutathione Conjugates by the Product of the Multidrug Resistance Protein (MRP) Gene. Mol. Pharmacol. 1997, 51, 1034–1041. [Google Scholar] [CrossRef]
- Owumi, S.; Najophe, E.S.; Farombi, E.O.; Oyelere, A.K. Gallic acid protects against Aflatoxin B1-induced oxidative and inflammatory stress damage in rats kidneys and liver. J. Food Biochem. 2020, 44, e13316. [Google Scholar] [CrossRef] [PubMed]
- Storvik, M.; Huuskonen, P.; Kyllonen, T.; Lehtonen, S.; El-Nezami, H.; Auriola, S.; Pasanen, M. Aflatoxin B1--a potential endocrine disruptor--up-regulates CYP19A1 in JEG-3 cells. Toxicol. Lett. 2011, 202, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Gugliandolo, E.; Peritore, A.F.; D’Amico, R.; Licata, P.; Crupi, R. Evaluation of Neuroprotective Effects of Quercetin against Aflatoxin B1-Intoxicated Mice. Animals 2020, 10, 898. [Google Scholar] [CrossRef]
- Wang, W.-J.; Xu, Z.-L.; Yu, C.; Xu, X.-H. Effects of aflatoxin B1 on mitochondrial respiration, ROS generation and apoptosis in broiler cardiomyocytes. Anim. Sci. J. 2017, 88, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
- Hinton, D.M.; Myers, M.J.; Raybourne, R.A.; Francke-Carroll, S.; Sotomayor, R.E.; Shaddock, J.; Warbritton, A.; Chou, M.W. Immunotoxicity of Aflatoxin B1 in Rats: Effects on Lymphocytes and the Inflammatory Response in a Chronic Intermittent Dosing Study. Toxicol. Sci. 2003, 73, 362–377. [Google Scholar] [CrossRef]
- Rooney, L.W.; Waniska, R.D. Sorghum Food and Industrial Utilization; John Wiley & Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Owumi, S.E.; Kazeem, A.I.; Wu, B.; Ishokare, L.O.; Arunsi, U.O.; Oyelere, A.K. Apigeninidin-rich Sorghum bicolor (L. Moench) extracts suppress A549 cells proliferation and ameliorate toxicity of aflatoxin B1-mediated liver and kidney derangement in rats. Sci. Rep. 2022, 12, 1–19. [Google Scholar] [CrossRef]
- Xu, J.; Wang, W.; Zhao, Y. Phenolic Compounds in Whole Grain Sorghum and Their Health Benefits. Foods 2021, 10, 1921. [Google Scholar] [CrossRef]
- de Morais Cardoso, L.; Pinheiro, S.S.; Martino, H.S.D.; Pinheiro-Sant’Ana, H.M. Sorghum (Sorghum bicolor L.): Nutrients, bioactive compounds, and potential impact on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 372–390. [Google Scholar] [CrossRef]
- Owumi, S.E.; Otunla, M.T.; Arunsi, U.O.; Oyelere, A.K. Apigeninidin-enriched Sorghum bicolor (L. Moench) extracts alleviate Aflatoxin B(1)-induced dysregulation of male rat hypothalamic-reproductive axis. Exp. Biol. Med. 2022, 247, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Ademiluyi, A.O.; Oboh, G.; Agbebi, O.J.; Boligon, A.A.; Athayde, M.L. Sorghum [Sorghum bicolor(L.) Moench] Leaf Sheath Dye Protects Against Cisplatin-Induced Hepatotoxicity and Oxidative Stress in Rats. J. Med. Food 2014, 17, 1332–1338. [Google Scholar] [CrossRef] [PubMed]
- Espitia-Hernández, P.; González, M.L.C.; Ascacio-Valdés, J.A.; Dávila-Medina, D.; Flores-Naveda, A.; Silva, T.; Chacón, X.R.; Sepúlveda, L. Sorghum (Sorghum bicolor L.) as a potential source of bioactive substances and their biological properties. Crit. Rev. Food Sci. Nutr. 2020, 62, 2269–2280. [Google Scholar] [CrossRef] [PubMed]
- Garrett, A.R.; Weagel, E.; Martinez, A.D.; Heaton, M.; Robison, R.A.; O’Neill, K.L. A novel method for predicting antioxidant activity based on amino acid structure. Food Chem. 2014, 158, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Benson, K.F.; Beaman, J.L.; Ou, B.; Okubena, A.; Okubena, O.; Jensen, G.S. West African Sorghum bicolor Leaf Sheaths Have Anti-Inflammatory and Immune-Modulating Properties In Vitro. J. Med. Food 2013, 16, 230–238. [Google Scholar] [CrossRef]
