Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules
Abstract
1. Introduction
2. Results and Discussion
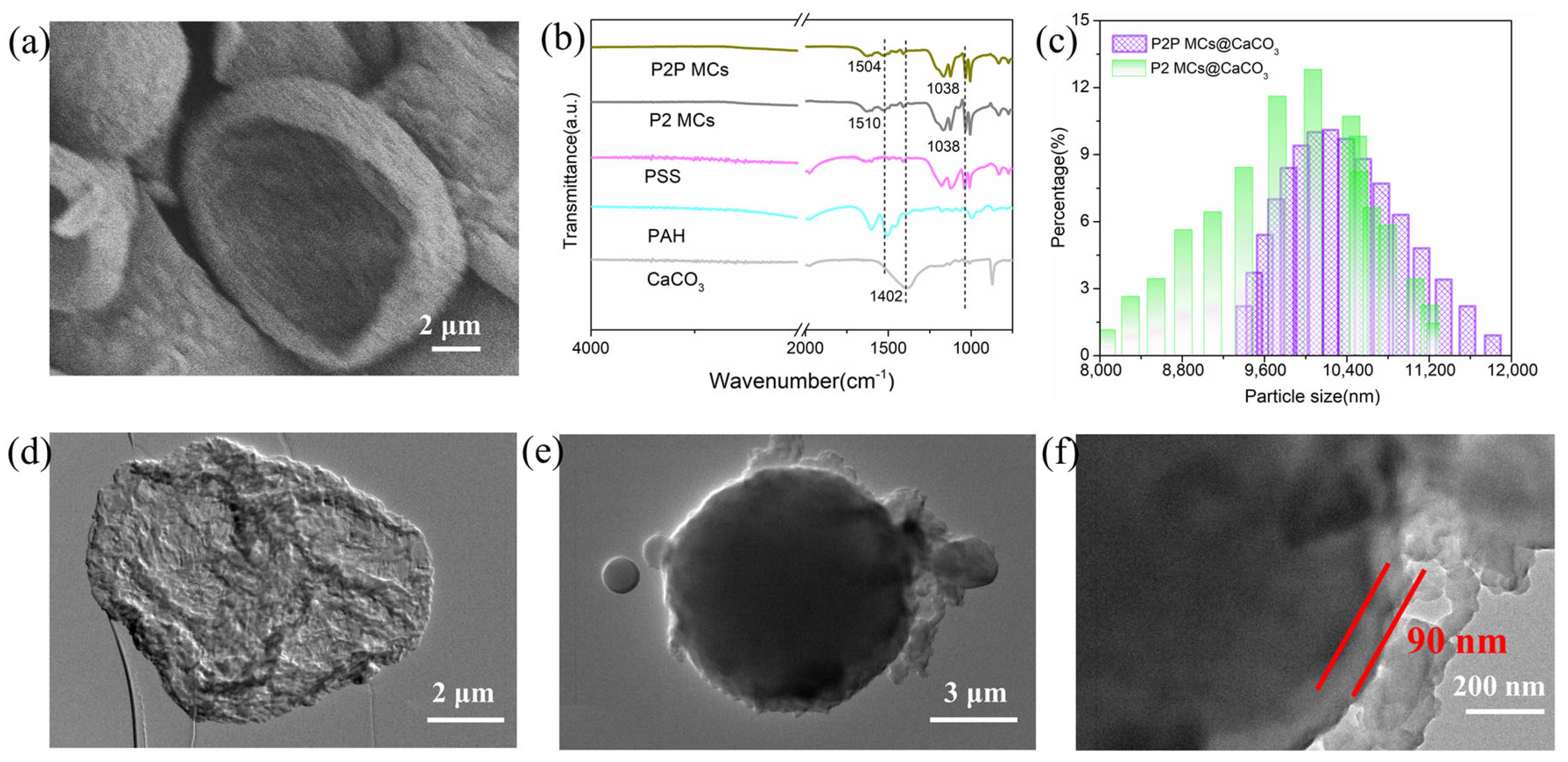
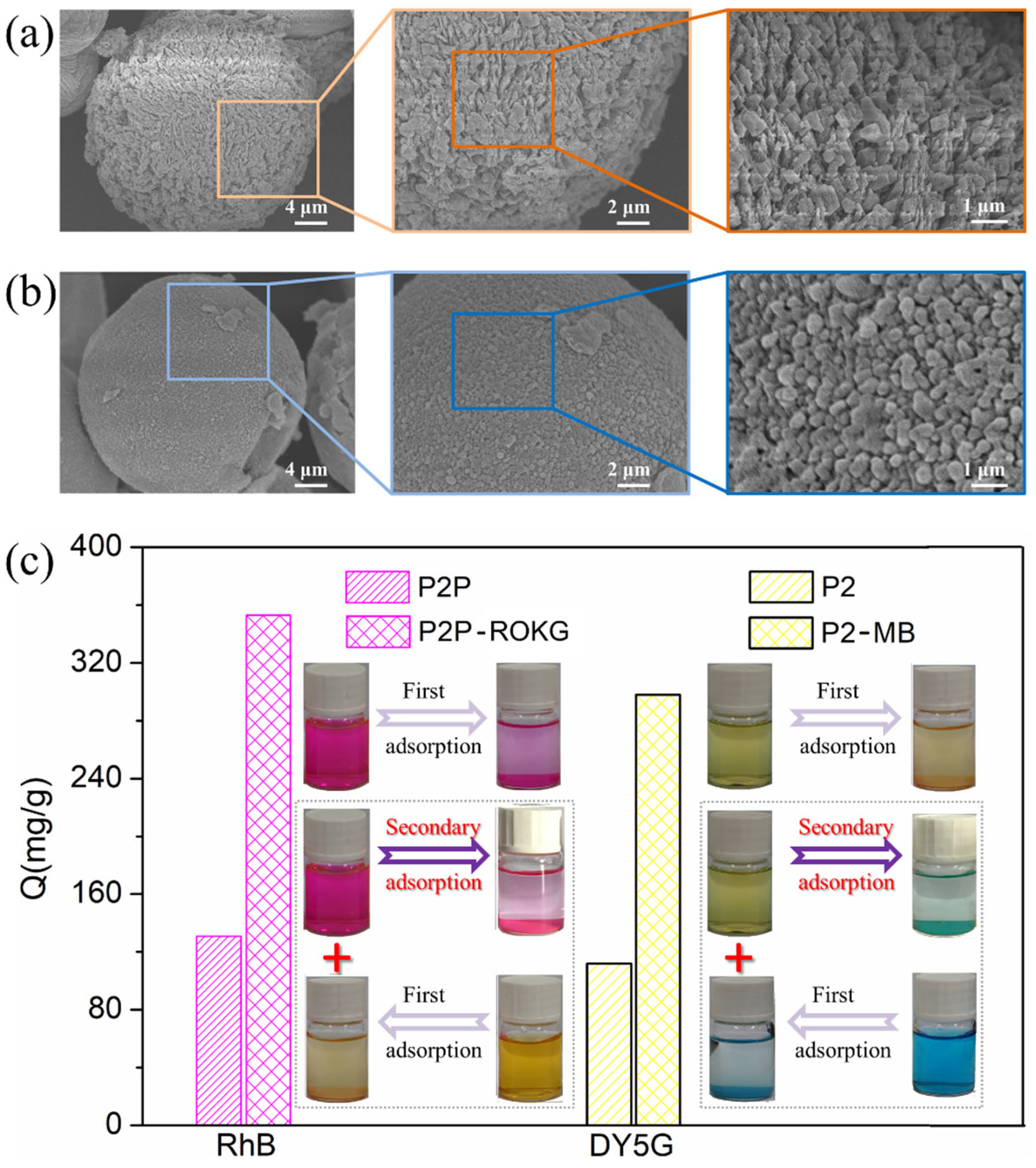
2.1. Microstructure and Morphology of P2P and P2 Microcapsules
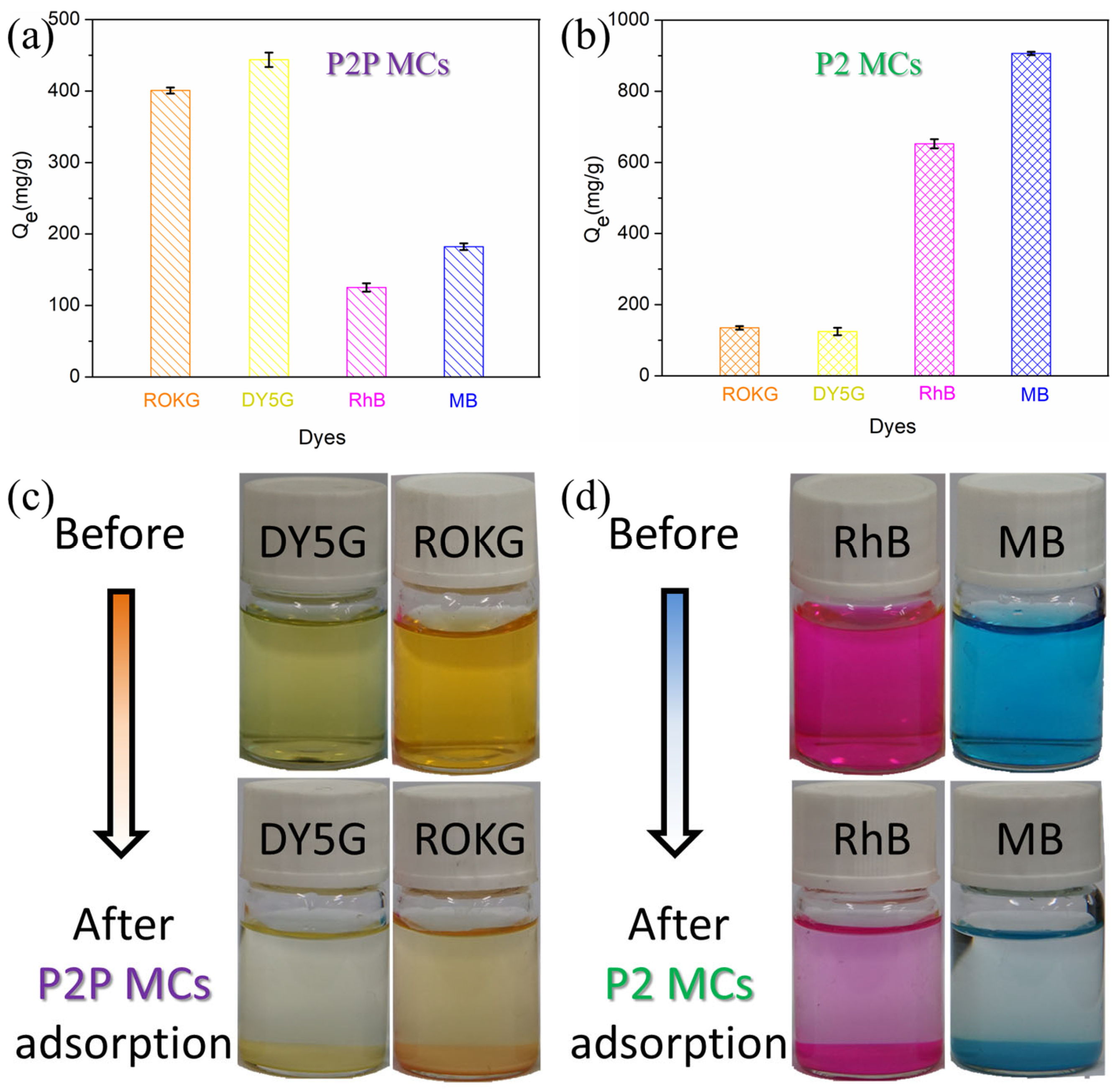
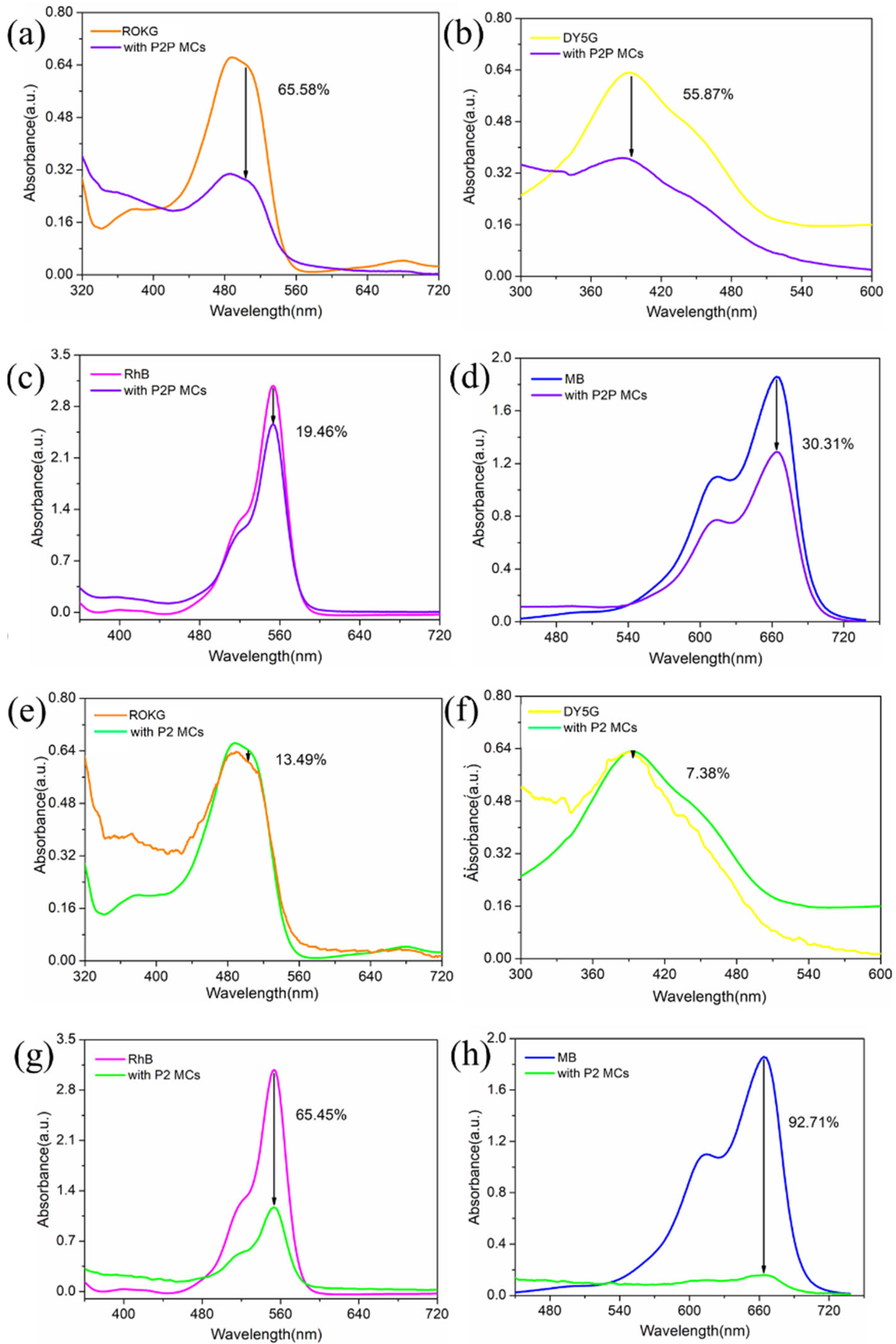
2.2. Adsorption of Dyes
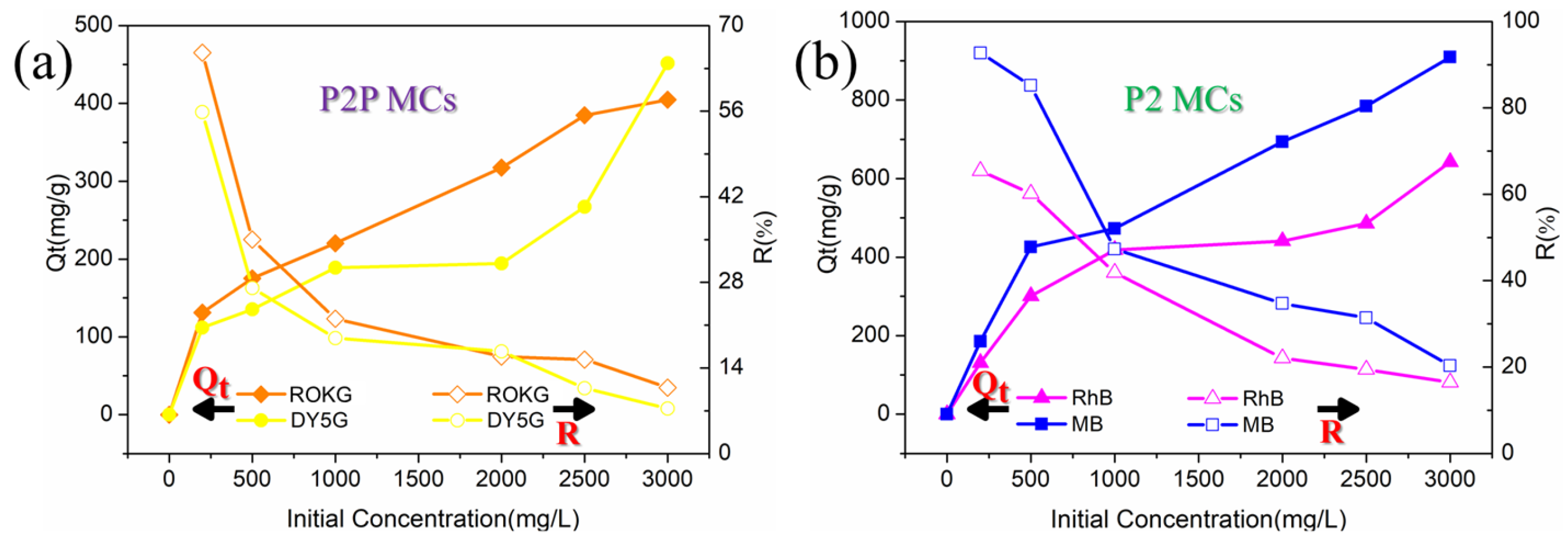
2.2.1. Effect of Initial Dye Concentrations on Dye Adsorption
2.2.2. Effect of Time of Dye on Adsorption
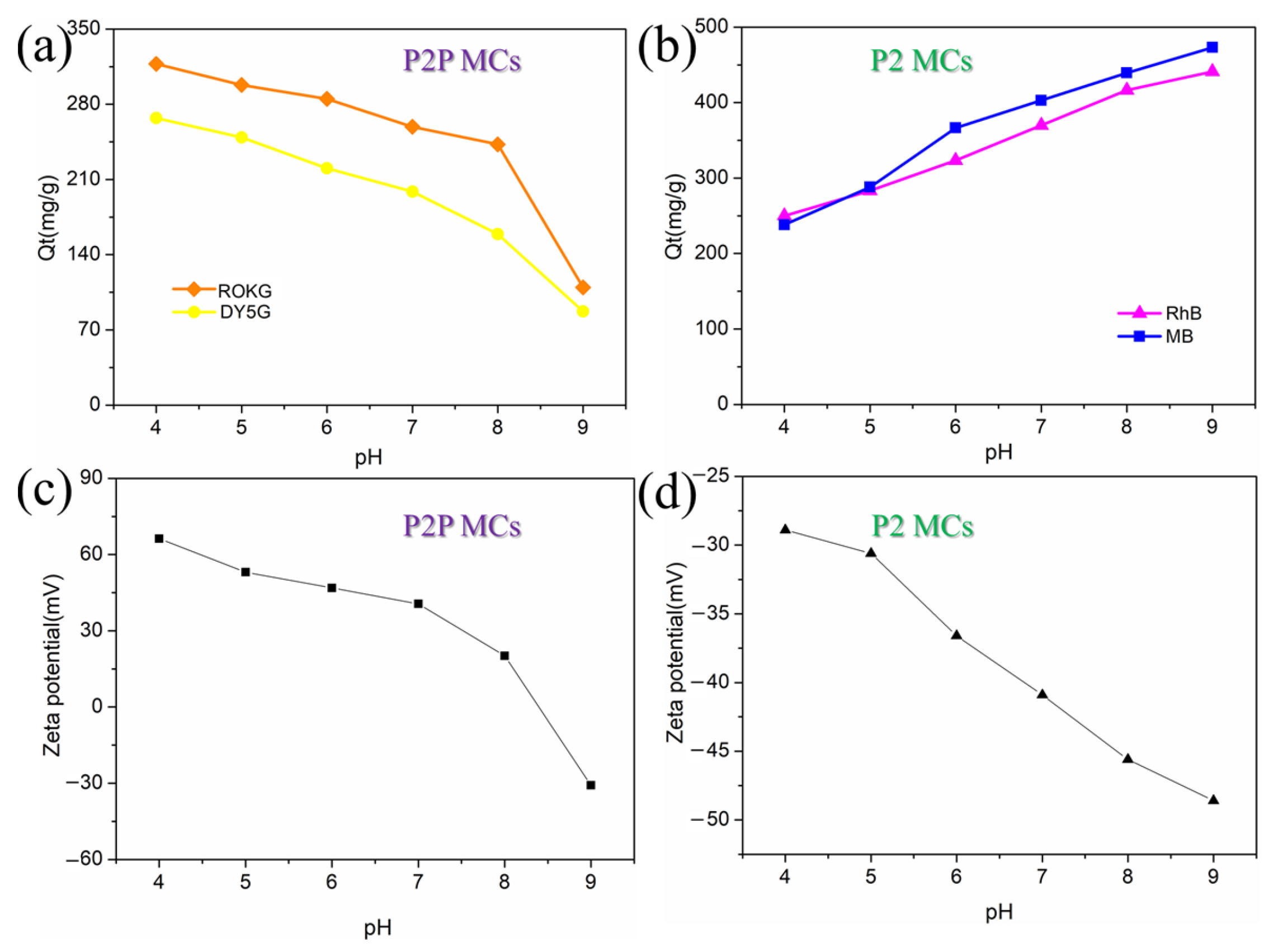
2.2.3. Effect of pH on Dye Adsorption
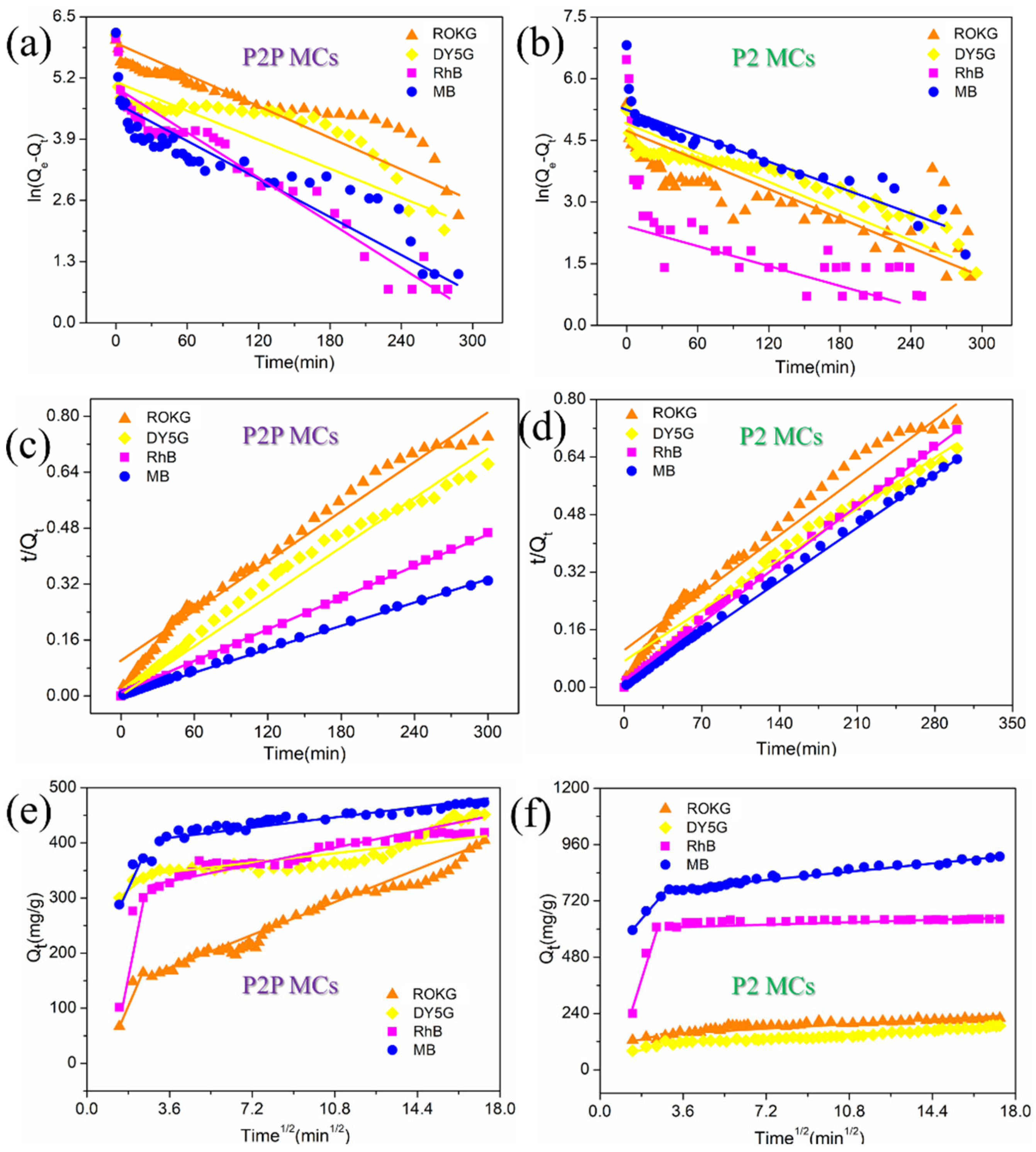
2.3. Adsorption Kinetic Study
2.4. Adsorption Isotherm
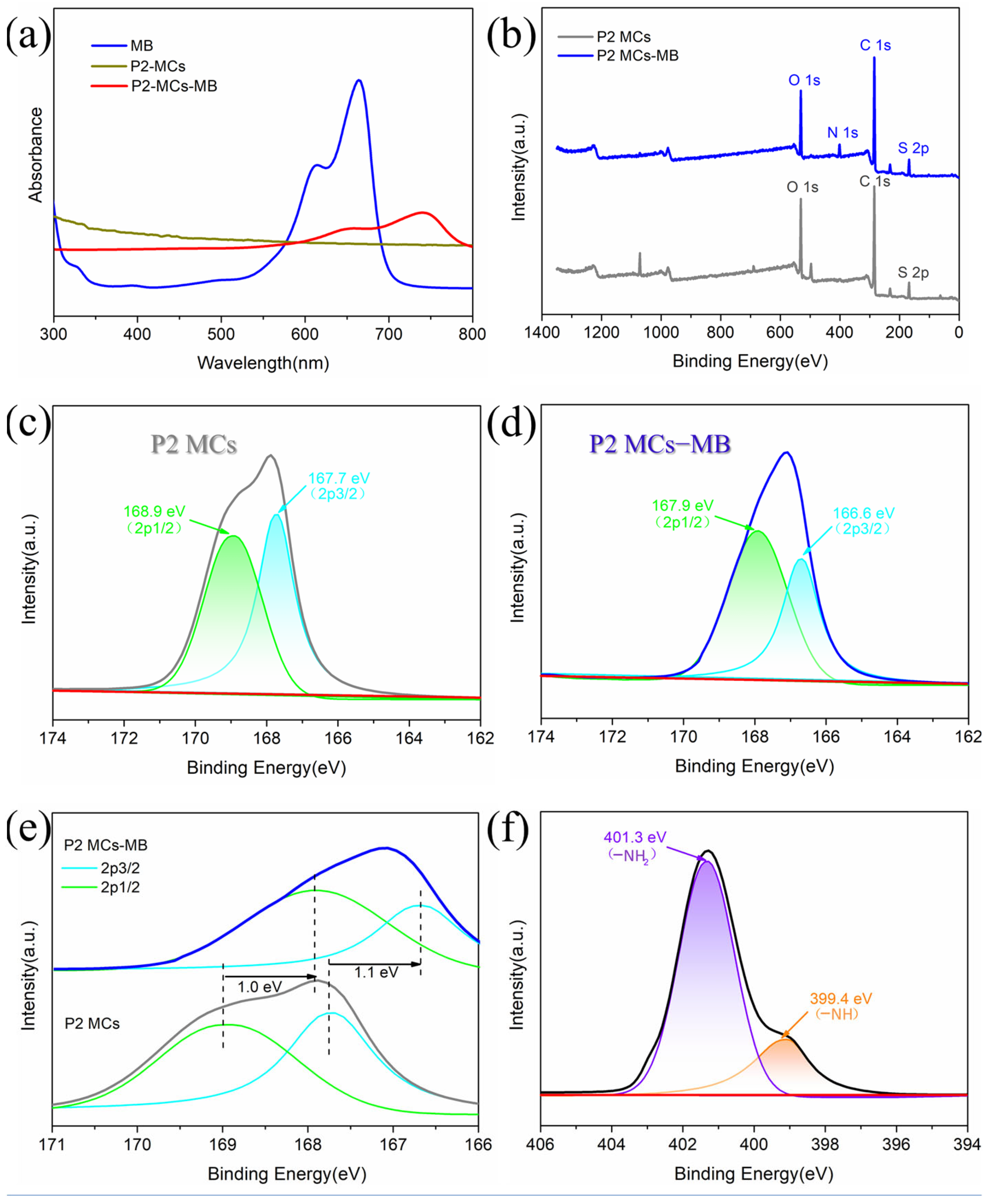
2.5. Adsorption Mechanisms
2.6. The Secondary Adsorption of P2 and P2P Microcapsules to RhB and DY5G
2.7. Desorption
3. Materials and Methods
3.1. Materials
3.2. Preparation of (PAH/PSS)2 and (PAH/PSS)2PAH
3.3. Characterization of (PAH/PSS)nPAH Microcapsules
3.4. Adsorption Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mahabeer, J.; Kumari, U.; Lokhat, D.; Carsky, M.; Meikap, B.C. Implementation of microplastics derived from waste plastic for uptake of mb dye: Performance and lca study. Desalination 2023, 546, 116214. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, S.; Wang, J.; Zhou, Z.; Wan, J.; Yang, J.; Zhang, H.; Zhang, J. Pigments, dyes and the restoration history of the painted figurines of the tang dynasty from the astana tombs revealed by comprehensive chemical analysis. ChemistrySelect 2022, 7, e202202342. [Google Scholar] [CrossRef]
- Kumar, S.; Tewari, C.; Sahoo, N.G.; Philip, L. Mechanistic insights into carbo-catalyzed persulfate treatment for simultaneous degradation of cationic and anionic dye in multicomponent mixture using plastic waste–derived carbon. J. Hazard. Mater. 2022, 435, 128956. [Google Scholar] [CrossRef]
- Han, L.; Ren, Y.; Fang, K.; Zhang, K.; Zhang, Y.; Wang, W.; Zhang, Z.; Xie, R. Short clean dyeing of two-component cotton/polyamide fabrics through adaptive adjustment of the dye solution. J. Clean. Prod. 2022, 333, 130077. [Google Scholar] [CrossRef]
- He, H.; Qin, Y.; Liu, J.; Wang, Y.; Wang, J.; Zhao, Y.; Zhu, Z.; Jiang, Q.; Wan, Y.; Qu, X.; et al. A wearable self-powered fire warning e-textile enabled by aramid nanofibers/mxene/silver nanowires aerogel fiber for fire protection used in firefighting clothing. Chem. Eng. J. 2023, 460, 141661. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, Y.; Zhao, H.; Han, L.; Yang, G.; Liu, Y.; Fang, K. Short wet-steaming low-carbon cleaner pad dyeing of cotton/polyamide/lyocell fabric with reactive dyes. Ind. Crop. Prod. 2023, 197, 116556. [Google Scholar] [CrossRef]
- Mpatani, F.M.; Aryee, A.A.; Kani, A.N.; Guo, Q.; Dovi, E.; Qu, L.; Li, Z.; Han, R. Uptake of micropollutant-bisphenol a, methylene blue and neutral red onto a novel bagasse-β-cyclodextrin polymer by adsorption process. Chemosphere 2020, 259, 127439. [Google Scholar] [CrossRef] [PubMed]
