Chemical Composition, Enantiomeric Distribution and Biological Activity of Essential Oil from Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur
Abstract
1. Introduction
2. Results
2.1. Essential Oil Obtained
2.2. Physical Properties of Essential Oil
2.3. Chemical Composition of Essential Oil
2.4. Enantiomeric Analysis
2.5. Antimicrobial Activity
2.6. Antioxidant Activity
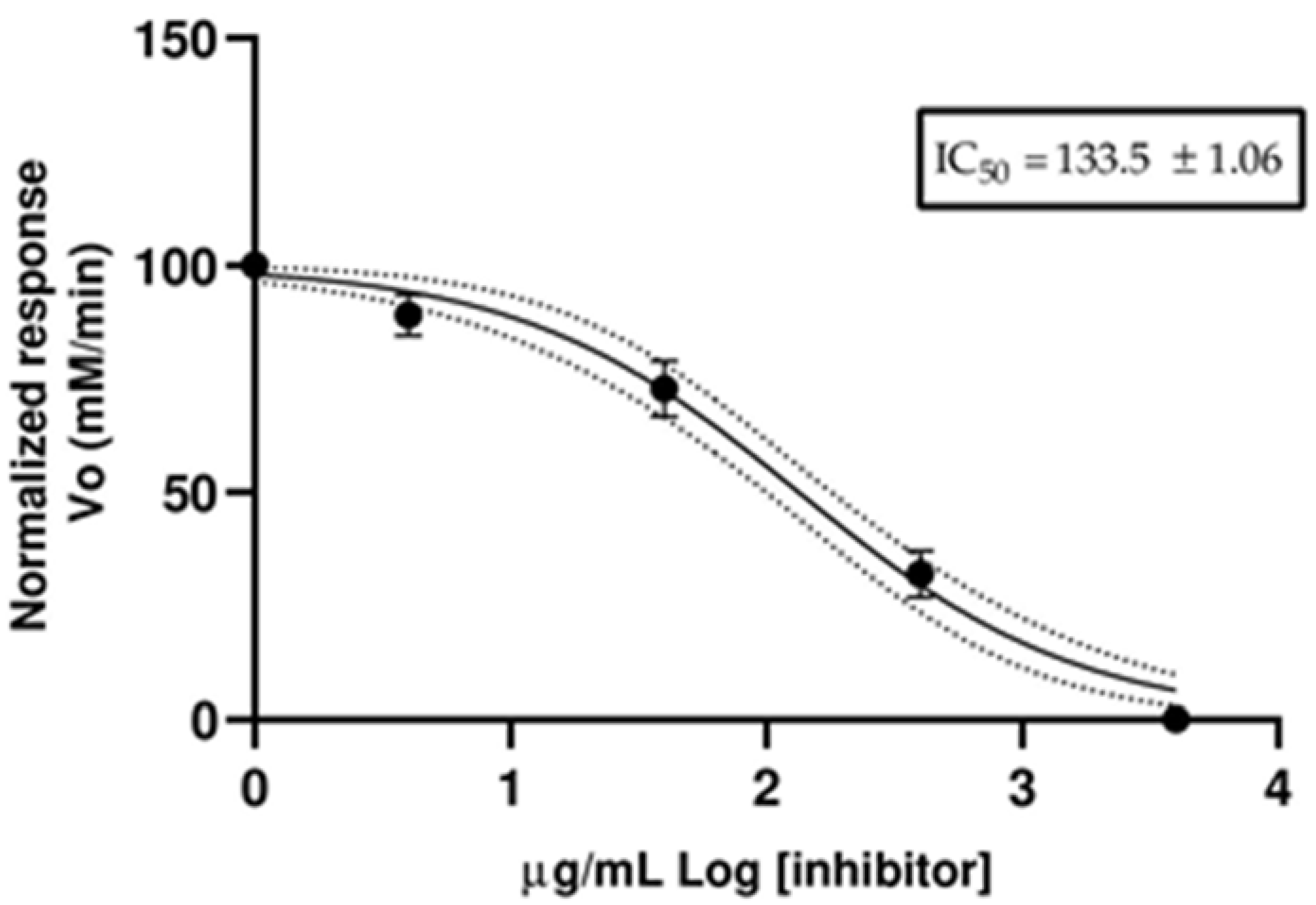
2.7. Anticholinesterase Activity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Plant Material
4.3. Essential Oil Isolation
4.4. Identification and Quantification of Essential Oil Compounds
4.5. Enantioselective Analysis
4.6. Antimicrobial Activity
4.7. Evaluation of Antioxidant Capacity
4.8. Anticholinesterase Activity
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Olaoluwa, O.; Taiwo, O.; Nahar, L.; Sarker, S.D. Ethnopharmacology, phytochemistry and biological activities of selected African species of the genus Ficus. Trends Phytochem. Res. 2022, 6, 46–69. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Venditti, A.; Akbarzadeh, A. The genus Perovskia Kar.: Ethnobotany, chemotaxonomy and phytochemistry: A review. Toxin Rev. 2021, 40, 484–505. [Google Scholar] [CrossRef]
