Abstract
The trifluoromethyl group is widely recognized for its significant role in the fields of medicinal chemistry and material science due to its unique electronic and steric properties that can alter various physiochemical properties of the parent molecule, such as lipophilicity, acidity, and hydrogen bonding capabilities. Compared to the well-established C-trifluoromethylation, N-trifluoromethylation has received lesser attention. Considering the extensive contribution of nitrogen to drug molecules, it is predicted that constructing N-trifluoromethyl (N-CF3) motifs will be of great significance in pharmaceutical and agrochemical industries. This review is mainly concerned with the synthesis of heterocycles containing this motif. In three-membered heterocycles containing the N-CF3 motif, the existing literature mostly demonstrated the synthetic strategy, as it does for four- and larger-membered heterocycles. Certain structures, such as oxaziridines, could serve as an oxidant or building blocks in organic synthesis. In five-membered heterocycles, it has been reported that N-CF3 azoles showed a higher lipophilicity and a latent increased metabolic stability and Caco-2-permeability compared with their N-CH3 counterparts, illustrating the potential of the N-CF3 motif. Various N-CF3 analogues of drugs or bioactive molecules, such as sildenafil analogue, have been obtained. In general, the N-CF3 motif is developing and has great potential in bioactive molecules or materials. Give the recent development in this motif, it is foreseeable that its synthesis methods and applications will become more and more extensive. In this paper, we present an overview of the synthesis of N-CF3 heterocycles, categorized on the basis of the number of rings (three-, four-, five-, six- and larger-membered heterocycles), and focus on the five-membered heterocycles containing the N-CF3 group.
1. Introduction
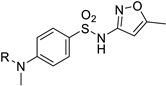
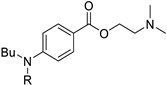
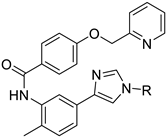
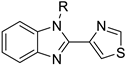
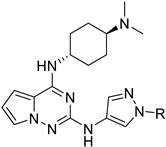
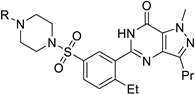
Organic fluorine chemistry, as a prominent research area, has garnered significant attention for several decades and has also become essential to the evolution of many different but interconnected research fields [1]. The introduction of the fluorine group, especially the trifluoromethyl group, into organic compounds has become known as one of the most efficient methods for modulating molecular properties, for example, lipophilicity and metabolic stability [2]. Due to its potential utility, many methods have been studied extensively [3,4]. However, in contrast to the well-developed C-trifluoromethylations, the N-trifluoromethyl (N-CF3) motif has rarely been investigated to date. Considering the widespread dissemination of nitrogen (especially nitrogen-containing heterocycles) in drug molecules [5,6,7], constructing the N-CF3 motif in molecules is of great significance in pharmaceutical and agrochemical industries. Drug analogues and potential agents containing the N-CF3 motif are partially shown in Figure 1 [8,9,10,11,12,13].
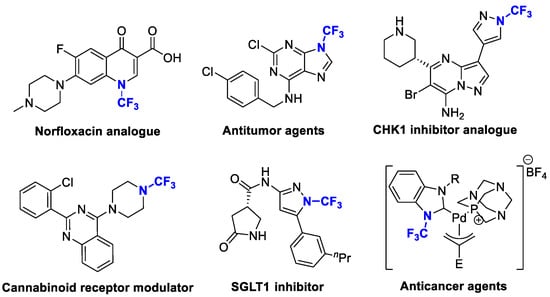
Figure 1.
Drug analogues containing N-trifluoromethyl motif.
Despite the great potential of the N-CF3 motif, the synthesis of this moiety and its relative chemistry have been rarely explored. The limited use of N-CF3 compounds is primarily due to the absence of scalable methods for their preparation [14,15,16]. Recently, thanks to the new reagents and methods, this motif has increasingly been featured in the literature.
In terms of constructing the N-CF3 motif in heterocycles, there are two main approaches. The first involves utilizing starting materials containing the N-CF3 motif to generate heterocycles directly, while the second strategy entails introducing the CF3 group via trifluoromethylation or fluorination of nitrogen-containing species. In this paper we review the construction of N-CF3 heterocycles on the basis of the size of the cycles, involving three-, four-, five-, six- and larger-membered heterocycles. This article covers as much literature as possible, from the 1960s to early 2023. The structures mentioned in this paper are shown in Figure 2.
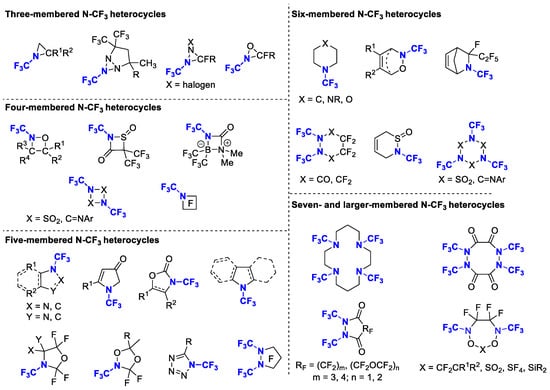
Figure 2.
Variably sized N-heterocycles containing the N-trifluoromethyl motif.
2. Three-Membered Heterocycles
In previous reports, the synthesis of three-membered N-CF3 heterocycles mainly relied on starting materials containing the N-CF3 motif. In 1964, Logothetis reported the aziridination of N-CF3 imines 1a in the presence of diazomethane, which obtained aziridine 2a in the yield of 64% (Scheme 1a) [17]. Subsequently, Coe et al. investigated the substructure scope of imines [18]. Unfortunately, poor selectivity was exhibited when R was replaced with iC3F7 (perfluoroisopropyl) or CF=CHCF3. When R = iC3F7, a mixture of three components in the ratio of 2b:2b″:2b′ = 6:9:1 was obtained and when R = CF=CHCF3, a mixture of two products was obtained in the ratio of 2d:2d′ = 71:29 by F shift and further CH insertion into a C-F bond. Kaupp et al. synthesized some stable triaziridines 4 which could be purified by fractional distillation by the irradiation of azimines 3 at room temperature (Scheme 1b) [19]. However, not much attention has been paid to N-CF3 triaziridine.
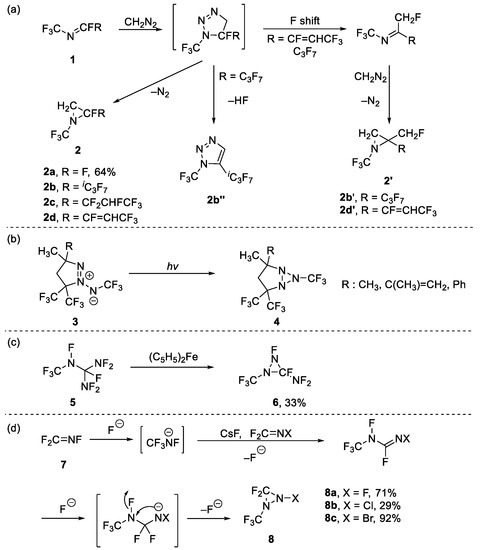
Scheme 1.
Synthesis of (a) aziridines, (b) triziridines and (c,d) diaziridines.
Compared to the structures mentioned above, diaziridine attracted more attention, and its synthesis could be divided into two strategies: Mitsch et al., while studying the reductive defluorination-cyclization of organic fluoronitrogens, found that diaziridine 6 could be generated from 1,1-bis(difluoramino)perfluoro-2-azapropan 5 in the presence of ferrocene (Scheme 1c) [20]. Later DesMarteau et al. obtained another diaziridine 6 by nucleophilic cyclization when studying perfluoroalkanamine ions (Scheme 1d) [21]. In the presence of CsF, CF2=NX (when X = F) yielded perfluoroalkanamine ion (CF3NF−), which underwent further reaction with another CF2=NX to form perfluoro-1-methylformamidine 8. On the basis of previous work, DesMarteau group achieved synthesis of other diaziridines (when X = Br, Cl) through perfIuoroalkanamine ions [22,23]. Meanwhile, other electrophilic species, such as N-CF3 imine 1a, could also be attacked by CF3NF−, leading to 3,3-difluoro-1,2-bis(trifluoromethyl)diaziridine [24].
Furthermore, perfluorinated oxaziridine 9 has drawn wide attention and its structure, synthesis and applications have been studied. Petrov and Resnati have already summarized the synthesis and reactivity of perfluorinated oxaziridines, in which N-CF3 oxaziridines are included [25]. In previous reports, the most common methods of synthesis were oxidative cyclization. In 1976, DesMarteau et al. reported that oxaziridine was obtained by the oxidation of a N-CF3 imines 1a by CF3OOH [26]. This reaction was performed in two steps: addition, and further cyclization mediated by NaF. Later, different metal fluorides were investigated by the authors of [27] and KHF2 was found to be the most suitable reagent for the yield of oxaziridine 7 (the yield was up to 92%). This method was difficult and the starting materials were potentially explosive. In order to make the reaction safer and more convenient, different oxidants, such as hydrogen peroxide [28] and chlorine gas in the presence of metal carbonate [29], etc., have been developed, but these methods were still difficult. Finally, using meta-chloroperbenzoic acid (mCPBA) as the oxidant was determined to be a safer and more attractive choice (Scheme 2) [30,31].
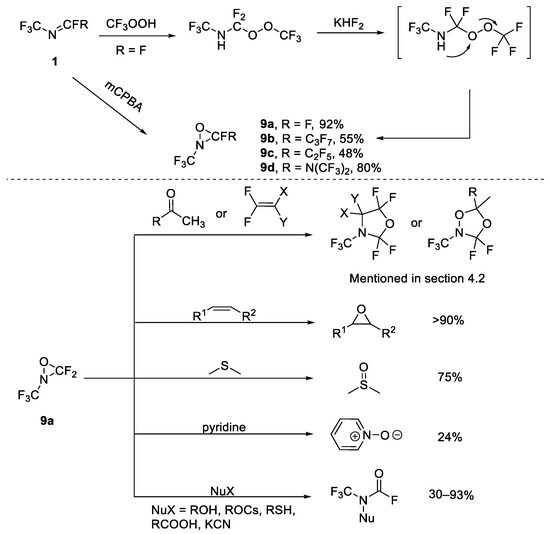
Scheme 2.
Synthesis and applications of oxaziridines.
To date, N-CF3 aziridine, triaziridine and diaziridine have still gained less attention and their properties and applications are less-developed. On the other hand, there have been studies into the structure, properties, and applications of oxaziridine [25] (shown in Scheme 2), such as its reaction with nucleophiles [32,33] and its use as an oxidant [34] or as building blocks. These studies are included in Section 4.2 of this paper.
3. Four-Membered Heterocycles
Similarly, the synthesis of four-membered N-CF3 heterocycles was based on starting materials containing the N-CF3 motif.
The predominant approach to oxazetidine was [2+2] cycloaddition reaction. Trifluoronitrosomethane (CF3NO) was the most common starting material. In the 1950s, Barr and Haszeldine reported that CF3NO reacted with tetrafluoroethylene 10a to give two products: an oxazetidine 11a and a copolymer (consisting of two monomers in a 1:1 ratio) [35,36]. The oxazetidine predominated in this reaction at a high temperature (ca. 100 °C) and the copolymer at room temperature (Scheme 3a). Since then, several halogenated tetrafluoroethylenes (CF2=CXY) have been investigated (Scheme 3b) [37,38]. It was found that the formation of an oxazetidine and the copolymer from CF3NO occurred most readily with the olefins CF2=CF2, CF2=CHF (adducts were a 99:1 mixture of 3,3,4-trifluoro-2-trifluoromethyl-1,2-oxazetidine and 3,4,4-trifluoro-2-trifluoromethyl-1,2-oxazetidine), CF2=CFCl, and CF2=CCl2, and less readily with perfluoro-olefins containing more than two carbon atoms, or with ethylenes containing two or more vinylic hydrogens [38].
