Naphth[1,2-d]imidazoles Bioactive from β-Lapachone: Fluorescent Probes and Cytotoxic Agents to Cancer Cells
Abstract
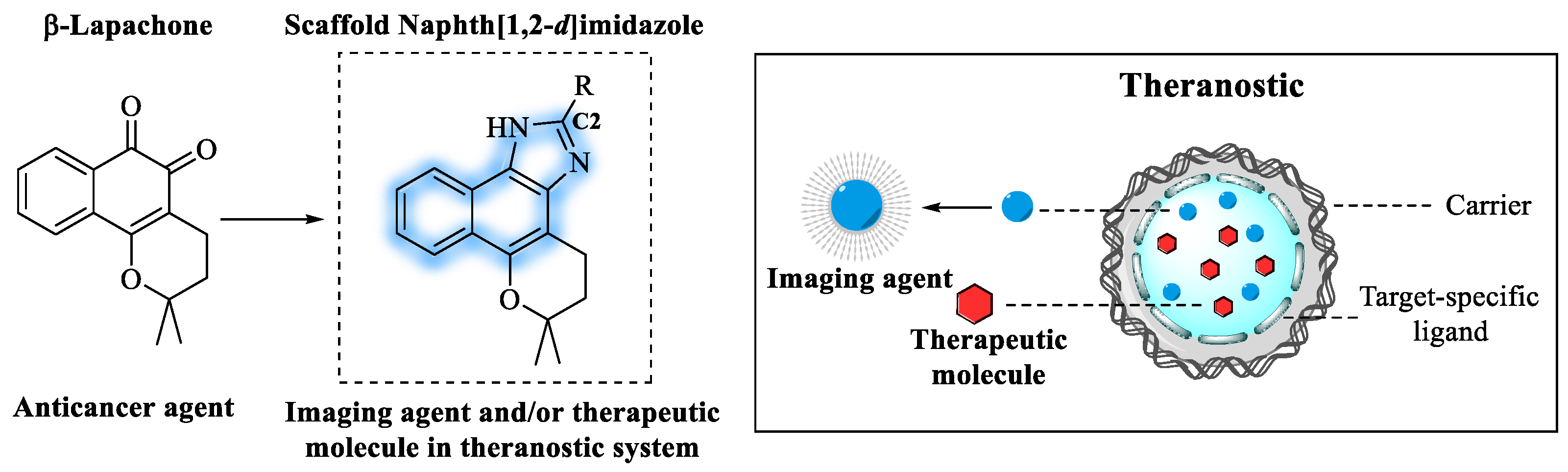
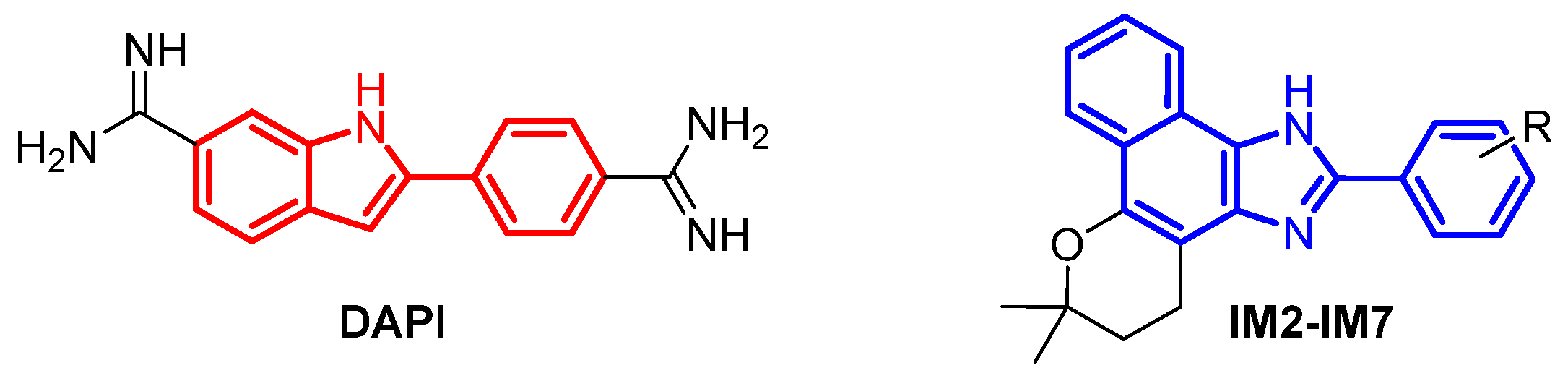
1. Introduction
2. Results and Discussion
2.1. Optical Properties—Studies of Absorption and Fluorescence Spectra
2.1.1. Solvatochromism Study
2.1.2. Molar Absorption Coefficient
| Compound | Solvent | λmax (nm) | εAbs a (104 M−1cm−1) | λemis b (nm) | ΔST c (nm) |
|---|---|---|---|---|---|
| IM1 | CH2Cl2 | 316 | 0.61 | 366 | 50 |
| IM2 | DMSO | 342 | 2.43 | 402 | 60 |
| IM3 | CH2Cl2 | 354 | 1.57 | 457 | 103 |
| IM4 | CH2Cl2 | 344 | 1.48 | 393 | 49 |
| IM5 | CH2Cl2 | 368 | 2.71 | 422 | 54 |
| IM6 | CH3OH | 411 | 1.85 | 391 | 20 |
| IM7 | Hexane | 337 | 1.35 | 385 | 48 |
| DAPI | H2O | 343 d | - | 452 | 112 |
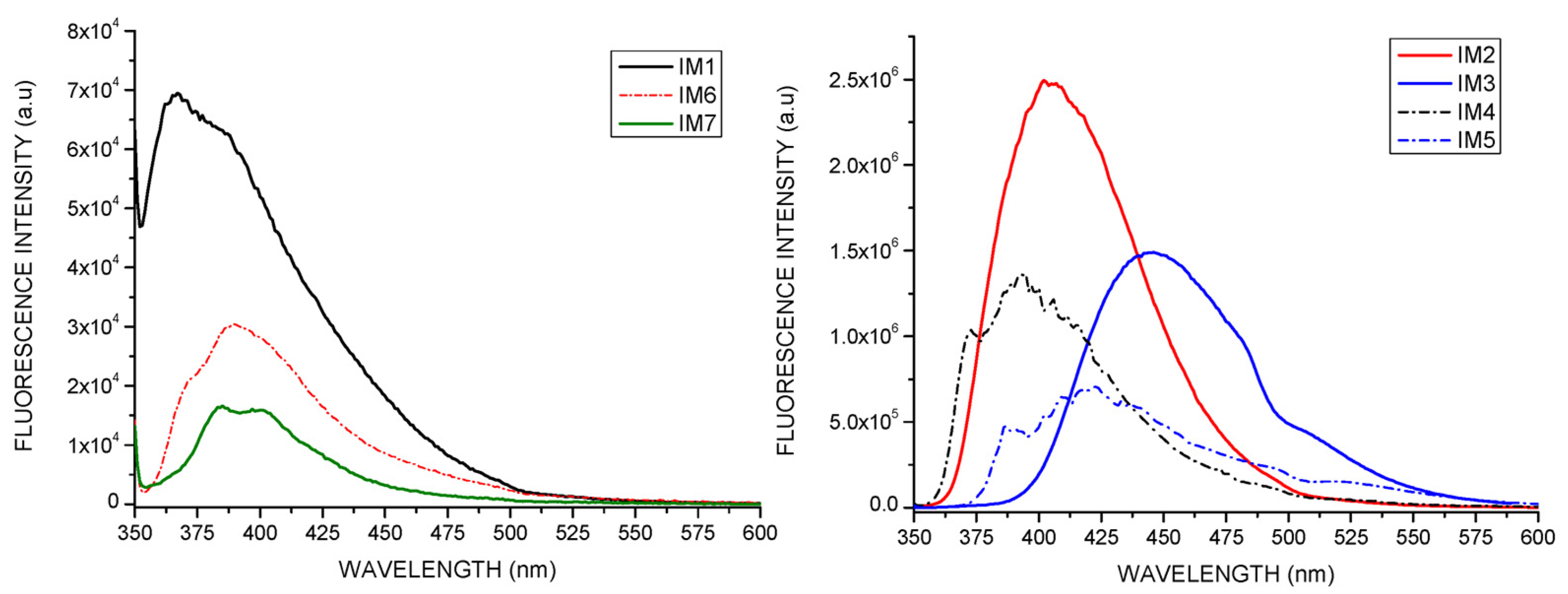
2.1.3. Fluorescence Spectroscopy Experiments
2.1.4. Stokes Shift
2.2. Cytotoxicity Assay
3. Materials and Methods
3.1. Materials
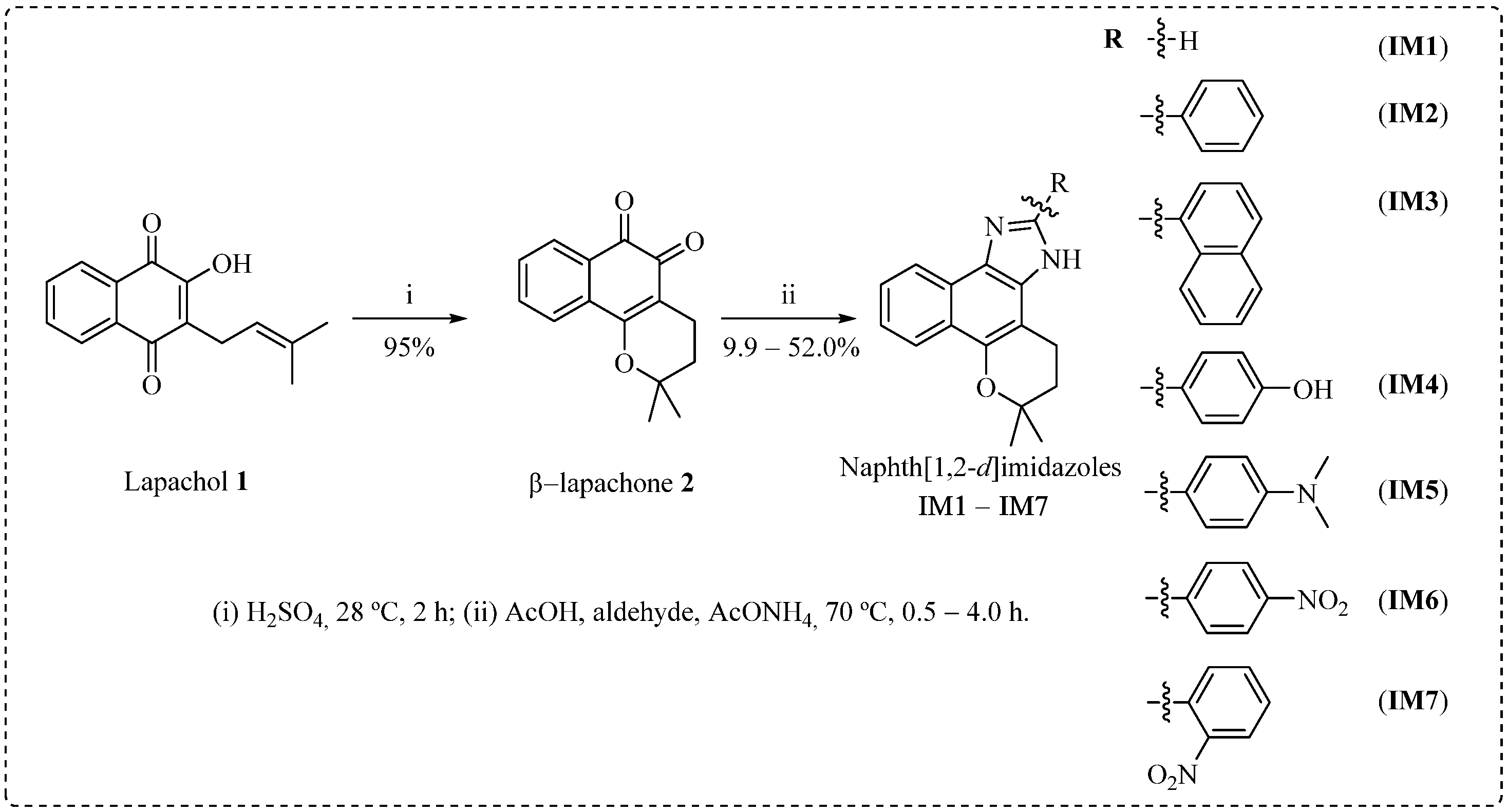
3.2. Synthesis of Naphth[1,2-d]imidazoles IM1–IM7
3.2.1. Synthesis of β-Lapachone 2
3.2.2. General Synthesis of the Naphth[1,2-d]imidazoles
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-pyran[b-4,3]naphth[1,2-d]imidazole (IM1)
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-(phenyl)-pyran[b-4,3]naphth[1,2-d]imidazole (IM2)
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-(naphthalenyl)-pyran[b-4,3]naphth[1,2-d]imidazole (IM3)
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-(4-hidroxyphenyl)-pyran[b-4,3]naphth[1,2-d]imidazole (IM4)
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-(4-dimethylaminophenyl)-pyran[b-4,3]naphth[1,2-d]imidazole (IM5)
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-(4-nitrophenyl)-pyran[b-4,3]naphth[1,2-d]imidazole (IM6)
Synthesis of 4,5-dihydro-6,6-dimethyl-6H-2-(2-nitrophenyl)-pyran[b-4,3]naphth[1,2-d]imidazole (IM7)
3.3. Evaluation of Photophysical Properties
3.3.1. Obtaining Visible Ultraviolet Absorption Spectra