- Mouthe Kemayou, G.P.; Fotsing Kache, S.; Dzouemo, L.C.; Happi, G.M.; Fogue Kouam, S.; Tchouankeu, J.C. Phytochemistry, traditional uses, and pharmacology of the genus Ekebergia (Meliaceae): A review. Trends Phytochem. Res. 2021, 5, 110–125. [Google Scholar] [CrossRef]
- WFO Plant List. Myricaceae Rich. ex Kunth. Available online: https://wfoplantlist.org/plant-list (accessed on 5 July 2022).
- Bornstein, A.J. Myricaceae Blume, Wax-Myrtle Family. Available online: www.efloras.org (accessed on 5 July 2022).
- Gonzalez-Villarreal, L.M. La Familia Myricaceae en el Estado de Jalisco, México; University of Guadalajara: Jalisco, México, 2004; p. 17. [Google Scholar]
- Jørgesen, P.M.; León-Yáñez, S. Catalogue of the Vascular Plants of Ecuador. Available online: http://legacy.tropicos.org/ProjectAdvSearch.aspx?projectid=2 (accessed on 11 July 2022).
- Silva, B.J.C.; Seca, A.M.L.; Barreto, M.D.; Pinto, D.C.G.A. Recent Breakthroughs in the Antioxidant and Anti-Inflammatory Effects of Morella and Myrica Species. Int. J. Mol. Sci. 2015, 16, 17160–17180. [Google Scholar] [CrossRef] [PubMed]
- Tropicos.org. Missouri Botanical Garden. Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur. Available online: https://www.tropicos.org/name/50172723 (accessed on 15 February 2023).