- Wuyts, J.; Van Dijck, P.; Holtappels, M. Fungal persister cells: The basis for recalcitrant infections? PLoS Pathog. 2018, 14, e1007301. [Google Scholar] [CrossRef]
- Smolensky, D.; Rhodes, D.; McVey, D.S.; Fawver, Z.; Perumal, R.; Herald, T.; Noronha, L. High-Polyphenol Sorghum Bran Extract Inhibits Cancer Cell Growth Through ROS Induction, Cell Cycle Arrest, and Apoptosis. J. Med. Food 2018, 21, 990–998. [Google Scholar] [CrossRef]
- Hua, Z.; Liu, R.; Chen, Y.; Liu, G.; Li, C.; Song, Y.; Cao, Z.; Li, W.; Li, W.; Lu, C.; et al. Contamination of Aflatoxins Induces Severe Hepatotoxicity Through Multiple Mechanisms. Front. Pharmacol. 2021, 11, 605823. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.R.; Han, E.H.; Kim, J.Y.; Oh, S.J.; Kim, S.K.; Woo, E.-R.; Jeong, H.G.; Kang, K.W. Potent protective effect of isoimperatorin against aflatoxin B1-inducible cytotoxicity in H4IIE cells: Bifunctional effects on glutathione S-transferase and CYP1A. Carcinogenesis 2006, 27, 2483–2490. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Otunla, M.T.; Najophe, E.S.; Oyelere, A.K. Decrease in reproductive dysfunction using aflatoxin B1 exposure: A treatment with 3-indolepropionic acid in albino Wistar rat. Andrologia 2021, 54, e14248. [Google Scholar] [CrossRef]
- Donato, M.T.; Jiménez, N.; Pelechá, M.; Tolosa, L. Oxidative-stress and long-term hepatotoxicity: Comparative study in Upcyte human hepatocytes and hepaRG cells. Arch. Toxicol. 2022, 96, 1021–1037. [Google Scholar] [CrossRef]
- Owumi, S.; Lewu, D.; Arunsi, U.; Oyelere, A. Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-H.; Zhang, N.-Y.; Zhu, M.-K.; Zhao, L.; Zhou, J.-C.; Qi, D.-S. Prevention of Aflatoxin B1 Hepatoxicity by Dietary Selenium Is Associated with Inhibition of Cytochrome P450 Isozymes and Up-Regulation of 6 Selenoprotein Genes in Chick Liver. J. Nutr. 2016, 146, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Vipin, A.V.; Rao, R.; Kurrey, N.K.; Anu, A.K.A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against Aflatoxin B(1)-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017, 91, 415–424. [Google Scholar]
- Akinrinde, A.S.; Adebiyi, O.E.; Asekun, A. Amelioration of Aflatoxin B1-induced gastrointestinal injuries by Eucalyptus oil in rats. J. Complement. Integr. Med. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.; Owumi, S.; Uwaifo, A.; Farombi, E. Aflatoxin B1 and ethanol co-exposure induces hepatic oxidative damage in mice. Toxicol. Ind. Health 2010, 26, 717–724. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.-M.; Yeon, S.-J.; Ham, H.J.; Kim, J.-H.; Han, S.-I.; Oh, D.-H. Flavonoids in Decorticated Sorghum Grains Exert Antioxidant, Antidiabetic and Antiobesity Activities. Molecules 2020, 25, 2854. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Pratomo, I.P.; Noor, D.R.; Kusmardi, K.; Rukmana, A.; Paramita, R.I.; Erlina, L.; Fadilah, F.; Gayatri, A.; Fitriani, M.; Purnomo, T.T.H.; et al. Xanthine Oxidase-Induced Inflammatory Responses in Respiratory Epithelial Cells: A Review in Immunopathology of COVID-19. Int. J. Inflamm. 2021, 2021, 1–10. [Google Scholar] [CrossRef]