- Ouachtak, H.; El Guerdaoui, A.; El Haouti, R.; Haounati, R.; Ighnih, H.; Toubi, Y.; Alakhras, F.; Rehman, R.; Hafid, N.; Addi, A.A.; et al. Combined molecular dynamics simulations and experimental studies of the removal of cationic dyes on the eco-friendly adsorbent of activated carbon decorated montmorillonite mt@ac. RSC Adv. 2023, 13, 5027–5044. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Yan, Q.; Liu, X.; Xia, G.; Shao, Q.; Liang, K.; Hong, L.; Chi, B.; Wang, H. Benzocaine-incorporated smart 1,3-squaraine dyes: Red emission, excellent stability and cell bioimaging. Dye. Pigment. 2020, 173, 107977. [Google Scholar] [CrossRef]
- Almeida, E.J.R.; Corso, C.R. Decolorization and removal of toxicity of textile azo dyes using fungal biomass pelletized. Int. J. Environ. Sci. Technol. 2019, 16, 1319–1328. [Google Scholar] [CrossRef]
- Sobhani-Nasab, A.; Pourmasoud, S.; Ahmadi, F.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Rahimi-Nasrabadi, M. Synthesis and characterization of mnwo4/tmvo4 ternary nano-hybrids by an ultrasonic method for enhanced photocatalytic activity in the degradation of organic dyes. Mater. Lett. 2019, 238, 159–162. [Google Scholar] [CrossRef]
- Naushad, M.; Sharma, G.; Alothman, Z.A. Photodegradation of toxic dye using gum arabic-crosslinked-poly(acrylamide)/Ni(OH)2/FeOOH nanocomposites hydrogel. J. Clean. Prod. 2019, 241, 118263. [Google Scholar] [CrossRef]
- Chen, B.; Long, F.; Chen, S.; Cao, Y.; Pan, X. Magnetic chitosan biopolymer as a versatile adsorbent for simultaneous and synergistic removal of different sorts of dyestuffs from simulated wastewater. Chem. Eng. J. 2020, 385, 123926. [Google Scholar] [CrossRef]
- Largo, F.; Haounati, R.; Ouachtak, H.; Hafid, N.; Jada, A.; Addi, A.A. Design of organically modified sepiolite and its use as adsorbent for hazardous malachite green dye removal from water. Water Air Soil Pollut. 2023, 234, 183. [Google Scholar] [CrossRef]
- Ouachtak, H.; Akhouairi, S.; Haounati, R.; Addi, A.A.; Jada, A.; Taha, M.L.; Douch, J. 3,4-dihydroxybenzoic acid removal from water by goethite modified natural sand column fixed-bed: Experimental study and mathematical modeling. Desalin. Water Treat. 2020, 194, 439–449. [Google Scholar] [CrossRef]
- Kang, H.; Zhang, D.; Chen, X.; Zhao, H.; Yang, D.; Li, Y.; Bao, M.; Wang, Z. Preparation of mof/polypyrrole and flower-like mno2 electrodes by electrodeposition: High-performance materials for hybrid capacitive deionization defluorination. Water Res. 2023, 229, 119441. [Google Scholar] [CrossRef]
- Zhang, P.; He, M.; Huo, S.; Li, F.; Li, K. Recent progress in metal-based composites toward adsorptive removal of phosphate: Mechanisms, behaviors, and prospects. Chem. Eng. J. 2022, 446, 137081. [Google Scholar] [CrossRef]
- Singh, M.; Nguyen, T.T.; Austreia, M.P.; Ngo, Q.P.; Kim, D.H.; Kim, N.H.; Lee, J.H. Metallic metastable hybrid 1t′/1t phase triggered co,p sns2 nanosheets for high efficiency trifunctional electrocatalyst. Small 2023, 19, 2206726. [Google Scholar] [CrossRef]
- Atallah Al-Asad, H.; Parniske, J.; Qian, J.; Alex, J.; Ramaswami, S.; Kaetzl, K.; Morck, T. Development and application of a predictive model for advanced wastewater treatment by adsorption onto powdered activated carbon. Water Res. 2022, 217, 118427. [Google Scholar] [CrossRef]