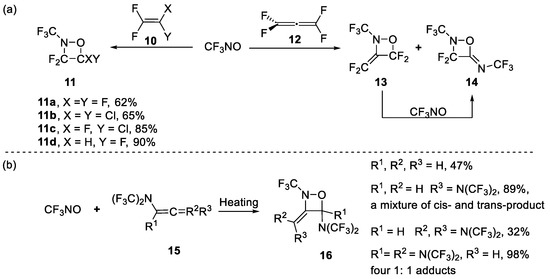
Scheme 3.
Synthesis of oxazetidines through trifluoronitrosomethane.
Meanwhile, it was reported that an attack on the substituted allene by CF3NO led to another form of oxazetidine. Banks et al. found that tetrafluoroallene 12 reacted with CF3NO to yield a complex mixture of oxazetidine 13 and 14 [39]. By adjusting the reaction conditions, the highest yields of oxazetidine 13 and 14 can reach 43% and 42%, respectively. They also found that compound 14 could be obtained (82% yield) when heating oxazetidine 13 with CF3NO. Later, Haszeldine et al. synthesized a series of oxazedines 16 with limited regioselectivity and stereoselectivity through the reaction of N, N-bistrifluoromethylamino-substituted allenes 15 with CF3NO (Scheme 3b) [40,41].
In addition to CF3NO, there were other reagents used in [2+2] cycloaddition. For example, in 1986, Sundermeyer and co-workers found that CF3N=S=O could react with ketene to generate thiazetidin 17 (Scheme 4a) [42]. Burger et al. reported that the reaction of CF3N=C=O and boranamine led to diazaboretidin 18 (Scheme 4b) [43].
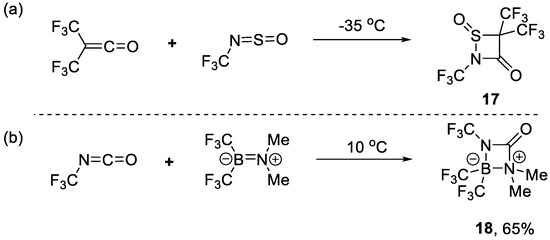
Scheme 4.
Synthesis of (a) thiazetidine and (b) diazaboretidin through [2+2] cycloaddition reaction.
4. Five-Membered Heterocycles
The literature on three- and four-membered N-CF3 heterocycles was more focused on their synthesis, with limited exploration of their properties and potential applications. However, in contrast, five- and six-membered heterocycles featuring the N-CF3 motif have recently been receiving more and more attention [8,44,45,46]. Their synthesis methods, as well as biological activities and derivatization, are gradually being studied.
To evaluate the suitability of the N-CF3 motif on amines and azoles in drug design, Schiesser et al. synthesized a series of N-CF3 amines and azoles (shown in Figure 3), and determined their stability in aqueous media and other properties [45]. For example, the stability of N-CF3 analogues of known bioactive compounds (sulfamethoxazole derivative 19a, tetracaine derivative 20a, inhibitors of hedgehog pathway 21a [47,48], inhibitors of methionine aminopeptidase 22a [49], inhibitors of interleukin-1 receptor associated kinase 4 (IRAK4) 23a [50], and sildenafil analogue 24a) were studied and are shown in Table 1.
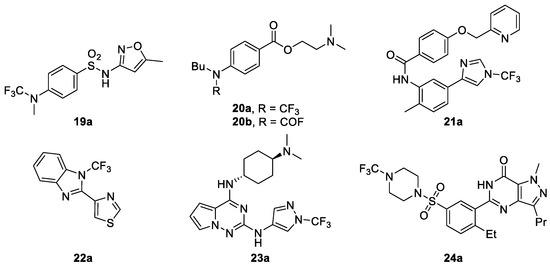
Figure 3.
Synthesis of N-trifluoromethyl compounds to determine their aqueous stability and additional key in vitro properties.

Table 1.
Half-life of N-CF3 compounds 19a–24a [a].
Two anilines 19a and 20a, as well as piperazine 24a, showed fast hydrolysis at all three pH values investigated, with half-lives of less than 1.5 days at 25 °C (Table 1). It should to be noted that for 20a, the corresponding carbamoyl fluoride 20b was the main product at pH 1.0, with a small amount of product where both the carbamoyl fluoride had been further hydrolyzed to the secondary amine and the ester bond had been cleaved. The latter compound was also the main product at pH 7.4 and pH 10.0. In contrast to the N-CF3 anilines and piperazine, for all the N-CF3 azoles they investigated no corresponding carbamoyl fluoride of free azole was detected in aqueous media at any of the three pH values studied.
Moreover, they then compared the key in vitro properties in medicinal chemistry (log D, experimentally determined polar surface area (ePSA) [51,52], permeability in human epithelial colorectal adenocarcinoma cells (Caco-2), and metabolic stability for the N-CF3 compounds 19a–24a and their N-CH3 counterparts (Table 2).

Table 2.
Overview of the change in key in vitro properties when exchanging N-CH3 and N-CF3 [f].
In the compounds investigated, the exchange of a methyl for trifluoromethyl led to the expected higher lipophilicity as proven by an increased log D7.4 and chromlog D7.4 and a decreased ePSA. Log D7.4 increases by on average 1.1 log units and chromlog D7.4 by 1.6 log units. However, the extent of this change can vary significantly and was dependent on both the individual compound and type of log D7.4 analysis used. Changes in permeability and metabolic stability were less consistent.Stability to human liver microsomes (HLMs) can be significantly increased for the trifluoromethyl analogue as seen for 22a (p = 0.004) or decreased as for 21a. The decreased metabolic stability of the latter two compounds could be due to an increased lipophilicity, rendering the potential metabolic soft spots (benzylic methyl group in 21a) more susceptible to metabolism.
According to Schiesser’s research [45], N-CF3 amines were prone to hydrolysis, whereas N-CF3 azoles have excellent aqueous stability. Compared to N-CH3 analogues, N-CF3 azoles showed a higher lipophilicity and a latent increase in metabolic stability and Caco-2-permeability, which illustrated the value and potentiality of N-CF3 diazole in medicinal chemistry.
In terms of synthesizing these five-membered N-CF3 structures, both types of constructing N-CF3 were included. In this section, the synthesis of five-membered heterocycles would be divided into the three parts: nucleophilic fluorination, cyclization based on N-CF3 starting materials, and electrophilic trifluoromethylation.
4.1. Nucleophilic Fluorination
4.1.1. Fluorine/Halogen Exchange
Fluorine/halogen exchange was one of the first reactions to obtain the trifluoromethyl group on the nitrogen atom (Scheme 5) [16]. Yagupolskii et al. achieved fluorine/halogen exchange with N-trichloromethyl derivatives in the presence of HF or Me4NF [53]. This strategy was also capable of generating N-CF3 pyrazoles [54,55] and N-CF3 1,2,4-triazoles [55]. However, highly toxic or environmentally unfriendly reagents (such as CF2Br2, a known ozone-depleting reagent [56]) would be used in this method for fluorine/halogen exchange or in the preparation of the precursors (such as 26) for fluorine/halogen exchange, which limited its use.
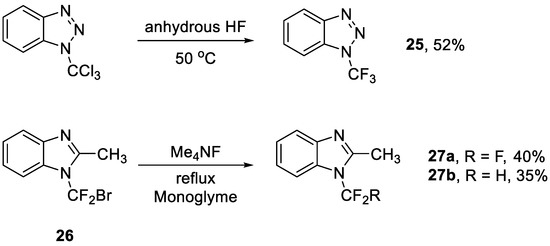
Scheme 5.
Synthesis of N-trifluoromethyl benzotriazole or benzimidazole through fluorine/halogen exchange.
4.1.2. Oxidative Desulfurization and Fluorination
Compared to fluorine/halogen exchange, this method has been much more thoroughly studied. This method allowed people to replace C-S bonds with C-F bods under extremely mild conditions compared to the fluorination of formamides [57] or fluorination induced by SF4 [58].
Hiyama et al. reported conversion from methyl dithiocarbamates to trifluoromethylamines in the presence of readily available fluoride ions (Scheme 6a) [59]. The reaction conditions were applicable to a wide range of disubstituted nitrogen, with substituents including phenyl, heteroaromatic or alkyl. Recently, Schindler et al. applied chlorodithiophenylformiate as an electrophile and successfully obtained phenyl aminodithioate 30 [60]. Then trifluoromethylamines 31 were generated after the desulfurization and fluorination. Additionally, Hagooly et al. used BrF3 for desulfurization and fluorination and obtained 2-(trifluoromethyl)isoindoline-1,3-dione and 1-(trifluoromethyl)azepan-2-one [61].
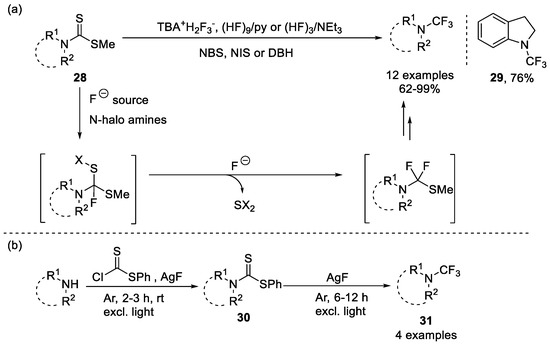
Scheme 6.
Synthesis of N-trifluoromethyl amines from (a) methyl dithiocarbamate or (b) phenyl dithiocarbamate.
Furthermore, Schoenebeck et al. reported that amines and SCF3- source (Me4N)SCF3 could generate the highly electrophilic thiocarbomoyl fluoride 32 followed by a reaction with AgF to yield trifluoromethyl amines 31 [62]. Furthermore, there were other approaches to the intermediate 32. Lin and Xiao et al. [63] generated this intermediate from difluorocarbene and sulfur (S8), while Jiang and Yi et al. [64] reported a method using CF3SO2Na (Scheme 7). These strategies have been used to synthesize interesting analogues of biologically active compounds (examples are shown in Section 5.1).
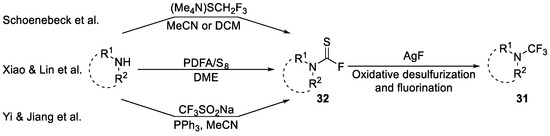
Scheme 7.
Synthesis of thiocarbomoyl fluoride intermediate and further oxidative desulfurization and fluorination to access N-trifluoromethyl amines.
4.1.3. Cyclization Induced by Fluoride Ion
Fluoro-olefins containing terminal double bonds have been shown to isomerize in the presence of fluoride ion (Scheme 8) [65]. There is a considerable amount of literature on the transformation of perfluoro-2,5-diazahexa-2,4-diene (CF2=NCF2CF2N=CF2, 36a) and its precursor CCl2=NCCl2CCl2N=CCl2, 35. In 1967, Ogden and Mitsch reported that isomerization of perfluoro-, -diazomethines with CsF could form a cyclic five-membered N-CF3 heterocycle as a minor product (34, 30% yield) [66].
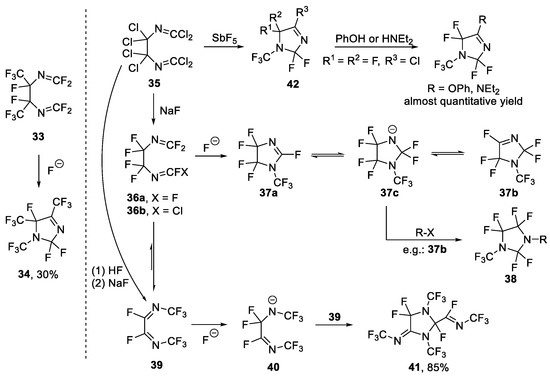
Scheme 8.