- Torre, L.D.L.; Navarrete, H.; Muriel, M.P.; Macía Barco, M.J.; Balslev, H. Enciclopedia de las Plantas Útiles del Ecuador; Herbario QCA de la Escuela de Ciencias Biológicas de la Pontificia Universidad Católica del Ecuador: Quito, Ecuador; Herbario AAU del Departamento de Ciencias Biológicas de la Universidad de Aarhus: Aarhus, Denmark, 2008. [Google Scholar]
- Aguilar, Z.; Ulloa, C.; Hidalgo, P. Guía de Plantas Útiles de los Páramos de Zuleta, Ecuador; PPA-EcoCiencia: Quito, Ecuador, 2009; p. 101. [Google Scholar]
- Sandoval, J.S.; Quijano, C.E.; Morales, G.; Pino, J.A. Composition of the Essential Oil from the Leaves and Fruits of Morella pubescens (Humb.et Bonpl. ex Willd.) Wilbur Grown in Colombia. J. Essent. Oil Res. 2010, 22, 133–134. [Google Scholar] [CrossRef]
- St-Gelais, A.; Roger, B.; Alsarraf, J.; Legault, J.; Massé, D.; Pichette, A. Aromas from Quebec. VI. Morella pensylvanica from the Magdalen Islands: A (-)-α-bisabolol-rich oil featuring a new bisabolane ether. J. Essent. Oil Res. 2018, 30, 319–329. [Google Scholar] [CrossRef]
- Dolveni, M.F.; Bladimiro, S.; Vanessa, H.; Beltran, R.L.; Juan, C. Chemical Composition of the essential oil of Morella parvifolia (Benth.) Parra-O. from the Venezuelan Andes. Emir. J. Food Agric. 2016, 28, 288–290. [Google Scholar] [CrossRef]
- Arango, O.; Hurtado, A.; Castillo, P.; Santacruz, M. Study of extraction conditions of “laurel de cera” (Morella pubescens) essential oil by means of steam distillation. Biotecnol. Sect. Agropecu. Agroind. 2009, 7, 40–48. [Google Scholar]
- Molares, S.; González, S.B.; Ladio, A.; Agueda Castro, M. Etnobotánica, anatomía y caracterización físico-química del aceite esencial de Baccharis obovata Hook. et Arn. (Asteraceae: Astereae). Acta Bot. Bras. 2009, 23, 578–589. [Google Scholar] [CrossRef]
- Peña Vega, K.M. Efecto Repelente in vivo de los Aceites Esenciales de Myrica pubescens (Laurel), Piper aduncum (Matico) y Ruta graveolens (ruda) Frente al Estadío Adulto de Aedes aegypti. Bachelor’s Thesis, Universidad Nacional Pedro Ruiz Gallo, Lambayeque, Perú, 2022. [Google Scholar]
- Delgado Ospina, J.; Grande Tovar, C.D.; Menjívar Flores, J.C.; Sánchez Orozco, M.S. Relationship between refractive index and thymol concentration in essential oils of Lippia origanoides Kunth. Chil. J. Agric. Anim. Sci. 2016, 32, 127–133. [Google Scholar] [CrossRef]
- Quijano Celis, C.E.; Pino, J.A. Constituyentes volátiles de las hojas de Morella pubescens (Humb. et Bonpl. ex Willd.) Wilbur. Rev. Cub. Quim. 2007, 19, 54–57. [Google Scholar]
- Barba, C.; Santa-María, G.; Herraiz, M.; Martínez, R.M. Direct enantiomeric analysis of Mentha essential oils. Food Chem. 2013, 141, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Van Vuuren, S.; Holl, D. Antimicrobial natural product research: A review from a South African perspective for the years 2009–2016. J. Ethnopharmacol. 2017, 208, 236–252. [Google Scholar] [CrossRef]