- Aryee, A.A.; Liu, Y.; Han, R.; Qu, L. Bimetallic adsorbents for wastewater treatment: A review. Environ. Chem. Lett. 2023, 279, 1–25. [Google Scholar] [CrossRef]
- Ahmadijokani, F.; Molavi, H.; Bahi, A.; Fernández, R.; Alaee, P.; Wu, S.; Wuttke, S.; Ko, F.; Arjmand, M. Metal-organic frameworks and electrospinning: A happy marriage for wastewater treatment. Adv. Funct. Mater. 2022, 32, 2207723. [Google Scholar] [CrossRef]
- Yu, C.; Liang, Y.; Xue, W.; Zhang, Z.; Jia, X.; Huang, H.; Qiao, Z.; Mei, D.; Zhong, C. Polymer-supported ultra-thin zif-67 membrane through in situ interface self-repair. J. Membr. Sci. 2021, 625, 119139. [Google Scholar] [CrossRef]
- Qazvini, O.T.; Babarao, R.; Telfer, S.G. Selective capture of carbon dioxide from hydrocarbons using a metal-organic framework. Nat. Commun. 2021, 12, 197. [Google Scholar] [CrossRef] [PubMed]
- Tochetto, G.A.; Simão, L.; de Oliveira, D.; Hotza, D.; Immich, A.P.S. Porous geopolymers as dye adsorbents: Review and perspectives. J. Clean. Prod. 2022, 374, 133982. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, J.; Muhammad, A.; Qin, R.; Tian, J.; Li, L.; Zhang, Q.; Chen, L.; Yang, P. An amyloid-like proteinaceous adsorbent for uranium extraction from aqueous medium. J. Mater. Chem. A 2022, 10, 14906–14916. [Google Scholar] [CrossRef]
- Zhao, X.; Su, Y.; Wang, H.; Lei, Z.; Hu, E.; Hu, F.; Wang, Q.; Xu, L.; Fan, S.; Liu, X.; et al. Modification of activated carbon from agricultural waste lotus leaf and its adsorption mechanism of beryllium. Korean J. Chem. Eng. 2023, 40, 255–266. [Google Scholar] [CrossRef]
- Han, Z.; Lu, Y.; Li, Y.; Wu, R.; Huang, Z. Strategy to combine two functional components: Efficient nano material development for iodine immobilization. Chemosphere 2022, 309, 136477. [Google Scholar] [CrossRef]
- Kou, X.; Zhang, X.; Cheng, Y.; Yu, M.; Meng, Q.; Ke, Q. Mannitol is a good anticaking agent for spray-dried hydroxypropyl-beta-cyclodextrin microcapsules. Molecules 2023, 28, 1119. [Google Scholar] [CrossRef]
- Yu, C.; Song, Y. Modified supporting materials to fabricate form stable phase change material with high thermal energy storage. Molecules 2023, 28, 1309. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Ferreira, W.H.; Goycoolea, F.M.; Murray, B.S.; Andrade, C.T. Improved antioxidant and mechanical properties of food packaging films based on chitosan/deep eutectic solvent, containing açaí-filled microcapsules. Molecules 2023, 28, 1507. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Chen, Z.; Yang, H.; Cai, Y.; Wang, S.; Chen, J.; Hu, B.; Huang, Q.; Shen, C.; et al. Advanced porous nanomaterials as superior adsorbents for environmental pollutants removal from aqueous solutions. Crit. Rev. Env. Sci. Tec. 2023, ahead-of-print. [Google Scholar] [CrossRef]
- Peng, W.; Du, S.; Shaoning, Z.; Xieyi, H.; Qingyuan, B.; Meng, Q.; Wei, Z.; Fuqiang, H. Constructing mesoporous phosphated titanium oxide for efficient cr(iii) removal. J. Hazard. Mater. 2020, 384, 121278. [Google Scholar] [CrossRef] [PubMed]