3.3.2. Molar Absorptivity Coefficient
3.3.3. Fluorescence Emission Spectrum and Stokes Shift
3.4. Evaluation of the Cytotoxic Activity
3.4.1. Cell Lines
3.4.2. Assessment of In Vitro Anticancer Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kelkar, S.S.; Reineke, T.M. Theranostics: Combining Imaging and Therapy. Bioconjugate Chem. 2011, 22, 1879–1903. [Google Scholar] [CrossRef]
- Xiao, S.; Tang, Y.; Lv, Z.; Lin, Y.; Chen, L. Nanomedicine—Advantages for their use in rheumatoid arthritis theranostics. J. Control. Release 2019, 316, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Golovin, Y.; Klyachko, N.; Majouga, A.; Kabanov, A. Modeling drug release from functionalized magnetic nanoparticles actuated by non-heating low frequency magnetic field. J. Nanoparticle Res. 2017, 64, 19. [Google Scholar] [CrossRef]
- Jain, T.; Kumar, S.; Dutta, P.K. Theranostics: A Way of Modern Medical Diagnostics and the Role of Chitosan. J. Mol. Genet. Med. 2014, 9, 1000159. [Google Scholar] [CrossRef]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [Google Scholar] [CrossRef]
- Santos, V.; Guimarães, D.; Nishimura, R.; Rolim, L.; Gonsalves, A.; Araújo, C. Naftoimidazóis e naftoxazóis—Promissores componentes de sistemas teranósticos. Quim. Nova 2022, 45, 560–577. [Google Scholar] [CrossRef]
- Kerru, N.; Bhaskaruni, S.V.H.S.; Gummidi, L.; Maddila, S.N.; Maddila, S.; Jonnalagadda, S.B. Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Synth. Commun. 2019, 49, 2437–2459. [Google Scholar] [CrossRef]
- Santos, V.; Gonsalves, A.; Araújo, C. Resgate da reação de debus-radziszewski: Ensino prático de reações multicomponentes na síntese da lofina. Quim. Nova 2020, 43, 1344–1349. [Google Scholar] [CrossRef]
- Bombaça, A.C.S.; Silva, L.A.; Chaves, O.A.; da Silva, L.S.; Barbosa, J.M.; da Silva, A.M.; Ferreira, A.B.; Menna-Barreto, R.F. Novel N,N-di-alkylnaphthoimidazolium derivative of β-lapachone impaired Trypanosoma cruzi mitochondrial electron transport system. Biomed. Pharmacother. 2021, 135, 111186. [Google Scholar] [CrossRef]