Synthesis of N-trifluoromethyl compounds through cyclization of fluoro-olefins induced by fluoride ion.
Scholl et al. synthesized 36a from 35 via two steps, and found that 36a could cyclize to form two isomers (37a and 37b) in the presence of fluoride ion [67]. Both isomers could form nitrogen anion 37c in the presence of fluoride ion. 37c could also react with 37b to generate substituted N-CF3 imidazolidine 38. Subsequently, the same group found another transformation of 35 and obtained 39 in two steps [68]. In the presence of fluoride ion, another nitrogen anion 40 could be generated, which subsequently reacted with another 39 to obtain imidazolidine 41.
Later in 1984, Banks et al. [69] investigated in some detail the effect of temperature and metal fluoride on the systems used by Scholl. The product composition depends on the reactivity of the fluoride source and the reaction conditions, i.e., the product may be under kinetic or equilibrium control. In addition to the substances reported earlier by Scholl, they detected others. Later, Chambers et al. [70,71] reacted the nitrogen anion 37c with different trapping agents including haloalkane, perfluoro azaarene, perfluoro cyclobutene, etc., and obtained diverse heterocycles 38.
Meanwhile, Pawelke et al. [72] investigated the transformation of 35 or its derivative in the presence of fluoride source. Cyclization of compound 35 led to the perfluorinated 1H-imidazole 42, whose chlorine atom could be further substituted by OPh or NEt2.
4.2. Cyclization Based on N-CF3 Starting Materials
4.2.1. [3+2] Cycloaddition
Utilization of starting materials containing the N-CF3 motif is a commonly employed strategy for achieving the target heterocyclic compounds. As mentioned in Section 2, perfluorinated oxaziridine 9a could serve as building blocks in organic chemistry. DesMarteau et al. reported that some cycloaddition of oxaziridine 9a with electron-rich alkenes and ketones resulted in oxazolidines or dioxazolidines (Scheme 9) [73,74]. However, perfluorinated oxaziridine 9a also had certain limitations as a building block: attempts to achieve the cycloaddition of 9a with CH2=CH2, CFCl=CFCl, perfluorocyclopentene, acrylonitrile, and acetylene have failed. Additionally, the reaction could not occur with fluorinated ketones such as hexafluoroacetone.
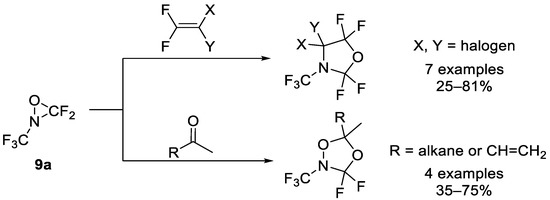
Scheme 9.
Synthesis of oxazolidines or dioxazolidines through [3+2] cycloaddition of perfluorinated oxaziridine.
In recent decades [3+2] cycloaddition between azide and alkyne has attracted significant attention, and copper-catalyzed azide-alkyne cycloaddition is one of the most widely used forms of this technique [75]. In 2017, Beier et al. reported the synthesis of azidoperfluoroalkanes 43 which could be synthesized from perfluoroalkyl trimethylsilane (TMSRF) with p-toluenesulfonyl azide (TsN3) in the presence of CsF, or synthesized from (perfluoroethyl)lithium (RF = C2F5) with TsN3 [76]. These azidoperfluoroalkanes could undergo [3+2] cycloaddition with alkynes catalyzed by copper to access diverse N-perfluoroalkyl 1,2,3-triazoles 44 (Scheme 10). Later in 2018, Beier et al. reported a mild and efficient and synthesis of highly functionalized 1-perfluoroalkyl-1H-1,2,3-triazoles 45 via in situ generated enamines in azide-ketone [3+2] cycloaddition (Scheme 10) [77].
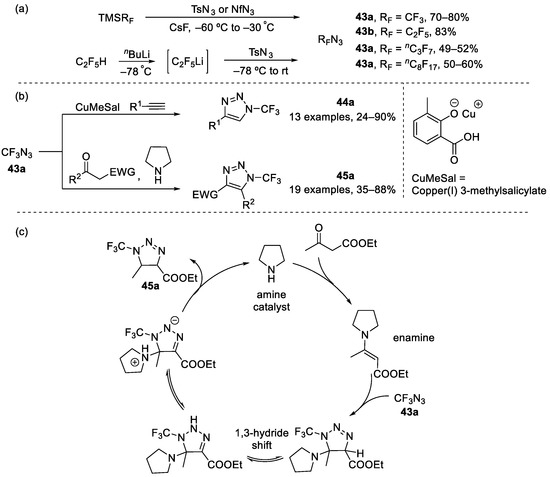
Scheme 10.
(a) Synthesis of perfluoroalkyl azides; (b) cycloaddition of trifluoromethyl azides with alkynes or ketones; (c) mechanism of the cycloaddition of trifluoromethyl azides with ketones.
In the same year, the Beier group developed a highly efficient method for the synthesis of a broad range of previously unreported N-fluoroalkyl-substituted five-membered heterocycles with microwave heating-assisted rhodium-catalyzed transannulation of N-fluoroalkyl-substituted 1,2,3-triazoles 44 [78]. Subsequently, the same group expanded this approach to acetylene substrates and successfully generated N-fluoroalkyl pyrrole (Scheme 11) [79]. The mechanism proposed by authors is shown in Scheme 12.
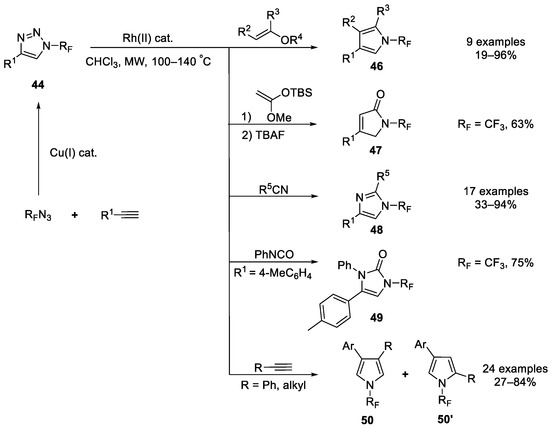
Scheme 11.
Synthesis of diverse N-trifluoromethyl heterocycles through transannulation catalyzed by rhodium.
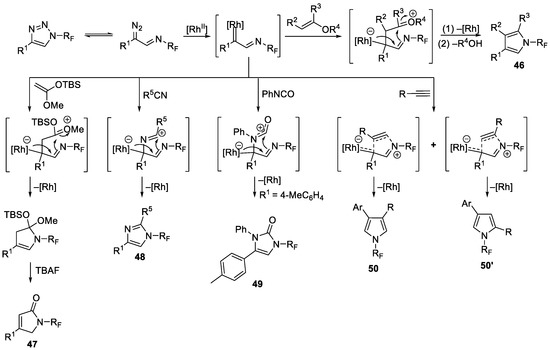
Scheme 12.
Mechanism of transannulation catalyzed by rhodium.
Xu, Guan, and Wang et al. [80] reported an alternative and scalable cyclization strategy based on N-CF3-containing synthons for constructing diverse N-CF3 azoles, including N-CF3 tetrazoles, N-CF3 imidazoles, and N-CF3 1,2,3-triazoles (Scheme 13). This method involved using a hypervalent iodine reagent for trifluoromethylation in combination with a base to efficiently carry out the reaction. Furthermore, estrone analogue 54 could be generated in two steps in a total of 74% yield. Subsequently, the authors’ group developed the reaction of the N-CF3 nitrilium ions 51 with N-, O-, and S-nucleophiles, resulting in various N-CF3 amidines, imidates, and Thioimidates [81]. Very recently, they utilized hypervalent iodine reagent for the trifluoromethylation of 4-alkylamino-pyridine to generate N-CF3 pyridinium salt which could be further translated to 2-functionlized nicotinaldehydes [82].
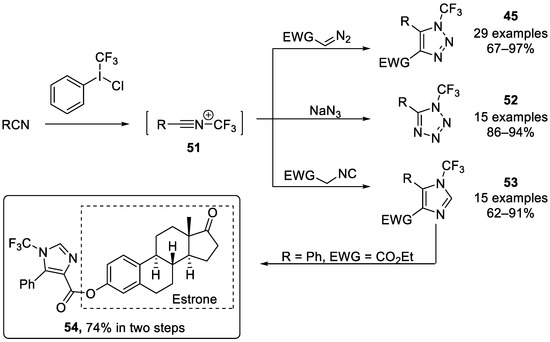
Scheme 13.
Synthesis of N-trifluoromethyl heterocycles through 1,3-dipoles generated by hypervalent iodine reagent with nitriles.
4.2.2. Other Cyclization
In addition to [3+2] cycloaddition, other cyclization pathways have been explored for the synthesis of N-CF3-containing five-membered heterocycles. For instance, Sundermeyer and co-workers reported access to the preparation of imidazolidinedione 55 through the reaction of trifluoromethyl isocyanate with trimethylsilyl cyanide, followed by hydrolysis [83]. In 1977, Rudiger Mews synthesized dioxazolidine 56 from CF3NO and bis(trifluoromethyl)diazomethane [84]. Lentz reported that trifluoromethyl isocyanide reacted with hexafluoroacetone to yield compound 57 [85]. Later, the same group reacted trifluoromethyl isocyanide with diphosphene, leading to the formation of azaphospholidine 58 [86]. In addition, Crousse et al. developed a direct approach to obtaining N-CF3 hydrazines from CF3SO2Na. Among the family of N-CF3 hydrazines, hydrazides 59 showed hydrolysis in the presence of HCl and reacted further with diketone, leading to N-CF3-1H-pyrazoles 60 in 44% yield totally (Scheme 14d) [87].
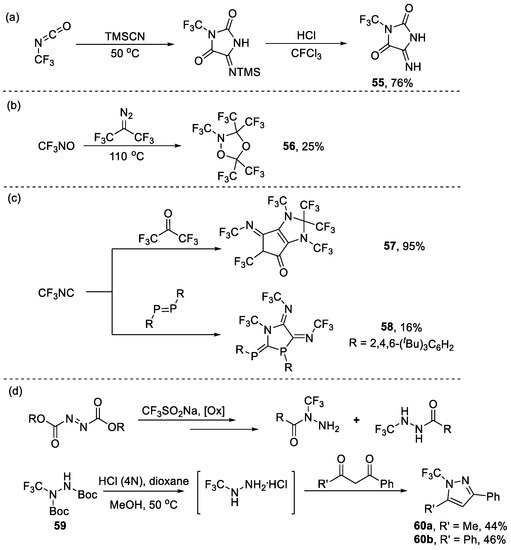
Scheme 14.
Synthesis of other N-trifluoromethyl heterocycles through cyclization of (a) trifluoromethyl isocyanate, (b) trifluoronitrosomethane, (c) trifluoromethyl isocyanide, and (d) N-trifluoromethyl hydrazides.
In 2019, Schoenebeck et al. reported straight access to N-CF3 amides, carbamates, thiocarbamates or ureas via N-CF3 carbomoyl building blocks 61 [88]. After that, the same group developed the transformation of the building blocks and generated non-cyclic or heterocyclic N-CF3 compounds as shown in Scheme 15 [89,90,91,92]. Additionally, antihistamine derivative oxatomide analogue 64a could be generated in 62% in two steps by N-H functionalization of 64.
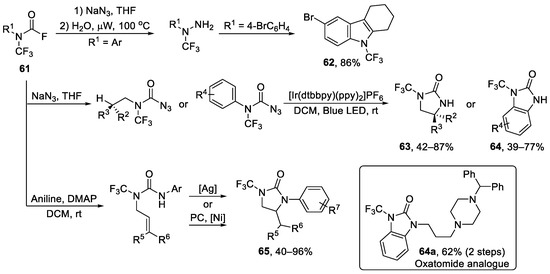
Scheme 15.