- Hernandes, P.T.; Franco, D.S.P.; Georgin, J.; Salau, N.P.G.; Dotto, G.L. Investigation of biochar from cedrella fissilis applied to the adsorption of atrazine herbicide from an aqueous medium. J. Environ. Chem. Eng. 2022, 10, 107408. [Google Scholar] [CrossRef]
- Ying, Z.; Huang, L.; Ji, L.; Li, H.; Liu, X.; Zhang, C.; Zhang, J.; Yi, G. Efficient removal of methylene blue from aqueous solutions using a high specific surface area porous carbon derived from soybean dreg. Materials 2021, 14, 1754. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Wang, C. Preparation of polyhydroxyl adsorbent and its application in the removal of ginkgolic acids. Ind. Crop. Prod. 2022, 184, 114998. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Gong, J.; Li, Z.; Zhang, J. A poly(allylamine hydrochloride)/poly(styrene sulfonate) microcapsule-coated cotton fabric for stimulus-responsive textiles. RSC Adv. 2020, 10, 17731–17738. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Q.; Gong, J.; Li, Z.; Zhang, J. Hybrid poly(allylamine hydrochloride)–graphene oxide microcapsules: Preparation, characterization and application in textiles with controlled release behavior. Mater. Adv. 2020, 1, 804–813. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, J.; Sun, C.; Liu, X.; Zhang, H. Fabrication of highly hydrophobic organic–inorganic hybrid magnetic polysulfone microcapsules: A lab-scale feasibility study for removal of oil and organic dyes from environmental aqueous samples. J. Hazard. Mater. 2016, 309, 65–76. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated performance of thin film nanocomposite membranes enabled by modified hydrophilic mofs for nanofiltration. ACS Appl. Mater. Interfaces 2017, 9, 1975–1986. [Google Scholar] [CrossRef]
- Tsirigotis-Maniecka, M.; Lamch, A.; Chojnacka, I.; Gancarz, R.; Wilk, K.A. Microencapsulation of hesperidin in polyelectrolyte complex microbeads: Physico-chemical evaluation and release behavior. J. Food Eng. 2017, 214, 104–116. [Google Scholar] [CrossRef]
- Göktepe, F.; Bozkurt, A.; Günday, S.T. Synthesis and proton conductivity of poly(styrene sulfonic acid)/heterocycle-based membranes. Polym. Int. 2008, 57, 133–138. [Google Scholar] [CrossRef]
- Luo, J.; Kong, F.; Ma, X. Role of aspartic acid in the synthesis of spherical vaterite by the Ca(OH)2-CO2 reaction. Cryst. Growth Des. 2016, 16, 728–736. [Google Scholar] [CrossRef]
- Chang, J.; Fang, Y.; Shang, X. The role of β-c2s and γ-c2s in carbon capture and strength development. Mater. Struct. 2016, 49, 4417–4424. [Google Scholar] [CrossRef]
- Fu, R.; Ren, Y.; Fang, K.; Sun, Y.; Zhang, Z.; Luo, A. Preparation, characterization and biocompatibility of chitosan/tempo-oxidized bacterial cellulose composite film for potential wound dressing applications. Fiber. Polym. 2021, 22, 1790–1799. [Google Scholar] [CrossRef]
- Ahmadi, Y.; Kim, K. Hyperbranched polymers as superior adsorbent for the treatment of dyes in water. Adv. Colloid Interface Sci. 2022, 302, 102633. [Google Scholar] [CrossRef]
- Zhu, C.; Xia, Y.; Zai, Y.; Dai, Y.; Liu, X.; Bian, J.; Liu, Y.; Liu, J.; Li, G. Adsorption and desorption behaviors of hpei and thermoresponsive hpei based gels on anionic and cationic dyes. Chem. Eng. J. 2019, 369, 863–873. [Google Scholar] [CrossRef]