- Setzer, W.N.; Schmidt, J.M.; Noletto, J.A.; Vogler, B. Leaf oil compositions and bioactivities of Abaco bush medicines. Pharmacologyonline 2006, 3, 794–802. [Google Scholar]
- Meniso, B.G.; Boru, A.D.; Ganjinaboyina, N.B. Phytochemical investigation and evaluation of antimicrobial activities of stem bark of Morella salicifolia. Bull. Chem. Soc. Ethiop. 2019, 33, 293–306. [Google Scholar] [CrossRef]
- Schmidt, E.; Bail, S.; Friedl, S.M.; Jirovetz, L.; Buchbauer, G.; Wanner, J.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Geissler, M. Antimicrobial activities of single aroma compounds. Nat. Prod. Commun. 2010, 5, 1365–1368. [Google Scholar] [CrossRef] [PubMed]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.K.; Ezzat, M.O.; Majid, A.S.A.; Majid, A.M.S.A. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Lis-Balcnin, M.; Ochocka, R.J.; Deans, S.G.; Asztemborska, M.; Hart, S. Differences in Bioactivity between the Enantiomers of α-Pinene. J. Essent. Oil Res. 1999, 11, 393–397. [Google Scholar] [CrossRef]
- Vuuren, S.F.v.; Viljoen, A.M. Antimicrobial activity of limonene enantiomers and 1,8-cineole alone and in combination. Flavour Fragr. J. 2007, 22, 540–544. [Google Scholar] [CrossRef]
- Anthony, K.P.; Deolu-Sobogun, S.A.; Saleh, M.A. Comprehensive Assessment of Antioxidant Activity of Essential Oils. J. Food Sci. 2012, 77, C839–C843. [Google Scholar] [CrossRef]
- Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities. Medicines 2017, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Karthikeyan, A.; Saravanan, R. Protective Effects of d-Limonene on Lipid Peroxidation and Antioxidant Enzymes in Streptozotocin-Induced Diabetic Rats. Basic Clin. Pharmacol. Toxicol. 2013, 112, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Valarezo, E.; Ludeña, J.; Echeverria-Coronel, E.; Cartuche, L.; Meneses, M.A.; Calva, J.; Morocho, V. Enantiomeric Composition, Antioxidant Capacity and Anticholinesterase Activity of Essential Oil from Leaves of Chirimoya (Annona cherimola Mill.). Plants 2022, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Valarezo, E.; Rivera, J.X.; Coronel, E.; Barzallo, M.A.; Calva, J.; Cartuche, L.; Meneses, M.A. Study of Volatile Secondary Metabolites Present in Piper carpunya Leaves and in the Traditional Ecuadorian Beverage Guaviduca. Plants 2021, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; Calva, J.; Cartuche, L.; Valarezo, E.; Armijos, C. Chemical Composition, Enantiomeric Distribution and Anticholinesterase and Antioxidant Activity of the Essential Oil of Diplosthephium juniperinum. Plants 2022, 11, 1188. [Google Scholar] [CrossRef]