Synthesis of diverse N-trifluoromethyl heterocycles through N-trifluoromethyl carbomoyl building blocks.
Meanwhile, Huang and Xu et al. reported the design and synthesis of novel N-CF3 hydroxylamine reagents 66 as well as their applications in preparation of N-CF3 compounds [93]. Some oxazolidinones 67 and 67′ could be generated from trifluoromethylamination/cyclization of styrenes or vinyl ether (Scheme 16a). For example, estrone analogue 67a, could be generated in 71% yield. Furthermore, ynamide 68 could be generated from reagent 66, which could further form heterocycles 69 via Pd-catalyzed cyclization (Scheme 16d). Subsequently, the same group employed reagent 66′ and trimethylsilyl cyanide to convert 1,3-enynes to trifluoromethylaminated allenes under a photoredox/copper-catalyzed 1,4-difunctionalization, in which allenes 70 could further yield oxazolidinones 71 in the presence of N-bromosuccinimide (NBS) or N-iodosuccinimide (NIS) (Scheme 16e) [94].
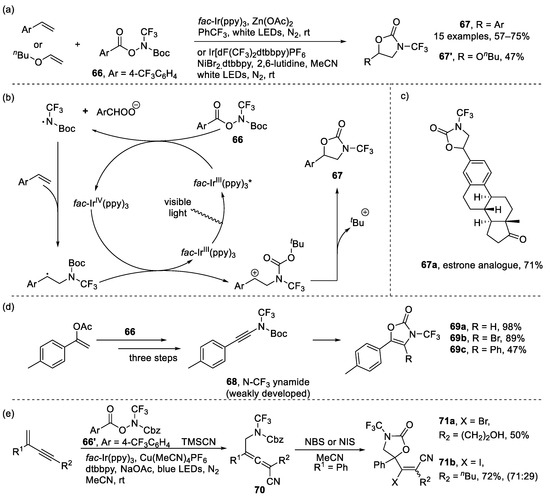
Scheme 16.
(a) Synthesis of N-trifluoromethyl oxazolidinones through trifluoromethylamination/cyclization of styrenes or vinyl; (b) Proposed reaction mechanism; (c) estrone analogue 65a; (d) Synthesis of N-trifluoromethyl oxazolidinones through trifluoromethylamination of vinyl acetate 66 and further cyclization; (e) Synthesis of oxazolidinones through cyclization of allenes.
4.3. Electrophilic Trifluoromethylation
In 2006, Togni et al. developed two new trifluoromethylation reagents (72 and 73) based on hypervalent iodine [95,96]. Subsequently, in 2011, the same group reported a Ritter-type direct electrophilic trifluoromethylation at nitrogen atoms using hypervalent iodine reagent 72 and obtained N-CF3 benzotriazole 74 as a side product (Scheme 17a) [97]. Subsequently, they further refined the method and successfully conducted the trifluoromethylation of a variety of heterocycles (Scheme 17b) [98].
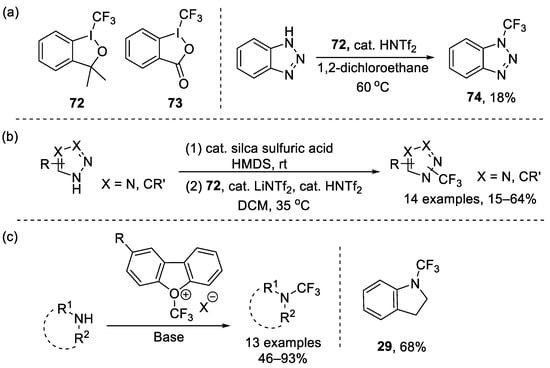
Scheme 17.
(a) Togni’s reagents and trifluoromethylation of benzotriazole; (b) Synthesis of various five-membered N-trifluoromethyl heterocycles through Togni’s reagent; (c) Synthesis of trifluoromethyl amines through Umemoto’s reagents.
Meanwhile, Umemoto developed diverse derivatives of (trifluoromethyl)dibenzofuranylium that could generate CF3+ anion at a low temperature, which facilitated the electrophilic trifluoromethylation of primary, secondary, or aromatic amines (Scheme 17c) [99]. For example, 1-(trifluoromethyl)indoline could be generated in 68% yield.
5. Six-Membered Heterocycles
From the existing literature, the synthetic methodologies of six-membered heterocycles were similar to the five-membered heterocycles containing the N-CF3 motif. Therefore, in this section, some examples are shown briefly.
5.1. Nucleophilic Trifluoromethylation
Similarly, oxidative desulfurization and subsequent fluorination was also an efficient way to achieve six-membered heterocycles containing the N-CF3 motif, such as piperazines and piperidines. For example, Tlili et al. [100] used carbon disulfide and (diethylamino)sulfur trifluoride (DAST) to generate thiocarbomoyl fluoride intermediate 32, and then synthesized a series of N-CF3 piperazines. Borbas et al. employed DAST and NBS to generate N-CF3 morpholine while studying N-fluoroalkylated nucleoside analogues [101]. In addition to AgF, pyridinium poly(hydrogen fluoride [8] could also be employed for oxidative desulfurization and fluorination to give the product 31c which exhibited antibacterial activity. These methods allowed the introduction of the CF3 group into the nitrogen of pharmaceuticals or their analogues, demonstrating the potential of bioactive molecule modification (Scheme 18) [8,62,64].
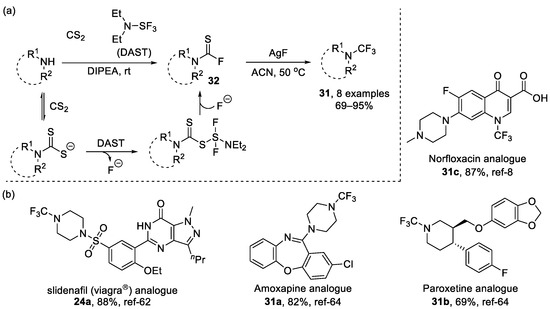
Scheme 18.
(a) Synthesis of thiocarbomoyl fluoride intermediate through DAST and CS2 and subsequently Oxidative desulfurization and fluorination; (b) examples of drug analogues obtained by Oxidative desulfurization and fluorination.
5.2. [4+2] Cycloaddition
As described in Section 3, CF3NO could serve as building blocks in cycloaddition. The application of CF3NO in [4+2] cycloaddition has been investigated [39,102,103,104,105,106,107], yielding various adducts 75 (Scheme 19a). Carson et al. have studied the nucleophilic displacements of fluorine atoms in perfluoro-1,2-oxazines, in particular amino-defluorination reactions [107]. It was established that perfluoro-(3,6-dihydro-2-methyl-2H-1,2-oxazine) reacted with ammonia at room temperature to give a mixture of 4- and 5-amino derivatives, while when reacted with disubstituted amines in diethyl ester at −78 °C it only gave 5-amino compounds. Furthermore, other starting materials featuring N-CF3 were utilized in [4+2] cycloaddition, as shown in Scheme 19b,c [108,109].
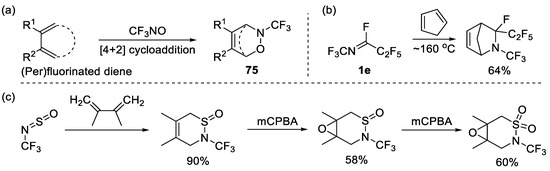
Scheme 19.
Synthesis of six-membered N-trifluoromethyl heterocycles through [2+2] cycloaddition: (a) trifluoronitrosomethane with substituted diene; (b) N-trifluoromethyl imines with cyclopentadiene; (c) Trifluoromethyl-substituted N-sulfinylamine with diene and further oxidation.
5.3. Other Approaches
In 1976, Haszeldine et al. reported the formation of triazine via unsymmetrical carbodiimide intermediate, which was subsequently dimerized, trimerized or intramolecular cyclized (Scheme 20a) [110]. Similarly, Mews et al. reported that RFN=S=O reacted with SO3, leading to the formation of sulfonimide [111]. The degree of oligomer depended on the size of the substituent, and at what time RF = CF3, dimer and trimer were formed in the ratio of 3:1 (Scheme 20b).
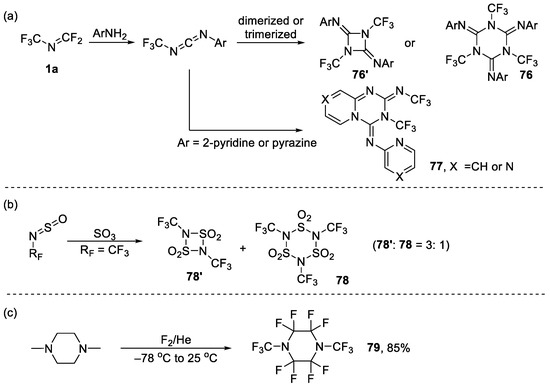
Scheme 20.
Synthesis of diverse six-membered heterocycles: (a) Dimerization or trimerization of N-trifluoromethyl carbodiimide; (b) Dimerization or trimerization of trifluoromethyl-substituted N-sulfinylamine; (c) direct fluorination of N, N-dimethyl piperidine.
Direct fluorination of hydrocarbons by fluorine gas was indeed also a method used to synthesize the corresponding fluorous compounds. Lagow et al. reported the synthesis of perfluoro highly-branched heterocyclic fluorine compounds by direct fluorination, and also reported that 1,4-bis(trifluoromethyl)piperazine 79 was highly generated in 85% yield (Scheme 20c) [112].
6. Seven- and Larger-Membered Heterocycles
Perfluoro-2,5-diazahexane-2,5-dioxyl 80 could readily attack nitric oxide and hydrogen-atom donors, giving adducts, such as its monofunctional analogue, bis-trifluoromethyl nitroxide ((CF3)2NO) [113,114], which could readily react with fluoro-olefins. In some cases, the reaction of bis-trifluoromethyl nitroxide with a variable valence element compound led to an increase in the oxidation state of that element.
Banks et al. reported that an attack by dioxyl 80 on tetrafluoroethylene or hexafluoropropene led mainly to the formation of copolymers in, and also to a smaller number of adducts (81a, 81b) [115]. It should be noted that the yield of 81b could rise to 63% if the reactants were mixed at room temperature and at ca. 25 mmHg pressure. In addition, Banks et al. pointed out that the formation of adducts would require a gas-phase reaction [113]. Subsequently, Tipping et al. investigated the scope of the cycloadduct formation by using fluoroalkenes and a wide variety of hydrogen-containing alkenes [116]. Their report clearly illustrated the limitations of gas-phase reaction. Such reactions must be restricted to simple ethenes or halogenated propenes due to the possibility of hydrogen abstraction occurring.
Later, Tipping et al. synthesized mercurial 84 from dioxyl 80 and investigated the reaction of mercurial 84 with halogenated alkanes, acid chlorides, and dichlorosilanes [117]. The reaction of mercurial 84 with dichlorodimethylsilane resulted in the formation of silicon-containing heterocycle in 93% yield, while with l,l-dichlorosilacyclobutane, the spiro compound was isolated in 64% yield. On the other hand, Booth et al. reported that the dioxyl 80 could react readily by oxidative addition to [Pt(PPh3)4] or [IrCl(CO)L2] (L = PPh3, AsPh3, PMePh2) to afford the corresponding metal–nitroso complex containing a seven-membered chelate ring [118]. The resulting complexes were stable in air for several days or in N2 atmosphere for several months.