- Moura, K.C.; Carneiro, P.F.; Pinto, M.D.C.F.; da Silva, J.A.; Malta, V.; de Simone, C.A.; Dias, G.; Jardim, G.A.; Cantos, J.; Coelho, T.S.; et al. 1,3-Azoles from ortho-naphthoquinones: Synthesis of aryl substituted imidazoles and oxazoles and their potent activity against Mycobacterium tuberculosis. Bioorganic Med. Chem. 2012, 20, 6482–6488. [Google Scholar] [CrossRef]
- Castellanos, J.R.G.; Prieto, J.M.; Heinrich, M. Red Lapacho (Tabebuia impetiginosa)—A global ethnopharmacological commodity? J. Ethnopharmacol. 2009, 121, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moon, D.-O.; Kang, C.-H.; Kim, M.-O.; Jeon, Y.-J.; Lee, J.-D.; Choi, Y.H.; Kim, G.-Y. β-Lapachone (LAPA) Decreases Cell Viability and Telomerase Activity in Leukemia Cells: Suppression of Telomerase Activity by LAPA. J. Med. Food 2010, 13, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Jardim, G.A.M.; Lima, D.J.B.; Valença, W.O.; Cavalcanti, B.C.; Pessoa, C.; Rafique, J.; Braga, A.L.; Jacob, C.; Da Silva Júnior, E.N.; Da Cruz, E.H.G. Synthesis of Selenium-Quinone Hybrid Compounds with Potential Antitumor Activity via Rh-Catalyzed C-H Bond Activation and Click Reactions. Molecules 2017, 23, 83. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.F.; Rangel, L.P.; Martins-Dinis, M.M.D.C.; Ferretti, G.D.S.; Ferreira, V.F.; Silva, J.L. Anticancer potential of resveratrol, β-lapachone and their analogues. Molecules 2020, 25, 893. [Google Scholar] [CrossRef]
- Khong, H.T.; Dreisbach, L.; Kindler, H.L.; Trent, D.F.; Jeziorski, K.G.; Bonderenko, I.; Popiela, T.; Yagovane, D.M.; Dombal, G. A phase 2 study of ARQ 501 in combination with gemcitabine in adult patients with treatment naïve, unresectable pancreatic adenocarcinoma. J. Clin. Oncol. 2007, 25, 15017. [Google Scholar] [CrossRef]
- Kawecki, A.; Adkins, D.R.; Cunningham, C.C.; Vokes, E.; Yagovane, D.M.; Dombal, G.; Koralewski, P.; Hotko, Y.; Vladimirov, V. A phase II study of ARQ 501 in patients with advanced squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2007, 25, 16509. [Google Scholar] [CrossRef]