- Benny, A.; Thomas, J. Essential Oils as Treatment Strategy for Alzheimer’s Disease: Current and Future Perspectives. Planta Med. 2019, 85, 239–248. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas—Liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST. NIST Chemistry WebBook, SRD 69. Base de Datos de Referencia Estándar del NIST Número 69. Available online: http://webbook.nist.gov (accessed on 19 May 2022).
- Morocho, V.; Hidalgo-Tapia, M.; Delgado-Loyola, I.; Cartuche, L.; Cumbicus, N.; Valarezo, E. Chemical Composition and Biological Activity of Essential Oil from Leaves and Fruits of Limoncillo (Siparuna muricata (Ruiz & Pav.) A. DC.). Antibiotics 2023, 12, 82. [Google Scholar] [CrossRef]
- Valarezo, E.; Benítez, L.; Palacio, C.; Aguilar, S.; Armijos, C.; Calva, J.; Ramírez, J. Volatile and non-volatile metabolite study of endemic ecuadorian specie Piper lanceifolium Kunth. J. Essent. Oil Res. 2021, 33, 182–188. [Google Scholar] [CrossRef]
| Morella pubescens EO | ||
|---|---|---|
| Mean | SD | |
| Density, ρ (g/cm3) | 0.8978 | 0.0039 |
| Refractive index, n20 | 1.4976 | 0.0006 |
| Specific rotation, [α] (°) | +1.04 | 0.05 |
| Subjective color | Yellow | |
| RGB color values | R:255, G:255, B:141 | |
| CMYK color values | C:0, M:0, Y:0.45, K:0 | |
| CN | RT | Compound | RIC | RIR | % a | SD | Type | CF | MM (Da) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 7.91 | α-Thujene | 924 | 924 | 0.1 | 0.0 | MH | C10H16 | 136.13 |
| 2 | 8.18 | α-Pinene | 933 | 932 | 4.0 | 0.2 | MH | C10H16 | 136.13 |
| 3 | 8.83 | Camphene | 946 | 946 | 0.1 | 0.0 | MH | C10H16 | 136.13 |
| 4 | 9.80 | Sabinene | 969 | 969 | 0.1 | 0.0 | MH | C10H16 | 136.13 |
| 5 | 9.98 | β-Pinene | 974 | 974 | 0.3 | 0.0 | MH | C10H16 | 136.13 |
| 6 | 10.56 | Myrcene | 987 | 988 | 0.4 | 0.0 | MH | C10H16 | 136.13 |
| 7 | 11.23 | n-Octanal | 1000 | 998 | 0.1 | 0.0 | OC | C8H16O | 128.12 |
| 8 | 11.73 | α-Terpinene | 1015 | 1014 | 0.1 | 0.0 | MH | C10H16 | 136.13 |
| 9 | 12.09 | ρ-Cymene | 1022 | 1020 | 1.2 | 0.1 | MH | C10H14 | 134.11 |
| 10 | 12.30 | Limonene | 1026 | 1024 | 11.8 | 0.6 | MH | C10H16 | 136.13 |
| 11 | 12.47 | 1,8-Cineole | 1029 | 1026 | 0.3 | 0.0 | OM | C10H18O | 154.14 |
| 12 | 13.63 | γ-Terpinene | 1056 | 1054 | 1.5 | 0.1 | MH | C10H16 | 136.13 |
| 13 | 14.89 | Terpinolene | 1083 | 1086 | 0.1 | 0.0 | MH | C10H16 | 136.13 |
| 14 | 15.72 | Linalool | 1099 | 1095 | 0.1 | 0.0 | OM | C10H18O | 154.14 |
| 15 | 20.68 | n-Decanal | 1205 | 1201 | tr | - | OM | C10H20O | 156.15 |
| 16 | 20.93 | Octanol acetate | 1212 | 1211 | tr | - | OM | C10H20O2 | 172.15 |
| 17 | 22.14 | Neral | 1239 | 1235 | 0.1 | 0.0 | OM | C10H16O2 | 152.12 |
| 18 | 23.50 | Geranial | 1268 | 1264 | 0.3 | 0.0 | OM | C10H16O2 | 152.12 |
| 19 | 24.24 | (E)-Anethole | 1285 | 1282 | 0.4 | 0.0 | OM | C10H12O | 137.00 |
| 20 | 27.03 | α-Cubebene | 1344 | 1348 | tr | - | SH | C15H24 | 204.19 |
| 21 | 27.22 | α-Terpinyl acetate | 1348 | 1346 | tr | - | OC | C12H20O2 | 196.15 |
| 22 | 28.34 | α-Copaene | 1372 | 1374 | 0.1 | 0.0 | SH | C15H24 | 204.19 |