Meanwhile, Smith et al. reported the reaction of SO2 and SF4 with dioxyl 80 and obtained two heterocycles 82a and 82b (Scheme 21) [119]. In neither case was a copolymer formed, something which differed from the results from the reaction of dioxyl 80 with tetrafluoroethene and hexafluoropropene in a previous report [113]. Compound 82a slowly reacted with PPh3 at room temperature giving deoxidation products 83 and 83′.
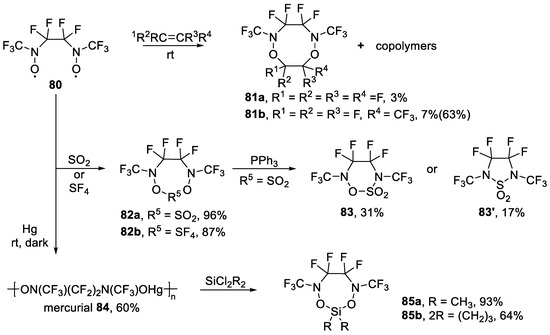
Scheme 21.
Synthesis of larger-membered N-trifluoromethyl heterocycles through addition reaction of dioxyl 80 or its mercurial 84.
Moreover, in 2018, Beier et al. reported another strategy based on N-perfluoroalkyl 1,2,3-triazoles (Scheme 22) [120]. A series of then-unknown N-perfluoroalkyl azepine derivatives were obtained via the aza-[4+3]-annulation of triazoles 44 with both (E)-1-subtituted and 2-substituted dienes. When silyloxy-substituted butadiene was employed, N-CF3 azepinone 86′ could be prepared.
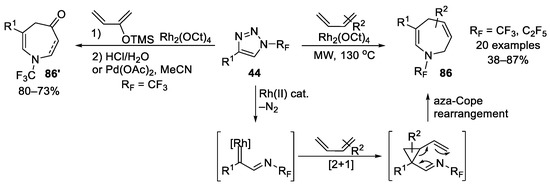
Scheme 22.
Synthesis of N-trifluoromethyl azepine and azepinone via rhodium-catalyzed annulation of 1,2,3-triazoles.
7. Other Methods
In addition to the methods mentioned above, various other synthetic routes have been explored for the generation of heterocycles containing the N-CF3 motif. However, considering the involvement of multiple cyclic structures in these methods, their classification is challenging. Therefore, these approaches are described in this section.
In 1971, Ogden [121] reported a route to some perfluoroheterocyclic compounds via fluoride ion. In his work, tetrafluoroformaldazine 87 reacted with oxalyl fluoride and other carbonyl fluorides and obtained the heterocycles 88, which could be further photolysis to smaller heterocycles 89 (Scheme 23).
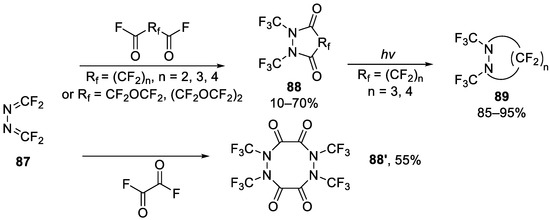
Scheme 23.
Synthesis of perfluoro heterocycles through reaction of tetrafluoroformaldazine with oxalyl fluoride or carbonyl fluoride.
In early organic chemistry, pyrolysis was an effective tool used to study the composition and properties of substances. For example, Banks et al. found that pyrolysis of perfluoropiperidine or perfluoromorpholine led to the generation of N-CF3 pyrrolidine 90a or N-CF3 oxazolidine 90b, respectively [102,122,123,124]. The pyrolysis of perfluorooxazinane in platinum at 480 °C led to the formation of perfluoro-(1-methylazetidine) 91 in 73% yield and trace perfluoro-(1-methyl-2-pyrrolidone) 92, while at 580 °C/19 mm, the yield of 91 and 92 changed to 48% and 24%, respectively (Scheme 24a).
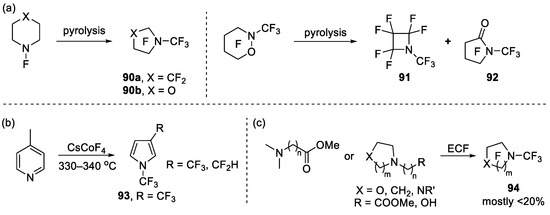
Scheme 24.
Synthesis of diverse N-trifluoromethyl heterocycles: (a) pyrolysis of perfluoropiperidine, perfluoromorpholine or perfluorooxazinane; (b) fluorination of 4-methylpyridine in the presence of caesium tetrafluorocobaltate; (c) electrochemical fluorination of various nitrogen-containing materials.
Tatlow et al. investigated the fluorination of 4-methylpyridine in the presence of caesium tetrafluorocobaltate (CsCoF4) and obtained perfluoro-(1,3dimethylpyrrolidine) 93 and its analogue, together with a range of (per)fluoro-pyridine bearing CF3, CHF2, and CH2F groups (Scheme 24b) [125]. Similar to CsCoF4, CoF3 was a useful reagent for the preparation of a wide array of highly fluorinated organic molecules including open chain/cyclic aliphatics and aromatics. However, the high reactivity of CoF3 meant that most of these reactions were relatively unselective with poor functional compatibility [126].
In addition, electrochemical fluorination (ECF) was one of the most commonly used methods for the fluorination of nitrogen-containing materials [127]. In the past few decades, many different nitrogen-containing materials [128,129,130,131,132,133] have been used in the ECF (Scheme 24c), but the yield was generally unsatisfactory (mostly < 20%) and side products were inevitable, which limited the scope.
8. Conclusions
Over the last decades, the chemistry of the N-CF3 motif has been weakly developed because of the limited approaches and an incompatibility with functionalized molecules. Very recently, some new simpler, safer, and powerful methods of obtaining this motif have been explored. In general, the existing literature mainly focuses on synthesis, with limited properties or applications. In three-membered heterocycles containing the N-CF3 motif, cycloaddition, reductive defluorination-cyclization, nucleophilic cyclization, and oxidative cyclization can reach the motif. Among them, properties and applications of oxaziridine were reported (oxidant or building blocks [25,34,73,74]), while other three-membered heterocycles were not reported. In four-membered heterocycles, cycloaddition was the predominant approach, while trifluoronitrosomethane was the most common starting material. Similarly, there were limited reports on the applications of four-membered heterocycles containing the N-CF3 motif. In five-membered heterocycles, Scheiesser et al. [45] studied, for example, the stability in aqueous media, lipophilicity and metabolic stability of various N-CF3 amines or azoles, illustrating the potential of the N-CF3 motif in medicinal chemistry. Generally, five-membered heterocycles containing this motif can be synthesized from nucleophilic fluorination, cyclization, and electrophilic trifluoromethylation. Furthermore, N-fluoroalkyl 1,2,3-triazoles could serve as the building blocks to access some other N-fluoroalkyl heterocycles [78,79,120]. In six-membered heterocycles, the synthetic approaches were similar. In larger-membered heterocycles, the perfluoro-2,5-diazahexane-2,5-dioxyl showed its potential in coordination chemistry. The dioxyl could react readily with [Pt(PPh3)4] or [IrCl(CO)L2} to form the corresponding metal–nitroso complex containing a seven-membered chelate ring which was stable in air for several days or in N2 atmosphere for several months [118].
Overall, the literature has concentrated on the synthesis of this motif in recent years, and research investigating its properties or applications is becoming more frequent. We believe that the chemistry of the motif will become more and more clear, thereby extending its fields of application. We expect that this review will help to inspire the development of new synthetic strategies or the application of certain structures.
Author Contributions
Conceptualization, Z.Z.; writing—original draft preparation, Z.L. and H.G.; writing—review and editing, W.C. and J.F.; visualization, Z.L. and J.F.; supervision, Z.Z. and J.F.; project administration, Z.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by [National Key Research and Development Program of China] grant number [2022YFD1700302] and [2115 Talent Development Program of China Agricultural University] grant number [201324].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Acena, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II-III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef] [PubMed]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Tomashenko, O.A.; Grushin, V.V. Aromatic trifluoromethylation with metal complexes. Chem. Rev. 2011, 111, 4475–4521. [Google Scholar] [CrossRef]
- Besset, T.; Schneider, C.; Cahard, D. Tamed Arene and Heteroarene Trifluoromethylation. Angew. Chem. Int. Ed. 2012, 51, 5048–5050. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef]
- Matiadis, D.; Sagnou, M. Pyrazoline hybrids as promising anticancer agents: An up-to-date overview. Int. J. Mol. Sci. 2020, 21, 5507. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Zacconi, F.; Gupta, G.; Aljabali, A.A.A.; Mishra, V.; Tambuwala, M.M.; Kapoor, D.N.; Negi, P.; de Jesus Andreoli Pinto, T.; et al. Synthesis and Anticancer Properties of ‘Azole’ Based Chemotherapeutics as Emerging Chemical Moieties: A Comprehensive Review. Curr. Org. Chem. 2021, 25, 654–668. [Google Scholar]
- Asahina, Y.; Araya, I.; Iwase, K.; Iinuma, F.; Hosaka, M.; Ishizaki, T. Synthesis and antibacterial activity of the 4-quinolone-3-carboxylic acid derivatives having a trifluoromethyl group as a novel N-1 substituent. J. Med. Chem. 2005, 48, 3443–3446. [Google Scholar] [CrossRef]
- Schow, S.R.; Mackman, R.L.; Blum, C.L.; Brooks, E.; Horsma, A.G.; Joly, A.; Kerwar, S.S.; Lee, G.; Shiffman, D.; Nelson, M.G.; et al. Synthesis and activity of 2,6,9-trisubstituted purines. Bioorg. Med. Chem. Lett. 1997, 7, 2697–2702. [Google Scholar] [CrossRef]
- Samadder, P.; Suchankova, T.; Hylse, O.; Khirsariya, P.; Nikulenkov, F.; Drapela, S.; Strakova, N.; Vanhara, P.; Vasickova, K.; Kolarova, H.; et al. Synthesis and Profiling of a Novel Potent Selective Inhibitor of CHK1 Kinase Possessing Unusual N-trifluoromethylpyrazole Pharmacophore Resistant to Metabolic N-dealkylation. Mol. Cancer Ther. 2017, 16, 1831–1842. [Google Scholar] [CrossRef]
- Gahman, T.C.; Thomas, D.J.; Lang, H.; Massari, M.E. Aminoquinazoline Cannabinoid Receptor Modulators for Treatment of Disease and Their Preparation. U.S. Patent WO2008157500 A1, 24 December 2008. [Google Scholar]