- Gerber, D.E.; Beg, M.S.; Fattah, F.; Frankel, A.E.; Fatunde, O.; Arriaga, Y.; Dowell, J.E.; Bisen, A.; Leff, R.D.; Meek, C.C.; et al. Phase 1 study of ARQ 761, a β-lapachone analogue that promotes NQO1-mediated programmed cancer cell necrosis. Br. J. Cancer 2018, 119, 928–936. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Cho, J.-Y.; Yoon, S.H.; Jang, I.-J.; Yu, K.-S. Pharmacokinetics and tolerability of MB12066, a beta-lapachone derivative targeting NAD(P)H:quinone oxidoreductase 1: Two independent, double-blind, placebo-controlled, combined single and multiple ascending dose first-in-human clinical trials. Drug Des. Dev. Ther. 2017, 11, 3187–3195. [Google Scholar] [CrossRef]
- Kim, I.; Kim, H.; Ro, J.; Jo, K.; Karki, S.; Khadka, P.; Yun, G.; Lee, J. Preclinical Pharmacokinetic Evaluation of β-Lapachone: Characteristics of Oral Bioavailability and First-Pass Metabolism in Rats. Biomol. Ther. 2015, 23, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Bey, E.A.; Khemtong, C.; Yang, S.-G.; Setti-Guthi, J.; Chen, H.; Kessinger, C.W.; Carnevale, K.A.; Bornmann, W.G.; Boothman, D.A.; et al. β-Lapachone Micellar Nanotherapeutics for Non–Small Cell Lung Cancer Therapy. Cancer Res 2010, 70, 3896–3904. [Google Scholar] [CrossRef]
- Pavoni, J.; Neves-Junior, W.; Spiropulos, M.; De Araujo, D. Uma montagem experimental para a medida de fluorescência. Rev. Bras. de Ensino de F?sica 2014, 36, 1–9. [Google Scholar] [CrossRef]
- Bozkurt, E.; Dogan, S.D. Photophysical behavior of a novel 4-aza-indole derivative in different solvents: Reverse solvatochromism. Res. Chem. Intermed. 2018, 45, 863–872. [Google Scholar] [CrossRef]
- de Rezende, L.C.D.; Vaidergorn, M.M.; Moraes, J.C.B.; Emery, F.D.S. Synthesis, Photophysical Properties and Solvatochromism of Meso-Substituted Tetramethyl BODIPY Dyes. J. Fluoresc. 2013, 24, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Telegin, F.Y.; Marfin, Y.S. New insights into quantifying the solvatochromism of BODIPY based fluorescent probes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119683. [Google Scholar] [CrossRef]