- Boukoussa, B.; Hakiki, A.; Moulai, S.; Chikh, K.; Kherroub, D.E.; Bouhadjar, L.; Guedal, D.; Messaoudi, K.; Mokhtar, F.; Hamacha, R. Adsorption behaviors of cationic and anionic dyes from aqueous solution on nanocomposite polypyrrole/sba-15. J. Mater. Sci. 2018, 53, 7372–7386. [Google Scholar] [CrossRef]
- Abid, Z.; Hakiki, A.; Boukoussa, B.; Launay, F.; Hamaizi, H.; Bengueddach, A.; Hamacha, R. Preparation of highly hydrophilic pva/sba-15 composite materials and their adsorption behavior toward cationic dye: Effect of pva content. J. Mater. Sci. 2019, 54, 7679–7691. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, X.; Niu, Y.; Pan, B. Spherical polystyrene-supported nano-fe3o4 of high capacity and low-field separation for arsenate removal from water. J. Hazard. Mater. 2012, 243, 319–325. [Google Scholar] [CrossRef]
- Cai, C.; Bakowsky, U.; Rytting, E.; Schaper, A.K.; Kissel, T. Charged nanoparticles as protein delivery systems: A feasibility study using lysozyme as model protein. Eur. J. Pharm. Biopharm. 2008, 69, 31–42. [Google Scholar] [CrossRef]
- Wei, Y.; Feng, K.; Zong, M.; Wu, H. Ph-responsive composite micro-capsule as an efficient intestinal-specific oral delivery system for lactoferrin. Food Hydrocoll. 2019, 95, 203–211. [Google Scholar] [CrossRef]
- Li, Y.; Yu, E.; Sun, S.; Liu, W.; Hu, R.; Xu, L. Fast and highly efficient adsorption of cationic dyes by phytic acid crosslinked β-cyclodextrin. Carbohyd. Polym. 2022, 284, 119231. [Google Scholar] [CrossRef]
- Kazemi, J.; Javanbakht, V. Alginate beads impregnated with magnetic chitosan@zeolite nanocomposite for cationic methylene blue dye removal from aqueous solution. Int. J. Biol. Macromol. 2020, 154, 1426–1437. [Google Scholar] [CrossRef]
- Marco-Brown, J.L.; Guz, L.; Olivelli, M.S.; Schampera, B.; Torres Sánchez, R.M.; Curutchet, G.; Candal, R. New insights on crystal violet dye adsorption on montmorillonite: Kinetics and surface complexes studies. Chem. Eng. J. 2018, 333, 495–504. [Google Scholar] [CrossRef]
- Khan, T.A.; Dahiya, S.; Ali, I. Use of kaolinite as adsorbent: Equilibrium, dynamics and thermodynamic studies on the adsorption of rhodamine b from aqueous solution. Appl. Clay Sci. 2012, 69, 58–66. [Google Scholar] [CrossRef]
- Usman, M.A.; Khan, A.Y. Selective adsorption of anionic dye from wastewater using polyethyleneimine based macroporous sponge: Batch and continuous studies. J. Hazard. Mater. 2022, 428, 128238. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shang, J.; Dou, B.; Lan, J.; Zhang, C.; Zou, R.; Xiao, H.; Lin, S. Co2-responsive functional cotton fibers decorated with ag nanoparticles for “smart” selective and enhanced dye adsorption. J. Hazard. Mater. 2022, 429, 128327. [Google Scholar] [CrossRef] [PubMed]
- Boughrara, L.; Zaoui, F.; Guezzoul, M.; Sebba, F.Z.; Bounaceur, B.; Kada, S.O. New alginic acid derivatives ester for methylene blue dye adsorption: Kinetic, isotherm, thermodynamic, and mechanism study. Int. J. Biol. Macromol. 2022, 205, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, X. Wrapping carbon nanotubes with poly (sodium 4-styrenesulfonate) for enhanced adsorption of methylene blue and its mechanism. Chem. Eng. J. 2014, 256, 85–92. [Google Scholar] [CrossRef]