- Miura, T.; Tamatani, Y. Preparation of a Methyllactam Ring Compound and Its Medicinal Uses. U.S. Patent WO2019168096 A1, 6 September 2019. [Google Scholar]
- Scattolin, T.; Bortolamiol, E.; Visentin, F.; Palazzolo, S.; Caligiuri, I.; Perin, T.; Canzonieri, V.; Demitri, N.; Rizzolio, F.; Togni, A. Palladium(II)-η3-Allyl Complexes Bearing N-Trifluoromethyl N-Heterocyclic Carbenes: A New Generation of Anticancer Agents that Restrain the Growth of High-Grade Serous Ovarian Cancer Tumoroids. Chem.-Eur. J. 2020, 26, 11868–11876. [Google Scholar] [CrossRef] [PubMed]
- Leroux, F.; Jeschke, P.; Schlosser, M. α-Fluorinated Ethers, Thioethers, and Amines: Anomerically Biased Species. Chem. Rev. 2005, 105, 827–856. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Neumann, C.N.; Ritter, T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem. Int. Ed. 2013, 52, 8214–8264. [Google Scholar] [CrossRef]
- Milcent, T.; Crousse, B. The main and recent syntheses of the N-CF3 motif. C. R. Chim. 2018, 21, 771–781. [Google Scholar] [CrossRef]
- Logothetis, A.L. Aziridines from diazomethane and fluorine-substituted imines. J. Org. Chem. 1964, 29, 3049–3052. [Google Scholar] [CrossRef]
- Coe, P.L.; Holton, A.G. Some reactions of diazomethane with polyfluoroazaolefins. J. Fluor. Chem. 1977, 10, 553–564. [Google Scholar] [CrossRef]
- Kaupp, G.; Dengler, O.; Burger, K.; Rottegger, S. Stable triaziridines. Angew. Chem. 1985, 97, 341–342. [Google Scholar] [CrossRef]
- Mitsch, R.A. Organic fluoronitrogens. X. Reductive defluorination-cyclization. J. Org. Chem. 1968, 33, 1847–1849. [Google Scholar] [CrossRef]
- Chang, S.C.; Desmarteau, D.D. Perfluoromethanamine ion. Polyhedron 1982, 1, 129–130. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Bauknight, C.W., Jr.; DesMarteau, D.D. Some novel reactions of N-chlorodifluoromethanimine. J. Org. Chem. 1984, 49, 3590–3595. [Google Scholar] [CrossRef]
- Bauknight, C.W., Jr.; DesMarteau, D.D. Reactions of N-bromodifluoromethanimine. J. Org. Chem. 1988, 53, 4443–4447. [Google Scholar] [CrossRef]
- Bauknight, C.W., Jr.; DesMarteau, D.D. Fluoride-promoted competitive reactions of cyanogen fluoride, perfluoromethanimine, and pentafluoro-2-azapropene. J. Am. Chem. Soc. 1990, 112, 728–733. [Google Scholar] [CrossRef]
- Petrov, V.A.; Resnati, G. Polyfluorinated Oxaziridines: Synthesis and Reactivity. Chem. Rev. 1996, 96, 1809–1823. [Google Scholar] [CrossRef] [PubMed]
- Falardeau, E.R.; DesMarteau, D.D. Direct synthesis of fluorinated peroxides. 6. The addition of fluorinated hydroperoxides to perfluoro-2-azapropene and the preparation of the first perfluorooxazirine. J. Am. Chem. Soc. 1976, 98, 3529–3532. [Google Scholar] [CrossRef]
- Sekiya, A.; DesMarteau, D.D. Reaction of metal fluorides with CF3OOCF2N(H)CF3. Inorg. Chem. 1979, 18, 919–920. [Google Scholar] [CrossRef]
- Navarrini, W.; Desmarteu, D.D. Preparation of Perfluoroalkylaminooxaziridines as Monomers and Intermediates for Nitrons and Photopolymerization Initiators. U.S. Patent US4874875 A, 17 October 1989. [Google Scholar]
- Ratcliffe, C.T. Epoxides. U.S. Patent US4287128 A, 1 September 1981. [Google Scholar]
- Petrov, V.A.; DesMarteau, D.D. A new method for the synthesis of perfluorooxaziridines. Preparation of perfluoro-cis-2,3-dialkyloxaziridines. J. Org. Chem. 1993, 58, 4754–4755. [Google Scholar] [CrossRef]
- Mlsna, T.E.; Young, J.A.; DesMarteau, D.D. Synthesis and chemistry of novel perhalogenated imines, oxaziridines, and oxazolidines. Z. Anorg. Allg. Chem. 2002, 628, 1789–1793. [Google Scholar] [CrossRef]
- Sekiya, A.; Desmarteau, D.D. The reaction of 2-(trifluoromethyl)-3,3-difluorooxaziridine with nucleophiles. J. Fluor. Chem. 1979, 14, 289–297. [Google Scholar] [CrossRef]
- Sekiya, A.; DesMarteau, D.D. Reaction of 2-trifluoromethyl-3,3-difluorooxaziridine with some fluorinated nucleophiles. J. Org. Chem. 1979, 44, 1131–1133. [Google Scholar] [CrossRef]
- Bragante, L.; Desmarteau, D.D. The chemistry of 3-(trifluoromethyl)perfluoroaza-2-butene and the synthesis of a new oxaziridine: 3,3-bis(trifluoromethyl)-2-(trifluoromethyl)oxaziridine. J. Fluor. Chem. 1991, 53, 181–197. [Google Scholar] [CrossRef]
- Barr, D.A.; Haszeldine, R.N. Perfluoroalkyl derivatives of nitrogen. I. Perfluoro-2-methyl-1,2-oxazetidine and perfluoro (alkylene alkylamines). J. Chem. Soc. 1955, 1881–1889. [Google Scholar] [CrossRef]
- Barr, D.A.; Haszeldine, R.N. Perfluoroalkyl derivatives of nitrogen. III. Heptafluoronitrosopropane, perfluoro-2-n-propyl-1,2-oxazetidine, perfluoro(methylene-n-propylamine), and related compounds. J. Chem. Soc. 1956, 3416–3428. [Google Scholar] [CrossRef]
- Barr, D.A.; Haszeldine, R.N.; Willis, C.J. Perfluoroalkyl derivatives of nitrogen. IX. Reaction of trifluoronitrosomethane with some unsymmetrical olefins. J. Chem. Soc. 1961, 1351–1362. [Google Scholar] [CrossRef]
- Banks, R.E.; Haszeldine, R.N.; Sutcliffe, H.; Willis, C.J. Perfluoroalkyl derivatives of nitrogen. XV. The reaction of trifluoronitrosomethane with trifluoroethylene, vinylidene fluoride, vinyl fluoride, and ethylene. J. Chem. Soc. 1965, 2506–2513. [Google Scholar] [CrossRef]
- Banks, R.E.; Haszeldine, R.N.; Taylor, D.R. Polyhaloallenes. II. Reaction of tetrafluoroallene with trifluoronitrosomethane. J. Chem. Soc. 1965, 5602–5612. [Google Scholar] [CrossRef]
- Coy, D.H.; Haszeldine, R.N.; Newlands, M.J.; Tipping, A.E. Poly(bistrifluoromethylamino)-compounds. Synthesis of NN-bistrifluoromethylamino-substituted allenes and their reaction with trifluoronitrosomethane. J. Chem. Soc. D 1970, 456–457. [Google Scholar] [CrossRef]
- Coy, D.H.; Haszeldine, R.N.; Newlands, M.J.; Tipping, A.E. Polyfluoroalkyl derivatives of nitrogen. XL. Reaction of trifluoronitrosomethane with NN-bis(trifluoromethyl)amino-substituted allenes. J. Chem. Soc. Perkin Trans. 1 1973, 1561–1564. [Google Scholar] [CrossRef]
- Jaeger, U.; Schwab, M.; Sundermeyer, W. Fluorine-substituted 1,2-thiazetan-3-one 1-oxides by reaction of bis(trifluoromethyl)ketene and N-sulfinylamines. Chem. Ber. 1986, 119, 1127–1132. [Google Scholar] [CrossRef]
- Ansorge, A.; Brauer, D.J.; Buerger, H.; Doerrenbach, F.; Hagen, T.; Pawelke, G.; Weuter, W. [2+2]-Cycloaddition reactions of (dialkylamino)bis(trifluoromethyl)boranes with isocyanates and isothiocyanates. Crystal structures of cyclic compounds (CF3)2BNMe2CONtBu, (CF3)2BNMe2CSNtBu, (CF3)2BNPhC(NMe2)S and (CF3)2BNMeC(NEt2)OCONMe. J. Organomet. Chem. 1991, 407, 283–300. [Google Scholar] [CrossRef]
- Meanwell, N.A. Fluorine and Fluorinated Motifs in the Design and Application of Bioisosteres for Drug Design. J. Med. Chem. 2018, 61, 5822–5880. [Google Scholar] [CrossRef]
- Schiesser, S.; Chepliaka, H.; Kollback, J.; Quennesson, T.; Czechtizky, W.; Cox, R.J. N-Trifluoromethyl Amines and Azoles: An Underexplored Functional Group in the Medicinal Chemist’s Toolbox. J. Med. Chem. 2020, 63, 13076–13089. [Google Scholar] [CrossRef] [PubMed]
- Kubo, O.; Takami, K.; Kamaura, M.; Watanabe, K.; Miyashita, H.; Abe, S.; Matsuda, K.; Tsujihata, Y.; Odani, T.; Iwasaki, S.; et al. Discovery of a novel series of GPR119 agonists: Design, synthesis, and biological evaluation of N-(Piperidin-4-yl)-N-(trifluoromethyl)pyrimidin-4-amine derivatives. Bioorg. Med. Chem. 2021, 41, 116208–116220. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hird, A.W.; Russell, D.J.; Fauber, B.P.; Dakin, L.A.; Zheng, X.; Su, Q.; Godin, R.; Brassil, P.; Devereaux, E.; et al. Discovery of novel hedgehog antagonists from cell-based screening: Isosteric modification of p38 bisamides as potent inhibitors of SMO. Bioorg. Med. Chem. Lett. 2012, 22, 4907–4911. [Google Scholar] [CrossRef] [PubMed]
- Dakin, L.; Fauber, B.; Hird, A.; Janetka, J.; Russell, D.J.; Su, Q.; Yang, B.; Zheng, X.L. Preparation of Amide Compounds Containing Heterocycle Moiety as Hedgehog Pathway Inhibitors. U.S. Patent WO2009027746 A1, 5 March 2009. [Google Scholar]
- Schiffmann, R.; Neugebauer, A.; Klein, C.D. Metal-mediated inhibition of Escherichia coli methionine aminopeptidase: Structure-activity relationships and development of a novel scoring function for metal-ligand interactions. J. Med. Chem. 2006, 49, 511–522. [Google Scholar] [CrossRef]
- Romero, D.; Robinson, S.; Greenwood, J.R. Preparation of IRAK Inhibitors and Their Uses in the Treatment of Diseases and Disorders. U.S. Patent WO2017004134 A1, 5 January 2017. [Google Scholar]
- Goetz, G.H.; Farrell, W.; Shalaeva, M.; Sciabola, S.; Anderson, D.; Yan, J.; Philippe, L.; Shapiro, M.J. High Throughput Method for the Indirect Detection of Intramolecular Hydrogen Bonding. J. Med. Chem. 2014, 57, 2920–2929. [Google Scholar] [CrossRef]
- Goetz, G.H.; Philippe, L.; Shapiro, M.J. EPSA: A Novel Supercritical Fluid Chromatography Technique Enabling the Design of Permeable Cyclic Peptides. ACS Med. Chem. Lett. 2014, 5, 1167–1172. [Google Scholar] [CrossRef]
- Yagupolskii, L.M.; Fedyuk, D.V.; Petko, K.I.; Troitskaya, V.I.; Rudyk, V.I.; Rudyuk, V.V. N-Trihalomethyl derivatives of benzimidazole, benzotriazole and indazole. J. Fluor. Chem. 2000, 106, 181–187. [Google Scholar] [CrossRef]