- Chen, S.; Hong, F. Palladium-Catalyzed C-H Functionalization of Amido-Substitued 1,4-Napthoquinone in the Presence of Amines toward the Formation of Pyrroles and Imidazoles. Chemistryselect 2017, 2, 10232–10238. [Google Scholar] [CrossRef]
- Nagarajan, N.; Vanitha, G.; Ananth, D.A.; Rameshkumar, A.; Sivasudha, T.; Renganathan, R. Bioimaging, antibacterial and antifungal properties of imidazole-pyridine fluorophores: Synthesis, characterization and solvatochromism. J. Photochem. Photobiol. B: Biol. 2013, 127, 212–222. [Google Scholar] [CrossRef]
- De Souza, V.P.; Vendrusculo, V.; Morás, A.M.; Steffens, L.; Santos, F.S.; Moura, D.J.; Rodembusch, F.S.; Russowsky, D. Synthesis and photophysical study of new fluorescent proton transfer dihydropyrimidinone hybrids as potential candidates for molecular probes. New J. Chem. 2017, 41, 15305–15311. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. Molecular fluorescence: Principles and applications, 2nd ed.; John Wiley & Sons: Weinheim, Germany, 2012. [Google Scholar]
- Liu, X.; Xu, Z.; Cole, J.M. Molecular Design of UV–vis Absorption and Emission Properties in Organic Fluorophores: Toward Larger Bathochromic Shifts, Enhanced Molar Extinction Coefficients, and Greater Stokes Shifts. J. Phys. Chem. C 2013, 117, 16584–16595. [Google Scholar] [CrossRef]
- Tariq, A.; Garnier, U.; Ghasemi, R.; Lefevre, J.P.; Mongin, C.; Brosseau, A.; Audibert, J.F.; Pansu, R.; Dauzères, A.; Leray, I. Perylene based PET fluorescent molecular probes for pH monitoring. J. Photochem. Photobiol. A Chem. 2022, 432, 114035. [Google Scholar] [CrossRef]
- Ghodbane, A.; Colléaux, J.; Saffon, N.; Mahiou, R.; Galaup, J.-P.; Fery-Forgues, S. Blue-Emitting Nanocrystals, Microcrystals, and Highly Oriented Nanofibers Prepared by Reprecipitation and Solvent Drop-Casting of 2-Phenyl-naphthoxazoles. Chempluschem 2012, 78, 185–191. [Google Scholar] [CrossRef]