- Zhou, G.; Jiang, W.; Xu, Z.; Yang, S.; Jia, X.; Tian, Y.; Luan, G. Synthesis and performance analysis of a mesoporous polydopamine-functionalized magnetic microcapsule adsorbent in water treatment. J. Water Process Eng. 2022, 48, 102894. [Google Scholar] [CrossRef]
- Dridi-Dhaouadi, S.; Mhenni, M.F. Effect of dye auxiliaries on chemical oxygen demand and colour competitive removal from textile effluents usingposidonia oceanica. Chem. Ecol. 2014, 30, 579–588. [Google Scholar] [CrossRef]
- Ecer, Ü.; Zengin, A.; Sahan, T. Magnetic clay\zeolitic imidazole framework nanocomposite (zif-8@fe3o4@bnt) for reactive orange 16 removal from liquid media. Colloids Surf. A: Physicochem. Eng. Asp. 2021, 630, 127558. [Google Scholar] [CrossRef]
- Essomba, J.S.; Alla, J.P.; Belibi, P.D.B.; Fathima, N.N. Clay/polymer nanocomposite material: A sustainable approach of leather industries wastewater treatment. Int. J. Environ. Sci. Te. 2022, 19, 5181–5194. [Google Scholar] [CrossRef]
- Fiorentin, L.D.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Trigueros, D.E.G.; Kroumov, A.D.; Manenti, D.R.; Borba, C.E. Biosorption of the reactive blue 5g dye in a fixed bed column packed with orange bagasse: Experimental and mathematical modelling. Sep. Sci. Technol. 2015, 50, 2267–2275. [Google Scholar] [CrossRef]
- Hayeeye, F.; Sattar, M.; Chinpa, W.; Sirichote, O. Kinetics and thermodynamics of rhodamine b adsorption by gelatin/activated carbon composite beads. Colloids Surf. A: Physicochem. Eng. Asp. 2017, 513, 259–266. [Google Scholar] [CrossRef]
- He, H.; Chai, K.; Wu, T.; Qiu, Z.; Wang, S.; Hong, J. Adsorption of rhodamine b from simulated waste water onto kaolin-bentonite composites. Materials 2022, 15, 4058. [Google Scholar] [CrossRef]
- Jiang, Q.; Han, Z.; Yu, X.; Yuan, Y.; Ren, Y.; Li, J.; Zhao, C.; Cheng, Z. Nh2-mil-125 (ti)/biochar fibers for enhanced direct dyes adsorption. J. Environ. Chem. Eng. 2021, 9, 106636. [Google Scholar] [CrossRef]
- Kirupa Sankar, M.; Muthu Kumar, K.; Ranganathan, B.V. Adsorption of anionic azo dye from aqueous solution using strychnos potatorum linn seeds: Isotherm and kinetic studies. Int. J. Environ. Sci. Te. 2015, 12, 2957–2964. [Google Scholar] [CrossRef]
- Li, H.; Lin, L.; Su, S.; Wen, X.; Yan, R.; Liu, H.; Tao, C. Enhanced photothermal effect of functionalized hmpda@aunps microcapsules for near-infrared theranostic treatment of tumor. J. Mater. Sci. 2022, 57, 7694–7705. [Google Scholar] [CrossRef]
- Malakootian, M.; Heidari, M.R. Reactive orange 16 dye adsorption from aqueous solutions by psyllium seed powder as a low-cost biosorbent: Kinetic and equilibrium studies. Appl. Water Sci. 2018, 8, 212. [Google Scholar] [CrossRef]
- Marrakchi, F.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous carbonaceous material from fish scales as low-cost adsorbent for reactive orange 16 adsorption. J. Taiwan Inst. Chem. E. 2017, 71, 47–54. [Google Scholar] [CrossRef]