- Sokolenko, T.M.; Petko, K.I.; Yagupolskii, L.M. N-trifluoromethylazoles. Chem. Heterocycl. Compd. 2009, 45, 430–435. [Google Scholar] [CrossRef]
- Morimoto, K.; Makino, K.; Yamamoto, S.; Sakata, G. Synthesis of fluoromethyl, difluoromethyl and trifluoromethyl analogs of pyrazosulfuron-ethyl as herbicides. J. Heterocycl. Chem. 1990, 27, 807–810. [Google Scholar] [CrossRef]
- Burkholder, J.B.; Wilson, R.R.; Gierczak, T.; Talukdar, R.; McKeen, S.A.; Orlando, J.J.; Vaghjiani, G.L.; Ravishankara, A.R. Atmospheric fate of CF3Br, CF2Br2, CF2ClBr, and CF2BrCF2Br. J. Geophys. Res. Atmos. 1991, 96, 5025–5043. [Google Scholar] [CrossRef]
- Dmowski, W.; Kaminski, M. Reaction of tertiary formamides with sulfur tetrafluoride. Direct synthesis of (trifluoromethyl)amines. J. Fluor. Chem. 1983, 23, 207–218. [Google Scholar] [CrossRef]
- Boswell, G.A., Jr.; Ripka, W.C.; Scribner, R.M.; Tullock, C.W. Fluorination by sulfur tetrafluoride. Org. React. 1974, 21, 1–124. [Google Scholar]
- Kuroboshi, M.; Hiyama, T. A facile synthesis of trifluoromethylamines by oxidative desulfurization-fluorination of dithiocarbamates. Tetrahedron Lett. 1992, 33, 4177–4178. [Google Scholar] [CrossRef]
- Schaub, S.; Becker, J.; Schindler, S. A Facile and Inexpensive Way to Synthesize N-trifluoromethyl Compounds. ChemistrySelect 2022, 7, e202201803. [Google Scholar] [CrossRef]
- Hagooly, Y.; Gatenyo, J.; Hagooly, A.; Rozen, S. Toward the Synthesis of the Rare N-(Trifluoromethyl)amides and the N-(Difluoromethylene)-N-(trifluoromethyl)amines [RN(CF3)CF2R’] Using BrF3. J. Org. Chem. 2009, 74, 8578–8582. [Google Scholar] [CrossRef]
- Scattolin, T.; Deckers, K.; Schoenebeck, F. Efficient Synthesis of Trifluoromethyl Amines through a Formal Umpolung Strategy from the Bench-Stable Precursor (Me4N)SCF3. Angew. Chem. Int. Ed. 2017, 56, 221–224. [Google Scholar] [CrossRef]
- Yu, J.; Lin, J.-H.; Xiao, J.-C. Reaction of Thiocarbonyl Fluoride Generated from Difluorocarbene with Amines. Angew. Chem. Int. Ed. 2017, 56, 16669–16673. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Wei, J.; Jiang, L.; Liu, J.; Mumtaz, Y.; Yi, W. One-pot synthesis of trifluoromethyl amines and perfluoroalkyl amines with CF3SO2Na and RfSO2Na. Chem. Commun. 2019, 55, 8536–8539. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.T., Jr.; Frass, W.; Resnick, P.R. Cesium fluoride catalyzed rearrangement of perfluorodienes to perfluorodialkylacetylenes. J. Am. Chem. Soc. 1961, 83, 1767–1768. [Google Scholar] [CrossRef]
- Ogden, P.H.; Mitsch, R.A. Isomerization of perfluoro-α, ω-bisazomethines. J. Am. Chem. Soc. 1967, 89, 5007–5011. [Google Scholar] [CrossRef]
- Scholl, H.J.; Klauke, E.; Lauerer, D. Azomethines. I. Fluorination of tetrachloroethane-1,2-bisisocyanide dichloride. J. Fluor. Chem. 1973, 2, 203–204. [Google Scholar] [CrossRef]
- Scholl, H.J.; Klauke, E.; Lauerer, D. Azomethines. II. New synthesis of perfluoro-2,5-diazahexa-2,4-diene and its dimerization. J. Fluor. Chem. 1973, 2, 205–206. [Google Scholar] [CrossRef]
- Barnes, R.N.; Chambers, R.D.; Silvester, M.J.; Hewitt, C.D.; Klauke, E. Reactions involving fluoride ion. Part 28. Cyclization and formation of dimers from perfluoro-2,5-diazahexa-2,4-diene. J. Fluor. Chem. 1984, 24, 211–218. [Google Scholar] [CrossRef]
- Barnes, R.N.; Chambers, R.D.; Hewitt, C.D.; Silvester, M.J.; Klauke, E. Reaction involving fluoride ion. Part 31. Cooligomers of perfluoro-1-methyl-1,3-diazacyclopent-2- and -3-ene. J. Chem. Soc. Perkin Trans. 1 1985, 53–56. [Google Scholar] [CrossRef]
- Chambers, R.D.; Hewitt, C.D.; Silvester, M.J.; Klauke, E. Reactions involving fluoride ion. Part 33. Perfluoroaza-alkylation of fluorinated heteroaromatics with perfluoro-1-methyl-1,3-diazacyclopent-2- and -3-ene. J. Fluor. Chem. 1986, 32, 389–402. [Google Scholar] [CrossRef]
- Pawelke, G.; Buerger, H.; Brauer, D.J.; Wilke, J. Fluorination with concomitant cyclization of Cl2C=NCCl2CCl2N=CCl2 with antimony pentafluoride and molecular structure of a 2-imidazolidinone derivative. J. Fluor. Chem. 1987, 36, 185–194. [Google Scholar] [CrossRef]
- Lam, W.Y.; DesMarteau, D.D. Unusual cycloaddition reactions with 2-(trifluoromethyl)-3,3-difluorooxaziridine. J. Am. Chem. Soc. 1982, 104, 4034–4035. [Google Scholar] [CrossRef]
- O’Brien, B.A.; Lam, W.Y.; DesMarteau, D.D. Cycloaddition and oxygen-transfer reactions of 2-(trifluoromethyl)-3,3-difluorooxaziridine. J. Org. Chem. 1986, 51, 4466–4470. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Q.; Ye, F.; Xia, Y.; Wu, G.; Hossain, M.L.; Zhang, Y.; Wang, J. Copper(I)-Catalyzed Three-Component Coupling of N-Tosylhydrazones, Alkynes and Azides: Synthesis of Trisubstituted 1,2,3-Triazoles. Adv. Synth. Catal. 2015, 357, 2277–2286. [Google Scholar] [CrossRef]
- Blastik, Z.E.; Voltrova, S.; Matousek, V.; Jurasek, B.; Manley, D.W.; Klepetarova, B.; Beier, P. Azidoperfluoroalkanes: Synthesis and Application in Copper(I)-Catalyzed Azide-Alkyne Cycloaddition. Angew. Chem. Int. Ed. 2017, 56, 346–349. [Google Scholar] [CrossRef]
- Blastik, Z.E.; Klepetarova, B.; Beier, P. Enamine-Mediated Azide-Ketone [3+2] Cycloaddition of Azidoperfluoroalkanes. ChemistrySelect 2018, 3, 7045–7048. [Google Scholar] [CrossRef]
- Motornov, V.; Markos, A.; Beier, P. A rhodium-catalyzed transannulation of N-(per)fluoroalkyl-1,2,3-triazoles under microwave conditions—A general route to N-(per)fluoroalkyl-substituted five-membered heterocycles. Chem. Commun. 2018, 54, 3258–3261. [Google Scholar] [CrossRef] [PubMed]
- Bakhanovich, O.; Khutorianskyi, V.; Motornov, V.; Beier, P. Synthesis of N-perfluoroalkyl-3,4-disubstituted pyrroles by rhodium-catalyzed transannulation of N-fluoroalkyl-1,2,3-triazoles with terminal alkynes. Beilstein J. Org. Chem. 2021, 17, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Z.; Zhang, R.X.; Wang, S.; Xu, C.; Guan, W.; Wang, M. An N-Trifluoromethylation/Cyclization Strategy for Accessing Diverse N-Trifluoromethyl Azoles from Nitriles and 1,3-Dipoles. Angew. Chem. Int. Ed. 2022, 61, e202110749. [Google Scholar]
- Zhang, R.Z.; Huang, W.; Zhang, R.X.; Xu, C.; Wang, M. Synthesis of N-CF3 Amidines/Imidates/Thioimidates via N-CF3 Nitrilium Ions. Org. Lett. 2022, 24, 2393–2398. [Google Scholar] [CrossRef]
- Liu, X.; Wang, S.; Gao, C.; Guan, W.; Wang, M. Reassembly and functionalization of N-CF3 pyridinium salts: Synthesis of nicotinaldehydes. Org. Chem. Front. 2022, 9, 4549–4553. [Google Scholar] [CrossRef]
- Lutz, W.; Sundermeyer, W. Synthesis and reactions of trifluoromethyl isocyanate. Chem. Ber. 1979, 112, 2158–2166. [Google Scholar] [CrossRef]
- Varwig, J.; Mews, R. Synthesis of a sTable 1,3,4-dioxazolidine. Angew. Chem. 1977, 89, 675. [Google Scholar] [CrossRef]
- Lentz, D.; Bruedgam, I.; Hartl, H. Trifluoromethyl isocyanide as a building block in synthesis. Reaction with trifluoroacetic acid and hexafluoroacetone. Angew. Chem. Int. Ed. Engl. 1987, 99, 921–923. [Google Scholar] [CrossRef]
- Lentz, D.; Marschall, R. Cycloaddition reactions of trifluoromethyl isocyanide with diphosphenes. Synthesis and structure of the new 2-phosphinidene-1,3-azaphospholidine derivative Mes*P=CN(CF3)C(=NCF3)C(=NCF3)-PMes*. Z. Anorg. Allg. Chem. 1992, 617, 53–58. [Google Scholar] [CrossRef]
- Cao, T.; Retailleau, P.; Milcent, T.; Crousse, B. Synthesis of N-CF3 hydrazines through radical trifluoromethylation of azodicarboxylates. Chem. Commun. 2021, 57, 10351–10354. [Google Scholar] [CrossRef]
- Scattolin, T.; Bouayad-Gervais, S.; Schoenebeck, F. Straightforward access to N-trifluoromethyl amides, carbamates, thiocarbamates and ureas. Nature 2019, 573, 102–107. [Google Scholar] [CrossRef]
- Bouayad-Gervais, S.; Scattolin, T.; Schoenebeck, F. N-Trifluoromethyl Hydrazines, Indoles and Their Derivatives. Angew. Chem. Int. Ed. 2020, 59, 11908–11912. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.D.T.; Zivkovic, F.G.; Schoenebeck, F. Synthesis of N-CF3 Alkynamides and Derivatives Enabled by Ni-Catalyzed Alkynylation of N-CF3 Carbamoyl Fluorides. J. Am. Chem. Soc. 2021, 143, 13029–13033. [Google Scholar] [CrossRef] [PubMed]
- Bouayad-Gervais, S.; Nielsen, C.D.T.; Turksoy, A.; Sperger, T.; Deckers, K.; Schoenebeck, F. Access to Cyclic N-Trifluoromethyl Ureas through Photocatalytic Activation of Carbamoyl Azides. J. Am. Chem. Soc. 2022, 144, 6100–6106. [Google Scholar] [CrossRef]
- Turksoy, A.; Bouayad-Gervais, S.; Schoenebeck, F. N-CF3 Imidazolidin-2-one Derivatives via Photocatalytic and Silver-Catalyzed Cyclizations. Chem.-Eur. J. 2022, 28, e202201435. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, Y.; Wang, J.; Qing, F.-L.; Xu, X.-H. General Synthesis of N-Trifluoromethyl Compounds with N-Trifluoromethyl Hydroxylamine Reagents. J. Am. Chem. Soc. 2022, 144, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, Y.; Qing, F.-L.; Xu, X.-H. Photoredox/Copper-Catalyzed Trifluoromethylamino-Cyanation of 1,3-Enynes. Eur. J. Org. Chem. 2023, 26, e202201061. [Google Scholar] [CrossRef]
- Eisenberger, P.; Gischig, S.; Togni, A. Novel 10-I-3 hypervalent iodine-based compounds for electrophilic trifluoromethylation. Chem.-Eur. J. 2006, 12, 2579–2586. [Google Scholar] [CrossRef]