- Farahat, A.A.; Kumar, A.; Say, M.; Wenzler, T.; Brun, R.; Paul, A.; Wilson, W.D.; Boykin, D.W. Exploration of DAPI analogues: Synthesis, antitrypanosomal activity, DNA binding and fluorescence properties. Eur. J. Med. Chem. 2017, 128, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Doğru, Ü.; Ürüt, G.; Bayramin, D. Synthesis and spectroscopic characterization of Y-shaped fluorophores with an imidazole core containing crown ether moieties. J. Lumin. 2015, 163, 32–39. [Google Scholar] [CrossRef]
- Chipem, F.A.S.; Mishra, A.; Krishnamoorthy, G. The role of hydrogen bonding in excited state intramolecular charge transfer. Phys. Chem. Chem. Phys. 2012, 14, 8775–8790. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, J.; Cao, J.; Zheng, H.; Zhan, C.; Liu, H.; Zhang, L.; Xiao, K.; Liu, S.; Xiang, D.; et al. Identification of coexistence of biological and non-biological aerosol particles with DAPI (4′,6-diamidino-2-phenylindole) stain. Particuology 2023, 72, 49–57. [Google Scholar] [CrossRef]
- Hu, J.; Guo, Y.; Geng, X.; Wang, J.; Li, S.; Sun, Y.; Qu, L.; Li, Z. Tuning asymmetric electronic structure endows carbon dots with unexpected huge stokes shift for high contrast in vivo imaging. Chem. Eng. J. 2022, 446, 136928. [Google Scholar] [CrossRef]
- Araneda, J.F.; Piers, W.E.; Heyne, B.; Parvez, M.; McDonald, R. High Stokes Shift Anilido-Pyridine Boron Difluoride Dyes. Angew. Chem. Int. Ed. 2011, 50, 12214–12217. [Google Scholar] [CrossRef]
- Shcherbakova, D.M.; Hink, M.A.; Joosen, L.; Gadella, T.W.J.; Verkhusha, V.V. An Orange Fluorescent Protein with a Large Stokes Shift for Single-Excitation Multicolor FCCS and FRET Imaging. J. Am. Chem. Soc. 2012, 134, 7913–7923. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Sun, X.; Guo, Z.; Tang, J.; Shen, Y.; James, T.D.; Tian, H.; Zhu, W. In Vivo and in Situ Tracking Cancer Chemotherapy by Highly Photostable NIR Fluorescent Theranostic Prodrug. J. Am. Chem. Soc. 2014, 136, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Wu, I.