| 23 | 28.95 | (Z)-β-Damascone | 1385 | 1386 | 0.1 | 0.0 | OC | C13H20O | 208.15 |
| 24 | 29.09 | β-Elemene | 1388 | 1389 | 1.3 | 0.0 | SH | C15H24 | 204.19 |
| 25 | 29.19 | Sativene | 1390 | 1390 | 0.2 | 0.0 | SH | C15H24 | 204.19 |
| 26 | 29.66 | β-Longipinene | 1400 | 1400 | 0.2 | 0.0 | SH | C15H24 | 204.19 |
| 27 | 30.40 | (E)-Caryophyllene | 1416 | 1417 | 27.5 | 1.3 | SH | C15H24 | 204.19 |
| 28 | 30.87 | (E)-α-Ionone | 1426 | 1428 | 4.2 | 0.2 | OC | C13H20O | 208.15 |
| 29 | 31.43 | 6,9-Guaiadiene | 1438 | 1442 | 0.1 | 0.0 | SH | C15H24 | 204.19 |
| 30 | 32.00 | α-Humulene | 1450 | 1452 | 0.7 | 0.0 | SH | C15H24 | 204.19 |
| 31 | 32.23 | allo-Aromadendrene | 1455 | 1458 | 0.1 | 0.0 | SH | C15H24 | 204.19 |
| 32 | 32.70 | 4,5-di-epi-Aristolochene | 1465 | 1471 | 0.2 | 0.0 | SH | C15H24 | 204.19 |
| 33 | 32.88 | β-Chamigrene | 1469 | 1476 | 1.1 | 0.0 | SH | C15H24 | 204.19 |
| 34 | 32.98 | trans-Cadina-1(6),4-diene | 1471 | 1475 | 0.3 | 0.0 | SH | C15H24 | 204.19 |
| 35 | 33.35 | ar-Curcumene | 1479 | 1479 | 0.2 | 0.0 | SH | C15H24 | 202.17 |
| 36 | 33.45 | γ-Himachalene | 1481 | 1481 | 0.2 | 0.0 | SH | C15H24 | 204.19 |
| 37 | 33.59 | β-Selinene | 1484 | 1489 | 8.0 | 0.2 | SH | C15H24 | 204.19 |
| 38 | 33.91 | δ-Selinene | 1491 | 1492 | 9.1 | 0.2 | SH | C15H24 | 204.19 |
| 39 | 34.10 | α-Muurolene | 1495 | 1500 | 0.2 | 0.0 | SH | C15H24 | 204.19 |
| 40 | 34.38 | γ-Patchoulene | 1501 | 1502 | 0.1 | 0.0 | SH | C15H24 | 204.19 |
| 41 | 34.57 | β-Bisabolene | 1505 | 1505 | 0.2 | 0.0 | SH | C15H24 | 204.19 |
| 42 | 34.76 | γ-Cadinene | 1509 | 1513 | 0.3 | 0.0 | SH | C15H24 | 204.19 |
| 43 | 34.99 | δ-Cadinene | 1514 | 1520 | 2.9 | 0.1 | SH | C15H24 | 204.19 |
| 44 | 35.65 | Lilial | 1528 | 1527 | 0.8 | 0.0 | OC | C14H20O | 204.15 |
| 45 | 35.83 | Zonarene | 1532 | 1528 | 4.7 | 0.2 | SH | C15H24 | 204.19 |
| 46 | 36.07 | Selina-3,7(11)-diene | 1537 | 1545 | 5.3 | 0.2 | SH | C15H24 | 204.19 |
| 47 | 36.35 | Occidentalol | 1543 | 1550 | 0.0 | 0.0 | OS | C15H24O | 220.18 |
| 48 | 36.82 | Germacrene B | 1553 | 1559 | 5.0 | 0.5 | SH | C15H24 | 204.19 |
| 49 | 37.00 | epi-Longipinanol | 1557 | 1562 | 0.3 | 0.0 | OS | C15H26O | 222.20 |
| 50 | 37.94 | Caryophyllene oxide | 1577 | 1582 | 1.8 | 0.1 | OS | C15H24O | 220.18 |
| 51 | 40.19 | 2-epi-α-Cedren-3-one | 1625 | 1626 | 0.3 | 0.0 | OS | C15H22O | 218.17 |
| 52 | 40.79 | epi-α-Cadinol | 1638 | 1638 | 0.2 | 0.0 | OS | C15H26O | 222.20 |
| 53 | 41.03 | Selina-3,11-dien-6-α-ol | 1643 | 1642 | 0.3 | 0.0 | OS | C15H24O | 220.18 |
| 54 | 41.54 | α-Cadinol | 1654 | 1652 | 0.7 | 0.0 | OS | C15H26O | 222.20 |
| 55 | 42.01 | Intermedeol | 1664 | 1665 | 0.2 | 0.0 | OS | C15H26O | 222.20 |
| 56 | 43.37 | Eudesm-7(11)-en-4-ol | 1693 | 1700 | 0.4 | 0.0 | OS | C15H26O | 222.20 |
| 57 | 43.98 | (E)-Apritone | 1706 | 1708 | 0.1 | 0.0 | OS | C15H24O | 220.18 |