- Eisenberger, P. The Development of New Hypervalent iodine Reagents for Electrophilic Trifluoromethylation. Ph.D. Thesis, Swiss Federal Institute of Technology, Zürich, Switzerland, 2007. [Google Scholar]
- Niedermann, K.; Frueh, N.; Vinogradova, E.; Wiehn, M.S.; Moreno, A.; Togni, A. A Ritter-type reaction: Direct electrophilic trifluoromethylation at nitrogen atoms using hypervalent iodine Reagents. Angew. Chem. Int. Ed. 2011, 50, 1059–1063. [Google Scholar] [CrossRef]
- Niedermann, K.; Frueh, N.; Senn, R.; Czarniecki, B.; Verel, R.; Togni, A. Direct Electrophilic N-Trifluoromethylation of Azoles by a Hypervalent Iodine Reagent. Angew. Chem. Int. Ed. 2012, 51, 6511–6515. [Google Scholar] [CrossRef] [PubMed]
- Umemoto, T.; Adachi, K.; Ishihara, S. CF3 Oxonium Salts, O-(Trifluoromethyl)dibenzofuranium Salts: In Situ Synthesis, Properties, and Application as a Real CF3+ Species Reagent. J. Org. Chem. 2007, 72, 6905–6917. [Google Scholar] [CrossRef] [PubMed]
- Onida, K.; Vanoye, L.; Tlili, A. Direct Synthesis of Thiocarbamoyl Fluorides and Trifluoromethylamines Through Fluorinative Desulfurization. Eur. J. Org. Chem. 2019, 2019, 6106–6109. [Google Scholar] [CrossRef]
- Debreczeni, N.; Hotzi, J.; Bege, M.; Lovas, M.; Mezo, E.; Bereczki, I.; Herczegh, P.; Kiss, L.; Borbas, A. N-Fluoroalkylated Morpholinos—A New Class of Nucleoside Analogues. Chem.-Eur. J. 2023, 29, e202203248. [Google Scholar] [CrossRef] [PubMed]
- Banks, R.E.; Barlow, M.G.; Haszeldine, R.N. Perfluoroalkyl derivatives of nitrogen. XVIII. Reaction of trifluoronitrosomethane with perfluorobutadiene and 3,4-dichlorohexafluorobut-1-ene. J. Chem. Soc. 1965, 6149–6163. [Google Scholar] [CrossRef]
- Banks, R.E.; Barlow, M.G.; Haszeldine, R.N. Perfluoroalkyl derivatives of nitrogen. XVI. Reaction of trifluoronitrosomethane with butadiene and with isobutene. J. Chem. Soc. 1965, 4714–4718. [Google Scholar] [CrossRef]
- Banks, R.E.; Harrison, A.C.; Haszeldine, R.N.; Orrell, K.G. Polyfluorocyclopentadienes. III. Diels-Alder reactions of perfluorocyclopentadiene. J. Chem. Soc. C 1967, 1608–1621. [Google Scholar] [CrossRef]
- Haszeldine, R.N.; Banks, R.E.; Bridge, M.; Roberts, D.W.; Tucker, N.I. Polyfluorocyclopentadienes. VI. Synthesis of 1- and 5-chloropentafluorocyclopentadiene. J. Chem. Soc. C 1970, 2531–2535. [Google Scholar] [CrossRef]
- Barlow, M.G.; Haszeldine, R.N.; Murray, K.W. Polyfluoroalkyl derivatives of nitrogen. Part 49. Ene reactions of trifluoronitrosomethane: Formation of N-trifluoromethylhydroxylamines. J. Chem. Soc. Perkin Trans. 1 1980, 1960–1964. [Google Scholar] [CrossRef]
- Carson, P.A.; Roberts, D.W. Nucleophilic substitution reactions of the heterocyclic fluoro-olefin perfluoro-(3,6-dihydro-2-methyl-2H-1,2-oxazine). Tetrahedron 1986, 42, 6495–6510. [Google Scholar] [CrossRef]
- Al’bekov, V.A.; Benda, A.F.; Gontar, A.F.; Sokol’skii, G.A.; Knunyants, I.L. [2+4]-Cycloaddition reactions of perfluoroazomethines. Izv. Akad. Nauk SSSR Ser. Khim. 1986, 1437–1440. [Google Scholar] [CrossRef]
- Jaeger, U.; Sundermeyer, W. [4+2]-Cycloaddition products of perfluoroorgano-N-sulfinylamines and their oxidation. Chem. Ber. 1986, 119, 3405–3410. [Google Scholar] [CrossRef]
- Flowers, W.T.; Franklin, R.; Haszeldine, R.N.; Perry, R.J. Reaction of amines with perfluoroazapropene: Formation of the novel 4H-pyrido [1,2-a]-s-triazine system via unsymmetrical carbodi-imides. J. Chem. Soc. Chem. Commun. 1976, 567–568. [Google Scholar] [CrossRef]
- Tesky, F.M.; Mews, R. 1,1,3,3-Tetraoxo-2,4-bis(perfluoroalkyl)cyclodiaza-λ6-thianes (RfNSO2)2. Chem.-Ztg. 1987, 111, 345–346. [Google Scholar]
- Lin, W.H.; Lagow, R.J. The synthesis of perfluoro highly branched heterocyclic fluorine compounds by direct fluorination. J. Fluor. Chem. 1990, 50, 15–30. [Google Scholar] [CrossRef]
- Banks, R.E.; Eapen, K.C.; Haszeldine, R.N.; Holt, A.V.; Myerscough, T.; Smith, S. Nitroxide chemistry. VII. Synthesis and reactions of perfluoro-2,5-diazahexane 2,5-dioxyl. J. Chem. Soc., Perkin Trans. 1 1974, 2532–2538. [Google Scholar] [CrossRef]
- Banks, R.E.; Haszeldine, R.N.; Stevenson, M.J. Perfluoroalkyl derivatives of nitrogen. XXI. Some reactions of bis(trifluoromethyl) nitroxide. J. Chem. Soc. C 1966, 901–904. [Google Scholar] [CrossRef]
- Banks, R.E.; Eapen, K.C.; Haszeldine, R.N.; Mitra, P.; Myerscough, T.; Smith, S. Perfluoro-2,5-diazahexane-2,5-dioxyl and its use in polymer chemistry and in polymer cross-linking. J. Chem. Soc. Chem. Commun. 1972, 833–834. [Google Scholar] [CrossRef]
- Green, M.J.; Tipping, A.E. The reaction of perfluoro-2,5-diazahexane 2,5-dioxyl with alkenes. J. Fluor. Chem. 1993, 65, 115–125. [Google Scholar] [CrossRef]
- Green, M.J.; Tipping, A.E. The reactions of mercury(II) perfluoro-2,5-diazahexane-2,5-dioxyl and perfluoro-2,5-diazahexane-2,5-diol with haloalkanes, acid chlorides, and dichlorosilanes. J. Fluor. Chem. 1994, 66, 271–277. [Google Scholar] [CrossRef]
- Booth, B.L.; Haszeldine, R.N.; Holmes, R.G.G. Some oxidative-addition reactions of the diradical, perfluoro-NN’-dimethylethane-1,2-bis(aminooxyl), CF3N(O)CF2CF2N(O)CF3, with iridium(I) and platinum(0) complexes. J. Chem. Soc. Dalton Trans. 1982, 671–672. [Google Scholar] [CrossRef]
- Arfaei, A.; Smith, S. Reactions of perfluoronitroxides with sulfur dioxide. J. Chem. Soc. Perkin Trans. 1 1984, 1791–1794. [Google Scholar] [CrossRef]
- Motornov, V.; Beier, P. Chemoselective Aza-[4+3]-annulation of N-Perfluoroalkyl-1,2,3-triazoles with 1,3-Dienes: Access to N-Perfluoroalkyl-Substituted Azepines. J. Org. Chem. 2018, 83, 15195–15201. [Google Scholar] [CrossRef]
- Ogden, P.H. Cyclizations via fluoride ion induced isomerizations. Novel perfluoroheterocyclic compounds. J. Chem. Soc. C 1971, 2920–2926. [Google Scholar] [CrossRef]
- Banks, R.E.; Cheng, W.M.; Haszeldine, R.N. Heterocyclic polyfluoro compounds. II. Reactions of undecafluoropiperidine. The preparation of perfluoro-2,3,4,5-tetrahydropyridine and perfluoro-(1-methylpyrrolidine). J. Chem. Soc. 1962, 3407–3416. [Google Scholar] [CrossRef]
- Banks, R.E.; Burling, E.D. Heterocyclic polyfluoro compounds. IX. Some reactions of perfluoro-N-fluoromorpholine. The preparation of perfluoro-5,6-dihydro-2H-1,4-oxazine and perfluoro-3-methyloxazolidine. J. Chem. Soc. 1965, 6077–6083. [Google Scholar] [CrossRef]
- Banks, R.E.; Haszeldine, R.N.; Matthews, V. Heterocyclic polyfluoro compounds. XIII. Thermal reactions of perfluorotetrahydro-2-methyl-2H-1,2-oxazine and perfluoro-3,6-dihydro-2-methyl-2H-1,2-oxazine. Synthesis and properties of perfluoro-1-methyl-2-pyrrolidone, perfluoro-1-methyl-2-oxo-3-pyrroline, and perfluoro-1-methylazetidine. J. Chem. Soc. C 1967, 2263–2267. [Google Scholar] [CrossRef]
- Plevey, R.G.; Rendell, R.W.; Tatlow, J.C. Fluorinations with complex metal fluorides. Part 7. Fluorinations of the methylpyridines with cesium tetrafluorocobaltate. J. Fluor. Chem. 1982, 21, 265–286. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Guo, P.; Han, J.-F.; Ye, K.-Y. Cobalt fluorides: Preparation, reactivity and applications in catalytic fluorination and C-F functionalization. Chem. Commun. 2020, 56, 8512–8523. [Google Scholar] [CrossRef]
- Meinert, H.; Fackler, R.; Mader, J.; Reuter, P.; Roehlke, W. The electrochemical fluorination of derivatives of morpholine, piperidine and carbazole. J. Fluor. Chem. 1992, 59, 351–365. [Google Scholar] [CrossRef]
- Abe, T.; Hayashi, E.; Baba, H.; Fukaya, H. The electrochemical fluorination of nitrogen-containing carboxylic acids. Fluorination of dimethylamino- or diethylamino-substituted carboxylic acid derivatives. J. Fluor. Chem. 1990, 48, 257–279. [Google Scholar] [CrossRef]
- Abe, T.; Hayashi, E.; Fukaya, H.; Hayakawa, Y.; Baba, H.; Ishikawa, S.; Asahino, K. The electrochemical fluorination of nitrogen-containing carboxylic acids. Fluorination of methyl esters of 3-(dialkylamino)propionic acids. J. Fluor. Chem. 1992, 57, 101–111. [Google Scholar] [CrossRef]
- Abe, T.; Baba, H.; Soloshonok, I. Electrochemical fluorination of 1-ethylpiperazine and 4-methyl- and/or 4-ethylpiperazinyl substituted carboxylic acid methyl esters. J. Fluor. Chem. 2001, 108, 21–35. [Google Scholar] [CrossRef]
- Abe, T.; Baba, H.; Fukaya, H.; Tamura, M.; Sekiya, A. Simons electrochemical fluorination of substituted homopiperazines(hexahydro-1,4-diazepines) and piperazines. J. Fluor. Chem. 2003, 119, 27–38. [Google Scholar] [CrossRef]
- Naito, Y.; Inoue, Y.; Ono, T.; Arakawa, Y.; Fukaya, C.; Yokoyama, K.; Kobayashi, Y.; Yamanouchi, K. Synthesis of perfluorochemicals for use as blood substitutes, part I. Electrochemical fluorination of N-methyldecahydroquinoline and N-methyldecahydroisoquinoline. J. Fluor. Chem. 1984, 26, 485–497. [Google Scholar] [CrossRef]
- Sartori, P.; Velayutham, D.; Ignat’ev, N.; Noel, M. Investigations on the product distribution pattern during the electrochemical fluorination of 2-fluoropyridine and pyridine. J. Fluor. Chem. 1998, 87, 31–36. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).