-C.; Yu, J.; Ye, F.; Rong, Y.; Gallina, M.E.; Fujimoto, B.S.; Zhang, Y.; Chan, Y.-H.; Sun, W.; Zhou, X.-H.; et al. Squaraine-Based Polymer Dots with Narrow, Bright Near-Infrared Fluorescence for Biological Applications. J. Am. Chem. Soc. 2014, 137, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, Post-Treatment Recovery, and Selectivity Analysis of Naturally Occurring Podophyllotoxins from Bursera fagaroides var. fagaroides on Breast Cancer Cell Lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, R.; Zhu, Z.; Adachi, C.; Zhang, X.; Lee, C.-S. Highly Stable Near-Infrared Fluorescent Organic Nanoparticles with a Large Stokes Shift for Noninvasive Long-Term Cellular Imaging. ACS Appl. Mater. Interfaces 2015, 7, 26266–26274. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.A.D.; Silva, A.R.D.; Ferreira, M.A.; Lemos, M.J.D.; Ramos, R.G.; Ferreira, A.B.B.; Souza, S.R.D. Atividade biológica do lapachol e de alguns derivados sobre o desenvolvimento fúngico e em germinação de sementes. Quim. Nova 2008, 31, 1670–1672. [Google Scholar] [CrossRef]
- Yu, D.; Chen, X.-L.; Ai, B.-R.; Zhang, X.-M.; Wang, J.-Y. Tetrabutylammonium iodide catalyzed hydroxylation of naphthoquinone derivatives with tert-butyl hydroperoxide as an oxidant. Tetrahedron Lett. 2018, 59, 3620–3623. [Google Scholar] [CrossRef]
- Singh, P.; Natani, K.; Jain, S.; Arya, K.; Dandia, A. Microwave-assisted rapid cyclization of lapachol, a main constituent of Heterophragma adenophyllum. Nat. Prod. Res. 2006, 20, 207–212. [Google Scholar] [CrossRef] [PubMed]
| Cell Line | |||||||
|---|---|---|---|---|---|---|---|
| Compound | L929 | SNB-19 | HCT-116 | HL-60 | |||
| IC50 (μM) a | IC50 (μM) a | SI b | IC50 (μM) a | SI b | IC50 (μM) a | SI b | |
| IM1 | 186.17 | 46.44 (38.59–55.76) | 4.00 | 62.11 (54.14–71.31) | 3.00 | 24.23 (22.32–21.41) | 7.68 |
| IM2 | 104.85 | 26.97 (24.44–26.96) | 3.89 | 21.69 (18.34–25.66) | 4.83 | 29.92 (14.44–19.87) | 3.50 |