- Romagnoli, M.; Gomez-Cabrera, M.C.; Perrelli, M.G.; Biasi, F.; Pallardo, F.V.; Sastre, J.; Poli, G.; Vina, J. Xanthine oxidase-induced oxidative stress causes activation of NF-kappaB and inflammation in the liver of type I diabetic rats. Free Radic. Biol. Med. 2010, 49, 171–177. [Google Scholar] [CrossRef]
- Owumi, S.E.; Otunla, M.T.; Arunsi, U.O.; Najophe, E.S. 3-Indolepropionic acid upturned male reproductive function by reducing oxido-inflammatory responses and apoptosis along the hypothalamic-pituitary-gonadal axis of adult rats exposed to chlorpyrifos. Toxicology 2021, 463, 152996. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Grimm, E.A.; Sikora, A.G.; Ekmekcioglu, S. Molecular Pathways: Inflammation-Associated Nitric-Oxide Production as a Cancer-Supporting Redox Mechanism and a Potential Therapeutic Target. Clin. Cancer Res. 2013, 19, 5557–5563. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Aliyu-Banjo, N.O.; Danso, O.F. Fluoride and diethylnitrosamine coexposure enhances oxido-inflammatory responses and caspase-3 activation in liver and kidney of adult rats. J. Biochem. Mol. Toxicol. 2019, 33, e22327. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative Stress, Molecular Inflammation and Sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef] [PubMed]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Turkmen, K. Inflammation, oxidative stress, apoptosis, and autophagy in diabetes mellitus and diabetic kidney disease: The Four Horsemen of the Apocalypse. Int. Urol. Nephrol. 2016, 49, 837–844. [Google Scholar] [CrossRef]
- Nakano, K.; Vousden, K.H. PUMA, a Novel Proapoptotic Gene, Is Induced by p53. Mol. Cell 2001, 7, 683–694. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Luo, H.; Xu, F.; Liang, J.; Ma, C.; Ren, L.; Wang, H.; Hou, Y. Aflatoxin B1 induces microglia cells apoptosis mediated by oxidative stress through NF-kappaB signaling pathway in mice spinal cords. Environ. Toxicol. Pharmacol. 2022, 90, 103794. [Google Scholar] [CrossRef]
- Mughal, M.J.; Xi, P.; Yi, Z.; Jing, F. Aflatoxin B1 invokes apoptosis via death receptor pathway in hepatocytes. Oncotarget 2017, 8, 8239–8249. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.-M.; Jang, G.Y.; Park, D.; Woo, K.S.; Kim, T.M.; Jeong, H.S.; Kim, D.J. Effect of sorghum ethyl-acetate extract on benign prostatic hyperplasia induced by testosterone in Sprague–Dawley rats. Biosci. Biotechnol. Biochem. 2018, 82, 2101–2108. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.M.K.; Nikolic-Paterson, D.J.; Lan, H.-Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Nalkurthi, C.; Schroder, W.A.; Melino, M.; Irvine, K.M.; Nyuydzefe, M.; Chen, W.; Liu, J.; Teng, M.W.; Hill, G.R.; Bertolino, P.; et al. ROCK2 inhibition attenuates profibrogenic immune cell function to reverse thioacetamide-induced liver fibrosis. JHEP Rep. 2021, 4, 100386. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Garud, M.S.; Kulkarni, Y.A. Gallic acid attenuates type I diabetic nephropathy in rats. Chem. Interact. 2018, 282, 69–76. [Google Scholar] [CrossRef]
- Javanbakht, S.; Shaabani, A. Carboxymethyl cellulose-based oral delivery systems. Int. J. Biol. Macromol. 2019, 133, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Adefisan, A.O.; Madu, J.C.; Owumi, S.E.; Adaramoye, O.A. Calliandra portoricensis ameliorates ovarian and uterine oxido-inflammatory responses in N-methyl-N-nitrosourea and benzo[a] pyrene-treated rats. Exp. Biol. Med. 2020, 245, 1490–1503. [Google Scholar] [CrossRef]