- Wawrzkiewicz, M.; Polska-Adach, E.; Hubicki, Z. Application of titania based adsorbent for removal of acid, reactive and direct dyes from textile effluents. Adsorption 2019, 25, 621–630. [Google Scholar] [CrossRef]
- Yefimova, S.L.; Bespalova, I.I.; Grygorova, G.V.; Sorokin, A.V.; Mateychenko, P.V.; Cui, X.Q.; Malyukin, Y.V. Synthesis and characterization of mesoporous caco3@pss microspheres as a depot system for sustained methylene blue delivering. Microporous Mesoporous Mater. 2016, 236, 120–128. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, X.; Yan, X.; Feng, R.; Zhou, M.; Xue, J. Enhanced adsorption of rhodamine b by magnetic nitrogen-doped porous carbon prepared from bimetallic zifs. Colloids Surf. A: Physicochem. Eng. Asp. 2019, 575, 10–17. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X.; Liu, X.; Fang, K.; Gong, J.; Si, J.; Gao, W.; Liu, D. In situ generated uio-66/cotton fabric easily recyclable for reactive dye adsorption. Langmuir 2022, 38, 12095–12102. [Google Scholar] [CrossRef]
- Liu, H.; Tian, D.; Ouyang, M.; Qian, Z.; Wang, X. Morphology-controlled fabrication of magnetic phase-change microcapsules for synchronous efficient recovery of wastewater and waste heat. J. Colloid Interface Sci. 2022, 608, 1497–1513. [Google Scholar] [CrossRef] [PubMed]
- Lima, G.; Morais, W.G.; Gomes, W.J.A.S.; Huguenin, F. Acid–base machines: Electrical work from neutralization reactions. Phys. Chem. Chem. Phys. 2017, 19, 31202–31215. [Google Scholar] [CrossRef]
- Mei, Y.; Liu, L.; Lu, Y.; Tang, C.Y. Reverse electrodialysis chemical cell for energy harvesting from controlled acid–base neutralization. Environ. Sci. Technol. 2019, 53, 4640–4647. [Google Scholar] [CrossRef]
- Chen, H.; Luo, J.; Wang, X.; Liang, X.; Zhao, Y.; Yang, C.; Baikenov, M.I.; Su, X. Synthesis of al2o3/carbon composites from wastewater as superior adsorbents for pb(ii) and cd(ii) removal. Microporous Mesoporous Mater. 2018, 255, 69–75. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, L.; Gu, P.; Tian, L.; Li, N.; Chen, D.; Marcomini, A.; Xu, Q.; Lu, J. Novel calixarene-based porous organic polymers with superfast removal rate and ultrahigh adsorption capacity for selective separation of cationic dyes. Chem. Eng. J. 2022, 433, 134442. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, C.; Lai, Z.; Chen, S.; He, D.; Mu, J. Honeycomb-like cork activated carbon with ultra-high adsorption capacity for anionic, cationic and mixed dye: Preparation, performance and mechanism. Bioresour. Technol. 2022, 357, 127363. [Google Scholar] [CrossRef] [PubMed]




| Cationic Dyes | Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe,cal (mg/g) | k1 (min−1) | R12 | Qe,cal (mg/g) | k2 (g·mg−1·min−1) | R22 | ki1 (mg·g−1·min−1/2) | c1 (mg/g) | Ri12 | ki2 (mg·g−1·min−1/2) | c2 (mg/g) | Ri22 | ||
| RhB | P2P | 394.5180 | 0.0508 | 0.9243 | 426.0395 | 2.706 × 10−4 | 0.9988 | 53.3927 | 169.2147 | 0.8647 | 6.5780 | 316.3695 | 0.9218 |
| P2 | 600.0253 | 0.04833 | 0.5156 | 644.1718 | 1.206 × 10−4 | 0.9999 | 244.7164 | 7.5673 | 0.9371 | 0.9043 | 626.7337 | 0.7801 | |
| MB | P2P | 459.6831 | 0.0305 | 0.8153 | 474.1180 | 2.705 × 10−4 | 0.9994 | 122.9298 | 114.1426 | 0.6103 | 3.6964 | 407.2721 | 0.9345 |