| IM4 | >363.21 | 31.90 (28.39–35.83) | >11.38 | 23.04 (21.56–24.67) | >15.76 | 8.71 (7.46–10.22) | >41.70 |
| IM5 | 52.84 | 21.05 (18.20–24.39) | 2.51 | 44.07 (37.42–51.87) | 1.20 | 12.35 (10.22–14.55) | 4.28 |
| IM6 | 151.55 | 22.27 (20.23–24.49) | 6.81 | 21.12 (19.56–22.78) | 7.18 | 13.67 (14.79–18.09) | 11.09 |
| IM7 | NE | >67.00 | NE | 35.11 (28.94–42.56) | NE | 14.90 (10.64–14.17) | NE |
| Doxorubicin | 3.16 | 2.21 (1.90–2.56) | 1.43 | 0.20 (0.15–0.26) | 15.80 | 0.04 (0.035–0.039) | 79.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, V.L.d.A.; Gonsalves, A.d.A.; Guimarães, D.G.; Simplicio, S.S.; Oliveira, H.P.d.; Ramos, L.P.S.; Costa, M.P.d.; Oliveira, F.d.C.E.d.; Pessoa, C.; Araújo, C.R.M. Naphth[1,2-d]imidazoles Bioactive from β-Lapachone: Fluorescent Probes and Cytotoxic Agents to Cancer Cells. Molecules 2023, 28, 3008. https://doi.org/10.3390/molecules28073008
Santos VLdA, Gonsalves AdA, Guimarães DG, Simplicio SS, Oliveira HPd, Ramos LPS, Costa MPd, Oliveira FdCEd, Pessoa C, Araújo CRM. Naphth[1,2-d]imidazoles Bioactive from β-Lapachone: Fluorescent Probes and Cytotoxic Agents to Cancer Cells. Molecules. 2023; 28(7):3008. https://doi.org/10.3390/molecules28073008
Chicago/Turabian StyleSantos, Victória Laysna dos Anjos, Arlan de Assis Gonsalves, Délis Galvão Guimarães, Sidney Silva Simplicio, Helinando Pequeno de Oliveira, Lara Polyana Silva Ramos, Marcília Pinheiro da Costa, Fátima de Cássia Evangelista de Oliveira, Claudia Pessoa, and Cleônia Roberta Melo Araújo. 2023. "Naphth[1,2-d]imidazoles Bioactive from β-Lapachone: Fluorescent Probes and Cytotoxic Agents to Cancer Cells" Molecules 28, no. 7: 3008. https://doi.org/10.3390/molecules28073008
APA StyleSantos, V. L. d. A., Gonsalves, A. d. A., Guimarães, D. G., Simplicio, S. S., Oliveira, H. P. d., Ramos, L. P. S., Costa, M. P. d., Oliveira, F. d. C. E. d., Pessoa, C., & Araújo, C. R. M. (2023). Naphth[1,2-d]imidazoles Bioactive from β-Lapachone: Fluorescent Probes and Cytotoxic Agents to Cancer Cells. Molecules, 28(7), 3008. https://doi.org/10.3390/molecules28073008