- Clairborne, A. Catalase Activity; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Owumi, S.E.; Danso, O.F.; Nwozo, S.O. Gallic acid and omega-3 fatty acids mitigate epididymal and testicular toxicity in manganese-treated rats. Andrologia 2020, 52, e13630. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Nwozo, S.O.; Effiong, M.E.; Najophe, E.S. Gallic acid and omega-3 fatty acids decrease inflammatory and oxidative stress in manganese-treated rats. Exp. Biol. Med. 2020, 245, 835–844. [Google Scholar] [CrossRef]
- Jollow, D.; Mitchell, J.; Zampaglione, N.; Gillette, J. Bromobenzene-Induced Liver Necrosis. Protective Role of Glutathione and Evidence for 3,4-Bromobenzene Oxide as the Hepatotoxic Metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.I.; Gawehn, K.; Grassl, M. Methods of Enzymatic Analysis, 2nd ed.; Academic Press Inc.: New York, NY, USA, 1974; Volume 1. [Google Scholar]
- Ohkawa, H.O.N.; Yagi, K. Assay for lipid peroxidation in animal tissues by Thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Owumi, S.E.; Dim, U.J. Manganese suppresses oxidative stress, inflammation and caspase-3 activation in rats exposed to chlorpyrifos. Toxicol. Rep. 2019, 6, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Granell, S.; Gironella, M.; Bulbena, O.; Panés, J.; Mauri, M.; Sabater, L.; Aparisi, L.; Gelpí, E.; Closa, D. Heparin mobilizes xanthine oxidase and induces lung inflammation in acute pancreatitis. Crit. Care Med. 2003, 31, 525–530. [Google Scholar] [CrossRef]
- Owumi, S.E.; Adedara, I.A.; Akomolafe, A.P.; Farombi, E.O.; Oyelere, A.K. Gallic acid enhances reproductive function by modulating oxido-inflammatory and apoptosis mediators in rats exposed to aflatoxin-B1. Exp. Biol. Med. 2020, 245, 1016–1028. [Google Scholar] [CrossRef]
- Owumi, S.E.; Nwozo, S.O.; Arunsi, U.O.; Oyelere, A.K.; Odunola, O.A. Co-administration of Luteolin mitigated toxicity in rats’ lungs associated with doxorubicin treatment. Toxicol. Appl. Pharmacol. 2020, 411, 115380. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practise of Histological Techniques, 6th ed.; Churchill Livingstone Elsevier: Philadelphia, PA, USA, 2008; pp. 83–134. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owumi, S.E.; Ajakaiye, B.; Akinwunmi, A.O.; Nwozo, S.O.; Oyelere, A.K. The Hydrophobic Extract of Sorghum bicolor (L. Moench) Enriched in Apigenin-Protected Rats against Aflatoxin B1-Associated Hepatorenal Derangement. Molecules 2023, 28, 3013. https://doi.org/10.3390/molecules28073013
Owumi SE, Ajakaiye B, Akinwunmi AO, Nwozo SO, Oyelere AK. The Hydrophobic Extract of Sorghum bicolor (L. Moench) Enriched in Apigenin-Protected Rats against Aflatoxin B1-Associated Hepatorenal Derangement. Molecules. 2023; 28(7):3013. https://doi.org/10.3390/molecules28073013
Chicago/Turabian StyleOwumi, Solomon E., Blessing Ajakaiye, Adenike O. Akinwunmi, Sarah O. Nwozo, and Adegboyega K. Oyelere. 2023. "The Hydrophobic Extract of Sorghum bicolor (L. Moench) Enriched in Apigenin-Protected Rats against Aflatoxin B1-Associated Hepatorenal Derangement" Molecules 28, no. 7: 3013. https://doi.org/10.3390/molecules28073013
APA StyleOwumi, S. E., Ajakaiye, B., Akinwunmi, A. O., Nwozo, S. O., & Oyelere, A. K. (2023). The Hydrophobic Extract of Sorghum bicolor (L. Moench) Enriched in Apigenin-Protected Rats against Aflatoxin B1-Associated Hepatorenal Derangement. Molecules, 28(7), 3013. https://doi.org/10.3390/molecules28073013