| P2 | 872.9607 | 0.0586 | 0.8588 | 912.4087 | 1.365 × 10−4 | 0.9995 | 96.1240 | 483.7519 | 0.9832 | 9.8377 | 740.1333 | 0.9676 | |
| Anionic Dyes | Adsorbent | Pseudo-First-Order | Pseudo-Second-Order | Intraparticle Diffusion | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Qe,cal (mg/g) | k1 (min−1) | R12 | Qe,cal (mg/g) | k2 (g·mg−1·min−1) | R22 | ki1 (mg·g−1·min−1/2) | c1 (mg/g) | Ri12 | ki2 (mg·g−1·min−1/2) | c2 (mg/g) | Ri22 | ||
| ROKG | P2 | 210.1818 | 0.03552 | 0.87635 | 217.3913 | 7.0323 × 10−4 | 0.9985 | 12.4521 | 109.7804 | 0.8944 | 30.9230 | 238.1153 | 0.6772 |
| P2P | 331.6909 | 0.01869 | 0.8822 | 384.0245 | 6.970 × 10−5 | 0.9771 | 25.9532 | 69.89 | 0.6226 | 16.3119 | 104.9623 | 0.9553 | |
| DY5G | P2 | 176.8772 | 0.0162 | 0.8979 | 188.75005 | 2.514 × 10−4 | 0.9858 | 30.7321 | 36.535 | 0.6652 | 1.8974 | 119.999 | 0.9615 |
| P2P | 401.7688 | 0.0233 | 0.69724 | 450.5465 | 1.222 × 10−4 | 0.9853 | 8.8988 | 314.2024 | 0.8589 | 6.3555 | 321.5930 | 0.8005 | |
| Dye | Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | ||
| (mg·g−1) | (L·g−1) | (mg·g−1) | |||||
| ROKG | P2P | 478.4689 | 1.8993 | 0.9979 | 14.4877 | 2.4 | 0.9492 |
| P2 | 478.43 | 1.25 | 0.9939 | 6.519 | 1.9723 | 0.9767 | |
| DY5G | P2P | 561.1672 | 1.2875 | 0.9949 | 7.8491 | 1.9743 | 0.966 |
| P2 | 433.0047 | 0.9277 | 0.9999 | 0.0311 | 0.8834 | 0.968 | |
| Dye | Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| qm | KL | R2 | KF | n | R2 | ||
| (mg·g−1) | (L·g−1) | (mg·g−1) | |||||
| RhB | P2P | 750.0694 | 1.0701 | 0.9989 | 7.7512 | 1.8677 | 0.9605 |
| P2 | 875.0628 | 0.8901 | 0.9982 | 0.1872 | 0.9835 | 0.9739 | |
| MB | P2P | 1217.879 | 0.8335 | 0.9891 | 7.51927 | 1.6864 | 0.931 |
| P2 | 1239.065 | 0.895 | 0.9989 | 0.3124 | 1.004 | 0.9733 | |
| Dye | Abbreviation | λmax a (nm) | Molecular Formula | Molecular Structure | Category |
|---|---|---|---|---|---|
| Reactive orange K-G | ROKG | 413 | C19H16N7Na3O10S3Cl | 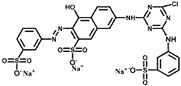 | Anionic |
| Direct yellow 5G | DY5G | 393 | C24H19N4NaO5S2 |  | Anionic |
| Rhodamine-B | RhB | 554 | C28H31N2O3Cl | 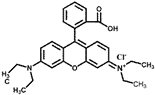 | cationic |
| Methylene Blue | MB | 664 | C16H18ClN3S | 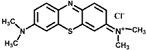 | cationic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Zhou, H.; Han, X.; Han, L.; Xu, Z.; Wang, P. Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules. Molecules 2023, 28, 3010. https://doi.org/10.3390/molecules28073010
Zhao Z, Zhou H, Han X, Han L, Xu Z, Wang P. Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules. Molecules. 2023; 28(7):3010. https://doi.org/10.3390/molecules28073010
Chicago/Turabian StyleZhao, Zhiqi, Hongbing Zhou, Xu Han, Lun Han, Zhenzhen Xu, and Peng Wang. 2023. "Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules" Molecules 28, no. 7: 3010. https://doi.org/10.3390/molecules28073010
APA StyleZhao, Z., Zhou, H., Han, X., Han, L., Xu, Z., & Wang, P. (2023). Rapid, Highly-Efficient and Selective Removal of Anionic and Cationic Dyes from Wastewater Using Hollow Polyelectrolyte Microcapsules. Molecules, 28(7), 3010. https://doi.org/10.3390/molecules28073010