| 58 | 44.12 | 14-hydroxy-α-Humulene | 1709 | 1713 | tr | - | OS | C15H24O | 220.18 |
| MH | 19.7 | ||||||||
| OM | 1.2 | ||||||||
| SH | 67.8 | ||||||||
| OS | 4.1 | ||||||||
| OC | 5.2 | ||||||||
| Total identified | 97.9 | ||||||||
| RT | Enantiomers | RI | ED (%) | e.e. (%) |
|---|---|---|---|---|
| 4.81 | (+)-α-Pinene (1R,5R) | 917 | 2.6 | 94.8 |
| 5.01 | (−)-α-Pinene (1R,5R) | 923 | 97.4 | |
| 9.56 | (−)-Limonene (4S) | 1037 | 4.3 | 91.3 |
| 9.71 | (+)-Limonene (4R) | 1040 | 95.7 | |
| 39.07 | (+)-δ-cadinene (1S,8aR) | 1539 | 17.6 | 64.8 |
| 39.17 | (−)-δ-cadinene (1R,8aS) | 1541 | 82.4 |
| Microorganism | Essential oil | Positive Control | Negative Control |
|---|---|---|---|
| MIC (µg/mL) | |||
| Gram-Positive Cocci | |||
| Enterococcus faecalis (ATCC 19433) | >4000 | 0.78 | + |
| Enterococcus faecium (ATCC 27270) | 250 | 0.39 | + |
| Staphylococcus aureus (ATCC 25923) | 2000 | 0.39 | + |
| Gram-positive bacilli | |||
| Listeria monocytogenes ATCC 19115 | 4000 | 1.56 | + |
| Gram-negative bacilli | |||
| Escherichia coli O157:H7 (ATCC 43888) | >4000 | 1.56 | + |
| Pseudomonas aeruginosa (ATCC 10145) | >4000 | 0.39 | + |
| Salmonella enterica subs enterica serovar Thypimurium WDCM 00031, derived (ATCC 14028) | >4000 | 0.39 | + |
| Sample | DPPH | ABTS |
|---|---|---|
| SC50 (µg/mL) ± SD | ||
| Morella pubescens essential oil | 237.1 ± 1.8 | 46.4 ± 1.0 |
| Trolox | 30.0 ± 1.1 | 23.3 ± 1.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valarezo, E.; Correa-Jaramillo, C.; Astudillo-Dávila, P.; Garzón-Yaguache, J.; Cartuche, L.; Meneses, M.A.; Morocho, V. Chemical Composition, Enantiomeric Distribution and Biological Activity of Essential Oil from Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur. Molecules 2023, 28, 2910. https://doi.org/10.3390/molecules28072910
Valarezo E, Correa-Jaramillo C, Astudillo-Dávila P, Garzón-Yaguache J, Cartuche L, Meneses MA, Morocho V. Chemical Composition, Enantiomeric Distribution and Biological Activity of Essential Oil from Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur. Molecules. 2023; 28(7):2910. https://doi.org/10.3390/molecules28072910
Chicago/Turabian StyleValarezo, Eduardo, Carlos Correa-Jaramillo, Paola Astudillo-Dávila, Julio Garzón-Yaguache, Luis Cartuche, Miguel Angel Meneses, and Vladimir Morocho. 2023. "Chemical Composition, Enantiomeric Distribution and Biological Activity of Essential Oil from Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur" Molecules 28, no. 7: 2910. https://doi.org/10.3390/molecules28072910
APA StyleValarezo, E., Correa-Jaramillo, C., Astudillo-Dávila, P., Garzón-Yaguache, J., Cartuche, L., Meneses, M. A., & Morocho, V. (2023). Chemical Composition, Enantiomeric Distribution and Biological Activity of Essential Oil from Morella pubescens (Humb. & Bonpl. ex Willd.) Wilbur. Molecules, 28(7), 2910. https://doi.org/10.3390/molecules28072910










